Hierarchical Y Zeolite-Based Catalysts for VGO Cracking: Impact of Carbonaceous Species on Catalyst Acidity and Specific Surface Area
Abstract
:1. Introduction
2. Results and Discussion
2.1. Zeolites and Catalyst Properties
2.2. Catalytic Performance
Conversion and Product Distribution
3. Materials and Methods
3.1. Alkaline Treatment and Hydrothermal Stabilization of Y Zeolites
3.2. Catalyst Synthesis
3.3. Physicochemical Characterization of Zeolites and Catalysts
3.4. VGO Characterization
SARA VGO Fractioning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oloruntoba, A.; Zhang, Y.; Hsu, C.S. State of-the-art review of fluid catalytic cracking (FCC) catalyst regeneration intensification technologies. Energies 2022, 15, 2061. [Google Scholar] [CrossRef]
- Ibarra, A.; Hita, I.; Arandes, J.M.; Bilbao, J. A Hybrid FCC/HZSM-5 Catalyst for the Catalytic Cracking of a VGO/Bio-Oil Blend in FCC Conditions. Catalysts 2020, 10, 1157. [Google Scholar] [CrossRef]
- Fernández, M.L.; Lacalle, A.; Bilbao, J.; Arandes, J.M.; de la Puente, G.; Sedran, U. Recycling hydrocarbon cuts into FCC units. Energy Fuels 2002, 16, 615–621. [Google Scholar] [CrossRef]
- Mizuno, T.; Yamazaki, H.; Takamiya, Y.; Hasegawa, H.; Tanaka, C.; Mitsui, T. Effects of the FCC catalyst binder type on propylene production during catalytic cracking of VGO. Appl. Catal. A Gen. 2023, 661, 119214. [Google Scholar] [CrossRef]
- Suganuma, S.; Katada, N. Innovation of catalytic technology for upgrading of crude oil in petroleum refinery. Fuel Process. Technol. 2020, 208, 106518. [Google Scholar] [CrossRef]
- Tanimu, A.; Tanimu, G.; Alasiri, H.; Aitani, A. Catalytic cracking of crude oil: Mini review of catalyst formulations of enhanced selectivity to light olefins. Energy Fuels 2022, 36, 5152–5166. [Google Scholar] [CrossRef]
- Fals, J.; García, J.R.; Falco, M.; Sedran, U. Performance of equilibrium FCC catalysts in the conversion of the SARA fractions in VGO. Energy Fuels 2020, 34, 16512–16521. [Google Scholar] [CrossRef]
- Fals, J.; García, J.R.; Falco, M.; Sedran, U. Coke from SARA fractions in VGO. Impact on Y zeolite acidity and physical properties. Fuel 2018, 225, 26–34. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, S.; Bari Siddiqui, M.A.; Al-Shammari, A. The Investigation of Zeolite to Matrix Ratio Effect on the Performance of FCC Catalysts during Catalytic Cracking of Hydrotreated VGO. Catalysts 2023, 13, 1255. [Google Scholar] [CrossRef]
- Fals, J.; Toloza, C.A.T.; Puello-Polo, E.; Márquez, E.; Méndez, F.J. A comprehensive study of product distributions and coke deposition during catalytic cracking of vacuum gas oil over hierarchical zeolites. Heliyon 2023, 9, 15408. [Google Scholar] [CrossRef] [PubMed]
- García, J.R.; Falco, M.; Sedran, U. Impact of the Desilication Treatment of Y Zeolite on the Catalytic Cracking of Bulky Hydrocarbon Molecules. Top. Catal. 2016, 59, 268–277. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Cho, K.-S.; Lee, Y.-K. Structure and activity of Ni2P/desilicated zeolite β catalysts for hydrocracking of pyrolysis fuel oil into benzene, toluene, and xylene. Catalysts 2020, 10, 47. [Google Scholar] [CrossRef]
- Su, L.; Liu, L.; Zhuang, J.; Wang, H.; Li, Y.; Shen, W.; Xu, Y.; Bao, X. Creating mesopores in ZSM-5 zeolite by alkali treatment: A new way to enhance the catalytic performance of methane dehydroaromatization on Mo/HZSM-5 catalysts. Catal. Lett. 2003, 91, 155–167. [Google Scholar] [CrossRef]
- Groen, J.; Zhu, W.; Brouwer, S.; Huynink, S.; Kapteijn, R.; Moulijn, J.; Pe’rez-Ramı´rez, J. Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication. J. Am. Chem. Soc. 2007, 129, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Sadrameli, S.M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Catalytic cracking review. Fuel 2016, 173, 285–297. [Google Scholar] [CrossRef]
- Bleken, F.L.; Barbera, K.; Bonino, F.; Olsbye, U.; Lillerud, K.P.; Bordiga, S.; Beato, P.; Janssens, T.V.; Svelle, S. Catalyst deactivation by coke formation in microporous and desilicated zeolite H-ZSM-5 during the conversion of methanol to hydrocarbons. J. Catal. 2013, 307, 62–73. [Google Scholar] [CrossRef]
- Yamazaki, H.; Hasegawa, H.; Tanaka, C.; Takamiya, Y.; Mitsui, T.; Mizuno, T. Al ion-exchanged USY in FCC catalyst for high LPG yield. Catal. Comm. 2021, 159, 106354. [Google Scholar] [CrossRef]
- Passamonti, F.J.; de la Puente, G.; Sedran, U. Comparison between MAT flow fixed bed and batch fluidized bed reactors in the evaluation of FCC catalysts. 1. conversion and yields of the main hydrocarbon groups. Energy Fuels 2009, 23, 1358–1363. [Google Scholar] [CrossRef]
- Passamonti, F.J.; de la Puente, G.; Gilbert, W.R.; Sedran, U. Comparison between fixed fluidized bed (FFB) and batch fluidized bed reactors in the evaluation of FCC catalysts. Chem. Eng. J. 2012, 183, 433–447. [Google Scholar] [CrossRef]
- Hussain, A.I.; Aitani, A.M.; Kubů, M.; Čejka, J.; Al-Khattaf, S. Catalytic cracking of Arabian Light VGO over novel zeolites as FCC catalyst additives for maximizing propylene yield. Fuel 2016, 167, 226–239. [Google Scholar] [CrossRef]
- Ibarra, Á.; Hita, I.; Azkoiti, M.J.; Arandes, J.M.; Bilbao, J. Catalytic cracking of raw bio-oil under FCC unit conditions over different zeolite-based catalysts. J. Ind. Eng. Chem. 2019, 78, 372–382. [Google Scholar] [CrossRef]
- García, J.R.; Falco, M.; Sedran, U. Intracrystalline mesoporosity over Y zeolites: Processing of VGO and resid-VGO mixtures in FCC. Catal Today 2017, 296, 247–253. [Google Scholar] [CrossRef]
- Gackowski, M.; Tarach, K.; Podobiński, J.; Jarczewski, S.; Kuśtrowski, P.; Datka, J. Hierarchical zeolites Y obtained by desilication: Porosity, acidity and catalytic properties. Microporous Mesoporous Mater. 2018, 263, 282–288. [Google Scholar] [CrossRef]
- Shen, B.; Qin, Z.; Gao, X.; Lin, F.; Zhou, S.; Shen, W.; Wang, B.; Zhao, H.; Liu, H. Desilication by Alkaline Treatment and Increasing the Silica to Alumina Ratio of Zeolite Y. Chin. J. Catal. 2012, 33, 152–163. [Google Scholar] [CrossRef]
- Li, W.; Zheng, J.; Luo, Y.; Da, Z. Effect of hierarchical porosity and phosphorus modification on the catalytic properties of zeolite Y. Appl. Surf. Sci. 2016, 382, 302–308. [Google Scholar] [CrossRef]
- Sadowska, K.; Wach, A.; Olejniczak, Z.; Kuśtrowski, P.; Datka, J. Hierarchic zeolites: Zeolite ZSM-5 desilicated with NaOH and NaOH/tetrabutylamine hydroxide. Microporous Mesoporous Mater. 2013, 167, 82–88. [Google Scholar] [CrossRef]
- Cruz, P.L.; Montero, E.; Datka, J.D. Modelling of co-processing of HDO-oil with VGO in a FCC unit. Fuel 2017, 196, 362–370. [Google Scholar] [CrossRef]
- Gong, J.-H.; Long, J.; Xu, Y.-H. Protolytic cracking in Daqing VGO catalytic cracking process. J. Fuel Chem. Technol. 2008, 36, 691–695. [Google Scholar] [CrossRef]
- Anderson, P.C.; Sharkey, J.M.; Walsh, R.P. Calculation of the research octane number of motor gasolines from gas chromatographic data and a Nerw approach to motor gasoline quality control. J. Inst. Pet 1972, 58, 83–94. [Google Scholar]
- Prakash, A.; Wang, C.; Janssen, A.; Aradi, A.; Cracknell, R. Impact of Fuel Sensitivity (RON-MON) on Engine Efficiency. J. Fuels Lubr. 2017, 10, 362–370. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Quitian, A.; Fernandez, Y.; Ancheyta, J. Viscosity reduction of heavy oil during slurryphase hydrocracking. Chem. Eng. Technol. 2019, 42, 148–155. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Kardasheva, Y.; Vinnikova, M.; Kulikov, L. Hydrotreating of light cycle oil over supported on porous aromatic framework catalysts. Catalysts 2018, 8, 397. [Google Scholar] [CrossRef]
- García, J.R.; Bertero, M.; Falco, M.; Sedran, U. Catalytic cracking of bio-oils improved by the formation of mesopores by means of Y zeolite desilication. Appl. Catal. A Gen. 2015, 503, 1–8. [Google Scholar] [CrossRef]
- Ochoa, A.; Valle, B.; Resasco, D.E.; Bilbao, J.; Gayubo, A.G.; CastaÑo, P. Temperature programmed oxidation coupled with in situ techniques reveal the nature and location of coke deposited on a Ni/La2O3-αAl2O3 catalyst in the steam reforming of bio-oil. ChemCatChem 2018, 10, 2311–2321. [Google Scholar] [CrossRef]
- Cumming, K.A.; Wojciechowski, B.W. Hydrogen Transfer, Coke Formation, and Catalyst Decay and Their Role in the Chain Mechanism of Catalytic Cracking. Catalysts 2018, 8, 397–411. [Google Scholar] [CrossRef]
- Al-Khattaf, S.; de Lasa, H. The role of diffusion in alkyl-benzenes catalytic cracking. Appl. Catal. A 2002, 226, 139–153. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, L.; Zhang, Z.; Zhang, H.; Li, Y. Coke Formation over Zeolite Catalysts in Light Alkanes Aromatization and Anti-Carbon-Deposition Strategies and Perspectives: A Review. Energy Fuels 2023, 37, 1657–1677. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Ateka, A.; Pérez-Uriarte, P.; Castaño, P.; Aguayo, A.T.; Bilbao, J. Insight into the deactivation and regeneration of HZSM-5 zeolite catalysts in the conversion of dimethyl ether to olefins. Ind. Eng. Chem. Res. 2018, 57, 13689–13702. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Corma, A.; Melo, F.V.; Sauvanaud, L. Attempts to improve the product slate quality: Influence of coke-on-catalyst content. Ind. Eng. Chem. Res. 2007, 46, 4100–4109. [Google Scholar] [CrossRef]
- Epelde, E.; Gayubo, A.G.; Olazar, M.; Bilbao, J.; Aguayo, A.T. Modified HZSM-5 zeolites for intensifying propylene production in the transformation of 1-butene. Chem. Eng. J. 2014, 251, 80–91. [Google Scholar] [CrossRef]
- Bauer, F.; Karge, H.G. Characterization of coke on zeolites. Mol. Sieves 2007, 5, 249–364. [Google Scholar] [CrossRef]
- Froment, G.F.; De Meyer, J.; Derouane, E.G. Deactivation of zeolite catalysts by coke formation. J. Catal. 1990, 124, 391–400. [Google Scholar] [CrossRef]
- Muhammad, I.; Makwashi, N.; Ahmed, T.G.; Manos, G.; Zhao, D. A Mechanistic Model on Catalyst Deactivation by Coke Formation in a CSTR Reactor. J. Catal. 2023, 11, 944. [Google Scholar] [CrossRef]
- Liu, B.; Slocombe, D.; AlKinany, M.; AlMegren, H.; Wang, J.; Arden, J.; Vai, A.; Gonzalez-Cortes, S.; Xiao, T.; Kuznetsov, V.; et al. Advances in the study of coke formation over zeolite catalysts in the methanol-to-hydrocarbon process. Appl. Petrochem. Res. 2016, 6, 209–215. [Google Scholar] [CrossRef]
- D3906-13; Standard Test Method for Determination of Relative X-ray Diffraction Intensities of Faujasite-Type Zeolite-Containing Materials. ASTM International: West Conshohocken, PA, USA, 2013.
- D3942-19; Standard Test Method for Determination of the Unit Cell Dimension of a Faujasite-Type Zeolite. ASTM International: West Conshohocken, PA, USA, 2019.
- Ibarra, A.; Veloso, A.; Bilbao, J.; Arandes, J.; Castaño, P. Dual coke deactivation pathways during the catalytic cracking of raw bio-oil and vacuum gasoil in FCC conditions. Appl. Catal. B 2016, 182, 336–346. [Google Scholar] [CrossRef]
- D287-12b; Standard Test Method for API Gravity of Crude Petroleum and Petroleum Products (Hydrometer Method). ASTM International: West Conshohocken, PA, USA, 2019.
- D4530-15; Standard Test Method for Determination of Carbon Residue (Micro Method). ASTM International: West Conshohocken, PA, USA, 2015.
- D1160-18; Standard Test Method for Distillation of Petroleum Products at Reduced Pressure. ASTM International: West Conshohocken, PA, USA, 2018.
- D2007-11; Standard Test Method for Characteristic Groups in Rubber Extender and Processing Oils and Other Petroleum-Derived Oils by the Clay-Gel Absorption Chromatographic Method. ASTM International: West Conshohocken, PA, USA, 2011.

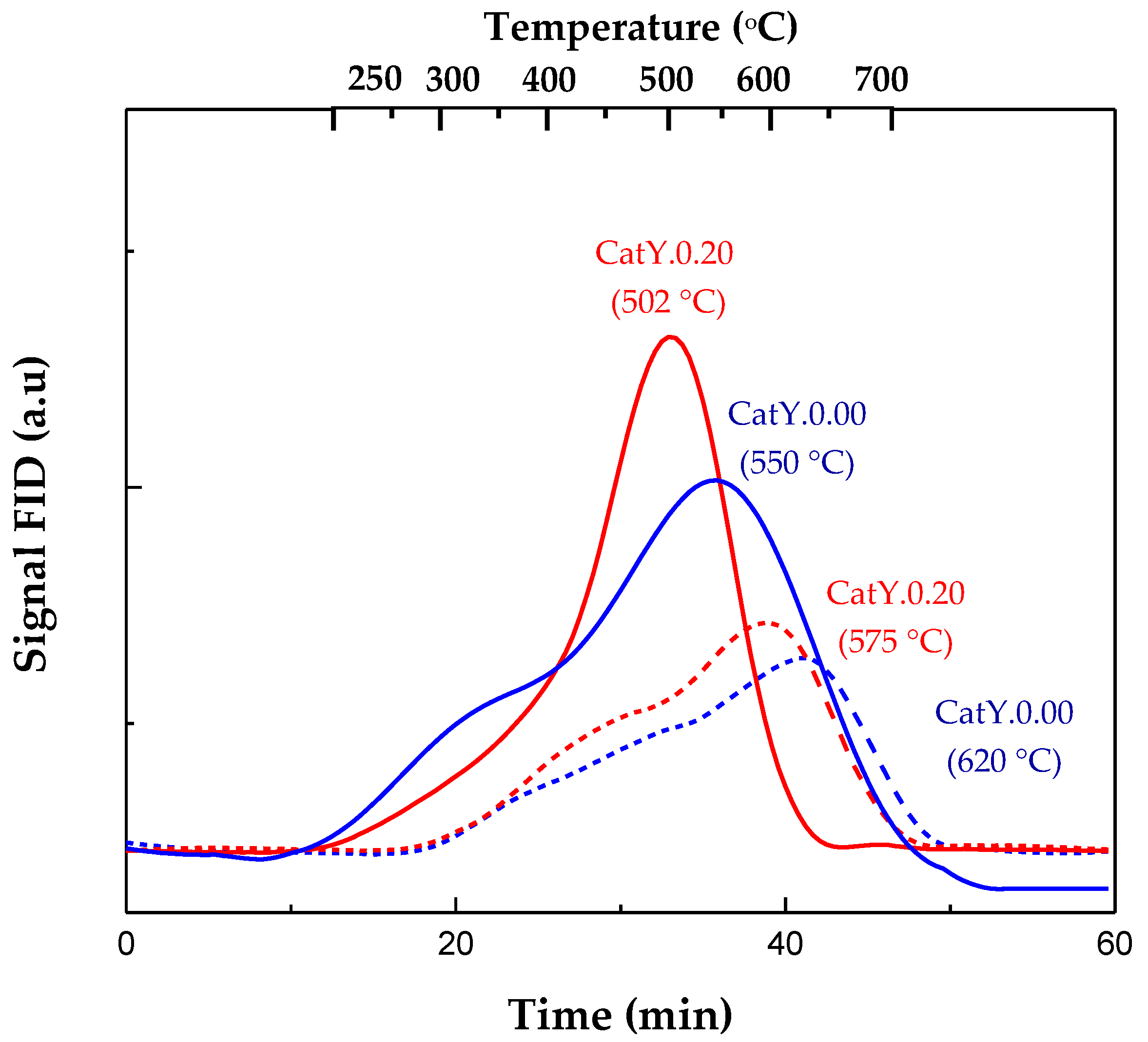


| Y-0.00-M | Y-0.20-M | CatY.0.00 | CatY.0.20 | |
|---|---|---|---|---|
| BET specific surface area, SBET (m2/g) | 693 | 436 | 362 | 355 |
| Mesopore specific surface area, Smeso (m2/g) | 124 | 210 | 311 | 332 |
| Total pore volume, VTP (cm3/g) | 0.582 | 0.771 | 0.602 | 0.699 |
| Micropore volume, Vmicro (cm3/g) | 0.283 | 0.094 | 0.051 | 0.003 |
| Mesopore volume, Vmeso (cm3/g) | 0.299 | 0.677 | 0.551 | 0.669 |
| Average mesopore diameter, (Å) | 64.2 | 84.3 | 66.7 | 99.7 |
| Crystallinity (%) | 93 | 29 | 13 | 5 |
| Unit cell size (Å) | 24.25 | 24.21 | 24.22 | 24.19 |
| Desorption Temperature (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 | 300 | 450 | |||||||
| B | L | B/L | B | L | B/L | B | L | B/L | |
| Y-0.00-M | 91 | 107 | 0.85 | 58 | 71 | 0.82 | 28 | 30 | 0.93 |
| Y-0.20-M | 93 | 135 | 0.70 | 61 | 84 | 0.73 | 29 | 48 | 0.61 |
| CatY.0.00 | 26 | 35 | 0.81 | 9 | 13 | 0.80 | 16 | 18 | 0.81 |
| CatY.0.20 | 35 | 45 | 0.79 | 20 | 27 | 0.79 | 13 | 15 | 0.77 |
| Conversion (wt%) | Higher | Lower | ||
|---|---|---|---|---|
| 83.4 | 69.2 | 60.3 | 50.2 | |
| Selectivities (wt%) | CatY.0.20 | CatY.0.00 | CatY.0.20 | CatY.0.00 |
| SDG | 21.5 | 28.5 | 13.8 | 20.5 |
| SLPG | 26.1 | 33.8 | 16.4 | 26.5 |
| SGASOLINE | 42.9 | 28.5 | 50.9 | 34.7 |
| Gasoline composition (%) | ||||
| Paraffins | 7.5 | 21.4 | 15.8 | 27.7 |
| Olefins | 30.4 | 32.1 | 22.5 | 25.1 |
| Naphthenes | 15.8 | 23.6 | 27.0 | 26.8 |
| Aromatics | 46.3 | 22.9 | 34.7 | 20.4 |
| RON | 88.2 | 79.8 | 86.4 | 81.1 |
| SCOKE (wt%) | 9.5 | 9.2 | 18.9 | 18.3 |
| °API | 19.7 |
|---|---|
| Aniline point (°C) | 78.5 |
| CCR (wt%) | 0.43 |
| Refractive index | 1.49 |
| Distillation curve (°C) | |
| Initial | 272 |
| 10 vol.% | 387 |
| 30 vol.% | 420 |
| 50 vol.% | 450 |
| 70 vol.% | 487 |
| 95 vol.% | 534 |
| Final | 582 |
| SARA fractions (wt%) | |
| Saturated | 47.4 |
| Aromatic | 50.0 |
| Resin | 2.10 |
| Asphaltene | 0.50 |
| Nickel (ppm) | 0.48 |
| Vanadium (ppm) | 0.97 |
| Sodium (ppm) | 0.83 |
| Iron (ppm) | 0.24 |
| Sulfur (wt%) | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fals, J.; Garcia-Valencia, J.F.; Puello-Polo, E.; Tuler, F.; Márquez, E. Hierarchical Y Zeolite-Based Catalysts for VGO Cracking: Impact of Carbonaceous Species on Catalyst Acidity and Specific Surface Area. Molecules 2024, 29, 3085. https://doi.org/10.3390/molecules29133085
Fals J, Garcia-Valencia JF, Puello-Polo E, Tuler F, Márquez E. Hierarchical Y Zeolite-Based Catalysts for VGO Cracking: Impact of Carbonaceous Species on Catalyst Acidity and Specific Surface Area. Molecules. 2024; 29(13):3085. https://doi.org/10.3390/molecules29133085
Chicago/Turabian StyleFals, Jayson, Juan Francisco Garcia-Valencia, Esneyder Puello-Polo, Fernando Tuler, and Edgar Márquez. 2024. "Hierarchical Y Zeolite-Based Catalysts for VGO Cracking: Impact of Carbonaceous Species on Catalyst Acidity and Specific Surface Area" Molecules 29, no. 13: 3085. https://doi.org/10.3390/molecules29133085
APA StyleFals, J., Garcia-Valencia, J. F., Puello-Polo, E., Tuler, F., & Márquez, E. (2024). Hierarchical Y Zeolite-Based Catalysts for VGO Cracking: Impact of Carbonaceous Species on Catalyst Acidity and Specific Surface Area. Molecules, 29(13), 3085. https://doi.org/10.3390/molecules29133085







