Protective Effect of Polyphenolic Extracts from Hippophae rhamnoides L. and Reynoutria japonica Houtt. on Erythrocyte Membrane
Abstract
1. Introduction
2. Results
2.1. Analysis of Extracts’ Total Phenolic Content (TPC) and Polyphenolic Composition
2.2. Determination of the Anti-Radical Activity of the Extracts
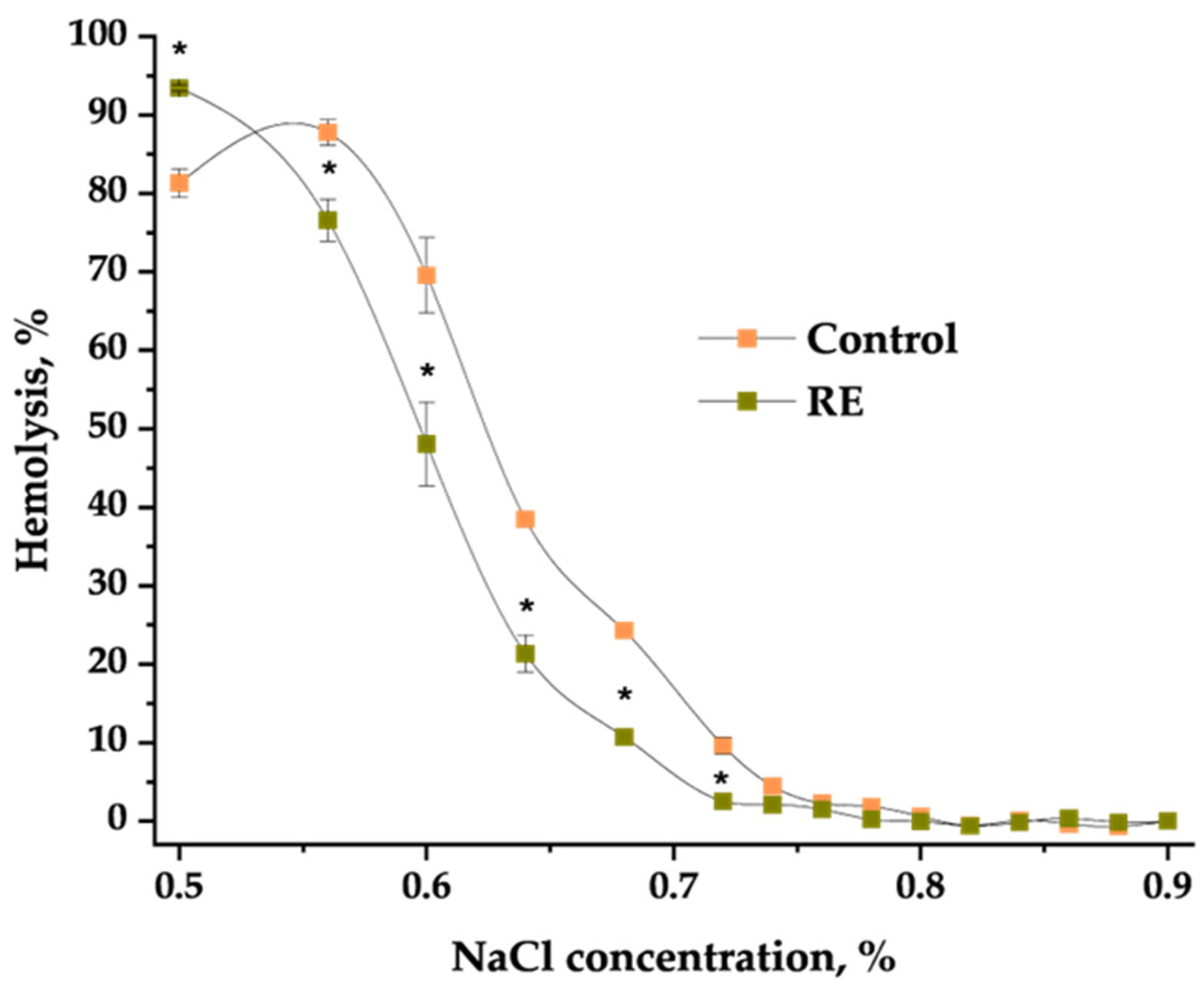
2.3. Hemolytic Activity of Extracts and Their Impact on Osmotic Fragility of Erythrocytes
2.4. Antioxidant Properties of Extracts against Erythrocytes
2.5. Microscopic Observations
2.6. Impact of Extracts on the Fluidity of the Erythrocyte Membrane
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Plant Material
4.3. Analysis of Extracts by UPLC-PDA-ESI-MS/MS
4.4. Determination of Total Phenolic Content (TPC) in Extracts
4.5. Investigation of the Anti-Radical Activity of Extracts
4.6. Preparation of Erythrocytes
4.7. Preparation of Erythrocyte Membranes (Ghosts)
4.8. Hemolytic Activity of Extracts and Their Impact on the Erythrocytes’ Osmotic Resistance
4.9. Antioxidant Activity of Extracts
4.9.1. Protection of Erythrocytes against AAPH
4.9.2. Protection of Erythrocyte Membranes against AAPH
4.10. Microscopic Preparations
4.11. Changes in the Fluidity of the Membrane
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Pogačnik, L.; Bergant, T.; Skrt, M.; Ulrih, N.P.; Viktorová, J.; Ruml, T. In Vitro Comparison of the Bioactivities of Japanese and Bohemian Knotweed Ethanol Extracts. Foods 2020, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Patočka, J.; Navrátilová, Z.; Ovando, M. Biologically Active Compounds of Knotweed (Reynoutria spp.). Mil. Med. Sci. Lett. 2017, 86, 17–31. [Google Scholar] [CrossRef]
- Zeb, A. Important Therapeutic Uses of Sea Buckthorn (Hippophae): A Review. J. Biol. Sci. 2004, 4, 687–693. [Google Scholar] [CrossRef]
- Rousi, A. The Genus Hippophaë L. A Taxonomic Study. Ann. Bot. Fenn. 1971, 8, 177–227. [Google Scholar]
- Yang, B.; Kallio, H. Composition and Physiological Effects of Sea Buckthorn (Hippophaë) lipids. Trends Food Sci. Technol. 2002, 13, 160–167. [Google Scholar] [CrossRef]
- Yang, B.; Kallio, H.P. Fatty Acid Composition of Lipids in Sea Buckthorn (Hippophaë rhamnoides L.) Berries of Different Origins. J. Agric. Food Chem. 2001, 49, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Criste, A.; Urcan, A.C.; Bunea, A.; Furtuna, F.R.P.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical Composition and Biological Activity of Berries and Leaves from Four Romanian Sea Buckthorn (Hippophae rhamnoides L.) Varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.T.Y.; Cenkowski, S.; Hydamaka, A. Effect of Drying on the Nutraceutical Quality of Sea Buckthorn (Hippophae rhamnoides L. ssp. sinensis) Leaves. J. Food Sci. 2006, 70, E514–E518. [Google Scholar] [CrossRef]
- Eccleston, C.; Baoru, Y.; Tahvonen, R.; Kallio, H.; Rimbach, G.H.; Minihane, A.M. Effects of an Antioxidant-Rich Juice (Sea buckthorn) on Risk Factors for Coronary Heart Disease in Humans. J. Nutr. Biochem. 2002, 13, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Süleyman, H.; Demirezer, L.; Büyükokuroglu, M.E.; Akcay, M.F.; Gepdiremen, A.; Banoglu, Z.N.; Göçer, F. Antiulcerogenic Effect of Hippophae rhamnoides L. Phytotherapy Res. 2001, 15, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.K.; Kumar, R.; Siddiqui, M.S.; Gupta, A. Mechanism of Wound-Healing Activity of Hippophae rhamnoides L. Leaf Extract in Experimental Burns. Evid. Based Complement. Altern. Med. 2011, 2011, 659705. [Google Scholar] [CrossRef]
- Bailey, J.P.; Bímová, K.; Mandák, B. Asexual Spread versus Sexual Reproduction and Evolution in Japanese Knotweed s.l. Sets the Stage for the “Battle of the Clones”. Biol. Invasions 2009, 11, 1189–1203. [Google Scholar] [CrossRef]
- Liu, L.-T.; Guo, G.; Wu, M.; Zhang, W.-G. The Progress of the Research on Cardio-Vascular Effects and Acting Mechanism of Polydatin. Chin. J. Integr. Med. 2012, 18, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gong, X.; Mo, Y.; Wu, S. Polydatin Inhibits the Oxidative Stress-Induced Proliferation of Vascular Smooth Muscle Cells by Activating the eNOS/SIRT1 Pathway. Int. J. Mol. Med. 2016, 37, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Resveratrol Mediates Its Anti-Cancer Effects by Nrf2 Signaling Pathway Activation. Cancer Cell Int. 2021, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.W.; Lai, J.H.; Wu, J.C.-C.; Tsai, Y.-R.; Chen, Y.-H.; Kang, S.-J.; Chiang, Y.-H.; Chang, C.-F.; Chen, K.-Y. Neuroprotective Effects of Emodin against Ischemia/Reperfusion Injury through Activating ERK-1/2 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 2899. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, T.; Bonarska-Kujawa, D.; Męczarska, K.; Cyboran-Mikołajczyk, S.; Oszmiański, J.; Kapusta, I. Analysis of the Polyphenolic Composition of Vaccinium L. Extracts and Their Protective Effect on Red Blood Cell Membranes. Membranes 2023, 13, 589. [Google Scholar] [CrossRef] [PubMed]
- Colleran, B.; Lacy, S.N.; Retamal, M.R. Invasive Japanese Knotweed (Reynoutria japonica Houtt.) and Related Knotweeds as Catalysts for Streambank Erosion. River Res. Appl. 2020, 36, 1962–1969. [Google Scholar] [CrossRef]
- Carter, B.; Curtis, T.G.F.; Sheehy-Skeffington, M.J. Coastal Dunes: Geomorphology, Ecology and Management for Conservation. In Proceedings of the Third European Dune Congress, Galway, Ireland, 17–21 June 1992; A. A. Balkema: Rotterdam, The Netherlands, 1992. [Google Scholar]
- Tsuzuku, S. Local Knowledge about Japanese Vegetables and Herbs among People of Japanese Descent in Southwest British Columbia. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2013. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Kleszczyńska, H.; Oszmiański, J.; Pasławski, R. Allium ursinum L. leaves components modified the physico-chemical properties of red blood cells protecting them from the effects of oxidative stress. Acta Pol. Pharm. 2019, 76, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Włoch, A.; Kapusta, I.; Bielecki, K.; Oszmiański, J.; Kleszczyńska, H. Activity of Hawthorn Leaf and Bark Extracts in Relation to Biological Membrane. J. Membr. Biol. 2013, 246, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Bessis, M. Red Cell Shapes. An Illustrated Classification and Its Rationale. Nouv. Rev. Fr. Hematol. 1972, 12, 721–746. [Google Scholar] [PubMed]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Reed, J.D. Nutritional Toxicology of Tannins and Related Polyphenols in Forage Legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar] [CrossRef]
- Murdiati, T.B.; McSweeney, C.S.; Campbell, R.S.F.; Stoltz, D.S. Prevention of Hydrolysable Tannin Toxicity in Goats Fed Clidemia hirta by Calcium Hydroxide Supplementation. J. Appl. Toxicol. 1990, 10, 325–331. [Google Scholar] [CrossRef]
- Hamada, S.; Yamamoto, T.; Koga, T.; McGhee, J.R.; Michalek, S.M.; Yamamoto, S. Chemical Properties and Immunobiological Activities of Streptococcal Lipoteichoic Acids. Zentralblatt Für Bakteriol. Mikrobiol. Und Hygiene. Ser. A Med. Microbiol. Infect. Dis. Virol. Parasitol. 1985, 259, 228–243. [Google Scholar] [CrossRef]
- Machado, T.; Pinto, A.; Pinto, M.; Leal, I.; Silva, M.; Amaral, A.; Kuster, R.; Netto-Dossantos, K. In Vitro Activity of Brazilian Medicinal Plants, Naturally Occurring Naphthoquinones and Their Analogues, against Methicillin-Resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2003, 21, 279–284. [Google Scholar] [CrossRef]
- Funatogawa, K.; Hayashi, S.; Shimomura, H.; Yoshida, T.; Hatano, T.; Ito, H.; Hirai, Y. Antibacterial Activity of Hydrolyzable Tannins Derived from Medicinal Plants against Helicobacter pylori. Microbiol. Immunol. 2004, 48, 251–261. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and Antiviral Activity of Hydrolysable Tannins. Mini-Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High Molecular Weight Plant Polyphenolics (Tannins) as Biological Antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Tang, L.; Lv, H.; Li, S.; Bi, H.; Gao, X.; Zhou, J. Protective Effects of Polyphenol Extracts from Sea Buckthorn (Hippophaë rhamnoides L.) on Rat Hearts. Open J. Mol. Integr. Physiol. 2016, 06, 10–18. [Google Scholar] [CrossRef][Green Version]
- Asofiei, I.; Calinescu, I.; Trifan, A.; Gavrila, A.I. A Semi-Continuous Process for Polyphenols Extraction From Sea Buckthorn Leaves. Sci. Rep. 2019, 9, 12044. [Google Scholar] [CrossRef]
- Asofiei, I.; Calinescu, I.; Trifan, A.; David, I.G.; Gavrila, A.I. Microwave-Assisted Batch Extraction of Polyphenols from Sea Buckthorn Leaves. Chem. Eng. Commun. 2016, 203, 1547–1553. [Google Scholar] [CrossRef]
- Heinäaho, M.; Pusenius, J.; Julkunen-Tiitto, R. Effects of Different Organic Farming Methods on the Concentration of Phenolic Compounds in Sea Buckthorn Leaves. J. Agric. Food Chem. 2006, 54, 7678–7685. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- van Dijk, C.; Driessen, A.J.; Recourt, K. The Uncoupling Efficiency and Affinity of Flavonoids for Vesicles. Biochem. Pharmacol. 2000, 60, 1593–1600. [Google Scholar] [CrossRef]
- Uchida, S.; Ohta, H.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Nonaka, G.; Nishioka, I.; Niwa, M.; Akashi, T.; Ozaki, M. Active Oxygen Free Radicals are Scavenged by Condensed Tannins. Prog. Clin. Biol. Res. 1988, 280, 135–138. [Google Scholar]
- Cossins, E.; Lee, R.; Packer, L. Esr Studies of Vitamin c Regeneration, Order of Reactivity of Natural Source Phytochemical Preparations. IUBMB Life 1998, 45, 583–597. [Google Scholar] [CrossRef]
- Blazsó, G.; Gábor, M.; Rohdewald, P. Antiinflammatory Activities of Procyanidin-Containing Extracts from Pinus Pinaster Ait. after Oral and Cutaneous Application. Pharmazie 1997, 52, 380–382. [Google Scholar]
- Facinó, R.; Carini, M.; Aldini, G.; Berti, F.; Rossoni, G.; Bombardelli, E.; Morazzoni, P. Procyanidines from Vitis vinifera Seeds Protect Rabbit Heart from Ischemia/Reperfusion Injury: Antioxidant Intervention and/or Iron and Copper Sequestering Ability. Planta Medica 1996, 62, 495–502. [Google Scholar] [CrossRef]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant Activity and Biologic Properties of a Procyanidin-Rich Extract from Pine (Pinus maritima) Bark, Pycnogenol. Free. Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef] [PubMed]
- Jug, U.; Naumoska, K.; Vovk, I. (−)-Epicatechin—An Important Contributor to the Antioxidant Activity of Japanese Knotweed Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation. Antioxidants 2021, 10, 133. [Google Scholar] [CrossRef]
- Chen, H.; Deng, Q.; Ji, X.; Zhou, X.; Kelly, G.; Zhang, J. Glucose Oxidase-Assisted Extraction of Resveratrol from Japanese Knotweed (Fallopia japonica). New J. Chem. 2016, 40, 8131–8140. [Google Scholar] [CrossRef]
- Bensa, M.; Glavnik, V.; Vovk, I. Leaves of Invasive Plants—Japanese, Bohemian and Giant Knotweed—The Promising New Source of Flavan-3-ols and Proanthocyanidins. Plants 2020, 9, 118. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin-Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley and Sons, Ltd.: Chichester, UK, 2017; pp. 107–115. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Cyboran, S.; Strugała, P.; Włoch, A.; Oszmiański, J.; Kleszczyńska, H. Concentrated Green Tea Supplement: Biological Activity and Molecular Mechanisms. Life Sci. 2015, 126, 1–9. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Radical Chemistry of Flavonoid Antioxidants. Adv. Exp. Med. Biol. 1990, 264, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Anggraini, T.; Wilma, S.; Syukri, D.; Azima, F. Total Phenolic, Anthocyanin, Catechins, DPPH Radical Scavenging Activity, and Toxicity of Lepisanthes alata (Blume) Leenh. Int. J. Food Sci. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging Effects of Tea Catechins and Their Derivatives on 1,1-Diphenyl-2-Picrylhydrazyl Radical. Free. Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Nariya, P.; Nariya, M.; Shukla, V.; Acharya, R.; Bhalodia, N. In Vitro Evaluation of Antioxidant Activity of Cordia Dichotoma (Forst f.) Bark. Ayu 2013, 34, 124–128. [Google Scholar] [CrossRef]
- Tanwar, H.; Shweta; Singh, D.; Singh, S.B.; Ganju, L.; Ananth, M.K.; Narsimhan, S. Anti-Inflammatory Activity of the Functional Groups Present in Hippophae rhamnoides (Seabuckthorn) Leaf Extract. Inflammopharmacology 2017, 26, 291–301. [Google Scholar] [CrossRef]
- Upadhyay, N.K.; Kumar, M.Y.; Gupta, A. Antioxidant, Cytoprotective and Antibacterial Effects of Sea buckthorn (Hippophae rhamnoides L.) Leaves. Food Chem. Toxicol. 2010, 48, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Kovářová, M.; Maděra, P.; Frantík, T.; Novák, J.; Vencl, Š. Effects of Knotweed-Enriched Feed on the Blood Characteristics and Fitness of Horses. Agriculture 2022, 12, 109. [Google Scholar] [CrossRef]
- Filho, V.M.B.; Waczuk, E.P.; Kamdem, J.P.; Abolaji, A.O.; Lacerda, S.R.; da Costa, J.G.M.; de Menezes, I.R.A.; Boligon, A.A.; Athayde, M.L.; da Rocha, J.B.T.; et al. Phytochemical Constituents, Antioxidant Activity, Cytotoxicity and Osmotic Fragility Effects of Caju (Anacardium microcarpum). Ind. Crop. Prod. 2014, 55, 280–288. [Google Scholar] [CrossRef]
- Oteiza, P. A Mechanism for the Stimulatory Effect of Aluminum on Iron-Induced Lipid Peroxidation. Arch. Biochem. Biophys. 1994, 308, 374–379. [Google Scholar] [CrossRef]
- Kolanjiappan, K.; Manoharan, S.; Kayalvizhi, M. Measurement of Erythrocyte Lipids, Lipid Peroxidation, Antioxidants and Osmotic Fragility in Cervical Cancer Patients. Clin. Chim. Acta 2002, 326, 143–149. [Google Scholar] [CrossRef]
- Olchowik, E.; Lotkowski, K.; Mavlyanov, S.; Abdullajanova, N.; Ionov, M.; Bryszewska, M.; Zamaraeva, M. Stabilization of Erythrocytes Against Oxidative and Hypotonic Stress by Tannins Isolated from Sumac Leaves (Rhus typhina L.) and Grape Seeds (Vitis vinifera L.). Cell. Mol. Biol. Lett. 2012, 17, 333–348. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Csonka, Á.; Molnar, J.; Szabó, D.; Oszmiański, J.; Kleszczyńska, H. In Vitro Studies of Anti-Hemolytic and Cytotoxic Activity of Procyanidin-Rich Extract from the Leaves of Actinidia Arguta. Pol. J. Food Nutr. Sci. 2018, 68, 171–177. [Google Scholar] [CrossRef]
- Peyrat-Maillard, M.N.; Cuvelier, M.E.; Berset, C. Antioxidant Activity of Phenolic Compounds in 2,2′-Azobis (2-Amidinopropane) Dihydrochloride (AAPH)-Induced Oxidation: Synergistic and Antagonistic Effects. J. Am. Oil Chem. Soc. 2003, 80, 1007–1012. [Google Scholar] [CrossRef]
- Puiggròs, F.; Sala, E.; Vaqué, M.; Ardévol, A.; Blay, M.; Fernández-Larrea, J.; Arola, L.; Bladé, C.; Pujadas, G.; Salvadó, M.J. In Vivo, in Vitro, and in Silico Studies of Cu/Zn-Superoxide Dismutase Regulation by Molecules in Grape Seed Procyanidin Extract. J. Agric. Food Chem. 2009, 57, 3934–3942. [Google Scholar] [CrossRef]
- Iglič, A.; Kralj-Iglič, V.; Hägerstrand, H. Amphiphile Induced Echinocyte-Spheroechinocyte Transformation of Red Blood Cell Shape. Eur. Biophys. J. 1998, 27, 335–339. [Google Scholar] [CrossRef]
- Deuticke, B. Properties and Structural Basis of Simple Diffusion Pathways in the Erythrocyte Membrane. Rev. Physiol. Biochem. Pharmacol. 1977, 78, 1–97. [Google Scholar] [CrossRef]
- Gedde, M.; Huestis, W. Membrane Potential and Human Erythrocyte Shape. Biophys. J. 1997, 72, 1220–1233. [Google Scholar] [CrossRef]
- Virtanen, V.; Karonen, M. Partition Coefficients (logP) of Hydrolysable Tannins. Molecules 2020, 25, 3691. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A Comprehensive Review Encompassing Structure Elucidation via Mass Spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- He, W. DPH Probe Method for Liposome-Membrane Fluidity Determination. Methods Mol. Biol. 2023, 2622, 241–244. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Cyboran-Mikołajczyk, S.; Kleszczyńska, H. Molecular Mechanism of Action of Chlorogenic Acid on Erythrocyte and Lipid Membranes. Mol. Membr. Biol. 2015, 32, 46–54. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Żyłka, R.; Jurkiewicz, P.; Pruchnik, H.; Oszmiański, J.; Hof, M.; Kleszczyńska, H. Interaction of Procyanidin B3 with Membrane Lipids—Fluorescence, DSC and FTIR Studies. Biochim. Biophys. Acta (BBA)—Biomembr. 2017, 1859, 1362–1371. [Google Scholar] [CrossRef]
- Gasiorowski, K.; Szyba, K.; Brokos, B.; Kołaczyńska, B.; Jankowiak-Włodarczyk, M.; Oszmiański, J. Antimutagenic Activity of Anthocyanins Isolated from Aronia Melanocarpa Fruits. Cancer Lett. 1997, 119, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Gerçek, E.; Zengin, H.; Erişir, F.E.; Yılmaz, Ö. Biochemical Changes and Antioxidant Capacity of Naringin and Naringenin against Malathion Toxicity in Saccharomyces Cerevisiae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108969. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Męczarska, K.; Solarska-Ściuk, K.; Ratajczak-Wielgomas, K.; Oszmiański, J.; Jencova, V.; Bonarska-Kujawa, D. Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts—The Mechanism of Action. Molecules 2022, 27, 5865. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The Preparation and Chemical Characteristics of Hemoglobin-Free Ghosts of Human Erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Męczarska, K.; Cyboran-Mikołajczyk, S.; Włoch, A.; Bonarska-Kujawa, D.; Oszmiański, J.; Kleszczynska, H. Polyphenol Content and Bioactivity of Saskatoon (Amelanchier alnifolia nutt.) Leaves and Berries. Acta Pol. Pharm. 2017, 74, 660–669. [Google Scholar]
- Kuhry, J.-G.; Fonteneau, P.; Duportail, G.; Maechling, C.; Laustriat, G. TMA-DPH: A Suitable Fluorescence Polarization Probe for Specific Plasma Membrane Fluidity Studies in Intact Living Cells. Cell Biophys. 1983, 5, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]


| Extract/Substance | EC50 [μg/mL ± SD] |
|---|---|
| BE | 6.28 ± 0.06 * |
| RE | 5.89 ± 0.47 * |
| LE | 6.03 ± 0.65 * |
| L (+)-ascorbic acid | 11.59 ± 0.27 |
| IC50 [μg/mL] ± SD | ||
|---|---|---|
| Extract/Object | Erythrocytes | Erythrocytes Membrane |
| BE | 13.7 ± 3.07 | 6.57 ± 1.12 |
| RE | 15.7 ± 1.34 | 6.42 ± 0.68 |
| LE | 6.00 ± 3.02 | 7.55 ± 2.90 |
| AA/Trolox® | 32.5 ± 4.2 * | 3.90 ± 0.60 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak, T.; Męczarska, K.; Lachowicz-Wiśniewska, S.; Cyboran-Mikołajczyk, S.; Oszmiański, J.; Bonarska-Kujawa, D. Protective Effect of Polyphenolic Extracts from Hippophae rhamnoides L. and Reynoutria japonica Houtt. on Erythrocyte Membrane. Molecules 2024, 29, 3090. https://doi.org/10.3390/molecules29133090
Kaźmierczak T, Męczarska K, Lachowicz-Wiśniewska S, Cyboran-Mikołajczyk S, Oszmiański J, Bonarska-Kujawa D. Protective Effect of Polyphenolic Extracts from Hippophae rhamnoides L. and Reynoutria japonica Houtt. on Erythrocyte Membrane. Molecules. 2024; 29(13):3090. https://doi.org/10.3390/molecules29133090
Chicago/Turabian StyleKaźmierczak, Teresa, Katarzyna Męczarska, Sabina Lachowicz-Wiśniewska, Sylwia Cyboran-Mikołajczyk, Jan Oszmiański, and Dorota Bonarska-Kujawa. 2024. "Protective Effect of Polyphenolic Extracts from Hippophae rhamnoides L. and Reynoutria japonica Houtt. on Erythrocyte Membrane" Molecules 29, no. 13: 3090. https://doi.org/10.3390/molecules29133090
APA StyleKaźmierczak, T., Męczarska, K., Lachowicz-Wiśniewska, S., Cyboran-Mikołajczyk, S., Oszmiański, J., & Bonarska-Kujawa, D. (2024). Protective Effect of Polyphenolic Extracts from Hippophae rhamnoides L. and Reynoutria japonica Houtt. on Erythrocyte Membrane. Molecules, 29(13), 3090. https://doi.org/10.3390/molecules29133090








