Polysaccharides from Porphyra haitanensis: A Review of Their Extraction, Modification, Structures, and Bioactivities
Abstract
:1. Introduction
2. Extraction of Polysaccharides from P. haitanensis
| Source | Method of Extraction | Extraction Times | Time (Min) | Temperature °C | Water/Material Ratio (mL/g) | Yield | References | |
| Xiamen China | HWE | 1 | 240 | Boiling | 40:1 | N/A | [19] | |
| Guangdong China | HWE | 1 | 300 | 100 | 50:1 | N/A | [42] | |
| Dongtou Zhejiang China | HWE | 3 | 120 | 95 | 20:1 | N/A | [10] | |
| Pingtan Island Fujian China | HWE | 1 | 120 | 80 | 20:1 | N/A | [15] | |
| Nan’ao Island Shantou Guangdong China | HWE | 1 | 120 | 90 | 30:1 | 3.3% | [11] | |
| Putian Fujian China | HWE | 2 | 90 | 80 | 20:1 | 3.8% | [29] | |
| Nan’ao Island, Shantou Guangdong China | HWE | 1 | 120 | 90 | 30:1 | 4.10 ± 0.11% | [30] | |
| Zhejiang and Fujian China | HWE | 1 | 118.2 | 88.4 | 40:1 | 15.19% | [28] | |
| China | HWE | 1 | 180 | 80 | 25:1 | 20.33% (±0.15) | [31] | |
| Source | Method of Extraction | Extraction times | Time (Min) | Temperature °C | Water/Material Ratio (mL/g) | Power (W) | Yield | References |
| Hangzhou and Zhejiang China | MAE | 1 | 8 | N/A | 50:1 | 300 | 3.6% | [32] |
| Jiangsu China | MAE | 1 | 14 | N/A | 30:1 | 78 | 5.01 ± 0.32% | [20] |
| Nan’ao Island Shantou Guangdong China | UMAE | 1 | 30 | 80 | 42:1 | 500 (Microwave) 50 (Ultrasonic) | 20.53% | [37] |
3. Purification of P. haitanensis Polysaccharides
4. Physicochemical and Structural Characteristics of Polysaccharides from P. haitanensis
4.1. Sulfate Content
4.2. Monosaccharide Composition
4.3. Molecular Weight
4.4. Chemical Structure
4.5. Morphological Traits
| No. | Polysaccharide Names | Molecular Weight (Da) | Sulfate Conent (%) | Monosaccharide Composition | Structural Characterization | Analysis Technique | References |
|---|---|---|---|---|---|---|---|
| 1 | PHP-KC | 2.01 × 105 | 12.61 ± 0.44% | Gal, Glu, and Man in a molar ratio of 94.85:3.18:1.97 | (1→4)-linked 3,6-anhydro-α-L-galactopyranose units or (1→4)-linked α-L-galactose 6 sulphate units | HPSEC-RID, HPLC FT-IR, NMR | [30,54] |
| 2 | PHP-KC-AC | 2.5 × 105 | 3.8 ± 0.3% | Gal and 3,6-AG in a molar ratio of 1.2:1.0 | →4–3,6-anhydro-α-L- galactopyranose (1→3) β-D-galactopyranose segments | HPSEC GC-MS, FT-IR, NMR | [11] |
| 3 | PP3-4a | 2 × 104 | 19.8% | Gal and 3,6-AG | →3) β-D-galactose (1→4) 3,6-anhydro-α-L-galactose (1→, and →3) β-D- galactose (1→4) α-L-galactose-6-S (1→, repeating structural units | HPGPC, GC-MS, NMR | [29] |
| 4 | PY1 | N/A | 5.4% | Gal and a little of Xyl and Ara | α-glycosidic bonds | HPLC-GPC,FT-IR, GC | [32] |
| 5 | PY2 | N/A | 3.7% | Gal and a little of Ara, Xyl, Glu, and Man. | β-amide pyranose | ||
| 6 | CPHP-TZ | 5.38 × 105 | 6.48% | Rha, Xyl, Man, Glu, and Gal in a molar ratio of 3.67:2.31:2.49:1:246.64 | N/A | HPGPC, GC-MS, FT-IR, UV–Vis, NMR | [14] |
| 7 | PHP1-TZ | 4.99 × 105 | 7.11% | Rha, Xyl, Man, Glu, and Gal in a molar ratio of 1.21:4.36:1.36:1:705.86 | Contains α-type and β-type glycosidic bonds | ||
| 8 | PHP2-TZ | 5.23 × 105 | 8.33% | Rha, Xyl, Man, Glu, and Gal in a molar ratio of 2:3:2.6:1:990.3 | |||
| 9 | PHP3-TZ | 7.97 × 105 | 11.96% | Rha, Xyl, Man, Glu, and Gal in a molar ratio of 9.1:6.3:2.4:1:960.8 | |||
| 10 | PHPD-IV-4 | 1.0 × 104 (Before purification) | N/A | Gal and 3,6-AG | repeat units of →3) β-D-galactose (1→4) 3,6-anhydro-α-L-galactose (1→, and→3) β-D-galactose (1→4) α-L-galactose-6-S (1→. | HPGPC, FT-IR, NMR | [7] |
| 11 | PHP1-BZ | 5.46 × 105 g/mol | 6.93 ± 0.05% | Gal | →3) β-D-galactose-4-sulfate (1→3) β-D-galactose (1→6) α-D-galactose-4-sulfate (1→4) 3,6-anhydro-α-L-galactose (→6) α-D-galactose-4-sulfate (1→ | GC-FID, GC-MS, FT-IR, NMR | [52] |
| 12 | PHP2-BZ | 1.14 × 106 (±3.44%) g/mol | 5.07 ± 0.04% | Gala, Man, and Glu in a molar ratio of 69.27:21.32:9.41 | →4) α-galactose (1→6) β-D-galactose-4-sulfate (1→4) β-glucose (1→ and a side chain of α-mannose (1→6) β-glucose | GC, FT-IR, NMR, GC-MS | [57] |
| 13 | LP-G2 | 8381 | 12.94% | Gal, GalA, Glu, and Ara in a molar ratio of 10.46:14.10:0.33:1.52:0.04 | →4) β-D-galactose→4) α-L-galactose-6-sulfate segments, with β-D-glucose and α-D-galactose unit substituted at the 6-position of α-L-galactose. | HP-GPC, HPLC, NMR, IR, GC-MS. | [12] |
| 14 | P1 | 3.003 × 105 | 8.42% | Gal, Glu, Man, Ara, Rha, Xyl, and Fuc in a molar ratio of 97.48:1.31:0.33:0.39:0.22:0.18:0.10 | repeat units of→3) β-D-galactose (1→4) 3,6-anhydro-α-L-galactose (1→, and→3) β-D-galactose (1→4) α-L-galactose-6-S (1→, and→3) 6-O-methyl-β-D-galactose (1→4) 3,6-anhydro-α-L- galactose (1→. | HPGPC, GC-MS, UV, NMR | [53] |
| 15 | P2 | 1.304 × 105 | 9.48% | Gal, Glu, Man, Ara, Rha, Xyl, and Fuc in a molar ratio of 97.1:1.18:1.39:0.18:0.07:0.06:0.03 | |||
| 16 | P3 | 1.115 × 105 | 13.68% | Gal, Glu, Man, Ara, Rha, Xyl, and Fuc in a molar ratio of 98.62:0.73:0.24:0.12:0.21:0.04:0.04 | |||
| 17 | PHPW | N/A | 12.79 ± 1.20% | GulUA, Man, Rib, Rha, GlcN, GalUA, GalN, Glc, Gal, Ara, and Fuc in a molar ratio of 0.01:0.33:0.34:0.04:0.08:0.21:0.44:0.65:33.19:0.07:3.27 | N/A | HPLC, GPC FTIR | [51] |
| 18 | PHPX | N/A | 8.00 ± 0.75% | GulUA, ManUA, Man, Rib, Rha, GlcN, GalUA, GalN, Glc, Gal, Ara, and Fuc in a molar ratio of 0.02:0.04:0.31:0.49:0.11:0.10:0.27:1.35:0.65:34.17:0.02:2.85 | N/A | ||
| 19 | PHPZ | N/A | 9.28 ± 0.15% | GulUA, Man, Rib, Rha, GlcN, GalUA, GalN, Glc, Gal, Ara, and Fuc in a molar ratio of 0.02:0.49:0.64:0.04:0.02:0.09:0.30:0.18:37.22:0.02:3.70 | N/A | ||
| 20 | PHPR | N/A | 14.38 ± 0.45% | GulUA, Man, Rib, GlcN, GalUA, GalN, Glc, Gal, and Fuc in a molar ratio of 0.05:0.11:0.28:0.04:0.07:0.47:0.62:24.39:4.4 | N/A | ||
| 21 | PHPN | N/A | 13.11 ± 1.05% | GulUA, Man, Rib, GlcN, GalUA, GalN, Glc, Gal, and Fuc in a molar ratio of 0.04:0.28:0.44:0.02:0.06:1.12:34.35:4.85 | N/A | ||
| 22 | PHPR01 | N/A | 10.45 ± 0.90% | Man, Rib, Rha, GlcN, GalUA, GalN, Glc, Gal, Ara, and Fuc in a molar ratio of 0.16:0.42:0.01:0.09:0.15:2.00:0.69:33.98:0.07:5.22 | N/A | ||
| 23 | PHPR02 | N/A | 13.75 ± 1.65% | Man, Rib, GlcN, GalUA, GalN, Glc, Gal, and Fuc in a molar ratio of 0.27:0.52:0.01:0.03:2.06:0.18:35.05:6.11 | N/A | ||
| 24 | PHPR03 | 6.7 × 105 | 14.70 ± 2.11% | Man, Rib, Rha, GlcN, GalUA, GalN, Glc, Gal, Ara, and Fuc in a molar ratio of 0.22:0.50:0.04:0.05:1.62:0.19:33.78:0.04:6.70 | N/A | ||
| 25 | PHPR04 | N/A | 12.90 ± 0.45% | Man, Rib., Glu, GalUA, Gal, Glu, Gal, Ara, and Fuc in a molar ratio of 0.10:0.41:0.02:0.04:2.45:0.14:29.88:0.03:7.55 | N/A | ||
| 26 | PHPR05 | N/A | 6.72 ± 0.15% | Man, Rib., Glu, GalUA, Gal, Glu, Gal, Ara, and Fuc in a molar ratio of 0.16:0.36:0.05:0.11:0.26:0.45:35.34:0.03:4.16 | N/A | ||
| 27 | PHP1-LF | 5.6705 × 105 | 8.36 ± 0.16% | Gal, Glc, Xyl, Man, Fru, and Glc-UA in a molar ratio of 98.10:0.54:0.19:0.36:0.15:0.66 | N/A | HPGPC, IC FT-IR UV-vis | [13] |
| 28 | PHP2-LF | 4.1409 × 105 | 7.53 ± 0.53% | Gal, Glc, Xyl, Man, Fru, and Glc-UA in a molar ratio of 94.27:3.95:0.28:0.46:0.26:0.78 | N/A | ||
| 29 | PHP3-LF | 3.2380 × 105 | 4.23 ± 0.59% | Gal, Glc, Xyl, Man, Fru, and Glc-UA in a molar ratio of 96.91:1.66:0.19:0.54:0.17:0.53 | N/A | ||
| 30 | PH | 5.23 × 105 | 5.28% | Gal, Glu, Man, Ara, Fuc, Xyl, and Rha in a molar ratio of 98.66:0.23:0.49:0.07:0.05:0.07:0.44 | N/A | HPLC GC-MS | [22] |
| 31 | CPH-TZ-AO | 5.24 × 105 | 8.42% | Gal, Glc, Man, Ara, Fuc, Xyl, and Rha in a molar ratio of 98.66:0.23:0.49:0.07:0.05:0.07:0.44 | N/A | HPGPC GC-MS FT-IR UV | [17] |
| 32 | DCHP-TZ-AO | 2.17 × 105 | 13.68% | Gal, Glc, Man, Ara, Fuc, Xyl, and Rha in a molar ratio of 95.60:2.01:1.34:0.20:0.08:0.09:0.70 | N/A | ||
| 33 | PHP-XJ | 6.3 × 105 | 2.7 mg/mL | Glu, Gal, and Fuc in a molar ratio of 2.1:76.2:1 | N/A | HPGPC LC | [31] |
5. Molecular Modifications of Polysaccharides from P. haitanensis
5.1. Degradation Modification
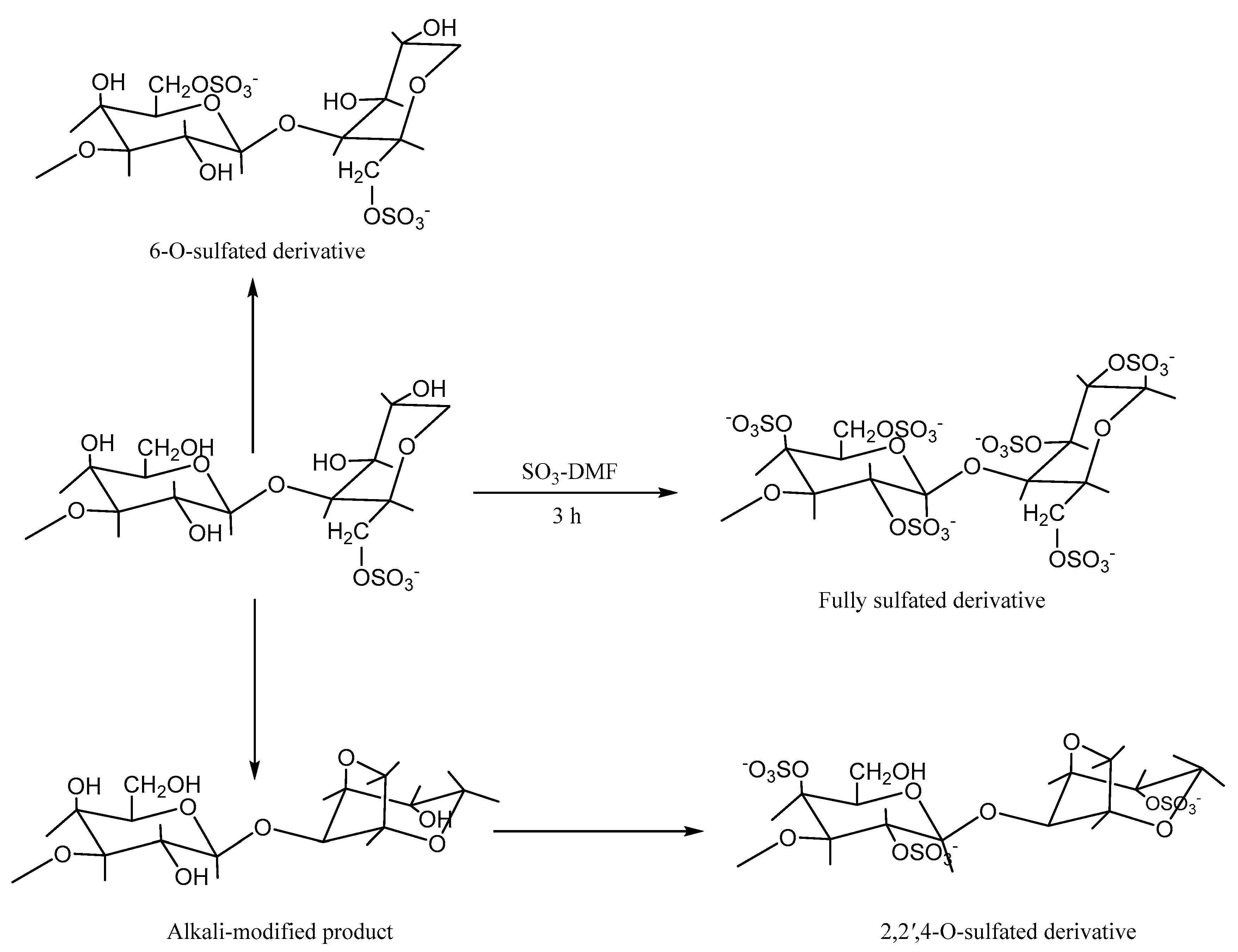
5.2. Sulfation Modification
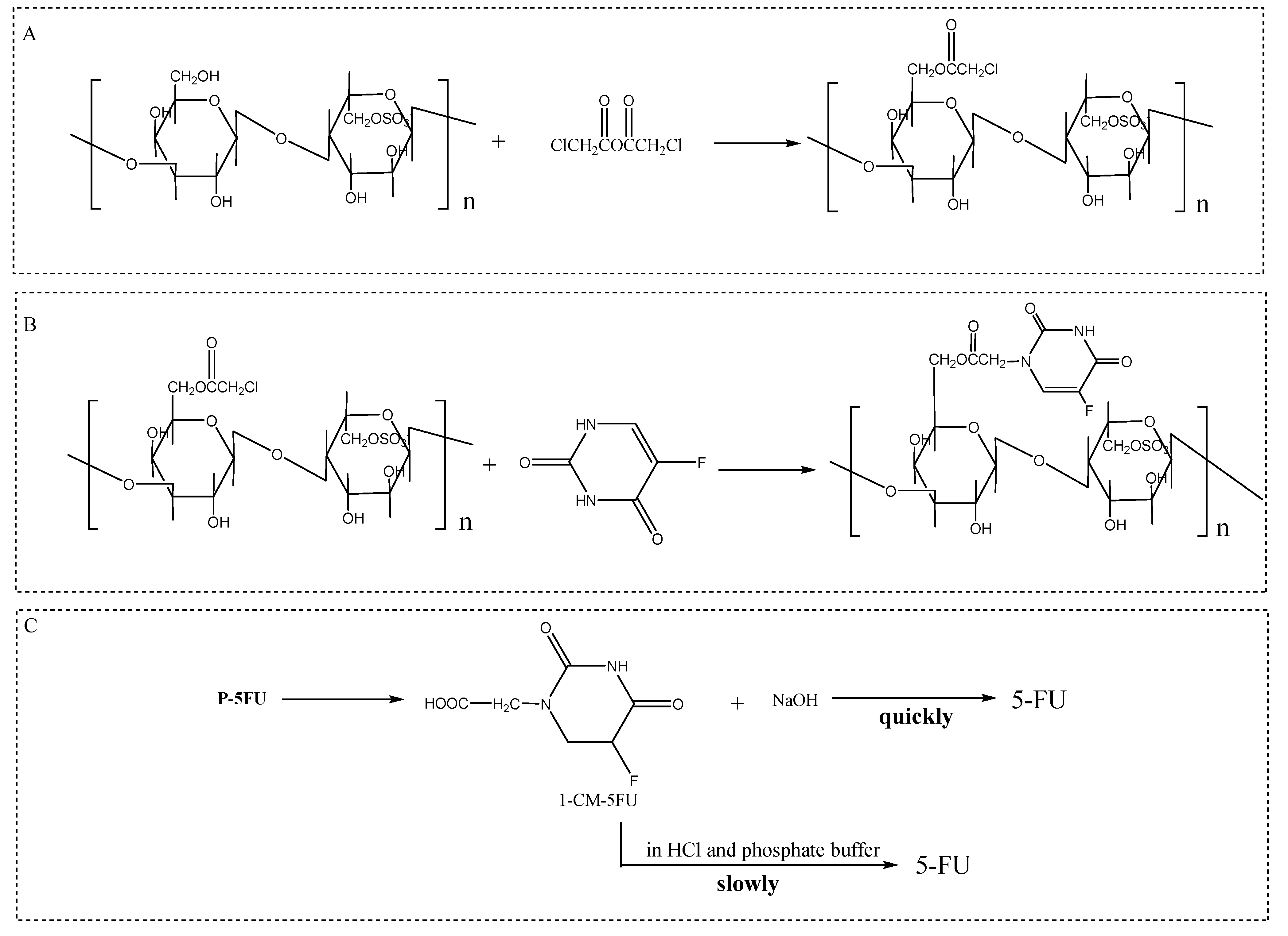
5.3. 5-Fluorouracil Polysaccharide
5.4. Other Modification Methods
| Classification of Modification | Method | Biological Activity | The Characteristic IR Absorption Peak | References |
|---|---|---|---|---|
| Degradation | Ascorbate and hydrogen peroxide method | Anti-aging, Antioxidant, and immunostimulatory | N/A | [7,62,63,64] |
| Pectinase degradation method | Antioxidant and immunomodulatory | [17,53]. | ||
| Sulfation | Chlorosulfonic acid and N, N-dimethylformamide method | Antioxidant | New peaks at 1225 and 817 cm−1 | [68] |
| Chlorosulfonic acid and N, N-dimethylformamide method (Regioselective modification of DMT as a protective group) | Antioxidant | New peaks at 1225 and 817 cm−1 | [70]. | |
| Anticoagulant | ||||
| 5-fluorouracil polysaccharide | Chloroacetylation and 5-fluorouracil substitution method | Anti-tumor | New peaks at 3428, 2963, 1715–1668 cm−1 | [77,78] |
| Acetylation | Acetic anhydride acylation method | Antioxidant | New peaks at 1726 or 1730 cm−1 | [68,80,82]. |
| Phosphorylation | Tributylamine and polyphosphoric acid method | Antioxidant | New peaks at 1268 and 988 cm−1 | [68,80] |
| Drug-loaded nanoparticles | Complex coacervation method. | Anti-tumor | N/A | [84] |
6. Biological Activities of P. haitanensis Polysaccharides
| Biological Activities | Polysaccharide Names | Types | Testing Subjects | Doses/Duration | Effects/ Mechanisms | Refs. |
|---|---|---|---|---|---|---|
| Antioxidant activity | PHP-KC-AC (HE) | In vitro | DPPH, hydroxyl, and ABTS radicals | 0.0625, 0.125, 0.25, 0.5, 1, and 2 mg/mL | At 2.0 mg/mL, the scavenging rates of DPPH, hydroxyl, and ABTS free radicals by PHP were 34.63%, 23.80%, and 53.16%, respectively. | [11] |
| PHP3—TZ (HE-DE-SE) | In vitro | DPPH, hydroxyl radical, superoxide anion, and reducing powers | 1 to 5 mg/mL | At 5 mg/mL, DPPH and superoxide anion scavenging rates of 52.1% and 56.39%, respectively. At 2 mg/mL, HO• scavenging rates of 22.84% | [14] | |
| PHPR03 (ME) | In vitro | DPPH, hydroxyl, ABTS, and superoxide anion radicals | 2, 4, 6, 8, and 10 mg/mL | Showed the strongest scavenging capability. | [51] | |
| P3 (D-DE) | In vitro | DPPH, hydroxyl radical and ferric reducing | 5 mg/mL | DPPH scavenging and hydroxyl radical rate of, respectively 48.4%, and 38.3%. Reductive ability test solution absorbance is 0.19. | [53] | |
| PP3–4 (HE-D-DE) | In vitro | DPPH, hydroxyl radical, superoxide anion, and reducing powers | 0.5 to 8 mg/mL | Showed the strongest scavenging capability and reducing ability. | [29] | |
| P3 (HE-D) | In vitro | DPPH radical and ferric reducing | 0 to 2.5 mg/mL | Better DPPH radical scavenging activity and reducing ability than original polysaccharide. | [62] | |
| PHP (HE) | in vitro | DPPH, hydroxyl, ABTS, superoxide radicals, and T-AOC | 4 mg/mL | Showed the highest level of hydroxyl radical scavenging ability. | [15] | |
| PHP0.5–1-UF (HE-DE-SE) | In vitro | DPPH, hydroxyl, ABTS, superoxide radicals, and ferric ion reducing | 4 mg/mL | Showed the highest levels of DPPH, superoxide anion radical, and ABTS+ radical, as well as T-AOC scavenging ability. | [15] | |
| P1 P2 (S) P3 (A) | In vitro | DPPH superoxide radicals and ferric ion reducing | 0 to 5 mg/mL | The sulfated derivative with certain DS showed stronger antioxidant activity. The acetylated derivative showed the most excellent antioxidant activity. | [82] | |
| AP (A) PP (P) BP (B) | In vitro | Hydroxyl, superoxide anion radicals, and reducing powers | N/A | The acetylation, phosphorylation, and benzoylation derivatives of P. haitanensis polysaccharides showed stronger antioxidant activity than the original polysaccharides. | [80] | |
| DCPH-TZ-AO (D) | In vitro | Ferric reducing power | 1 to 5 mg/mL | The total antioxidant capacity is significant. | [17] | |
| PHP3—TZ (HE-DE-SE) | In vitro | In H2O2-stimulated RAW264.7 cells | 0, 25, 50, 100, 200, and 400 μg/mL for 1 h | SOD, CAT and GSH-Px ↑; MDA and ROS level ↓; | [14] | |
| DCPH-TZ-AO (D) | In vitro | In H2O2-treated RAW264.7 cells | 0, 25, 50, 100, 200, and 400 μg/mL for 1 h | MDA and ROS↓; CAT ↑; | [17] | |
| F1 (HE-S) | In vivo | Aging Kunming mice (20 months old, 35–45 g) | 50, 100, and 200 mg/kg/d, i.p., for 20 days | SOD, GSH-Px ↑; MDA ↓; | [56] | |
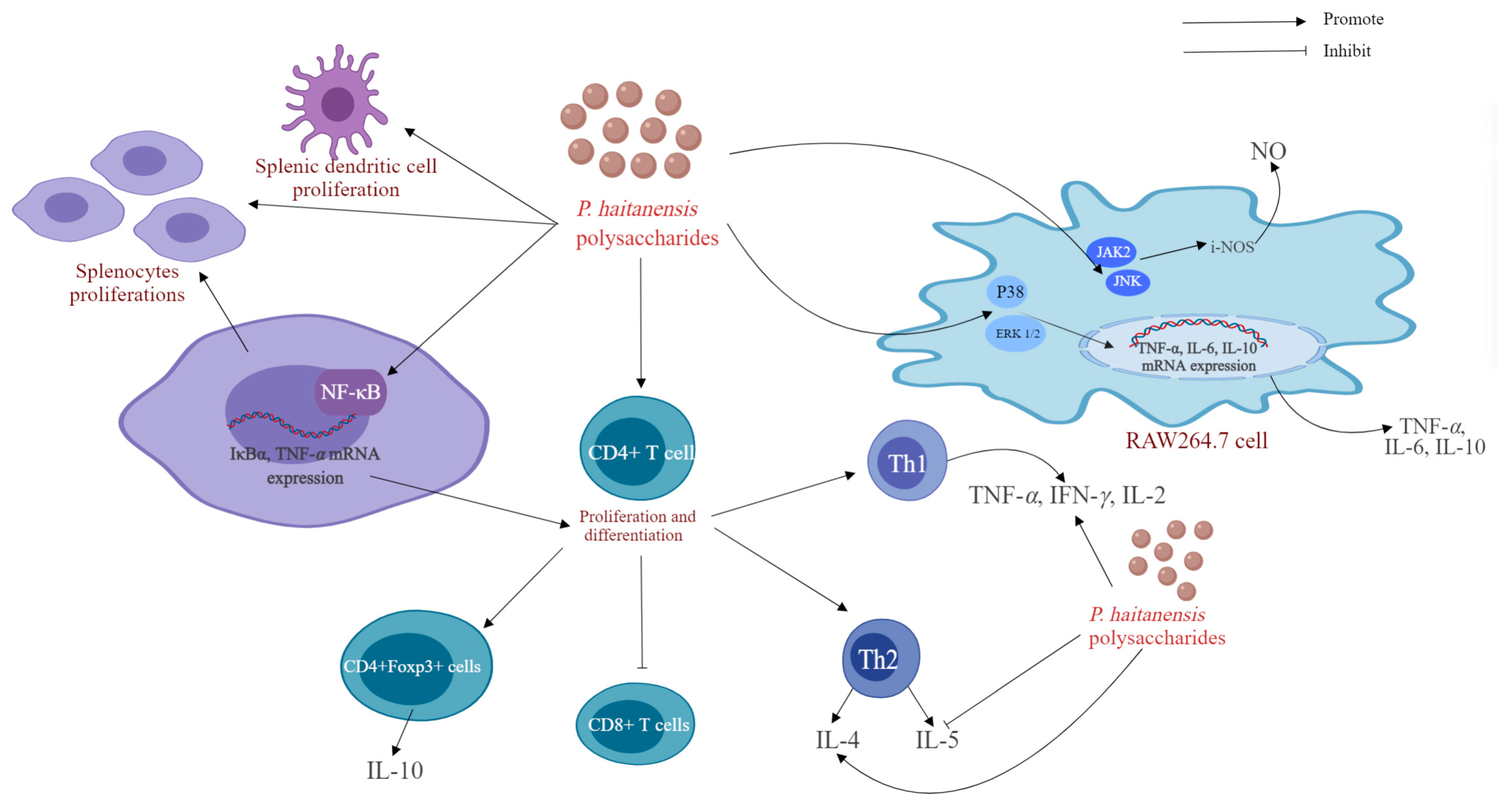
| Immunomodulating activity | PHPD-IV-4 (HE-D-SE) | In vitro | RAW264.7 cells | 0~200 µg/mL for 24 h | Phagocytic uptake, NO, ERK1/2, JNK, and P38, ↑; | [7] |
| DCPH-TZ-AO (D) | In vitro | RAW264.7 cells | 25, 50, 100, 200, and 400 μg/mL for 24 h | Phagocytic uptake, NO ↑; | [17] | |
| PHPS (HE) | In vitro | RAW264.7 cells | 5, 20, 40, 80 and 100 μg/mL for 12 h or 24 h, | Phagocytic uptake, NO, (IL)-6, IL-10, TNF-α, JNK and JAK2 ↑; | [16] | |
| PHPS (HE) | In vivo | BALB/c mice | 50, 150, and 250 mg/kg/d for i.g.,14 days | Lymphocytes proliferation, splenocytes proliferations TNF-α, IL-10, CD4+ Th cells, DCs, and Tregs ↑; CD8+ T cells ↓; | [16] | |
| PH (HE) | In vivo | BALB/c mice | 5 mg/d i.g., for three days a week, four weeks in total | NF-κB, IFN-γ, TNF-α, IL-4 and IL-10 and CD4+CD25+ Tregs ↑; IL-5 ↓; | [10] | |
| Anti-allergy activity | PHPS (HE-DE) | In vitro | In tropomyosin-sensitized splenic lymphocytes | IL-4, IL-5 and IL-13↓; Th1, IFN-γ, IL-10, JNK and JNK2↑; | [19] | |
| PHPS (HE-DE) | In vivo | In tropomyosin-sensitized mice | 100 μg/mouse, i.p., | IgE, IgG1 ↓; IgG2a ↑; | [19] | |
| PHPS (HE-DE) | In vivo | In tropomyosin-sensitized mice | 100 μg/mouse, i.g | Histamine levels ↓; | [19] | |
| PP (HE) | In vivo | In OVA-sensitized mice | 25, 150, or 250 mg/kg/d, i.g., for 31 days | IgE, IgG1, IL-2, IL-4, and IL-17 ↓; IL-10 ↑; | [85] | |
| DPHSP (HE-D) | In vivo | In OVA-induced food allergy mouse | 50, 150, or 250 mg/kg/d, i.g., for 13 days | IgE, IL-4, IL-13 ↓; Differentiation of Treg cells↑; | [18] | |
| Anti-tumor activity | PHP (ME) | In vitro | Human gastric carcinoma SGC-7901 cells | 10, 20, 100, 200, 500 μg/mL for 48 h | Within the concentration range of 10 to 500 μg/mL, inhibition rates increased from 8.25% to 70.40%. | [20] |
| PHPs (UME-DE) | In vitro | Human colon cancer HT-29, LoVo, and SW-480 cells | 0, 300 and 600 μg/mL for 72 h | Cell hyperplasia and cell G0–G1 phase ↓; | [21] | |
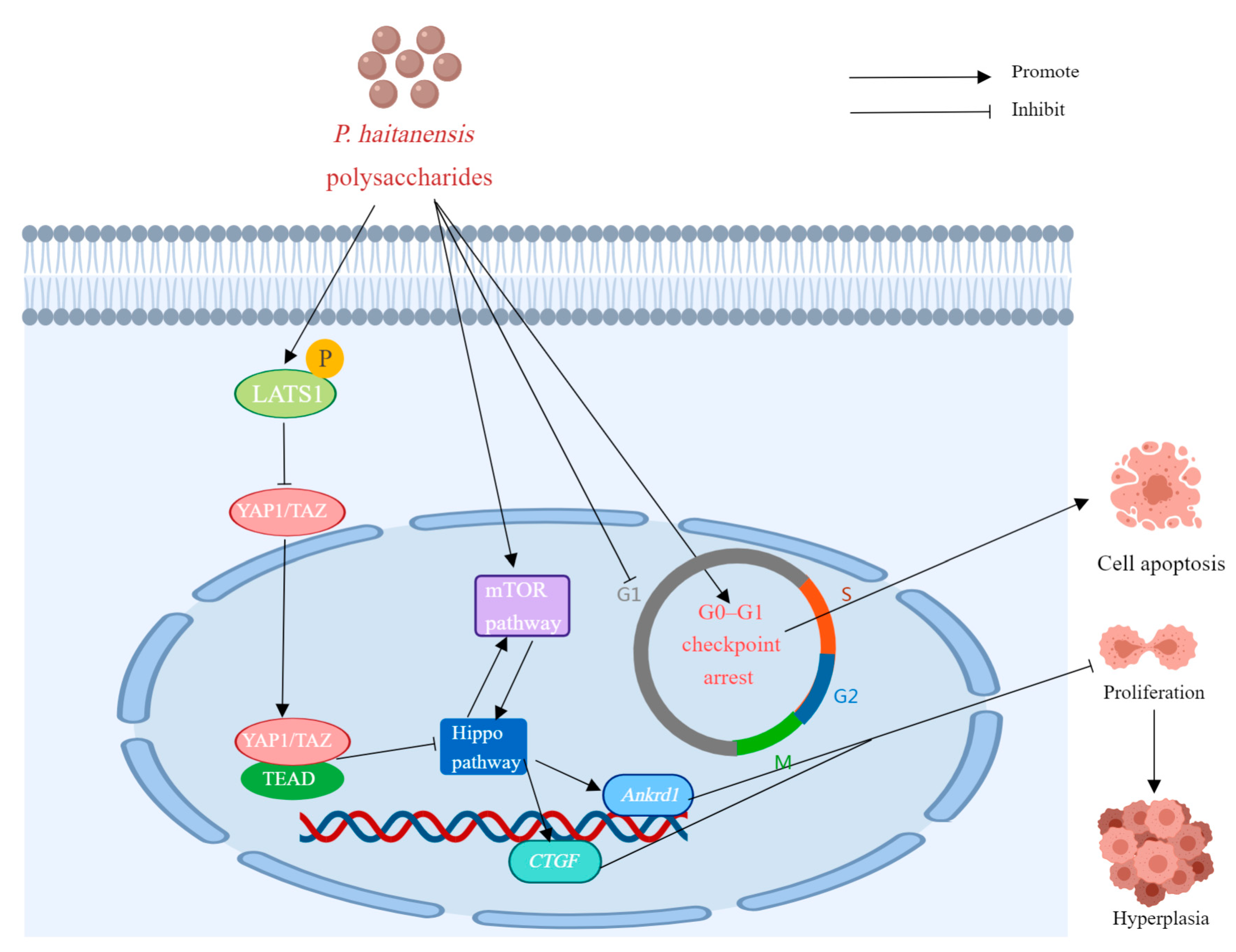
| PH (HE-DE-SE) | In vitro | Mouse colon cancer CMT93 cells | 0.2, 0.4, or 0.8 mg/mL for 24, 48, or 72 h. | Cell hyperplasia, TAZ, YAP1, CTGF and Ankrd1 ↓; LATS1 ↑; | [22] | |
| PHP (ME) | In vivo | SGC-7901 tumor-bearing mice | 40, 80, and 160 mg/kg/d, i.g., for 25 days | Inhibition rates of high-, middle-, and low-dose groups were 57.26%, 67.55% and 77.04%, respectively | [20] | |
| Anti-aging activity | PHP (HE) | In vitro | In H2O2-treated WI-38 cells | 10 g/mL for 2 h | p53-p21 pathways and SAHF-like foci ↓; SA-β-gal positive cells decreases from 53% to 23% in the cultures at 30 PDs. | [24] |
| DP (HE-D) | In vivo | Kunming male mice (Aβ1−40 induction) | 75, 150 and 300 mg/kg/d for 16 days | Cerebral acetylcholine and ChAT ↑; AchE ↓; | [64] | |
| P (1,2,3) (HE-D) | In vivo | Drosophila melanogaster | 0.01 to 2 mg/mL | P1 and P2 on 1% diet significantly increased mean lifespan by 8.60% and 6.68%, respectively. For P, flies kept on 0.2% diet significantly increased mean life span by 6.10%. | [23] | |
| Prebiotic activity | PHP-KC (HE) | In vitro | Fermentation of healthy human feces | 100 mg/9 mL | Bacteroides thetaiotaomicron, Bacteroides ovatus, Defluviitalea saccharophila, and Faecalibacterium prausnitzii ↑; Putrefying bacteria ↓; | [30] |
| PHP (UME) | In vitro | Fermentation of healthy human feces | 100 mg | Coprococcus_3, Bacteroides, Sutterella, Lachnospiraceae_UCG_006, and Bacteroidales_S24_7_group ↑; Escherichia_Shigella and Dorea ↓; | [37] | |
| PHP1 (HE-DE-SE) | In vitro | 10-week-old SPF rats fecal material (10 g) | 10.00 g/L for 24 h | Escherichia-Shigella ↓; Propionate-producing, Ruminococcaceae_NK4A214, the norank_f_Ruminococcaceae, Christensenellaceae_R-7, Fusicatenibacter, Ruminiclostridium_5, Blautia, E.coprostanoligenes, Desulfovibrio, Lactobacillus and Parasutterella ↑; | [52] | |
| PHP2-BZ (HE-DE-SE) | In vitro | Sprague Dawley rat fecal material (~10 g) | 10 g/L 24 h | Ruminococcaceae_UCG-005, Ruminococcus_2, Lactobacillus and Escherichia-Shigella ↑; | [57] | |
| Hypolipidemic activity | APHP (HE) | In vivo | In alloxan-induced diabetic mice | 100, 200 and 400 mg/kg/d, i.g., for 21 days | TC, TG and LDL ↓; HDL ↑; | [42] |
| PHP (HE) | In vivo | In diet-induced high-fat Mesocricetus auratus | 100, 200 mg/kg/d for 4 weeks | TC, TG, LDLC, Abhd5, Me1, Elovl6, Fasn, and Pnpla3 ↓; Muribaculaceae, Faecalibaculum, CD36, Acacb, and PPARg ↑; | [86] | |
| Hypoglycemic activity | APHP (HE) | In vivo | In alloxan-induced diabetic mice | 100, 200, and 400 mg/kg/d, i.g., for 21 days | TC, TG, and LDL ↓; β-cell proliferation and HDL ↑; | [42] |
| Anticoagulant activity | Sulfated porphyrans | In vitro | Citrated normal chicken plasma | 1 to 20 μg/mL | APTT, TT, and PT ↑; | [70] |
| Anti-complement activity | LP-G2 (HE-D-DE-SE) | In vitro | 0.5% rabbit erythrocytes | 0 to 3.5 mg/mL 0 to 10 mg/mL | Block hemolysis of SRBC; Selectively interact with C1q, C2, C4, and C9; Classical approach and alternative approach ↓; | [12] |
| Anti-diarrhea activity | PHSP(hp) (HE) | In vivo | In ETEC-K88 infected mice | 10 mg/kg/d, i.g., for 1 week | TNF-α, IL-6 ↑; Nitroblue tetrazolium, B cells↓ | [58] |
6.1. Antioxidant Activity
6.2. Immunomodulatory Activity
6.3. Anti-Allergy Activity
6.4. Anti-Tumor Activity
6.5. Anti-Aging Activity
6.6. Prebiotic Activity
6.7. Other Activities
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P. haitanensis | Porphyra haitanensis |
| HWE | Hot water extraction |
| MAE | Microwave-assisted extraction |
| UMAE | Ultrasonic–microwave-assisted extraction |
| HPSEC | High-performance size exclusion chromatography |
| HPGPC | High-performance gel permeation chromatography |
| GC-MS | Gas chromatography-mass spectrometry |
| HPLC | High-performance liquid chromatography |
| FT-IR | Fourier transform infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
| UV | Ultraviolet analysis |
| Gal | Galactose |
| Glu | Glucose |
| Man | Mannose |
| Ara | Arabinose |
| Fuc | Fucose |
| Xyl | Xylose |
| Rha | Rhamnose |
| Rib | Ribose |
| GalN | Galactosamine |
| GalUA | Galacturonic acid |
| GlcN | Glucosamine |
| GulUA | Guluronic |
| ManUA | Mannuronic |
| GlcUA | Glucuronic acid |
| 3,6-AG | 3,6-anhydro galactose |
| H2O2-VC | Hydrogen peroxide–Vitamin C |
| DMF | N,N-dimethylformamide |
| 5-FU | 5-Fluorouracil |
| AFM | Atomic force microscopy |
| SEM | Scanning electron microscopy |
| ROS | Reactive oxygen species |
References
- Li, Y.; Shen, S.; He, L.; Xu, P.; Lu, S. Sequence Analysis of rDNA Intergenic Spacer (IGS) of Porphyra haitanensis. J. Appl. Phycol. 2010, 22, 187–193. [Google Scholar] [CrossRef]
- Venkatraman, K.L.; Mehta, A. Health Benefits and Pharmacological Effects of Porphyra Species. Plant Foods Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-E.; Lu, Q.-Q.; Brodie, J. A Review of the Bladed Bangiales (Rhodophyta) in China: History, Culture and Taxonomy. Eur. J. Phycol. 2017, 52, 251–263. [Google Scholar] [CrossRef]
- Xu, N.; Xu, K.; Wang, W.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Nutrient Enrichment Improves Growth and Food Quality of Two Strains of the Economic Seaweed Pyropia haitanensis. Front. Mar. Sci. 2020, 7, 544582. [Google Scholar] [CrossRef]
- Pan, C.; Ma, J.; Tao, F.; Ji, C.; Zhao, Y.; Chen, S.; Yang, X. Novel Insight into the Antioxidant Proteins Derived from Laver (Porphyra haitanensis) by Proteomics Analysis and Protein Based Bioinformatics. Food Biosci. 2021, 42, 101134. [Google Scholar] [CrossRef]
- Ou, Y.; Guo, Y.; Xu, L.; Lu, X.; Guo, Z.; Zheng, B. Structural Characterization and Hypoglycemic Activity of Glycoproteins Extracted from Porphyra haitanensis by Ammonium Sulfate and Ethanol Extraction Methods. Food Biosci. 2023, 54, 102868. [Google Scholar] [CrossRef]
- Gong, G.; Dang, T.; Fang, J.; Deng, Y.; Liu, Q.; Dai, W.; Sun, J.; Wang, L.; Liu, Y.; Sun, T.; et al. Preparation, Structural Characterization, and Bioactivity of PHPD-IV-4 Derived from Porphyra haitanensis. Food Chem. 2020, 329, 127042. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A Review of Bioactive Plant Polysaccharides: Biological Activities, Functionalization, and Biomedical Applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Fu, L.; Qian, Y.; Wang, C.; Xie, M.; Huang, J.; Wang, Y. Two Polysaccharides from Porphyra Modulate Immune Homeostasis by NF-κB-Dependent Immunocyte Differentiation. Food Funct. 2019, 10, 2083–2093. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.-M.; Xu, S.-Y.; Liu, Y.; Cheong, K.-L. Physicochemical Characterization and Antioxidant Activity of Sulphated Polysaccharides Derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Lv, F.; Xie, X.; Zhang, S.; Cai, C.; Jia, R.; Pan, Y.; Liu, F. Anti-Complementary Activity of a Degraded Sulfated Heterogalactan from Red Alga Pyropia haitanensis. Int. J. Biol. Macromol. 2020, 147, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Z.; Wang, Y.; Fu, L. Effect of the Harvest Period on the Structure and Anti-Allergic activity of Porphyra haitanensis Polysaccharides. Food Funct. 2022, 13, 10034–10045. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Huo, Y.-F.; Xu, L.; Xu, Y.-Y.; Wang, X.-L.; Zhou, T. Purification, Characterization and Antioxidant Activity of Polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 165, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xu, Y.; Yang, S.; Chang, Q.; Zheng, B.; Zhang, Y.; Hu, X.; Zeng, H. Application of X-ray Diffraction and Energy Dispersive Spectroscopy in the Isolation of Sulfated Polysaccharide from Porphyra haitanensis and Its Antioxidant Capacity under In Vitro digestion. J. Sci. Food Agric. 2021, 101, 6452–6462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, S.; Li, L.; Pan, T.; Shi, C.; Liu, H.; Cao, M.; Su, W.; Liu, G. In Vitro and In Vivo Immunomodulatory Activity of Sulfated Polysaccharide from Porphyra haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huo, Y.; Wang, F.; Wang, C.; Zhu, Q.; Wang, Y.; Fu, L.; Zhou, T. Improved Antioxidant and Immunomodulatory Activities of Enzymatically Degraded Porphyra haitanensis Polysaccharides. J. Food Biochem. 2020, 44, e13189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Gao, Y.; Shu, Z.; Zhang, J.; Liu, H.; Cao, M.; Liu, G.; Sun, J. Degraded Porphyra haitanensis Sulfated Polysaccharide Relieves Ovalbumin-Induced Food Allergic Response by Restoring the Balance of T Helper Cell Differentiation. Food Funct. 2021, 12, 4707–4719. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pan, T.; Cao, M.; Liu, Q.; Zhang, L.; Liu, G. Suppression of Th2 Immune Responses by the Sulfated Polysaccharide from Porphyra haitanensis in Tropomyosin-Sensitized Mice. Int. Immunopharmacol. 2015, 24, 211–218. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y. Optimization of Microwave Assisted Extraction, Chemical Characterization and Antitumor Activities of Polysaccharides from porphyra haitanensis. Carbohydr. Polym. 2019, 206, 179–186. [Google Scholar] [CrossRef]
- Yao, W.-Z.; Veeraperumal, S.; Qiu, H.-M.; Chen, X.-Q.; Cheong, K.-L. Anti-Cancer Effects of Porphyra haitanensis Polysaccharides on Human Colon Cancer Cells via Cell Cycle Arrest and Apoptosis without Causing Adverse Effects In Vitro. 3 Biotech 2020, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, W.; Sun, Z.; Sun, Y.; Wang, Y.; Fu, L. Porphyra haitanensis Polysaccharide (PH) Attenuates Cell Hyperplasia via Remodeling the Cross-Talk between Hippo/YAP and mTOR Pathways. Food Sci. Hum. Wellness 2023, 12, 424–430. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Q.; Qi, H.; Liu, X.; Li, Z. Extension of Life Span and Improvement of Vitality of Drosophila Melanogaster by Long-Term Supplementation with Different Molecular Weight Polysaccharides from Porphyra haitanensis. Pharmacol. Res. 2008, 57, 67–72. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Su, H.; Pan, Y.; Han, J.; Zhang, T.; Mao, G. Effect of Sulfated Galactan from Porphyra haitanensis on H2O2-Induced Premature Senescence in WI-38 Cells. Int. J. Biol. Macromol. 2018, 106, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of Polysaccharides from Edible Mushrooms: Emerging Technologies and Recent Advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, G. Preparation, Structural Characteristics, and Application of Taro Polysaccharides in Food. J. Sci. Food Agric. 2022, 102, 6193–6201. [Google Scholar] [CrossRef]
- Zhou, S.; Rahman, A.; Li, J.; Wei, C.; Chen, J.; Linhardt, R.J.; Ye, X.; Chen, S. Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef]
- Cai, C.; Yang, Y.; Zhao, M.; Jia, R.; Jiao, B.; He, P. Extraction and Antioxidation of Polysaccharide from Porphyra haitanensis using response surface method. Pak. J. Bot. 2017, 49, 1137–1141. [Google Scholar]
- Gong, G.; Zhao, J.; Wang, C.; Wei, M.; Dang, T.; Deng, Y.; Sun, J.; Song, S.; Huang, L.; Wang, Z. Structural Characterization and Antioxidant Activities of the Degradation Products from Porphyra haitanensis polysaccharides. Process Biochem. 2018, 74, 185–193. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Aweya, J.J.; Li, N.; Deng, R.-Y.; Chen, W.-Y.; Tang, J.; Cheong, K.-L. Microbial Catabolism of Porphyra haitanensis Polysaccharides by Human Gut Microbiota. Food Chem. 2019, 289, 177–186. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, Y.; Wang, C.; Yang, Q.; Jiang, X.; Zhu, C. Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis. Mar. Drugs 2020, 18, 539. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, Y. Separation and Purification of Porphyra haitanensis polysaccharide and Its Preliminary Structural Characterization. Sep. Sci. Technol. 2017, 52, 1835–1842. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Zhang, R.; Parniakov, O.; Grimi, N.; Lebovka, N.; Marchal, L.; Vorobiev, E. Emerging Techniques for Cell Disruption and Extraction of Valuable Bio-Molecules of Microalgae Nannochloropsis sp. Bioprocess. Biosyst. Eng. 2019, 42, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L. (Eds.) Natural Products Isolation; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 864, ISBN 978-1-61779-623-4. [Google Scholar]
- Chuo, S.C.; Nasir, H.M.; Mohd-Setapar, S.H.; Mohamed, S.F.; Ahmad, A.; Wani, W.A.; Muddassir, M.; Alarifi, A. A Glimpse into the Extraction Methods of Active Compounds from Plants. Crit. Rev. Anal. Chem. 2022, 52, 667–696. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Chen, X.-Q.; Liu, Y.; Cheong, K.-L. Ultrasonic/Microwave-Assisted Extraction, Simulated Digestion, and Fermentation In Vitro by Human Intestinal Flora of Polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 152, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; An, X.; Wang, S.; Sun, M.; Zhou, H. Basil Polysaccharides: A Review on Extraction, Bioactivities and Pharmacological Applications. Bioorg. Med. Chem. 2020, 28, 115179. [Google Scholar] [CrossRef] [PubMed]
- Bhotmange, D.U.; Wallenius, J.H.; Singhal, R.S.; Shamekh, S.S. Enzymatic Extraction and Characterization of Polysaccharide from Tuber Aestivum. Bioact. Carbohydr. Diet. Fibre 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Zhao, H.-K.; Wei, X.-Y.; Xie, Y.-M. Supercritical CO2 Extraction, Structural Analysis and Bioactivity of Polysaccharide from Grifola frondosa. J. Food Compos. Anal. 2021, 102, 104067. [Google Scholar] [CrossRef]
- Chen, R.; Luo, S.; Wang, C.; Bai, H.; Lu, J.; Tian, L.; Gao, M.; Wu, J.; Bai, C.; Sun, H. Effects of Ultra-High Pressure Enzyme Extraction on Characteristics and Functional Properties of Red Pitaya (Hylocereus polyrhizus) Peel Pectic Polysaccharides. Food Hydrocoll. 2021, 121, 107016. [Google Scholar] [CrossRef]
- Cao, C.; Chen, M.; Liang, B.; Xu, J.; Ye, T.; Xia, Z. Hypoglycemic Effect of Abandoned Porphyra Haitanensis Polysaccharides in Alloxan-Induced Diabetic Mice. Bioact. Carbohydr. Diet. Fibre 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Zhang, K.; Lu, Y.; Wu, Q.; Chen, J.; Li, Y.; Wu, Q.; Chen, Y. Extraction, Purification, Structural Characterization, and Gut Microbiota Relationship of Polysaccharides: A Review. Int. J. Biol. Macromol. 2022, 213, 967–986. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, Isolation and Purification Methods of Polysaccharides from Natural Products: A Review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Tang, W.; Liu, D.; Yin, J.-Y.; Nie, S.-P. Consecutive and Progressive Purification of Food-Derived Natural Polysaccharide: Based on Material, Extraction Process and Crude Polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar] [CrossRef]
- Chen, S.; Guan, X.; Yong, T.; Gao, X.; Xiao, C.; Xie, Y.; Chen, D.; Hu, H.; Wu, Q. Structural Characterization and Hepatoprotective Activity of an Acidic Polysaccharide from Ganoderma lucidum. Food Chem. X 2022, 13, 100204. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, S.; Hopson, C.; Gorman, J.; Gabriel, R.; Singer, S.W. Purification and Characterization of a Native LYTIC polysaccharide Monooxygenase from Thermoascus aurantiacus. Biotechnol. Lett. 2020, 42, 1897–1905. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel LOW-Molecular-Mass Pumpkin Polysaccharide: Structural Characterization, Antioxidant Activity, and Hypoglycemic Potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Cheng, Y.; Lv, D.; Li, M.; Qi, Y.; Lan, J.; Zhao, Q.; Li, Z. Marine Sulfated Polysaccharides: Preventive and Therapeutic Effects on Metabolic Syndrome: A Review. Mar. Drugs 2021, 19, 608. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.-M.; Aweya, J.J.; Liu, X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.-L. Bioactive Polysaccharides from Red Seaweed as Potent Food Supplements: A Systematic Review of Their Extraction, Purification, and Biological Activities. Carbohydr. Polym. 2022, 275, 118696. [Google Scholar] [CrossRef]
- Ji, C.; Pan, C.; Huang, H.; Tao, F.; Lin, S.; Chen, S.; Qi, B.; Hu, X.; Yang, X. Effects of Origin and Harvest Period on Characterisation, Structure and Antioxidant Activity of Polysaccharides Derived from Porphyra haitanensis. Int. J. Food Sci. Technol. 2022, 57, 123–136. [Google Scholar] [CrossRef]
- Chen, P.; Tong, M.; Zeng, H.; Zheng, B.; Hu, X. Structural Characterization and In Vitro Fermentation by Rat Intestinal Microbiota of a Polysaccharide from Porphyra haitanensis. Food Res. Int. 2021, 147, 110546. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.-F.; Li, Y.-T.; Xia, W.; Wang, C.; Xie, Y.-Y.; Wang, Y.-B.; Zhou, T.; Fu, L.-L. Degraded Polysaccharides from Porphyra haitanensis: Purification, Physico-Chemical Properties, Antioxidant and Immunomodulatory Activities. Glycoconj. J. 2021, 38, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.-M.; Veeraperumal, S.; Lv, J.-H.; Wu, T.-C.; Zhang, Z.-P.; Zeng, Q.-K.; Liu, Y.; Chen, X.-Q.; Aweya, J.J.; Cheong, K.-L. Physicochemical Properties and Potential Beneficial Effects of Porphyran from Porphyra haitanensis on Intestinal Epithelial Cells. Carbohydr. Polym. 2020, 246, 116626. [Google Scholar] [CrossRef] [PubMed]
- Hongfeng, G.; Minghou, J.; Wenda, C. Comparative Studies on Structural Feature of Agar Polysaccharides from Porphyra haitanensis Grown in South and North China. Chin. J. Ocean. Limnol. 1993, 11, 25–33. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The Structure of a Sulfated Galactan from Porphyra haitanensis and Its In Vivo Antioxidant Activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, L.; Cheng, Z.; Zhang, Y.; Zheng, B.; Hu, X.; Zeng, H. Structure Elucidation and In Vitro Rat Intestinal Fermentation Properties of a Novel Sulfated Glucogalactan from Porphyra haitanensis. Food Sci. Hum. Wellness 2023, 12, 596–606. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.-M.; Li, G.-L.; Sun, L.-C.; Gao, Y.-Y.; Zhang, Y.-F.; Liu, H.; Cao, M.-J.; Liu, G.-M. The Anti-Diarrhea Activity of Red Algae-Originated Sulphated Polysaccharides on ETEC-K88 Infected mice. RSC Adv. 2019, 9, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Y.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Chemically Modified Polysaccharides: Synthesis, Characterization, Structure Activity Relationships of Action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Huang, H.; Huang, G. Extraction, Separation, Modification, Structural Characterization, and Antioxidant Activity of Plant Polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef]
- Huang, S.; Chen, F.; Cheng, H.; Huang, G. Modification and Application of Polysaccharide from Traditional Chinese Medicine Such as Dendrobium Officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Q.; Qi, H.; Zhang, H.; Niu, X.; Xu, Z.; Li, Z. Degradation of Porphyran from Porphyra haitanensis and the Antioxidant Activities of the Degraded Porphyrans with Different Molecular Weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef]
- Yi, L.; Zhang, M.; Cheng, J.; Wan, H.; Li, C.; Zhu, J.; Zhang, Q.; Liu, Q.; Xu, G. Antidepressant-like Effects of Degraded Porphyran Isolated from Porphyra Haitanensis. Mol. Nutr. Food Res. 2021, 65, 2000869. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Pan, Y.; Wang, G.; Mao, G. The Degraded Polysaccharide from Pyropia haitanensis Represses Amyloid Beta Peptide-Induced Neurotoxicity and Memory In Vivo. Int. J. Biol. Macromol. 2020, 146, 725–729. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L. Sulfated Modification, Characterization, and Potential Bioactivities of Polysaccharide from the Fruiting Bodies of Russula Virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated Modification, Characterization and Bioactivities of an Acidic Polysaccharide Fraction from an Edible Mushroom Pleurotus Eous (Berk.) Sacc. Heliyon 2021, 7, e05964. [Google Scholar] [CrossRef]
- Huang, R.; Shen, M.; Yu, Y.; Liu, X.; Xie, J. Physicochemical Characterization and Immunomodulatory Activity of Sulfated Chinese Yam Polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Zhang, H.; Niu, X.; Li, P. Preparation of the Different Derivatives of the Low-Molecular-Weight Porphyran from Porphyra Haitanensis and Their Antioxidant Activities in Vitro. Int. J. Biol. Macromol. 2009, 45, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, Z.; Chen, J.; Wang, D.; Zhang, Y. Recent Advances in Antiviral Activities and Potential Mechanisms of Sulfated Polysaccharides. Carbohydr. Polym. 2021, 272, 118526. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Song, H.; Zhang, H.; Niu, X. Regioselective Syntheses of Sulfated Porphyrans from Porphyra Haitanensis and Their Antioxidant and Anticoagulant Activities in Vitro. Carbohydr. Polym. 2010, 79, 1124–1129. [Google Scholar] [CrossRef]
- Haggag, Y.A.; Osman, M.A.; El-Gizawy, S.A.; Goda, A.E.; Shamloula, M.M.; Faheem, A.M.; McCarron, P.A. Polymeric Nano-Encapsulation of 5-Fluorouracil Enhances Anti-Cancer Activity and Ameliorates Side Effects in Solid Ehrlich Carcinoma-Bearing Mice. Biomed. Pharmacother. 2018, 105, 215–224. [Google Scholar] [CrossRef]
- Naren, G.; Wang, L.; Zhang, X.; Cheng, L.; Yang, S.; Yang, J.; Guo, J.; Nashun, B. The Reversible Reproductive Toxicity of 5-Fluorouracil in Mice. Reprod. Toxicol. 2021, 101, 1–8. [Google Scholar] [CrossRef]
- Barary, M.; Hosseinzadeh, R.; Kazemi, S.; Liang, J.J.; Mansoori, R.; Sio, T.T.; Hosseini, M.; Moghadamnia, A.A. The Effect of Propolis on 5-Fluorouracil-Induced Cardiac Toxicity in Rats. Sci. Rep. 2022, 12, 8661. [Google Scholar] [CrossRef]
- Liu, J.-J.; Chen, J.; Wang, Y.; Yan, C.-C.; Zhang, C.; Mehmood, S.; Pan, W.-J.; Zhang, W.; Lu, Y.-M.; Wu, Q.-X.; et al. Reduction of 5-Fluorouracil-Induced Toxicity by Sarcodon Aspratus Polysaccharides in Lewis Tumor-Bearing Mice. Int. J. Biol. Macromol. 2020, 163, 232–239. [Google Scholar] [CrossRef]
- Wzorek França Dos Santos, I.; Sauruk Da Silva, K.; Regis Bueno, L.; Suzane Schneider, V.; Silva Schiebel, C.; Mulinari Turin De Oliveira, N.; Cristine Malaquias Da Silva, L.; Soares Fernandes, E.; Biondaro Gois, M.; Mach Cortes Cordeiro, L.; et al. Polysaccharide Fraction from Campomanesia Adamantium and Campomanesia Pubescens Attenuates 5-Fluorouracil-Induced Intestinal Mucosal Inflammation in Mice. Nutr. Cancer 2023, 75, 1382–1398. [Google Scholar] [CrossRef]
- Cai, B.; Pan, J.; Chen, H.; Chen, X.; Ye, Z.; Yuan, H.; Sun, H.; Wan, P. Oyster Polysaccharides Ameliorate Intestinal Mucositis and Improve Metabolism in 5-Fluorouracil-Treated S180 Tumour-Bearing Mice. Carbohydr. Polym. 2021, 256, 117545. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Shi, X.; Zhang, J.; Song, H. Synthesis and Drug Release in Vitro of Porphyran Carrying 5-Fluorouracil. Carbohydr. Polym. 2010, 79, 628–632. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z. The Antitumor Activity of a Red Alga Polysaccharide Complexes Carrying 5-Fluorouracil. Int. J. Biol. Macromol. 2014, 69, 542–545. [Google Scholar] [CrossRef]
- Xia, S.; Zhai, Y.; Wang, X.; Fan, Q.; Dong, X.; Chen, M.; Han, T. Phosphorylation of Polysaccharides: A Review on the Synthesis and Bioactivities. Int. J. Biol. Macromol. 2021, 184, 946–954. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Shi, X.; Song, H.; Zhang, J. In Vitro Antioxidant Activities of Acetylated, Phosphorylated and Benzoylated Derivatives of Porphyran Extracted from Porphyra Haitanensis. Carbohydr. Polym. 2009, 78, 449–453. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Lei, L.; Li, F.; Tang, Y.; Yuan, Y.; Zhang, Y.; Wu, S.; Yin, R.; Ming, J. Acetylation of Polysaccharide from Morchella Angusticeps Peck Enhances Its Immune Activation and Anti-Inflammatory Activities in Macrophage RAW264.7 Cells. Food Chem. Toxicol. 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Song, H.; Zhang, H.; Niu, X. Chemical Modification and Influence of Function Groups on the in Vitro-Antioxidant Activities of Porphyran from Porphyra Haitanensis. Carbohydr. Polym. 2010, 79, 290–295. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.P. Drug Encapsulating Polysaccharide-loaded Metal Nanoparticles: A Perspective Drug Delivery System. Drug Dev. Res. 2021, 82, 145–148. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Huo, Y.-F.; Xu, L.; Zhu, Y.-Z.; Wu, Y.-T.; Wei, X.-Y.; Zhou, T. Resveratrol-Loaded Ovalbumin/Porphyra Haitanensis Polysaccharide Composite Nanoparticles: Fabrication, Characterization and Antitumor Activity. J. Drug Deliv. Sci. Technol. 2021, 66, 102811. [Google Scholar] [CrossRef]
- Wei, Y.-J.; Fang, R.-E.; Ou, J.-Y.; Pan, C.-L.; Huang, C.-H. Modulatory Effects of Porphyra-Derived Polysaccharides, Oligosaccharides and Their Mixture on Antigen-Specific Immune Responses in Ovalbumin-Sensitized Mice. J. Funct. Foods 2022, 96, 105209. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, P.; Wang, Z.; Hu, X.; Zhang, Y.; Zheng, B. Porphyra Haitanensis Polysaccharides Attenuates Blood Lipid via Gut-Liver Axis in Diet-Induced High-Fat Mesocricetus Auratus through Multiple Integrated Omics. Mol. Nutr. Food Res. 2023, 67, 2200638. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Bai, L.; Xu, D.; Zhou, Y.-M.; Zhang, Y.-B.; Zhang, H.; Chen, Y.-B.; Cui, Y.-L. Antioxidant Activities of Natural Polysaccharides and Their Derivatives for Biomedical and Medicinal Applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.; Huang, G. Antioxidant Activities and Mechanisms of Polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative Stress Mitigation by Antioxidants—An Overview on Their Chemistry and Influences on Health Status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory Effects of Polysaccharides from Edible Fungus: A Review. Food Sci. Human Wellness. 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Kiddane, A.T.; Kim, G.-D. Anticancer and Immunomodulatory Effects of Polysaccharides. Nutr. Cancer 2021, 73, 2219–2231. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, J.; Wu, W.; Wen, Y.; Lu, S.; El-Seedi, H.R.; Zhao, C. Structure–Immunomodulatory Activity Relationships of Dietary Polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341. [Google Scholar] [CrossRef]
- Lee, H.; Fessler, M.B.; Qu, P.; Heymann, J.; Kopp, J.B. Macrophage Polarization in Innate Immune Responses Contributing to Pathogenesis of Chronic Kidney Disease. BMC Nephrol. 2020, 21, 270. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in Immunoregulation and Therapeutics. Sig Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, C.; Xue, W.; Huang, L.; Wang, Z. Natural Immunomodulating Substances Used for Alleviating Food Allergy. Crit. Rev. Food Sci. Nutr. 2023, 63, 2407–2425. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Han, Y.; Yang, B.; Lin, H.; Li, Z. The Natural Substances with Anti-Allergic Properties in Food Allergy. Trends Food Sci. Technol. 2022, 128, 53–67. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as Potential Anti-Tumor Biomacromolecules —A Review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Q.; Xu, X.; Li, G.; Tian, C.; Zhang, T. Molecular Mechanisms of Anti-Cancer Bioactivities of Seaweed Polysaccharides. Chin. Herb. Med. 2022, 14, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Zhang, C. Recent Advances on Bioactivity of Seaweed Polysaccharides. Med. Res. 2019, 3, 200003. [Google Scholar] [CrossRef]
- Warraich, U.-A.; Hussain, F.; Kayani, H.U.R. Aging-Oxidative Stress, Antioxidants and Computational Modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef]
- Harman, M.F.; Martín, M.G. Epigenetic Mechanisms Related to Cognitive Decline during Aging. J. Neurosci. Res. 2020, 98, 234–246. [Google Scholar] [CrossRef]
- Sadrekarimi, H.; Gardanova, Z.R.; Bakhshesh, M.; Ebrahimzadeh, F.; Yaseri, A.F.; Thangavelu, L.; Hasanpoor, Z.; Zadeh, F.A.; Kahrizi, M.S. Emerging Role of Human Microbiome in Cancer Development and Response to Therapy: Special Focus on Intestinal Microflora. J. Transl. Med. 2022, 20, 301. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Zhang, Y.; Gao, W.; He, Y.; Wang, Y.; Sun, Y.; Kuang, H. Polysaccharides from Porphyra haitanensis: A Review of Their Extraction, Modification, Structures, and Bioactivities. Molecules 2024, 29, 3105. https://doi.org/10.3390/molecules29133105
Sun M, Zhang Y, Gao W, He Y, Wang Y, Sun Y, Kuang H. Polysaccharides from Porphyra haitanensis: A Review of Their Extraction, Modification, Structures, and Bioactivities. Molecules. 2024; 29(13):3105. https://doi.org/10.3390/molecules29133105
Chicago/Turabian StyleSun, Minghao, Yuping Zhang, Wuyou Gao, Yujia He, Yu Wang, Yanping Sun, and Haixue Kuang. 2024. "Polysaccharides from Porphyra haitanensis: A Review of Their Extraction, Modification, Structures, and Bioactivities" Molecules 29, no. 13: 3105. https://doi.org/10.3390/molecules29133105
APA StyleSun, M., Zhang, Y., Gao, W., He, Y., Wang, Y., Sun, Y., & Kuang, H. (2024). Polysaccharides from Porphyra haitanensis: A Review of Their Extraction, Modification, Structures, and Bioactivities. Molecules, 29(13), 3105. https://doi.org/10.3390/molecules29133105







