Cs-Doped WO3 with Enhanced Conduction Band for Efficient Photocatalytic Oxygen Evolution Reaction Driven by Long-Wavelength Visible Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization and Influencing Factors of Structure of Cs-WO3
2.2. The Optical Properties of Cs-WO3
2.3. Photocatalytic Activity
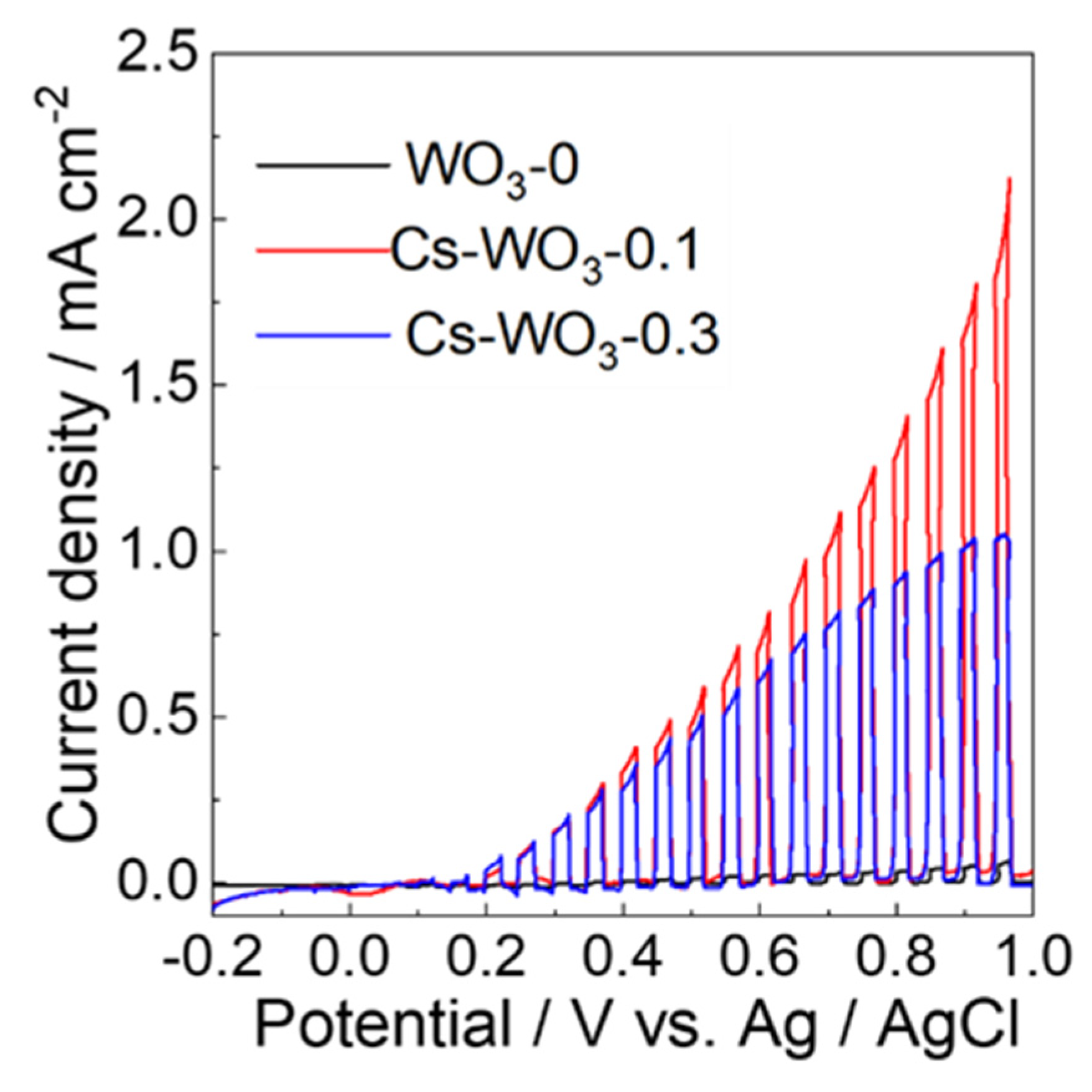
2.4. Photoelectrocatalytic Properties
3. Experimental Section
3.1. Materials
3.2. Synthesis of WO3 Powders
3.3. Fabrication of Electrodes
3.4. Characterization of the Photocatalysts
3.5. Photocatalytic Activity Measurement
3.6. Photoelectrocatalytic Property Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Mebrahtu, C.; Liao, L.; Beine, A.K.; Palkovits, R. Stability and deactivation of OER electrocatalysts: A review. J. Energy Chem. 2022, 69, 301–329. [Google Scholar] [CrossRef]
- Bignozzi, C.A.; Caramori, S.; Cristino, V.; Argazzi, R.; Meda, L.; Tacca, A. Nanostructured photoelectrodes based on WO3: Applications to photooxidation of aqueous electrolytes. Chem. Soc. Rev. 2013, 42, 2228–2246. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lian, W.; Liu, B.; Lv, H.; Zhang, Y.; Wu, X.; Wang, T.; Gong, J.; Chen, T.; Xu, H. A transparent, high-performance, and stable Sb2S3 photoanode enabled by heterojunction engineering with conjugated polycarbazole frameworks for unbiased photoelectrochemical overall water splitting devices. Adv. Mater. 2022, 34, 2200723. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Mao, Y. Ultrafine iridium oxide nanorods synthesized by molten salt method toward electrocatalytic oxygen and hydrogen evolution reactions. Electrochim. Acta 2016, 212, 686–693. [Google Scholar] [CrossRef]
- Li, H.; Zhu, B.; Sun, J.; Gong, H.; Yu, J.; Zhang, L. Photocatalytic hydrogen production from seawater by TiO2/RuO2 hybrid nanofiber with enhanced light absorption. J. Colloid Interface Sci. 2024, 654, 1010–1019. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Ke, G.; Liu, B.; Dong, F.; Yang, L.; He, H.; Zhou, Y. WO3 homojunction photoanode: Integrating the advantages of WO3 different facets for efficient water oxidation. J. Energy Chem. 2021, 56, 37–45. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, X.; Nawaz, M.; Fan, X.; Tao, R. Boosting the photoelectrochemical water splitting of Fe2O3 by surface-state regulation. Inorg. Chem. Front. 2024, 11, 526–533. [Google Scholar] [CrossRef]
- del Olmo, L.; Dommett, M.; Oevreeide, I.H.; Walsh, A.; Di Tommaso, D.; Crespo-Otero, R. Water oxidation catalysed by quantum-sized BiVO4. J. Mater. Chem. A 2018, 6, 24965–24970. [Google Scholar] [CrossRef]
- He, Y.; Thorne, J.E.; Wu, C.H.; Ma, P.; Du, C.; Dong, Q.; Guo, J.; Wang, D. What limits the performance of Ta3N5 for solar water splitting? Chem 2016, 1, 640–655. [Google Scholar] [CrossRef]
- Maeda, K.; Lu, D.; Domen, K. Solar-Driven Z-scheme water splitting using modified BaZrO3–BaTaO2N solid solutions as photocatalysts. Acs Catal. 2013, 3, 1026–1033. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Chen, L.; Cao, F.; Guo, J.; Li, L. Three-dimensional WO3 nanoplate/Bi2S3 nanorod heterojunction as a highly efficient photoanode for improved photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2017, 9, 40235–40243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wygant, B.R.; Mabayoje, O.; Lin, J.; Kawashima, K.; Kim, J.-H.; Li, W.; Li, J.; Mullins, C.B. Interface engineering and its effect on WO3-based photoanode and tandem cell. ACS Appl. Mater. Interfaces 2018, 10, 12639–12650. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Q.; Zhao, Z.; Gao, L.; Li, X. Synthesis and photocatalytic activity study of S-doped WO3 under visible light irradiation. Environ. Sci. Pollut. Res. 2020, 27, 15103–15112. [Google Scholar] [CrossRef] [PubMed]
- Mi, Q.; Ping, Y.; Li, Y.; Cao, B.; Brunschwig, B.S.; Khalifah, P.G.; Galli, G.A.; Gray, H.B.; Lewis, N.S. Thermally stable N2-intercalated WO3 photoanodes for water oxidation. J. Am. Chem. Soc. 2012, 134, 18318–18324. [Google Scholar] [CrossRef] [PubMed]
- Kalanur, S.S.; Yoo, I.-H.; Eom, K.; Seo, H. Enhancement of photoelectrochemical water splitting response of WO3 by Means of Bi doping. J. Catal. 2018, 357, 127–137. [Google Scholar] [CrossRef]
- Radecka, M.; Sobas, P.; Wierzbicka, M.; Rekas, M. Photoelectrochemical properties of undoped and Ti-doped WO3. Phys. B Condens. Matter 2005, 364, 85–92. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Lou, Z.; Xiao, M.; Hu, L.; Ye, Z.; Zhu, L. Synthesis of Fe-doped WO3 nanostructures with high visible-light-driven photocatalytic activities. Appl. Catal. B Environ. 2015, 166, 112–120. [Google Scholar] [CrossRef]
- Song, Z.; Ma, J.; Sun, H.; Wang, W.; Sun, Y.; Sun, L.; Liu, Z.; Gao, C. Synthesis of NiWO4 nano-particles in low-temperature molten salt medium. Ceram. Int. 2009, 35, 2675–2678. [Google Scholar] [CrossRef]
- Quyen, V.T.; Kim, J.; Park, P.-M.; Huong, P.T.; Viet, N.M.; Thang, P.Q. Enhanced the visible light photocatalytic decomposition of antibiotic pollutant in wastewater by using Cu doped WO3. J. Environ. Chem. Eng. 2021, 9, 104737. [Google Scholar] [CrossRef]
- Cheng, X.; Leng, W.; Liu, D.; Zhang, J.; Cao, C. Enhanced photoelectrocatalytic performance of Zn-doped WO3 photocatalysts for nitrite ions degradation under visible light. Chemosphere 2007, 68, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.; Zhang, Z.; Goh, T.G.; Subramanian, G.; Seng, H.D.; Hor, T.A.; Luo, H.-K.; Chi, D. Yb-doped WO3 photocatalysts for water oxidation with visible light. Int. J. Hydrogen Energy 2014, 39, 4291–4298. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Rare earth doped metal oxide nanoparticles for photocatalysis: A perspective. Nanotechnology 2022, 33, 142001. [Google Scholar] [CrossRef] [PubMed]
- Sudrajat, H.; Babel, S. Rapid photocatalytic degradation of the recalcitrant dye amaranth by highly active N-WO3. Environ. Chem. Lett. 2016, 14, 243–249. [Google Scholar] [CrossRef]
- Sun, Y.; Murphy, C.J.; Reyes-Gil, K.R.; Reyes-Garcia, E.A.; Thornton, J.M.; Morris, N.A.; Raftery, D. Photoelectrochemical and structural characterization of carbon-doped WO3 films prepared via spray pyrolysis. Int. J. Hydrogen Energy 2009, 34, 8476–8484. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wang, X.; Chen, Q. Preparation and water-splitting photocatalytic behavior of S-doped WO3. Appl. Surf. Sci. 2012, 263, 157–162. [Google Scholar] [CrossRef]
- Sun, J.; Li, B.; Wang, Q.; Zhang, P.; Zhang, Y.; Gao, L.; Li, X. Preparation of phosphorus-doped tungsten trioxide nanomaterials and their photocatalytic performances. Environ. Technol. 2021, 42, 4104–4114. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, J.; Li, R. Facile synthesis of Cs 0.3 WO3 nanofibers by hydrothermal method and their optical properties. Opt. Mater. 2020, 107, 110147. [Google Scholar] [CrossRef]
- Kako, T.; Zou, Z.; Ye, J. Photocatalytic oxidation of 2-propanol in the gas phase over cesium bismuth niobates under visible light irradiation. Res. Chem. Intermed. 2005, 31, 359–364. [Google Scholar] [CrossRef]
- Friesen, D.A.; Morello, L.; Headley, J.V.; Langford, C.H. Factors influencing relative efficiency in photo-oxidations of organic molecules by Cs3PW12O40 and TiO2 colloidal photocatalysts. J. Photochem. Photobiol. A Chem. 2000, 133, 213–220. [Google Scholar] [CrossRef]
- Qiao, X.; Seo, H.J. A visible-light-driven photocatalyst of cesium vanadate Cs2V4O11 nanowires by hydrothermal method. Mater. Lett. 2014, 136, 322–324. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, J.; Hu, C.; Liu, X.; Wei, D.; Seo, H.J. Cs-doped α-Bi2O3 microplates: Hydrothermal synthesis and improved photochemical activities. Appl. Surf. Sci. 2019, 473, 401–408. [Google Scholar] [CrossRef]
- Rajput, H.; Changotra, R.; Sangal, V.K.; Mahla, S.K.; Dhir, A. A facile synthesis of Cs loaded TiO2 nanotube photoelectrode for the removal of 4-chloroguaiacol. Chemosphere 2019, 218, 687–695. [Google Scholar] [CrossRef]
- Guan, D.Q.; Shi, C.L.; Xu, H.Y.; Gu, Y.X.; Zhong, J.; Sha, Y.C.; Hu, Z.W.; Ni, M.; Shao, Z.P. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Jiang, X.; Liu, L.; Li, X.; Li, H.; Liang, W. In-situ self-assembly of plant polyphenol-coated Fe3O4 particles for oleaginous microalgae harvesting. J. Environ. Manag. 2018, 214, 335–345. [Google Scholar] [CrossRef]
- Sivakarthik, P.; Thangaraj, V.; Parthibavarman, M. A facile and one-pot synthesis of pure and transition metals (M=Co & Ni) doped WO3 nanoparticles for enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2017, 28, 1–7. [Google Scholar]
- Wang, Y.; Zhang, F.; Zhao, G.; Zhao, Y.; Ren, Y.; Zhang, H.; Zhang, L.; Du, J.; Han, Y.; Kang, D.J. Porous WO3 monolith-based photoanodes for high-efficient photoelectrochemical water splitting. Ceram. Int. 2019, 45, 7302–7308. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.N.; Song, Y.F.; Guan, D.Q.; Xu, M.G.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z.P. The BaCe0.16Y0.04Fe0.8O3-δnanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, D.; Li, R.X. Synthesis ofCs0.3WO3 with visible transparency and near-infrared absorption from commercial WO3. J. Solid State Chem. 2022, 306, 122768. [Google Scholar] [CrossRef]
- Windisch, C.F., Jr.; Exarhos, G.J.; Yao, C.; Wang, L.Q. Raman study of the influence of hydrogen on defects in ZnO. J. Appl. Phys. 2007, 101, 1012. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, W.; Li, W.; Bai, X.; Zhao, J.; Tse, E.C.M.; Phillips, D.L.; Zhu, Y. Steering Electron-Hole Migration Pathways Using Oxygen Vacancies in Tungsten Oxides to Enhance Their Photocatalytic Oxygen Evolution Performance. Angew. Chem. 2021, 60, 8236–8242. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chandra, D.; Takeuchi, R.; Togashi, T.; Kurihara, M.; Saito, K.; Yui, T.; Yagi, M. Dual-Functional Surfactant-Templated Strategy for Synthesis of an In Situ N2-Intercalated Mesoporous WO3 Photoanode for Efficient Visible-Light-Driven Water Oxidation. Chem. A Eur. J. 2017, 23, 6596–6604. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, D.; Lin, Q.; Zhang, W.; Shao, Y.; Chen, Y.; Sun, M.; Fu, X. Efficient degradation of benzene over LaVO4/TiO2 nanocrystalline heterojunction photocatalyst under visible light irradiation. Environ. Sci. Technol. 2009, 43, 4164–4168. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, C.; Che, H.; Hu, H.; Hu, W.; Liu, C.; Ai, J.; Dong, H. Decoration of mesoporous Co3O4 nanospheres assembled by monocrystal nanodots on g-C3N4 to construct Z-scheme system for improving photocatalytic performance. Appl. Surf. Sci. 2018, 440, 308–319. [Google Scholar] [CrossRef]
- Ye, M.; Gong, J.; Lai, Y.; Lin, C.; Lin, Z. High-efficiency photoelectrocatalytic hydrogen generation enabled by palladium quantum dots-sensitized TiO2 nanotube arrays. J. Am. Chem. Soc. 2012, 134, 15720–15723. [Google Scholar] [CrossRef]
- Bertoluzzi, L.; Bisquert, J. Equivalent circuit of electrons and holes in thin semiconductor films for photoelectrochemical water splitting applications. J. Phys. Chem. Lett. 2012, 3, 2517–2522. [Google Scholar] [CrossRef]









| Samples | Molar Ratio of Cs/W (a) | Crystallite Diameter (b) (nm) | Lattice Parameters | Surface Area (c) (m2 g−1) | ||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | ||||
| WO3-0 | 0:1 | 27.5 | 7.3125 | 7.5384 | 7.5964 | 9.2 |
| Cs-WO3-0.1 | 0.12:1 | 25.2 | 7.2997 | 7.5009 | 7.5625 | 16.1 |
| Cs-WO3-0.3 | 0.15:1 | 20.9 | 7.2453 | 7.4813 | 7.4953 | 10.6 |
| Cs-WO3-0.5 | 0.17:1 | 19.1 | 7.2046 | 7.4364 | 7.4678 | 8.9 |
| Samples | Absorption Energies | EFB | ND (1019 cm−3) | ECB | EVB |
|---|---|---|---|---|---|
| WO3-0 | 2.81 | 0.66 | 3.68 | 0.56 | 3.37 |
| Cs-WO3-0.1 | 2.38 | 0.21 | 3.78 | 0.11 | 2.49 |
| Cs-WO3-0.3 | 2.47 | 0.43 | 3.82 | 0.33 | 2.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Tian, S.; Qian, Q.; Gao, C.; Shen, H.; Han, F. Cs-Doped WO3 with Enhanced Conduction Band for Efficient Photocatalytic Oxygen Evolution Reaction Driven by Long-Wavelength Visible Light. Molecules 2024, 29, 3126. https://doi.org/10.3390/molecules29133126
Li D, Tian S, Qian Q, Gao C, Shen H, Han F. Cs-Doped WO3 with Enhanced Conduction Band for Efficient Photocatalytic Oxygen Evolution Reaction Driven by Long-Wavelength Visible Light. Molecules. 2024; 29(13):3126. https://doi.org/10.3390/molecules29133126
Chicago/Turabian StyleLi, Dong, Siyu Tian, Qiuhua Qian, Caiyun Gao, Hongfang Shen, and Fei Han. 2024. "Cs-Doped WO3 with Enhanced Conduction Band for Efficient Photocatalytic Oxygen Evolution Reaction Driven by Long-Wavelength Visible Light" Molecules 29, no. 13: 3126. https://doi.org/10.3390/molecules29133126





