Easy and Green Method to Fabricate Highly Thermally Conductive Poly(decamethylene terephthalamide)/Graphite Nanoplatelets Nanocomposite with Aligned Structure
Abstract
:1. Introduction
2. Experimental
2.1. Materials
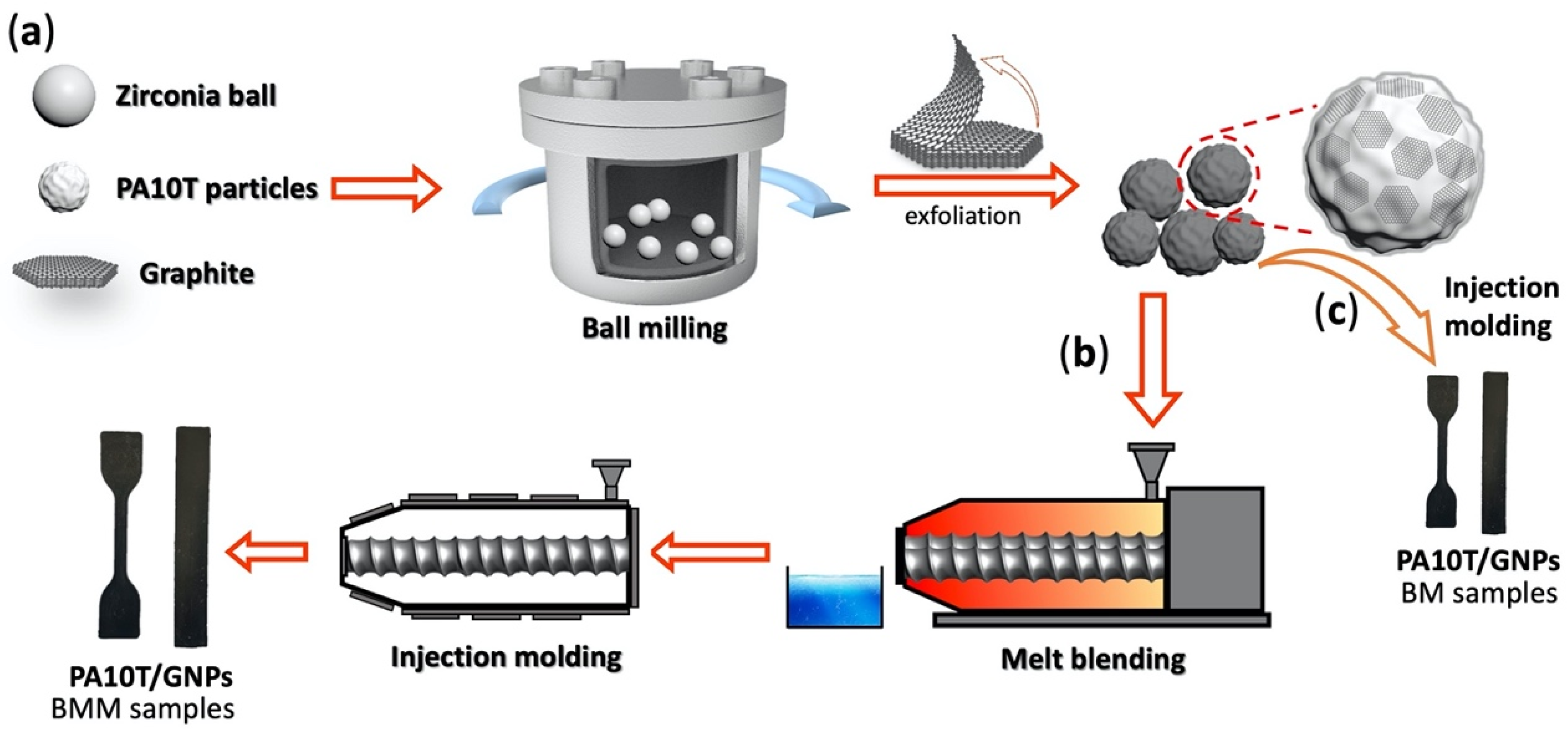
2.2. Fabrication of PA10T/GNP Composites
2.3. Characterization and Measurement
3. Results and Discussion
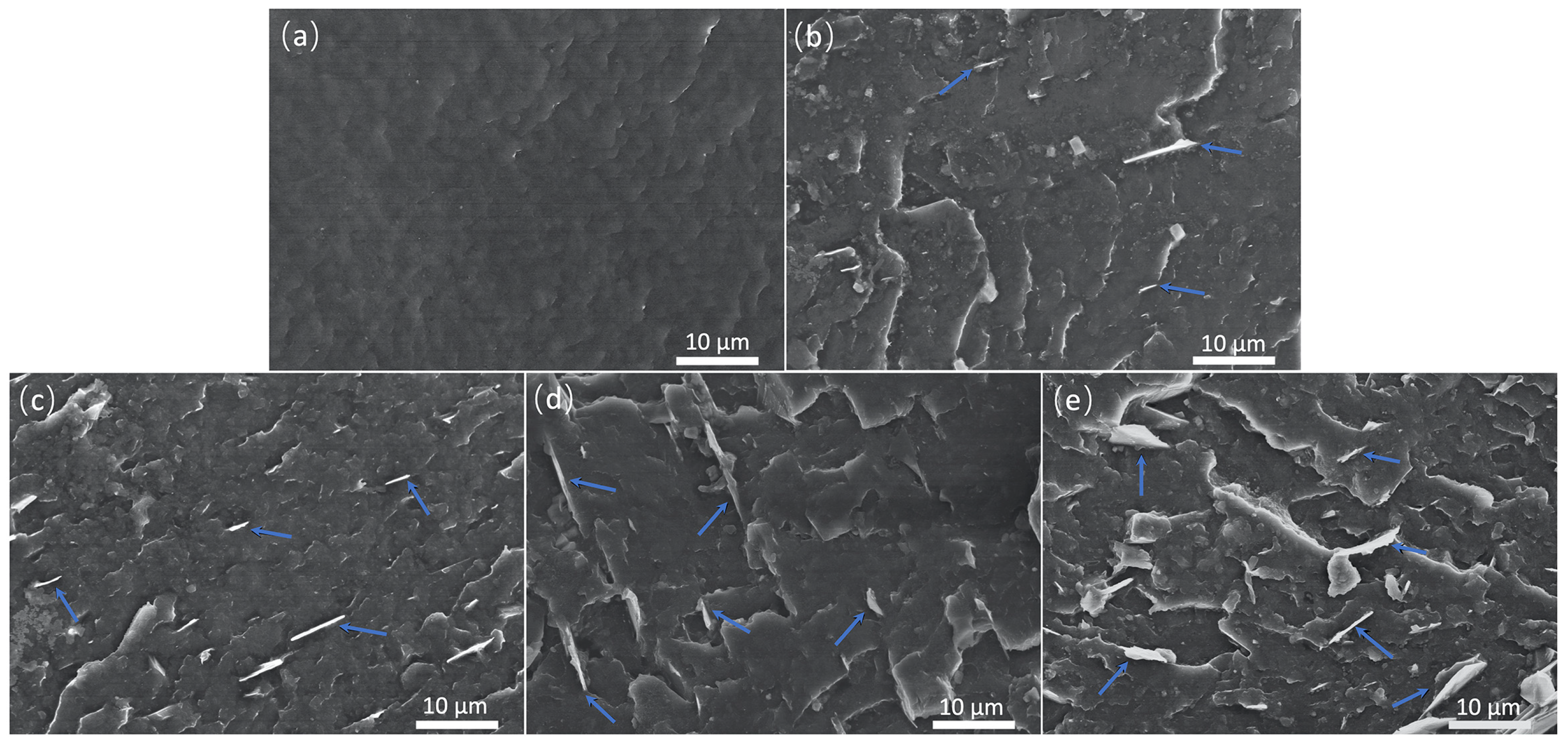
3.1. Characterization of GNPs and Their Dispersion
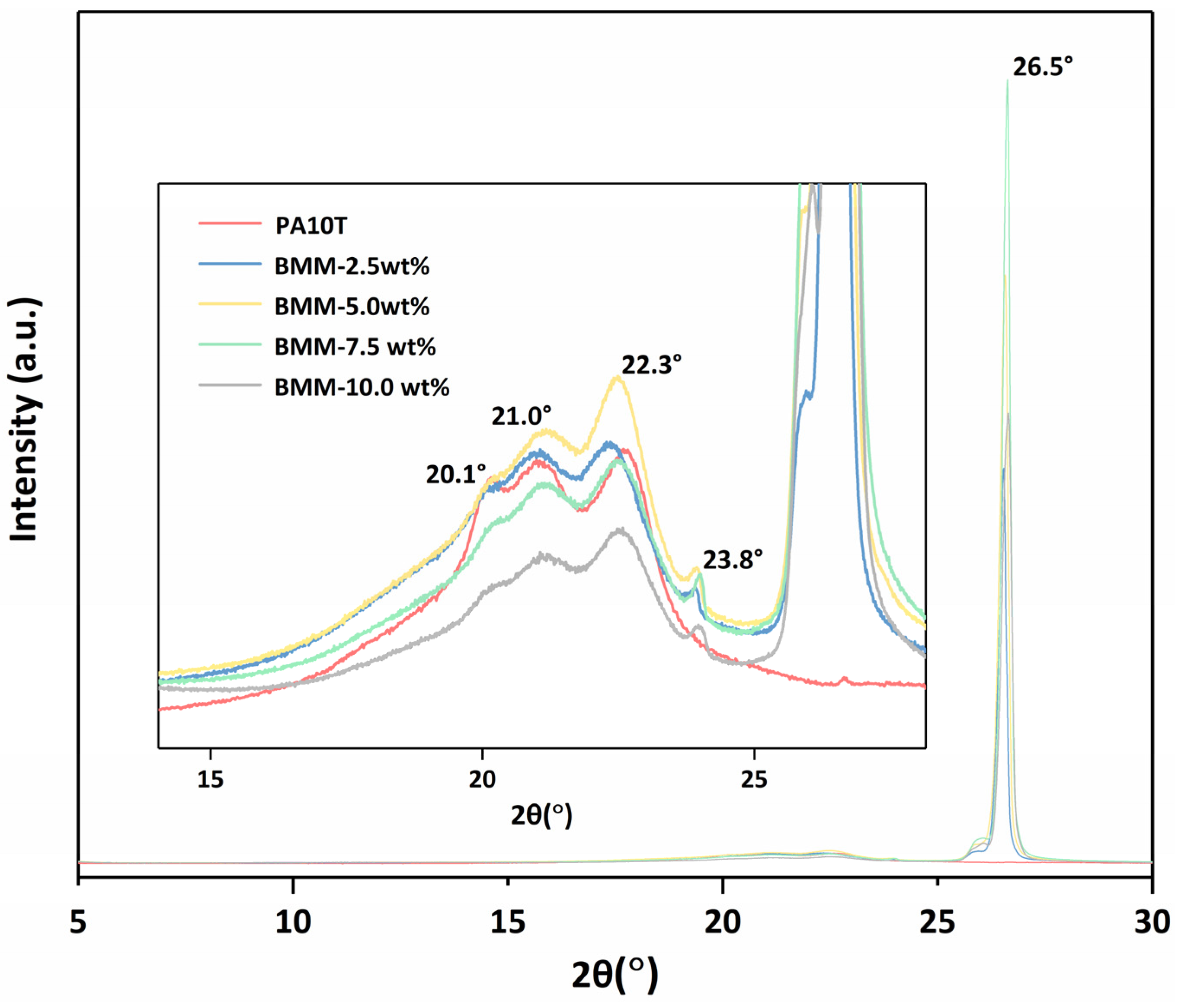
3.2. XRD Analysis
3.3. DSC and TGA Analysis
3.4. DMA Analysis
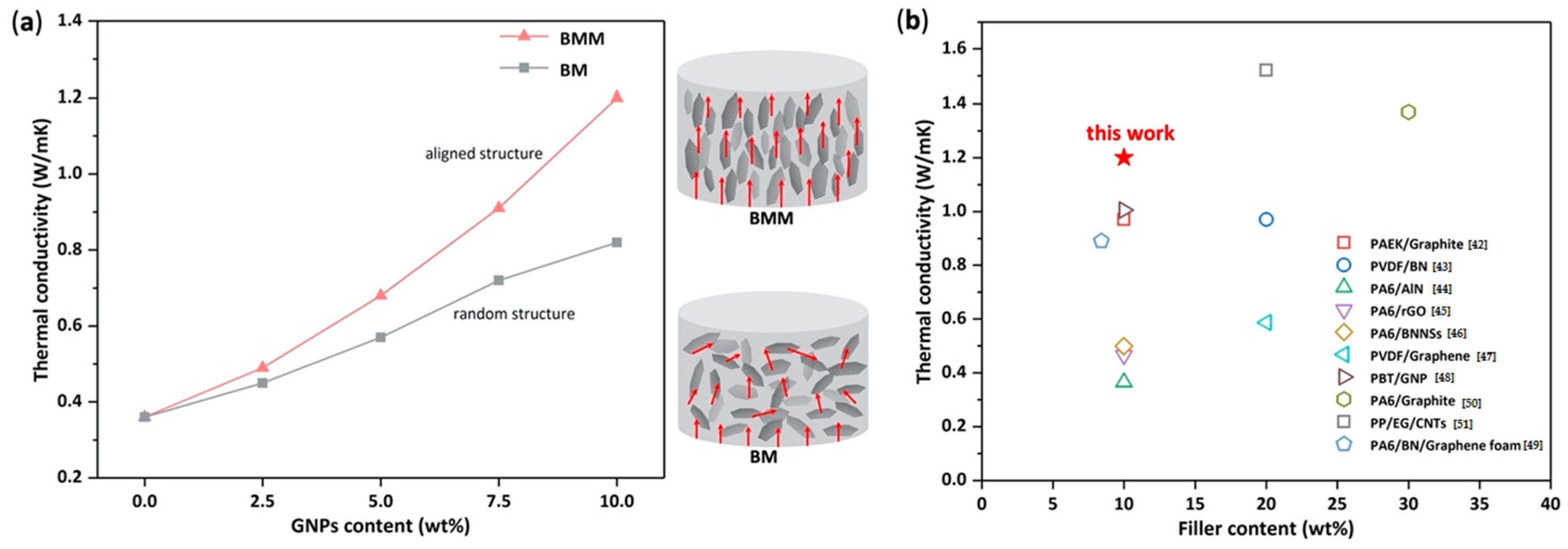
3.5. Thermal Conductivity
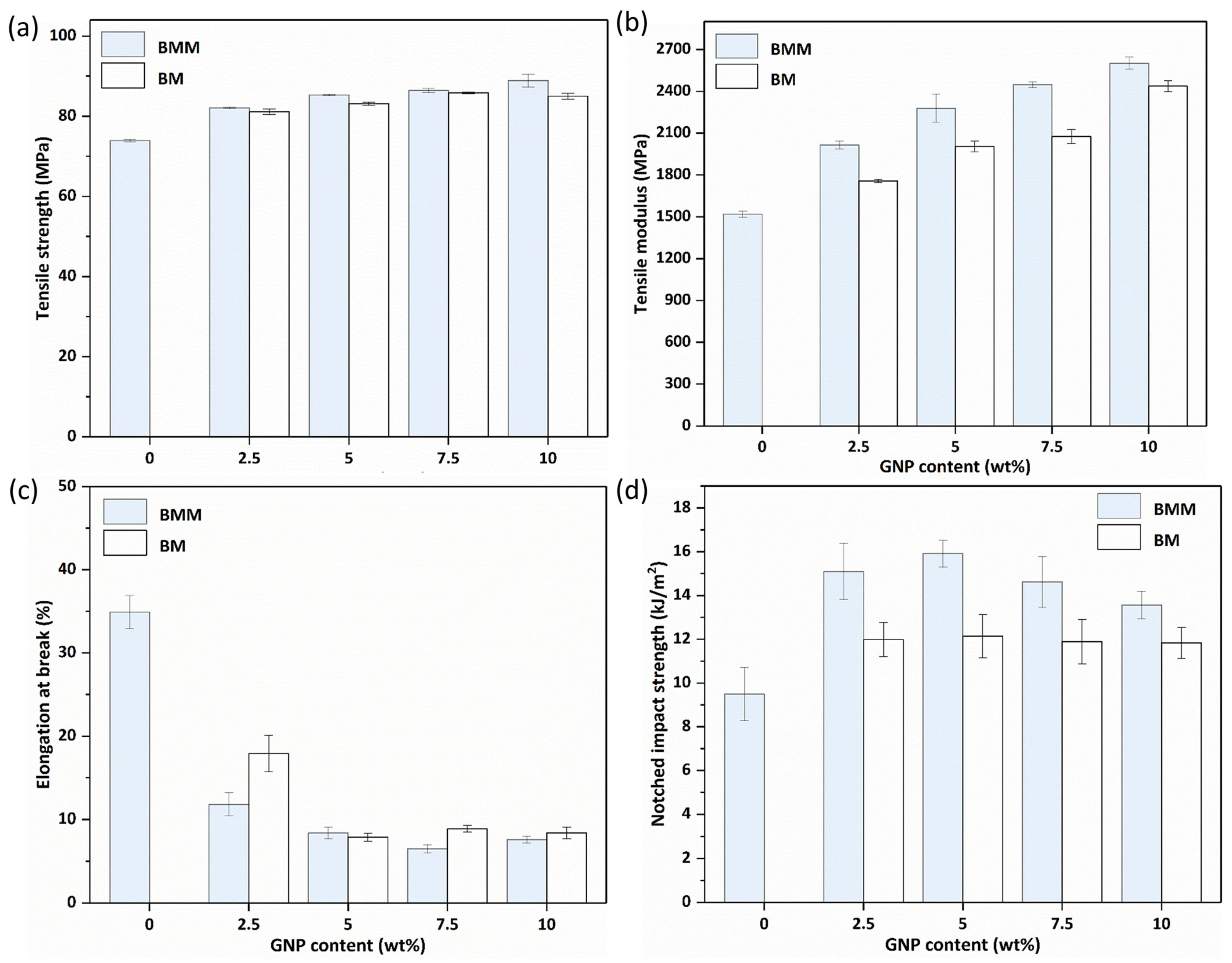
3.6. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Im, H.; Kim, J. Thermal conductivity of a graphene oxide-carbon nanotube hybrid/epoxy composite. Carbon 2012, 50, 5429–5440. [Google Scholar] [CrossRef]
- Song, N.; Cao, D.; Luo, X.; Guo, Y.; Gu, J.; Ding, P. Aligned cellulose/nanodiamond plastics with high thermal conductivity. J. Mater. Chem. C 2018, 6, 13108–13113. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal Conductivity of Polymers and Their Nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef]
- Ai, T.; Zou, G.; Feng, W.; Wang, P.; Lu, B.; Ren, Z.; Ji, J. Biobased Semiaromatic Polyamides Derived from Dimethyl Furan-2,5-Dicarboxylate: Influence of the Composition on Their Properties. ACS Sustain. Chem. Eng. 2022, 10, 13792–13804. [Google Scholar] [CrossRef]
- Ai, T.; Zou, G.; Feng, W.; Ren, Z.; Li, F.; Wang, P.; Lu, B.; Ji, J. Synthesis and properties of biobased copolyamides based on polyamide 10T and polyamide 56 through one-pot polymerization. New J. Chem. 2021, 45, 14677–14686. [Google Scholar] [CrossRef]
- Feng, W.; Zou, G.; Ding, Y.; Ai, T.; Wang, P.; Ren, Z.; Ji, J. Effect of Aliphatic Diacid Chain Length on Properties of Semiaromatic Copolyamides Based on PA10T and Their Theoretical Study. Ind. Eng. Chem. Res. 2019, 58, 7217–7226. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Wang, Y.L.; Wan, Y.Z.; Zhou, F.G.; Dong, X.H. Preparation and properties of in situ polymerized fiber-reinforced nylon composites. J. Mater. Sci. Lett. 2002, 21, 987–989. [Google Scholar] [CrossRef]
- Ren, Y.; Ren, L.; Li, J.; Lv, R.; Wei, L.; An, D.; Maqbool, M.; Bai, S.; Wong, C.-P. Enhanced thermal conductivity in polyamide 6 composites based on the compatibilization effect of polyether-grafted graphene. Compos. Sci. Technol. 2020, 199, 108340. [Google Scholar] [CrossRef]
- He, X.; Wang, Y. Highly Thermally Conductive Polyimide Composite Films with Excellent Thermal and Electrical Insulating Properties. Ind. Eng. Chem. Res. 2020, 59, 1925–1933. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Wei, X.; Bai, M.-H.; Lei, J.; Xu, L.; Huang, H.-D.; Du, J.; Dai, K.; Xu, J.-Z.; Li, Z.-M. Green Production of Covalently Functionalized Boron Nitride Nanosheets via Saccharide-Assisted Mechanochemical Exfoliation. ACS Sustain. Chem. Eng. 2021, 9, 11155–11162. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Wang, Y.; Song, Y.; Alam, F.E.; Nishimura, K.; Lin, C.-T.; Jiang, N. Enhanced thermal conductivity for polyimide composites with a three-dimensional silicon carbide nanowire@graphene sheets filler. J. Mater. Chem. A 2015, 3, 4884–4891. [Google Scholar] [CrossRef]
- Ouyang, Y.; Bai, L.; Tian, H.; Li, X.; Yuan, F. Recent progress of thermal conductive ploymer composites: Al2O3 fillers, properties and applications. Compos. Part A-Appl. Sci. Manuf. 2022, 152, 106685. [Google Scholar] [CrossRef]
- Zhuang, Y.F.; Cao, X.Y.; Zhang, J.N.; Ma, Y.Y.; Shang, X.X.; Lu, J.X.; Yang, S.L.; Zheng, K.; Ma, Y.M. Monomer casting nylon/graphene nanocomposite with both improved thermal conductivity and mechanical performance. Compos. Part A-Appl. Sci. Manuf. 2019, 120, 49–55. [Google Scholar] [CrossRef]
- Song, S.; Hu, B.; Qu, G.; Wang, Z.; Qi, G.; Tang, K.; Li, B. Reinforced Interfacial Interaction to Fabricate Poly(vinylidene fluoride) Composites with High Thermal Conductivity for Heat Exchangers. Ind. Eng. Chem. Res. 2020, 59, 17845–17855. [Google Scholar] [CrossRef]
- Liu, H.; Gu, S.; Cao, H.; Li, X.; Li, Y. A dense packing structure constructed by flake and spherical graphite: Simultaneously enhanced in-plane and through-plane thermal conductivity of polypropylene/graphite composites. Compos. Commun. 2020, 19, 25–29. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Sun, J.; Zhao, Z.; Zhan, S.; Guo, Y.; Zhou, H.; Liu, W.; Wang, J.; Zhao, T. Thermally conductive and electrically insulating alumina-coated graphite/phthalonitrile composites with thermal stabilities. Compos. Sci. Technol. 2021, 202, 108558. [Google Scholar] [CrossRef]
- Qian, T.; Zhu, S.; Wang, H.; Li, A.; Fan, B. Comparative Study of Single-Walled Carbon Nanotubes and Graphene Nanoplatelets for Improving the Thermal Conductivity and Solar-to-Light Conversion of PEG-Infiltrated Phase-Change Material Composites. ACS Sustain. Chem. Eng. 2019, 7, 2446–2458. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Guo, H.-L.; Wang, X.-F.; Qian, Q.-Y.; Wang, F.-B.; Xia, X.-H. A Green Approach to the Synthesis of Graphene Nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Ong, T.S.; Yang, H. Effect of atmosphere on the mechanical milling of natural graphite. Carbon 2000, 38, 2077–2085. [Google Scholar] [CrossRef]
- Wei, P.; Cui, S.; Bai, S. In situ exfoliation of graphite in solid phase for fabrication of graphene/polyamide-6 composites. Compos. Sci. Technol. 2017, 153, 151–159. [Google Scholar] [CrossRef]
- Rosehr, A.; Griebe, D.; Luinstra, G.A. Graphite nanosheets—Polypropylene composites from in toluene delaminated graphite using atactic polypropylene as dispersant. Compos. Sci. Technol. 2018, 156, 28–38. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Mortazavi, B.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Alignments and network of graphite fillers to improve thermal conductivity of epoxy-based composites. Int. J. Heat Mass Transf. 2015, 89, 505–513. [Google Scholar] [CrossRef]
- Yuan, F.; Jiao, W.; Yang, F.; Liu, W.; Xu, Z.; Wang, R. Surface modification and magnetic alignment of hexagonal boron nitride nanosheets for highly thermally conductive composites. RSC Adv. 2017, 7, 43380–43389. [Google Scholar] [CrossRef]
- Luo, F.; Wu, K.; Huang, X.; Hu, W.; Lu, M. Encapsulation of Graphite Nanoflakes for Improving Thermal Conductivity of Mesogenic Epoxy Composites. Ind. Eng. Chem. Res. 2017, 56, 489–494. [Google Scholar] [CrossRef]
- GB/T 1042.2-1992; Method for Field Test of Natural Durability of Wood. Ministry of Forestry of the People Republic of China: Beijing, China, 1992.
- GB/T 1843.1-2008; Plastics. Determination of Izod Impact Strength. China Petroleum and Chemical Industry Association: Beijing, China, 2008.
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. In situ Polymerization Approach to Graphene-Reinforced Nylon-6 Composites. Macromolecules 2010, 43, 6716–6723. [Google Scholar] [CrossRef]
- Jin, J.; Rafiq, R.; Gill, Y.Q.; Song, M. Preparation and characterization of high performance of graphene/nylon nanocomposites. Eur. Polym. J. 2013, 49, 2617–2626. [Google Scholar] [CrossRef]
- Ho, Q.B.; Osazuwa, O.; Modler, R.; Daymond, M.; Gallerneault, M.T.; Kontopoulou, M. Exfoliation of graphite and expanded graphite by melt compounding to prepare reinforced, thermally and electrically conducting polyamide composites. Compos. Sci. Technol. 2019, 176, 111–120. [Google Scholar] [CrossRef]
- Song, H.D.; Im, Y.-K.; Baek, J.-B.; Jeon, I.-Y. Heptene-functionalized graphitic nanoplatelets for high-performance composites of linear low-density polyethylene. Compos. Sci. Technol. 2020, 199, 108380. [Google Scholar] [CrossRef]
- Zou, G.; Wang, P.; Feng, W.; Ren, Z.; Ji, J. Poly(decamethylene terephthalamide) copolymerized with long-chain alkyl dodecanedioic acid: Toward bio-based polymer and improved performances. J. Appl. Polym. Sci. 2018, 135, 46531. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Li, H.; Yan, C. Facile preparation route for graphene oxide reinforced polyamide 6 composites via in situ anionic ring-opening polymerization. J. Mater. Chem. 2012, 22, 24081–24091. [Google Scholar] [CrossRef]
- Shen, J.; Zeng, C.C.; Lee, L.J. Synthesis of poly styrene-carbon nanofibers nanocomposite foams. Polymer 2005, 46, 5218–5224. [Google Scholar] [CrossRef]
- Rudzinski, S.; Haeussler, L.; Harnisch, C.; Maeder, E.; Heinrich, G. Glass fibre reinforced polyamide composites: Thermal behaviour of sizings. Compos. Part A-Appl. Sci. Manuf. 2011, 42, 157–164. [Google Scholar] [CrossRef]
- Li, J.; Tong, L.; Fang, Z.; Gu, A.; Xu, Z. Thermal degradation behavior of multi-walled carbon nanotubes/polyamide 6 composites. Polym. Degrad. Stab. 2006, 91, 2046–2052. [Google Scholar] [CrossRef]
- Zhang, X.; Loo, L.S. Study of Glass Transition and Reinforcement Mechanism in Polymer/Layered Silicate Nanocomposites. Macromolecules 2009, 42, 5196–5207. [Google Scholar] [CrossRef]
- Kafi, A.; Huson, M.; Creighton, C.; Khoo, J.; Mazzola, L.; Gengenbach, T.; Jones, F.; Fox, B. Effect of surface functionality of PAN-based carbon fibres on the mechanical performance of carbon/epoxy composites. Compos. Sci. Technol. 2014, 94, 89–95. [Google Scholar] [CrossRef]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.-S.; Paik, K.-W.; Jeon, S. Enhanced Thermal Conductivity of EpoxyGraphene Composites by Using Non-Oxidized Graphene Flakes with Non-Covalent Functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Asmatulu, R.; Soltani, S.A.; Le, L.N.; Kumar, S.S.A. Mechanical and Thermal Properties of Hierarchical Composites Enhanced by Pristine Graphene and Graphene Oxide Nanoinclusions. J. Appl. Polym. Sci. 2014, 131, 40826. [Google Scholar] [CrossRef]
- Lee, M.; Son, K.; Kim, J.; Kim, D.; Min, B.H.; Kim, J.H. Effect of PA6T on morphology and electrical conductivity in PA66/PA6T/PPE/multiwalled carbon nanotube nanocomposites. Compos. Sci. Technol. 2018, 164, 260–266. [Google Scholar] [CrossRef]
- Panda, J.N.; Bijwe, J.; Pandey, R.K. Variation in size of graphite particles and its cascading effect on the performance properties of PAEK composites. Compos. Part B-Eng. 2020, 182, 107641. [Google Scholar] [CrossRef]
- Xiao, Y.-j.; Wang, W.-y.; Lin, T.; Chen, X.-j.; Zhang, Y.-t.; Yang, J.-h.; Wang, Y.; Zhou, Z.-w. Largely Enhanced Thermal Conductivity and High Dielectric Constant of Poly(vinylidene fluoride)/Boron Nitride Composites Achieved by Adding a Few Carbon Nanotubes. J. Phys. Chem. C 2016, 120, 6344–6355. [Google Scholar] [CrossRef]
- Wang, F.; Shi, W.; Mai, Y.; Liao, B. Effect of Thermal Conductive Fillers on the Flame Retardancy, Thermal Conductivity, and Thermal Behavior of Flame-Retardant and Thermal Conductive Polyamide 6. Materials 2019, 12, 4114. [Google Scholar] [CrossRef]
- Ding, P.; Su, S.; Song, N.; Tang, S.; Liu, Y.; Shi, L. Highly thermal conductive composites with polyamide-6 covalently-grafted graphene by an in situ polymerization and thermal reduction process. Carbon 2014, 66, 576–584. [Google Scholar] [CrossRef]
- Guo, H.; Xu, T.; Zhou, S.; Jiang, F.; Jin, L.; Song, N.; Ding, P. A technique engineered for improving thermal conductive properties of polyamide-6 composites via hydroxylated boron nitride masterbatch-based melt blending. Compos. Part B-Eng. 2021, 212, 108716. [Google Scholar] [CrossRef]
- Guo, H.; Li, X.; Li, B.; Wang, J.; Wang, S. Thermal conductivity of graphene/poly(vinylidene fluoride) nanocomposite membrane. Mater. Des. 2017, 114, 355–363. [Google Scholar] [CrossRef]
- Colonna, S.; Monticelli, O.; Gomez, J.; Novara, C.; Saracco, G.; Fina, A. Effect of morphology and defectiveness of graphene-related materials on the electrical and thermal conductivity of their polymer nanocomposites. Polymer 2016, 102, 292–300. [Google Scholar] [CrossRef]
- Shao, L.; Shi, L.; Li, X.; Song, N.; Ding, P. Synergistic effect of BN and graphene nanosheets in 3D framework on the enhancement of thermal conductive properties of polymeric composites. Compos. Sci. Technol. 2016, 135, 83–91. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, Y.; Zou, H.; Liang, M. Thermally conductive composites obtained by flake graphite filling immiscible Polyamide 6/Polycarbonate blends. Thermochim. Acta 2013, 566, 84–91. [Google Scholar] [CrossRef]
- Wu, K.; Xue, Y.; Yang, W.; Chai, S.; Chen, F.; Fu, Q. Largely enhanced thermal and electrical conductivity via constructing double percolated filler network in polypropylene/expanded graphite—Multi-wall carbon nanotubes ternary composites. Compos. Sci. Technol. 2016, 130, 28–35. [Google Scholar] [CrossRef]
- Wang, X.; Jin, J.; Song, M. An investigation of the mechanism of graphene toughening epoxy. Carbon 2013, 65, 324–333. [Google Scholar] [CrossRef]
- Abel, G.A.; Viamajala, S.; Varanasi, S.; Yamamoto, K. Toward Sustainable Synthesis of PA12 (Nylon-12) Precursor from Oleic Acid Using Ring-Closing Metathesis. ACS Sustain. Chem. Eng. 2016, 4, 5703–5710. [Google Scholar] [CrossRef]


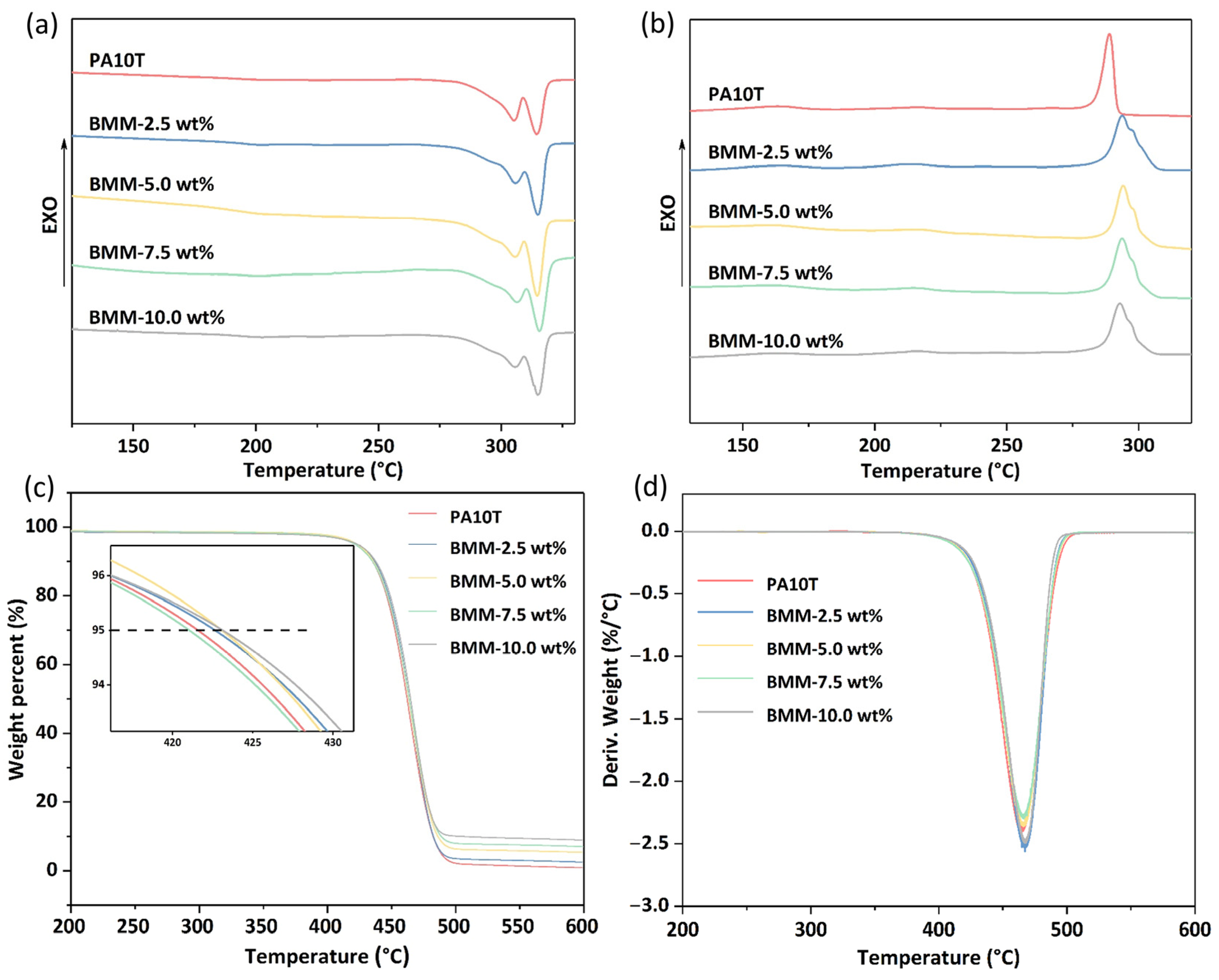

| Samples | Tm (°C) | Tc (°C) | ΔHm (J/g) | Td,5% (°C) | Td,10% (°C) | Td,max (°C) |
|---|---|---|---|---|---|---|
| PA10T | 314.5 | 288.9 | 59.2 | 421.6 | 435 | 464.6 |
| BMM-2.5 wt% | 314.9 | 293.8 | 61.2 | 422.7 | 436.5 | 467.3 |
| BMM-5.0 wt% | 314.7 | 294.1 | 60.7 | 423.1 | 435.8 | 466.1 |
| BMM-7.5 wt% | 315.6 | 293.8 | 58.7 | 421.1 | 435.1 | 466.2 |
| BMM-10.0 wt% | 314.9 | 292.8 | 56.6 | 423.1 | 437.6 | 467.2 |
| Samples | Tm (°C) | Tc (°C) | ΔHm (J/g) | Td,5% (°C) | Td,10% (°C) | Td,max (°C) |
|---|---|---|---|---|---|---|
| PA10T | 314.5 | 288.9 | 59.2 | 421.6 | 435 | 464.6 |
| BMM-2.5 wt% | 314.9 | 293.8 | 61.2 | 422.7 | 436.5 | 467.3 |
| BMM-5.0 wt% | 314.7 | 294.1 | 60.7 | 423.1 | 435.8 | 466.1 |
| BMM-7.5 wt% | 315.6 | 293.8 | 58.7 | 421.1 | 435.1 | 466.2 |
| BMM-10.0 wt% | 314.9 | 292.8 | 56.6 | 423.1 | 437.6 | 467.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Ai, T.; Wang, P.; Ji, J. Easy and Green Method to Fabricate Highly Thermally Conductive Poly(decamethylene terephthalamide)/Graphite Nanoplatelets Nanocomposite with Aligned Structure. Molecules 2024, 29, 3141. https://doi.org/10.3390/molecules29133141
Xu P, Ai T, Wang P, Ji J. Easy and Green Method to Fabricate Highly Thermally Conductive Poly(decamethylene terephthalamide)/Graphite Nanoplatelets Nanocomposite with Aligned Structure. Molecules. 2024; 29(13):3141. https://doi.org/10.3390/molecules29133141
Chicago/Turabian StyleXu, Pengyuan, Tianhao Ai, Pingli Wang, and Junhui Ji. 2024. "Easy and Green Method to Fabricate Highly Thermally Conductive Poly(decamethylene terephthalamide)/Graphite Nanoplatelets Nanocomposite with Aligned Structure" Molecules 29, no. 13: 3141. https://doi.org/10.3390/molecules29133141




