Characterisation of New Zealand Propolis from Different Regions Based on Its Volatile Organic Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Resin Analyses
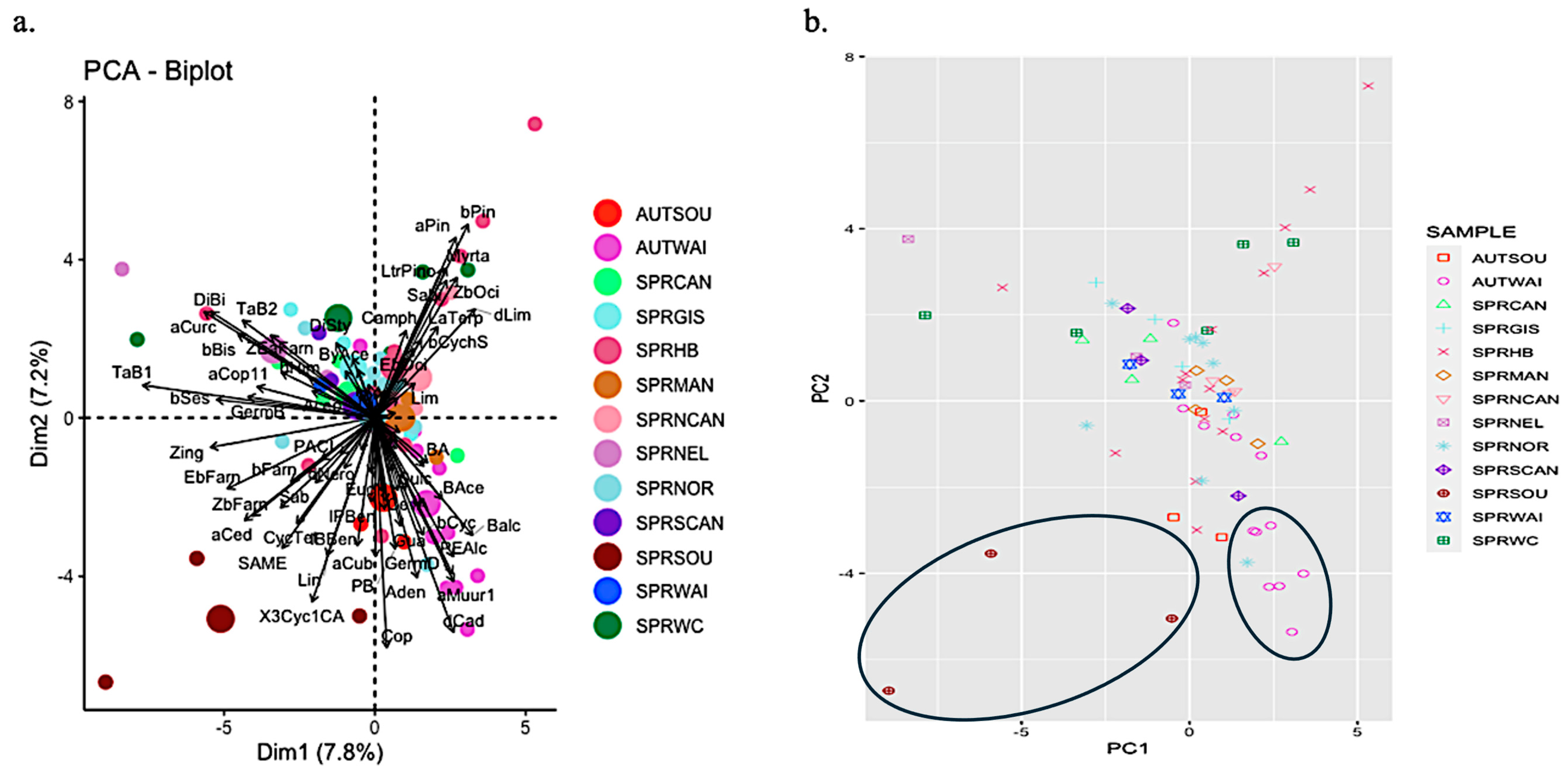
2.2. Propolis Analyses

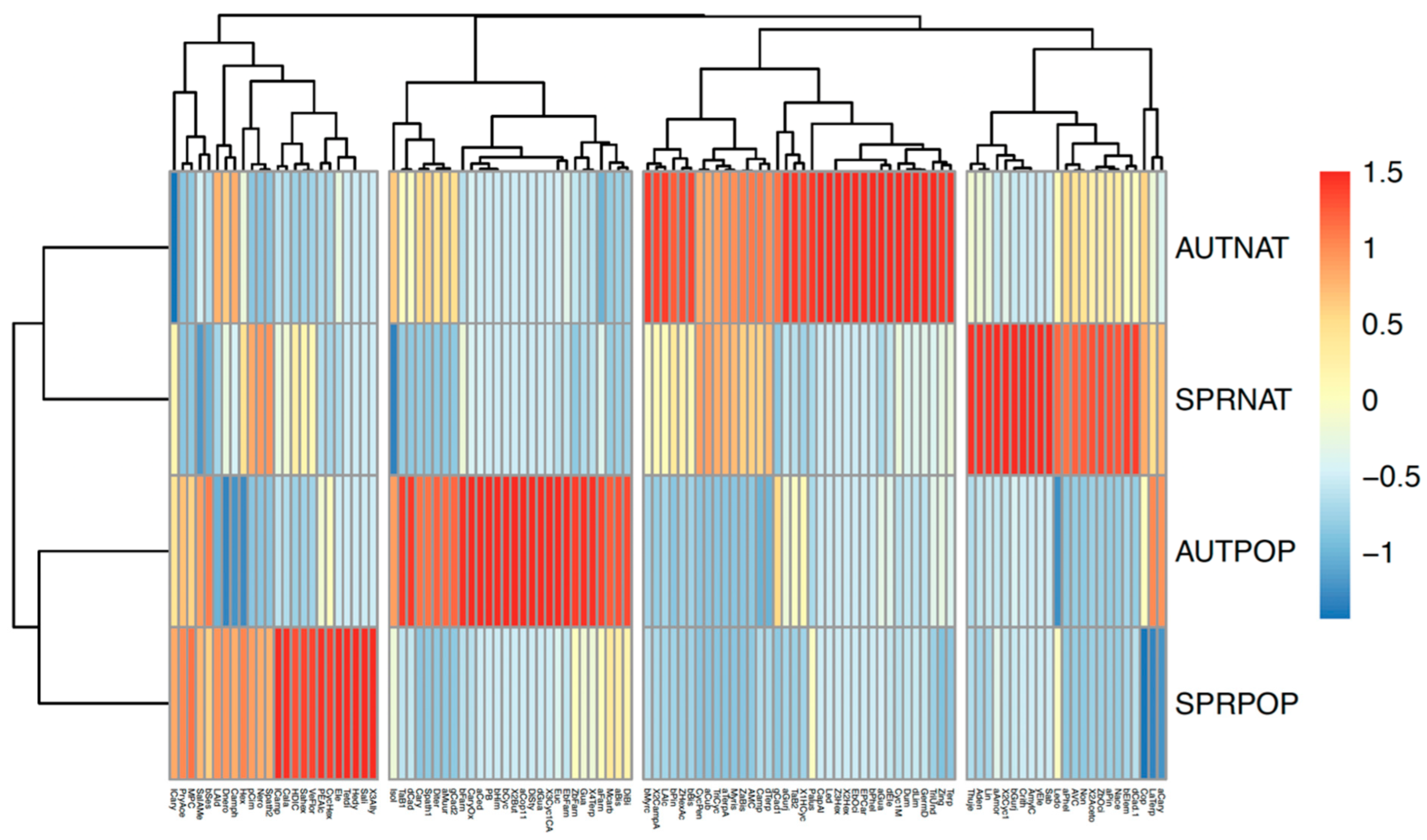
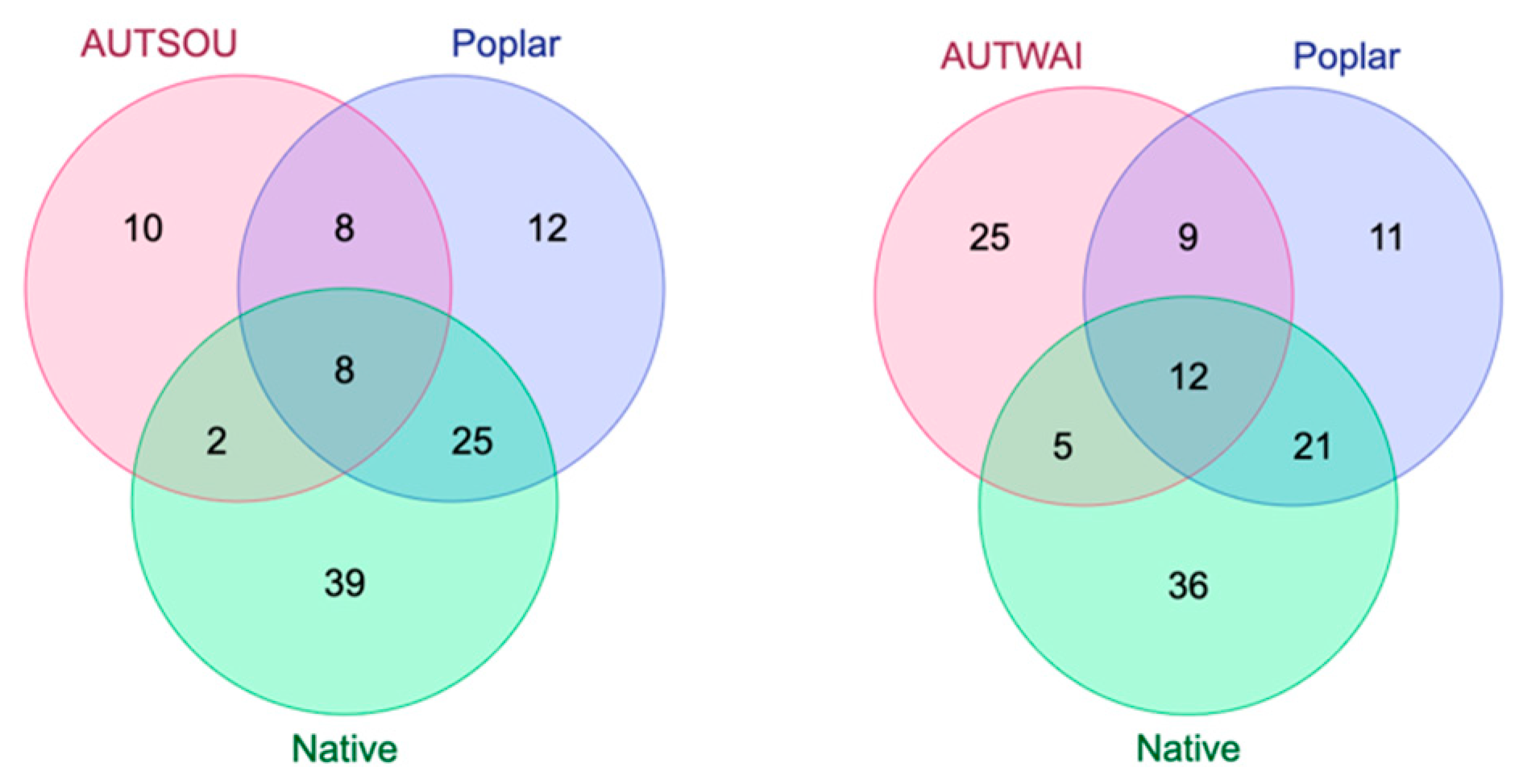
2.3. Comparison of Resin and Propolis Samples
2.4. Discussion
3. Materials and Methods
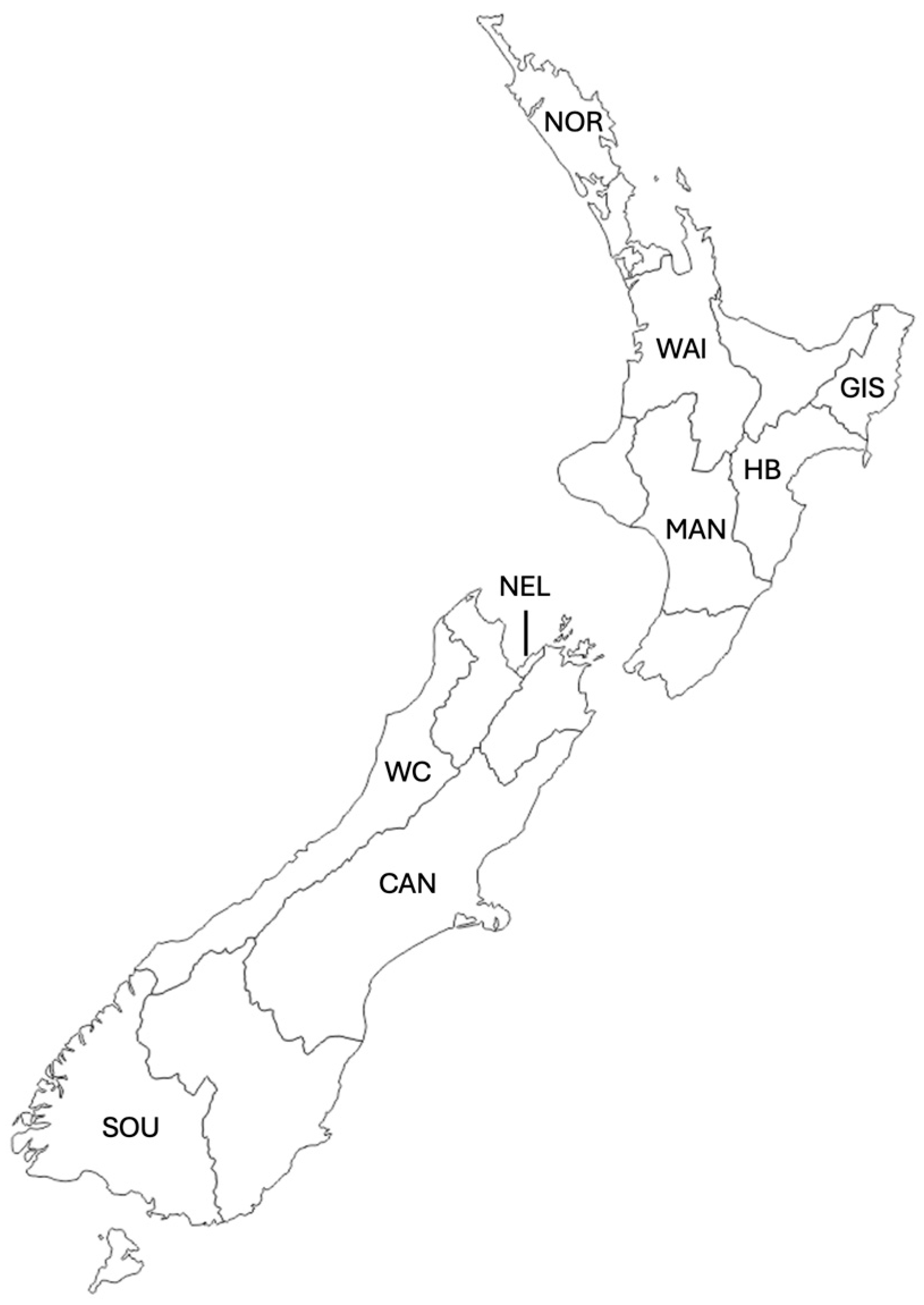
3.1. Resin Sample Collection and Extraction
3.2. Propolis Sample Collection
3.3. Headspace Solid-Phase Microextraction (HS-SPME) Procedure for Propolis Samples
3.4. Gas Chromatography–Mass Spectrometry Analyses for Resin and Propolis Samples
3.5. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mountford-McAuley, R.; Prior, J.; Clavijo McCormick, A. Factors affecting propolis production. J. Apic. Res. 2023, 62, 162–170. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M. Bee Products—Chemical and Biological Properties; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Crane, E. Bee products. In Encyclopedia of Insects; Elsevier: Amsterdam, The Netherlands, 2009; pp. 71–75. [Google Scholar]
- Ghisalberti, E. Propolis: A review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Borba, R.S.; Klyczek, K.K.; Mogen, K.L.; Spivak, M. Seasonal benefits of a natural propolis envelope to honey bee immunity and colony health. J. Exp. Biol. 2015, 218, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Borba, R.S.; Wilson, M.B.; Spivak, M. Hidden benefits of honey bee propolis in hives. In Beekeeping—From Science to Practice; Springer: Cham, Switzerland, 2017; pp. 17–38. [Google Scholar]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, individual, and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Harriet, J.; Gende, L.; Maggi, M.; Eguaras, M.; Zunino, P. Efficacy of natural propolis extract in the control of American Foulbrood. Vet. Microbiol. 2008, 131, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Bastos, E.M.A.; Simone, M.; Jorge, D.M.; Soares, A.E.E.; Spivak, M. In vitro study of the antimicrobial activity of Brazilian propolis against Paenibacillus larvae. J. Invertebr. Pathol. 2008, 97, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Borba, R.S.; Wilson, M.; Spivak, M. Propolis counteracts some threats to honey bee health. Insects 2017, 8, 46. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Cheng, P.C.; Wong, G. Honey bee propolis: Prospects in medicine. Bee World 1996, 77, 8–15. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Garcıa-Viguera, C.; Bankova, V.; De Castro, S.; Dantas, A.P.; Valente, P.; Paulino, N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Cardile, V.; Sanchez, F.; Troncoso, N.; Vanella, A.; Garbarino, J. Chilean propolis: Antioxidant activity and antiproliferative action in human tumor cell lines. Life Sci. 2004, 76, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.A.; Libério, S.A.; Guerra, R.N.; Ribeiro, M.N.S.; Nascimento, F.R. Mechanisms of action underlying the anti-inflammatory and immunomodulatory effects of propolis: A brief review. Rev. Bras. Farmacogn. 2012, 22, 208–219. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, W.; Scaysbrook, T.; Whatley, F. The composition and plant origins of propolis: A report of work at Oxford. Bee World 1990, 71, 107–118. [Google Scholar] [CrossRef]
- Letullier, C.; Manduchet, A.; Dlalah, N.; Hugou, M.; Georgé, S.; Sforcin, J.M.; Cardinault, N. Comparison of the antibacterial efficiency of propolis samples from different botanical and geographic origins with and without standardization. J. Apic. Res. 2020, 59, 19–24. [Google Scholar] [CrossRef]
- Cracco, P.; Cabrera, M.C.; Galietta, G. Physicochemical characterization of georeferenced propolis from 14 locations of Uruguay. Agrociencia Urug. 2023, 27, 1–12. [Google Scholar]
- Grassi, G.; Capasso, G.; Gambacorta, E.; Perna, A.M. Chemical and functional characterization of propolis collected from different areas of South Italy. Foods 2023, 12, 3481. [Google Scholar] [CrossRef]
- Herrera-López, M.G.; Richomme, P.; Peña-Rodríguez, L.M.; Calvo-Irabien, L.M. Bee species, botanical sources and the chemical composition of propolis from Yucatan, Mexico. J. Chem. Ecol. 2023, 49, 408–417. [Google Scholar] [CrossRef]
- Kyomya, J.; Kirabo, M.K.; Mayoka, W.J.; Namunyenga, R.; Jaggwe, R.; Imanirampa, L.; Tusiimire, J. Variation of antioxidant and antibacterial activities of ethanolic extracts of propolis in three bee-keeping agro-ecological zones of Uganda. Afr. J. Pharm. Pharmacol. 2023, 17, 118–127. [Google Scholar]
- Lima, A.B.D.; Oliveira, T.F.; Silva, M.V.D.; Ferrão, S.P.; Almeida, V.V.D.; Santos, L.S. Red propolis: Chemical and mid-infrared spectroscopic characterization and classification by geographic origin. J. Braz. Chem. Soc. 2024, 35, e-20240055. [Google Scholar]
- Sahinler, N.; Kaftanoglu, O. Natural product propolis: Chemical composition. Nat. Prod. Res. 2005, 19, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How diverse is the chemistry and plant origin of Brazilian propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Bankova, V.; Christov, R.; Popov, S.; Pureb, O.; Bocari, G. Volatile constituents of propolis. Z. Für Naturforschung C 1994, 49, 6–10. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Krofczik, S.; Menzel, R.; Nawrot, M.P. Rapid odor processing in the honey bee antennal lobe network. Front. Comput. Neurosci. 2009, 2, 287. [Google Scholar]
- Velikova, M.; Bankova, V.; Sorkun, K.; Houcine, S.; Tsvetkova, I.; Kujumgiev, A. Propolis from the Mediterranean region: Chemical composition and antimicrobial activity. Z. Für Naturforschung C 2000, 55, 790–793. [Google Scholar] [CrossRef]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.; Umsza-Guez, M.A.; Barbosa, J.D.; Portela, R.D.; Machado, B.A. Propolis: Types, composition, biological activities, and veterinary product patent prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, A.C.H.F.; Barbosa da Silva Cunha, I.; Marcucci, M.C. Analytical methods applied to diverse types of Brazilian propolis. Chem. Cent. J. 2011, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- López, B.G.-C.; Schmidt, E.M.; Eberlin, M.N.; Sawaya, A.C. Phytochemical markers of different types of red propolis. Food Chem. 2014, 146, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical diversity and challenges in quality control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Antiproliferative activity of New Zealand propolis and phenolic compounds vs human colorectal adenocarcinoma cells. Fitoterapia 2015, 106, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S.; Catchpole, O.; Mitchell, K.; Webby, R.; Davis, P. Antiproliferative acylated glycerols from New Zealand propolis. J. Nat. Prod. 2019, 82, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, L.; Zhang, H.; Yang, X.; Lv, Y.; Song, H. Common aroma-active components of propolis from 23 regions of China. J. Sci. Food Agric. 2010, 90, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, L.G.; Rodríguez, S.A.; Nazareno, M.A. Comparative analysis of volatile compound profiles of propolis from different provenances. J. Sci. Food Agric. 2018, 98, 3409–3415. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Stratis, E.; Chinou, I. Volatile constituents of propolis from various regions of Greece—Antimicrobial activity. Food Chem. 2007, 103, 375–380. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Tun, K.M.; Minor, M.; Jones, T.; McCormick, A.C. Volatile profiling of fifteen willow species and hybrids and their responses to giant willow aphid infestation. Agronomy 2020, 10, 1404. [Google Scholar] [CrossRef]
- Effah, E.; Min Tun, K.; Rangiwananga, N.; Clavijo McCormick, A. Mānuka clones differ in their volatile profiles: Potential implications for plant defence, pollinator attraction and bee products. Agronomy 2022, 12, 169. [Google Scholar] [CrossRef]
- Kumeroa, F.; Komahan, S.; Sofkova-Bobcheva, S.; Clavijo McCormick, A. Characterization of the volatile profiles of six industrial hemp (Cannabis sativa L.) cultivars. Agronomy 2022, 12, 2651. [Google Scholar] [CrossRef]
- Simionatto, E.; Facco, J.T.; Morel, A.F.; Giacomelli, S.R.; Linares, C.E. Chiral analysis of monoterpenes in volatile oils from propolis. J. Chil. Chem. Soc. 2012, 57, 1240–1243. [Google Scholar] [CrossRef]
- Falcão, S.; Freire, C.; Figueiredo, A.C.; Vilas-Boas, M. The volatile composition of Portuguese propolis towards its origin discrimination. Rec. Nat. Prod. 2015, 10, 176–188. [Google Scholar]
- Giovanini de Oliveira Sartori, A.; Papa Spada, F.; Pena Ribeiro, V.; Rosalen, P.L.; Ikegaki, M.; Kenupp Bastos, J.; de Alencar, S.M. An insight into the botanical origins of propolis from permanent preservation and reforestation areas of southern Brazil. Sci. Rep. 2021, 11, 22043. [Google Scholar] [CrossRef]
- Pellati, F.; Prencipe, F.P.; Benvenuti, S. Headspace solid-phase microextraction-gas chromatography–mass spectrometry characterization of propolis volatile compounds. J. Pharm. Biomed. Anal. 2013, 84, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mountford-McAuley, R. Characterising Poplar and New Zealand Native Plant Resins and New Zealand Propolis Using Volatile Organic Compounds. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2022. [Google Scholar]
- Drescher, N.; Klein, A.-M.; Schmitt, T.; Leonhardt, S.D. A clue on bee glue: New insight into the sources and factors driving resin intake in honey bees (Apis mellifera). PLoS ONE 2019, 14, e0210594. [Google Scholar] [CrossRef]
- Agüero, M.B.; Svetaz, L.; Sánchez, M.; Luna, L.; Lima, B.; López, M.L.; Zacchino, S.; Palermo, J.; Wunderlin, D.; Feresin, G.E. Argentinean Andean propolis associated with the medicinal plant Larrea nitida Cav.(Zygophyllaceae). HPLC–MS and GC–MS characterization and antifungal activity. Food Chem. Toxicol. 2011, 49, 1970–1978. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, Z.; Guo, X.; Hu, X.; Wu, J. Geographical origin identification of propolis using GC–MS and electronic nose combined with principal component analysis. Food Res. Int. 2013, 51, 813–822. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; Barreto, G.d.A.; Costa, S.S.; Silva, D.F.d.; Brandao, H.N.; Rocha, J.L.C.d.; Dellagostin, O.A.; Henriques, J.A.P.; Umsza-Guez, M.A. Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef] [PubMed]
- de Pontes, M.L.C.; Vasconcelos, I.R.A.; de Melo, M.d.F.F.; Pessôa, H.d.L.F. Chemical characterization and pharmacological action of Brazilian red propolis. Acta Bras. 2018, 2, 34–39. [Google Scholar] [CrossRef]
- Chang, R.; Piló-Veloso, D.; Morais, S.A.; Nascimento, E.A. Analysis of a Brazilian green propolis from Baccharis dracunculifolia by HPLC-APCI-MS and GC-MS. Rev. Bras. Farmacogn. 2008, 18, 549–556. [Google Scholar] [CrossRef]
- Nalbantsoy, A.; Sarıkahya, N.B.; Özverel, C.S.; Barlas, A.B.; Kırcı, D.; Akgün, İ.H.; Yalçın, T.; Düven, G.; Kışla, D.; Demirci, B. Chemical composition and biological activities of Cypriot propolis. J. Apic. Res. 2022, 61, 233–245. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LD Jayaweera, S.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Widiakongko, P.D.; Alisaputra, D.; Kangkamano, T. Molecular docking study of active compunds in ginger as inhibitor against COVID-19. In Proceedings of the International Conference on Science and Engineering (ICSE-UIN-SUKA 2021), Yogyakarta, Indonesia, 27 October 2021; pp. 61–68. [Google Scholar]
- Dastan, D.; Mahmoudi, R.; Saidijam, M.; Khoei, S.G.; Abdali, M.H.; Afshar, S. Evaluation of chemical composition of Hamadan Propolis as a potential anticancer agent. Jentashapir J. Cell. Mol. Biol. 2021, 12, e115348. [Google Scholar] [CrossRef]
- Annaz, H.; El Fakhouri, K.; Ben Bakrim, W.; Mahdi, I.; El Bouhssini, M.; Sobeh, M. Bergamotenes: A comprehensive compile of their natural occurrence, biosynthesis, toxicity, therapeutic merits and agricultural applications. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef]


| Sample ID | Region | Number of Replicates |
|---|---|---|
| AUTWAI | Waikato | 13 |
| AUTSOU | Southland | 3 |
| SPRNOR | Northland | 9 |
| SPRWAI | Waikato | 3 |
| SPRGIS | Gisborne | 4 |
| SPRHB | Hawke’s Bay | 14 |
| SPRMAN | Manawatū-Whanganui | 4 |
| SPRNEL | Nelson | 3 |
| SPRWC | West Coast | 5 |
| SPRNCAN | North Canterbury | 4 |
| SPRCAN | Central Canterbury | 4 |
| SPRSCAN | South Canterbury | 4 |
| SPRSOU | Southland | 3 |
| Sample ID | Common Name of Plant | Scientific Name of Plant * |
|---|---|---|
| Introduced | ||
| AUTA1-3 | Argyle | Populus × euramericana ‘Argyle’ |
| AUTF1-3 | Fraser | Populus × euramericana ‘Fraser’ |
| AUTP1-3 | Pakaraka | Populus × euramericana ‘Pakaraka’ |
| AUTS1-3 | Selwyn | Populus × euramericana ‘Selwyn’ |
| AUTV1-3 | Veronese | Populus × euramericana ‘Veronese’ |
| AUTW1-3 | Weraiti | Populus × euramericana ‘Weraiti’ |
| Native | ||
| AUTC1 | Pūriri | Vitex lucens |
| AUTG1 | Karaka | Corynocarpus laevigatus |
| AUTH1-2 | Whau | Entelea arborescens |
| AUTI1 | Pukatea | Laurelia novae-zelandiae |
| AUTK1-2 | Kawakawa | Piper excelsum |
| AUTL1 | Lemonwood | Pittosporum eugenioides |
| AUTN1 | Kānuka | Kunzea ericoides |
| AUTO1 | Kōhūhū | Pittosporum tenuifolium |
| AUTQ1 | Kauri | Agathis australis |
| AUTR1 | Ngaio | Myoporum laetum |
| AUTT1 | Māpou | Myrsine australis |
| Introduced | ||
| SPRA1-3 | ‘Argyle’ | Populus × euramericana ‘Argyle’ |
| SPRF1-3 | ‘Fraser’ | Populus × euramericana ‘Fraser’ |
| SPRP1-3 | ‘Pakaraka’ | Populus × euramericana ‘Pakaraka’ |
| SPRS1-3 | ‘Selwyn’ | Populus × euramericana ‘Selwyn’ |
| SPRV1-3 | ‘Veronese’ | Populus × euramericana ‘Veronese’ |
| SPRW1-3 | ‘Weraiti’ | Populus × euramericana ‘Weraiti’ |
| Native | ||
| SPRE1 | Tōtara | Podocarpus totara |
| SPRD1 | Rewarewa | Knightia excelsa |
| SPRH1 | Whau | Entelea arborescens |
| SPRK1 | Kawakawa | Piper excelsum |
| SPRM1 | Māhoe | Melicytus ramiflorus |
| SPRI1 | Pukatea | Laurelia novae-zelandiae |
| SPRJ1 | Mānuka | Leptospermum scoparium |
| SPRN1 | Kānuka | Kunzea ericoides |
| SPRO1 | Kōhūhū | Pittosporum tenuifolium |
| SPRQ1 | Kauri | Agathis australis |
| SPRR1 | Ngaio | Myoporum laetum |
| SPRT1 | Māpou | Myrsine australis |
| SPRU1 | Tī Kōuka | Cordyline australis |
| SPRX1 | Harakeke | Phormium tenax |
| SPRC1 | Pūriri | Vitex lucens |
| SPRZ1 | Kōwhai | Sophora microphylla |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mountford-McAuley, R.; Robertson, A.; Taylor, M.; Clavijo McCormick, A. Characterisation of New Zealand Propolis from Different Regions Based on Its Volatile Organic Compounds. Molecules 2024, 29, 3143. https://doi.org/10.3390/molecules29133143
Mountford-McAuley R, Robertson A, Taylor M, Clavijo McCormick A. Characterisation of New Zealand Propolis from Different Regions Based on Its Volatile Organic Compounds. Molecules. 2024; 29(13):3143. https://doi.org/10.3390/molecules29133143
Chicago/Turabian StyleMountford-McAuley, Ruby, Alastair Robertson, Michelle Taylor, and Andrea Clavijo McCormick. 2024. "Characterisation of New Zealand Propolis from Different Regions Based on Its Volatile Organic Compounds" Molecules 29, no. 13: 3143. https://doi.org/10.3390/molecules29133143







