Molecular and Phytochemical Characteristics of Flower Color and Scent Compounds in Dog Rose (Rosa canina L.)
Abstract
1. Introduction
2. Results
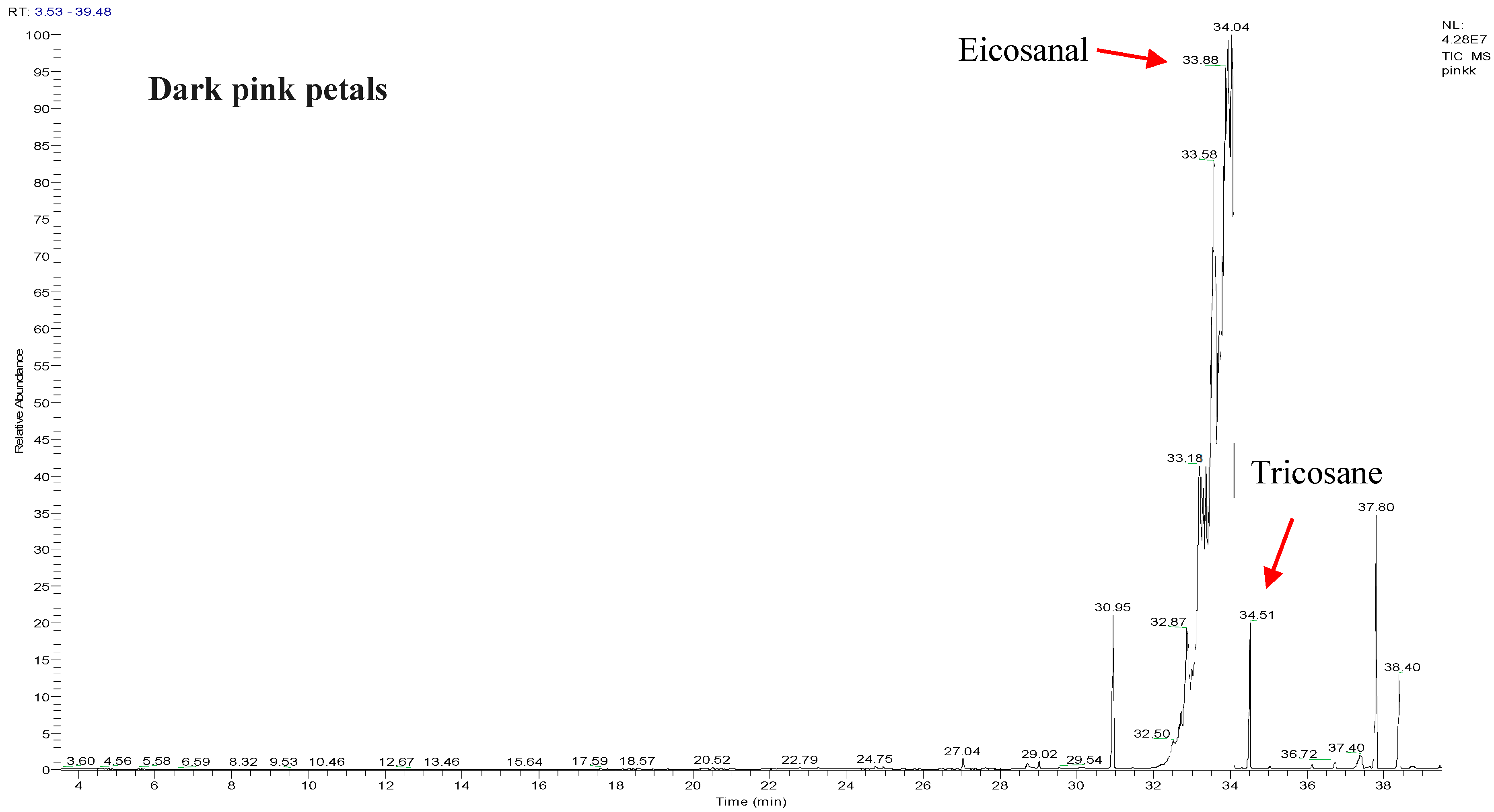
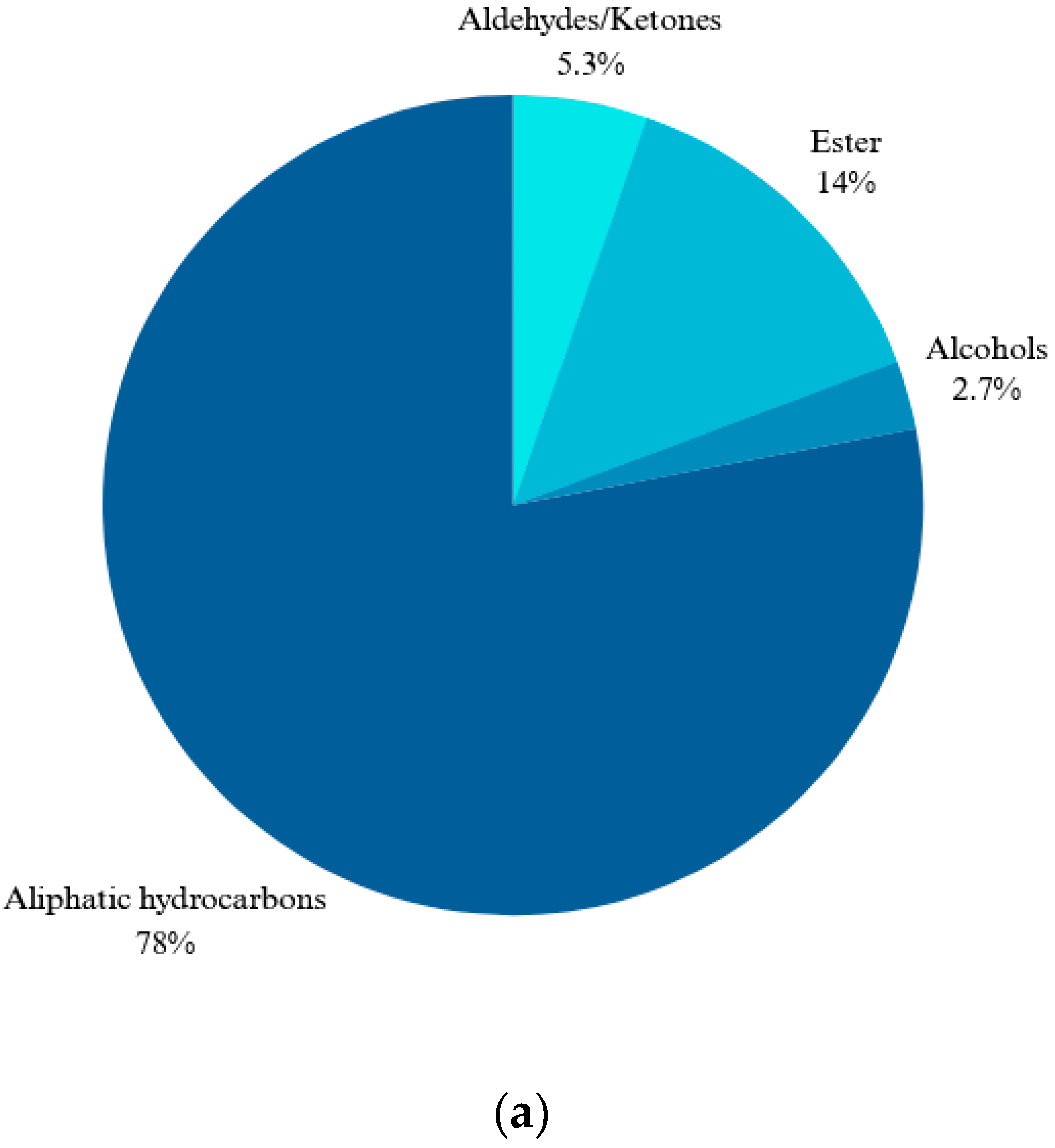
2.1. Chemical Compositions of the R. canina Essential Oil by GC-MS Analysis
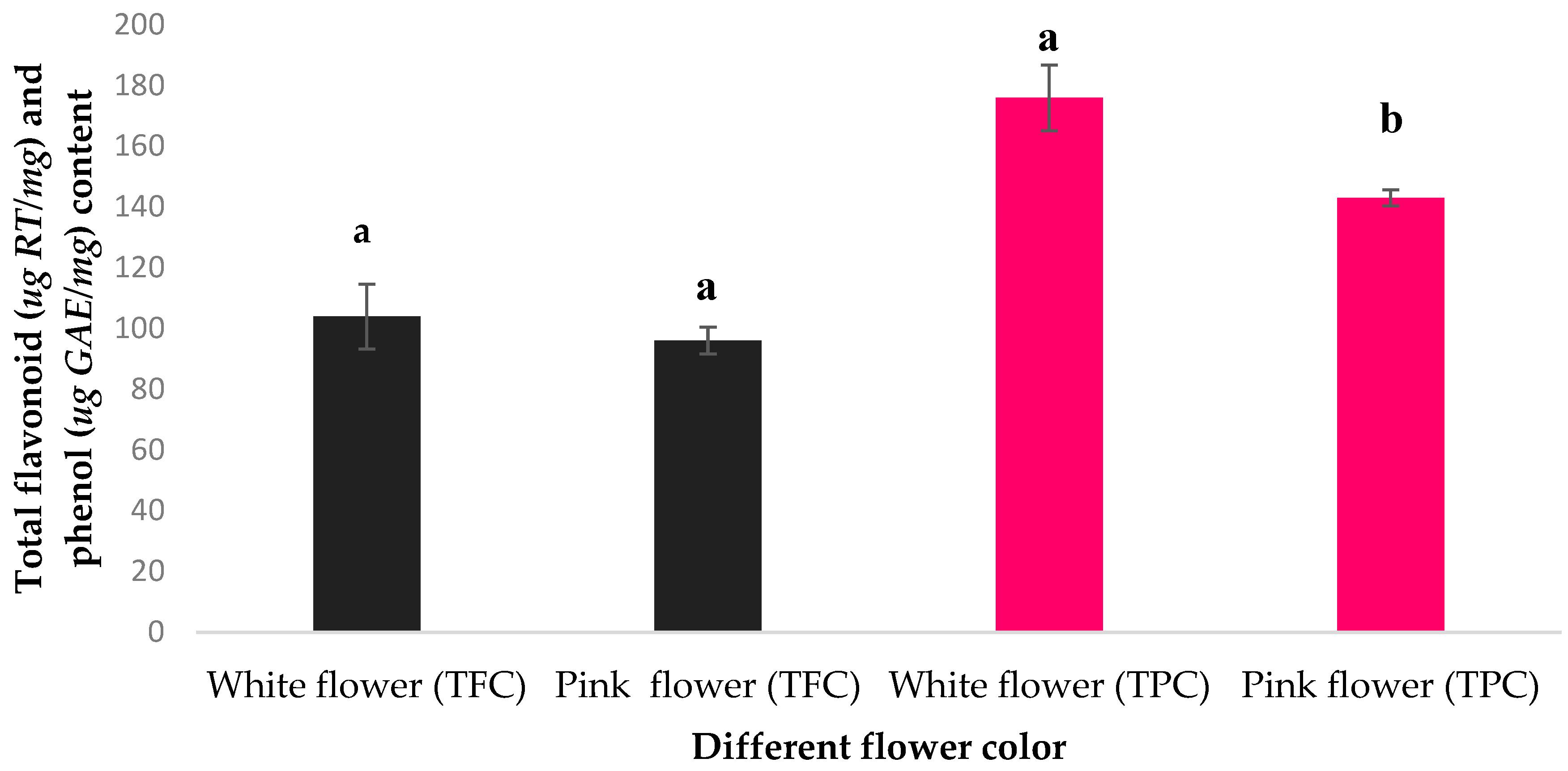
2.2. Total Phenolic and Flavonoid Content of the Petal Extracts
2.3. Total Anthocyanin Content (TAC)
2.4. Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry (LC-ESI-MS) Phenolic Profile of R. canina Methanolic Extract
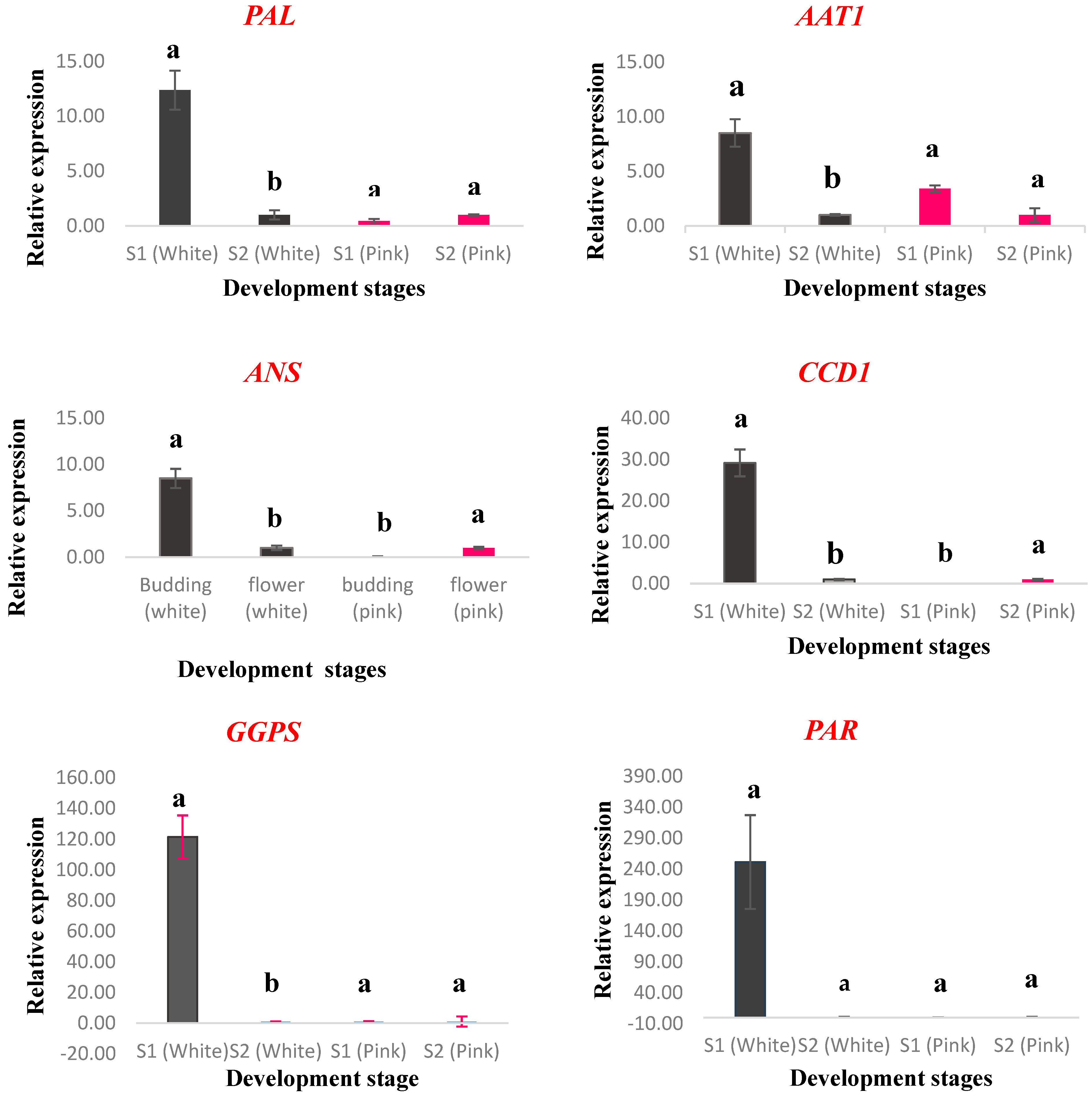
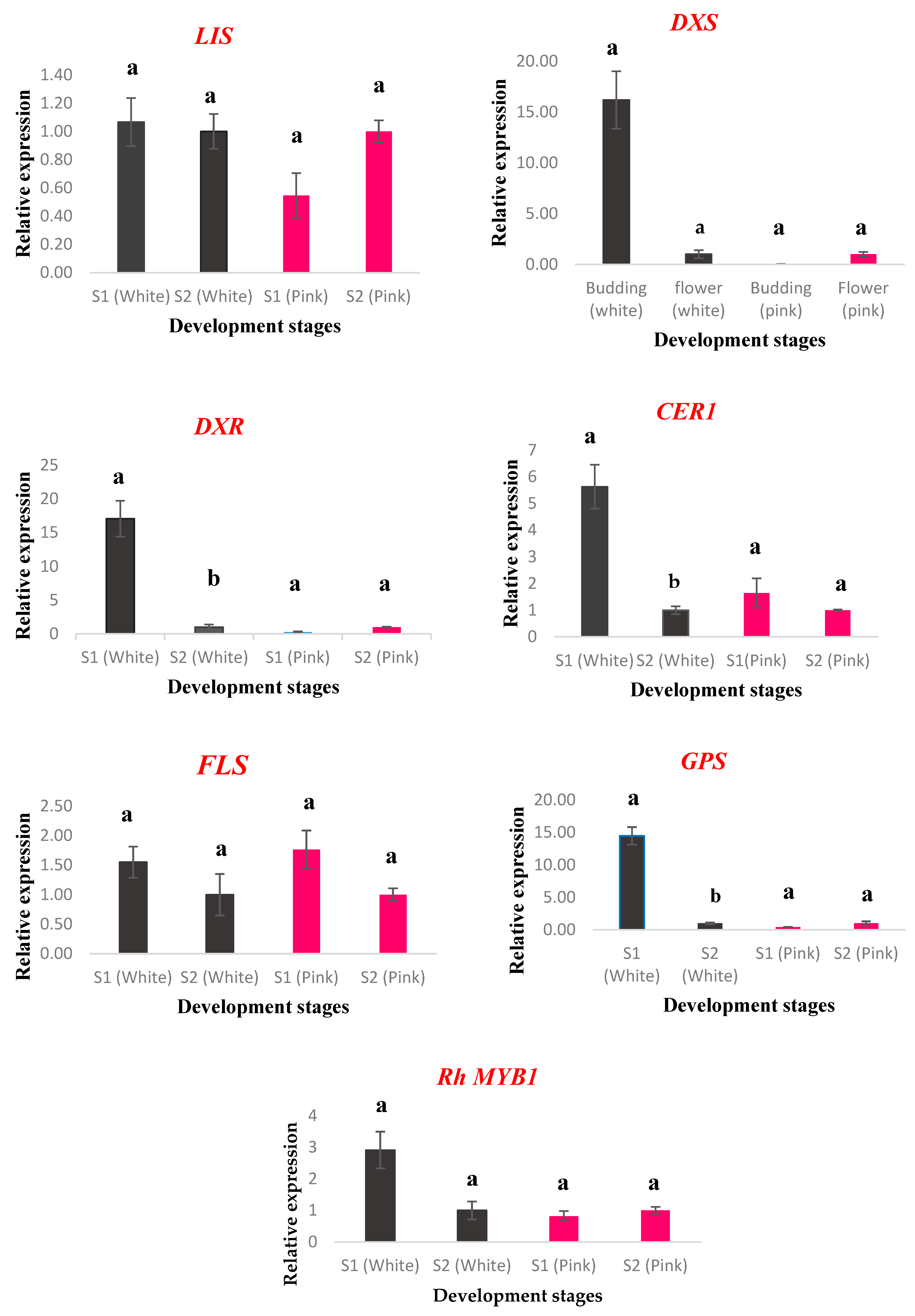
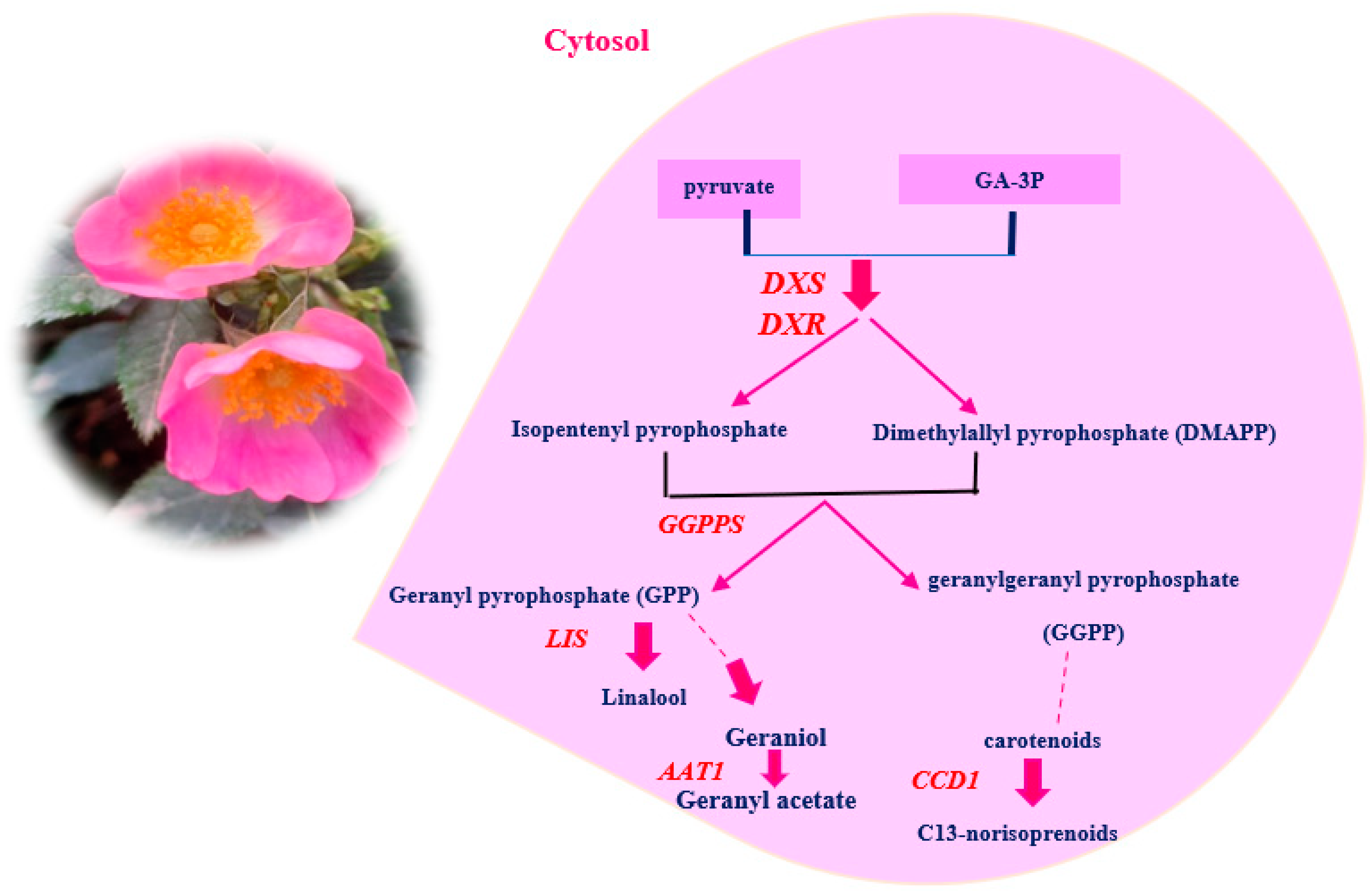
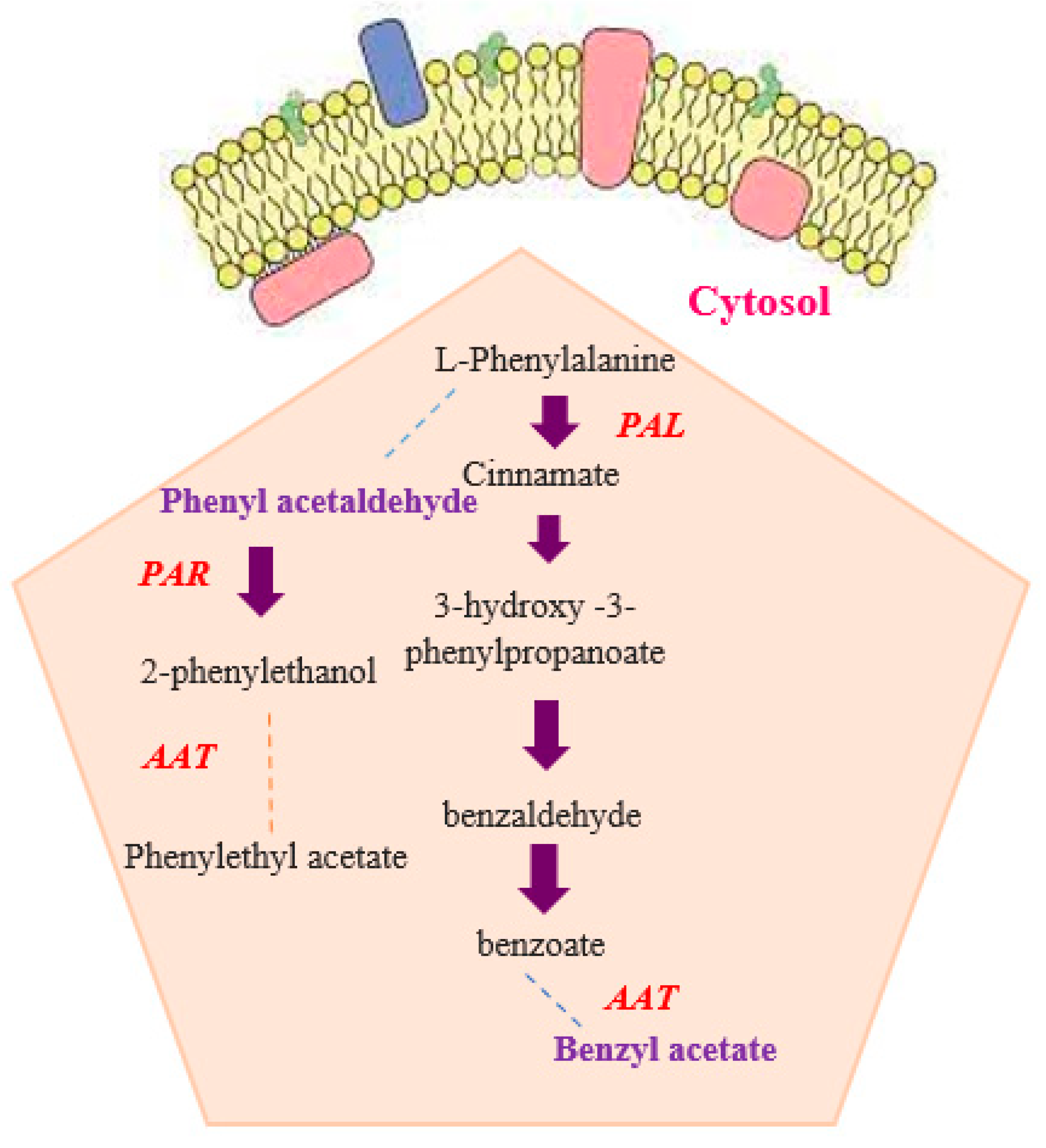
2.5. Gene Expression Analysis
2.6. Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction of the Essential Oils
4.3. Chemical Compositions of the R. canina Essential Oil by GC-MS Analysis
4.4. Measurement of Total Phenol Content
4.5. Measurement of Total Flavonoid Content
4.6. Measurement of Total Anthocyanin Content
4.7. UPLC–Electrospray Ionization Mass Spectrometry (ESI-MS) Analysis
4.8. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baudino, S.; Sun, P.; Caissard, J.C.; Nairaud, B.; Moja, S.; Magnard, J.L.; Bony, A.; Jullien, F.; Schuurink, R.C.; Vergne, P.; et al. Rose Floral Scent. Acta Hortic. 2019, 1232, 69–80. [Google Scholar] [CrossRef]
- Tabaei-Aghdaei, S.R.; Babaei, A.; Khosh-Khui, M.; Jaimand, K.; Rezaee, M.B.; Assareh, M.H.; Naghavi, M.R. Morphological and Oil Content Variations amongst Damask Rose (Rosa damascena Mill.) Landraces from Different Regions of Iran. Sci. Hortic. 2007, 113, 44–48. [Google Scholar] [CrossRef]
- Feng, L.; Chen, C.; Li, T.; Wang, M.; Tao, J.; Zhao, D.; Sheng, L. Flowery Odor Formation Revealed by Differential Expression of Monoterpene Biosynthetic Genes and Monoterpene Accumulation in Rose (Rosa rugosa Thunb.). Plant Physiol. Biochem. 2014, 75, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, Z. Genetic and Biochemical Aspects of Floral Scents in Roses. Int. J. Mol. Sci. 2022, 23, 8014. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Tabaei-Aghdaei, S.R.; Khosh-Khui, M.; Omidbaigi, R.; Naghavi, M.R.; Esselink, G.D.; Smulders, M.J.M. Microsatellite Analysis of Damask Rose (Rosa damascena Mill.) Accessions from Various Regions in Iran Reveals Multiple Genotypes. BMC Plant Biol. 2007, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Anwar, F.; Gilani, A.-u.-H. Rose Hip (Rosa canina L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 667–675. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Rhimi, A.; Achour, H.; Dhaouadi, K.; Khebour Allouche, F.; Chaar, H.; Sebei, H. Ecological Distribution, Phytochemistry and Biological Properties of Rosa Species in Tunisia. In Agriculture Productivity in Tunisia Under Stressed Environment; Springer: Berlin/Heidelberg, Germany, 2021; pp. 73–136. [Google Scholar] [CrossRef]
- Schanzer, I.A.; Vagina, A. V ISSR (Inter Simple Sequence Repeat) Markers Reveal Natural Intersectional Hybridization in Wild Roses [Rosa L., Sect. Caninae (DC.) Ser. and Sect. Cinnamomeae (DC.) Ser.]. Wulfenia 2007, 14, 1–14. [Google Scholar]
- Ercisli, S. Rose (Rosa Spp.) Germplasm Resources of Turkey. Genet. Resour. Crop Evol. 2005, 52, 787–795. [Google Scholar] [CrossRef]
- Czyzowska, A.; Klewicka, E.; Pogorzelski, E.; Nowak, A. Polyphenols, Vitamin C and Antioxidant Activity in Wines from Rosa canina L. and Rosa rugosa Thunb. J. Food Compos. Anal. 2015, 39, 62–68. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of Volatiles, Phenolic Compounds and Antioxidant Activities of Rose Hip (Rosa L.) Fruits in Turkey. LWT—Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Najgebauer-Lejko, D. The Content of Selected Phytochemicals and in Vitro Antioxidant Properties of Rose Hip (Rosa canina L.) Tinctures. NFS J. 2020, 21, 50–56. [Google Scholar] [CrossRef]
- Živković, J.; Stojković, D.; Petrović, J.; Zdunić, G.; Glamočlija, J.; Soković, M. Rosa canina L.—New Possibilities for an Old Medicinal Herb. Food Funct. 2015, 6, 3687–3692. [Google Scholar] [CrossRef]
- Yeon, J.Y.; Kim, W.S. Biosynthetic Linkage between the Color and Scent of Flowers: A Review. Hortic. Sci. Technol. 2021, 39, 697–713. [Google Scholar] [CrossRef]
- Li, Y.; Gao, R.; Zhang, J.; Wang, Y.; Kong, P.; Lu, K.; Adnan; Liu, M.; Ao, F.; Zhao, C.; et al. The Biochemical and Molecular Investigation of Flower Color and Scent Sheds Lights on Further Genetic Modification of Ornamental Traits in Clivia Miniata. Hortic. Res. 2022, 9, uhac114. [Google Scholar] [CrossRef] [PubMed]
- Ramya, M.; Jang, S.; An, H.R.; Lee, S.Y.; Park, P.M.; Park, P.H. Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation. Int. J. Mol. Sci. 2020, 21, 1160. [Google Scholar] [CrossRef]
- Mohd-Hairul, A.R.; Namasivayam, P.; Cheng Lian, G.E.; Abdullah, J.O. Terpenoid, Benzenoid, and Phenylpropanoid Compounds in the Floral Scent of Vanda Mimi Palmer. J. Plant Biol. 2010, 53, 358–366. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, S.; Wu, J.; Liu, X.; Zhang, Z. Identification of Long Non-Coding RNAs Involved in Floral Scent of Rosa hybrida. Front. Plant Sci. 2022, 13, 996474. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M. Phenolic Content and Antioxidant Activity of Some Spices. World Appl. Sci. J. 2009, 6, 373–377. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of Plant Volatiles: Nature’s Diversity and Ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral Volatiles: From Biosynthesis to Function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Zvi, M.M.B.; Negre-Zakharov, F.; Masci, T.; Ovadis, M.; Shklarman, E.; Ben-Meir, H.; Tzfira, T.; Dudareva, N.; Vainstein, A. Interlinking Showy Traits: Co-Engineering of Scent and Colour Biosynthesis in Flowers. Plant Biotechnol. J. 2008, 6, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, T.; Zhang, M.; Zhang, Y.; Irfan, M.; Chen, L.; Zhang, L. Role of Core Structural Genes for Flavonoid Biosynthesis and Transcriptional Factors in Flower Color of Plants. Biotechnol. Biotechnol. Equip. 2021, 35, 1214–1229. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.A.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the Rose Flavonoid Biosynthetic Pathway Successfully Generated Blue-Hued Flowers Accumulating Delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Ogata, J.; Kanno, Y.; Itoh, Y.; Tsugawa, H.; Suzuki, M. Anthocyanin Biosynthesis in Roses. Nature 2005, 435, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N.; Ouahab, A. LC–DAD/ESI–MS/MS Characterization of Phenolic Constituents in Rosa canina L. and Its Protective Effect in Cells. Biomed. Chromatogr. 2020, 34, e4961. [Google Scholar] [CrossRef]
- Behnamnia, S.; Rahimmalek, M.; Haghighi, M.; Nikbakht, A.; Gharibi, S.; Pachura, N.; Szumny, A.; Łyczko, J. Variation in Flavonoid Compounds, Volatiles and Yield Related Traits in Different Iranian Rosa damascena Mill. Cultivars Based on SPME Arrow and LC-MS/MS. Foods 2024, 13, 668. [Google Scholar] [CrossRef]
- Salehi, M.; Karimzadeh, G.; Naghavi, M.R. Synergistic Effect of Coronatine and Sorbitol on Artemisinin Production in Cell Suspension Culture of Artemisia annua L. Cv. Anamed. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 3, 587–597. [Google Scholar] [CrossRef]
- Sauvageau, D.; Sauvageau, D. Microbial Esterase and the Degradation of Plasticizers. Master’s Thesis, McGill University, Montreal, QC, Canada, 2004. [Google Scholar]
- Wu, Y.; Han, X.; Yuan, W.Q.; Wang, X.X.; Meng, D.H.; Hu, J.Z.; Lv, Z.L. Salt Intervention for the Diversities of Essential Oil Composition, Aroma and Antioxidant Activities of Kushui Rose (R. Setate × R. Rugosa). Ind. Crops Prod. 2020, 150, 112417. [Google Scholar] [CrossRef]
- Khazayi, M.; Afshari, H.; Hashemi-Moghaddam, H. Evaluation of Extraction Method and Chemical Modifier on Chemical Composition of the Essential Oils from the Roots of Rosa canina L. J. Essent. Oil Bear. Plants 2019, 22, 131–140. [Google Scholar] [CrossRef]
- Tanjga, B.B.; Lončar, B.; Aćimović, M.; Kiprovski, B.; Šovljanski, O.; Tomić, A.; Travičić, V.; Cvetković, M.; Raičević, V.; Zeremski, T. Volatile Profile of Garden Rose (Rosa hybrida) Hydrosol and Evaluation of Its Biological Activity In Vitro. Horticulturae 2022, 8, 895. [Google Scholar] [CrossRef]
- Hosseini, H.; Zahedi, B.; Jowkar, A.; Kermani, M.J.; Karami, A. Investigation of Floral Scent and Essential Oil of Rosa Iberica Petals. J. Ornam. Plants 2021, 11, 89–97. [Google Scholar]
- Yari, E.; Sari, S.; Kelidari, H.; Asare-Addo, K.; Nokhodchi, A. Effect of Rosa damascena Essential Oil Loaded in Nanostructured Lipid Carriers on the Proliferation of Human Breast Cancer Cell Line MDA-MB-231 in Comparison with Cisplatin. J. Pharm. Innov. 2024, 19, 4. [Google Scholar] [CrossRef]
- Dani, K.G.S.; Fineschi, S.; Michelozzi, M.; Trivellini, A.; Pollastri, S.; Loreto, F. Diversification of Petal Monoterpene Profiles during Floral Development and Senescence in Wild Roses: Relationships among Geraniol Content, Petal Colour, and Floral Lifespan. Oecologia 2020, 197, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Sinakos, Z.; Papageorgiou, V.P. Radical Scavenging Activity of Crocus Sativus L. Extract and Its Bioactive Constituents. Phytother. Res. 2005, 19, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Rock, C.D. Abscisic Acid Biosynthesis and Response. Arab. Book/Am. Soc. Plant Biol. 2002, 1, e0058. [Google Scholar] [CrossRef]
- Mohsen, E.; Younis, I.Y.; Farag, M.A. Metabolites Profiling of Egyptian Rosa damascena Mill. Flowers as Analyzed via Ultra-High-Performance Liquid Chromatography-Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry in Relation to Its Anti-Collagenase Skin Effect. Ind. Crops Prod. 2020, 155, 112818. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-Derived Flavor Compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Elsharif, S.A.; Banerjee, A.; Buettner, A. Structure-Odor Relationships of Linalool, Linalyl Acetate and Their Corresponding Oxygenated Derivatives. Front. Chem. 2015, 3, 57. [Google Scholar] [CrossRef]
- Sterrett, F.S. The Nature of Essential Oils. II. Chemical Constituents, Analysis. J. Chem. Educ. 1962, 39, 246–251. [Google Scholar] [CrossRef]
- Qian, Y.; Bian, S.; Zhao, P.; Wang, T.; Hua, Y.; Liu, Y.; Teng, Q.; Tao, C. The Investigation of Evaporation Behavior of Lubricating Oil Droplet at High Ambient Temperature. Int. Commun. Heat Mass Transf. 2020, 117, 104689. [Google Scholar] [CrossRef]
- Restrepo-Cano, J.; Ordonez-Loza, J.; Guida, P.; Roberts, W.L.; Chejne, F.; Sarathy, S.M.; Im, H.G. Evaporation, Break-up, and Pyrolysis of Multi-Component Arabian Light Crude Oil Droplets at Various Temperatures. Int. Commun. Heat Mass Transf. 2022, 183, 122175. [Google Scholar] [CrossRef]
- Pawar, K.R.; Babu, R.J. Polymeric and Lipid-Based Materials for Topical Nanoparticle Delivery Systems. Crit. Rev. Trade Ther. Drug Carr. Syst. 2010, 27, 419–459. [Google Scholar] [CrossRef] [PubMed]
- Piechulla, B.; Effmert, U. Biosynthesis and Regulation of Flower Scent. Plant Dev. Biol. 2010, 2, 189–205. [Google Scholar] [CrossRef]
- Cseke, L.J.; Kaufman, P.B.; Kirakosyan, A. The Biology of Essential Oils in the Pollination of Flowers. Nat. Prod. Commun. 2019, 2, 1317–1336. [Google Scholar] [CrossRef]
- Palmqvist, B.; Brazeau, H.A.; Parachnowitsch, A.L. Differences in Floral Scent and Petal Reflectance Between Diploid and Tetraploid Chamerion Angustifolium. Front. Ecol. Evol. 2021, 9, 734128. [Google Scholar] [CrossRef]
- Guterman, I.; Shalit, M.; Menda, N.; Piestun, D.; Dafny-Yelin, M.; Shalev, G.; Bar, E.; Davydov, O.; Ovadis, M.; Emanuel, M.; et al. Rose Scent Genomics Approach to Discovering Novel Floral Fragrance–Related Genes. Plant Cell 2002, 14, 2325–2338. [Google Scholar] [CrossRef]
- Ghazghazi, H.; Miguel, M.G.; Hasnaoui, B.; Sebei, H.; Ksontini, M.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G. Phenols, Essential Oils and Carotenoids of Rosa canina from Tunisia and Their Antioxidant Activities. Afr. J. Biotechnol. 2012, 9, 2709–2716. [Google Scholar]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Roman, I.; Stǎnilǎ, A.; Stǎnilǎ, S. Bioactive Compounds and Antioxidant Activity of Rosa canina L. Biotypes from Spontaneous Flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Buchwald, W.; Zieliński, J.; Mielcarek, S. Flavonoid and Organic Acid Content in Rose Hips (Rosa L., Sect. Caninae Dc. Em. Christ.). Acta Biol. Crac. Ser. Bot. 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Wang, Z.; Wang, J. The Antioxidant Activity, α-Glucosidase and Acetylcholinesterase Inhibition Activity, and Chemical Composition of Paeonia delavayi Petal. Food Qual. Saf. 2022, 6, fyac020. [Google Scholar] [CrossRef]
- Manivannan, A.; Narasegowda, S.; Prakash, T. Comparative Study on Color Coordinates, Phenolics, Flavonoids, Carotenoids, and Antioxidant Potential of Marigold (Tagetes Sp.) with Diverse Colored Petals. J. Food Meas. Charact. 2021, 15, 4343–4353. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Ahadi, H.; Shokrpour, M.; Fatahi, R.; Naghavi, M.R.; Mirjalili, M.H. Essential Oil, Flavonoids and Anthocyanins Profiling of Some Iranian Damask Rose (Rosa damascena Mill.) Genotypes. Ind. Crops Prod. 2023, 205, 117579. [Google Scholar] [CrossRef]
- Zheng, J.; Meenu, M.; Xu, B. A Systematic Investigation on Free Phenolic Acids and Flavonoids Profiles of Commonly Consumed Edible Flowers in China. J. Pharm. Biomed. Anal. 2019, 172, 268–277. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Fattahi, M. Essential Oil, Total Phenolic, Flavonoids, Anthocyanins, Carotenoids and Antioxidant Activity of Cultivated Damask Rose (Rosa damascena) from Iran: With Chemotyping Approach Concerning Morphology and Composition. Sci. Hortic. 2021, 288, 110341. [Google Scholar] [CrossRef]
- Rasouli, O.; Ahmadi, N.; Rashidi Monfared, S.; Sefidkon, F. Physiological, Phytochemicals and Molecular Analysis of Color and Scent of Different Landraces of Rosa damascena during Flower Development Stages. Sci. Hortic. 2018, 231, 144–150. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Zhao, L.; Zheng, C.; Ma, F. Changes in Volatile Organic Compounds and Differential Expression of Aroma-Related Genes during Flowering of Rosa rugosa ‘Shanxian’. Hortic. Environ. Biotechnol. 2019, 60, 741–751. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Kader, A.A.; Dandekar, A.M. Apple Aroma: Alcohol Acyltransferase, a Rate Limiting Step for Ester Biosynthesis, Is Regulated by Ethylene. Plant Sci. 2005, 168, 1199–1210. [Google Scholar] [CrossRef]
- Wei, Y.L.; Li, J.N.; Lu, J.; Tang, Z.L.; Pu, D.C.; Chai, Y.R. Molecular Cloning of Brassica napus TRANSPARENT TESTA 2 Gene Family Encoding Potential MYB Regulatory Proteins of Proanthocyanidin Biosynthesis. Mol. Biol. Rep. 2007, 34, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Gang, D.R. Evolution of flavors and scents. Annu. Rev. Plant Biol. 2005, 56, 301–325. [Google Scholar] [CrossRef] [PubMed]
- Rameneni, J.J.; Choi, S.R.; Chhapekar, S.S.; Man-Sun, K.; Singh, S.; Yi, S.Y.; Heon, O.S.; Kim, H.; Lee, C.Y.; Man-Ho, O.; et al. Red Chinese Cabbage Transcriptome Analysis Reveals Structural Genes and Multiple Transcription Factors Regulating Reddish Purple Color. Int. J. Mol. Sci. 2020, 21, 2901. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The Tomato Carotenoid Cleavage Dioxygenase 1 Genes Contribute to the Formation of the Flavor Volatiles β-Ionone, Pseudoionone, and Geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and Their Cleavage Products: Biosynthesis and Functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, H.; Wang, Q.; Jian, H.; Qiu, X.; Wang, J.; Tang, K. Isolation and Identification of a Putative Scent-Related Gene RhMYB1 from Rose. Mol. Biol. Rep. 2011, 38, 4475–4482. [Google Scholar] [CrossRef]
- Zhu, C.; Bai, C.; Sanahuja, G.; Yuan, D.; Farré, G.; Naqvi, S.; Shi, L.; Capell, T.; Christou, P. The Regulation of Carotenoid Pigmentation in Flowers. Arch. Biochem. Biophys. 2010, 504, 132–141. [Google Scholar] [CrossRef]
- Liu, J.; Osbourn, A.; Ma, P. MYB Transcription Factors as Regulators of Phenylpropanoid Metabolism in Plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, L.; Wang, H.; Chen, X.; Wang, Y.; Yang, H.; Wei, C.; Wan, X.; Xia, T. The R2R3-MYB, BHLH, WD40, and Related Transcription Factors in Flavonoid Biosynthesis. Funct. Integr. Genom. 2013, 13, 75–98. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and Molecular Genetic Aspects of Floral Scents. Plant Physiol. 2000, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Cseke, L.; Blanc, V.M.; Pichersky, E. Evolution of Floral Scent in Clarkia: Novel Patterns of S-Linalool Synthase Gene Expression in the C. Breweri Flower. Plant Cell 1996, 8, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, W.; Zhang, D.; Wang, G.; Cao, F. Flowering Stage and Daytime Affect Scent Emission of Malus Ioensis “Prairie Rose”. Molecules 2019, 24, 2356. [Google Scholar] [CrossRef] [PubMed]
- Kantsa, A.; Garcia, J.E.; Raguso, R.A.; Dyer, A.G.; Steen, R.; Tscheulin, T.; Petanidou, T. Intrafloral Patterns of Color and Scent in Capparis spinosa L. and the Ghosts of Its Selection Past. Am. J. Bot. 2023, 110, e16098. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, C.; Cheng, B.; Wan, H.; Luo, L.; Pan, H.; Zhang, Q. Volatile Compound Analysis and Aroma Evaluation of Tea-Scented Roses in China. Ind. Crops Prod. 2020, 155, 112735. [Google Scholar] [CrossRef]
- Yeon, J.Y.; Kim, W.S. Floral Pigment-Scent Associations in Eight Cut Rose Cultivars with Various Petal Colors. Hortic. Environ. Biotechnol. 2020, 61, 633–641. [Google Scholar] [CrossRef]
- Feng, L.; Wang, M.; Wang, J.; Zang, S.; Xia, W.; Sheng, L. Isolation of 2-Phenylethanol Biosynthesis Related Genes and Their Relationship with 2-Phenylethanol Accumulation in Rosa rugosa. Acta Physiol. Plant 2015, 37, 256. [Google Scholar] [CrossRef]
- Jariani, P.; Shahnejat-Bushehri, A.A.; Naderi, R.; Naghavi, M.R.; Mofidi, S.S.H. Identification of MiRNAs and Their Target Genes Involved in the Biosynthesis of Flower Color and Scent in Rosa canina L. Iran. J. Sci. 2024, 48, 31–43. [Google Scholar] [CrossRef]
- Ahmadian, M.; Ahmadi, N.; Babaei, A.; Naghavi, M.R.; Ayyari, M. Comparison of Volatile Compounds at Various Developmental Stages of Tuberose (Polianthes tuberosa L. Cv. Mahallati) Flower with Different Extraction Methods. J. Essent. Oil Res. 2018, 30, 197–206. [Google Scholar] [CrossRef]
- Ranjbar, M.; Naghavi, M.R.; Alizadeh, H.; Soltanloo, H. Expression of Artemisinin Biosynthesis Genes in Eight Artemisia Species at Three Developmental Stages. Ind. Crops Prod. 2015, 76, 836–843. [Google Scholar] [CrossRef]
- Rasouli, O.; Ahmadi, N.; Rashidi Monfared, S. Molecular Characterization and Expression Pattern of RhPAR, RhMYB1 and RhANS Genes Involving in Scent and Color Production in Rosa damascena. Sci. Hortic. 2020, 272, 109399. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Color and Phenolic Content Changes during Flower Development in Groundcover Rose. J. Am. Soc. Hortic. Sci. 2010, 135, 195–202. [Google Scholar] [CrossRef]
- Han, M.; Yang, C.; Zhou, J.; Zhu, J.; Meng, J.; Shen, T.; Xin, Z.; Li, H. Analysis of Flavonoids and Anthocyanin Biosynthesis-Related Genes Expression Reveals the Mechanism of Petal Color Fading of Malus hupehensis (Rosaceae). Braz. J. Bot. 2020, 43, 81–89. [Google Scholar] [CrossRef]
- Koobaz, P.; Hosseini, Z.S.; Khatamsaz, M.; Jafarkhani Kermani, M. Taxonomical Study of Section Caninae (Rosa) and Their Hybrids in Iran. Rostaniha 2019, 20, 85–97. [Google Scholar] [CrossRef]
- Shameh, S.; Hosseini, B.; Alirezalu, A.; Maleki, R. Phytochemical Composition and Antioxidant Activity of Petals of Six Rosa Species from Iran. J. AOAC Int. 2018, 101, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.F.R.; De Portella, R.O.; Bufalo, J.; Marques, M.O.M.; Facanali, R.; Frei, F. Non-Oxygenated Sesquiterpenes in the Essential Oil of Copaifera langsdorffii Desf. Increase during the Day in the Dry Season. PLoS ONE 2016, 11, e0149332. [Google Scholar] [CrossRef]
- Hazrati, S.; Beidaghi, P.; Beyraghdar Kashkooli, A.; Hosseini, S.J.; Nicola, S. Effect of Harvesting Time Variations on Essential Oil Yield and Composition of Sage (Salvia officinalis). Horticulturae 2022, 8, 149. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, H.; Chen, M.; Jian, H.; Baudino, S.; Caissard, J.C.; Bendahmane, M.; Li, S.; Zhang, T.; Zhou, N.; et al. Transcriptome and Gene Expression Analysis during Flower Blooming in Rosa chinensis ‘Pallida’. Gene 2014, 540, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gargi, A.; Singh, J.; Rasane, P.; Kaur, S.; Kaur, J.; Kumar, M.; Sowdhanya, D.; Gunjal, M.; Choudhary, R.; Ercisli, S. Effect of Drying Methods on the Nutritional and Phytochemical Properties of Pumpkin Flower (Cucurbita maxima) and Its Characterization. J. Food Meas. Charact. 2023, 17, 5330–5343. [Google Scholar] [CrossRef]
- Sivaraj, C.; Abhirami, R.; Deepika, M.; Sowmiya, V.; Saraswathi, K.; Arumugam, P. Antioxidant, Antibacterial Activities and GC-MS Analysis of Fresh Rose Petals Aqueous Extract of Rosa damascena Mill L. J. Drug Deliv. Ther. 2019, 9, 68–77. [Google Scholar] [CrossRef]
- Maciąg, A.; Kalemba, D. Composition of Rugosa Rose (Rosa rugosa Thunb.) Hydrolate According to the Time of Distillation. Phytochem. Lett. 2015, 11, 373–377. [Google Scholar] [CrossRef]
- Mohamadi, M.; Mostafavi, A.; Shamspur, T. Effect of Storage on Essential Oil Content and Composition of Rosa damascena Mill. Petals under Different Conditions. J. Essent. Oil Bear. Plants 2013, 14, 430–441. [Google Scholar] [CrossRef]
- Naquvi, K.J.; Ansari, S.H.; Ali, M.; Najmi, A.K.; Kamran, C.; Naquvi, J. Volatile Oil Composition of Rosa damascena Mill. (Rosaceae). J. Pharmacogn. Phytochem. 2014, 2, 177–181. [Google Scholar]
- Kanehisa, M. Subramaniam The KEGG Database. Novartis Found. Symp. 2002, 247, 91–103. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Midford, P.E.; Ong, Q.; Ong, W.K.; et al. The MetaCyc Database of Metabolic Pathways and Enzymes. Nucleic Acids Res. 2018, 46, D633–D639. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.; Kothari, A.; Krummenacker, M.; Midford, P.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P. MetaCyc: A Database of Enzymes and Pathways with a Broad Coverage of Natural Products Biosynthesis. In Proceedings of the 3rd International Conference on Natural Products Discovery and Development in the Genomic Era. SIMB, San Diego, CA, USA, 12–16 January 2020. [Google Scholar]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New Approach for Understanding Genome Variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Q.D.; He, Q.; Sun, Q.; Zeng, W.C. A Theoretical and Experimental Study: The Influence of Different Standards on the Determination of Total Phenol Content in the Folin–Ciocalteu Assay. J. Food Meas. Charact. 2019, 13, 1349–1356. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic Content and Antioxidant Activity of Olive Extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated Methods for the Quantification of Biologically Active Constituents of Poplar-Type Propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Al-Salman, F.; Ali Redha, A.; Salem, M.; Saeed, Z. Phytochemical investigations of 10 edible plants and their antioxidant and antidiabetic activity. Int. J. Res. Pharm. Chem. 2020, 10, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Sakurai, M.; Shigeta, J.-I.; Yoshida, K.; Kondo, T. Formation of Anthocyanins from Cells Cultured from Different Parts of Strawberry Plants. J. Food Sci. 1993, 58, 788–792. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ozer, M.S.; Tlili, N. Comparison of the Influence of the Solvent on the Extraction of the Bioactive Compounds from Marrubium Lutescens Using Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry (LC-ESI-MS/MS). Anal. Lett. 2020, 53, 2222–2234. [Google Scholar] [CrossRef]
- Tlili, N.; Sarikurkcu, R.T.; Ozer, M.S.; Sarikurkcu, C. Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry (LC-ESI-MS/MS) Identification of Phytochemicals and the Effects of Solvents on Phenolic Constituents, Antioxidant Capacity, Skin-Whitening and Anti-Diabetic Activity of Onosma Mitis. Anal. Lett. 2022, 55, 32–46. [Google Scholar] [CrossRef]
- Morante-Carriel, J.; Sellés-Marchart, S.; Martínez-Márquez, A.; Martínez-Esteso, M.J.; Luque, I.; Bru-Martínez, R. RNA Isolation from Loquat and Other Recalcitrant Woody Plants with High Quality and Yield. Anal. Biochem. 2014, 452, 46–53. [Google Scholar] [CrossRef]
- Zarei, A.; Zamani, Z.; Mousavi, A.; Fatahi, R.; Karimi Alavijeh, M.; Dehsara, B.; Alireza Salami, S. An Effective Protocol for Isolation of High-Quality RNA from Pomegranate Seeds. Asian Australas. J. Plant Sci. Biotechnol. 2012, 6, 32–37. [Google Scholar]
- Aranda, P.S.; Lajoie, D.M.; Jorcyk, C.L. Bleach Gel: A Simple Agarose Gel for Analyzing RNA Quality. Electrophoresis 2012, 33, 366–369. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA Integrity and the Effect on the Real-Time QRT-PCR Performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and Modifications of Primer Design Program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Jozefczuk, J.; Adjaye, J. Quantitative Real-Time PCR-Based Analysis of Gene Expression. Methods Enzym. 2011, 500, 99–109. [Google Scholar] [CrossRef]
- Salehi, M.; Karimzadeh, G.; Naghavi, M.R.; Naghdi Badi, H.; Rashidi Monfared, S. Expression of Artemisinin Biosynthesis and Trichome Formation Genes in Five Artemisia Species. Ind. Crops Prod. 2018, 112, 130–140. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Balasubramaniyan, R.; Hüllermeier, E.; Weskamp, N.; Kämper, J. Clustering of Gene Expression Data Using a Local Shape-Based Similarity Measure. Bioinformatics 2005, 21, 1069–1077. [Google Scholar] [CrossRef]
- Jiang, D.; Tang, C.; Zhang, A. Cluster Analysis for Gene Expression Data: A Survey. IEEE Trans. Knowl. Data Eng. 2004, 16, 1370–1386. [Google Scholar] [CrossRef]
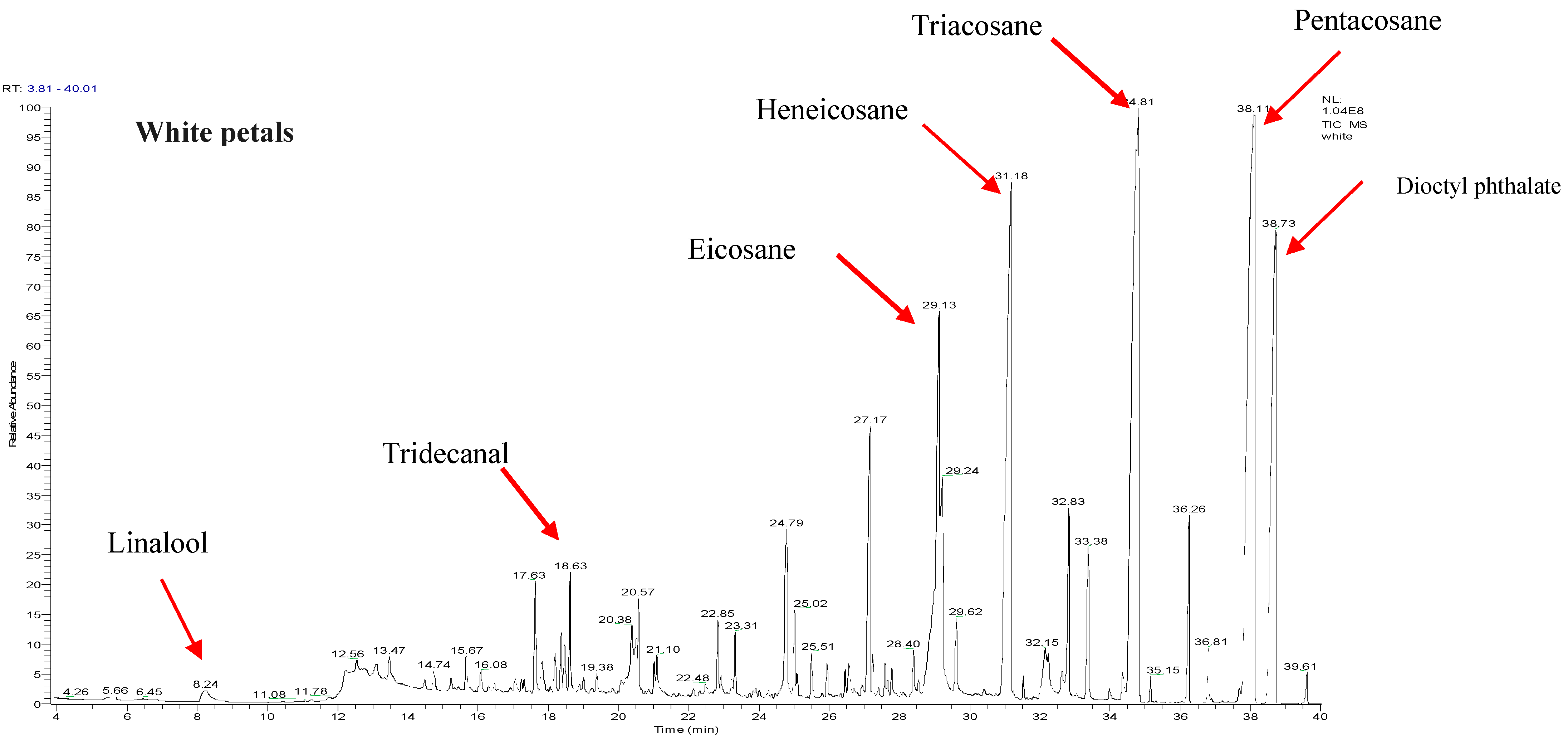

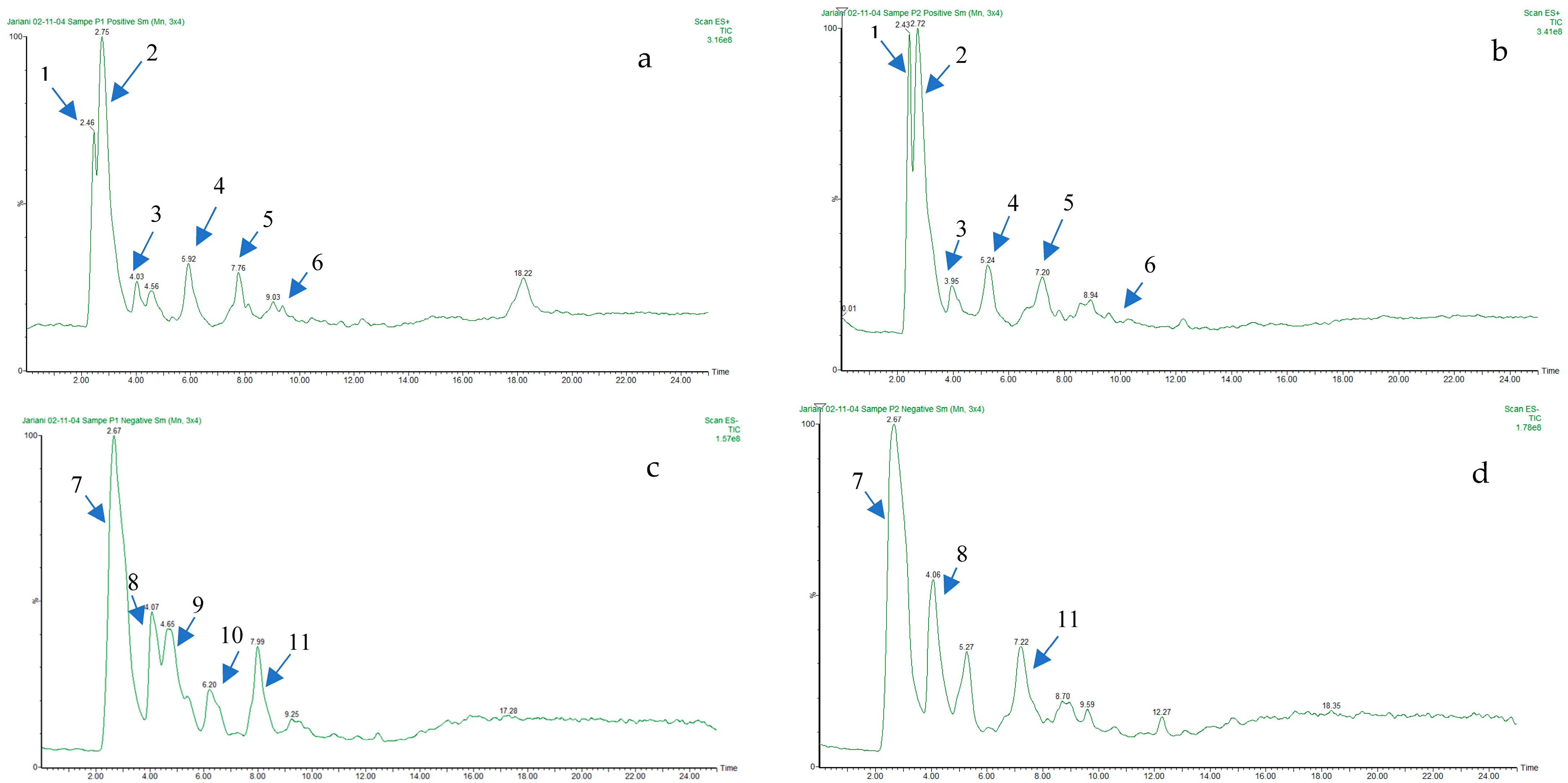



| No. | Compound Name | RI | Percentage Peak Area |
|---|---|---|---|
| 1 | Nonanal | 1116 | 0.21 |
| 2 | Linalool | 1118 | 0.31 |
| 3 | Linalool acetate | 1268 | 1.06 |
| 4 | Geranial | 1279 | 0.82 |
| 5 | Undecanal | 1313 | 0.14 |
| 6 | Citronellyl acetate | 1352 | 0.24 |
| 7 | Tetradecane | 1397 | 0.21 |
| 8 | Dauca-5,8-diene | 1472 | 0.91 |
| 9 | γ-Himachalene | 1480 | 0.25 |
| 10 | 2-Tridecanone | 1497 | 0.3 |
| 11 | Isodaucene | 1504 | 0.41 |
| 12 | Tridecanal | 1515 | 0.83 |
| 13 | Dodecanoic acid | 1588 | 1.03 |
| 14 | Hexadecane | 1596 | 0.73 |
| 15 | Heptadecane | 1696 | 0.48 |
| 16 | Pentadecanal | 1718 | 0.41 |
| 17 | Benzyl benzoate | 1789 | 2.03 |
| 18 | Octadecane | 1800 | 0.54 |
| 19 | Nonadecane | 1903 | 3.49 |
| 20 | Eicosane | 2003 | 8.16 |
| 21 | Hexadecenoic acid | 2009 | 2.77 |
| 22 | Octadecanal | 2029 | 0.48 |
| 23 | Heneicosane | 2114 | 13.84 |
| 24 | Linoleic acid | 2166 | 0.73 |
| 25 | 4 Docosane | 2203 | 1.69 |
| 26 | Eicosanal | 2234 | 1.26 |
| 27 | Triacosane | 2317 | 19.74 |
| 28 | Tetracosane | 2396 | 1.61 |
| 29 | 1-Docosanol | 2432 | 0.32 |
| 30 | Pentacosane | 2496 | 22.34 |
| 31 | Dioctyl phthalate | 2532 | 12.49 |
| 32 | Hexacosane | 2595 | 0.17 |
| No. | Compound Name | RI | Percentage Peak Area |
|---|---|---|---|
| 1 | Benzyl benzoate | 1787 | 0.04 |
| 2 | Octadecane | 1797 | 0.02 |
| 3 | Nonadecane | 1897 | 0.09 |
| 4 | Hexadecenoic acid | 1982 | 0.13 |
| 5 | Eicosane | 1997 | 0.06 |
| 6 | Heneicosane | 2101 | 1.11 |
| 7 | Octadecanoic acid | 2185 | 1.12 |
| 8 | Docosane | 2223 | 92.63 |
| 9 | Tricosane | 2299 | 1.2 |
| 10 | Tetracosane | 2396 | 0.08 |
| 11 | Docosanal | 2432 | 0.11 |
| 12 | Data MS | 2472 | 0.43 |
| 13 | Pentacosane | 2496 | 2 |
| 14 | Diisooctyl phthalate | 2532 | 0.88 |
| 15 | Hexacosane | 2595 | 0.1 |
| Peak | Rt (min) | MW | Ion Mode | Compounds | Chemical Formula | Peak Intensity in White Flower | Peak Intensity in Dark Pink Flower | Maximum Absorbance | Mass Fragments | |
|---|---|---|---|---|---|---|---|---|---|---|
| [M + H]+ | [M − H]− | |||||||||
| 1 | 2.43 | 772 | 773 | - | Quercetin-3-O-rutinoside-7-O-glucoside | C33H40O21 | 2.18 × 105 | 4.26 × 104 | 3.23 × 105 | 343, 344, 361, 366, 193, 194 |
| 2 | 2.49 | 533 | 534 | - | Pelargonidin 3-O-(6″-succinyl glucoside) | C25H25O13 | 1.15 × 105 | 3.10 × 105 | 1.34 × 105 | 136, 343, 193 |
| 3 | 2.52 | 290 | 291 | - | Epicatechin | C15H14O6 | 6.26 × 105 | 9.81 × 105 | 4.16 × 107 | 136, 193, 209 |
| 4 | 2.67 | 534 | - | 533 | Kaempferol 3-O-(6″-malonyl- glucoside) | C24H22O14 | 1.86 × 106 | 1.27 × 106 | 7.95 × 105 | 180, 192, 226, 372 |
| 5 | 2.81 | 314 | 315 | - | Dihydroxy-dimethoxy flavone | C17H14O6 | 4.84 × 105 | 3.50 × 105 | 1.43 × 105 | 104, 116, 118, 183, 187 |
| 6 | 2.85 | 270 | 271 | 269 | Apigenin | C15H10O5 | 4.41 × 107 | 5.71 × 107 | 4.03 × 105 | 104, 116, 118 |
| 7 | 3.04 | 566 | - | 565 | Quercetin-3-O-dipentoside | C25H26O15 | 8.90 × 104 | 6.62 × 104 | 9.69 × 104 | 133 |
| 8 | 3.05 | 298 | 299 | - | Apigenin-7,4′-dimethyl ether | C17H14O5 | 1.12 × 106 | 7.26 × 105 | 1.26 × 106 | 174, 183, 329 |
| 9 | 3.9 | 170 | - | 169 | Gallic acid | C7H6O5 | 8.57 × 1066 | 1.90 × 107 | 1.19 × 107 | 169, 171 |
| 10 | 4.06 | 788 | - | 787 | Tetra-O-galloyl-hexoside | 1.14 × 106 | 3.12 × 106 | 1.54 × 106 | 169, 392, 393, 477, 786, 787 | |
| 11 | 4.23 | 470 | - | 469 | Valoneic acid dilactone | C21H10O13 | 1.03 × 107 | 1.61 × 106 | 7.47 × 105 | 316, 317, 610, 611, 470, 484 |
| 12 | 4.64 | 318 | - | 317 | Myricetin | C15H10O8 | 8.33 × 105 | 9.23 × 106 | 1.08 × 106 | 316, 484, 485 |
| 13 | 4.70 | 434 | 435 | - | Delphinidin-O-pentoside | C29H33O18 | 1.65 × 106 | 2.39 × 106 | 1.92 × 106 | 612, 613, 435, 450, 288, |
| 14 | 4.85 | 610 | - | 609 | Rutin | C27H30O16 | 7.76 × 106 | 1.15 × 106 | 8.30 × 106 | 609, 610, 612 |
| 15 | 4.89 | 448 | 449 | - | Kaempferol-3-O glucoside | C21H20O | 2.36 × 106 | 1.96 × 106 | 2.64 × 106 | 612, 613, 392, 288, 289, 449,135, 245, 183 |
| 16 | 4.89 | 448 | 449 | - | Cyanidin-O-hexoside | C21H21ClO11 | 2.36 × 106 | 1.96 × 106 | 2.64 × 106 | |
| 17 | 4.89 | 448 | 449 | - | Quercitrin | C21H20O11 | 2.36 × 106 | 1.96 × 106 | ||
| 18 | 5.89 | 302 | 303 | - | Delphinidin | C15H11O7 | 1.97 × 107 | 1.38 × 107 | 2.23 × 107 | 303, 286, 142, 205, 164 |
| 19 | 5.92 | 286 | 287 | - | Cyanidin | C15H11O6 | 1.15 × 106 | 1.06 × 106 | 1.37 × 106 | 303, 286, 304, 205, 164 |
| 20 | 6.21 | 302 | - | 301 | Ellagic acid | C14H6O8 | 7.04 × 105 | 2.87 × 106 | 7.68 × 105 | 301, 302, 603, 604 |
| 21 | 7.17 | 302 | - | 301 | Quercetin | C15H10O7 | 1.60 × 106 | 4.32 × 105 | 7.68 × 105 | |
| 22 | 7.68 | 600 | - | 599 | Flavonol diglycoside | 1.26 × 107 | 1.14 × 107 | 1.63 × 106 | 599, 600, 601 | |
| 23 | 7.74 | 464 | 465 | - | Quercetin-3-O-hexoside | C21H20O12 | 1.32 × 106 | 1.76 × 106 | 1.54 × 106 | 288, 289, 450, 464, 465, 288, 289 |
| 24 | 7.94 | 448 | - | 447 | Kaempferol-3-O-glucoside | C21H20O11 | 3.73 × 106 | 5.04 × 106 | 4.20 × 106 | 447, 448 |
| 25 | 8.87 | 418 | - | 417 | Kaempferol-3-O-pentoside | - | 1.20 × 106 | 1.37 × 106 | 2.68 × 105 | 416, 417, 625, 626 |
| 26 | 9.90 | 594 | - | 593 | Kaempferol-3-O-rutinoside | C27H30O15 | 8.47 × 105 | 2.60 × 106 | 3.83 × 105 | 449, 450, 593, 594, 595, 596 |
| 27 | 9.90 | 594 | - | 593 | Kaempferol-3,7-hexose-rhamnoside | - | 8.47 × 105 | 2.60 × 106 | 3.83 × 105 | |
| 28 | 11.85 | 286 | - | 285 | Kaempferol | C15H10O6 | 1.14 × 106 | 3.49 × 105 | 1.61 × 105 | 190, 192, 372 |
| 29 | 12.41 | 290 | - | 289 | Catchin | C15H14O6 | 2.44 × 106 | 7.35 × 106 | 3.98 × 105 | 288, 289 |
| 30 | 14.82 | 592 | 593 | - | Cyanidin 3-O-(6″-dioxalyl-glucoside) | C25H20O17 | 8.26 × 105 | 7.06 × 104 | 1.12 × 105 | 225, 236, 237, 360, 519, 521 |
| 31 | 14.91 | 432 | 433 | - | Cyanidin-O-deoxyhexoside | - | 1.16 × 105 | 4.29 × 105 | 1.63 × 105 | 236, 237, 330, 446 |
| 32 | 14.91 | 432 | 433 | - | Apigenin-5-O-glucoside | C21H20O10 | 1.16 × 105 | 4.29 × 105 | 1.63 × 105 | 236, 237, 330, 446 |
| 33 | 15.06 | 616 | 617 | - | Cyanidin 3-O-sambubioside | C26H29ClO15 | 6.10 × 105 | 3.66 × 105 | 2.79 × 105 | 236, 618, 330, 360 |
| 34 | 15.31 | 610 | 611 | - | Cyanidin 3,5-O-diglucoside | C27H31O16Cl | 2.52 × 105 | 2.25 × 105 | 1.24 × 105 | 142, 183, 225, 230, 236 |
| Target Gene | Gene Bank Accession Number | Primer Sequences (Sequence in 5′-3′ Direction) |
|---|---|---|
| PAL | MG922976.1 | F: GAGTACAGGAAGCCAGTGGT R: CCATAGCTGTCCGTACCCTT |
| FLS | AB038247.1 | F: AAGGGTGGGTGGATCATCTG R: CATCACCACCAACTGCCTTC |
| CER1 | XM_024319816.2 | F: GGGAGATGGGTTGGTCATGA R: CGATCAACAGAGTTGCCACC |
| AAT1 | MG820126.1 | F: GCCCTCACTGGTTTTCTCTG R: GCTCCCTGGTGCTGTATCAT |
| MYB1 | EU082130.1 | F: CTATGTCAAGACTCGCACGC R: CAACGAGTGCAGGTGAGATG |
| GGPPS | KX661005.1 | F: CACAAAACTGCGGCTCTTCT R: AGTCCTTCCCAGCAGTCTTC |
| PAR | AB426519 | F: ACAGACCCAAAGGCAGAAC R: TCATCAACCACTACATCAGGAG |
| ANS | BI977949 | F: GCTCGTCAACAAGGAGAAGG R: GGTAGAGG CGAGAGCTTCCT |
| CCD1 | EU327776.1 | F: CGAAAATTGAGGTTGGAGGA R: GCATGGAA CCCATATGGAAC |
| DXR | JX518618.1 | F: GTGACCTCACCTTCCCTC R: CTACGCCACATCTACCAG |
| GPS | DQ286930 | F: TGGCAACTGTTGTGGAACAT R: AGCACGAGACTTCAGCACT |
| LIS1 | AGB14629 | F: ATGGCTGAGTGTGAGTGTGA R: AGCTTTTGTTTATGGCCGGC |
| DXS | ACD70396 | F: GGGTTACCTTGATTCCGACA R: CAACTTTTGCTGCCAGTTCA |
| beta-actin | RXHM01003052.1 | F: GGGGAAAATATGGCATCACACG R: GATTGCGACATACATTGCTGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jariani, P.; Shahnejat-Bushehri, A.-A.; Naderi, R.; Zargar, M.; Naghavi, M.R. Molecular and Phytochemical Characteristics of Flower Color and Scent Compounds in Dog Rose (Rosa canina L.). Molecules 2024, 29, 3145. https://doi.org/10.3390/molecules29133145
Jariani P, Shahnejat-Bushehri A-A, Naderi R, Zargar M, Naghavi MR. Molecular and Phytochemical Characteristics of Flower Color and Scent Compounds in Dog Rose (Rosa canina L.). Molecules. 2024; 29(13):3145. https://doi.org/10.3390/molecules29133145
Chicago/Turabian StyleJariani, Parisa, Ali-Akbar Shahnejat-Bushehri, Roohangiz Naderi, Meisam Zargar, and Mohammad Reza Naghavi. 2024. "Molecular and Phytochemical Characteristics of Flower Color and Scent Compounds in Dog Rose (Rosa canina L.)" Molecules 29, no. 13: 3145. https://doi.org/10.3390/molecules29133145
APA StyleJariani, P., Shahnejat-Bushehri, A.-A., Naderi, R., Zargar, M., & Naghavi, M. R. (2024). Molecular and Phytochemical Characteristics of Flower Color and Scent Compounds in Dog Rose (Rosa canina L.). Molecules, 29(13), 3145. https://doi.org/10.3390/molecules29133145







