Application of Bimetallic Hydroxide/Graphene Composites in Wastewater Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations
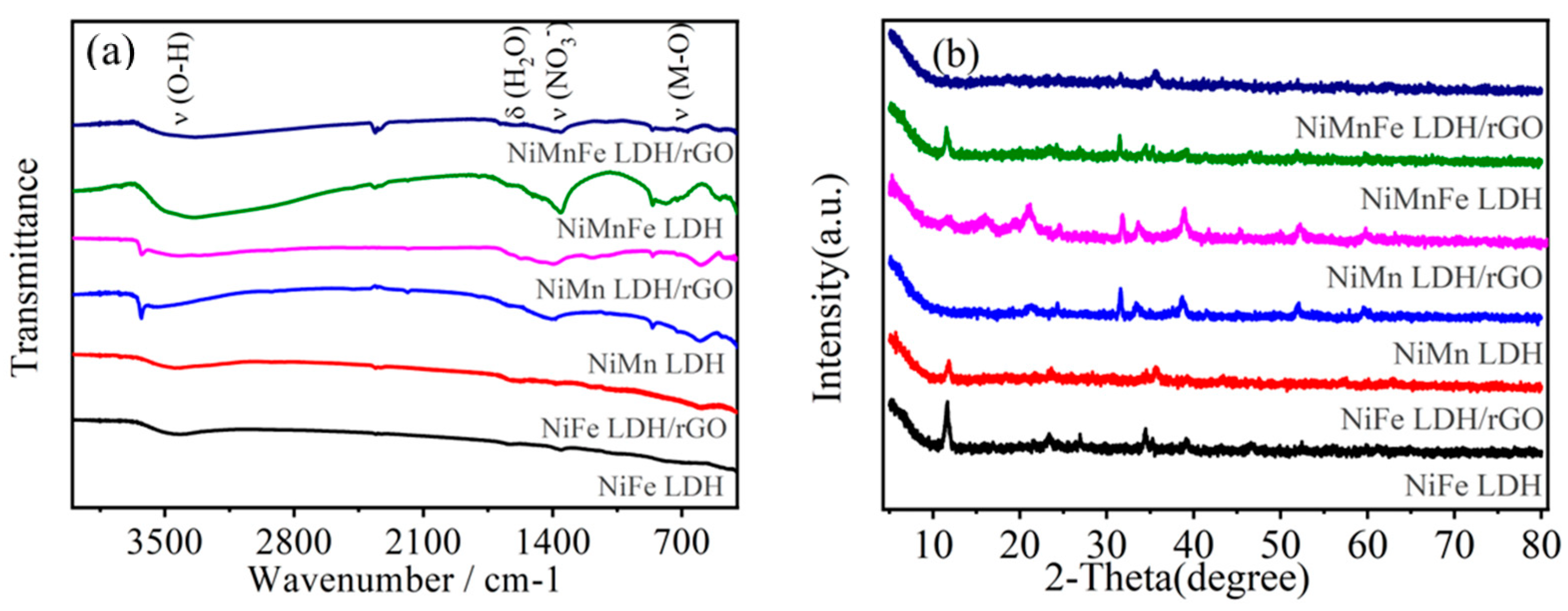
2.1.1. FT-IR and XRD Analysis
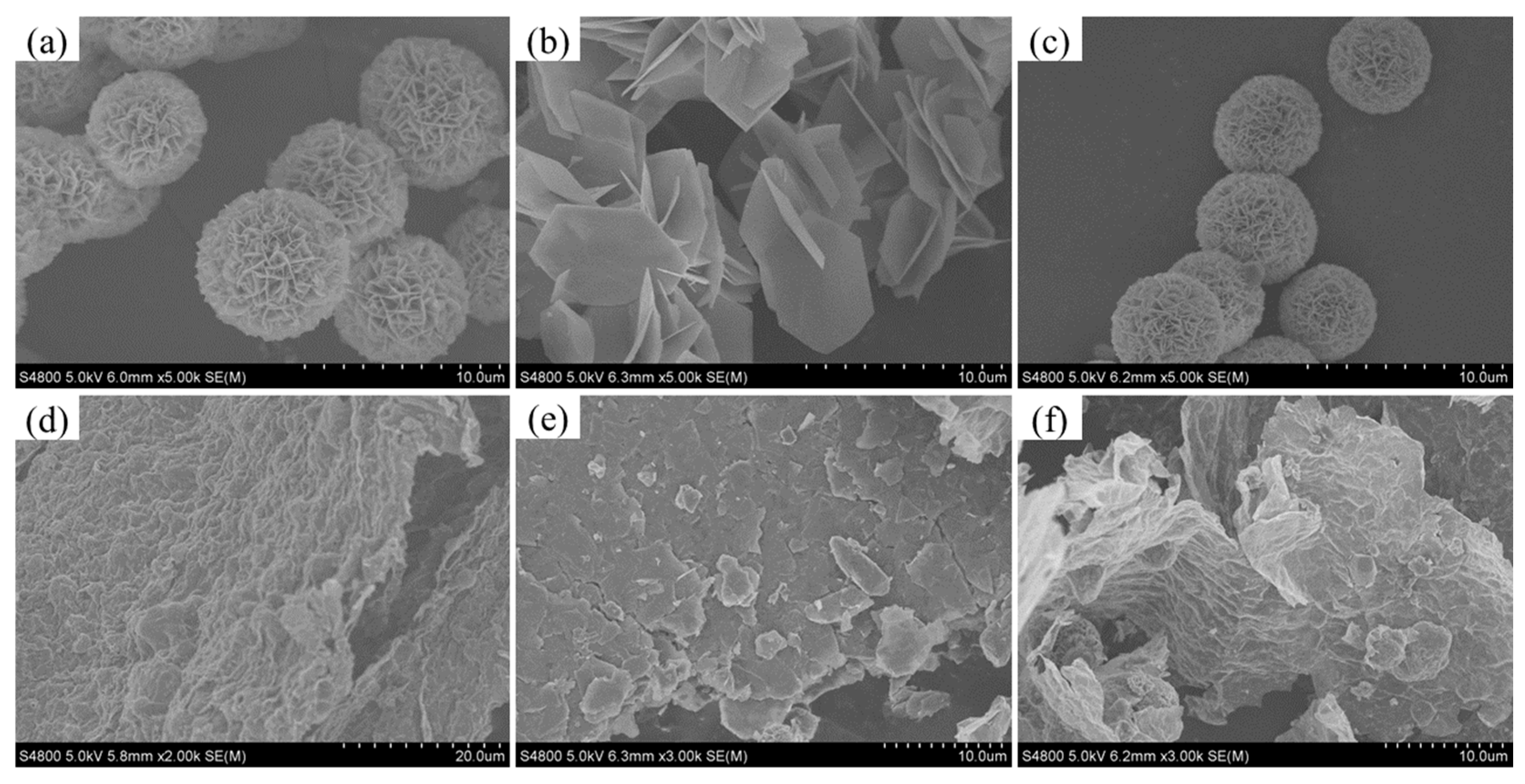
2.1.2. SEM Analysis
2.1.3. TEM Analysis
2.2. Electrocatalytic Oxygen Reduction Synthesis of H2O2 Performance
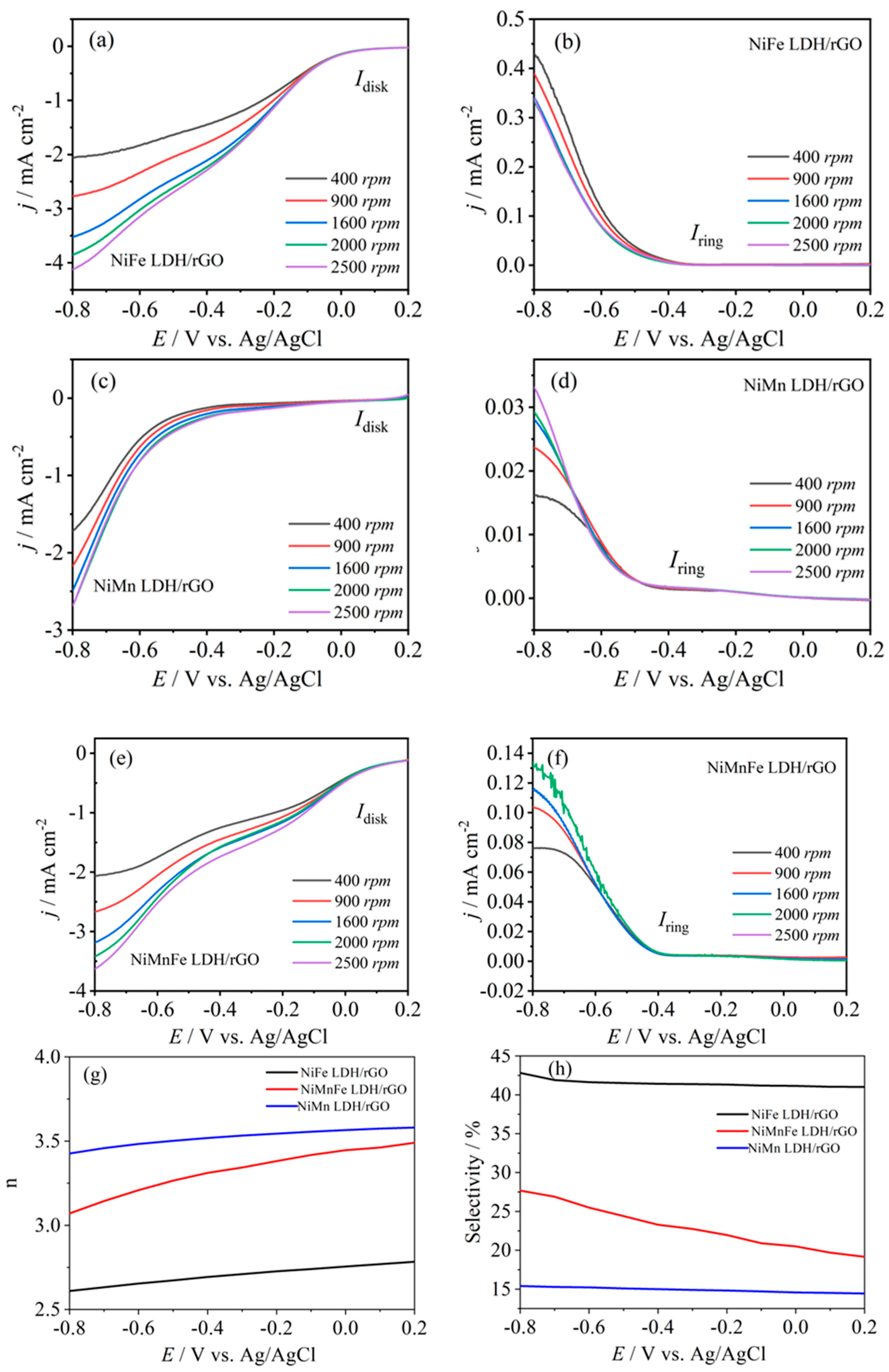
2.2.1. LSV Analysis
2.2.2. Electrochemical Active Area Analysis
2.3. Optimization of Electro-Fenton Process Conditions
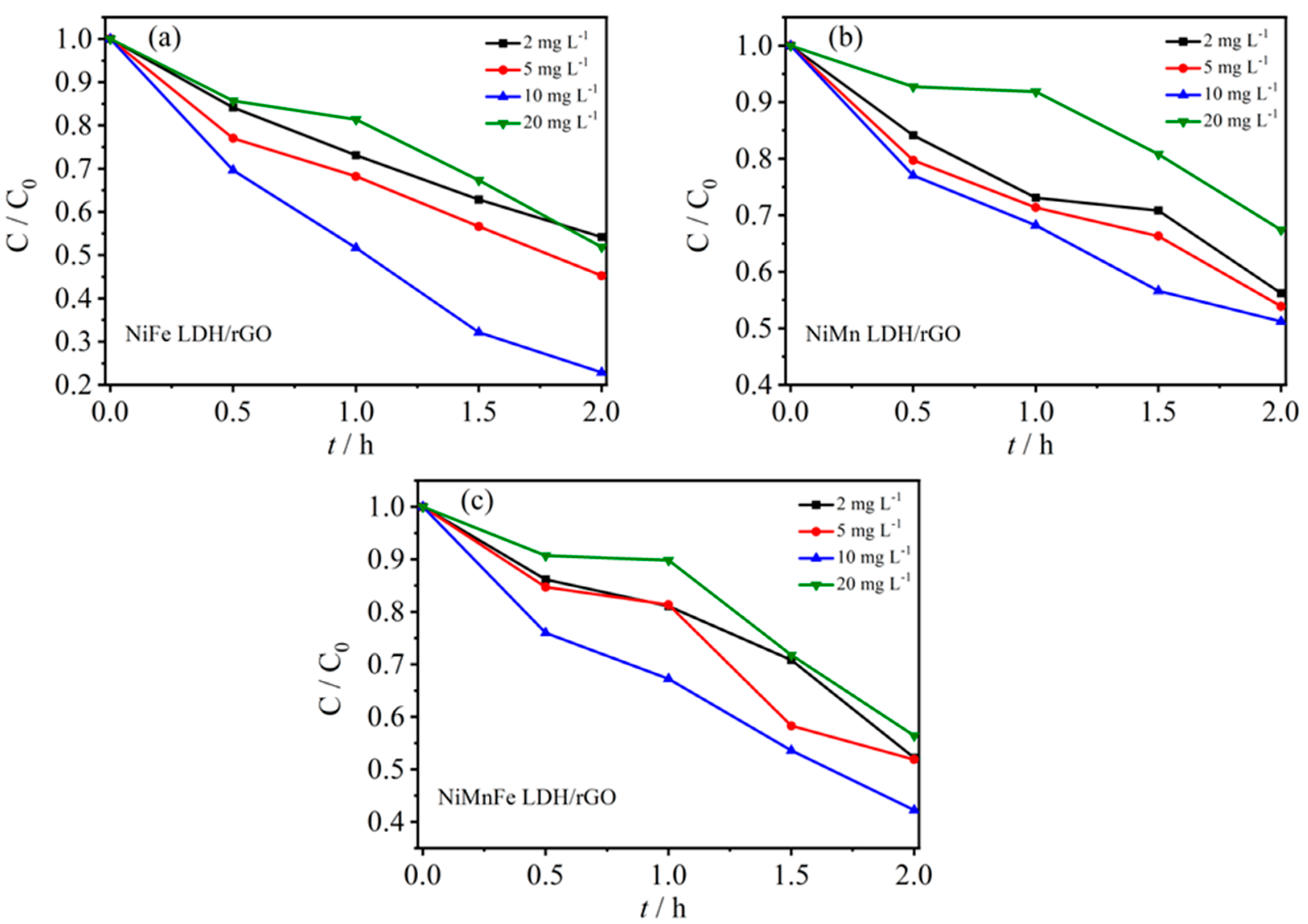
2.3.1. Effect of Initial Concentration
2.3.2. Effect of Solution pH
2.3.3. Effect of Current Density
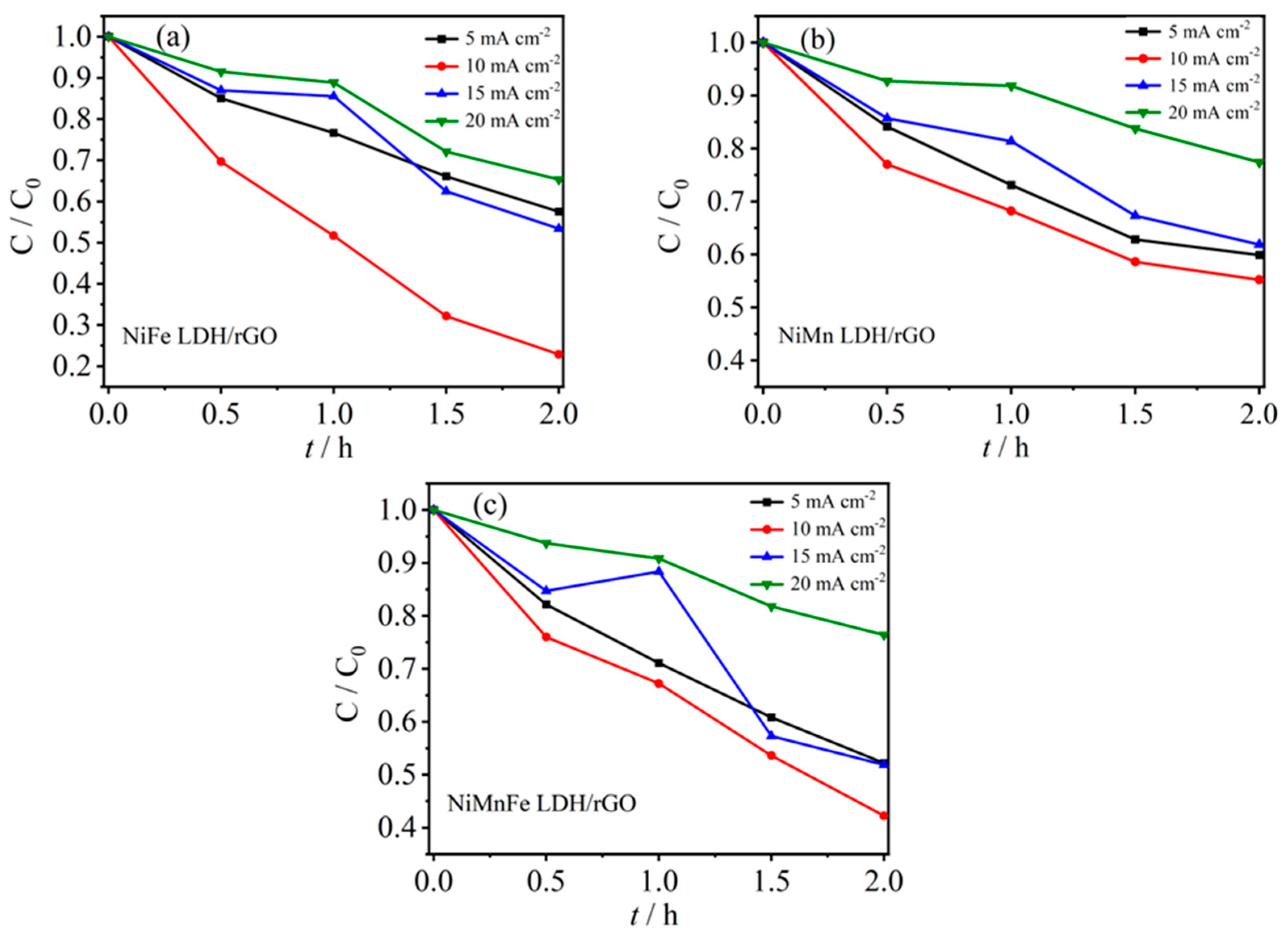
2.3.4. Stability Analysis of Electro-Fenton Cathode Materials
3. Experimental Section
3.1. Experimental Materials
3.2. Preparation of LDH
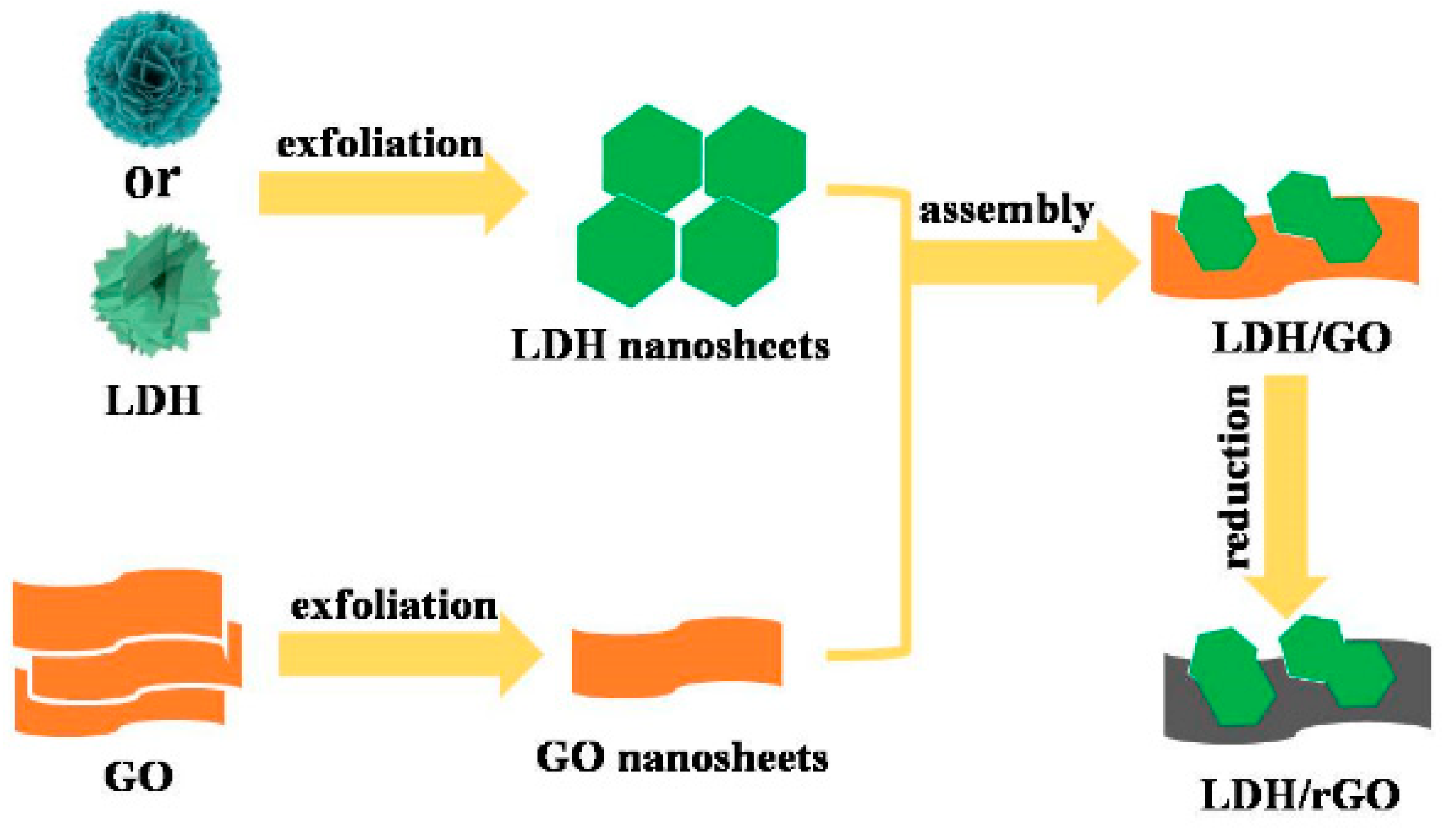
3.3. Stripping of LDH
3.4. Preparation of LDHs/rGO Composites
4. Conclusions
5. Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Hazmi, H.E.; Mohammadi, A.; Hejna, A.; Majtacz, J.; Esmaeili, A.; Habibzadeh, S.; Saeb, M.R.; Badawi, M.; Lima, E.C.; Mąkinia, J. Wastewater Reuse in Agriculture: Prospects and Challenges. Environ. Res. 2023, 236, 116711. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.; Al-Makishah, N.H.; Barakat, M.A. Wastewater Sludge Stabilization Using Pre-Treatment Methods. Process Saf. Environ. Prot. 2016, 102, 615–632. [Google Scholar] [CrossRef]
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.-D. Performance Evaluation of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Sun, H.; Tang, W.; Wang, Q. A Review on Three-Dimensional Electrochemical Technology for the Antibiotic Wastewater Treatment. Environ. Sci. Pollut. Res. 2023, 30, 73150–73173. [Google Scholar] [CrossRef] [PubMed]
- Akhil, D.; Lakshmi, D.; Senthil Kumar, P.; Vo, D.-V.N.; Kartik, A. Occurrence and Removal of Antibiotics from Industrial Wastewater. Environ. Chem. Lett. 2021, 19, 1477–1507. [Google Scholar] [CrossRef]
- Hui, X.; Fang, W.; Wang, G.; Liu, H.; Dai, X. Waste Recycling of Antibiotic Mycelial Residue: The Feasible Harmless Treatment and Source Control of Antibiotic Resistance. J. Clean. Prod. 2023, 401, 136786. [Google Scholar] [CrossRef]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial Use and Resistance in Animals. Clin. Infect. Dis. 2002, 34 (Suppl. S3), S93–S106. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of Antibiotic Resistance in China and Its Environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Shi, H.; Ni, J.; Zheng, T.; Wang, X.; Wu, C.; Wang, Q. Remediation of Wastewater Contaminated by Antibiotics. A review. Environ. Chem. Lett. 2020, 18, 345–360. [Google Scholar] [CrossRef]
- Feng, G.; Huang, H.; Chen, Y. Effects of Emerging Pollutants on the Occurrence and Transfer of Antibiotic Resistance Genes: A Review. J. Hazard. Mater. 2021, 420, 126602. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The Effects of Antibiotics on the Microbiome Throughout Development and Alternative Approaches for Therapeutic Modulation. Genome Med. 2016, 8, 1–16. [Google Scholar] [CrossRef]
- Iwu, C.D.; Patrick, S.M. An Insight into the Implementation of the Global Action Plan on Antimicrobial Resistance in the Who African Region: A Roadmap for Action. Int. J. Antimicrob. Agents 2021, 58, 106411. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Chaplin, B.P. The Prospect of Electrochemical Technologies Advancing Worldwide Water Treatment. Acc. Chem. Res. 2019, 52, 596–604. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Vieira dos Santos, E.; Tossi de Araújo Costa, E.C.; Martínez-Huitle, C.A. Electrochemical Advanced Oxidation Processes (Eaops) as Alternative Treatment Techniques for Carwash Wastewater Reclamation. Chemosphere 2018, 211, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Li, G.; Zhang, F. Scaling up Floating Air Cathodes for Energy-Efficient H2O2 Generation and Electrochemical Advanced Oxidation Processes. Electrochim. Acta 2019, 299, 273–280. [Google Scholar] [CrossRef]
- Zhang, M.-h.; Dong, H.; Zhao, L.; Wang, D.-x.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Vorontsov, A.V. Advancing Fenton and Photo-Fenton Water Treatment through the Catalyst Design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar] [CrossRef]
- Jose, N.A.; Zeng, H.C.; Lapkin, A.A. Hydrodynamic Assembly of Two-Dimensional Layered Double Hydroxide Nanostructures. Nat. Commun. 2018, 9, 4913. [Google Scholar] [CrossRef]
- Nadeema, A.; Dhavale, V.M.; Kurungot, S. NiZn Double Hydroxide Nanosheet-Anchored Nitrogen-Doped Graphene Enriched with the Γ-Niooh Phase as an Activity Modulated Water Oxidation Electrocatalyst. Nanoscale 2017, 9, 12590–12600. [Google Scholar] [CrossRef]
- Lu, P.; Liu, Y.; Zhou, T.; Wang, Q.; Li, Y. Recent Advances in Layered Double Hydroxides (Ldhs) as Two-Dimensional Membrane Materials for Gas and Liquid Separations. J. Membr. Sci. 2018, 567, 89–103. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of Two Dimensional Layered Double Hydroxide Nanosheets and Their Applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Shen, X.; Ma, L.; Ji, Z.; Xu, C.; Yuan, A. Solvothermal Synthesis of Nico-Layered Double Hydroxide Nanosheets Decorated on Rgo Sheets for High Performance Supercapacitor. Chem. Eng. J. 2015, 268, 251–259. [Google Scholar] [CrossRef]
- Hou, T.; Yan, L.; Li, J.; Yang, Y.; Shan, L.; Meng, X.; Li, X.; Zhao, Y. Adsorption Performance and Mechanistic Study of Heavy Metals by Facile Synthesized Magnetic Layered Double Oxide/Carbon Composite from Spent Adsorbent. Chem. Eng. J. 2020, 384, 123331. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.; Wang, J.; Yu, Y.; Wen, J.; Li, R. Exfoliation-Restacking Synthesis of Coal-Layered Double Hydroxide Nanosheets/Reduced Graphene Oxide Composite for High Performance Supercapacitors. Electrochim. Acta 2015, 152, 117–125. [Google Scholar] [CrossRef]
- Zhang, P.; Deng, X.; Li, W.; Ma, Z.; Wang, X. Electrochemical-Induced Surface Reconstruction to Nife-Ldhs-Based Heterostructure as Novel Positive Electrode for Supercapacitors with Enhanced Performance in Neutral Electrolyte. Chem. Eng. J. 2022, 449, 137886. [Google Scholar] [CrossRef]
- Chen, D.; He, Z.; Pei, S.; Huang, L.; Shao, H.; Jin, Y.; Wang, J. Pd nanoparticles supported on N and P dual-doped graphene as an excellent composite catalyst for methanol electro-oxidation. J. Alloys Compd. 2019, 785, 781–788. [Google Scholar] [CrossRef]
- Shahzad, A.; Ali, J.; Ifthikar, J.; Aregay, G.G.; Zhu, J.Y.; Chen, Z.L.; Chen, Z.Q. Non-radical PMS activation by the nanohybrid material with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Unifying the 2e− and 4e− Reduction of Oxygen on Metal Surfaces. J. Phys. Chem. Lett. 2012, 3, 2948–2951. [Google Scholar] [CrossRef]
- Nava-Andrade, K.; Carbajal-Arízaga, G.G.; Obregón, S.; Rodríguez-González, V. Layered Double Hydroxides and Related Hybrid Materials for Removal of Pharmaceutical Pollutants from Water. J. Environ. Manag. 2021, 288, 112399. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Zhang, M.; Zhang, B.-T.; Yu, Y.-G. The Electrochemical Degradation of Ciprofloxacin Using a SnO2-Sb/Ti Anode: Influencing Factors, Reaction Pathways and Energy Demand. Chem. Eng. J. 2016, 296, 79–89. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Schmidt, T.C. Determination of Acid Dissociation Constants (PKA) of Cephalosporin Antibiotics: Computational and Experimental Approaches. Chemosphere 2017, 169, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhao, Q.; Xue, Y.; Xu, W.; Ye, Z. Removal of Dinitrotoluene Sulfonate from Explosive Wastewater by Electrochemical Method Using Ti/Iro2 as Electrode. J. Clean. Prod. 2018, 188, 732–740. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Zhang, G.; Yang, F.; He, P. Nio-NiFe2O4-rGO Magnetic nanomaterials for activated peroxymonosulfate degradation of rhodamine B. Water 2019, 11, 384. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Chen, W.; Feng, X. Fast determination of rutin on a biosensor made using a layered double hydroxide nanocomposite modified electrode. Biosensors 2024, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tang, R.; Liu, M.; Xie, S.; Zhang, D.; Kong, X.; Jin, S.; Ji, H.; Zhang, T. Enhancing the catalytic activity of layered double hydroxide supported on graphene for lithium-sulfur redox reactions. Batteries 2022, 8, 200. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Crivoi, D.G.; Medina, F.; Tichit, D. Synthesis of chalcone using LDH/graphene nanocatalysts of different compositions. ChemEngineering 2019, 3, 29. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Wang, J.; Li, N.; Luo, X.; Yu, H.; Fu, H.; Chen, Z.; Yu, B.; Jin, Y.; Kopchuk, D.S. Application of Bimetallic Hydroxide/Graphene Composites in Wastewater Treatment. Molecules 2024, 29, 3157. https://doi.org/10.3390/molecules29133157
Chen D, Wang J, Li N, Luo X, Yu H, Fu H, Chen Z, Yu B, Jin Y, Kopchuk DS. Application of Bimetallic Hydroxide/Graphene Composites in Wastewater Treatment. Molecules. 2024; 29(13):3157. https://doi.org/10.3390/molecules29133157
Chicago/Turabian StyleChen, Dan, Jiao Wang, Nana Li, Xiaoqin Luo, Hua Yu, Haichang Fu, Zhangxin Chen, Binbin Yu, Yanxian Jin, and Dmitry S. Kopchuk. 2024. "Application of Bimetallic Hydroxide/Graphene Composites in Wastewater Treatment" Molecules 29, no. 13: 3157. https://doi.org/10.3390/molecules29133157
APA StyleChen, D., Wang, J., Li, N., Luo, X., Yu, H., Fu, H., Chen, Z., Yu, B., Jin, Y., & Kopchuk, D. S. (2024). Application of Bimetallic Hydroxide/Graphene Composites in Wastewater Treatment. Molecules, 29(13), 3157. https://doi.org/10.3390/molecules29133157





