Synthesis, Characterization, X-ray Molecular Structure, Antioxidant, Antifungal, and Allelopathic Activity of a New Isonicotinate-Derived meso-Tetraarylporphyrin
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of H2TMIPP (1)
2.2. Photophysical Properties of H2TMIPP (1)
2.2.1. UV/Vis Spectroscopy
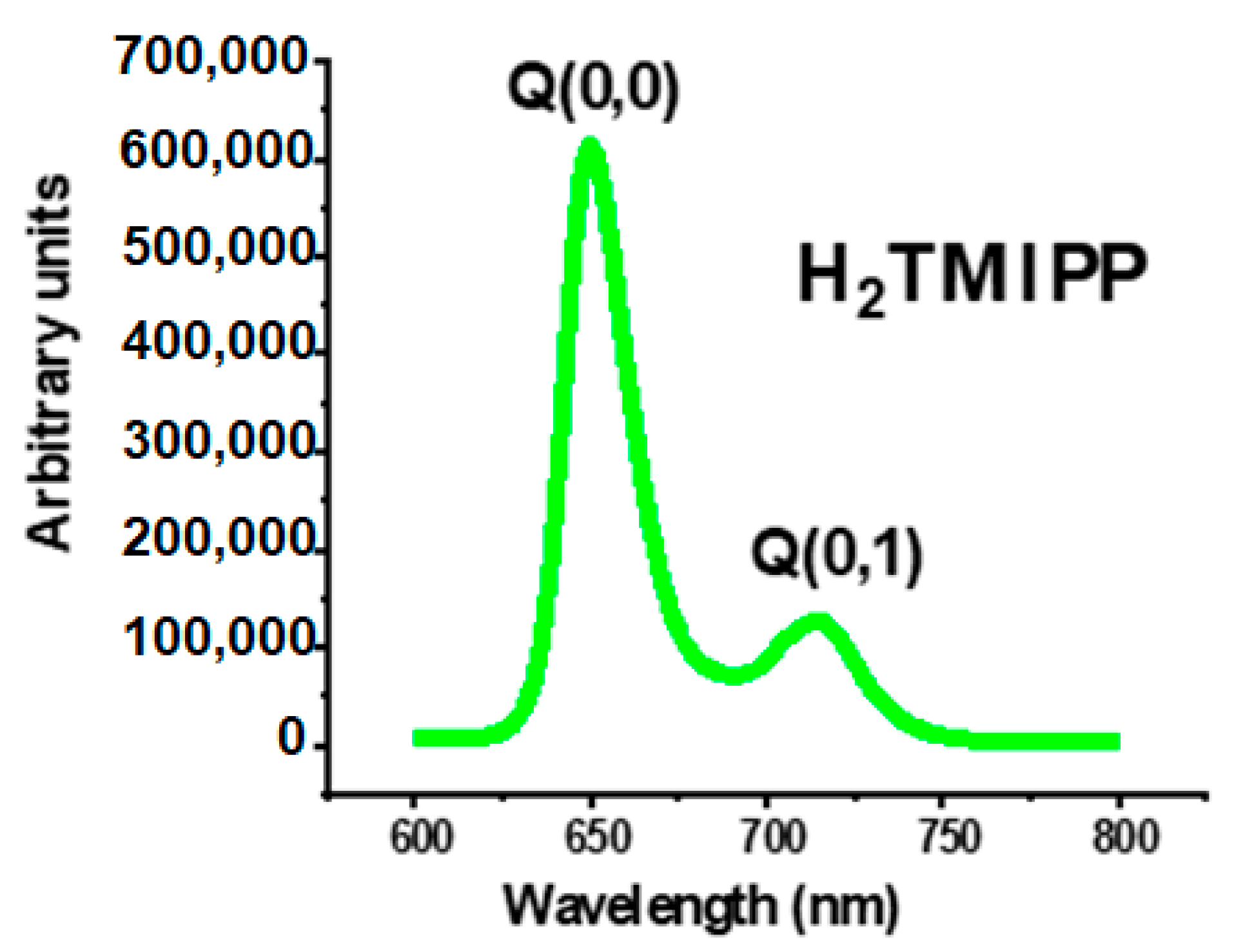
2.2.2. Fluorescence Spectroscopy
2.3. Biological Properties of H2TMIPP (1)
2.3.1. Antioxidant Activity
2.3.2. Antifungal Activity
The Disk Diffusion Method
The Microdilution Method
2.3.3. Allelopathic Activity
3. Materials and Methods
3.1. General Methods
3.2. Synthetic Procedures and Product Characterization
3.2.1. Synthesis of 4-Formyl-2-methoxyphenyl Isonicotinate 4
3.2.2. Synthesis of Porphyrin-5,10,15,20-tetrayltetrakis(2-methoxybenzene-4,1-diyl) Tetraisonicotinate 1
3.3. Antioxidant Activity
3.4. Antifungal Activity
3.5. Allelopathic Activity of H2TIMPP
3.6. X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.-J.; Hong, G.; Gao, L.-J.; Liu, T.-J.; Cao, W.-J. In vitro and in vivo antitumor activity of a novel porphyrin-based photosensitizer for photodynamic therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Rafiee, L.; Hassanzadeh, F.; Dadrass, A.R.; Khodarahmi, G.A. Synthesis of some new porphyrins and their metalloderivatives as potential sensitizers in photo-dynamic therapy. Res. Pharm. Sci. 2015, 10, 504–513. [Google Scholar] [PubMed] [PubMed Central]
- Campestrini, S.; Tonellato, U. Photoinitiated Olefin Epoxidation with Molecular Oxygen, Sensitized by Free Base Porphyrins and Promoted by Hexacarbonylmolybdenum in Homogeneous Solution. Eur. J. Org. Chem. 2002, 2002, 3827–3832. [Google Scholar] [CrossRef]
- Costa e Silva, R.; da Silva, L.O.; Bartolomeu, A.A.; Brocksom, T.J.; de Oliveira, K.T. Recent applications of porphyrins as photocatalysts in organic synthesis: Batch and continuous flow approaches. Beilstein J. Org. Chem. 2020, 16, 917–955. [Google Scholar] [CrossRef] [PubMed]
- Norvaiša, K.; Kielmann, M.; Senge, M.O. Porphyrins as Colorimetric and Photometric Biosensors in Modern Bioanalytical Systems. ChemBioChem 2020, 21, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.O.; Maia, L.B.; Cordas, C.; Delerue-Matos, C.; Moura, I.; Moura, J.J.G.; Morais, S. Nitric Oxide Detection Using Electrochemical Third-generation Biosensors—Based on Heme Proteins and Porphyrins. Electroanalysis 2018, 30, 2485–2503. [Google Scholar] [CrossRef]
- Tang, F.; Wu, J.; Lin, Z.; Wu, H.; Peng, X. A free base porphyrin as an effective modifier of the cathode interlayer for organic solar cells. Appl. Surf. Sci. 2023, 635, 157720. [Google Scholar] [CrossRef]
- Brogdon, P.; Cheema, H.; Delcamp, J.H. Near-Infrared-Absorbing Metal-Free Organic, Porphyrin, and Phthalocyanine Sensitizers for Panchromatic Dye-Sensitized Solar Cells. ChemSusChem 2018, 11, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Roucan, M.; Kielmann, M.; Connon, S.J.; Bernhard, S.S.R.; Senge, M.O. Conformational control of nonplanar free base porphyrins: Towards bifunctional catalysts of tunable basicity. Chem. Commun. 2018, 54, 26–29. [Google Scholar] [CrossRef]
- Arlegui, A.; El-Hachemi, Z.; Crusats, J.; Moyano, A. 5-Phenyl-10,15,20-Tris(4-sulfonatophenyl)porphyrin: Synthesis, Catalysis, and Structural Studies. Molecules 2018, 23, 3363. [Google Scholar] [CrossRef]
- Arlegui, A.; Soler, B.; Galindo, A.; Orteaga, O.; Canillas, A.; Ribó, J.M.; El-Hachemi, Z.; Crusats, J.; Moyano, A. Spontaneous mirror-symmetry breaking coupled to top-bottom chirality transfer: From porphyrin self-assembly to scalemic Diels–Alder adducts. Chem. Commun. 2019, 55, 12219–12222. [Google Scholar] [CrossRef]
- Arlegui, A.; Torres, P.; Cuesta, V.; Crusats, J.; Moyano, A. A pH-Switchable Aqueous Organocatalysis with Amphiphilic Secondary Amine–Porphyrin Hybrids. Eur. J. Org. Chem. 2020, 2020, 4399–4407. [Google Scholar] [CrossRef]
- Arlegui, A.; Torres, P.; Cuesta, V.; Crusats, J.; Moyano, A. Chiral Amphiphilic Secondary Amine-Porphyrin Hybrids for Aqueous Organocatalysis. Molecules 2020, 25, 3420. [Google Scholar] [CrossRef]
- Rybicka-Jasinska, K.; Shan, W.; Zawada, K.; Kadish, K.M.; Gryko, D. Porphyrins as Photoredox Catalysts: Experimental and Theoretical Studies. J. Am. Chem. Soc. 2016, 138, 15451–15458. [Google Scholar] [CrossRef]
- Torres, P.; Guillén, M.; Escribà, M.; Crusats, J.; Moyano, A. Synthesis of New Amino-Functionalized Porphyrins: Preliminary Study of Their Organophotocatalytic Activity. Molecules 2023, 28, 1997. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- Wagner, R.W.; Lawrence, D.S.; Lindsey, J.S. An improved synthesis of tetramesitylporphyrin. Tetrahedron Lett. 1987, 28, 3069–3070. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Prathapan, S.; Johnson, T.E.; Wagner, R.W. Porphyrin Building Blocks for Modular Construction of Bioorganic Model Systems. Tetrahedron 1994, 50, 8941–8968. [Google Scholar] [CrossRef]
- Amiri, N.; Taheur, F.B.; Chevreux, S.; Rodrigues, C.M.; Dorcet, V.; Lemercier, G.; Nasri, H. Syntheses, crystal structures, photo-physical properties, antioxidant and antifungal activities of Mg(II) 4,4′-bipyridine and Mg(II) pyrazine complexes of the 5,10,15,20 tetrakis(4–bromophenyl)porphyrin. Inorg. Chim. Acta 2021, 525, 120466. [Google Scholar] [CrossRef]
- Keskin, B.; Peksel, A.; Avciata, U.; Gül, A. Radical scavenging and in vitro antifungal activities of Cu(II) and Co(II) complexes of the t-butylphenyl derivative of porphyrazine. J. Coord. Chem. 2010, 63, 3999–4006. [Google Scholar] [CrossRef]
- Mkacher, H.; Taheur, F.B.; Amiri, N.; Almahri, A.; Loiseau, F.; Molton, F.; Vollbert, E.M.; Roisnel, T.; Turowska-Tyrk, I.; Nasri, H. DMAP and HMTA manganese(III) meso-tetraphenylporphyrin-based coordination complexes: Syntheses, physicochemical properties, structural and biological activities. Inorg. Chim. Acta 2023, 545, 121278. [Google Scholar] [CrossRef]
- Jabli, S.; Hrichi, S.; Chaabane-Banaoues, R.; Molton, F.; Loiseau, F.; Roisnel, T.; Turowska-Tyrk, I.; Babba, H.; Nasri, H. Study on the synthesis, physicochemical, electrochemical properties, molecular structure and antifungal activities of the 4-pyrrolidinopyridine Mg(II) meso-tetratolylporphyrin complex. J. Mol. Struct. 2022, 1261, 132882. [Google Scholar] [CrossRef]
- Fadda, A.A.; El-Gendy, E.; Refat, H.M.; Tawfik, E.H. Utility of dipyrromethane in the synthesis of some new A2B2 porphyrins and their related porphyrin-like derivatives with their evaluation as antimicrobial and antioxidant agents. Dye. Pigment. 2021, 191, 109008. [Google Scholar] [CrossRef]
- Potkin, V.I.; Bumagin, N.A.; Dikusar, E.A.; Petkevich, S.K.; Kurman, P.V. Functional Derivatives of 4-Formyl-2-methoxyphenyl Pyridine-4-carboxylate. Russ. J. Org. Chem. 2019, 55, 1483–1494. [Google Scholar] [CrossRef]
- CCDC 2309201 Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 22 November 2023).
- Ezzayani, K.; Denden, Z.; Najmudin, S.; Bonifácio, C.; Saint-Aman, E.; Loiseau, F.; Nasri, H. Exploring the Effects of Axial Pseudohalide Ligands on the Photophysical and Cyclic Voltammetry Properties and Molecular Structures of Mg II Tetraphenyl/porphyrin Complexes. Eur. J. Inorg. Chem. 2014, 31, 5348–5361. [Google Scholar] [CrossRef]
- Guergueb, M.; Nasri, S.; Brahmi, J.; Loiseau, F.; Molton, F.; Roisnel, T.; Guerineau, V.; Turowska-Tyrk, I.; Aouadi, K.; Nasri, H. Effect of the coordination of π-acceptor 4-cyanopyridine ligand on the structural and electronic properties of meso -tetra(para -methoxy) and meso -tetra(para -chlorophenyl) porphyrin cobalt(ii) coordination compounds. Application in the catalytic degradation of methylene blue dye. RSC Adv. 2020, 10, 6900–6918. [Google Scholar] [CrossRef] [PubMed]
- Dardouri, N.E.; Mkacher, H.; Ghalla, H.; Amor, F.B.; Hamdaoui, N.; Nasri, S.; Roisnel, T.; Nasri, H. Synthesis and characterization of a new cyanato-N cadmium(II) Meso-arylporphyrin complex by X-ray diffraction analysis, IR, UV/vis, 1H MNR spectroscopies and TDDFT calculations, optical and electrical properties. J. Mol. Struct. 2023, 1287, 135559. [Google Scholar] [CrossRef]
- Nasri, S.; Zahou, I.; Turowska-Tyrk, I.; Roisnel, T.; Loiseau, F.; Saint-Amant, E.; Nasri, H. Synthesis, Electronic Spectroscopy, Cyclic Voltammetry, Photophysics, Electrical Properties and X-ray Molecular Structures of meso -{Tetrakis [4-(benzoyloxy)phenyl]porphyrinato}zinc(II) Complexes with Aza Ligands. Eur. J. Inorg. Chem. 2016, 2016, 5004–5019. [Google Scholar] [CrossRef]
- Colladet, K.; Nicolas, M.; Goris, L.; Lutsen, L.; Vanderzande, D. Low-band gap polymers for photovoltaic applications. Thin Solid Films 2004, 451–452, 7–11. [Google Scholar] [CrossRef]
- Thangam, R.; Suresh, V.; Kannan, S. Optimized extraction of polysaccharides from Cymbopogon citratus and its biological activities. Int. J. Biol. Macromol. 2014, 65, 415–423. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; García-Moreno, M.V.; Igartuburu, J.M.; Garcia Barroso, C. Simplification of the DPPH assay for estimating the antioxidant activity of wine and wine by-products. Food Chem. 2014, 165, 198–204. [Google Scholar] [CrossRef]
- Lee, C.Y.; Nanah, C.N.; Held, R.A.; Clark, A.R.; Huynh, U.G.T.; Maraskine, M.C.; Uzarski, R.L.; McCracken, J.; Sharma, A. Effect of electron donating groups on polyphenol-based antioxidant dendrimers. Biochimie 2015, 111, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hrichi, S.; Chaabane-Banaoues, R.; Bayar, S.; Flamini, G.; Oulad El Majdoub, Y.; Mangraviti, D.; Mondello, L.; El Mzoughi, R.; Babba, H.; Mighri, Z.; et al. Botanical and Genetic Identification Followed by Investigation of Chemical Composition and Biological Activities on the Scabiosa atropurpurea L. Stem from Tunisian Flora. Molecules 2020, 25, 5032. [Google Scholar] [CrossRef] [PubMed]
- Hrichi, S.; Chaabane-Banaoues, R.; Giuffrida, D.; Mangraviti, D.; Majdoub, Y.O.E.; Rigano, F.; Mondello, L.; Babba, H.; Mighri, Z.; Cacciola, F. Effect of seasonal variation on the chemical composition and antioxidant and antifungal activities of Convolvulus althaeoides L. leaf extracts. Arab. J. Chem. 2020, 13, 5651–5668. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. Fundam. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- McArdle, P. SORTX—A program for on-screen stick-model editing and autosorting of SHELX files for use on a PC. J. Appl. Crystallogr. 1995, 28, 65. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP -3 for Windows—A version of ORTEP -III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]

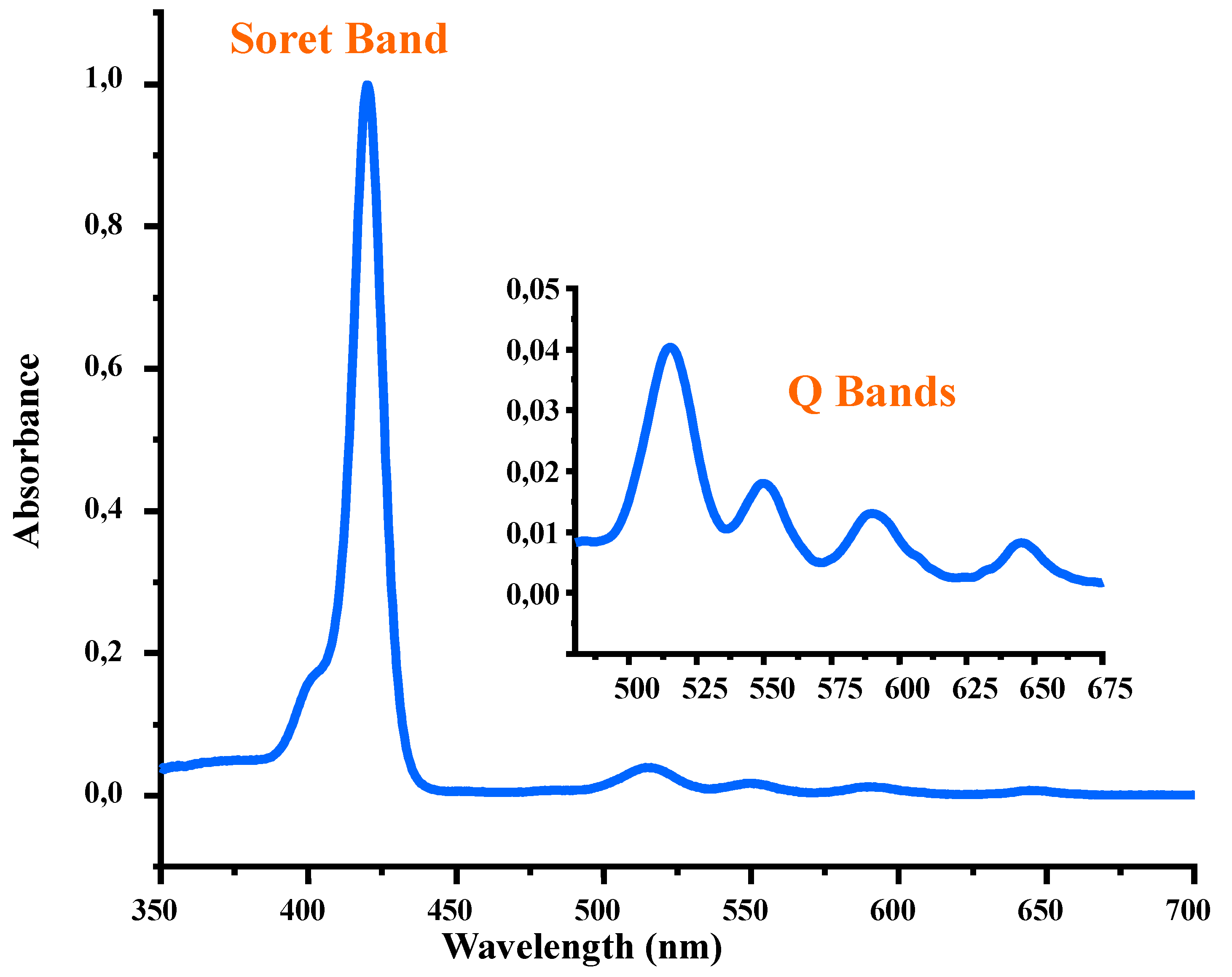


| Porphyrin | Soret Band | Q Bands | Ref. |
|---|---|---|---|
| λmax [nm] (logε) | |||
| H2TPP a | 416 (6.10) | 513 (5.70), 528 (4.36), 590 (4.24), 646 (4.19) | [26] |
| H2TClPP b | 417 (5.05) | 515 (4.93), 548 (4.56), 590 (2.90), 646 (2.60) | [27] |
| H2TBrPP c | 419 (6.58) | 515 (5.24), 549 (4.93), 590 (4.77), 648 (4.68) | [19] |
| H2TMPP d | 420 (5.49) | 517 (4.20), 553 (3.96), 589 (4.38), 645 (3.61) | [28] |
| H2TPBP e | 420 (5.72) | 516 (4.23), 552 (3.84), 591 (3.70), 646 (3.40) | [29] |
| H2TMIPP (1) | 420 (5.73) | 516 (4.33), 551 (3.97), 591 (3.87), 647 (3.68) | this work |
| Porphyrin | λmax Excitation Radiation (nm) | Emission Bands (nm) | ϕf | Ref. | |
|---|---|---|---|---|---|
| Q(0,0) nm | Q(0,1) nm | ||||
| H2TPP a | 420 | 652 | 715 | 0.11 | [26] |
| H2TBrPP b | 420 | 654 | 720 | 0.04 | [19] |
| H2TPBPc | 417 | 653 | 715 | 0.04 | [29] |
| H2TMPP d | 522 | 655 | 719 | 0.05 | [28] |
| H2TMIPP (1) | 420 | 650 | 716 | 0.04 | this work |
| Inhibition Diameters (mm) | |||
|---|---|---|---|
| Inhibitor | C. albicans (ATCC 90028) | C. glabrata (ATCC 64677) | C. tropicalis (ATCC 66029) |
| H2TMIPP (1) | 12 ± 0.5 | 8 ± 0.3 | 12 ± 0.4 |
| Amphotericin B (0.1 mg) | 20 ± 1 | 15 ± 0.5 | 20 ± 0.6 |
| C. albicans (ATCC 90028) | C. glabrata (ATCC 64677) | C. tropicalis (ATCC 66029) | ||||
|---|---|---|---|---|---|---|
| MIC | MIF | MIC | MIF | MIC | MIF | |
| H2TMIPP 1 (μg/L) | 1.25 × 103 | 2.5 × 103 | 5 × 103 | >5 × 103 | 1.25 × 103 | 2.5 × 103 |
| Amphotericin B (µg/L) | 0.25 | 0.5 | 0.5 | 1 | 0.25 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dardouri, N.E.; Hrichi, S.; Torres, P.; Chaâbane-Banaoues, R.; Sorrenti, A.; Roisnel, T.; Turowska-Tyrk, I.; Babba, H.; Crusats, J.; Moyano, A.; et al. Synthesis, Characterization, X-ray Molecular Structure, Antioxidant, Antifungal, and Allelopathic Activity of a New Isonicotinate-Derived meso-Tetraarylporphyrin. Molecules 2024, 29, 3163. https://doi.org/10.3390/molecules29133163
Dardouri NE, Hrichi S, Torres P, Chaâbane-Banaoues R, Sorrenti A, Roisnel T, Turowska-Tyrk I, Babba H, Crusats J, Moyano A, et al. Synthesis, Characterization, X-ray Molecular Structure, Antioxidant, Antifungal, and Allelopathic Activity of a New Isonicotinate-Derived meso-Tetraarylporphyrin. Molecules. 2024; 29(13):3163. https://doi.org/10.3390/molecules29133163
Chicago/Turabian StyleDardouri, Nour Elhouda, Soukaina Hrichi, Pol Torres, Raja Chaâbane-Banaoues, Alessandro Sorrenti, Thierry Roisnel, Ilona Turowska-Tyrk, Hamouda Babba, Joaquim Crusats, Albert Moyano, and et al. 2024. "Synthesis, Characterization, X-ray Molecular Structure, Antioxidant, Antifungal, and Allelopathic Activity of a New Isonicotinate-Derived meso-Tetraarylporphyrin" Molecules 29, no. 13: 3163. https://doi.org/10.3390/molecules29133163
APA StyleDardouri, N. E., Hrichi, S., Torres, P., Chaâbane-Banaoues, R., Sorrenti, A., Roisnel, T., Turowska-Tyrk, I., Babba, H., Crusats, J., Moyano, A., & Nasri, H. (2024). Synthesis, Characterization, X-ray Molecular Structure, Antioxidant, Antifungal, and Allelopathic Activity of a New Isonicotinate-Derived meso-Tetraarylporphyrin. Molecules, 29(13), 3163. https://doi.org/10.3390/molecules29133163






