The Significance of Lignocellulosic Raw Materials on the Pore Structure of Activated Carbons Prepared by Steam Activation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of the Raw Materials
2.1.1. Proximate and Ultimate Analysis of Charcoals
2.1.2. Environmental Scanning Electron Microscope (ESEM) Images of Charcoals
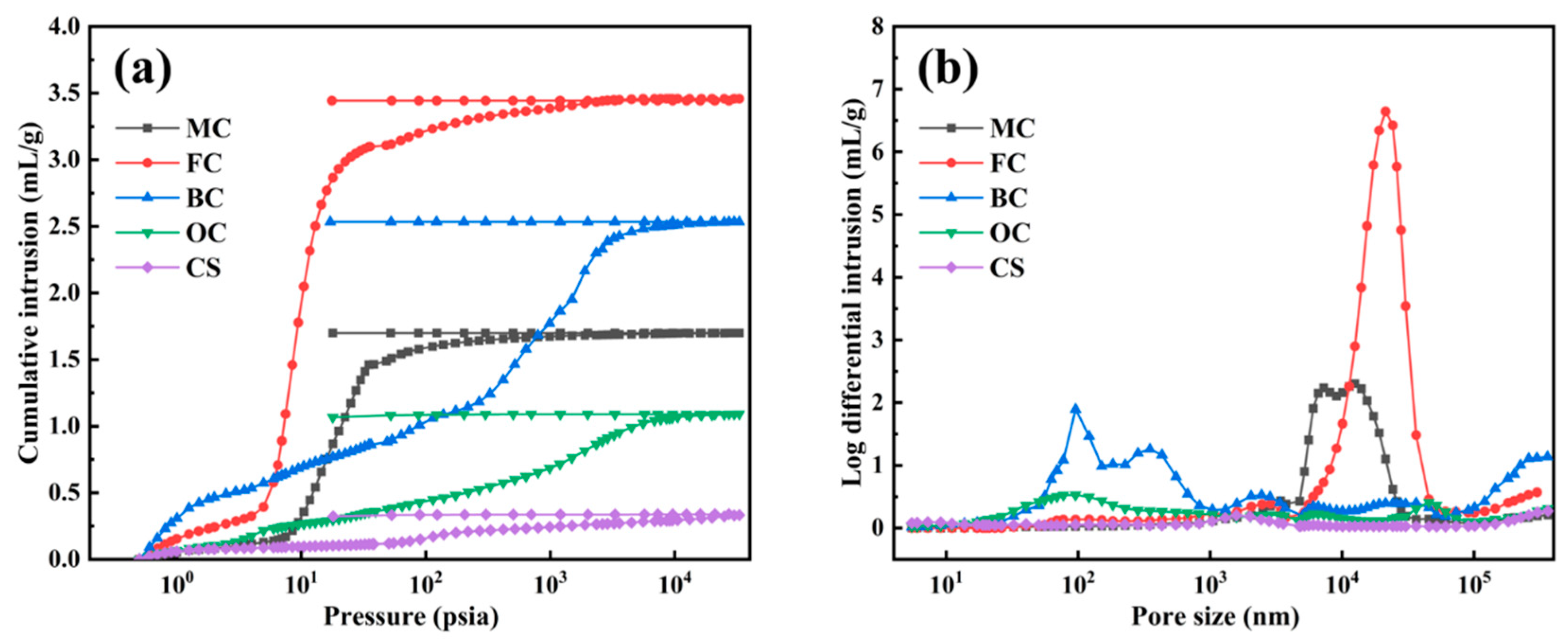
2.1.3. Mercury Injection Porosimetry (MIP) Results
2.2. Progression of Charcoal Burn-Off with Activation Time
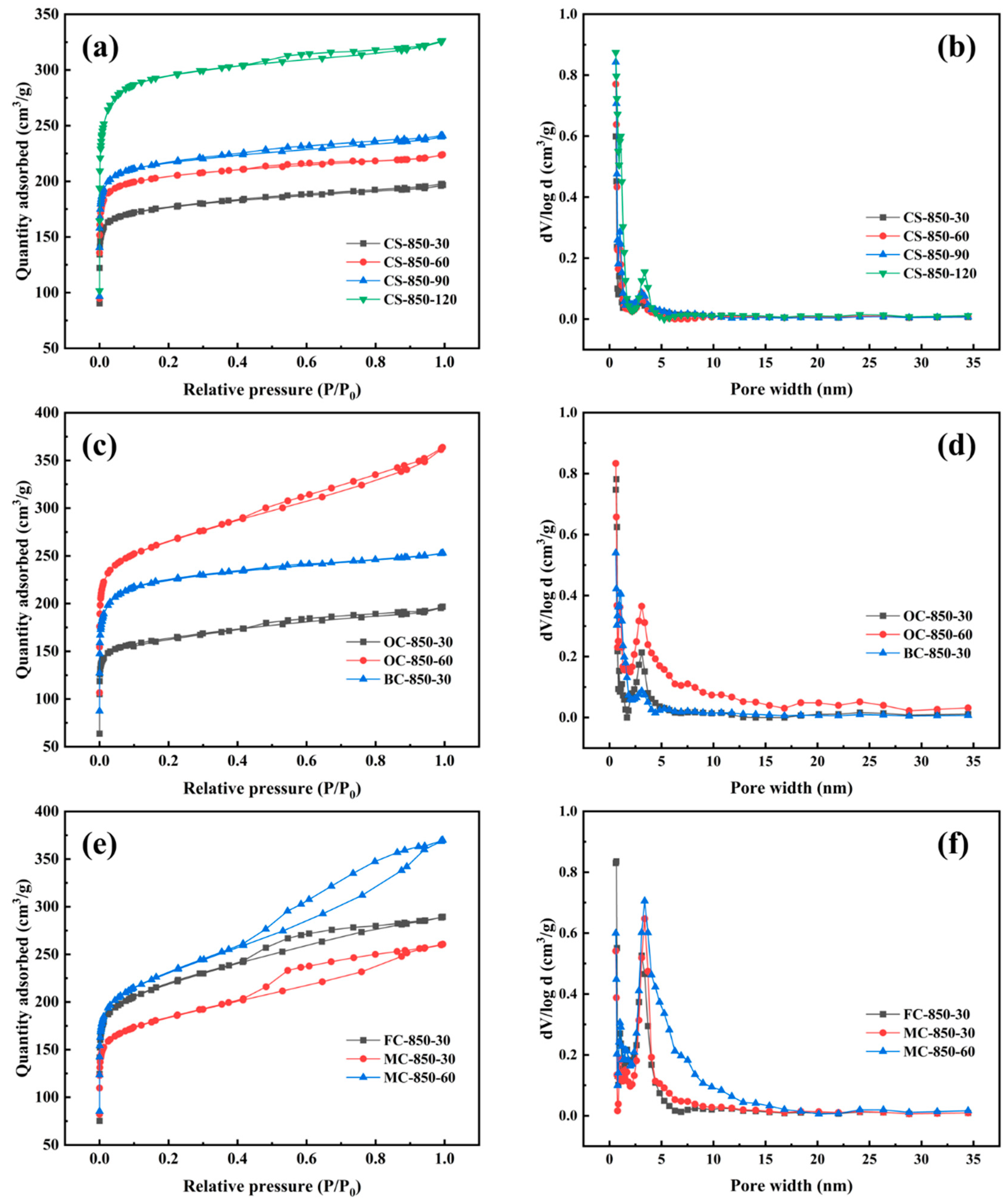
2.3. Development of Pore Structure at Burn-Off of Less Than 50%
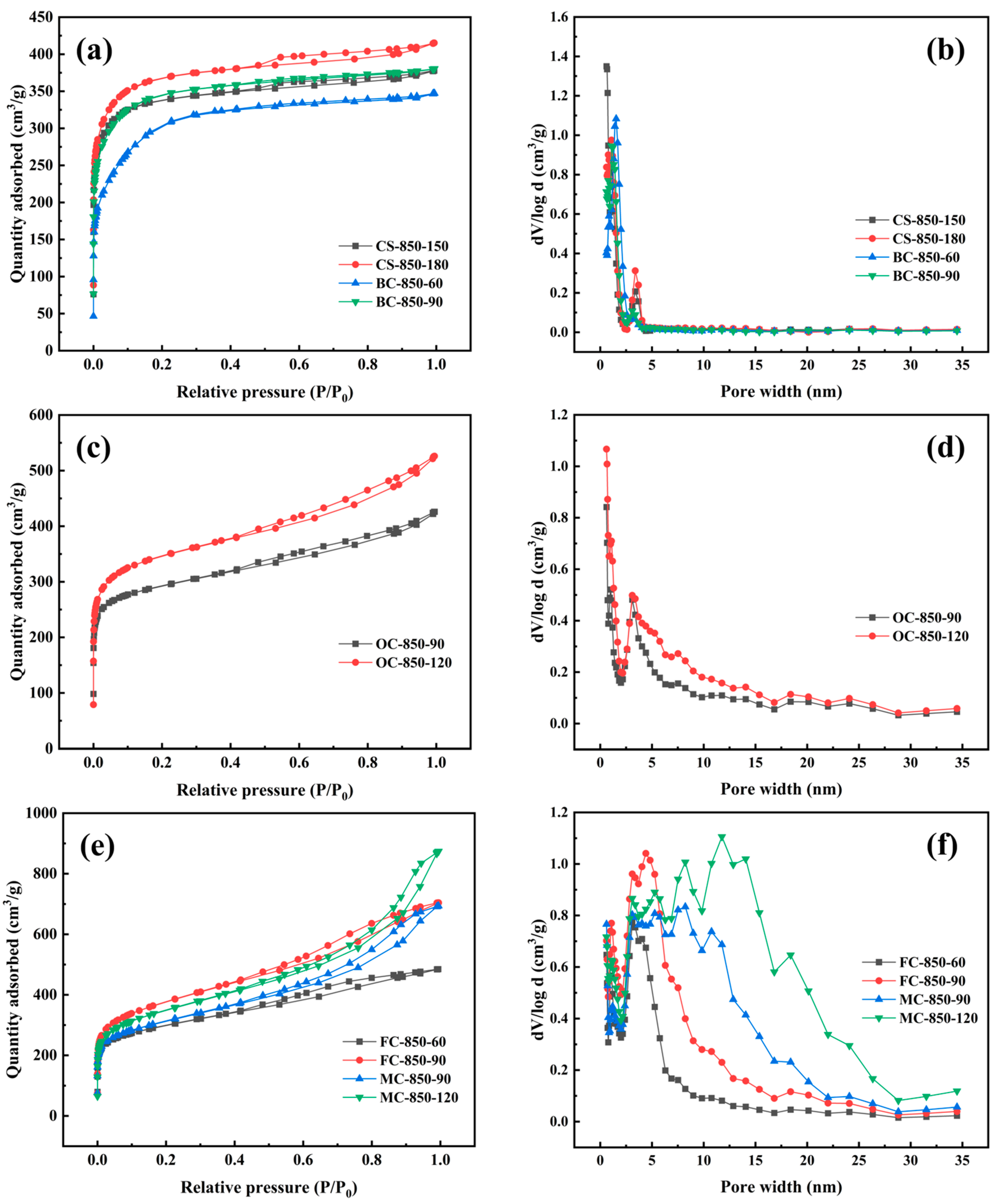
2.4. Pore Structures of Activated Carbons at High Burn-Off
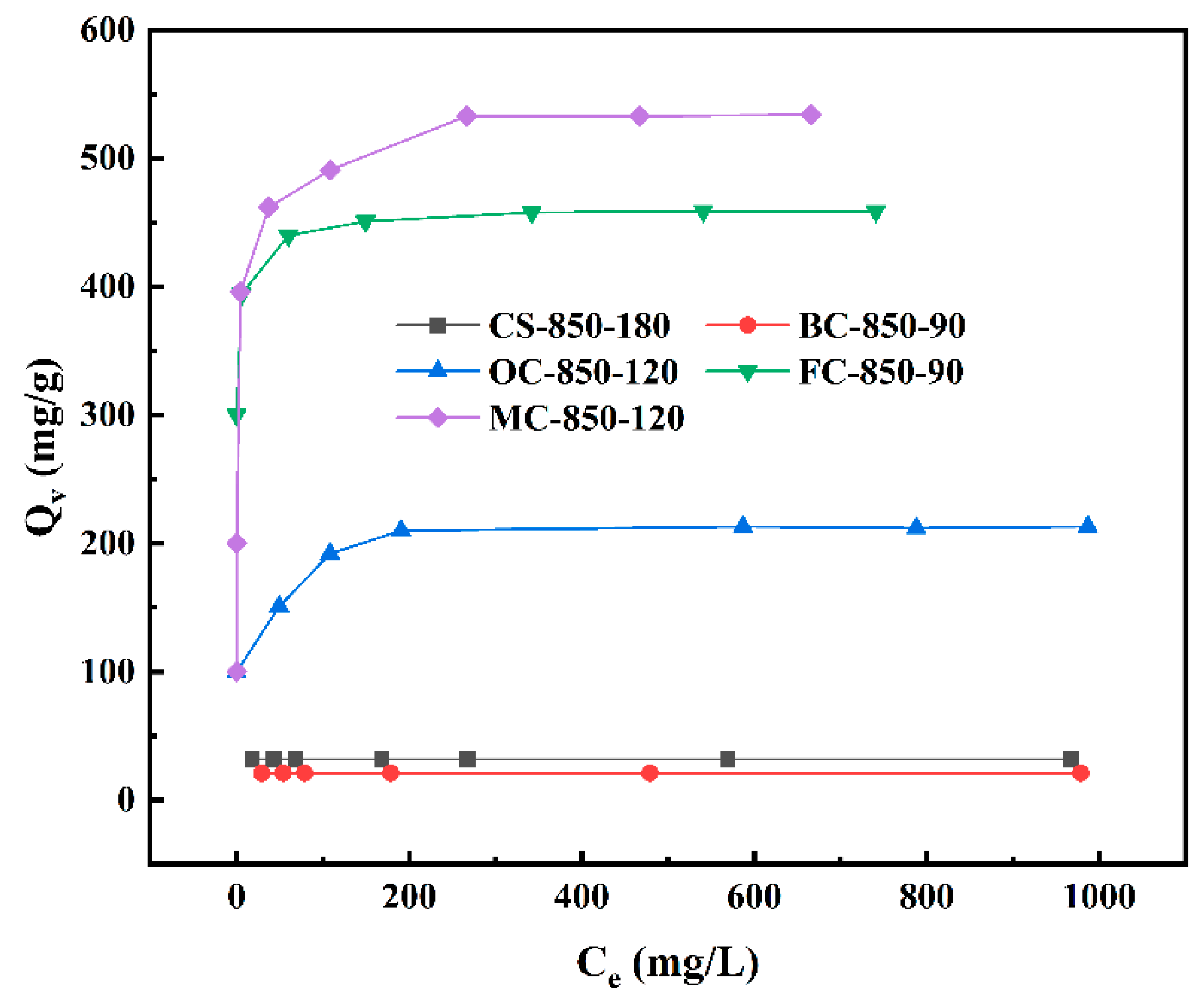
2.5. Vitamin B12 Adsorption on Steam-Activated Carbons
3. Experimental Section
3.1. Preparation of Activated Carbon Materials
3.2. Characterization
3.3. Vitamin B12 Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Gonzalez, J.C.; Gonzalez, M.T.; Molina-Sabio, M.; Rodriguez-Reinoso, F.; Sepulveda-Escribano, A. Porosity of activated carbons prepared from different lignocellulosic materials. Carbon 1995, 33, 1175–1177. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Caturla, F.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Preparation of activated carbon by chemical activation with ZnCl2. Carbon 1991, 29, 999–1007. [Google Scholar] [CrossRef]
- Zuo, S.; Xiao, Z.; Yang, J. Evolution of gaseous products from biomass pyrolysis in the presence of phosphoric acid. J. Anal. Appl. Pyrolysis 2012, 95, 236–240. [Google Scholar] [CrossRef]
- Xie, L.; Meng, Y.; Wang, Q.; Zhang, G.; Xie, H.; Zhou, G. Zanthoxylum bungeanum branches activated carbons with rich micropore structure prepared by low temperature H3PO4 hydrothermal pretreatment method for toluene adsorption. Diam. Relat. Mater. 2022, 130, 109474. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, Y.; Sun, S.; Feng, D.; Zhang, W. Preparation of corn straw-based carbon by “carbonization-KOH activation” two-step method: Gas–solid product characteristics, activation mechanism and hydrogen storage potential. Fuel 2024, 358, 130134. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Qiu, L.; Xiao, J.; Wu, F.; Cao, J.; Bai, Y.; Liu, F. Insights into the KOH activation parameters in the preparation of corncob-based microporous carbon for high-performance supercapacitors. Diam. Relat. Mater. 2022, 129, 109331. [Google Scholar] [CrossRef]
- Gergova, K.; Petrov, N.; Eser, S. Adsorption properties and microstructure of activated carbons produced from agricultural by-products by steam pyrolysis. Carbon 1994, 32, 693–702. [Google Scholar] [CrossRef]
- Nabais, J.O.M.V.; Nunes, P.; Carrott, P.J.M.; Carrott, M.M.L.R.; García, A.M.; Díaz-Díez, M.A. Production of activated carbons from coffee endocarp by CO2 and steam activation. Fuel Process. Technol. 2008, 89, 262–268. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Xia, H.; Zhang, L.; Srinivasakannan, C.; Guo, S. Textural characteristics of activated carbon by single step CO2 activation from coconut shells. J. Taiwan Inst. Chem. Eng. 2010, 41, 367–372. [Google Scholar] [CrossRef]
- González, M.T.; Rodríguez-Reinoso, F.; García, Á.N.; Marcilla, A. CO2 activation of olive stones carbonized under different experimental conditions. Carbon 1997, 35, 159–162. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Daud, W.M.A.W.; Ali, W.S.W.; Sulaiman, M.Z. The effects of carbonization temperature on pore development in palm-shell-based activated carbon. Carbon 2000, 38, 1925–1932. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, R.C. Influence of carbonization conditions on the gasification of acacia and eucalyptus wood chars by carbon dioxide. Fuel 1994, 73, 1922–1925. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crops Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Tsai, W.; Jiang, T. Mesoporous activated carbon produced from coconut shell using a single-step physical activation process. Biomass- Convers. Biorefinery 2018, 8, 711–718. [Google Scholar] [CrossRef]
- Nowicki, P.; Pietrzak, R. Carbonaceous adsorbents prepared by physical activation of pine sawdust and their application for removal of NO2 in dry and wet conditions. Bioresour. Technol. 2010, 101, 5802–5807. [Google Scholar] [CrossRef]
- Tseng, R.; Wu, F.; Juang, R. Liquid-phase adsorption of dyes and phenols using pinewood-based activated carbons. Carbon 2003, 41, 487–495. [Google Scholar] [CrossRef]
- Sánchez, A.R.; Elguézabal, A.A.; de La Torre Saenz, L. CO2 activation of char from Quercus agrifolia wood waste. Carbon 2001, 39, 1367–1377. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; González-García, C.M.; Valente Nabais, J.M.; Ortiz, A.L. Porosity development in activated carbons prepared from walnut shells by carbon dioxide or steam activation. Ind. Eng. Chem. Res. 2009, 48, 7474–7481. [Google Scholar] [CrossRef]
- Liu, X.; Zuo, S.; Cui, N.; Wang, S. Investigation of ammonia/steam activation for the scalable production of high-surface area nitrogen-containing activated carbons. Carbon 2022, 191, 581–592. [Google Scholar] [CrossRef]
- Hattingh, B.B.; Everson, R.C.; Neomagus, H.W.J.P.; Bunt, J.R. Assessing the catalytic effect of coal ash constituents on the CO2 gasification rate of high ash, South African coal. Fuel Process. Technol. 2011, 92, 2048–2054. [Google Scholar] [CrossRef]
- Aworn, A.; Thiravetyan, P.; Nakbanpote, W. Preparation and characteristics of agricultural waste activated carbon by physical activation having micro- and mesopores. J. Anal. Appl. Pyrolysis 2008, 82, 279–285. [Google Scholar] [CrossRef]
- Mermoud, F.; Salvador, S.; Van de Steene, L.; Golfier, F. Influence of the pyrolysis heating rate on the steam gasification rate of large wood char particles. Fuel 2006, 85, 1473–1482. [Google Scholar] [CrossRef]
- Gludovatz, B.; Walsh, F.; Zimmermann, E.A.; Naleway, S.E.; Ritchie, R.O.; Kruzic, J.J. Multiscale structure and damage tolerance of coconut shells. J. Mech. Behav. Biomed. Mater. 2017, 76, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Schmier, S.; Hosoda, N.; Speck, T. Hierarchical structure of the cocos nucifera (coconut) endocarp: Functional morphology and its influence on fracture toughness. Molecules 2020, 25, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Esteban, L.G.; de Palacios, P.; Heinz, I.; Gasson, P.E.; García-Iruela, A.; García-Fernández, F. Softwood Anatomy: A Review. Forests 2023, 14, 2–68. [Google Scholar] [CrossRef]
- Grosser, D.; Liese, W. On the anatomy of Asian bamboos, with special reference to their vascular bundles. Wood Sci. Technol. 1971, 5, 290–312. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, L.; Cai, Y. Combining mercury intrusion porosimetry and fractal theory to determine the porous characteristics of wood. Wood Sci. Technol. 2020, 55, 109–124. [Google Scholar] [CrossRef]
- Plötze, M.; Niemz, P. Porosity and pore size distribution of different wood types as determined by mercury intrusion porosimetry. Eur. J. Wood Wood Prod. 2010, 69, 649–657. [Google Scholar] [CrossRef]
- Abe, I.; Hitorni, M.; Ikuta, N.; Tatsumoto, H.; Kera, Y. Pore structural analysis of charcoals by mercury intrusion porosimetry. Tanso 1996, 1996, 77–82. [Google Scholar] [CrossRef]
- McDougall, G.J. The physical nature and manufacture of activated carbon. J. S. Afr. Inst. Min. Metall. 1991, 91, 109–120. [Google Scholar] [CrossRef]
- Karaca, M.; Kaya, D.; Yozgatligil, A.; Gökalp, I. Modeling and numerical simulations of lignite char gasification with CO2: The effect of gasification parameters on internal transport phenomena. Fuel 2021, 285, 119067. [Google Scholar] [CrossRef]
- Dufourny, A.; Van, D.S.L.; Humbert, G.; Guibal, D.; Martin, L.; Blin, J. Influence of pyrolysis conditions and the nature of the wood on the quality of charcoal as a reducing agent. J. Anal. Appl. Pyrolysis 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F. Controlled gasification of carbon and pore structure development. In Fundamental Issues in Control of Carbon Gasification Reactivity; Springer: Dordrecht, The Netherlands, 1991; Volume 192, pp. 533–571. [Google Scholar] [CrossRef]
- González, M.T.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Steam activation of olive stone chars, development of porosity. Carbon 1994, 32, 1407–1413. [Google Scholar] [CrossRef]
- Jung, S.H.; Oh, S.J.; Choi, G.G.; Kim, J.S. Production and characterization of microporous activated carbons and metallurgical bio-coke from waste shell biomass. J. Anal. Appl. Pyrolysis 2014, 109, 123–131. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, L.F.; Gao, Z.; Zhang, W.W. Equilibrium and kinetic studies on the adsorption of VB12 onto CMK-3. Chin. Chem. Lett. 2007, 18, 233–236. [Google Scholar] [CrossRef]
- Guo, B.; Gong, Q.; Wang, J.; Huang, Y.; Zhuang, D.; Liang, J. Fabrication of uniform porous CNTs/activated carbon composite spheres by oil-drop method in stratified oils and their adsorption of VB12. RSC Adv. 2015, 5, 46997–47003. [Google Scholar] [CrossRef]
- Lupaşcu, T.; Petuhov, O.; Ţîmbaliuc, N.; Cibotaru, S.; Rotaru, A. Adsorption capacity of vitamin B12 and creatinine on highly-mesoporous activated carbons obtained from lignocellulosic raw materials. Molecules 2020, 25, 3095. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zuo, S.; Wang, S. Simple and green synthesis of high-quality macroscopic mesoporous carbon spheres facilitated by graphitic crystallite nanomaterials. Chem. Eng. J. 2023, 462, 142171. [Google Scholar] [CrossRef]
- Zuo, S.; Yang, J.; Liu, J.; Cai, X. Significance of the carbonization of volatile pyrolytic products on the properties of activated carbons from phosphoric acid activation of lignocellulosic material. Fuel Process. Technol. 2009, 90, 994–1001. [Google Scholar] [CrossRef]
- GB/T 12496.4-1999; National Forestry and Grassland Administration. Test Methods of Wooden Activated Carbon-Determination of Moisture Content. Standards Press of China: Beijing, China, 1999.
- GB/T 12496.3-1999; National Forestry and Grassland Administration. Test Methods of Wooden Activated Carbon-Determination of Ash Content. Standards Press of China: Beijing, China, 1999.
- GB/T 17664-1999; National Forestry and Grassland Administration. Wood Charcoal and Test Methods of Wood Charcoal. Standards Press of China: Beijing, China, 1999.
- Washburn, E.W. Note on a method of determining the distribution of pore sizes in a porous material. Proc. Natl. Acad. Sci. USA 1921, 7, 115–116. Available online: http://www.jstor.org/stable/84084 (accessed on 9 December 2023). [CrossRef]
- Shen, W.; Zheng, J.; Zhang, Y.; Wang, J.; Qin, Z. The effect of pore structure of activated carbon on the adsorption of Congo red and vitamin B12. Stud. Surf. Ence Catal. 2002, 146, 779–782. [Google Scholar] [CrossRef]
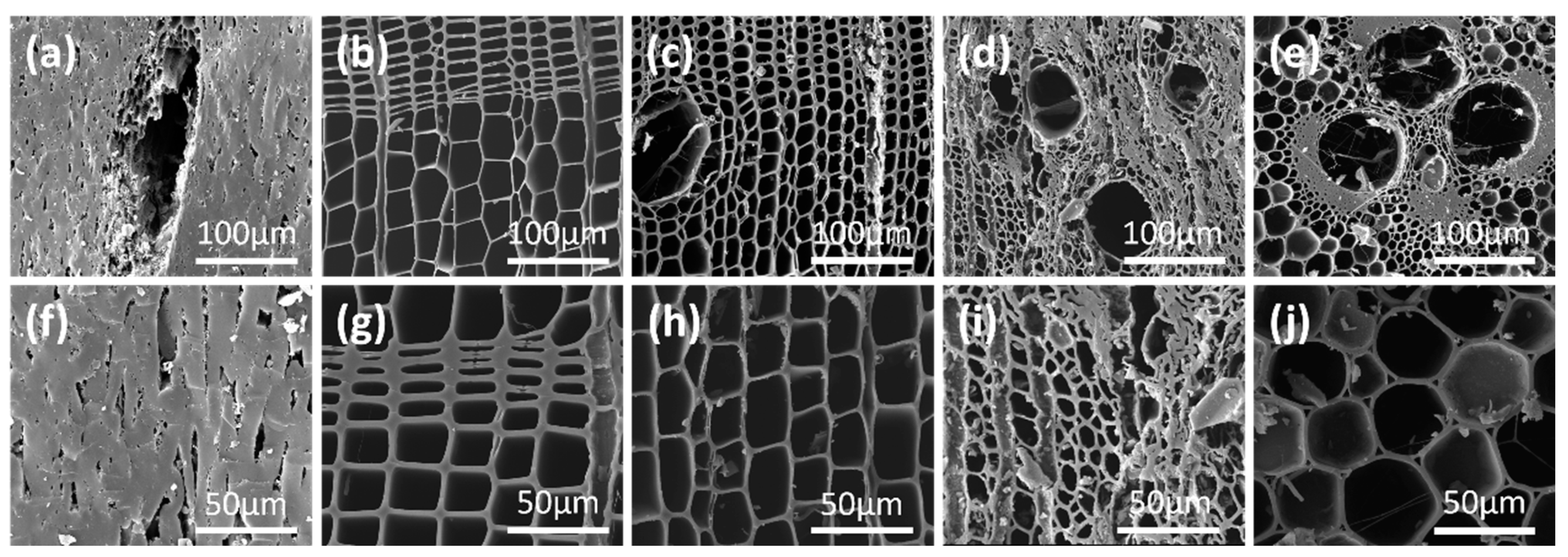

| Samples | Proximate Analysis (%) | Ultimate Analysis (%) | C/N | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatile Matters | Fixed Carbon | C | H | N | O | S | ||
| CS | 5.40 | 1.63 | 21.75 | 76.62 | 90.94 | 3.39 | 3.52 | 1.97 | 0.17 | 25.84 |
| MC | 2.69 | 1.47 | 7.11 | 91.42 | 90.50 | 3.16 | 3.64 | 2.51 | 0.17 | 24.86 |
| FC | 1.22 | 0.72 | 6.66 | 92.62 | 90.72 | 3.52 | 3.62 | 1.99 | 0.12 | 25.06 |
| OC | 5.76 | 3.86 | 27.35 | 68.79 | 88.49 | 4.33 | 3.87 | 3.13 | 0.15 | 22.87 |
| BC | 5.40 | 4.26 | 6.78 | 88.96 | 88.00 | 2.44 | 3.83 | 5.57 | 0.14 | 22.98 |
| Samples | Total Intrusion Volume (mL/g) | Average Pore Diameter (nm) | Bulk Density (g/mL) | Porosity (%) |
|---|---|---|---|---|
| CS | 0.3326 | 59.58 | 0.8751 | 29.10 |
| OC | 1.0912 | 115.81 | 0.5201 | 56.76 |
| BC | 2.5339 | 189.23 | 0.2771 | 70.21 |
| FC | 3.4573 | 2553.83 | 0.2191 | 75.77 |
| MC | 1.6990 | 1719.94 | 0.3816 | 64.83 |
| Samples | Burn-Off (%) | SBET (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) | Mesopore Rate (%) |
|---|---|---|---|---|---|---|
| CS-850-30 | 19.8 | 701 | 0.304 | 0.268 | 0.036 | 11.84 |
| CS-850-60 | 25.0 | 816 | 0.346 | 0.312 | 0.034 | 9.83 |
| CS-850-90 | 38.8 | 861 | 0.372 | 0.331 | 0.041 | 11.02 |
| CS-850-120 | 48.2 | 1162 | 0.505 | 0.446 | 0.059 | 11.68 |
| OC-850-30 | 35.3 | 639 | 0.304 | 0.242 | 0.062 | 20.39 |
| OC-850-60 | 49.7 | 1011 | 0.562 | 0.388 | 0.174 | 30.96 |
| BC-850-30 | 36.7 | 873 | 0.391 | 0.334 | 0.057 | 14.58 |
| FC-850-30 | 36.6 | 820 | 0.448 | 0.313 | 0.135 | 30.13 |
| MC-850-30 | 27.7 | 691 | 0.403 | 0.264 | 0.139 | 34.49 |
| MC-850-60 | 48.5 | 855 | 0.572 | 0.326 | 0.246 | 43.01 |
| Samples | Burn-Off (%) | SBET (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) | Mesopore Rate (%) |
|---|---|---|---|---|---|---|
| CS-850-150 | 58.4 | 1310 | 0.585 | 0.491 | 0.094 | 16.07 |
| CS-850-180 | 66.2 | 1415 | 0.642 | 0.522 | 0.120 | 18.69 |
| OC-850-90 | 54.5 | 1110 | 0.659 | 0.425 | 0.234 | 35.51 |
| OC-850-120 | 71.7 | 1301 | 0.814 | 0.493 | 0.321 | 39.43 |
| BC-850-60 | 59.0 | 1122 | 0.537 | 0.361 | 0.176 | 32.77 |
| BC-850-90 | 74.6 | 1312 | 0.588 | 0.471 | 0.117 | 19.90 |
| FC-850-60 | 58.0 | 1090 | 0.750 | 0.411 | 0.339 | 45.20 |
| FC-850-90 | 73.5 | 1360 | 1.089 | 0.501 | 0.588 | 53.99 |
| MC-850-90 | 62.7 | 1122 | 1.073 | 0.481 | 0.592 | 55.17 |
| MC-850-120 | 78.0 | 1260 | 1.352 | 0.465 | 0.887 | 65.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zuo, S. The Significance of Lignocellulosic Raw Materials on the Pore Structure of Activated Carbons Prepared by Steam Activation. Molecules 2024, 29, 3197. https://doi.org/10.3390/molecules29133197
Zhang L, Zuo S. The Significance of Lignocellulosic Raw Materials on the Pore Structure of Activated Carbons Prepared by Steam Activation. Molecules. 2024; 29(13):3197. https://doi.org/10.3390/molecules29133197
Chicago/Turabian StyleZhang, Li, and Songlin Zuo. 2024. "The Significance of Lignocellulosic Raw Materials on the Pore Structure of Activated Carbons Prepared by Steam Activation" Molecules 29, no. 13: 3197. https://doi.org/10.3390/molecules29133197
APA StyleZhang, L., & Zuo, S. (2024). The Significance of Lignocellulosic Raw Materials on the Pore Structure of Activated Carbons Prepared by Steam Activation. Molecules, 29(13), 3197. https://doi.org/10.3390/molecules29133197







