Surface Functionalization of Bamboo via Photo-Grafting Tannic Acid for Enhanced Silver Ion Loading Properties
Abstract
1. Introduction
2. Results and Discussion
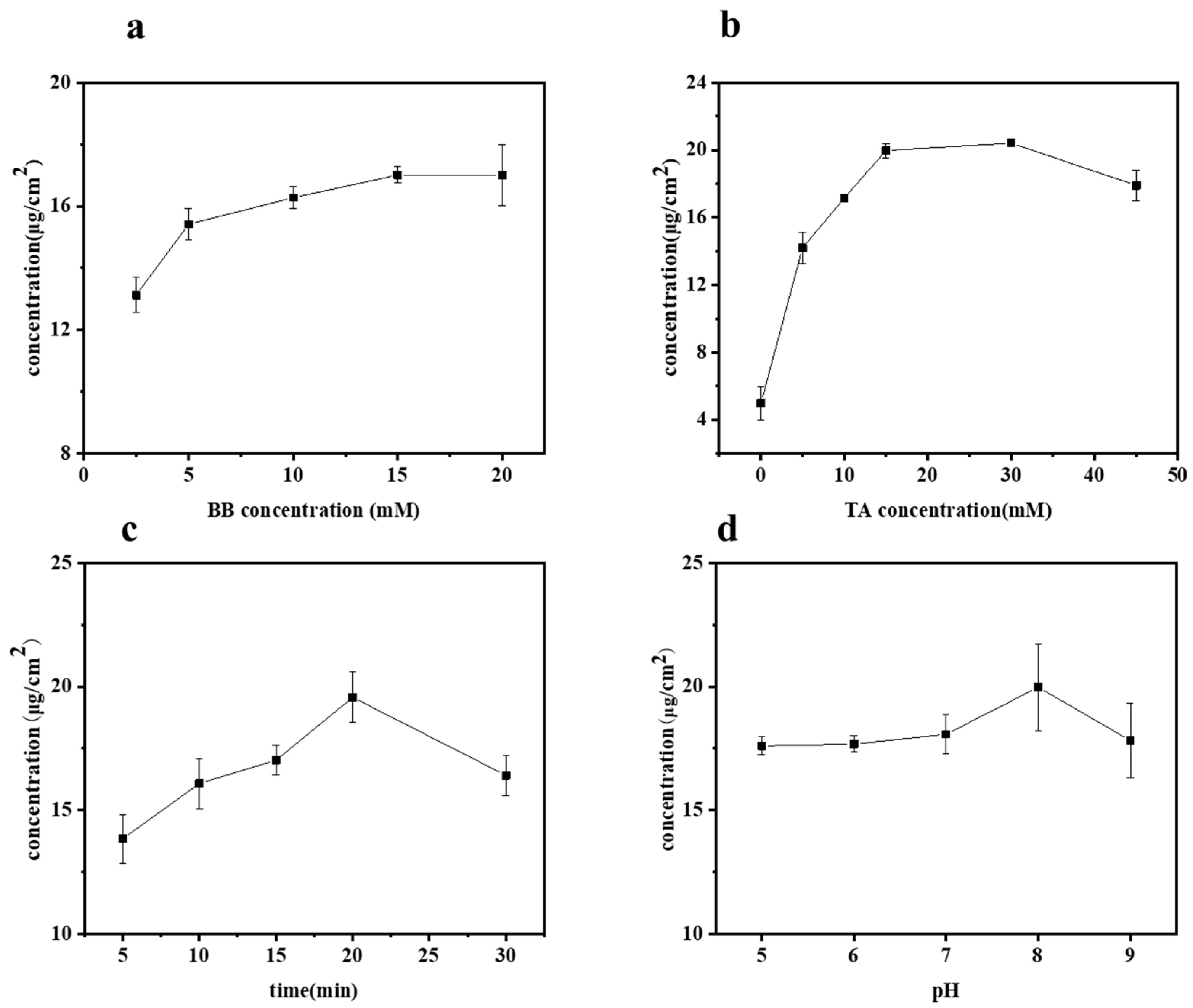
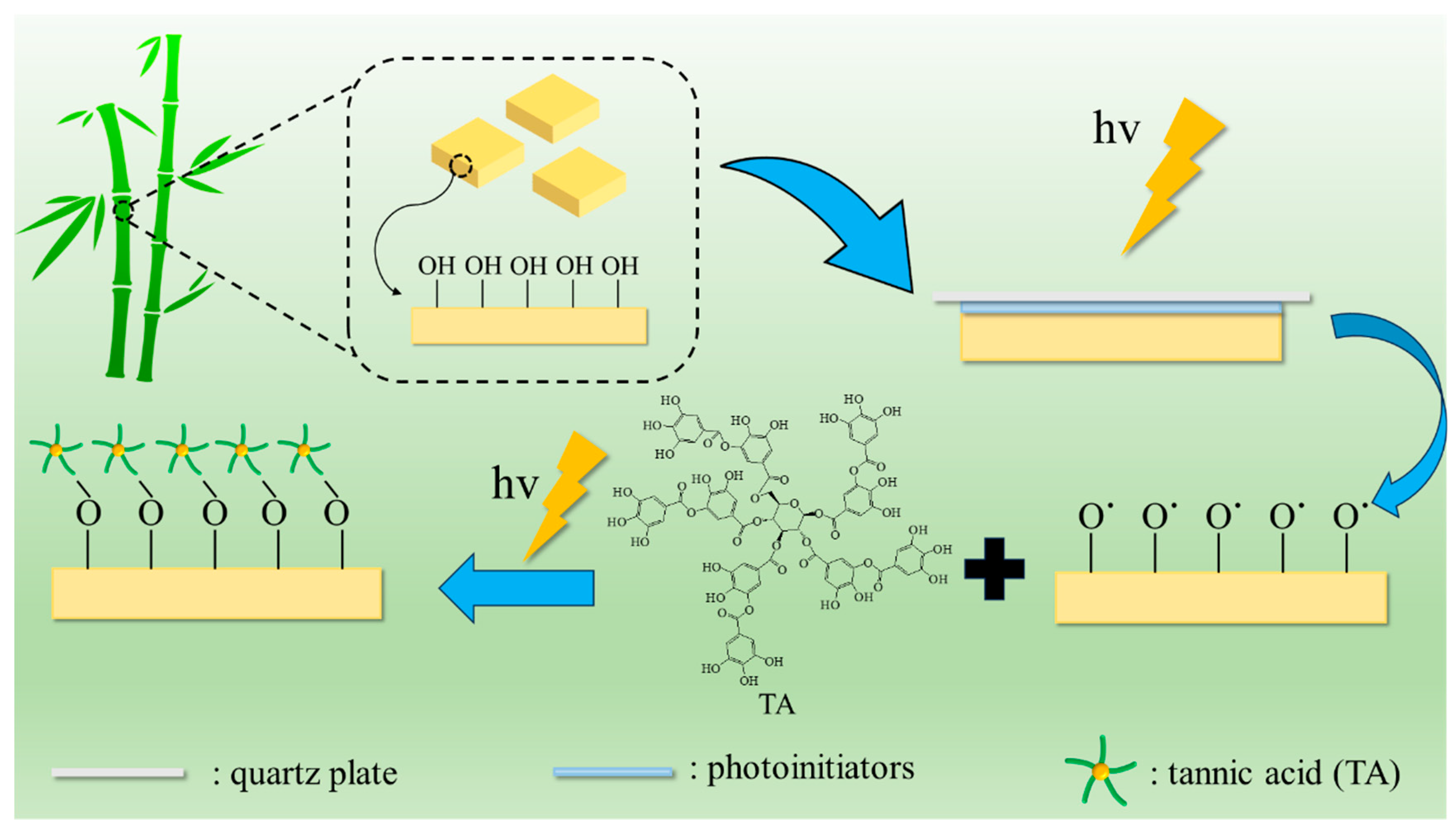
2.1. Surface Modification of Bamboo by TA Photo-Grafting
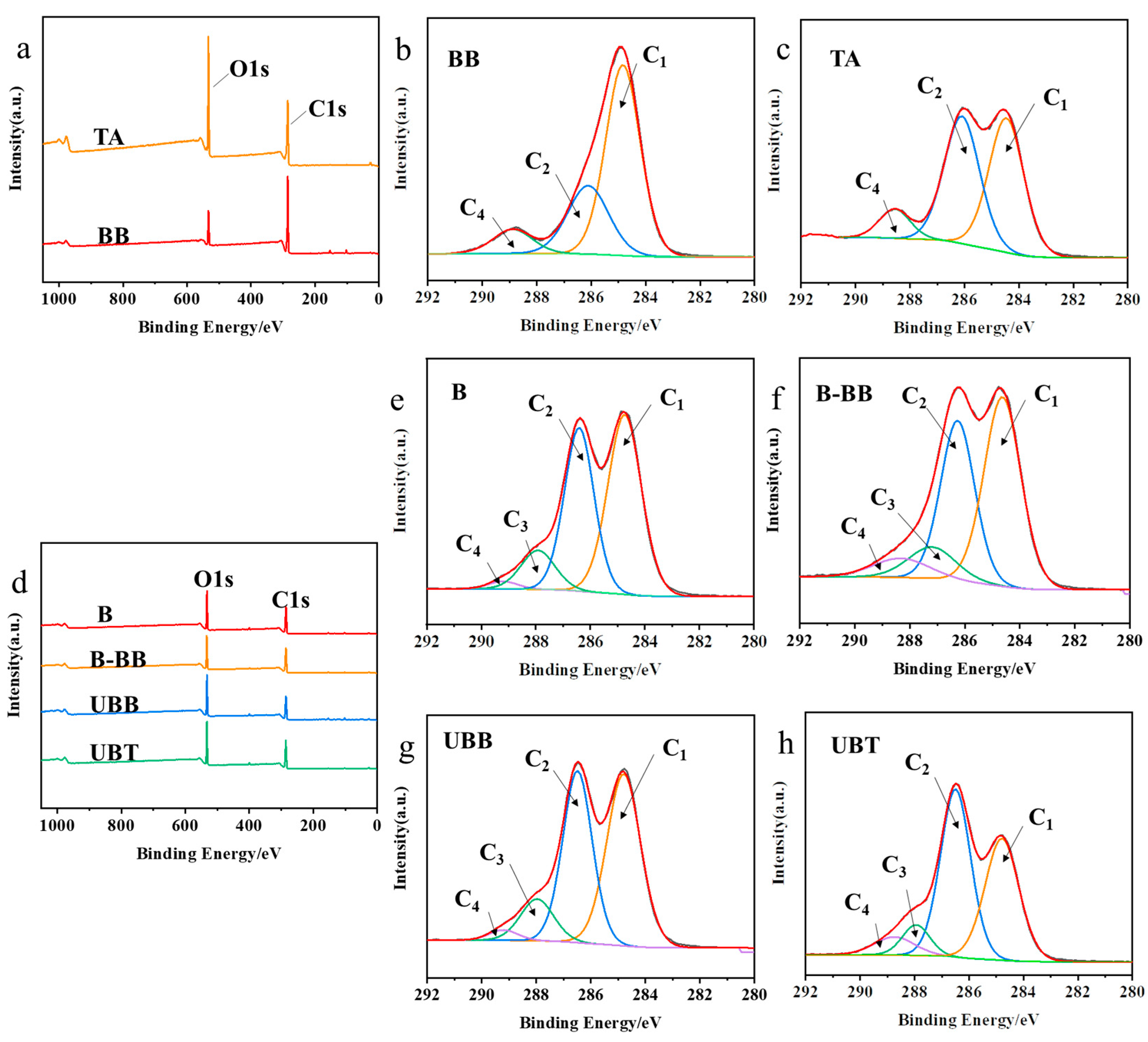
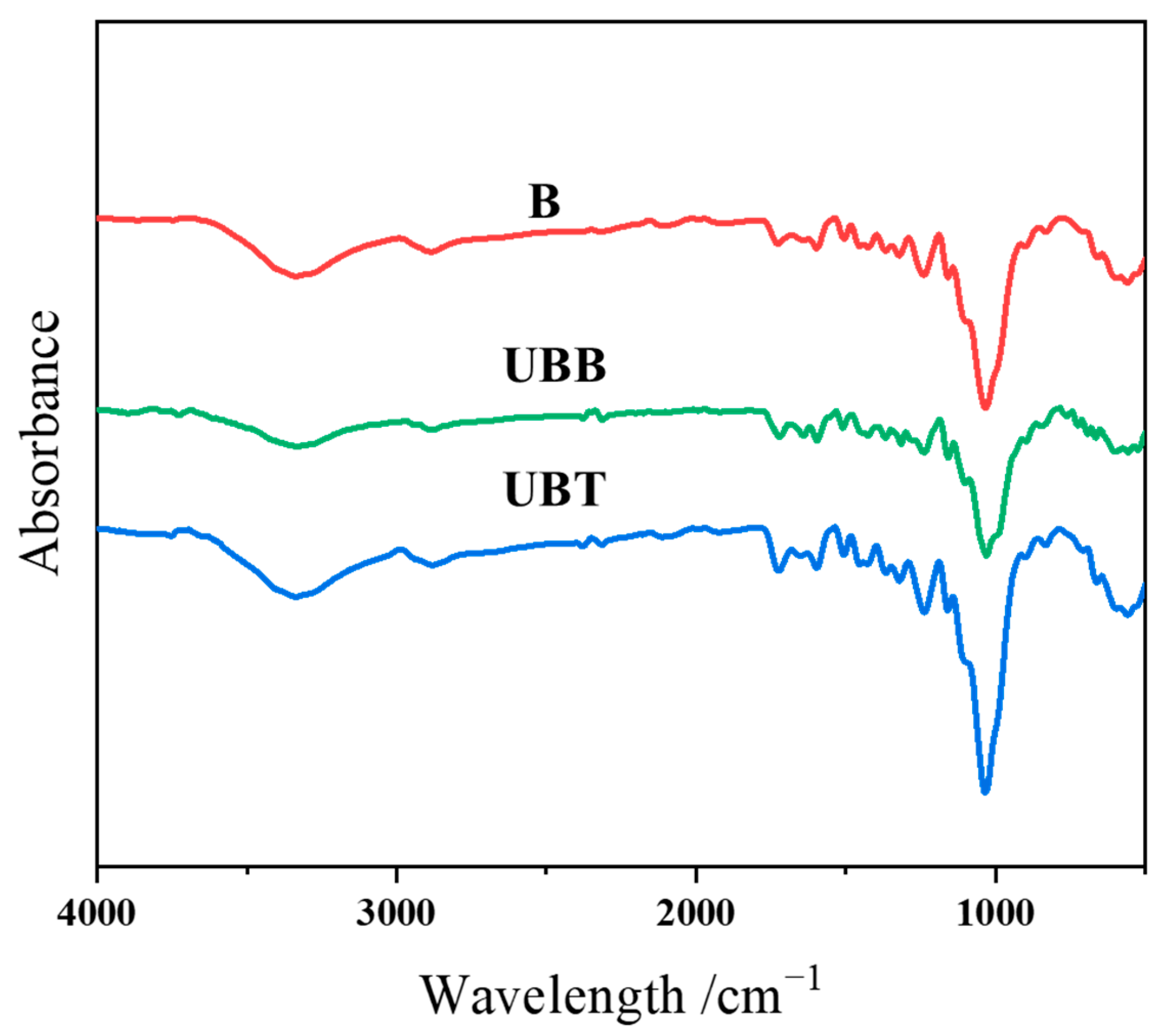
2.2. Chemical Changes Due to UV Photo-Grafting Process
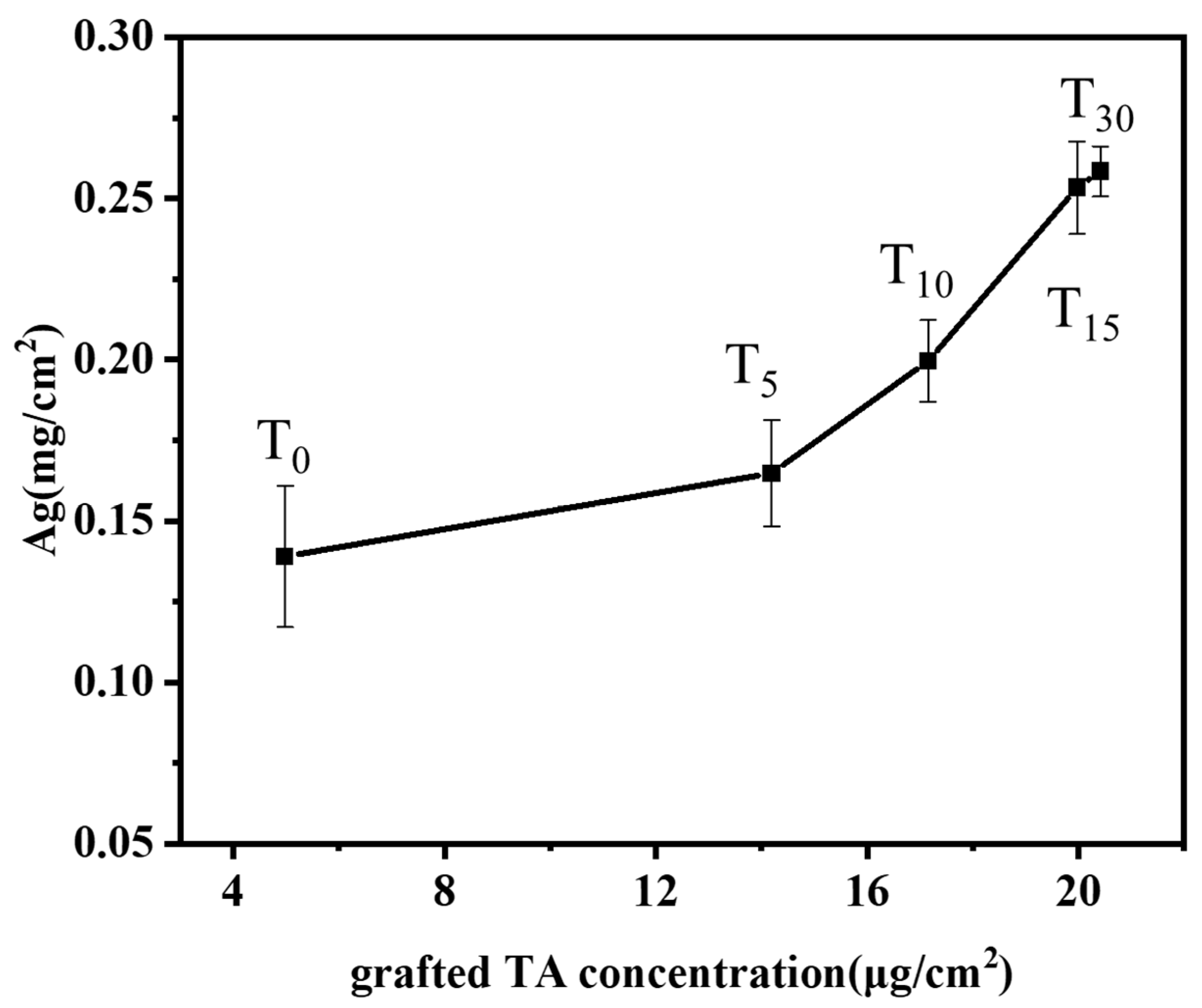
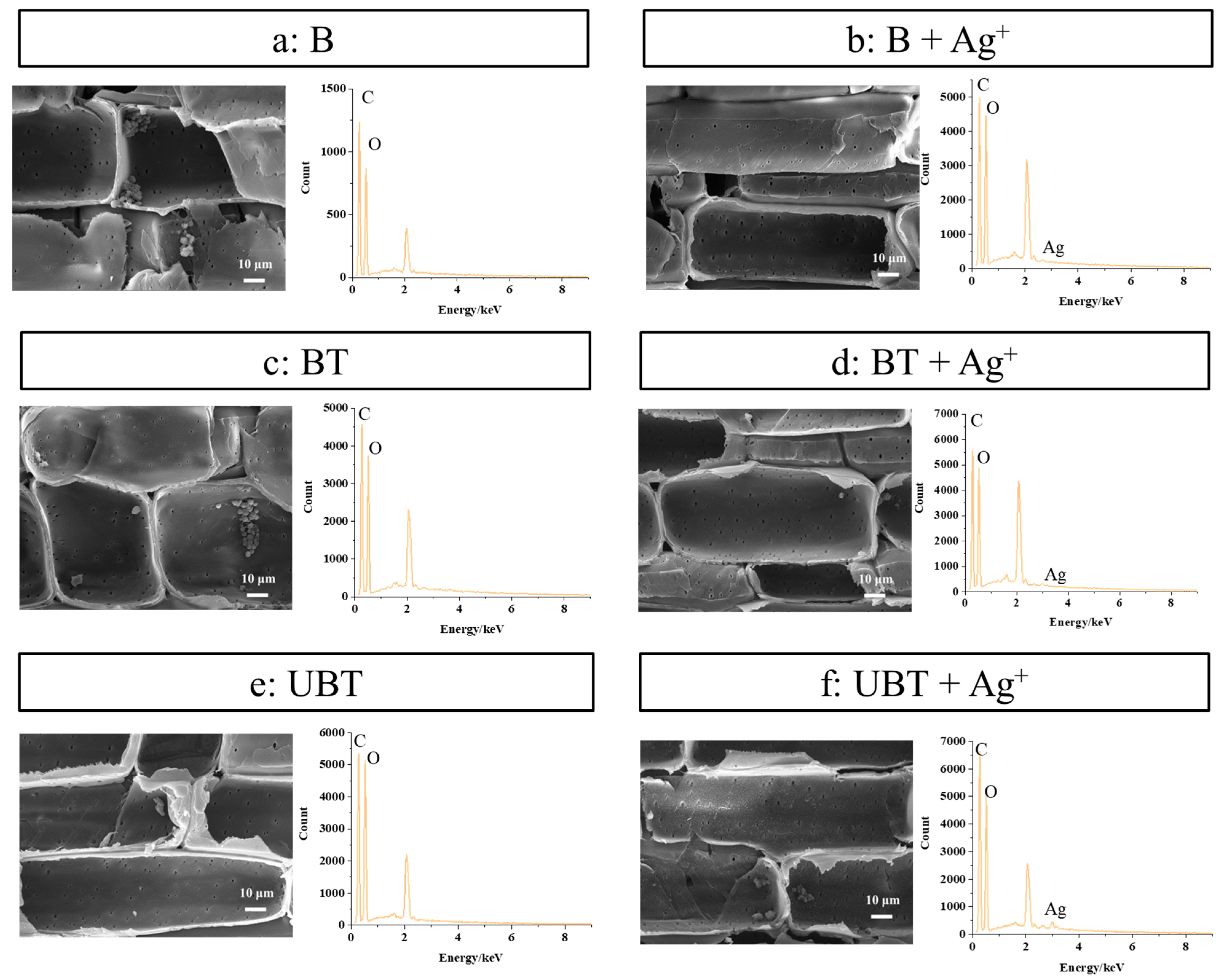
2.3. Analysis of Silver Ion Loading Capacity on Modified Bamboo Surface
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Surface Modification of Bamboo by TA Photo-Grafting
3.3. Folin-Ciocalteu Quantification of TA on Samples’ Surface
3.4. Analysis of Silver Ion Loading Capacity on Modified Bamboo Surface
3.5. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dezanet, C.; Dragoe, D.; Marie, P.; Harfouche, N.; Froissart, S.; Fouchet, A.; Rouden, J.; Lecourt, J.; Harnois, C.; Thébault, P.; et al. Zirconia Surface Polymer Grafting via Dopamine-Assisted Co-Deposition and Radical Photopolymerization. Prog. Org. Coat. 2022, 173, 107202. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Li, J.; Li, L.; Hu, W. Surface-Grafting Polymers: From Chemistry to Organic Electronics. Mater. Chem. Front. 2020, 4, 692–714. [Google Scholar] [CrossRef]
- Ren, L.; Chen, J.; Lu, Q.; Han, J.; Wu, H. Anti-Biofouling Nanofiltration Membrane Constructed by in-Situ Photo-Grafting Bactericidal and Hydrophilic Polymers. J. Membr. Sci. 2021, 617, 118658. [Google Scholar] [CrossRef]
- Shu, H.; Yang, L.; Wang, C.; Song, C.; Chen, D.; Zhang, X.; Ma, Y.; Yang, W. Fabrication and Properties of Asymmetric PET Fabrics with High-Permeation Flux for on-Demand Oil/Water Separation. J. Membr. Sci. 2024, 692, 122271. [Google Scholar] [CrossRef]
- Hammoud, F.; Pavlou, A.; Petropoulos, A.; Graff, B.; Siskos, M.G.; Hijazi, A.; Morlet-Savary, F.; Dumur, F.; Lalevée, J. Naphthoquinone-Based Imidazolyl Esters as Blue-Light-Sensitive Type I Photoinitiators. Polym. Chem. 2022, 13, 4817–4831. [Google Scholar] [CrossRef]
- Fang, L.; Feng, J.; Ye, D. Dual-Functional Photoinitiators and Photo-Grafting of Cotton Cellulose Nanowhiskers. Ind. Crops Prod. 2017, 107, 149–158. [Google Scholar] [CrossRef]
- Mueller, M.; Bandl, C.; Kern, W. Surface-Immobilized Photoinitiators for Light Induced Polymerization and Coupling Reactions. Polymers 2022, 14, 608. [Google Scholar] [CrossRef]
- Müller, M.; Drusgala, M.; Fischer, R.C.; Torvisco, A.; Kern, W.; Haas, M.; Bandl, C. Surface-Initiated Polymerizations Mediated by Novel Germanium-Based Photoinitiators. ACS Appl. Mater. Interfaces 2023, 15, 31836–31848. [Google Scholar] [CrossRef]
- Salmi-Mani, H.; Terreros, G.; Barroca-Aubry, N.; Aymes-Chodur, C.; Regeard, C.; Roger, P. Poly(Ethylene Terephthalate) Films Modified by UV-Induced Surface Graft Polymerization of Vanillin Derived Monomer for Antibacterial Activity. Eur. Polym. J. 2018, 103, 51–58. [Google Scholar] [CrossRef]
- Sanai, Y.; Ninomiya, T.; Arimitsu, K. Improvements in the Physical Properties of UV-Curable Coating by Utilizing Type II Photoinitiator. Prog. Org. Coat. 2021, 151, 106038. [Google Scholar] [CrossRef]
- Lian, H.; Li, P.; Xu, Y.; Zhang, X. A Simple and Sustainable Method for Preparing High-Strength, Lightweight, Dimensional Stable, and Mildew Resistant Multifunctional Bamboo. Constr. Build. Mater. 2024, 415, 135027. [Google Scholar] [CrossRef]
- Ju, Z.; Zhan, T.; Cui, J.; Brosse, N.; Zhang, H.; Hong, L.; Lu, X. Eco-Friendly Method to Improve the Durability of Different Bamboo (Phyllostachys Pubescens, Moso) Sections by Silver Electrochemical Treatment. Ind. Crops Prod. 2021, 172, 113994. [Google Scholar] [CrossRef]
- Zea Escamilla, E.; Habert, G. Environmental Impacts of Bamboo-Based Construction Materials Representing Global Production Diversity. J. Clean. Prod. 2014, 69, 117–127. [Google Scholar] [CrossRef]
- Hong, C.; Li, H.; Xiong, Z.; Lorenzo, R.; Corbi, I.; Corbi, O.; Wei, D.; Yuan, C.; Yang, D.; Zhang, H. Review of Connections for Engineered Bamboo Structures. J. Build. Eng. 2020, 30, 101324. [Google Scholar] [CrossRef]
- Yuan, T.; Yin, X.; Huang, Y.; Li, X.; Wang, X.; Chen, L.; Li, Y. Hydrothermal Treatment of Bamboo and Its Effect on Nano-Mechanic and Anti-Mildew Property. J. Clean. Prod. 2022, 380, 135189. [Google Scholar] [CrossRef]
- Silva, M.F.; Menis-Henrique, M.E.; Felisberto, M.H.; Goldbeck, R.; Clerici, M.T. Bamboo as an Eco-Friendly Material for Food and Biotechnology Industries. Curr. Opin. Food Sci. 2020, 33, 124–130. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; Paz San Andrés, M.; Díez-Pascual, A.M. Surface Functionalization of Graphene Oxide with Tannic Acid: Covalent vs Non-Covalent Approaches. J. Mol. Liq. 2022, 357, 119104. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, Y.; Xu, J.; Liu, L.; Ma, J.; Zhang, W.; Li, K.; Zhang, H.; Li, K. Tannic Acid-A Universal Immobilization and Fixation Agent for Nanocarbon Materials: A Novel Strategy for Aqueous Fabrication of Functional Nanocarbon Coating onto Silicon-Based Substances. ACS Sustain. Chem. Eng. 2019, 7, 18534–18541. [Google Scholar] [CrossRef]
- Tescione, F.; Tammaro, O.; Bifulco, A.; Del Monaco, G.; Esposito, S.; Pansini, M.; Silvestri, B.; Costantini, A. Silica Meets Tannic Acid: Designing Green Nanoplatforms for Environment Preservation. Molecules 2022, 27, 1944. [Google Scholar] [CrossRef]
- Zhan, K.; Li, Z.; Chen, J.; Hou, Y.; Zhang, J.; Sun, R.; Bu, Z.; Wang, L.; Wang, M.; Chen, X.; et al. Tannic Acid Modified Single Nanopore with Multivalent Metal Ions Recognition and Ultra-Trace Level Detection. Nano Today 2020, 33, 100868. [Google Scholar] [CrossRef]
- Meleán Brito, R.S.; Milanesio, J.; Oviedo, M.B.; Padró, J.M.; Strumia, M.C.; Mattea, F. Tannic Acid-Modified Poly(Acrylamide- Co -Acrylic Acid): A Versatile Approach for Aqueous Viscosity Modulation. ACS Appl. Polym. Mater. 2024, 6, 4462–4474. [Google Scholar] [CrossRef]
- Dultz, S.; Mikutta, R.; Kara, S.N.M.; Woche, S.K.; Guggenberger, G. Effects of Solution Chemistry on Conformation of Self-Aggregated Tannic Acid Revealed by Laser Light Scattering. Sci. Total Environ. 2021, 754, 142119. [Google Scholar] [CrossRef]
- Khosravi, Z.; Kharaziha, M.; Goli, R.; Karimzadeh, F. Antibacterial Adhesive Based on Oxidized Tannic Acid-Chitosan for Rapid Hemostasis. Carbohydr. Polym. 2024, 333, 121973. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Z.; Liu, T.; Du, G.; Ni, K.; Yang, H.; Su, H.; Liu, S.; Yin, C.; Ran, X.; et al. Xylan-Tannic Acid Adhesive Combined Activated Wood Interface to Construct Ultrastrong Cross-Linking Network Bonding Interface. Constr. Build. Mater. 2023, 398, 132556. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Liu, J.; Wu, J. FTIR and XPS Analysis of the Changes in Bamboo Chemical Structure Decayed by White-Rot and Brown-Rot Fungi. Appl. Surf. Sci. 2013, 280, 799–805. [Google Scholar] [CrossRef]
- Zhu, E.-Q. Rosin Acid Modification of Bamboo Powder and Thermoplasticity of Its Products Based on Hydrothermal Pretreatment. Adv. Compos. Hybrid Mater. 2021, 4, 584–590. [Google Scholar] [CrossRef]
- Bao, X.; Fan, X.; Yu, Y.; Wang, Q.; Wang, P.; Yuan, J. Graft Modification of Lignin-Based Cellulose via Enzyme-Initiated Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization and Free-Radical Coupling. Int. J. Biol. Macromol. 2020, 144, 267–278. [Google Scholar] [CrossRef]
- Joram Mendoza, D.; Mouterde, L.M.M.; Browne, C.; Singh Raghuwanshi, V.; Simon, G.P.; Garnier, G.; Allais, F. Grafting Nature-Inspired and Bio-Based Phenolic Esters onto Cellulose Nanocrystals Gives Biomaterials with Photostable Anti-UV Properties. ChemSusChem 2020, 13, 6552–6561. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, F.; Yu, Z.; Cao, M.; Wang, H.; Yang, R.; Yu, Y.; Salmén, L. Why Do Bamboo Parenchyma Cells Show Higher Nanofibrillation Efficiency than Fibers: An Investigation on Their Hierarchical Cell Wall Structure. Biomacromolecules 2022, 23, 4053–4062. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.-Y.; Wang, D.-W.; Yang, J.; Wu, C.-H.; Xu, J.; Yang, H.-Y.; Shi, Z.-J. Stepwise Modification with 2,3-Epoxypropyltrimethylammonium Chloride Cationization and Rosin Acid Impregnation to Improve Water Repellency and Mold-Proof Property of Bamboo. Ind. Crops Prod. 2023, 193, 116248. [Google Scholar] [CrossRef]
- Roy, A.; Chakraborty, S.; Kundu, S.P.; Majumder, S.B.; Adhikari, B. Surface Grafting of Corchorus Olitorius Fibre: A Green Approach for the Development of Activated Bioadsorbent. Carbohydr. Polym. 2013, 92, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose Nanowhiskers from Coconut Husk Fibers: Effect of Preparation Conditions on Their Thermal and Morphological Behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Liu, S.; Ji, W.; Wu, T.; He, Y.; Huang, Y.; Yu, Y.; Yu, W. Unraveling the Intricate Interaction: Bonding Mechanism between Tannic Acid and Wood Fibers. ACS Sustain. Chem. Eng. 2024, 12, 4224–4235. [Google Scholar] [CrossRef]
- Shan, S.; Ji, W.; Zhang, S.; Huang, Y.; Yu, Y.; Yu, W. Insights into the Immobilization Mechanism of Tannic Acid on Bamboo Cellulose Fibers. Ind. Crops Prod. 2022, 182, 114836. [Google Scholar] [CrossRef]
- Ferraris, S.; Zhang, X.; Prenesti, E.; Corazzari, I.; Turci, F.; Tomatis, M.; Vernè, E. Gallic Acid Grafting to a Ferrimagnetic Bioactive Glass-Ceramic. J. Non-Cryst. Solids 2016, 432, 167–175. [Google Scholar] [CrossRef]
- Xiao, S.; Wei, J.; Jin, S.; Xia, X.; Yuan, L.; Zou, Q.; Zuo, Y.; Li, J.; Li, Y. A Multifunctional Coating Strategy for Promotion of Immunomodulatory and Osteo/Angio-Genic Activity. Adv. Funct. Mater. 2023, 33, 2208968. [Google Scholar] [CrossRef]

| Photoinitiators | TA-Grafted Bamboo Samples | C (%) | O (%) | O/C (%) |
|---|---|---|---|---|
| / | B | 68.30 | 31.70 | 46.41 |
| BB | UBT | 64.86 | 35.14 | 54.18 |
| BBP | UBPT | 67.09 | 32.91 | 49.05 |
| BBO | UBOT | 68.95 | 31.05 | 45.03 |
| DMPA | UDT | 69.98 | 30.02 | 42.90 |
| HP | UHT | 70.69 | 29.31 | 41.46 |
| PPO | UPT | 69.52 | 30.48 | 43.84 |
| Samples | Carbon Components | O/C (%) | |||
|---|---|---|---|---|---|
| C1/% | C2/% | C3/% | C4/% | ||
| BB | 63.72 | 27.12 | / | 9.17 | 21.77 |
| TA | 46.50 | 44.54 | / | 8.96 | 56.57 |
| B | 48.56 | 38.99 | 10.69 | 1.75 | 43.58 |
| B-BB | 46.51 | 36.30 | 10.04 | 7.15 | 42.63 |
| UBB | 46.38 | 40.18 | 11.06 | 2.38 | 49.60 |
| UBT | 38.40 | 46.96 | 7.79 | 6.85 | 51.51 |
| Position of Bands (cm−1) | Possible Assignments | B (Iv/I898) | UBT (Iv/I898) |
|---|---|---|---|
| 3340 | -OH stretching | 6.67 | 7.67 |
| 2920 | -CH stretching in methyl and methylene | 2.07 | 2.33 |
| 1725 | Non-conjugated C=O stretching | 2.27 | 4.33 |
| 1600 | Aromatic -C=C- stretching | 2.33 | 3.27 |
| 1506 | Aromatic -C=C- stretching | 2.33 | 3.20 |
| 1240 | C–O stretching | 5.40 | 7.07 |
| 1160 | C–O–C stretching | 3.13 | 3.87 |
| 1030 | Aromatic C–H, symmetrical C–O stretching | 16.13 | 24.47 |
| Samples | C (%) | O (%) | Ag (%) |
|---|---|---|---|
| B | 63.85 | 36.15 | / |
| B + Ag+ | 60.21 | 39.76 | 0.03 |
| BT | 61.58 | 38.39 | / |
| BT + Ag+ | 60.42 | 39.41 | 0.18 |
| UBT | 60.21 | 39.76 | / |
| UBT + Ag+ | 62.23 | 37.38 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, L.; Ma, J.; Tang, B.; Shi, Z.; Zhang, H. Surface Functionalization of Bamboo via Photo-Grafting Tannic Acid for Enhanced Silver Ion Loading Properties. Molecules 2024, 29, 3203. https://doi.org/10.3390/molecules29133203
Xu J, Liu L, Ma J, Tang B, Shi Z, Zhang H. Surface Functionalization of Bamboo via Photo-Grafting Tannic Acid for Enhanced Silver Ion Loading Properties. Molecules. 2024; 29(13):3203. https://doi.org/10.3390/molecules29133203
Chicago/Turabian StyleXu, Juan, Lanxiang Liu, Jinju Ma, Baoshan Tang, Zhengjun Shi, and Hong Zhang. 2024. "Surface Functionalization of Bamboo via Photo-Grafting Tannic Acid for Enhanced Silver Ion Loading Properties" Molecules 29, no. 13: 3203. https://doi.org/10.3390/molecules29133203
APA StyleXu, J., Liu, L., Ma, J., Tang, B., Shi, Z., & Zhang, H. (2024). Surface Functionalization of Bamboo via Photo-Grafting Tannic Acid for Enhanced Silver Ion Loading Properties. Molecules, 29(13), 3203. https://doi.org/10.3390/molecules29133203






