Enhancing Sustainable Analytical Chemistry in Liquid Chromatography: Guideline for Transferring Classical High-Performance Liquid Chromatography and Ultra-High-Pressure Liquid Chromatography Methods into Greener, Bluer, and Whiter Methods
Abstract
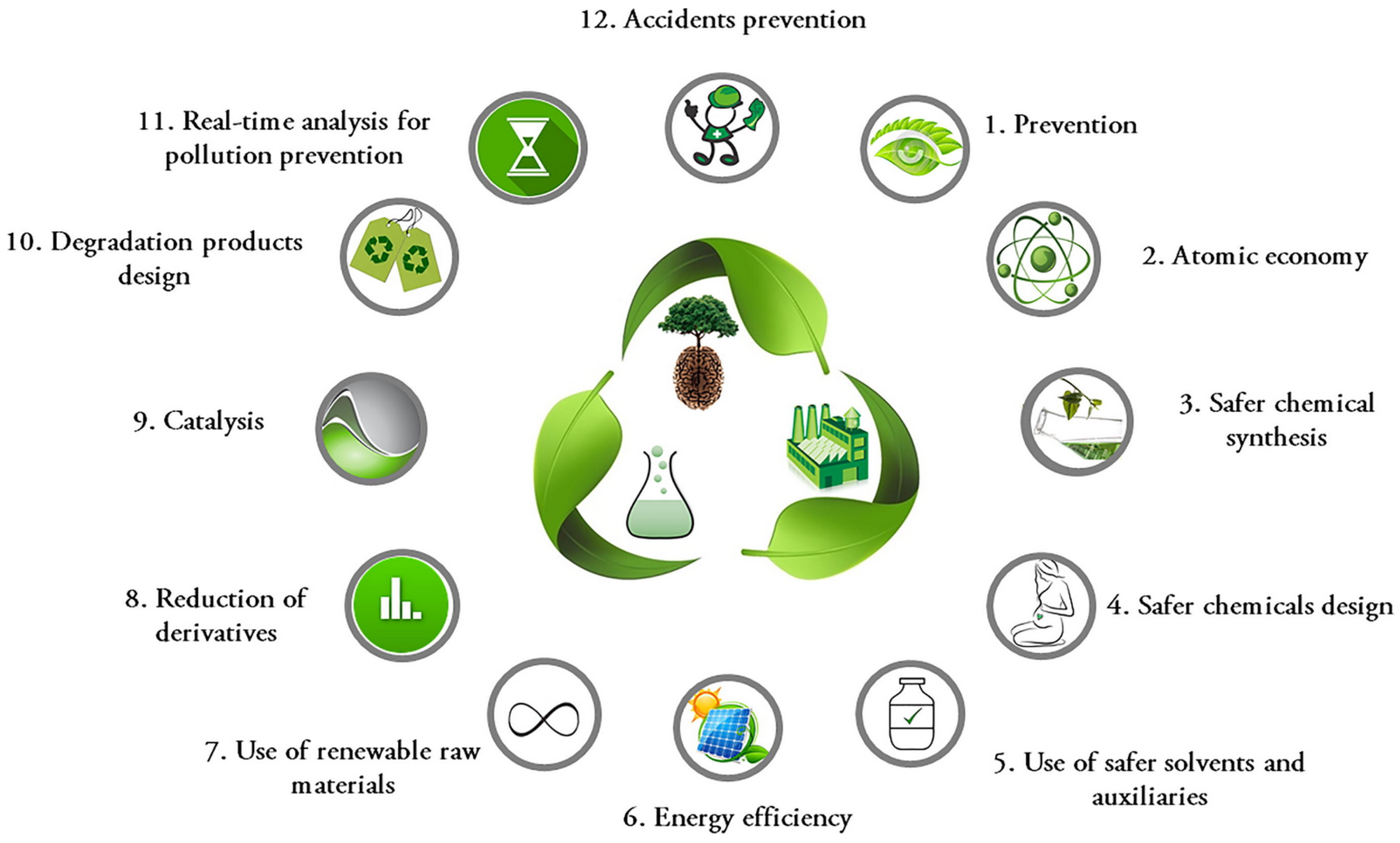
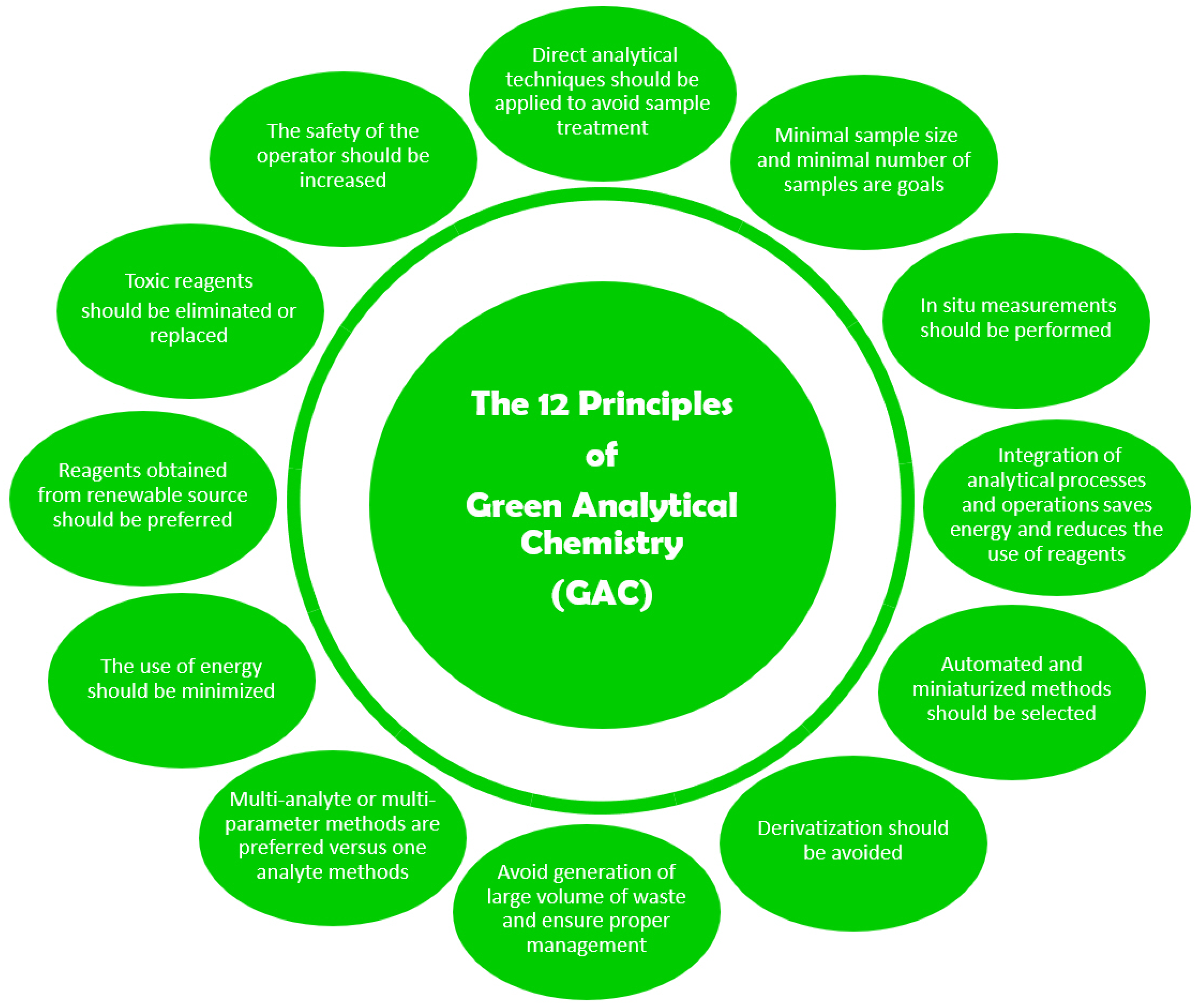
:1. Introduction
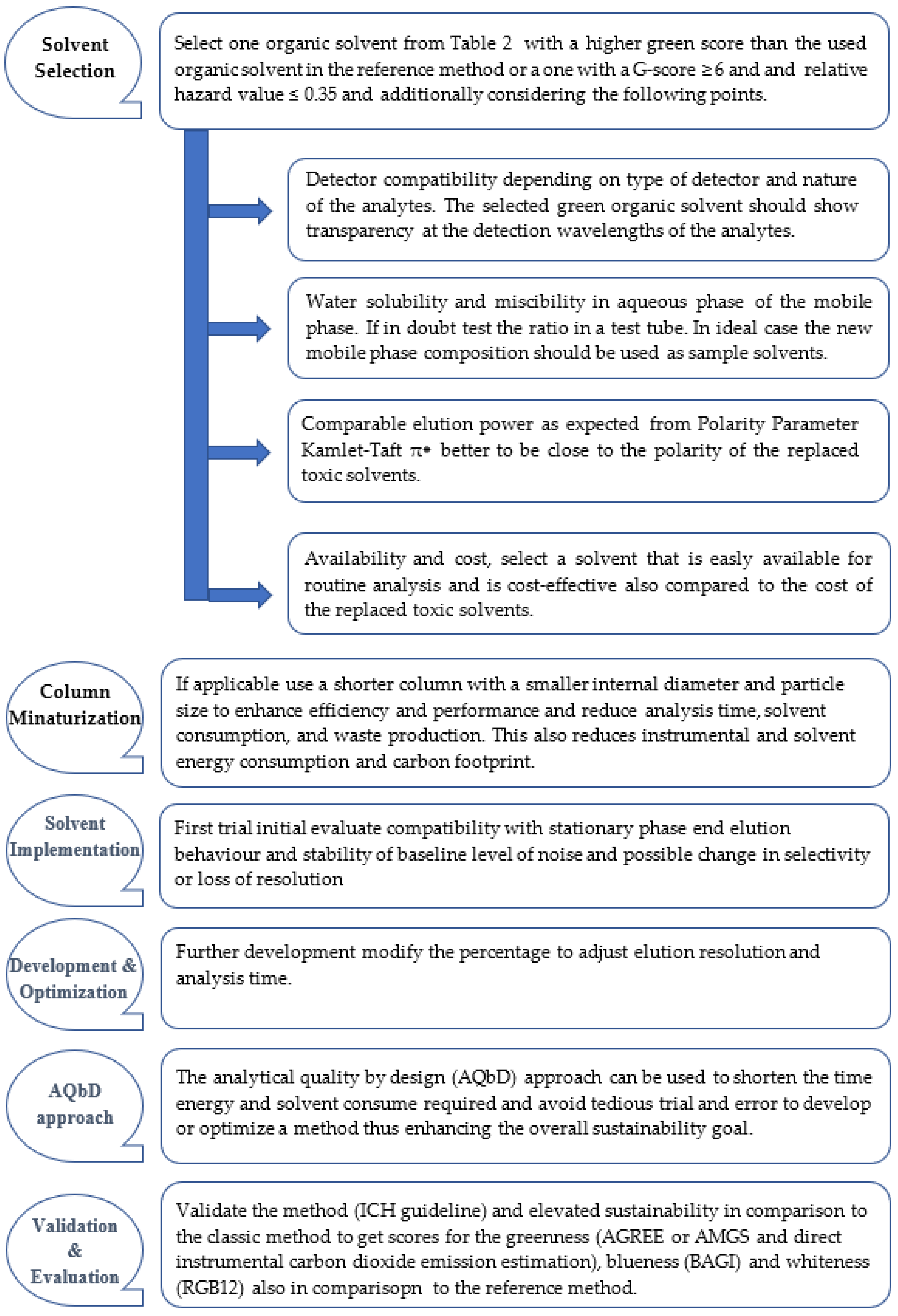
2. Solvent Selection
2.1. Solvent Selection Guidelines
2.2. G-Score
2.3. Relative Hazard
2.4. Consideration of Chromatographic Suitability
2.5. Liquid Chromatography Sustainability Guideline
3. Post-Greening Method Evaluation
3.1. Greening Evaluation
3.2. Blueness Evaluation
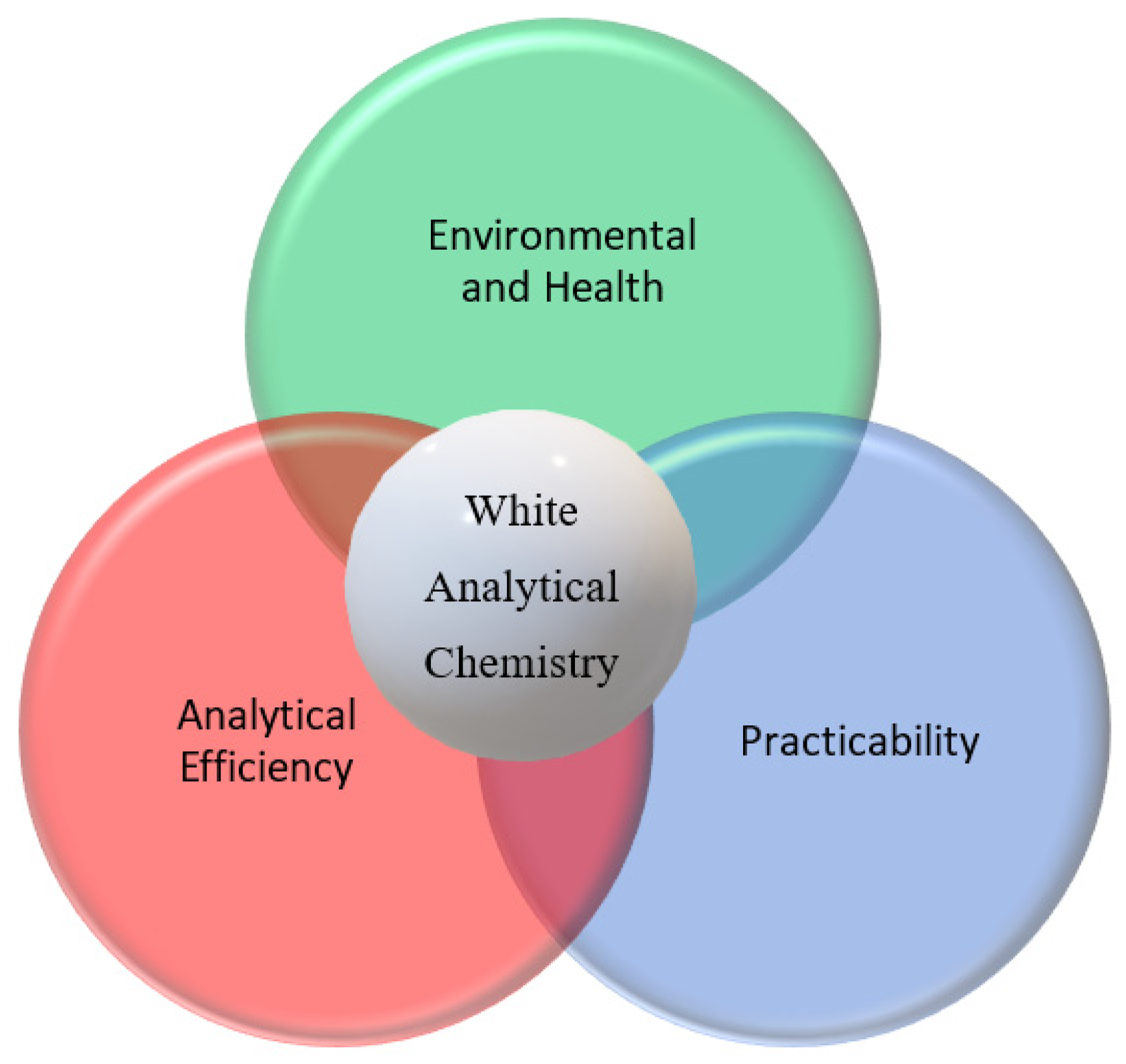
3.3. Whiteness Evaluation
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wennersten, R.; Qie, S. United Nations Sustainable Development Goals for 2030 and Resource Use. In Handbook of Sustainability Science and Research; Springer: Berlin/Heidelberg, Germany, 2018; pp. 317–339. [Google Scholar]
- Green Chemistry By Paul, T. Anastas and John C. Warner. Oxford University Press: Oxford. 2000. Paperback. 135 Pp. £14.99. ISBN 0-19-850698-9. Org. Process Res. Dev. 2000, 4, 437–438. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of Green Chemistry and Its Multidimensional Impacts: A Review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Cizmarova, I.; Parrak, V.; Secnik jr, P.; Secnik, P.; Sopko, L.; Nemergutova, K.; Kovac, A.; Mikus, P.; Piestansky, J. A Simple and Green Capillary Electrophoresis-Mass Spectrometry Method for Therapeutic Drug Monitoring of Colistin in Clinical Plasma Samples. Heliyon 2023, 9, e23111. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, S.; Wätzig, H.; El-Hady, D.A. Capillary Electrophoresis to Investigate Biopharmaceuticals and Pharmaceutically-Relevant Binding Properties. TrAC Trends Anal. Chem. 2013, 48, 112–131. [Google Scholar] [CrossRef]
- Datta, S.; Ghosh Auddy, R.; De, A. Supercritical Fluid Chromatography: A Green Approach for Separation and Purification of Organic and Inorganic Analytes. In Green Chromatographic Techniques; Springer: Dordrecht, The Netherlands, 2014; pp. 55–80. [Google Scholar]
- Ali, M.F.B.; Saraya, R.E.; El Deeb, S.; Ibrahim, A.E.; Salman, B.I. An Innovative Polymer-Based Electrochemical Sensor Encrusted with Tb Nanoparticles for the Detection of Favipiravir: A Potential Antiviral Drug for the Treatment of COVID-19. Biosensors 2023, 13, 243. [Google Scholar] [CrossRef]
- Yabré, M.; Ferey, L.; Somé, I.; Gaudin, K. Greening Reversed-Phase Liquid Chromatography Methods Using Alternative Solvents for Pharmaceutical Analysis. Molecules 2018, 23, 1065. [Google Scholar] [CrossRef]
- Solomon, K.R.; Tang, X.; Wilson, S.R.; Zanis, P.; Bais, A.F. Changes in Tropospheric Composition and Air Quality Due to Stratospheric Ozone Depletion. Photochem. Photobiol. Sci. 2003, 2, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A. Principles into Practice Setting the Bar for Green Chemistry. Environ. Health Perspect. 2010, 118, A254–A257. [Google Scholar] [CrossRef]
- Loste, N.; Roldán, E.; Lomba, L.; Giner, B. Green Chemistry and Environmental Management Systems: Relationships, Synergies, Advantages and Barriers of Joint Implementation at Universities. Environ. Manag. 2019, 64, 783–793. [Google Scholar] [CrossRef]
- Brice, R.W.; Zhang, X.; Colón, L.A. Fused-core, Sub-2 Μm Packings, and Monolithic HPLC Columns: A Comparative Evaluation. J. Sep. Sci. 2009, 32, 2723–2731. [Google Scholar] [CrossRef]
- Nowak, P.M.; Bis, A.; Rusin, M.; Woźniakiewicz, M. Carbon Footprint of the Analytical Laboratory and the Three-Dimensional Approach to Its Reduction. Green Anal. Chem. 2023, 4, 100051. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Abd Elmonem, H.M.; Al-Harrasi, A.; El Deeb, S. Comparative Evaluation of Reversed Stationary Phase Geometries and Greener Systems on HPLC and UHPLC Using Five Recent Hepatitis-C Antivirals. J. AOAC Int. 2023, 106, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAC Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hussain, C.G.; Keçili, R. White Analytical Chemistry Approaches for Analytical and Bioanalytical Techniques: Applications and Challenges. TrAC Trends Anal. Chem. 2023, 159, 116905. [Google Scholar] [CrossRef]
- de la Guardia, M.; Garrigues, S. The Concept of Green Analytical Chemistry. In Handbook of Green Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2012; pp. 1–16. [Google Scholar]
- Sajid, M.; Płotka-Wasylka, J. Green Analytical Chemistry Metrics: A Review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef]
- Al-Shatti, B.J.; Alsairafi, Z.; Al-Tannak, N.F. Green Chemistry and Its Implementation in Pharmaceutical Analysis. Rev. Anal. Chem. 2023, 42, 20230069. [Google Scholar] [CrossRef]
- Turner, C. Sustainable Analytical Chemistry—More than Just Being Green. Pure Appl. Chem. 2013, 85, 2217–2229. [Google Scholar] [CrossRef]
- Nanda, B.P.; Chopra, A.; Kumari, Y.; Narang, R.K.; Bhatia, R. A Comprehensive Exploration of Diverse Green Analytical Techniques and Their Influence in Different Analytical Fields. Sep. Sci. Plus 2024, 7, 2400004. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kaya, S.I.; Ozkan, S.A. An Overview of the Current Progress in Green Analytical Chemistry by Evaluating Recent Studies Using Greenness Assessment Tools. TrAC Trends Anal. Chem. 2023, 168, 117330. [Google Scholar] [CrossRef]
- Psillakis, E.; Pena-Pereira, F. The Twelve Goals of Circular Analytical Chemistry. TrAC Trends Anal. Chem. 2024, 175, 117686. [Google Scholar] [CrossRef]
- Jankech, T.; Gerhardtova, I.; Stefanik, O.; Chalova, P.; Jampilek, J.; Majerova, P.; Kovac, A.; Piestansky, J. Current Green Capillary Electrophoresis and Liquid Chromatography Methods for Analysis of Pharmaceutical and Biomedical Samples (2019–2023)—A Review. Anal. Chim. Acta 2024, 342889. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Diorazio, L.J.; Hose, D.R.J.; Adlington, N.K. Toward a More Holistic Framework for Solvent Selection. Org. Process Res. Dev. 2016, 20, 760–773. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Impurities: Guideline for Residual Solvents Q3C(R8). Available online: https://database.ich.org/sites/default/files/ICH_Q3C-R8_Guideline_Step4_2021_0422_1.pdf (accessed on 1 June 2024).
- El Deeb, S.; Abdelsamad, K.; Parr, M.K. Greener and Whiter Analytical Chemistry Using Cyrene as a More Sustainable and Eco-Friendlier Mobile Phase Constituent in Chromatography. Pharmaceuticals 2023, 16, 1488. [Google Scholar] [CrossRef]
- Benazzouz, A.; Moity, L.; Pierlot, C.; Sergent, M.; Molinier, V.; Aubry, J.-M. Selection of a Greener Set of Solvents Evenly Spread in the Hansen Space by Space-Filling Design. Ind. Eng. Chem. Res. 2013, 52, 16585–16597. [Google Scholar] [CrossRef]
- Fayaz, T.K.S.; Chanduluru, H.K.; Obaydo, R.H.; Sanphui, P. Propylene Carbonate as an Ecofriendly Solvent: Stability Studies of Ripretinib in RPHPLC and Sustainable Evaluation Using Advanced Tools. Sustain. Chem. Pharm. 2024, 37, 101355. [Google Scholar] [CrossRef]
- Larsen, C.; Lundberg, P.; Tang, S.; Ràfols-Ribé, J.; Sandström, A.; Mattias Lindh, E.; Wang, J.; Edman, L. A Tool for Identifying Green Solvents for Printed Electronics. Nat. Commun. 2021, 12, 4510. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and Further Expanding GSK’s Solvent Sustainability Guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Nowak, P.M.; Bis, A.; Zima, A. ChlorTox Base—A Useful Source of Information on Popular Reagents in Terms of Chemical Hazards and Greenness Assessment. Green Anal. Chem. 2023, 6, 100065. [Google Scholar] [CrossRef]
- Islam, T.; Islam Sarker, M.Z.; Uddin, A.H.; Yunus, K.B.; Prasad, R.; Mia, M.A.R.; Ferdosh, S. Kamlet Taft Parameters: A Tool to Alternate the Usage of Hazardous Solvent in Pharmaceutical and Chemical Manufacturing/Synthesis—A Gateway towards Green Technology. Anal. Chem. Lett. 2020, 10, 550–561. [Google Scholar] [CrossRef]
- Guillarme, D.; Heinisch, S.; Rocca, J.L. Effect of Temperature in Reversed Phase Liquid Chromatography. J. Chromatogr. A 2004, 1052, 39–51. [Google Scholar] [CrossRef]
- Karmaus, A.L.; Mansouri, K.; To, K.T.; Blake, B.; Fitzpatrick, J.; Strickland, J.; Patlewicz, G.; Allen, D.; Casey, W.; Kleinstreuer, N. Evaluation of Variability Across Rat Acute Oral Systemic Toxicity Studies. Toxicol. Sci. 2022, 188, 34–47. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency Technical Overview of Volatile Organic Compounds. Available online: https://www.epa.gov/indoor-air-quality-iaq/what-are-volatile-organic-compounds-vocs (accessed on 1 June 2024).
- World Health Organization Indoor Air Quality: Organic Pollutants. Environ. Technol. Lett. 1989, 10, 855–858. [CrossRef]
- Flinders Shire Council Classification of Flammable and Combustible Liquids. Available online: https://www.flinders.qld.gov.au/our-environment/environmental-health/flammable-and-combustible-materials (accessed on 1 June 2024).
- Lombardo, A.; Roncaglioni, A.; Boriani, E.; Milan, C.; Benfenati, E. Assessment and Validation of the CAESAR Predictive Model for Bioconcentration Factor (BCF) in Fish. Chem. Cent. J. 2010, 4, S1. [Google Scholar] [CrossRef] [PubMed]
- Dembek, M.; Bocian, S. Pure Water as a Mobile Phase in Liquid Chromatography Techniques. TrAC Trends Anal. Chem. 2020, 123, 115793. [Google Scholar] [CrossRef]
- Kowtharapu, L.P.; Katari, N.K.; Muchakayala, S.K.; Marisetti, V.M. Green Metric Tools for Analytical Methods Assessment Critical Review, Case Studies and Crucify. TrAC Trends Anal. Chem. 2023, 166, 117196. [Google Scholar] [CrossRef]
- Sinzervinch, A.; Torres, I.M.S.; Kogawa, A.C. Tools to Evaluate the Eco-Efficiency of Analytical Methods in the Context of Green and White Analytical Chemistry: A Review. Curr. Pharm. Des. 2023, 29, 2442–2449. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Tobiszewski, M.; Wojnowski, W.; Psillakis, E. A Tutorial on AGREEprep an Analytical Greenness Metric for Sample Preparation. Adv. Sample Prep. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Hicks, M.B.; Farrell, W.; Aurigemma, C.; Lehmann, L.; Weisel, L.; Nadeau, K.; Lee, H.; Moraff, C.; Wong, M.; Huang, Y.; et al. Making the Move towards Modernized Greener Separations: Introduction of the Analytical Method Greenness Score (AMGS) Calculator. Green Chem. 2019, 21, 1816–1826. [Google Scholar] [CrossRef]
- Ballester-Caudet, A.; Campíns-Falcó, P.; Pérez, B.; Sancho, R.; Lorente, M.; Sastre, G.; González, C. A New Tool for Evaluating and/or Selecting Analytical Methods: Summarizing the Information in a Hexagon. TrAC Trends Anal. Chem. 2019, 118, 538–547. [Google Scholar] [CrossRef]


| Classification | Solvent Name | CAS Number | Composite Colour | Boiling Point (°C) | Incineration | Recycling | Biotreatment | VOC Emissions | Aquatic Impact | Air Impact | Health Hazard | Exposure Potential | Flammability and Exlosion | Reactivity and Stability |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Halogenated | 1,2,4-Trichlorobenzene | 120-82-179-11-8 | 214 | 3 | 7 | 7 | 10 | 1 | 9 | 4 | 6 | 9 | 10 | |
| Chlorobenzene | 108-90-7 | 132 | 4 | 9 | 7 | 7 | 2 | 7 | 4 | 4 | 8 | 10 | ||
| 1,2-Dichlorobenezen | 95-50-1 | 180 | 4 | 8 | 6 | 7 | 1 | 6 | 7 | 6 | 8 | 10 | ||
| Trichloroacetonitrile | 545-06-2 | 83 | 4 | 8 | 5 | 4 | 3 | 4 | 4 | 3 | 7 | 10 | ||
| Perfluorotoluene | 434-64-0 | 104 | 4 | 4 | 6 | 6 | 1 | 7 | 4 | 4 | 5 | 10 | ||
| Flurobenzene | 462-06-6 | 85 | 4 | 7 | 5 | 4 | 3 | 4 | 4 | 3 | 4 | 10 | ||
| Perflurocyclic ether | 335-6-4 | 103 | 4 | 4 | 6 | 6 | 1 | 7 | 4 | 4 | 5 | 10 | ||
| Dichloromethane | 75-09-2 | 40 | 2 | 10 | 4 | 1 | 8 | 6 | 7 | 4 | 4 | 10 | ||
| 1,2-Dichloromethane | 107-06-2 | 84 | 2 | 7 | 5 | 5 | 9 | 7 | 1 | 2 | 5 | 10 | ||
| Perflurocyclohexane | 355-68-0 | 53 | 4 | 9 | 5 | 2 | 4 | 7 | 4 | 2 | 3 | 10 | ||
| Chloroform | 67-66-3 | 61 | 3 | 9 | 5 | 3 | 7 | 5 | 4 | 1 | 5 | 10 | ||
| Trichloroacetic acid | 76-03-9 | 197 | 1 | 4 | 3 | 9 | 2 | 5 | 4 | 6 | 10 | 6 | ||
| Chloroacetic acid | 79-11-8 | 189 | 1 | 4 | 4 | 9 | 6 | 4 | 1 | 5 | 10 | 6 | ||
| Trifluoroacetic acid | 76-05-1 | 72 | 1 | 5 | 2 | 4 | 4 | 4 | 4 | 3 | 7 | 6 | ||
| Perfluorohexane | 355-42-0 | 57 | 4 | 10 | 5 | 2 | 1 | 7 | 4 | 2 | 4 | 10 | ||
| Carbon tetrachloride | 56-23-5 | 77 | 3 | 7 | 5 | 4 | 4 | 1 | 4 | 1 | 4 | 10 | ||
| 2,2,2-Trifluoroethanol | 75-89-8 | 74 | 1 | 5 | 2 | 4 | 5 | 4 | 1 | 1 | 6 | 9 | ||
| Other | Furfural | 98-01-1 | 162 | 7 | 8 | 8 | 8 | 8 | 4 | 4 | 6 | 9 | 9 | |
| N,N-Dimethyldecanamide | 14433-76-2 | 291 | 6 | 7 | 6 | 10 | 4 | 6 | 10 | 10 | 10 | 10 | ||
| Dihydrolevoglucosenone | 1087696-49-8 | 203 | 4 | 4 | 5 | 10 | 9 | 6 | 4 | 8 | 10 | 10 | ||
| N,N-Dimethyloctanamide | 1118-92-9 | 261 | 5 | 6 | 5 | 8 | 7 | 5 | 4 | 6 | 9 | 10 | ||
| N,N-Dimethylaniline | 121-69-7 | 194 | 7 | 7 | 6 | 9 | 3 | 4 | 4 | 8 | 9 | 9 | ||
| Acetic anhydride | 108-24-7 | 140 | 4 | 6 | 4 | 8 | 8 | 7 | 4 | 4 | 8 | 6 | ||
| Nitromethane | 75-52-5 | 101 | 2 | 1 | 4 | 6 | 6 | 8 | 7 | 5 | 7 | 1 | ||
| Triethylamine | 121-44-8 | 89 | 4 | 4 | 1 | 5 | 8 | 3 | 4 | 3 | 5 | 6 |
| Solvent | UV Cut-Off Value (nm) | Water Solubility | Density (g/cm3) at 20 °C | Polarity Parameter Kamlet- Taft π * | Partition Coefficient n-Octanol/Water (log p Value) | Boiling Point °C | Flash Point °C at 1.013 hPa (c.c.) | Vapor Pressure hPa at 20 °C | LD50 Oral-Rat mg/kg | Composite Color of GSK Solvent Sustainability Guide | Relative Hazard (WHN) | G-Score Hansen Space |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetone | 330 | miscible in any proportion | 0.79 | 0.71 | −0.23 | 56.05 | −18 | 240 | 5800 | 0.35 | 5.9 | |
| Acetonitrile | 190 | miscible in any proportion | 0.7822 | 0.75 | −0.34 | 82 | 2 | 94.51 | 469 | 0.39 | 5.8 | |
| Butanol | 215 | ca. 77 g/L at 20 °C | 0.81 | 0.47 | 0.78 | 117.6 | 37 | 6.3 | 700 | 0.43 | 6.7 | |
| Chloroform | 245 | ca. 8 g/L at 20 °C | 1.498 | 0.58 | 1.97 | 62 | 9.7 | 210 | 908 | 1.00 | 4.4 | |
| Cyclohexane | 210 | ca. 0.1 g/L at 20 °C | 0.779 | 0.00 | 3.44 | 83 | −20 | 104 | >5000 | 0.87 | 5.3 | |
| Dihydrolevoglucosenone (Cyrene) | 350 | ca.52.6 g/L at 20 °C | 1.25 | 0.93 | −1.52 | 227 | 108 | 0.28 | >2000 | 0.13 | 6.9 | |
| Ethanol | 210 | ≥1000 g/L at 20 °C | 0.81 | 0.54 | −0.31 | 78 | 9.7 | 59 | 10.470 | 0.26 | 6.7 | |
| Ethyl acetate | 255 | ca. 87 g/L at 20 °C | 0.894 | 0.54 | 0.73 | 77 | −4 | 97 | 5620 | 0.35 | 6.8 | |
| Ethyl lactate | miscible | 1.03 | 0.82 | 0.70 | 154 | 46 | 2.7 | >2000 | 0.39 | 6.4 | ||
| Hexane | 195 | ca. 0.014 g/L at 20 °C | 0.655 | −0.04 | 3.90 | 69 | −22 | 160 | 25,000 | 0.78 | 4.8 | |
| Isopropanol | 205 | miscible in any proportion | 0.786 | 0.48 | 0.05 | 82.4 | 12 | 43 | 5840 | 0.35 | 6.5 | |
| Methanol | 205 | 1000 g/L at 20 °C—completely miscible | 0.7913 | 0.61 | −0.77 | 64.7 | 12 | 128 | 5628 | 0.57 | 5.8 | |
| Propanol | 210 | miscible in any proportion | 0.803 | 0.52 | 0.25 | 97 | 23.5 | 22 | 1870 | 0.39 | 6.6 | |
| Propylene carbonate | 220 | 175 g/L at 25 °C | 1.2047 | 0.9 | −0.41 | 240 | 132 | 0.04 | >5000 | 0.13 | 8.8 | |
| Tetrahydrofuran | 212 | miscible in any proportion | 0.883 | 0.58 | 0.45 | 65 | −21.2 | 170 | 1650 | 0.61 | 4.8 | |
| Toluene | 286 | ca. 0.573 g/L at 20 °C | 0.867 | 0.50 | 2.73 | 110.6 | 4.4 | 29 | 5580 | 0.86 | 6.0 | |
| Water | 190 | Not applied | 0.9982 | 1.28 | Not applied | 100 | Not applied | 17.535 | ≥90,000 | Not applied | 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Deeb, S. Enhancing Sustainable Analytical Chemistry in Liquid Chromatography: Guideline for Transferring Classical High-Performance Liquid Chromatography and Ultra-High-Pressure Liquid Chromatography Methods into Greener, Bluer, and Whiter Methods. Molecules 2024, 29, 3205. https://doi.org/10.3390/molecules29133205
El Deeb S. Enhancing Sustainable Analytical Chemistry in Liquid Chromatography: Guideline for Transferring Classical High-Performance Liquid Chromatography and Ultra-High-Pressure Liquid Chromatography Methods into Greener, Bluer, and Whiter Methods. Molecules. 2024; 29(13):3205. https://doi.org/10.3390/molecules29133205
Chicago/Turabian StyleEl Deeb, Sami. 2024. "Enhancing Sustainable Analytical Chemistry in Liquid Chromatography: Guideline for Transferring Classical High-Performance Liquid Chromatography and Ultra-High-Pressure Liquid Chromatography Methods into Greener, Bluer, and Whiter Methods" Molecules 29, no. 13: 3205. https://doi.org/10.3390/molecules29133205







