Evaluation of Terpene Decomposition in Kaffir Lime Juice during Storage Using Gas Chromatography–Mass Spectrometry and Proton Transfer Reaction–Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Potential Degradation Products
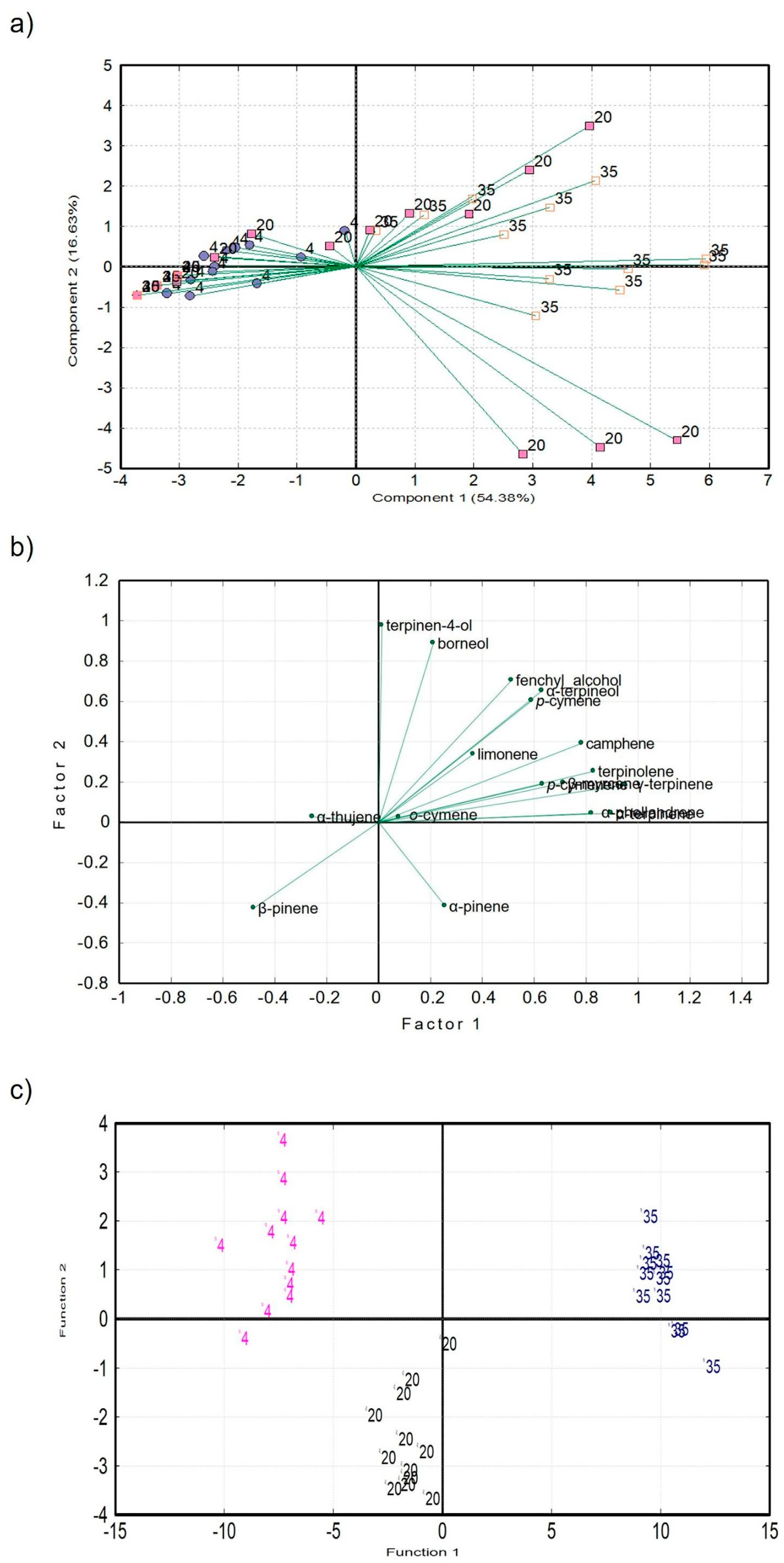
2.2. SPME-GC–MS Analysis
2.2.1. Determination of Terpenes and their Degradation Products Using GC–MS
2.2.2. Statistical Analysis
2.3. PTR–MS Analysis
2.3.1. Fragmentation Pattern
2.3.2. High-Throughput Headspace Analysis of Kaffir Lime Juice Using PTR–MS
3. Materials and Methods
3.1. Chemicals
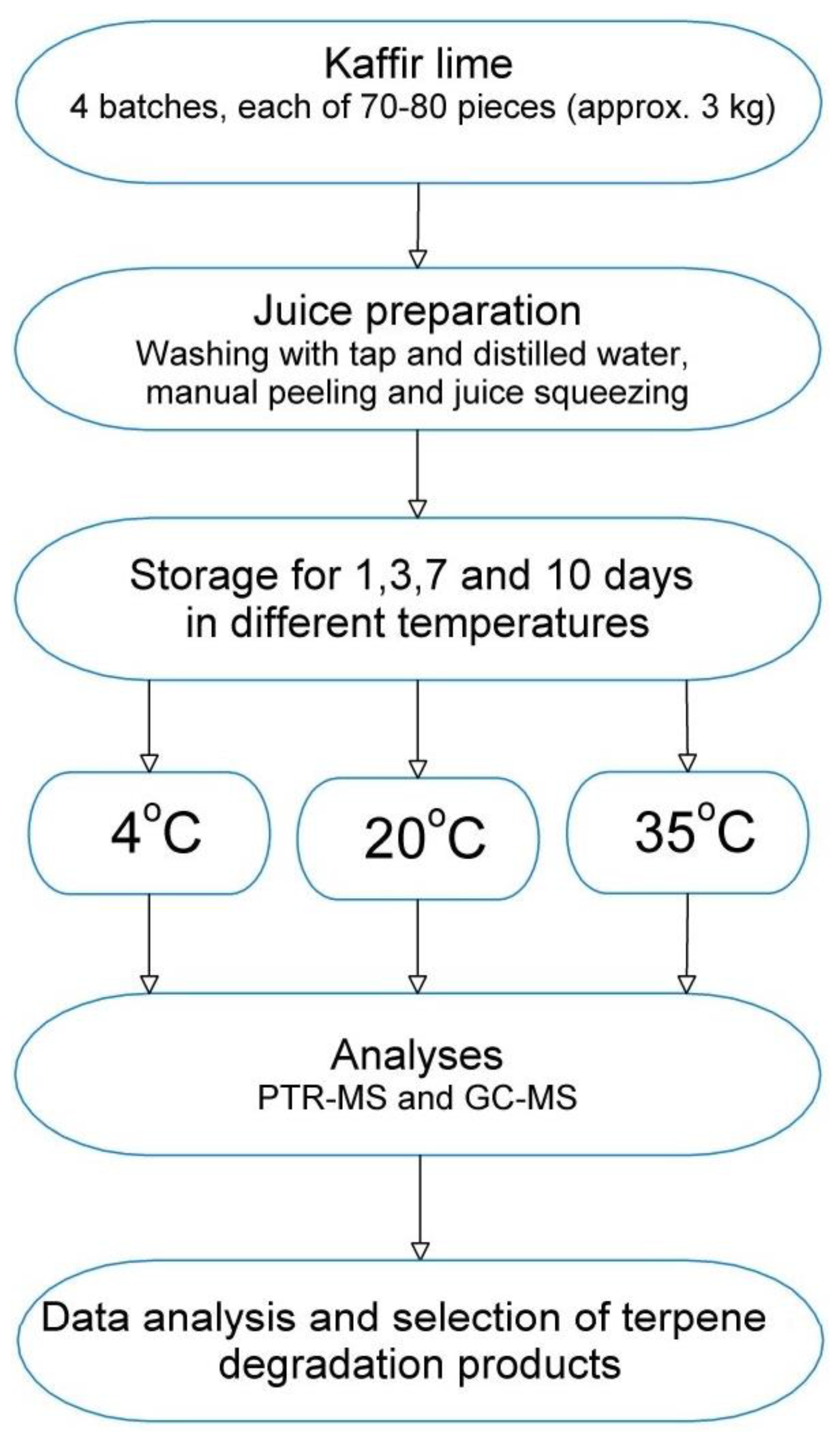
3.2. Fruit Samples
3.3. SPME-GC Analysis
3.3.1. SPME Extraction
3.3.2. GC–MS
3.4. PTR–MS Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakshi, M.P.S.; Wadhwa, M.; Makkar, H.P.S. Waste to worth: Vegetable wastes as animal feed. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2016, 11, 1–26. [Google Scholar] [CrossRef]
- Lubinska-Szczygeł, M.; Różańska, A.; Dymerski, T.; Namieśnik, J.; Katrich, E.; Gorinstein, S. A novel analytical approach in the assessment of unprocessed Kaffir lime peel and pulp as potential raw materials for cosmetic applications. Ind. Crops Prod. 2018, 120, 313–321. [Google Scholar] [CrossRef]
- Shafreen, R.B.R.B.; Lubinska-Szczygeł, M.; Różańska, A.; Dymerski, T.; Namieśnik, J.; Katrich, E.; Gorinstein, S. Human serum interactions with phenolic and aroma substances of Kaffir (Citrus hystrix) and Key lime (Citrus aurantifolia) juices. J. Lumin. 2018, 201, 115–122. [Google Scholar] [CrossRef]
- Lubinska-Szczygeł, M.; Polkowska, Ż.; Rutkowska, M.; Gorinstein, S. Chemical, Aroma and Pro-Health Characteristics of Kaffir Lime Juice—The Approach Using Optimized HS-SPME-GC-TOFMS, MP-OES, 3D-FL and Physiochemical Analysis. Int. J. Mol. Sci. 2023, 24, 12410. [Google Scholar] [CrossRef]
- Budiarto, R.; Poerwanto, R.; Santosa, E.; Efendi, D.; Agusta, A. Production, Post-Harvest and Marketing of Kaffir Lime (Citrus hystrix DC) in Tulungagung, Indonesia. J. Trop. Crop Sci. 2019, 6, 138–143. [Google Scholar] [CrossRef]
- Houng, P.; Ly, K.; Lay, S. Valorization of kaffir lime peel through extraction of essential oil and process optimization for phenolic compounds. J. Chem. Technol. Biotechnol. 2023, 98, 2745–2753. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Ethnic Culinary Herbs: A Guide to Identification and Cultivation in Hawai’i. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. The medicinal and nutritional role of underutilized citrus fruit—Citrus hystrix (Kaffir lime): A review. Drug Invent. Today 2014, 6, 1–5. [Google Scholar]
- Lubinska-Szczygieł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T.; Shafreen, R.B.; Weisz, M.; Ezra, A.; Gorinstein, S. Quality of limes juices based on the aroma and antioxidant properties. Food Control 2018, 89, 270–279. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.N.; Fan, G.; Pan, S.Y. Changes of aroma compounds and qualities of freshly-squeezed orange juice during storage. J. Food Sci. Technol. 2018, 55, 4530–4543. [Google Scholar] [CrossRef]
- Coudour, B.; Chetehouna, K.; Lemée, L.; Bertin, P.; Garo, J.P. Thermal degradation of α-pinene using a Py–GC/MS. J. Therm. Anal. Calorim. 2019, 137, 1315–1328. [Google Scholar] [CrossRef]
- Esmaeili, A.; Hashemi, E. Biotransformation of myrcene by Pseudomonas aeruginosa. Chem. Cent. J. 2011, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Mcgraw, G.W.; Hemingway, R.W.; Ingram, L.L.; Canady, C.S.; Mcgraw, W.B. Thermal degradation of terpenes: Camphene, Δ3-carene, limonene, and α-terpinene. Environ. Sci. Technol. 1999, 33, 4029–4033. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Wasik, A. Revealing dynamic changes of the volatile profile of food samples using PTR–MS. Food Chem. 2021, 364, 130404. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review. Anal. Chim. Acta 2018, 1035, 1–13. [Google Scholar] [CrossRef]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef] [PubMed]
- Schuhfried, E.; Romano, A.; Märk, T.D.; Biasioli, F. Proton Transfer Reaction-Mass Spectrometry (PTR-MS) as a tool for the determination of mass transfer coefficients. Chem. Eng. Sci. 2016, 141, 205–213. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Jakab, E.; Blazsó, M.; Barta-Rajnai, E.; Babinszki, B.; Sebestyén, Z.; Czégény, Z.; Nicol, J.; Clayton, P.; McAdam, K.; Liu, C. Thermo-oxidative decomposition of lime, bergamot and cardamom essential oils. J. Anal. Appl. Pyrolysis 2018, 134, 552–561. [Google Scholar] [CrossRef]
- Tani, A.; Hayward, S.; Hewitt, C.N. Measurement of monoterpenes and related compounds by proton transfer reaction-mass spectrometry (PTR-MS). Int. J. Mass Spectrom. 2003, 223–224, 561–578. [Google Scholar] [CrossRef]
- Tani, A.; Hayward, S.; Hansel, A.; Hewitt, C.N. Effect of water vapour pressure on monoterpene measurements using proton transfer reaction-mass spectrometry (PTR-MS). Int. J. Mass Spectrom. 2004, 239, 161–169. [Google Scholar] [CrossRef]
- Kari, E.; Miettinen, P.; Yli-Pirilä, P.; Virtanen, A.; Faiola, C.L. PTR-ToF-MS product ion distributions and humidity-dependence of biogenic volatile organic compounds. Int. J. Mass Spectrom. 2018, 430, 87–97. [Google Scholar] [CrossRef]
- Tani, A. Fragmentation and Reaction Rate Constants of Terpenoids Determined by Proton Transfer Reaction-mass Spectrometry. Environ. Control Biol. 2013, 51, 23–29. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Mączka, W.; Żołnierczyk, A.K.; Wińska, K. Transformations of Monoterpenes with the p-Menthane Skeleton in the Enzymatic System of Bacteria, Fungi and Insects. Molecules 2020, 25, 4840. [Google Scholar] [CrossRef]
- Miyazawa, M.; Wada, T. Biotransformation of γ-terpinene and (-)-α-phellandrene by the larvae of common cutworm (Spodoptera litura). J. Agric. Food Chem. 2000, 48, 2893–2895. [Google Scholar] [CrossRef]
- Taltal, E.L.F.D.E.; Patr, E.L.; Local, D.E.A. Formation of a-terpineol in Citrus Juices, Model and Buffer Solutions. J. Food Sci. 1999, 64, 165–176. [Google Scholar] [CrossRef]
- Comelli, N.A.; Ponzi, E.N.; Ponzi, M.I. Isomerization of α-pinene, limonene, α-terpinene, and terpinolene on sulfated zirconia. JAOCS, J. Am. Oil Chem. Soc. 2005, 82, 531–535. [Google Scholar] [CrossRef]
- Chang, H.T.; Lin, C.Y.; Hsu, L.S.; Chang, S.T. Thermal degradation of linalool-chemotype cinnamomum osmophloeum leaf essential oil and its stabilization by microencapsulation with β-cyclodextrin. Molecules 2021, 26, 409. [Google Scholar] [CrossRef]
- Hausen, B.M.; Reichling, J.; Harkenthal, M. Degradation products of monoterpenes are the sensitizing agents in tea tree oil. Am. J. Contact Dermat. 1999, 10, 68–77. [Google Scholar] [CrossRef]
- Degenhardt, A.; Liebig, M.; Kohlenberg, B.; Hartmann, B.; Brennecke, S.; Schäfer, U.; Schrader, D.; Kindel, G.; Trautzsch, S.; Krammer, G.E. Studies on Stability of Citrus Flavors and Insights into Degradation Pathways of Key Aroma Compounds. In Flavour Science; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]


| Chemical Compound | ID | Total Peak Area (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 20 °C | 35 °C | ||||||||||||
| Control | Day 1 | Day 3 | Day 7 | Day 10 | Day 1 | Day 3 | Day 7 | Day 10 | Day 1 | Day 3 | Day 7 | Day 10 | ||
| α-thujene | RT, MS | 0.12122 ± 0.013 | 0.136 ± 0.033 | 0.137 ± 0.022 | 0.113 ± 0.020 | 0.126 ± 0.017 | 0.283 ± 0.036 | 0.065 ± 0.068 | 0.095 ± 0.013 | 0.0978 ± 0.0011 | 0.0884 ± 0.0042 | 0.08 ± 0.02 | nd. | nd. |
| α-pinene | RT, MS | 8.20 ± 0.48 | 7.61 ± 0.73 | 8.54 ± 0.81 | 7.4 ± 1.5 | 8.8 ± 2.1 | 8.0 ± 1.0 | 7.8 ± 1.0 | 12.6 ± 2.0 | 2.30 ± 0.55 | 10.10 ± 0.52 | 7.21 ± 1.15 | 4.78 ± 0.31 | 2.97 ± 0.49 |
| camphene | RT, MS | 1.175 ± 0.069 | 1.12 ± 0.23 | 1.34 ± 0.04 | 1.75 ± 0.37 | 1.56 ± 0.18 | 1.18 ± 0.25 | 1.81 ± 0.30 | 3.29 ± 0.60 | 2.26 ± 0.51 | 2.53 ± 0.10 | 2.40 ± 0.44 | 2.07 ± 0.40 | 1.61 ± 0.32 |
| β-pinene | RT, MS | 41.3 ± 1.4 | 42.53 ± 4.0 | 42.7 ± 1.9 | 40.6 ± 3.2 | 43.3 ± 1.1 | 42.8 ± 2.7 | 29.1 ± 3.6 | 20.2 ± 5.1 | 1.15 ± 0.87 | 25.5 ± 1.5 | 14.56 ± 0.62 | 2.82 ± 0.48 | 0.362 ± 0.072 |
| β-myrcene | RT, MS | nd. | nd. | nd. | nd. | nd. | nd. | 0.530 ± 0.019 | 3.01 ± 0.16 | 1.95 ± 0.22 | 3.04 ± 0.21 | 3.16 ± 0.31 | 2.80 ± 0.24 | 4.08 ± 0.99 |
| α-phellandrene | RT, MS | 0.81 ± 0.10 | 0.89 ± 0.11 | 1.335 ± 0.033 | 1.41 ± 0.27 | 1.22 ± 0.17 | 1.40 ± 0.18 | 1.57 ± 0.14 | 2.09 ± 0.46 | 1.38 ± 0.11 | 1.604 ± 0.085 | 1.89 ± 0.17 | 2.09 ± 0.12 | 1.83 ± 0.29 |
| α-terpinene | RT, MS | 0.68 ± 0.15 | 2.53 ± 0.41 | 1.89 ± 0.19 | 1.59 ± 0.27 | 2.49 ± 0.40 | 3.55 ± 0.71 | 2.61 ± 0.65 | 3.21 ± 0.44 | 1.79 ± 0.15 | 2.66 ± 0.40 | 3.32 ± 0.50 | 4.83 ± 0.29 | 4.53 ± 0.29 |
| o-cymene | MS | 1.90 ± 0.15 | 1.80 ± 0.13 | 1.74 ± 0.10 | 1.61 ± 0.27 | 0.97 ± 0.24 | 0.82 ± 0.09 | 2.35 ± 0.17 | 1.60 ± 0.25 | 1.70 ± 0.28 | 1.93 ± 0.30 | 2.88 ± 0.09 | 1.33 ± 0.10 | 1.482 ± 0.094 |
| limonene | RT, MS | 17.9 ± 2.4 | 13.9 ± 3.3 | 12.7 ± 2.6 | 21.9 ± 3.5 | 16.66 ± 1.7 | 13.1 ± 1.9 | 13.1 ± 2.1 | 23.32 ± 3.2 | 28.5 ± 3.2 | 17.9 ± 1.6 | 16.0 ± 1.6 | 28.94 ± 5.77 | 36.8 ± 6.2 |
| p-cymene | MS | nd. | 2.23 ± 0.34 | 2.61 ± 0.13 | 2.64 ± 0.86 | 1.79 ± 0.05 | 2.19 ± 0.29 | 2.77 ± 0.46 | 2.61 ± 0.29 | 4.14 ± 0.41 | 2.77 ± 0.13 | 3.56 ± 0.47 | 2.80 ± 0.55 | 2.285 ± 0.086 |
| γ-terpinene | RT, MS | 13.9 ± 1.3 | 13.24 ± 0.70 | 15.22 ± 0.65 | 15.71 ± 0.90 | 12.51 ± 0.49 | 15.8 ± 2.0 | 17.01 ± 0.71 | 19.8 ± 1.9 | 14.4 ± 2.0 | 17.0 ± 2.2 | 19.4 ± 1.9 | 19.4 ± 2.0 | 18.64 ± 3.1 |
| p-cymenene | MS | nd. | nd. | nd. | nd. | nd. | nd. | 0.0508 ± 0.0016 | 0.045 ± 0.002 | 0.052 ± 0.021 | 0.0432 ± 0.0043 | 0.09 ± 0.0025 | 0.0785 ± 0.0055 | 0.121 ± 0.012 |
| terpinolene | RT, MS | 2.49 ± 0.20 | 2.36 ± 0.45 | 4.0 ± 1.1 | 4.39 ± 0.76 | 3.63 ± 0.24 | 3.99 ± 0.42 | 6.88 ± 0.29 | 10.09 ± 0.55 | 7.4 ± 1.7 | 7.60 ± 0.88 | 10.3 ± 1.1 | 11.8 ± 1.2 | 11.1 ± 1.6 |
| fenchyl alcohol | MS | 0.32 ± 0.20 | 0.31 ± 0.05 | 0.401 ± 0.091 | 0.490 ± 0.077 | 0.435 ± 0.066 | 0.361 ± 0.075 | 0.64 ± 0.12 | 1.021 ± 0.072 | 1.65 ± 0.39 | 0.72 ± 0.16 | 1.018 ± 0.087 | 1.30 ± 0.12 | 1.11 ± 0.066 |
| borneol | RT, MS | 0.28 ± 0.20 | 0.301 ± 0.042 | 0.282 ± 0.043 | 0.349 ± 0.039 | 0.311 ± 0.066 | 0.229 ± 0.034 | 0.437 ± 0.073 | 0.665 ± 0.045 | 1.72 ± 0.15 | 0.535 ± 0.057 | 0.647 ± 0.037 | 0.816 ± 0.041 | 0.732 ± 0.051 |
| terpinen-4-ol | RT, MS | 4.72 ± 0.83 | 5.00 ± 0.64 | 5.01 ± 1.0 | 5.20 ± 0.61 | 4.57 ± 0.44 | 6.8 ± 1.1 | 4.81 ± 0.77 | 5.75 ± 0.71 | 17.5 ± 2.3 | 6.1 ± 1.0 | 5.9 ± 1.0 | 6.8 ± 1.4 | 6.0 ± 1.2 |
| α-terpineol | RT, MS | 1.85 ± 0.15 | 2.280 ± 0.052 | 2.44 ± 0.26 | 2.40 ± 0.25 | 2.47 ± 0.38 | 2.52 ± 0.48 | 4.52 ± 0.66 | 6.55 ± 0.97 | 8.2 ± 1.5 | 4.58 ± 0.65 | 6.48 ± 0.26 | 6.9 ± 1.6 | 5.19 ± 0.67 |
| Parameter | Comparison a | Difference | Standard Error | Probability |
|---|---|---|---|---|
| α-thujene | 20–35 | 0.0760 | 0.0114 | 0.0001 |
| 4–35 | 0.0700 | 0.0101 | 0.0002 | |
| camphene | 35–4 | 0.5680 | 0.1307 | 0.0102 |
| 20–4 | 0.5540 | 0.1348 | 0.0124 | |
| β-pinene | 4–35 | 25.1560 | 0.1566 | 0.0000 |
| 20–35 | 11.0080 | 0.1564 | 0.0000 | |
| 4–20 | 14.1480 | 0.1559 | 0.0000 | |
| β-myrcene | 35–4 | 2.0780 | 0.2460 | 0.0000 |
| 35–20 | 1.5180 | 0.2459 | 0.0002 | |
| α-phellandrene | 35–4 | 0.5100 | 0.0889 | 0.0006 |
| 35–20 | 0.1940 | 0.0889 | 0.0413 | |
| α-terpinene | 35–4 | 1.3680 | 0.2317 | 0.0004 |
| 35–20 | 0.8360 | 0.2329 | 0.0377 | |
| limonene | 35–4 | 6.9000 | 1.0472 | 0.0001 |
| 35–20 | 4.3500 | 1.0468 | 0.0145 | |
| γ-terpinene | 35–4 | 3.5340 | 0.5663 | 0.0002 |
| 20–4 | 2.0680 | 0.5598 | 0.0350 | |
| p-cymenene | 35–4 | 0.0660 | 0.0040 | 0.0000 |
| 20–4 | 0.0300 | 0.0044 | 0.0000 | |
| 35–20 | 0.0360 | 0.0038 | 0.0000 | |
| terpinolene | 35–4 | 5.2880 | 0.4622 | 0.0000 |
| 20–4 | 2.7940 | 0.4578 | 0.0003 | |
| 35–20 | 2.4940 | 0.4601 | 0.0013 | |
| fenchyl alcohol | 35–4 | 0.5020 | 0.0445 | 0.0000 |
| 20–4 | 0.4060 | 0.0436 | 0.0000 | |
| borneol | 20–4 | 0.3620 | 0.0405 | 0.0000 |
| 35–4 | 0.2980 | 0.0387 | 0.0000 | |
| terpinen-4-ol | 20–4 | 3.0060 | 0.5401 | 0.0009 |
| 20–35 | 1.9800 | 0.5412 | 0.0342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubinska-Szczygeł, M.; Polkowska, Ż.; Tobolkova, B.; Majchrzak, T.; Polovka, M.; Promchote, P.; Gorinstein, S. Evaluation of Terpene Decomposition in Kaffir Lime Juice during Storage Using Gas Chromatography–Mass Spectrometry and Proton Transfer Reaction–Mass Spectrometry. Molecules 2024, 29, 3241. https://doi.org/10.3390/molecules29133241
Lubinska-Szczygeł M, Polkowska Ż, Tobolkova B, Majchrzak T, Polovka M, Promchote P, Gorinstein S. Evaluation of Terpene Decomposition in Kaffir Lime Juice during Storage Using Gas Chromatography–Mass Spectrometry and Proton Transfer Reaction–Mass Spectrometry. Molecules. 2024; 29(13):3241. https://doi.org/10.3390/molecules29133241
Chicago/Turabian StyleLubinska-Szczygeł, Martyna, Żaneta Polkowska, Blanka Tobolkova, Tomasz Majchrzak, Martin Polovka, Parichart Promchote, and Shela Gorinstein. 2024. "Evaluation of Terpene Decomposition in Kaffir Lime Juice during Storage Using Gas Chromatography–Mass Spectrometry and Proton Transfer Reaction–Mass Spectrometry" Molecules 29, no. 13: 3241. https://doi.org/10.3390/molecules29133241







