Dynamic Covalent Bond-Based Polymer Chains Operating Reversibly with Temperature Changes
Abstract
:1. Introduction
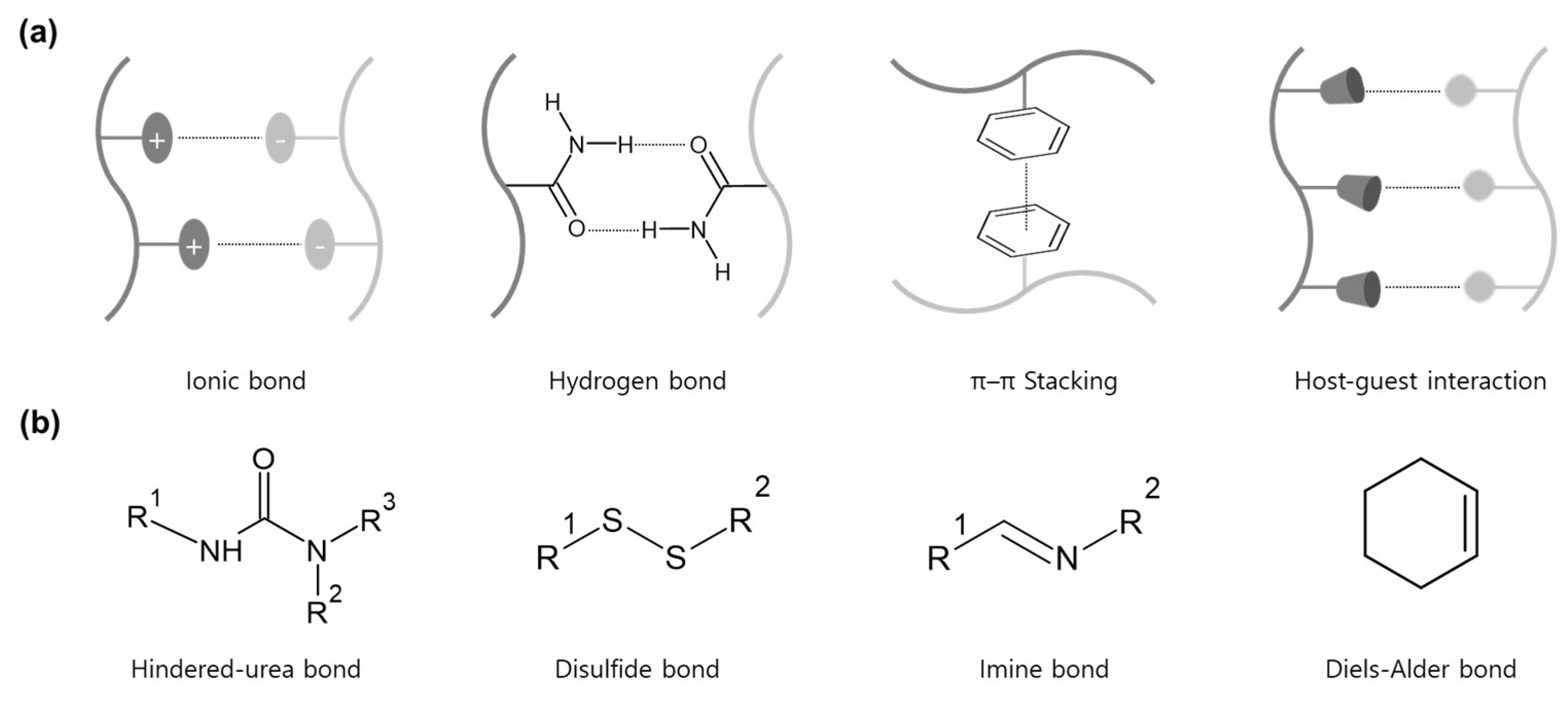
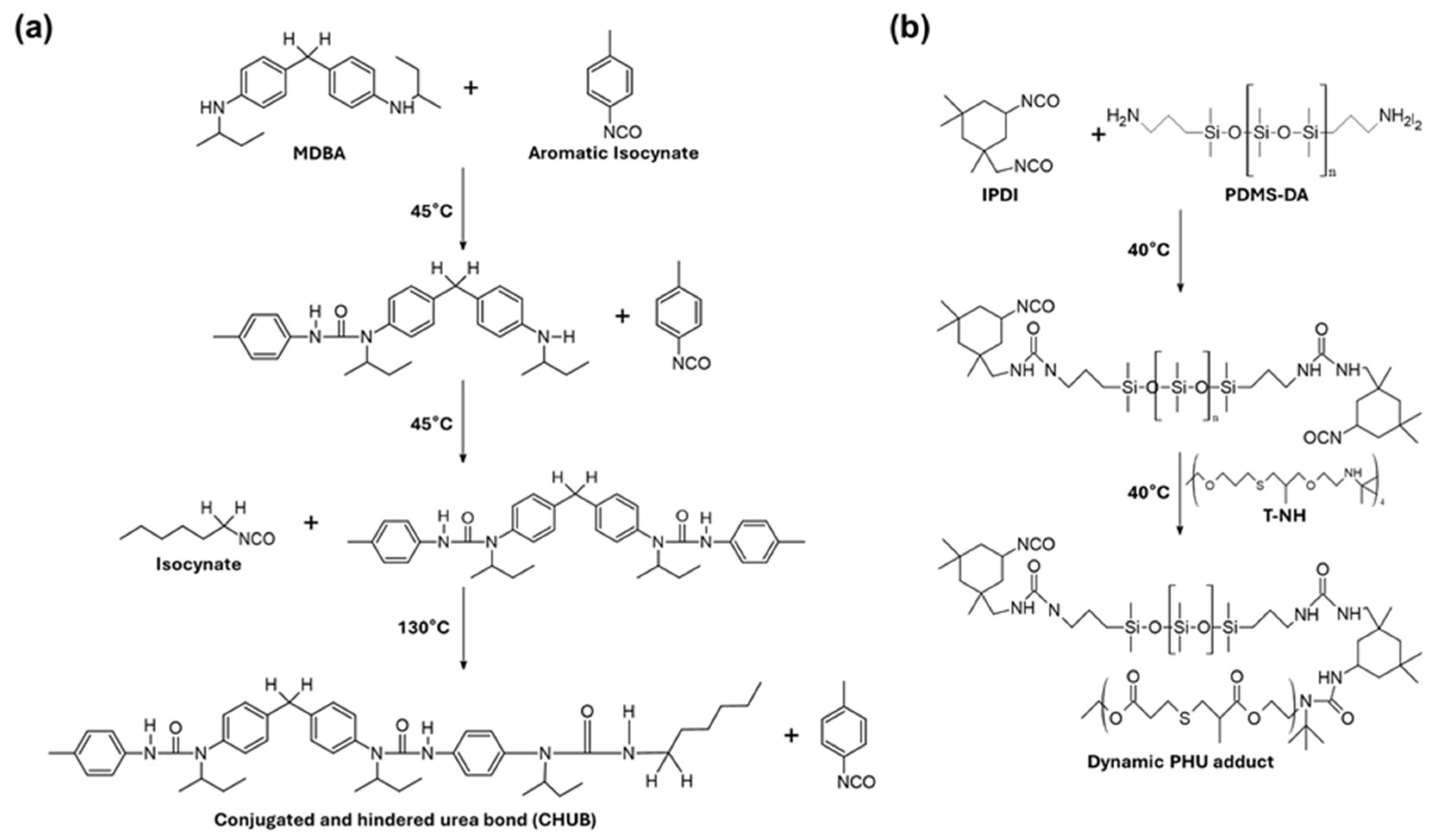
2. Hindered-Urea Bond
2.1. Bonding Mechanism and Kinetic
2.2. Synthesis
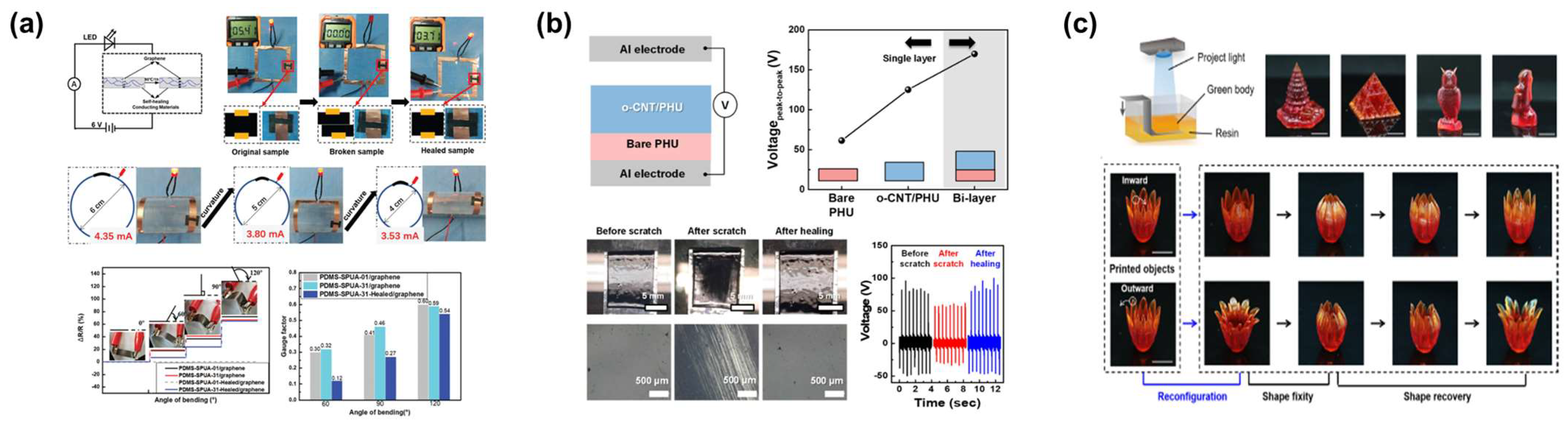
2.3. Application
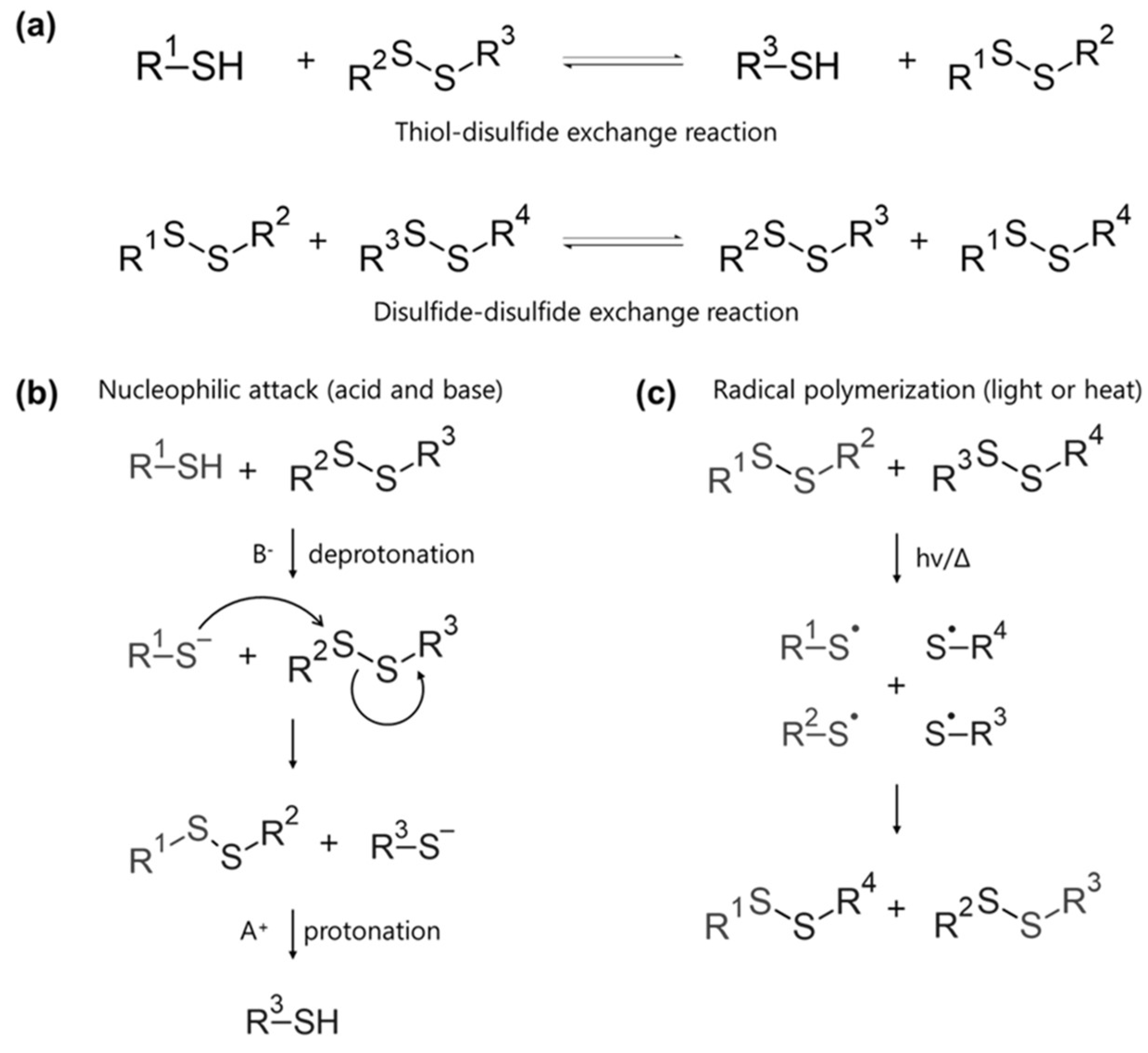
3. Disulfide Bond
3.1. Bonding Mechanism and Kinetic
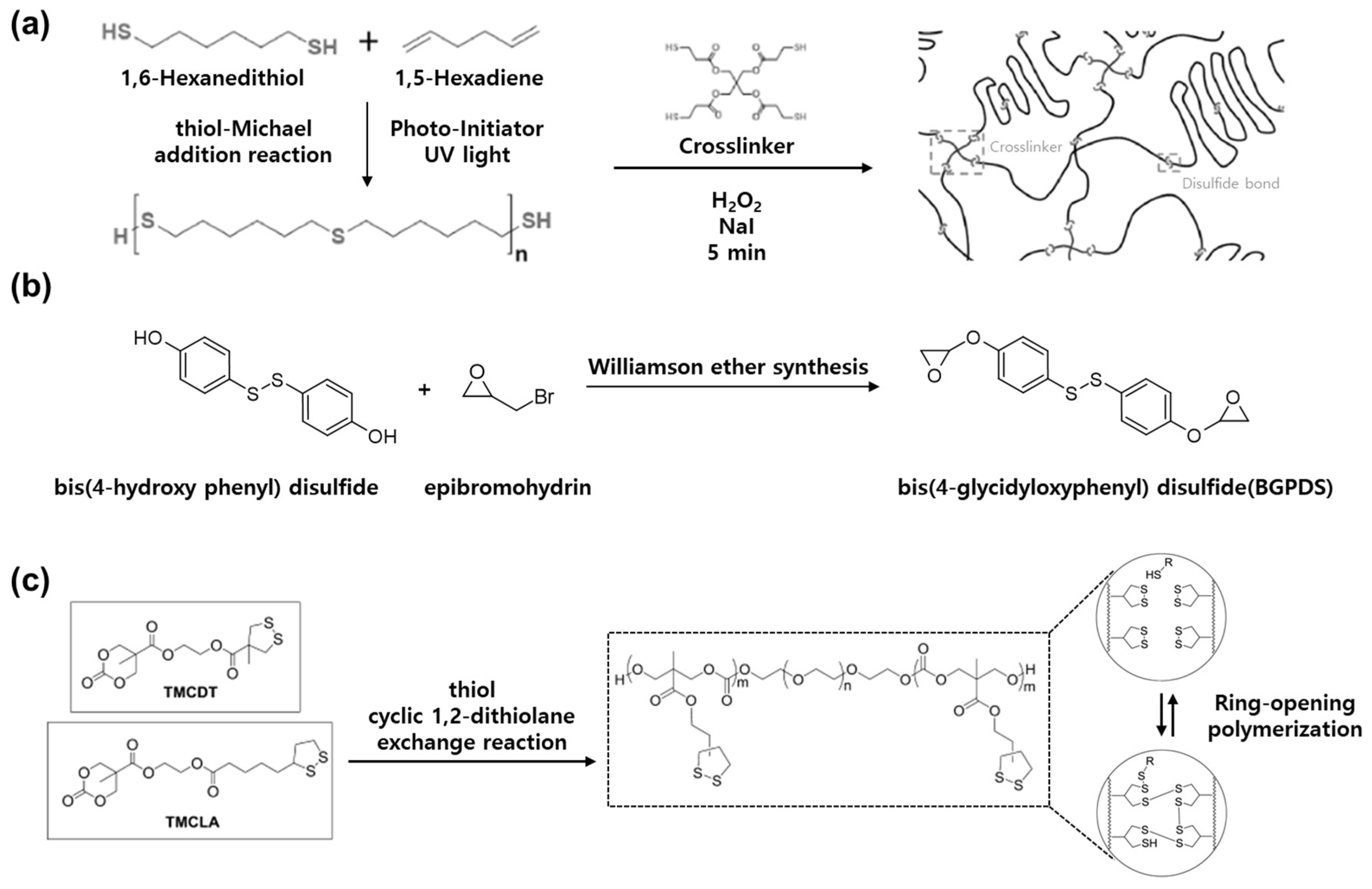
3.2. Synthesis
3.3. Application
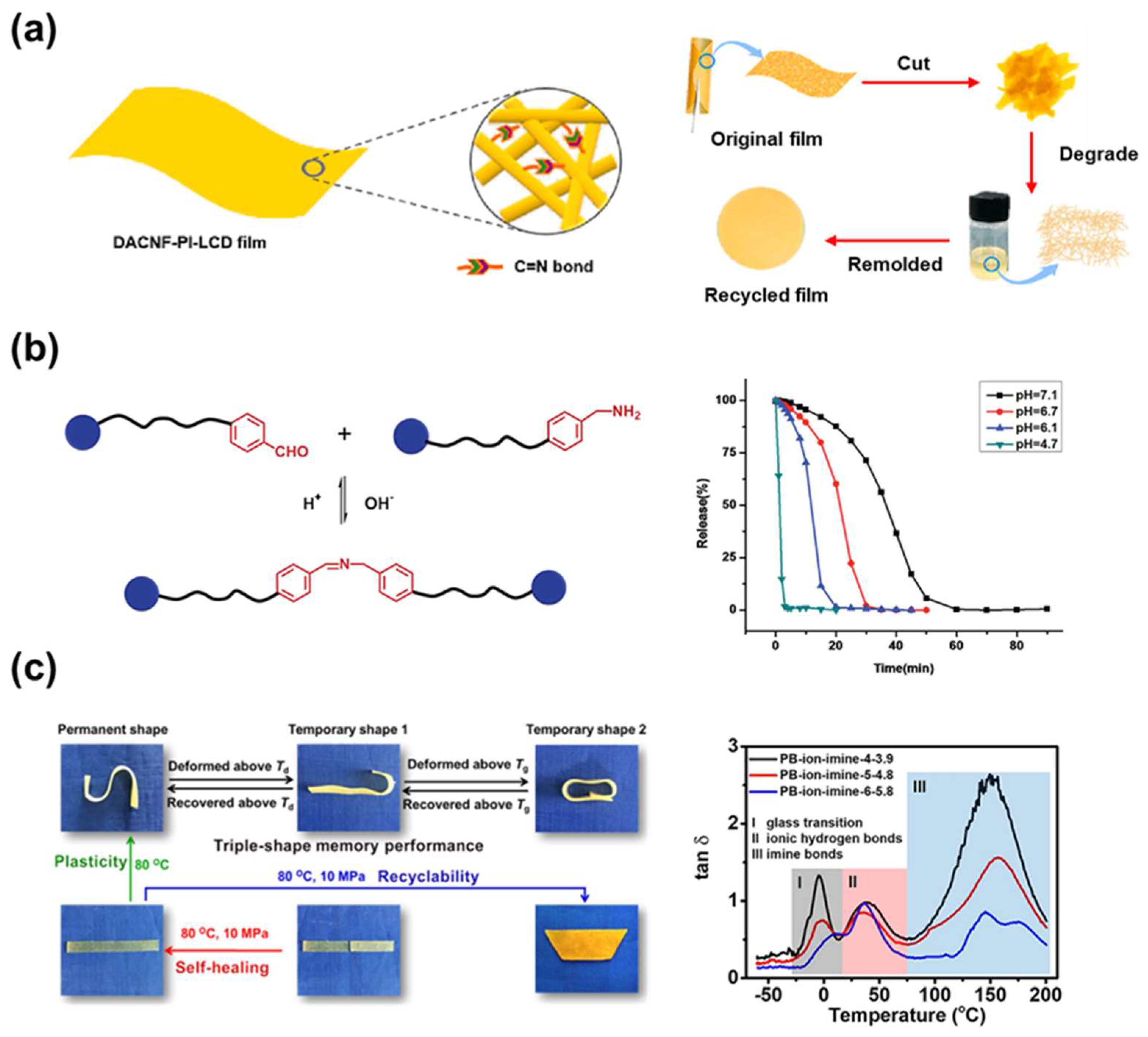
4. Imine Bond
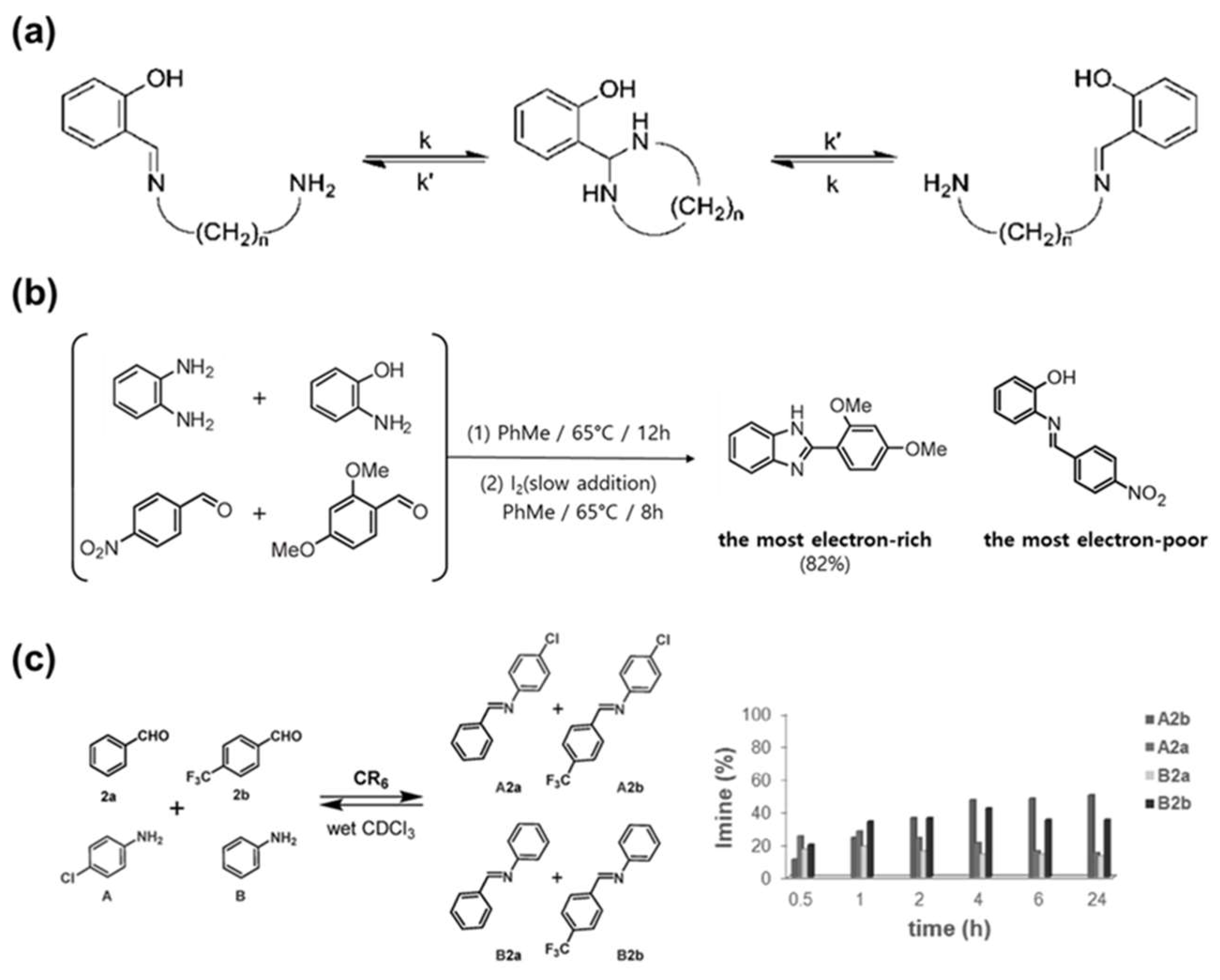
4.1. Bonding Mechanism and Kinetic
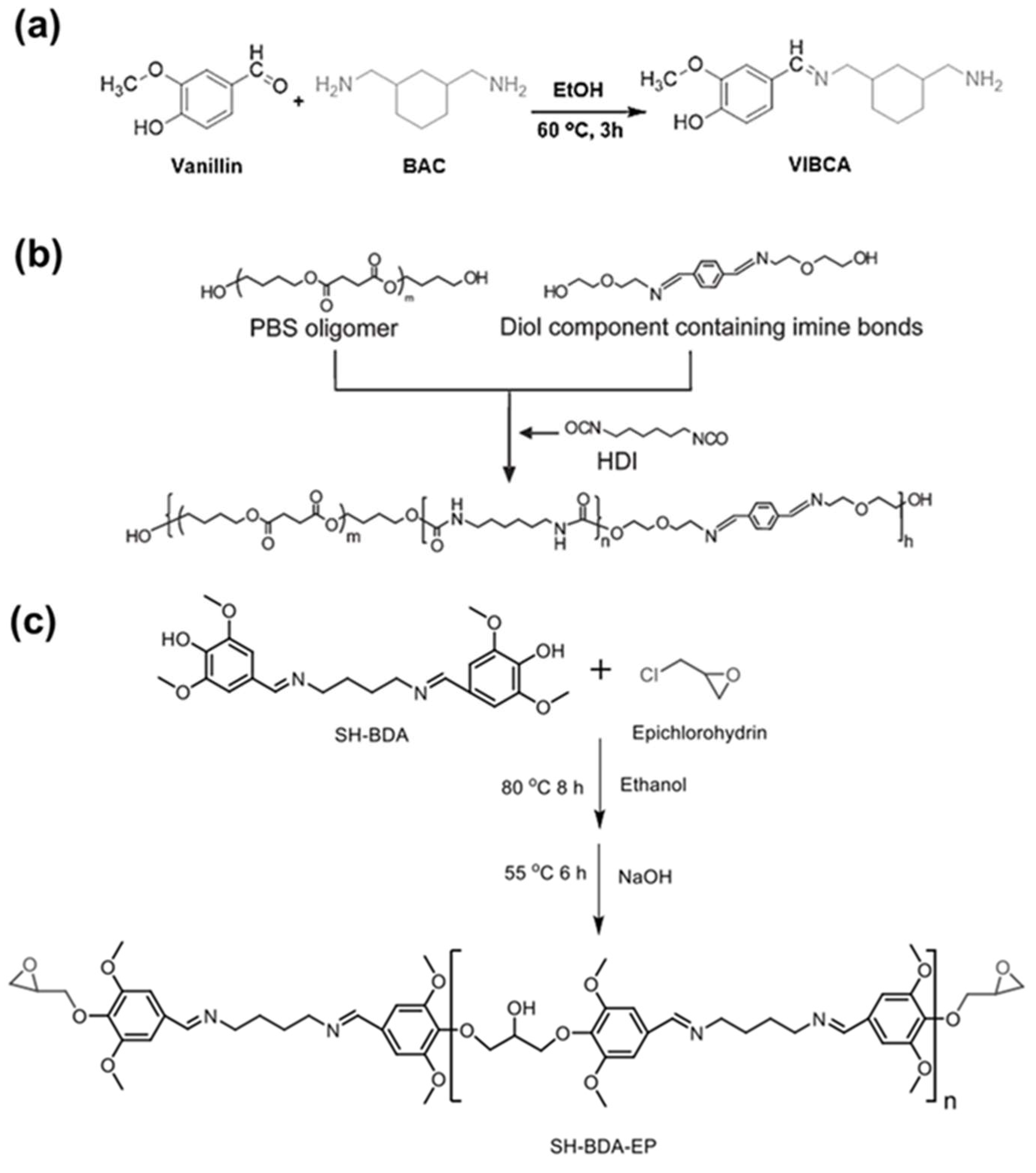
4.2. Synthesis
4.3. Application
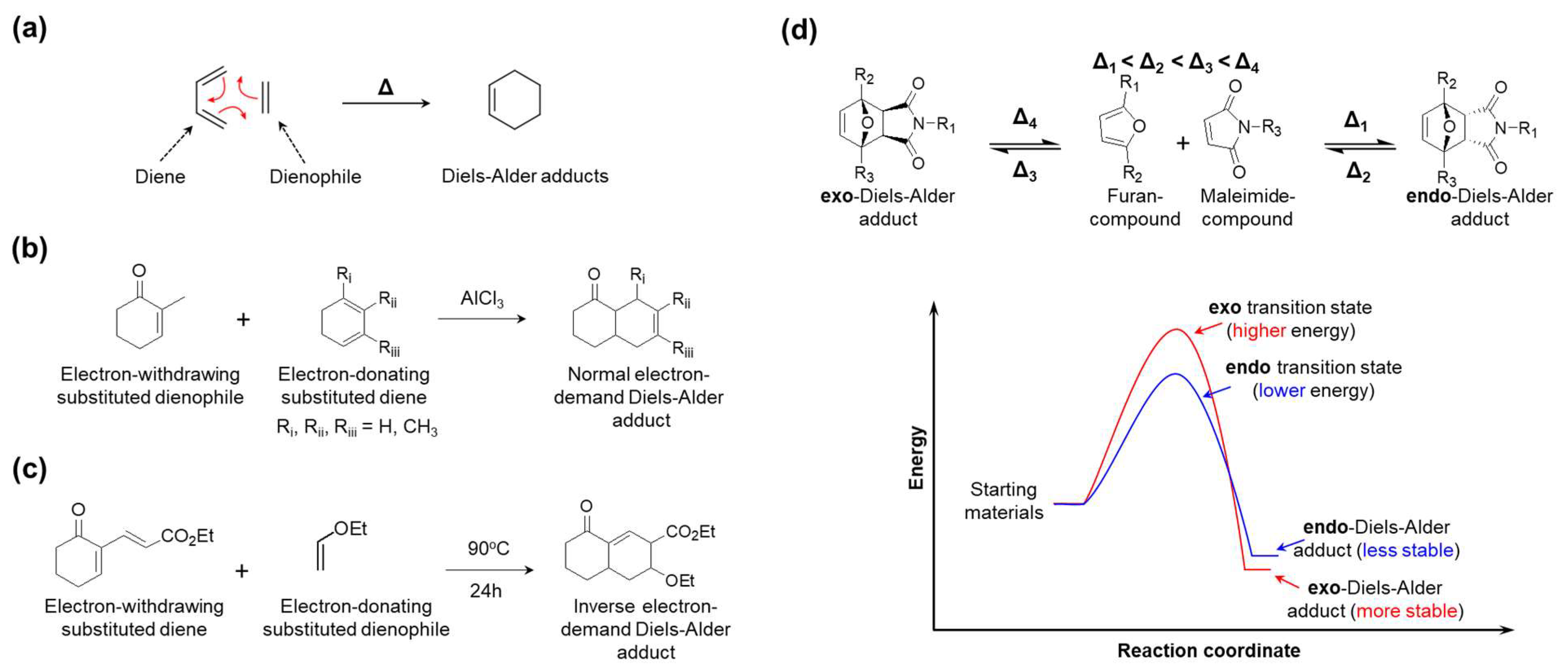
5. Diels-Alder Bond
5.1. Bonding Mechanism and Kinetic
5.2. Synthesis
5.3. Application

6. Others
6.1. Acylhydrazone Bond
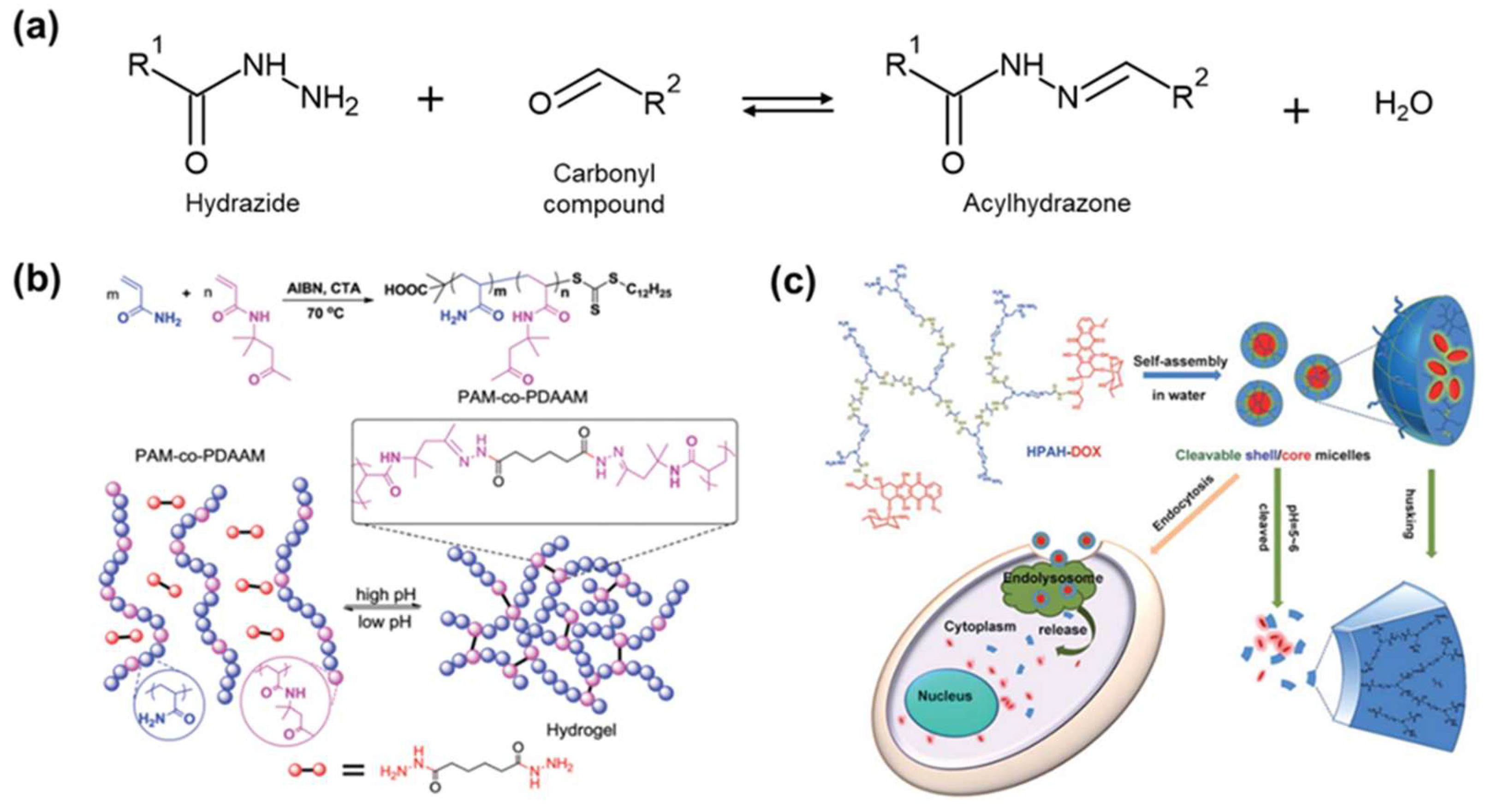
6.2. Boronic/Boronate Ester Bond

7. Summary and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, S.; Zhang, Y.; Chi, Z.; Xu, J. A pH-responsive polymer based on dynamic imine bonds as a drug delivery material with pseudo target release behavior. Polym. Chem. 2018, 9, 878–884. [Google Scholar] [CrossRef]
- Sun, P.; Huang, T.; Wang, X.; Wang, G.; Liu, Z.; Chen, G.; Fan, Q. Dynamic-Covalent Hydrogel with NIR-Triggered Drug Delivery for Localized Chemo-Photothermal Combination Therapy. Biomolecules 2020, 21, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fu, S.; Zhou, S.; Li, M.; Li, K.; Sun, W.; Zhai, Y. Advances in hydrogels based on dynamic covalent bonding and prospects for its biomedical application. Eur. Polym. J. 2020, 139, 110024. [Google Scholar] [CrossRef]
- Zhu, G.; Houck, H.A.; Spiegel, C.A.; Selhuber-Unkel, C.; Hou, Y.; Blasco, E. Introducing Dynamic Bonds in Light-based 3D Printing. Adv. Funct. Mater. 2024, 34, 2300456. [Google Scholar] [CrossRef]
- Samanta, S.; Kim, S.; Saito, T.; Sokolov, A.P. Polymers with Dynamic Bonds: Adaptive Functional Materials for a Sustainable Future. J. Phys. Chem. B 2021, 125, 9189–9401. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cao, Y.; Lei, H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels 2022, 8, 577. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, L.; Ren, X.; Gao, G. Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur. Polym. J. 2019, 112, 660–669. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Y.; You, B.; Zhao, D.; Ruan, Q.; Zeng, Y.; Ding, C. Smart hydrogels Co-switched by Hydrogen Bonds and π–π Stacking for Continuously Regulated Controlled-Release System. Adv. Funct. Mater. 2010, 20, 669–676. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, G. Polymeric Emissive Materials Based on Dynamic Covalent Bonds. Molecules 2022, 27, 6635. [Google Scholar] [CrossRef]
- Jackson, A.W.; Fulton, D.A. Making polymeric nanoparticles stimuli-responsive with dynamic covalent bonds. Polym. Chem. 2013, 4, 31–45. [Google Scholar] [CrossRef]
- Li, L.; Peng, X.; Zhu, D.; Zhang, J.; Xiao, P. Recent Progress in Polymers with Dynamic Covalent bonds. Macromol. Chem. Phys. 2023, 224, 2300224. [Google Scholar] [CrossRef]
- Zhang, K.D.; Matile, S. Complex Functional Systems with Three Different Types of Dynamic Covalent Bonds. Angew. Chem. 2015, 54, 8980–8983. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.M.; Ayres, N. Dynamic covalent bonds in self-healing, shape memory, and controllable stiffness hydrogels. Polym. Chem. 2020, 11, 1410–1423. [Google Scholar] [CrossRef]
- Rashid, M.A.; Zhu, S.; Jiang, Q.; Wei, Y.; Liu, W. Developing Easy Processable, Recyclable, and Self-Healable Biobased Epoxy Resin through Dynamic Covalent Imine Bonds. ACS Appl. Polym. Mater. 2023, 5, 279–289. [Google Scholar] [CrossRef]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: From Old Chemistry to Modern Day Innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, A.J.; Wendell, C.I.; Cencer, M.M.; Laffoon, S.D.; Moore, J.S. Kinetic and Thermodynamic Control in Dynamic Covalent Synthesis. Trends Chem. 2020, 2, 1043–1051. [Google Scholar] [CrossRef]
- Long, H.; Zhang, J.; Jia, Z.; He, N.; Zou, Y.; Han, Z.; Li, Y.; Ma, L. Controllable Switch of Thermodynamic and Kinetic Growing Paths in Two-Dimensional Covalent Organic Frameworks. Chem. Mater. 2024, 36, 666–674. [Google Scholar] [CrossRef]
- Gambaro, S.; Talotta, C.; Sala, P.D.; Soriente, A.; Rosa, M.D.; Gaeta, C.; Neri, P. Kinetic and Thermodynamic Modulation of Dynamic Imine Libraries Driven by the Hexameric Resorcinarene Capsule. J. Am. Chem. Soc. 2020, 142, 14914–14923. [Google Scholar] [CrossRef]
- Yu, J.; Gaedke, M.; Schaufelberger, F. Dynamic Covalent Chemistry for Synthesis and Co-conformational Control of Mechanically Interlocked Molecules. Eur. J. Org. Chem. 2023, 26, e202201130. [Google Scholar] [CrossRef]
- Diemer, S.L.; Kristenesen, M.; Rasmussen, B.; Beeren, S.R.; Pittelkow, M. Simultaneous Disulfide and Boronic Acid Ester Exchange in Dynamic Combinatorial Libraries. Int. J. Mol. Sci. 2015, 16, 21858–21872. [Google Scholar] [CrossRef] [PubMed]
- Gastón Orrillo, A.; Escalante, A.M.; Furlan, R.L.E. Covalent double level dynamic combinatorial libraries: Selectively addressable exchange processes. Chem. Commun. 2008, 42, 5298–5300. [Google Scholar] [CrossRef] [PubMed]
- Liguori, A.; Hakkarainen, M. Designed from Biobased Materials for Recycling: Imine-Based Covalent Adaptable Networks. Macromol. Rapid Commun. 2022, 43, 2100816. [Google Scholar] [CrossRef] [PubMed]
- Arias-Ugarte, R.; Wekesa, F.S.; Findlater, M. Selective aldol condensation or cyclotrimerization reactions catalyzed by FeCl3. Tetrahedron Lett. 2015, 56, 2406–2411. [Google Scholar] [CrossRef]
- Jia, Y.; Ying, H.; Zhang, Y.; He, H.; Cheng, J. Reconfigurable Poly(urea-urethane) Thermoset Based on Hindered Urea Bonds with Triple-Shape-Memory Performance. Macroml. Chem. Phys. 2019, 220, 1900148. [Google Scholar] [CrossRef]
- Zholdassov, Y.S.; Yuan, L.; Garcia, S.R.; Kwok, R.W.; Braunschweig, A.B. Acceleration of Diels-Alder reactions by mechanical distortion. Science 2023, 380, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Houck, H.A.; Yin, Q.; Xu, Y.; Huang, Y.; Lan, Y.; Yang, L.; Du Prez, F.E.; Chang, G. Force-reversible chemical reaction at ambient temperature for designing toughened dynamic covalent polymer networks. Nat. Commun. 2022, 13, 3231. [Google Scholar] [CrossRef] [PubMed]
- Wanasinghe, S.V.; Dodo, O.J.; Knokolewicz, D. Dynamic Bonds: Adaptable Timescales for Responsive Materials. Angew. Chem. 2022, 134, e202206938. [Google Scholar] [CrossRef]
- Crolais, A.E.; Dolinski, N.D.; Boynton, N.R.; Radhakrishnan, J.M.; Snyder, S.A.; Rowan, S.J. Enhancing the Equilibrium of Dynamic Thia-Michael Reactions through Heterocyclic Design. J. Am. Chem. Soc. 2023, 145, 14427–14434. [Google Scholar] [CrossRef]
- Zhang, V.; Kang, B.; Accardo, J.V.; Kalow, J.A. Structure-Reactivity-Property Relationships in Covalent Adaptable Networks. J. Am. Chem. Soc. 2022, 144, 22358–22377. [Google Scholar] [CrossRef]
- Zhang, Z.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- You, Y.; Fu, M.R.; Rong, M.Z.; Zhang, M.Q. Improving creep resistance while maintaining reversibility of covalent adaptive networks via constructing reversibly interlocked polymer networks. Mater. Today Chem. 2022, 23, 100687. [Google Scholar] [CrossRef]
- You, Y.; Peng, W.L.; Xie, P.; Rong, M.Z.; Zhang, M.Q.; Liu, D. Topological rearrangement-derived homogeneous polymer networks capable of reversibly interlocking: From phantom to reality and beyond. Mater. Today 2020, 33, 45–55. [Google Scholar] [CrossRef]
- Bin Rusayyis, M.A.; Torkelson, J.M. Reprocessable covalent adaptable networks with excellent elevated-temperature creep resistance: Facilitation by dynamic, diisociative bis(hindered amino) disulfide bonds. Polym. Chem. 2021, 12, 2760–2771. [Google Scholar] [CrossRef]
- Mondal, S.; Wong, A.J.; Wagh, M.A.; Alperstein, L.; Sanja, G.J.; Sumerlin, B.S. Creep resistance in doubly crosslinked dynamic covalent networks. Polym. Chem. 2024, 15, 1826–1832. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Lee, H.M.; Ahmed, S.; Kim, G.Y.; Kim, J.C.; Cheong, I.W. Effect of diisocyanate structure on steric restructuring of hindered urea bonds for self-healable coating. Prog. Org. Coat. 2022, 165, 106730. [Google Scholar] [CrossRef]
- Li, W.; Wang, J. Interactions between lignin and urea researched by molecular simulation. Mol. Simul. 2012, 38, 1048–1054. [Google Scholar] [CrossRef]
- Wang, Z.; Gangarapu, S.; Escorihuela, J.; Fei, G.; Zuilhof, H.; Xia, H. Dynamic covalent urea bonds and their potential for development of self-healing polymer materials. J. Mater. Chem. A 2019, 7, 15933. [Google Scholar] [CrossRef]
- Liu, W.X.; Yang, Z.; Qiao, Z.; Zhang, L.; Zhao, N.; Luo, S.; Xu, J. Dynamic multiphase semi-crystalline polymers based on thermally reversible pyrazole-urea bonds. Nat. Commun. 2019, 10, 4753. [Google Scholar] [CrossRef]
- Basso, A.; Martin, L.D.; Ebert, C.; Gardossi, L.; Linda, p. High isolated yields in thermodynamically controlled peptide synthesis in toluene catalysed by thermolysin adsorbed on Celite R-640. Chem. Commun. 2000, 467–468. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Rao, B.; Chen, X.; Ma, L.; Cui, C.; Zhong, Q.; Li, Z.; Cheng, Y.; Zhang, Y. Hindered urea bonds for dynamic polymers: An overview. React. Funct. Polym. 2021, 159, 104807. [Google Scholar] [CrossRef]
- Ying, H.; Cheng, J. Hydrolyzable Polyureas Bearing Hindered Urea Bonds. J. Am. Chem. Soc. 2014, 49, 16974–16977. [Google Scholar] [CrossRef] [PubMed]
- Hutchby, M.; Houlden, C.E.; Haddow, M.F.; Tyler, S.N.G.; Lloyd-Jones, G.C.; Booker-Milburn, K.I. Switching Pathways: Room-Temperature Neutral Solvolysis and Substitution of Amides. Angew. Chem. 2012, 51, 548–551. [Google Scholar] [CrossRef]
- Hutchby, M.; Houlden, C.E.; Gair Ford, J.; Tyler, S.G.; Gagné, M.; Lloyd-Jones, G.C.; Booker-Milburn, K.I. Hindered Ureas as Masked Isocyanates: Facile Carbamoylation of Nucelophiles under Neutral Conditions. Angew. Chem. 2009, 48, 8721–8724. [Google Scholar] [CrossRef]
- McCormick, L.J.; McDonnell-Worth, C.; Platts, J.A.; Edwards, A.J.; Turner, D.R. Investigation of steric influences on hydrogen bonding motifs in cyclic ureas by using X-ray, neutron, and computational methods. Chem. Asian J. 2013, 8, 2642–2651. [Google Scholar] [CrossRef]
- Malik, J.; Goldslager, B.A.; Clarson, S.J. Thermally controlled molecular disassembly of a crosslinked polymer network by the incorporation of sterically hindered urea linkages. J. Appl. Polym. Sci. 2022, 85, 856–864. [Google Scholar] [CrossRef]
- Park, J.I.; Choe, A.; Kim, M.P.; Ko, H.; Lee, T.H.; Noh, S.M.; Kim, J.C.; Cheong, I.W. Water-adaptive and repeatable self-healing polymers bearing bulky urea bonds. Polym. Chem. 2018, 9, 11–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, H.; Hart, K.R.; Wu, Y.; Hsu, A.J.; Coppola, A.M.; Kim, T.A.; Yang, K.; Sottos, N.R.; White, S.R.; et al. Malleable and Recyclable Poly(urea-urethane) Thermosets bearing Hindered Urea Bonds. Adv. Mater. 2016, 28, 7646–7651. [Google Scholar] [CrossRef]
- Lee, H.M.; Perumal, S.; Kim, G.Y.; Kim, J.C.; Kim, Y.R.; Kim, M.P.; Ko, H.; Rho, Y.; Cheong, I.W. Enhanced thermomechanical property of a self-healing polymer via self-assembly of a reversibly cross-linkable block copolymer. Polym. Chem. 2020, 11, 3701–3708. [Google Scholar] [CrossRef]
- Kim, G.Y.; Sung, S.; Kim, M.P.; Kim, S.C.; Lee, S.H.; Park, Y.I.; Noh, S.M.; Cheong, I.W.; Kim, J.C. Reversible polymer networks based on the dynamic hindered urea bond for scratch healing in automotive clearcoats. Appl. Surf. Sci. 2020, 505, 144546. [Google Scholar] [CrossRef]
- Fang, Z.; Shi, Y.; Mu, H.; Lu, R.; Wu, J.; Xie, T. 3D printing of dynamic covalent polymer network with on-demand geometric and mechanical reprogrammability. Nat. Commun. 2023, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Song, H.; Zhang, Y.; Jin, B.; Wu, J.; Zhao, Q.; Xie, T. Modular 4D Printing via Interfacial Welding of Digital Light-Controllable Dynamic Covalent Polymer Networks. Matter 2020, 2, 1187–1197. [Google Scholar] [CrossRef]
- Patel, T.; Park, J.; Kim, M.P.; Ye, Z.; Ko, H.; Jung, H.W.; Oh, J.K. Dynamic poly(hindered urea) hybrid network materials crosslinked with reactive methacrylate polymer. Polym. Chem. 2023, 14, 5115–5124. [Google Scholar] [CrossRef]
- Zhao, D.; Moore, J.S. Nucleation-elongation: A mechanism for cooperative supramolecular polymerization. Org. Biomol. Chem. 2003, 1, 3471–3491. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, S.; Ye, Z.; Bian, C.; Xing, X.; Hong, T.; Jing, X. Highly Tunable and Robust Dynamic Polymer Networks via Conjugated-Hindered Urea Bonds. Macromolecules 2022, 50, 9091–9102. [Google Scholar] [CrossRef]
- Murphy, E.; Wudl, F. The world of smart healable materials. Prog. Polym. Sci. 2010, 35, 223–251. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Xing, X.; Zhang, G.; Jing, X. Fully recyclable and high performance phenolic resin based on dynamic urethane bonds and its application in self-repairable composites. Polymer 2021, 229, 124022. [Google Scholar] [CrossRef]
- Patel, T.; Kim, M.P.; Park, J.; Lee, T.H.; Nellepalli, P.; Noh, S.M.; Jung, H.W.; Ko, H.; Oh, J.K. Self-Healable Reprocessable Triboelectric Nanogenerators Fabricated with Vitrimeric Poly(hindered Urea) Networks. ACS Nano 2020, 14, 11442–11451. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Z.; Lei, Y.; Yuan, Y.; Wu, B.; Lei, J. Sustainable Thermosetting Polyurea Vitrimers Based on a Catalyst-Free Process with Reprocessability, Permanent Shape Reconfiguration and Self-Healing Performance. ACS Appl. Polym. Mater. 2019, 1, 3261–3268. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, L.; Wang, S.; Mao, J.; Luo, J.; Chen, Y.; Cheng, Y. Mechanically enhanced healable and recyclable silicone with dynamic hindered urea bond for flexible electronics. J. Mater. Chem. C 2021, 9, 8579. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Liu, S.; Ning, N.; Zhang, L.; Tian, M.; Wang, Y. Interfacial polarization and dielectric properties of aligned carbon nanotubes/polymer composites: The role of molecular polarity. Compos. Sci. Technol. 2018, 154, 145–153. [Google Scholar] [CrossRef]
- Ning, N.; Bai, X.; Zhang, L.; Lu, Y.; Nishi, T.; Tian, M. Dramatically improved dielectric properties of polymer composites by controlling the alignment of carbon nanotubes in matrix. RSC Adv. 2014, 4, 4543–4551. [Google Scholar] [CrossRef]
- Feng, Y.; Zheng, Y.; Zhang, G.; Wang, D.; Zhou, F.; Liu, W. A new protocol toward high output TENG with polyimide as charge storage layer. Nano Energy 2017, 38, 467–476. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, Z.; Zou, W.; Zhao, Q.; Xie, T. Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation. Angew. Chem. Int. Ed. Engl. 2016, 55, 11421–11425. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tan, Y.; He, C.; Ji, S.; Xu, H. Unconstrained 3D Shape Programming with Light-Induced Stress Gradient. Adv. Mater. 2021, 33, 2105194. [Google Scholar] [CrossRef] [PubMed]
- Worrell, B.T.; McBride, M.K.; Lyon, G.B.; Cox, L.M.; Wang, C.; Mavila, S.; Lim, C.H.; Coley, H.M.; Musgrave, C.B.; Ding, Y.; et al. Bistable and photoswitchable states of matter. Nat. Commun. 2018, 9, 2804. [Google Scholar] [CrossRef] [PubMed]
- Atesan, R.; Dykstra, A.B.; Banerjee, A.; Agrawal, N.J. Heterogeneity in Disulfide Bond Reduction in IgG1 Antibodies Is Governed by Solvent Accessibility of the Cysteines. Antibodies 2023, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Cupp-Sutton, K.A.; Ashby, M.T. Biological Chemistry of Hydrogen Selenide. Antioxidants 2016, 5, 42. [Google Scholar] [CrossRef]
- Bebiano, L.B.; Lourenço, B.N.; Granja, P.L.; Pereira, R.F. Hydrogels as dynamic covalent networks for skin repair. In Hydrogels for Tissue Engineering and Regenerative Medicine; Oliveira, J.M., Silva-Correia, J., Reis, R.L., Eds.; Academic Press: Cambridge, MA, USA, 2024; Chapter 30; pp. 605–624. [Google Scholar]
- Black, S.P.; Sanders, J.K.M.; Stefankiewicz, A.R. Disulfide exchange: Exposing supramolecular reactivity through dynamic covalent chemistry. Chem. Soc. Rev. 2014, 43, 1861–1872. [Google Scholar] [CrossRef]
- Nagy, P. Kinetics and Mechanisms of Thiol–Disulfide Exchange Covering Direct Substitution and Thiol Oxidation-Mediated Pathways. Antioxid. Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Singh, S.P.; Bowman, C.N.; Anseth, K.S. Photodegradable, Photoadaptable Hydrogels via Radical-Mediated Disulfide Fragmentation Reaction. Macromolecules 2011, 44, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.; Schmidt, B.V.K.J.; Schlaad, H. Aminolysis induced functionalization of (RAFT) polymer-dithioester with thiols and disulfides. Polym. Chem. 2020, 11, 7677–7684. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar]
- Moreno, A.; Garcia, D.; Galià, M.; Ronda, J.C.; Cádiz, V.; Lligadas, G.; Percec, V. SET-LRP in the Neoteric Ethyl Lactate Alcohol. Biomacromolecules 2017, 18, 3447–3456. [Google Scholar] [CrossRef] [PubMed]
- Tsarevsky, N.V.; Matyjaszewski, K. Combining Atom Transfer Radical Polymerization and Disulfide/Thiol Redox Chemistry: A Route to Well-Defined (Bio)degradable Polymeric Materials. Macromolecules 2005, 38, 3087–3092. [Google Scholar] [CrossRef]
- Bongiardina, N.J.; Soars, S.M.; Podgorski, M.; Bowman, C.N. Radical-disulfide exchange in thiol–ene–disulfidation polymerizations. Polymer Chem. 2022, 13, 3991–4003. [Google Scholar] [CrossRef]
- Buback, M.; Hutchinson, R.A.; Lacík, I. Radical polymerization kinetics of water-soluble monomers. Prog. Polym. Sci. 2023, 138, 101645. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Rowan, S.J. Trapping Dynamic Disulfide Bonds in the Hard Segments of Thermoplastic Polyurethane Elastomers. Macromol. Chem. Phys. 2017, 218, 1600320. [Google Scholar] [CrossRef]
- Troussicot, L.; Vallet, A.; Molin, M.; Burmann, B.M.; Schanda, P. Disulfide-Bond-Induced Structural Frustration and Dynamic Disorder in a Peroxiredoxin from MAS NMR. J. Am. Chem. Soc. 2023, 145, 10700–10711. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Solera, G.; Azcarate-Ascasua, I.; Boucher, V.; Grande, H.-J.; Rekondo, A. Chemical control of the aromatic disulfide exchange kinetics for tailor-made epoxy vitrimers. Polymer 2022, 239, 124457. [Google Scholar] [CrossRef]
- Netto, L.E.S.; de Oliveira, M.A.; Monteiro, G.; Demasi, A.P.D.; Cussiol, J.R.R.; Discola, K.F.; Demasi, M.; Silva, G.M.; Alves, S.V.; Faria, V.G.; et al. Reactive cysteine in proteins: Protein folding, antioxidant defense, redox signaling and more. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, O.; Thorpe, C.; Bach, R.D. Mechanism of SN2 Disulfide Bond Cleavage by Phosphorus Nucleophiles. Implications for Biochemical Disulfide Reducing Agents. J. Org. Chem. 2007, 72, 8298–8307. [Google Scholar] [PubMed]
- Sobczak, S.; Ratajczyk, P.; Katrusiak, A. Squeezing Out the Catalysts: A Sustainable Approach to Disulfide Bond Exchange in Aryl Disulfides. ACS Sustain. Chem. Eng. 2021, 9, 7171–7178. [Google Scholar] [CrossRef]
- Soars, S.M.; Bongiardina, N.J.; Fairbanks, B.D.; Podgórski, M.; Bowman, C.N. Spatial and Temporal Control of Photomediated Disulfide–Ene and Thiol–Ene Chemistries for Two-Stage Polymerizations. Macromolecules 2022, 55, 1811–1821. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Phan, T.T.T.; Lee, J.S. Fabrication and Characterization of Self-Healable Polydisulfide Network-Based Composites. ACS Appl. Polym. Mater. 2023, 5, 485–493. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohishi, T.; Goseki, R.; Otsuka, H. Degradable epoxy resins prepared from diepoxide monomer with dynamic covalent disulfide linkage. Polymer 2016, 82, 319–326. [Google Scholar] [CrossRef]
- Zhang, X.; Waymouth, R.M. 1,2-Dithiolane-Derived Dynamic, Covalent Materials: Cooperative Self-Assembly and Reversible Cross-Linking. J. Am. Chem. Soc. 2017, 139, 3822–3833. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, Y.; Zhu, Z.; Zhang, J.; Xu, W. Dynamic disulfide bonds facilitated fabrication of a multifunctional liquid-free elastomer for recyclable soft electronics. J. Mater. Chem. C 2023, 11, 15759–15766. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X.; Chen, L.; Du, J.; Ji, L.; Peng, Y.; Liu, L.; Xu, Z.; Lin, X.; Lin, W.; et al. Rapid Self-Healing and High-Mechanical-Strength Epoxy Resin Coatings Incorporating Dynamic Disulfide Bonds. ACS Appl. Polym. Mater. 2024, 6, 4778–4788. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Qin, J.; Chen, L.; Zhang, L.; Wei, Y.; Liu, W. Hemiaminal dynamic covalent networks with rapid stress relaxation, reprocessability and degradability endowed by the synergy of disulfide and hemiaminal bonds. RSC Adv. 2023, 13, 28658–28665. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhong, Y.; Zhao, L.; Hu, C.; Shen, L.; Yang, Y.; Zhong, J. Self-healing polyacrylates based on dynamic disulfide and quadruple hydrogen bonds. Soft Matter 2024, 20, 3612–3619. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Torkelson, J.M. Covalent Adaptive Networks for Enhanced Adhesion: Exploiting Disulfide Dynamic Chemistry and Annealing during Application. ACS Appl. Polym. Mater. 2020, 2, 4658–4665. [Google Scholar] [CrossRef]
- Kong, W.; Yang, Y.; Wang, Y.; Cheng, H.; Yan, P.; Huang, L.; Ning, J.; Zeng, F.; Cai, X.; Wang, M. An ultra-low hysteresis, self-healing and stretchable conductor based on dynamic disulfide covalent adaptable networks. J. Mater. Chem. A 2022, 10, 2012–2020. [Google Scholar] [CrossRef]
- Ma, Y.; Qin, R.; Xu, M.; Jiang, X.; Sheng, Y.; Wang, M.; Zhang, W.; Lu, X. Wide temperature range damping polyurethane elastomer based on dynamic disulfide bonds. J. Appl. Polym. Sci. 2022, 139, 51453. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef] [PubMed]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef]
- Wayner, D.D.M.; Clark, K.B.; Rauk, A.; Yu, D.; Armstrong, D.A. C−H Bond Dissociation Energies of Alkyl Amines: Radical Structures and Stabilization Energies. J. Am. Chem. Soc. 1997, 119, 8925–8932. [Google Scholar] [CrossRef]
- Hu, J.; Mo, R.; Sheng, X.; Zhang, X. A self-healing polyurethane elastomer with excellent mechanical properties based on phase-locked dynamic imine bonds. Polym. Chem. 2020, 11, 2585–2594. [Google Scholar] [CrossRef]
- Wu, P.; Wang, X.; Shi, R.; Cheng, H.; Zhao, F. A self-healing and recyclable poly(urea-imine) thermoset synthesized from CO2. Green Chem. 2022, 24, 1561–1569. [Google Scholar] [CrossRef]
- Meger, F.S.; Murphy, J.A. Recent Advances in C–H Functionalisation through Indirect Hydrogen Atom Transfer. Molecules 2023, 28, 6127. [Google Scholar] [CrossRef]
- Van Dam, A.; van Schendel, R.; Gangarapu, S.; Zuilhof, H.; Smulders, M.M.J. DFT Study of Imine-Exchange Reactions in Iron(II)-Coordinated Pincers. Chem. A Eur. J. 2023, 29, e202301795. [Google Scholar] [CrossRef]
- Montag, S.D. Utilizing the Imine Condensation in Organic Chemistry Teaching Laboratories to Reinforce Steric Effects, Electronic Effects, and Green Chemistry Principles. J. Chem. Educ. 2023, 100, 4456–4461. [Google Scholar] [CrossRef]
- Godoy-Alcántar, C.; Yatsimirsky, A.K.; Lehn, J.-M. Structure-stability correlations for imine formation in aqueous solution. J. Phys. Org. Chem. 2005, 18, 979–985. [Google Scholar] [CrossRef]
- Soniya, K.; Awasthi, S.; Nair, N.N.; Chandra, A. Transimination Reaction at the Active Site of Aspartate Aminotransferase: A Proton Hopping Mechanism through Pyridoxal 5′-Phosphate. ACS Catal. 2019, 9, 6276–6283. [Google Scholar] [CrossRef]
- Ciaccia, M.; Di Stefano, S. Mechanisms of imine exchange reactions in organic solvents. Org. Biomol. Chem. 2015, 13, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ye, H.; Hai, Y.; Zhang, L.; You, L. n → π* interactions as a versatile tool for controlling dynamic imine chemistry in both organic and aqueous media. Chem. Sci. 2020, 11, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Derrick, S.D.; Boehme, R.; Wong, K.M.; Nemeth, F.; Tanaka, K.; Rumberg, B.; Beekman, R.A.; Dibble, P.W. Steric and electronic effects in imine-hemiaminal ring-chain tautomerism. Tetrahedron 1996, 52, 7679–7690. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Z.; Xu, J. Temperature-dependent annuloselectivity and stereochemistry in the reactions of methanesulfonyl sulfene with imines. Org. Biomol. Chem. 2016, 14, 7258–7267. [Google Scholar] [CrossRef]
- Schoustra, S.K.; Asadi, V.; Smulders, M.M.J. Probing the Solubility of Imine-Based Covalent Adaptable Networks. ACS Applied Polym. Mater. 2024, 6, 79–89. [Google Scholar] [CrossRef]
- Kovaříček, P.; Lehn, J.-M. Merging Constitutional and Motional Covalent Dynamics in Reversible Imine Formation and Exchange Processes. J. Am. Chem. Soc. 2012, 134, 9446–9455. [Google Scholar] [CrossRef] [PubMed]
- Osowska, K.; Miljanić, O.Š. Oxidative Kinetic Self-Sorting of a Dynamic Imine Library. J. Am. Chem. Soc. 2011, 133, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Shimoda, M.; Sukegawa, M.; Nobori, T.; Lehn, J.-M. Doubly degradable dynamers: Dynamic covalent polymers based on reversible imine connections and biodegradable polyester units. Green Chem. 2012, 14, 2907–2911. [Google Scholar] [CrossRef]
- Xie, W.; Huang, S.; Liu, S.; Zhao, J. Imine-functionalized biomass-derived dynamic covalent thermosets enabled by heat-induced self-crosslinking and reversible structures. Chem. Eng. J. 2021, 404, 126598. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Zeng, J.; Li, J.; Wang, B.; Gao, W.; Xu, J.; Chen, K. Dynamic covalent bond enabled strong Bio-based polyimide materials with Thermally-driven Adaptivity, healability and recycling. Chem. Eng. J. 2023, 465, 143017. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Wang, Z.; Zhang, X. Bolaform Superamphiphile Based on a Dynamic Covalent Bond and Its Self-Assembly in Water. Langmuir 2011, 27, 12375–12380. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Wu, N.; Li, C.; Zhu, C.; Zhao, N.; Xu, J. Recyclable, Self-Healing, Thermadapt Triple-Shape Memory Polymers Based on Dual Dynamic Bonds. ACS Appl. Mater. Interfaces 2020, 12, 9833–9841. [Google Scholar] [CrossRef] [PubMed]
- Bibiao, J.; Jianjun, H.; Wenyun, W.; Luxia, J.; Xinxian, C. Synthesis and properties of novel polybismaleimide oligomers. Eur. Polym. J. 2001, 37, 463–470. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels-Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Orozco, F.; Li, J.; Ezekiel, U.; Niyazov, Z.; Floyd, L.; Lima, G.M.R.; Winkelman, J.G.M.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Diels-Alder-based thermo-reversibly crosslinked polymers: Interplay of crosslinking density, network mobility, kinetics and stereoisomerism. Eur. Polym. J. 2020, 135, 109882. [Google Scholar] [CrossRef]
- Feitosa, L.F.; Campos, R.B.; Richter, W.E. Energetics and electronics of polar Diels-Alder reactions at the atomic level: QTAIM and IQA analyses of complete IRC paths. J. Mol. Graph. Model. 2023, 118, 108326. [Google Scholar] [CrossRef] [PubMed]
- Fringuelli, F.; Minuti, L.; Pizzo, F.; Taticchi, A. Reactivity and selectivity in Lewis-acid-catalyzed Diels-Alder reactions of 2-cyclohexenones. Acta Chem. Scand. 1993, 47, 255. [Google Scholar] [CrossRef]
- Bodwell, G.J.; Pi, Z. Electron deficient dienes I. Normal and inverse electron demand Diels-Alder reaction of the same carbon skeleton. Tetrahedron Lett. 1997, 38, 309–312. [Google Scholar] [CrossRef]
- Fringuelli, F.; Taticchi, A. The Diels-Alder Reaction: Selected Practical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels-Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef]
- Reinecke, M.; Ritter, H. Renewable resources, 1. Branching and crosslinking of an unsaturated oligoester with furfurylamides and sorbic acid amides via diels-alder additions. Die Makromol. Chem. 1993, 194, 2385–2393. [Google Scholar] [CrossRef]
- Woodroffe, J.-D.; Harvey, B.G. A Simple Process for the Dimerization and Cross-Coupling of Isoprene and Myrcene to High-Performance Jet and Diesel Blendstocks. Energy Fuels 2022, 36, 2630–2638. [Google Scholar] [CrossRef]
- Gambarotti, C.; Lauria, M.; Righetti, G.I.C.; Leonardi, G.; Sebastiano, R.; Citterio, A.; Truscello, A. Synthesis of Functionalized Aromatic Carboxylic Acids from Biosourced 3-Hydroxy-2-pyrones through a Base-Promoted Domino Reaction. ACS Sustain. Chem. Eng. 2020, 8, 11152–11161. [Google Scholar] [CrossRef]
- Zelisko, N.; Karpenko, O.; Muzychenko, V.; Gzella, A.; Lesyk, R. Citraconic acid and its anhydride-based hetero-Diels-Alder reactions in the synthesis of new thiopyrano [2,3-d][1,3]thiazole derivatives. Synth. Commun. 2021, 51, 964–970. [Google Scholar] [CrossRef]
- Samba, W.K.; Tia, R.; Adei, E. Regio-, enantio-, peri-, and stereo-selectivities of the reactions of five-membered cyclodiene derivatives with itaconic anhydride toward the formation of norbornene lactones. J. Phys. Org. Chem. 2021, 34, e4132. [Google Scholar] [CrossRef]
- Lording, W.J.; Fallon, T.; Sherburn, M.S.; Paddon-Row, M.N. The simplest Diels-Alder reactions are not endo-selective. Chem. Sci. 2020, 11, 11915–11926. [Google Scholar] [CrossRef]
- Froidevaux, V.; Borne, M.; Laborbe, E.; Auvergne, R.; Gandini, A.; Boutevin, B. Study of the Diels-Alder and retro-Diels-Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 2015, 5, 37742–37754. [Google Scholar] [CrossRef]
- Ratwani, C.R.; Kamali, A.R.; Abdelkader, A.M. Self-healing by Diels-Alder cycloaddition in advanced functional polymers: A review. Prog. Mater. Sci. 2023, 131, 101001. [Google Scholar] [CrossRef]
- Tesoro, G.C.; Sastri, V.R. Synthesis of siloxane-containing bis(furans) and polymerization with bis(maleimides). Ind. Eng. Chem. Prod. Res. Dev. 1986, 25, 444–448. [Google Scholar] [CrossRef]
- Hou, I.C.-Y.; Hu, Y.; Narita, A.; Müllen, K. Diels-Alder polymerization: A versatile synthetic method toward functional polyphenylenes, ladder polymers and graphene nanoribbons. Polym. J. 2018, 50, 3–20. [Google Scholar] [CrossRef]
- Jiang, Y.; Hadjichristidis, N. Diels-Alder Polymer Networks with Temperature-Reversible Cross-Linking-Induced Emission. Angew. Chem. 2021, 133, 335–341. [Google Scholar] [CrossRef]
- Dolci, E.; Michaud, G.; Simon, F.; Boutevin, B.; Fouquay, S.; Caillol, S. Remendable thermosetting polymers for isocyanate-free adhesives: A preliminary study. Polym. Chem. 2015, 6, 7851–7861. [Google Scholar] [CrossRef]
- Banella, M.B.; Giacobazzi, G.; Vannini, M.; Marchese, P.; Colonna, M.; Celli, A.; Gandini, A.; Gioia, C. A Novel Approach for the Synthesis of Thermo-Responsive Co-Polyesters Incorporating Reversible Diels-Alder Adducts. Macromol. Chem. Phys. 2019, 220, 1900247. [Google Scholar] [CrossRef]
- Restrepo-Montoya, A.C.; Larraza, I.; Echeverria-Altuna, O.; Harismendy, I.; Saralegi, A.; Eceiza, A. Emerging Reprocessable and Recyclable Biobased Cross-Linked Polyurethanes Through Diels-Alder Chemistry. ACS Appl. Polym. Mater. 2024, 6, 4475–4486. [Google Scholar] [CrossRef]
- Lacerda, T.M.; Carvalho, A.J.F.; Gandini, A. A minimalist furan–maleimide AB-type monomer and its thermally reversible Diels-Alder polymerization. RSC Adv. 2016, 6, 45696–45700. [Google Scholar] [CrossRef]
- Alharbi, H.Y.; Alnoman, R.B.; Aljohani, M.S.; Al-Anazia, M.; Monier, M. Synthesis and characterization of gellan gum-based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2024, 258, 128828. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Song, B.; Yang, K.; Hao, X.; Ma, J. Polyurethane toughened covalent adaptive networks epoxy composite based on thermoreversible Diels-Alder reaction: Self-healable, shape memory, and recyclable. J. Appl. Polym. Sci. 2024, 141, e54762. [Google Scholar] [CrossRef]
- Yu, C.; Wang, Z.; Fei, G.; Zhang, X.; de Luna, M.S.; Lavorgna, M.; Xia, H. Robust self-healing waterborne polyurethane coatings via dynamic covalent Diels-Alder bonds for corrosion protection. J. Polym. Sci. 2024, 62, 815–825. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Quiles-Díaz, S.; Seyler, H.; Ellis, G.J.; Shuttleworth, P.S.; Gómez-Fatou, M.A. Remotely triggered reversible bonds in adhesives for sustainable multi-layered packaging. Sustain. Mater. Technol. 2023, 36, e00632. [Google Scholar] [CrossRef]
- Lian, T.; Zhang, S.; Xu, Q.; Wang, K.; Li, B.; Qin, X.; Jiang, M.; Liu, P. Self-Healing and Flame-Retardant Modifications of Epoxy Resins by the Diels-Alder Release-Delivery Strategy for a High-Efficiency and Green Application. Ind. Eng. Chem. Res. 2023, 62, 6019–6031. [Google Scholar] [CrossRef]
- Gu, S.; Xiao, Y.F.; Tan, S.H.; Liu, B.W.; Guo, D.M.; Wang, Y.Z.; Chen, L. Neighboring Molecular Engineering in Diels-Alder Chemistry Enabling Easily Recyclable Carbon Fiber Reinforced Composites. Angew. Chem. 2023, 135, e202312638. [Google Scholar] [CrossRef]
- Jung, S.; Kim, S.Y.; Kim, J.C.; Noh, S.M.; Oh, J.K. Ambient temperature induced Diels-Alder crosslinked networks based on controlled methacrylate copolymers for enhanced thermoreversibility and self-healability. RSC Adv. 2017, 7, 26496–26506. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Lin, C.-H.; Han, T.-Y.; Xiao, Y.-T.; Chen, Y.-A.; Chou, H.-H. Disulfide bond and Diels-Alder reaction bond hybrid polymers with high stretchability, transparency, recyclability, and intrinsic dual healability for skin-like tactile sensing. J. Mater. Chem. A 2021, 9, 6109–6116. [Google Scholar] [CrossRef]
- Zhao, W.; Han, X.; Lu, Y.; Zhang, Z.; Zhang, Q.; Peng, P.; Wu, H. Fabrication of mechanically robust urushiol-based polymer coatings with excellent self-healing property and hydrophobicity. Prog. Org. Coat. 2023, 174, 107237. [Google Scholar] [CrossRef]
- Raut, S.K.; Mondal, P.; Parameswaran, B.; Sarkar, S.; Dey, P.; Gilbert, R.; Bhadra, S.; Naskar, K.; Nair, S.; Singha, N.K. Self-healable ultrahydrophobic modified bio-based elastomer using Diels-Alder ‘click chemistry’. Eur. Polym. J. 2021, 146, 110204. [Google Scholar] [CrossRef]
- Zhonglin, C.; Xiaoling, Z. Bio-Based Self-Healing Polymeric Materials Derived from Furfuryl Alcohol Based on the Diels-Alder Reversible Reaction. Polym. Sci. Ser. B 2023, 65, 450–456. [Google Scholar] [CrossRef]
- Sancar, T.; Altinbasak, I.; Sanyal, R.; Sanyal, A. Electrospun photothermally active graphene-based nanofibers with a Retro-Diels-Alder reaction to initiate drug release. Eur. Polym. J. 2024, 210, 112946. [Google Scholar] [CrossRef]
- Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Socea, L.-I.; Barbuceanu, S.-F.; Pahontu, E.M.; Dumitru, A.-C.; Nitulescu, G.M.; Sfetea, R.C.; Apostol, T.-V. Acylhydrazones and Their Biological Activity: A Review. Molecules 2022, 27, 8719. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.-H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, W.; Gu, H.; Feng, Y.; He, Z.; Chen, Q.; Mao, X.; Zhang, J.; Zheng, L. pH-Switchable and self-healable hydrogels based on ketone type acylhydrazone dynamic covalent bonds. Soft Matter 2017, 13, 7371–7380. [Google Scholar] [CrossRef]
- Ni, W.; Song, H.; Wang, L.; Liu, Y.; Wang, Q. Design, Synthesis and Various Bioactivity of Acylhydrazone-Containing Matrine Analogues. Molecules 2023, 28, 4163. [Google Scholar] [CrossRef]
- Zhu, L.; Tu, C.; Zhu, B.; Su, Y.; Pang, Y.; Yan, D.; Wu, J.; Zhu, X. Construction and application of pH-triggered cleavable hyperbranched polyacylhydrazone for drug delivery. Polym. Chem. 2011, 2, 1761–1768. [Google Scholar] [CrossRef]
- Xu, P.; Liu, J.; Wang, S.; Chen, J.; Han, B.; Meng, Y.; Yang, S.; Xie, L.; Yang, M.; Jia, R.; et al. Dynamic covalent polymer engineering for stable and self-healing perovskite solar cells. Mater. Horiz. 2023, 10, 5223–5234. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, B.; Chai, Q.; Zhang, M.; Wei, J.; Wang, H.; Li, M. A colorimetric and fluorescent sensor for the detection of both fluoride ions and trifluoroacetic acid based on acylhydrazone derivatives. Soft Matter 2019, 15, 6690–6695. [Google Scholar] [CrossRef]
- Chatterjee, S.; Anslyn, E.V.; Bandyopadhyay, A. Boronic acid based dynamic click chemistry: Recent advances and emergent applications. Chemical Science 2021, 12, 1585–1599. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.Y.; Oh, D.X.; Park, J. Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: Boronic/boronate esters, borax, and benzoxaborole. J. Mater. Chem. A 2021, 9, 14630–14655. [Google Scholar] [CrossRef]
- Teotonico, J.; Mantione, D.; Ballester-Bayarri, L.; Ximenis, M.; Sardon, H.; Ballard, N.; Ruipérez, F. A combined computational and experimental study of metathesis and nucleophile-mediated exchange mechanisms in boronic ester-containing vitrimers. Polym. Chem. 2024, 15, 181–192. [Google Scholar] [CrossRef]
- Martínez-Aguirre, M.A.; Villamil-Ramos, R.; Guerrero-Alvarez, J.A.; Yatsimirsky, A.K. Substituent Effects and pH Profiles for Stability Constants of Arylboronic Acid Diol Esters. J. Org. Chem. 2013, 78, 4674–4684. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Shinkai, S.; James, T.D. Boronic Acids in Molecular Self-Assembly. Chem. Asian J. 2008, 3, 1076–1091. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Wang, D.-P.; Zuo, J.-L.; Li, C.-H. A Tough and Self-Healing Polymer Enabled by Promoting Bond Exchange in Boronic Esters with Neighboring Hydroxyl Groups. ACS Mater. Lett. 2021, 3, 1328–1338. [Google Scholar] [CrossRef]
- Silva, M.P.; Saraiva, L.; Pinto, M.; Sousa, M.E. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules 2020, 25, 4323. [Google Scholar] [CrossRef] [PubMed]
- Scarano, W.; Lu, H.; Stenzel, M.H. Boronic acid ester with dopamine as a tool for bioconjugation and for visualization of cell apoptosis. Chem. Commun. 2014, 50, 6390–6393. [Google Scholar] [CrossRef]
- Kojima, T.; Nishida, J.-i.; Tokito, S.; Yamashita, Y. Development of Organic Electronic Devices Using Boronate Esters and Related Heterocycles. Chem. Lett. 2008, 37, 1122–1123. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Zhao, P.-C.; Zhao, Y.; Zuo, J.-L.; Li, C.-H. An Underwater Long-Term Strong Adhesive Based on Boronic Esters with Enhanced Hydrolytic Stability. Adv. Funct. Mater. 2022, 32, 2201959. [Google Scholar] [CrossRef]
- Kong, Z.; Boahen, E.K.; Kim, D.J.; Li, F.; Kim, J.S.; Kweon, H.; Kim, S.Y.; Choi, H.; Zhu, J.; Bin Ying, W.; et al. Ultrafast underwater self-healing piezo-ionic elastomer via dynamic hydrophobic-hydrolytic domains. Nat. Commun. 2024, 15, 2129. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, J.; Qin, J.; Wang, D.; Liang, L. Reprocessed, shape-memory and self-healing robust epoxy resin by hindered urea bond. Polymer 2024, 290, 126565. [Google Scholar] [CrossRef]
- Ren, S.; Zhou, W.; Song, K.; Gao, X.; Zhang, X.; Fang, H.; Li, X.; Ding, Y. Robust, self-healing, anti-corrosive waterborne polyurethane urea composite coatings enabled by dynamic hindered urea bonds. Prog. Org. Coat. 2023, 180, 107571. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Guo, A.; Yu, H.; Yu, C.; Zhou, Z.; Zhang, F. Intrinsically self-healing crosslinked elastomer with mechanically robust based on dynamic urea bond and hydrogen bond for smart humidty sensor. React. Funct. Polym. 2023, 192, 105737. [Google Scholar] [CrossRef]
- Zhou, J.H.; Tang, L.M. Investigation on UV-curing Reprocessable Thermosets Bearing Hindered Urea Bonds and Their Composites with Modified Zinc Oxide Nanoparticles. Chinese. J. Polym. Sci. 2024, 42, 751–765. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Xu, X.; Ma, X.; Cui, M.; Zhao, H.; Stott, N.E.; Zhu, J.; Yan, N.; Chen, J. Fully biomass-derived polyurethane based on dynamic imine with self-healing, rapid degradability, and editable shape memory capabilities. J. Chem. Eng. 2024, 479, 147823. [Google Scholar] [CrossRef]
- Qu, Y.; Lu, X.; Xin, Z. Biobased Polybenzoxazine Vitrimer with Imine Bonds: Shape Memory, Reprocessing, and Degradation. ACS Sustain. Chem. Eng. 2024, 12, 7739–7747. [Google Scholar] [CrossRef]
- Luo, T.; Lu, C.; Qi, J.; Wang, C.; Chu, F.; Wang, J. Fabrication of well-defined lignin-derived elastomers by atom transfer radical polymerization and Diels-Alder reaction towards sustainable, super-tough and high temperature-resistant hot-melt adhesives. J. Chem. Eng. 2024, 479, 147729. [Google Scholar] [CrossRef]
- Imato, K.; Ohishi, T.; Nishihara, M.; Takahara, A.; Otsuka, H. Network Reorganization of Dynamic Covalent Polymer Gels with Exchangeable Diarylbibenzofuranone at Ambient Temperature. J. Am. Chem. Soc. 2014, 136, 11839–11845. [Google Scholar] [CrossRef]






| Dynamic Covalent Bond | Polymer Matrix | Stimulus | Properties | Application | Ref. |
|---|---|---|---|---|---|
| Hindered-urea Bond | PtB-HDI 1 and PtB-IPDI 2 | 5 h at 37 °C in the presence of water (self-healing) | Water-adaptability, self-healable | Self-healable tube for flowing water | [48] |
| HUBM-co-PPGA 3 | 1 h at 80 °C (homolytic bond exchange) ~4 h at 120 °C (heterolytic bond exchange) | Shape memory, reprocessable, reprogrammable, thermally switchable | 3D printing ink | [52] | |
| HUG-EP 4 | 4 h at 80 °C (crosslinked) 20 min at 160 °C (self-healing) | Shape memory, reprocessable, biodegradable recycling | Electronic packaging | [174] | |
| WPUU 5 | 2 h at 80 °C | Anti-corrosion, self-healable | Protective coating layer | [175] | |
| PG 6 | 30 min at ambient temperature, followed by 3 h UV light (36 w, 365 nm) | Mechanically robust, self-healable, weldability, anti-corrosion | Humidity sensor | [176] | |
| PUT 7 | 2 h at 50 °C followed by UV curing | Reprocessable, self-healable, solubility, thermal stability | UV-shielding | [177] | |
| Disulfide Bond | PDSN 8 | Heat at 80 °C, followed by UV light | Shape memory, self-healable, adhesive force | - | [87] |
| Epoxy resin from BGPDS 9 | 30–60 min with DBU 10 at 100 °C or photo-irradiation (245 nm, 2 mW/cm2) | Rigid, degradable | - | [88] | |
| EPO345 11-CTAM 12 | well crosslinked above 80 °C | Adaptable network, reshapable, shear strength adhesive | Adaptable thermoset adhesive | [94] | |
| PU 13 polymers with TEA 14 | 56 °C (disulfide exchange), 1 h at 130 °C (self-healing) | Low hysteresis, stretchable, self-healable, crystallinity | EMG monitoring | [95] | |
| Polyurethane | 70 °C (chain expansion) | Energy dissipation, segmental mobility, rigid | Damping elastomer | [96] | |
| Epoxy resin | 1 h at 60 °C | Self-healable, high-strength, reprocessable | - | [91] | |
| Epoxy resin reinforced with fiber | 5 min at 200 °C under 100 bar | Reprocessable, reparability, recyclable | Thermosets | [178] | |
| Imine Bond | Vanilin-based epoxy resin | 40 min at 170 °C under 0.3 MPa (self-healing) | Thermal and solvent resistivity, self-healable, recyclable | Bio-based curing agent | [15] |
| PBS 15 oligomer | 240 h at 80 °C | Biodegradable, Self-healable | - | [114] | |
| SH-BDA-EP 16 | 25 h at 50 °C under the acidic condition | Bio-derived, fire-resistance, degradable | Thermosets | [115] | |
| DACNF 17-PI 18-LCD 19 | 15 min at 80 °C under 3 MPa | Self-healable, Recyclable, water barrier property, solvent resistance | Bio-degradable plastic | [116] | |
| FDP 20/AMDP 21 | pH = 12.1 (association), pH = 7.0 (dissociation) | Self-assemble, pH-sensitive, guest molecule loading | Bolaform superamphiphile | [117] | |
| Polybutadiene network | 10 min at 80 °C (Recycle, Self-healing) | triple-shape memory effect, plasticity, recyclable, self-healable | multi-shape memory polymer | [118] | |
| Betulin-based polyurethane | 40 min at 80 °C (Self-repairing) | Self-healable, reprocessable, degradable | editable shape memory polymer | [179] | |
| Benzoxazine monomer | 5 min at 100 °C (Reshaping), 30 min at 140 °C under 2 MPa (Reprocessing) | Shape memory, reprocessable, degradable | shape memory polymer | [180] | |
| Diels-Alder Bond | Crosslinked Polyurethanes | 3 h at 160 °C, followed by 20 h at 70 °C | Recyclable | - | [140] |
| DOPO 22-FU 23-BMI 24 | 1 h at 150 °C, followed by 12 h at 95 °C. | Self-healable, flame retardancy | - | [146] | |
| DOPO-TMP 25-EP 26 | 30 min at 180 °C | Self-healable, flame retardancy, recyclable | - | [147] | |
| Urushiol-based coating | 30 min at 125 °C | Self-healable, hydrophobicity, hardness | Self-healing coatings | [150] | |
| Photothermally activated nanofibers | NIR light for 15 min | Drug release controllable | Drug delivery | [153] | |
| Lignin-derived elastomers | 20 min at 120 °C | Self-healable, thermal stability, shape memory | Hot-melt adhesives | [181] | |
| PEG crosslinked by DABBF 27 | 40 °C | Recyclable, plasticity, self-healable | - | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, S.; Nam, Y.; Nguyen, M.T.N.; Han, J.-H.; Lee, J.S. Dynamic Covalent Bond-Based Polymer Chains Operating Reversibly with Temperature Changes. Molecules 2024, 29, 3261. https://doi.org/10.3390/molecules29143261
Roh S, Nam Y, Nguyen MTN, Han J-H, Lee JS. Dynamic Covalent Bond-Based Polymer Chains Operating Reversibly with Temperature Changes. Molecules. 2024; 29(14):3261. https://doi.org/10.3390/molecules29143261
Chicago/Turabian StyleRoh, Sojeong, Yeonjeong Nam, My Thi Ngoc Nguyen, Jae-Hee Han, and Jun Seop Lee. 2024. "Dynamic Covalent Bond-Based Polymer Chains Operating Reversibly with Temperature Changes" Molecules 29, no. 14: 3261. https://doi.org/10.3390/molecules29143261
APA StyleRoh, S., Nam, Y., Nguyen, M. T. N., Han, J.-H., & Lee, J. S. (2024). Dynamic Covalent Bond-Based Polymer Chains Operating Reversibly with Temperature Changes. Molecules, 29(14), 3261. https://doi.org/10.3390/molecules29143261








