Inhibitory Effect of Selected Guaianolide and Germacranolide Sesquiterpene Lactones on Nitric Oxide Production
Abstract
:1. Introduction
2. Results
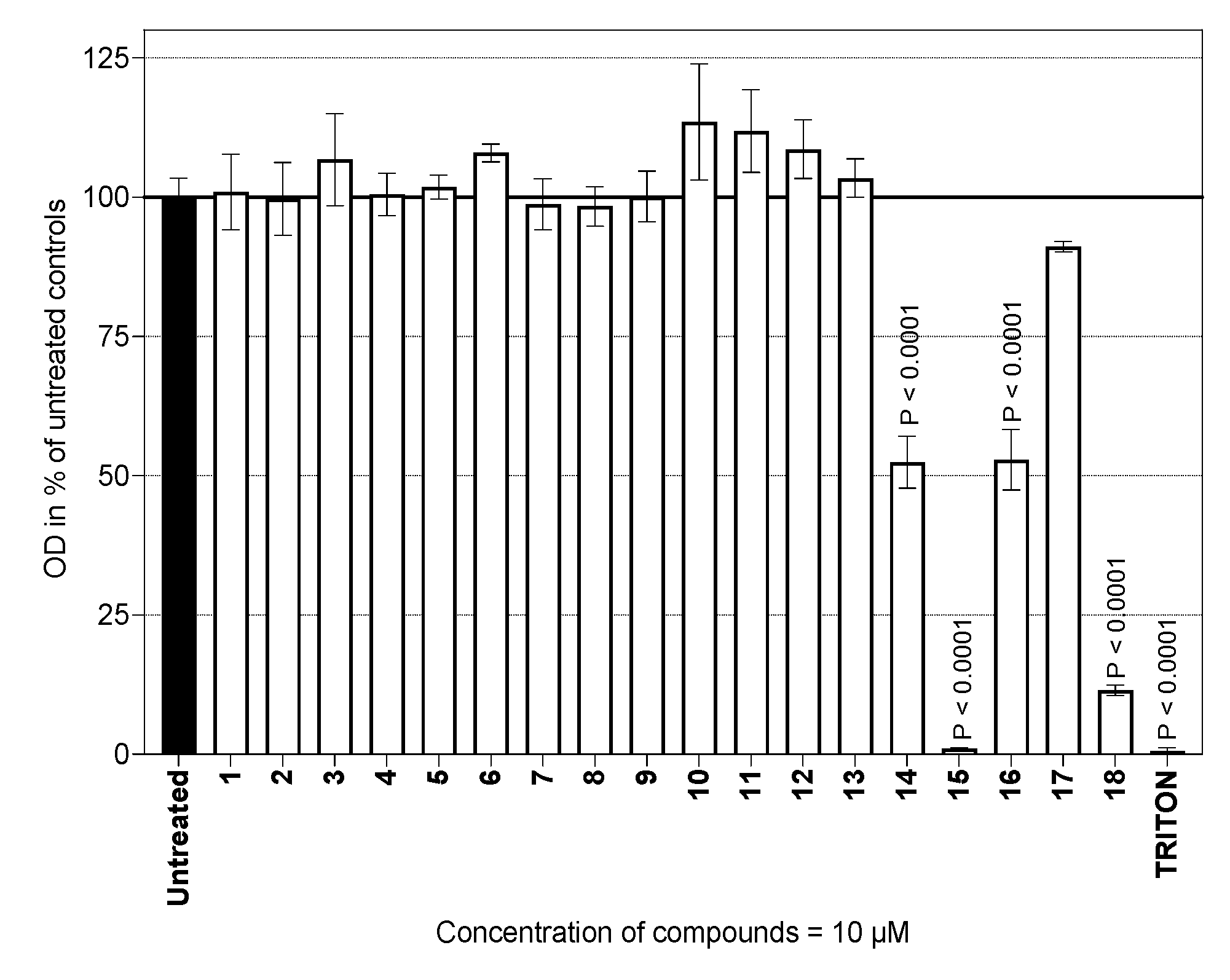
2.1. Determination of Viability
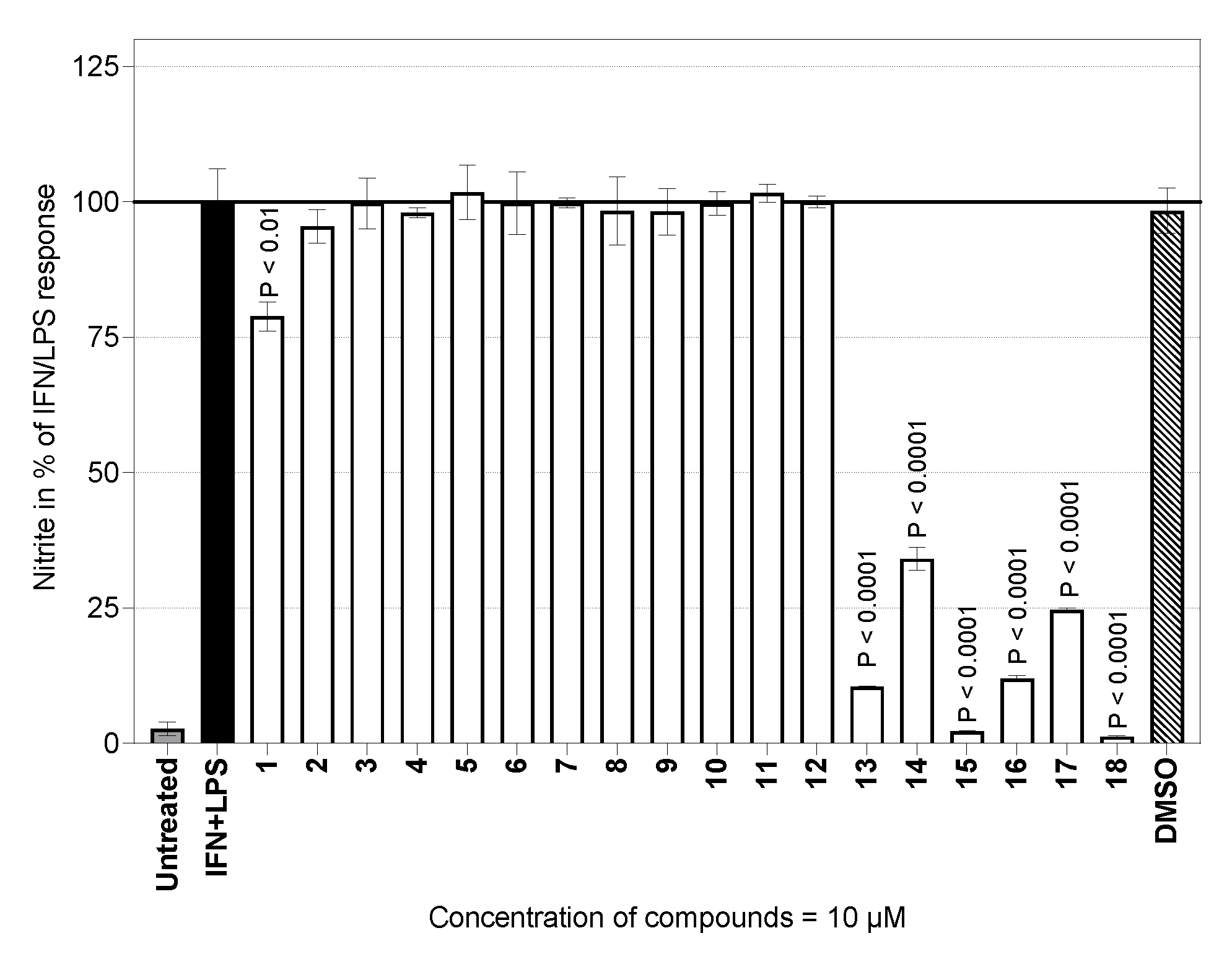
2.2. Nitric Oxide Production
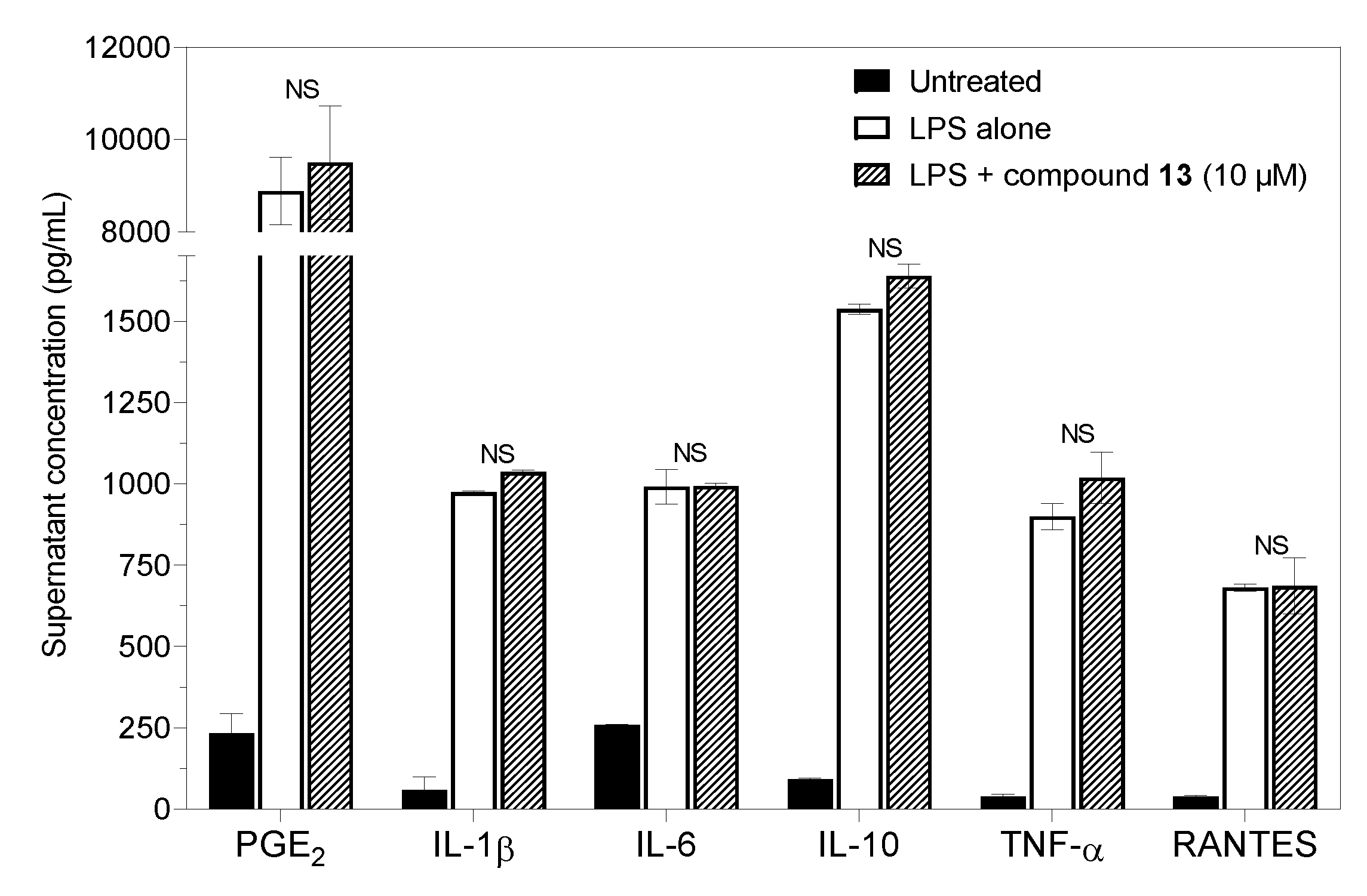
2.3. Prostaglandin E2 Production and Cytokine Secretion
3. Experimental Section
3.1. Chemicals
General Methods
3.2. Biological Assays
3.2.1. Compounds
3.2.2. Animals and Cell Culture
3.2.3. Cytotoxicity Assay
3.2.4. Nitric Oxide Assay
3.2.5. Prostaglandin E2 and Cytokine Assays
3.2.6. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, N.H.; Olivier, E.J.; Fischer, H.D. The biogenesis and chemistry of sesquiterpene lactones. Prog. Chem. Org. Nat. Prod. 1979, 38, 47–390. [Google Scholar]
- Fischer, N.H. Sesquiterpene Lactones: Biogenesis and Biomimetic Transformations. In Biochemistry of the Mevalonic Acid Pathway to Terpenoids. Recent Advances in Phytochemistry; Towers, G.H.N., Stafford, H.A., Eds.; Springer: Boston, MA, USA, 1990; Volume 24. [Google Scholar] [CrossRef]
- Picman, A.K. Biological activities of sesquiterpene lactones. Biochem. Syst. Ecol. 1986, 14, 255–281. [Google Scholar] [CrossRef]
- Holub, M.; Buděšínský, M. Sesquiterpene lactones of the Umbelliferae. Phytochemistry 1986, 25, 2015–2026. [Google Scholar] [CrossRef]
- Samek, Z.; Harmatha, J. Use of structural changes for stereochemical assignments of natural α-exomethylene γ-lactones of the germacra-1(10), 4-dienolide type on the basis of allylic and vicinal couplings of bridgehead protons. Hydrogenation of endocyclic double bonds. Collect. Czech Chem. Commun. 1978, 43, 2779–2799. [Google Scholar] [CrossRef]
- Holub, M.; Toman, J.; Herout, V. The phylogenetic relationships of the Asteraceae and Apiaceae based on phytochemical characters. Biochem. Syst. Ecol. 1987, 15, 321–326. [Google Scholar] [CrossRef]
- Herout, V. A chemical compound as a taxonomic character. In Chemistry in Botenical Classification, Proceedings of the Nobel Symposium, Ödeshög, Sweden, 20–25 August 1973; Bendz, G., Santesson, J., Eds.; Almquist & Wiksell: Uppsala, Sweden, 1973; pp. 55–62. [Google Scholar]
- Harmatha, J.; Nawrot, J. Comparison of the feeding deterrent activity of some sesquiterpene lactones and a lignan lactone towards selected insect storage pests. Biochem. Syst. Ecol. 1984, 12, 95–98. [Google Scholar] [CrossRef]
- Nawrot, J.; Harmatha, J.; Novotny, L. Insect feeding deterrent activity of bisabolangelone and of some sesquiterpenes of eremophilane type. Biochem. Syst. Ecol. 1984, 12, 99–101. [Google Scholar] [CrossRef]
- Nawrot, J.; Bloszyk, B.; Harmatha, J.; Novotny, L.; Drozdz, B. Action of antifeedants of plant origin on beetles infesting stored products. Acta Entomol. Bohemoslov. 1986, 83, 327–335. [Google Scholar]
- Nawrot, J.; Harmatha, J. Phytochemical feeding deterrents for stored product insect pests. Phytochem. Rev. 2012, 11, 543–566. [Google Scholar] [CrossRef]
- Drew, D.P.; Krichau, N.; Reichwald, K.; Simonsen, H.T. Guaianolides in apiaceae: Perspectives on pharmacology and biosynthesis. Phytochem. Rev. 2009, 8, 581–599. [Google Scholar] [CrossRef]
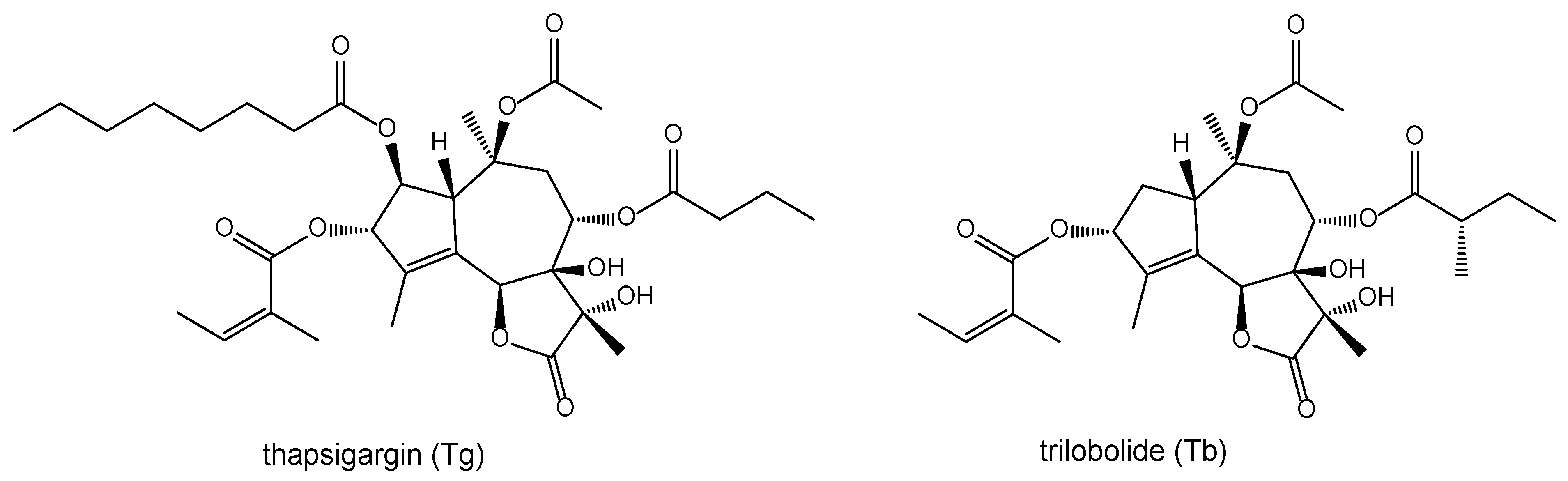
- Christensen, S.B.; Andersen, A.; Smitt, U.W. Sesquiterpenoids from Thapsia species and medicinal chemistry of the thapsigargins. Prog. Chem. Org. Nat. Prod. 1997, 71, 129–167. [Google Scholar]
- Kmoníčková, E.; Melkusová, P.; Harmatha, J.; Vokáč, K.; Farghali, H.; Zídek, Z. Inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPase thapsigargin stimulates production of nitric oxide and secretion of interferon-gamma. Eur. J. Pharmacol. 2008, 588, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kmoníčková, E.; Harmatha, J.; Vokáč, K.; Kostecká, P.; Farghali, H.; Zídek, Z. Sesquiterpene lactone trilobolide activates production of interferon-gamma and nitric oxide. Fitoterapia 2010, 81, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Harmatha, J.; Buděšínský, M.; Vokáč, K.; Kostecká, P.; Kmoníčková, E.; Zídek, Z. Trilobolide and related sesquiterpene lactones from Laser trilobum possessing immunobiological properties. Fitoterapia 2013, 89, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Harmatha, J.; Vokáč, K.; Buděšínský, M.; Zídek, Z.; Kmoníčková, E. Immunobiological properties of sesquiterpene lactones obtained by chemically transformed structural modifications of trilobolide. Fitoterapia 2015, 107, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Harmatha, J.; Buděšínský, M.; Jurášek, M.; Zimmermann, T.; Drašar, P.; Zídek, Z.; Kmoníčková, E.; Vejvodová, L. Structural modification of trilobolide for upgrading its immunobiological properties and reducing its cytotoxic action. Fitoterapia 2019, 134, 88–95. [Google Scholar] [CrossRef]
- Rimpelová, S.; Jurášek, M.; Peterková, L.; Bejček, J.; Spiwok, V.; Majdl, M.; Jirásko, M.; Buděšínský, M.; Harmatha, J.; Kmoníčková, E.; et al. Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica. Belstein J. Org. Chem. 2019, 15, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Harmatha, J.; Kmoníčková, E.; Zídek, Z. Immunopharmacological potential of the leading chemical constituents from Leuzea carthamoides. Planta Med. 2009, 75, PA5. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakashima, S.; Nakamura, S.; Hattori, Y.; Ando, T.; Matsuda, H. Inhibitory effects of cynaropicrin and related sesquiterpene lactones from leaves of artichoke (Cynara scolymus L.) on induction of iNOS in RAW264.7 cells and its high-affinity proteins. J. Nat. Med. 2021, 75, 381–392. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, A.R.; Jung, J.H.; Chun, T.; Rhee, M.H.; Yoo, E.S. Cytotoxic and pro-apoptotic activities of cynaropicrin, a sesquiterpene lactone, on the viability of leukocyte cancer cell lines. Eur. J. Pharmacol. 2004, 492, 85–94. [Google Scholar] [CrossRef]
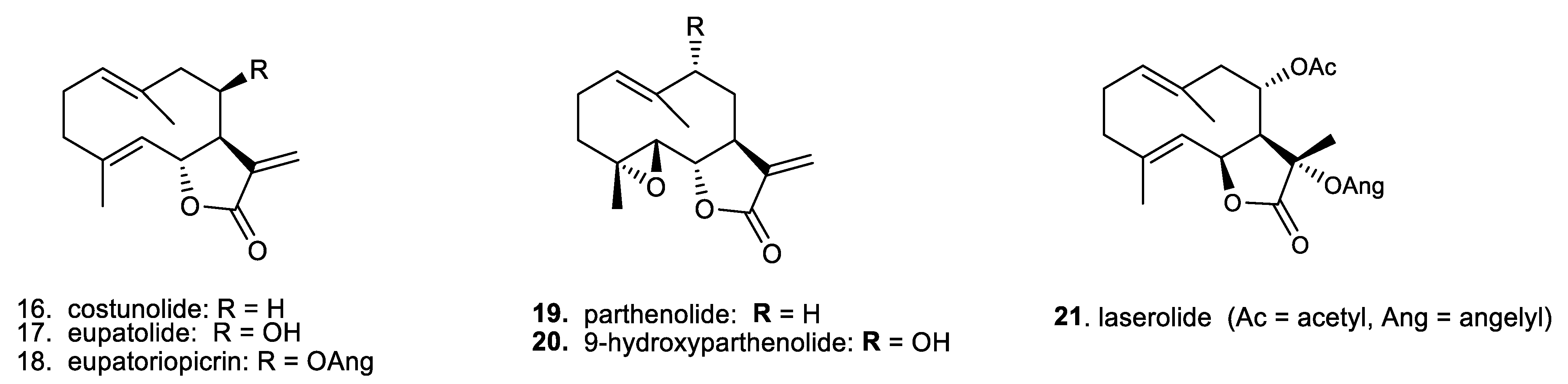
- Pae, H.O.; Jeong, G.S.; Kim, H.S.; Woo, W.H.; Rhew, H.Y.; Kim, H.S.; Sohn, D.H.; Kim, Y.C.; Chung, H.T. Costunolide inhibits production of tumor necrosis factor-alpha and interleukin-6 by inducing heme oxygenase-1 in RAW264.7 macrophages. Inflamm. Res. 2007, 56, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.G.; Do, T.T.; Nguyen, T.N.; Do, T.V.H.; Dong, N.P.; Vu, M.T. Chemical Constituents of Eupatorium japonicum and Anti-Inflammatory, Cytotoxic, and Apoptotic Activities of Eupatoriopicrin on Cancer Stem Cells. Evid. Based Complement. Altern. Med. 2021, 2021, 6610347. [Google Scholar] [CrossRef] [PubMed]
- Michalak, B.; Piwowarski, J.P.; Granica, S.; Waltenberger, B.; Atanasov, A.G.; Khan, S.Y.; Breuss, J.M.; Uhrin, P.; Żyżyńska-Granica, B.; Stojakowska, A.; et al. Eupatoriopicrin Inhibits Pro-inflammatory Functions of Neutrophils via Suppression of IL-8 and TNF-alpha Production and p38 and ERK 1/2 MAP Kinases. J. Nat. Prod. 2019, 82, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Elso, O.G.; Bivona, A.E.; Sanchez, A.A.; Cerny, N.; Fabian, L.; Morales, C.; Catalán, C.A.N.; Malchiodi, E.L.; Cazorla, S.I.; Sülsen, V.P. Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species. Molecules 2020, 25, 2014. [Google Scholar] [CrossRef] [PubMed]
- Dirsch, V.M.; Stuppner, H.; Ellmerer-Müller, E.P.; Vollmar, A.M. Structural requirements of sesquiterpene lactones to inhibit LPS-induced nitric oxide synthesis in RAW 264.7 macrophages. Bioorg. Med. Chem. 2000, 8, 2747–2753. [Google Scholar] [CrossRef]
- Jurášek, M.; Džubák, P.; Rimpelová, S.; Sedlák, D.; Konečný, P.; Frydrych, I.; Gurská, S.; Hajdúch, M.; Bogdanová, K.; Kolář, M.; et al. Trilobolide-steroid hybrids: Synthesis, cytotoxic and antimycobacterial activity. Steroids 2017, 117, 97–104. [Google Scholar] [CrossRef]
- Zídek, Z.; Harmatha, J.; Vokáč, K.; Kmoníčková, E. Immunosuppressive effects of sesquiterpene lactones from Laser trilobum (L.). Borkh. Planta Med. 2009, 75, PA10. [Google Scholar] [CrossRef]
- Kisiel, W.; Jakupoic, J.; Huneck, S. Guaianolides from Crepis crocea. Phytochemistry 1994, 35, 269–270. [Google Scholar] [CrossRef]
- Rychlewska, U.; Kisiel, W. Structure of the Naturally Occuring Sesquiterpene Lactone 8-epiisolipidiol. Acta Cryst. 1991, C47, 129–132. [Google Scholar]
- Kisiel, W. NMR studies of isomeric dehydrocostuslactone derivatives and structure revision of dentatins A–C. Phytochem. Lett. 2011, 4, 311–314. [Google Scholar] [CrossRef]
- Kisiel, W.; Michalska, K. Sesquiterpenoids and phenolics from Taraxacum hondoense. Fitoterapia 2005, 76, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucine-like guaianilides in mice. J. Ethnopharm 2006, 107, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, W.; Zielinska, K. Guaianolides from Cichorium intybus and structure revision of Cichorium sesquiterpene lactones. Phytochemistry 2001, 57, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Sesquiterpene lactones in hairy root culture of Cichorium intibus. Z. Naturfirschung C 2002, 57, 993–997. [Google Scholar]
- Kisiel, W.; Kohlmünzer, S. Ixerin F from Crepis biennis. Planta Med. 1987, 53, 390. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Kisiel, W. Sesquiterpene lactones from Taraxacum obovatum. Planta Med. 2003, 69, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, W.; Barszcz, B. Sesquiterpene lactone glycosides from Crepis pyrenaica. Phytochemistry 1995, 39, 1395–1397. [Google Scholar] [CrossRef]
- Kisiel, W.; Barszcz, B. Further sesquiterpenoids and phenolics from Taraxacum officinale. Fitoterapia 2000, 71, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Cis, J.; Nowak, G.; Kisiel, W. Antifeedant properties and chemotaxonomic implications of sesquiterpene lactones and syringin from Rhaponticum pulchrum. Biochem. Syst. Ecol. 2006, 34, 862–867. [Google Scholar] [CrossRef]
- Malarz, J.; Stojakowska, A.; Dohnal, B.; Kisiel, W. Helenalin acetate in in vitro propagated plants of Arnica montana. Planta Med. 1993, 59, 51–53. [Google Scholar] [CrossRef]
- Drozdz, B.; Grabarczyk, H.; Samek, Z.; Holub, M.; Herout, V.; Šorm, F. Sesquiterpenic lactones from Eupatorium cannabinum L. Revision of the structure of eupatoriopicrin. Collect. Czech Chem. Commun. 1972, 37, 1546–1554. [Google Scholar] [CrossRef]
- Harmatha, J.; Samek, Z. Diethylamine addition to natural sesquiterpenic α-exomethylene-γ-lactones and its use for chemical transformations of these compounds. Collect. Czech Chem. Commun. 1982, 47, 2779–2785. [Google Scholar] [CrossRef]
- Buděšínský, M.; Šaman, D.; Nowak, G.; Drozdz, H.M. 9α-Hydroxyparthenolide from Zoegea baldschuanica C. WINKL., and its absolute configuration. Collect. Czech Chem. Commun. 1984, 49, 637–641. [Google Scholar] [CrossRef]
- Holub, M.; Buděšínský, M.; Smítalová, Z.; Šaman, D.; Rychlewska, U. Structure of laserolide and isolaserolide: Revision of constitution and determination of relative and absolute configuration. Collect. Czech Chem. Commun. 1985, 50, 1878–1887. [Google Scholar] [CrossRef]
- Valente, A.H.; de Roode, M.; Ernst, M.; Peña-Espinoza, M.; Bornancin, L.; Bonde, C.S.; Martínez-Valladares, M.; Ramünke, S.; Krücken, J.; Simonsen, H.T.; et al. Identification of compounds responsible for the anthelmintic effects of chicory (Cichorium intybus) by molecular networking and bio-guided fractionation. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Schmidt, B.M.; Ilic, N.; Poulev, A.; Dey, M.; Kurmukov, A.G.; Raskin, I. Anti-inflammatory Effects of a Sesquiterpene Lactone Extract from Chicory (Cichorium intybus L.) Roots. Nat. Prod. Commun. 2007, 2, 717–722. Available online: https://journals.sagepub.com/doi/abs/10.1177/1934578X0700200702 (accessed on 14 February 2024). [CrossRef]
- Cankar, K.; Hakkert, J.C.; Sevenier, R.; Papastolopoulou, C.; Schipper, B.; Baixinho, J.P.; Fernández, N.; Matos, M.S.; Serra, A.T.; Santos, C.N.; et al. Lactucin Synthase Inactivation Boosts the Accumulation of Anti-inflammatory 8-Deoxylactucin and Its Derivatives in Chicory (Cichorium intybus L.). J. Agric. Food Chem. 2023, 71, 6061–6072. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Ding, L.F.; Tu, W.C.; Yang, H.; Su, J.; Peng, L.Y.; Li, Y.; Zhao, Q.S. Bioactive sesquiterpenoids from the flowers of Inula japonica. Phytochemistry 2016, 129, 68–76. [Google Scholar] [CrossRef]
- Lee, J.; Tae, N.; Lee, J.J.; Kim, T.; Lee, J.H. Eupatolide inhibits lipopolysaccharide-induced COX-2 and iNOS expression in RAW264.7 cells by inducing proteasomal degradation of TRAF6. Eur. J. Pharmacol. 2010, 636, 173–180. [Google Scholar] [CrossRef]
- Jin, H.Z.; Lee, D.; Lee, J.H.; Lee, K.; Hong, Y.S.; Choung, D.H.; Kim, Y.H.; Lee, J.J. New sesquiterpene dimers from Inula britannica inhibit NF-kappaB activation and NO and TNF-alpha production in LPS-stimulated RAW264.7 cells. Planta Med. 2006, 72, 40–45. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, P.; Bennett, S.; Charlesworth, O.; Tan, R.; Peng, X.; Gu, Q.; Kujan, O.; Xu, J. The therapeutic effect and mechanism of parthenolide in skeletal disease, cancers, and cytokine storm. Front. Pharmacol. 2023, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Cavin, C.; Delannoy, M.; Malnoe, A.; Debefve, E.; Touáché, A.; Courtois, D.; Schilter, B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun. 2005, 327, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Baixinho, J.P.; Anastácio, J.D.; Ivasiv, V.; Cankar, K.; Bosch, D.; Menezes, R.; de Roode, M.; Santos, C.N.D.; Matias, A.A.; Fernández, N. Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium intybus L. Roots. Molecules 2021, 26, 2583. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J. Toxic activities of sesquiterpene lactones: Structural and biochemical aspects. Curr. Org. Chem. 1999, 3, 577–608. [Google Scholar]

| Compound | IC50 (µM) | 95% Confidence Interval | References * |
|---|---|---|---|
| 1. 8-epiisoamberboin | 23.76 | 16.72–33.76 | [30] |
| 2. 8-epiisolippidiol | >100 | – | [31,32] |
| 3. deacetylmatricarin | >100 | – | [33] |
| 4. 11β,13-dihydrolactucin | >100 | – | [34] |
| 5. 4,8-diepiisolippidiol glucoside | >100 | – | [34] |
| 6. deacetylmatricarin glucoside | >100 | – | [33] |
| 7. crepidiaside B | >100 | – | [35,36] |
| 8. ixerin F | >100 | – | [37] |
| 9. 11β-hydroxyleukodin glucoside | >100 | – | [38] |
| 10. 11β-hydroxydeacetylmatricarin glucoside | >100 | – | [33] |
| 11. 11,13-dehydro-8-epiisolippidiol glucoside | >100 | – | [39] |
| 12. ixerin D | >100 | – | [40] |
| 13. 8-deoxylactucin | 2.81 | 2.08–3.79 | [34,35] |
| 14. cynaropicrin | 9.87 | 3.99–24.39 | [20,41] |
| 15. helenalin | 0.74 | 0.45–1.22 | [29,42] |
| 16. costunolide | 3.20 | 2.51–4.08 | [43] |
| 17. eupatolide | 4.38 | 2.85–6.73 | [43,44] |
| 18. eupatoriopicrin | 1.65 | 0.76–3.59 | [43,44] |
| 19. parthenolide | 2.79 | 1.84–4.22 | [45] |
| 20. 9-hydroxyparthenolide | 5.64 | 3.09–10.27 | [45] |
| 21. laserolide | 90.16 | 1.07–2.61 | [16,46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmatha, J.; Zídek, Z.; Kmoníčková, E. Inhibitory Effect of Selected Guaianolide and Germacranolide Sesquiterpene Lactones on Nitric Oxide Production. Molecules 2024, 29, 3289. https://doi.org/10.3390/molecules29143289
Harmatha J, Zídek Z, Kmoníčková E. Inhibitory Effect of Selected Guaianolide and Germacranolide Sesquiterpene Lactones on Nitric Oxide Production. Molecules. 2024; 29(14):3289. https://doi.org/10.3390/molecules29143289
Chicago/Turabian StyleHarmatha, Juraj, Zdeněk Zídek, and Eva Kmoníčková. 2024. "Inhibitory Effect of Selected Guaianolide and Germacranolide Sesquiterpene Lactones on Nitric Oxide Production" Molecules 29, no. 14: 3289. https://doi.org/10.3390/molecules29143289
APA StyleHarmatha, J., Zídek, Z., & Kmoníčková, E. (2024). Inhibitory Effect of Selected Guaianolide and Germacranolide Sesquiterpene Lactones on Nitric Oxide Production. Molecules, 29(14), 3289. https://doi.org/10.3390/molecules29143289







