Principal Bioactive Properties of Oleanolic Acid, Its Derivatives, and Analogues
Abstract
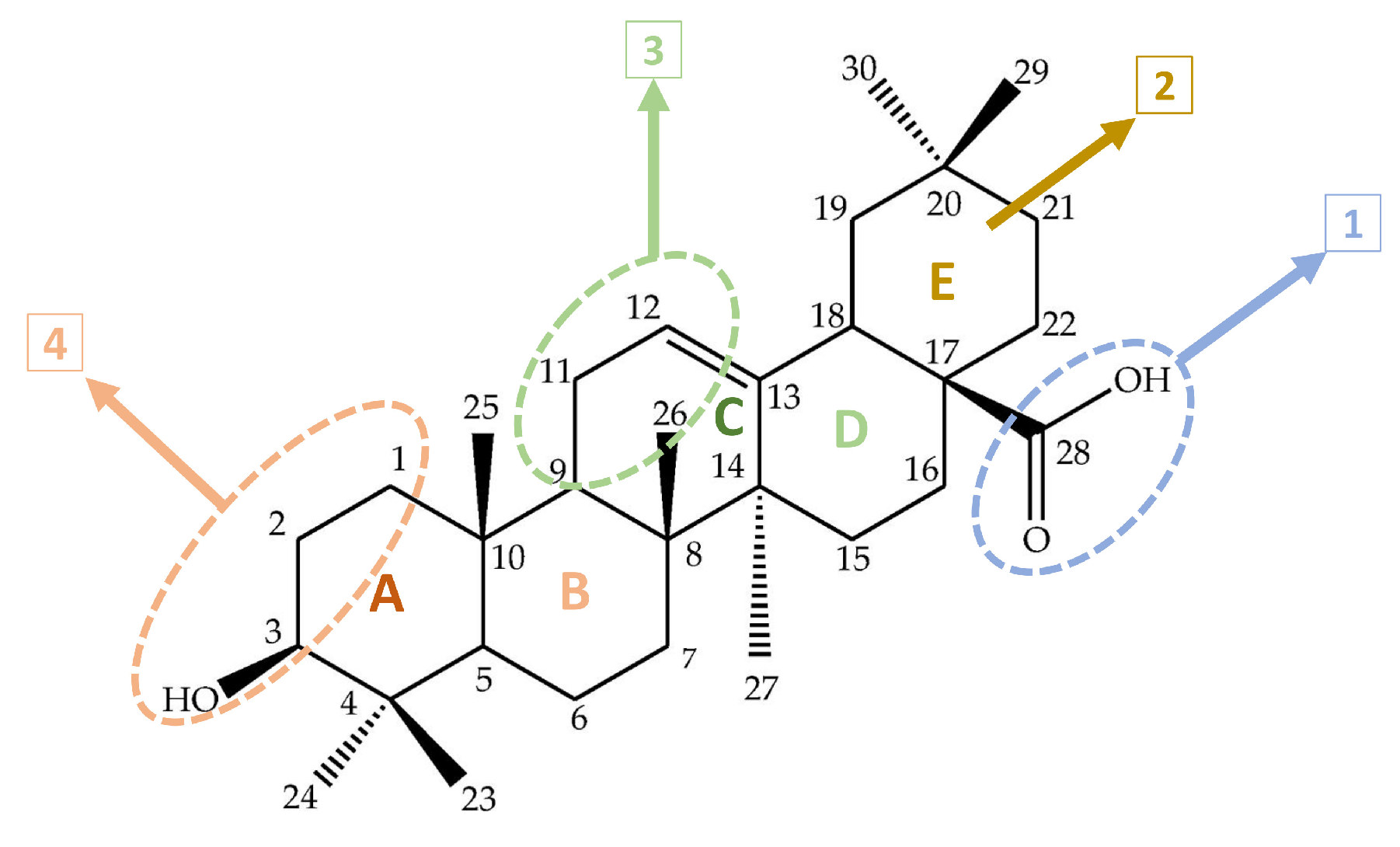
1. Introduction
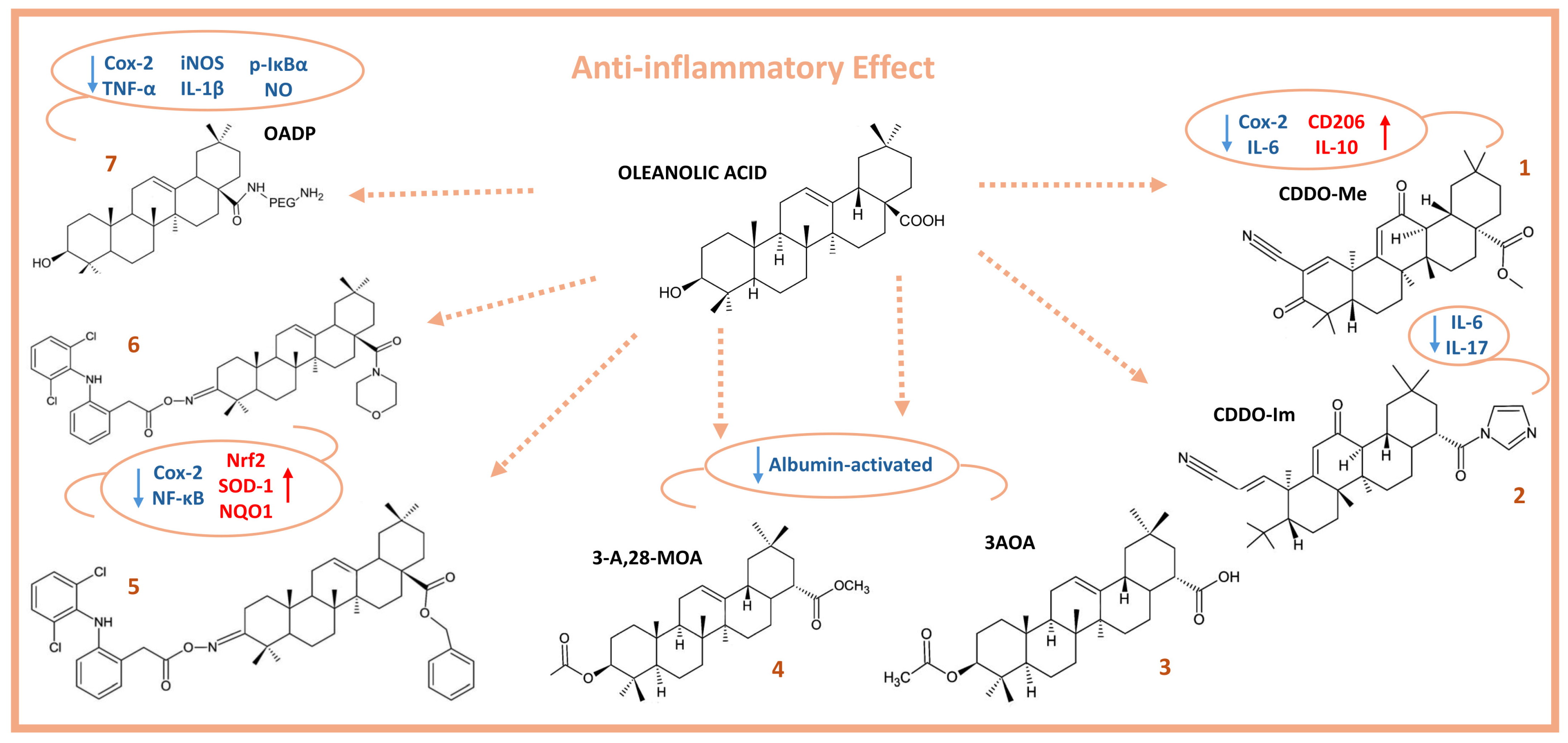
2. Anti-Inflammatory Activity of Oleanolic Acid
3. Antibacterial Activity of Oleanolic Acid
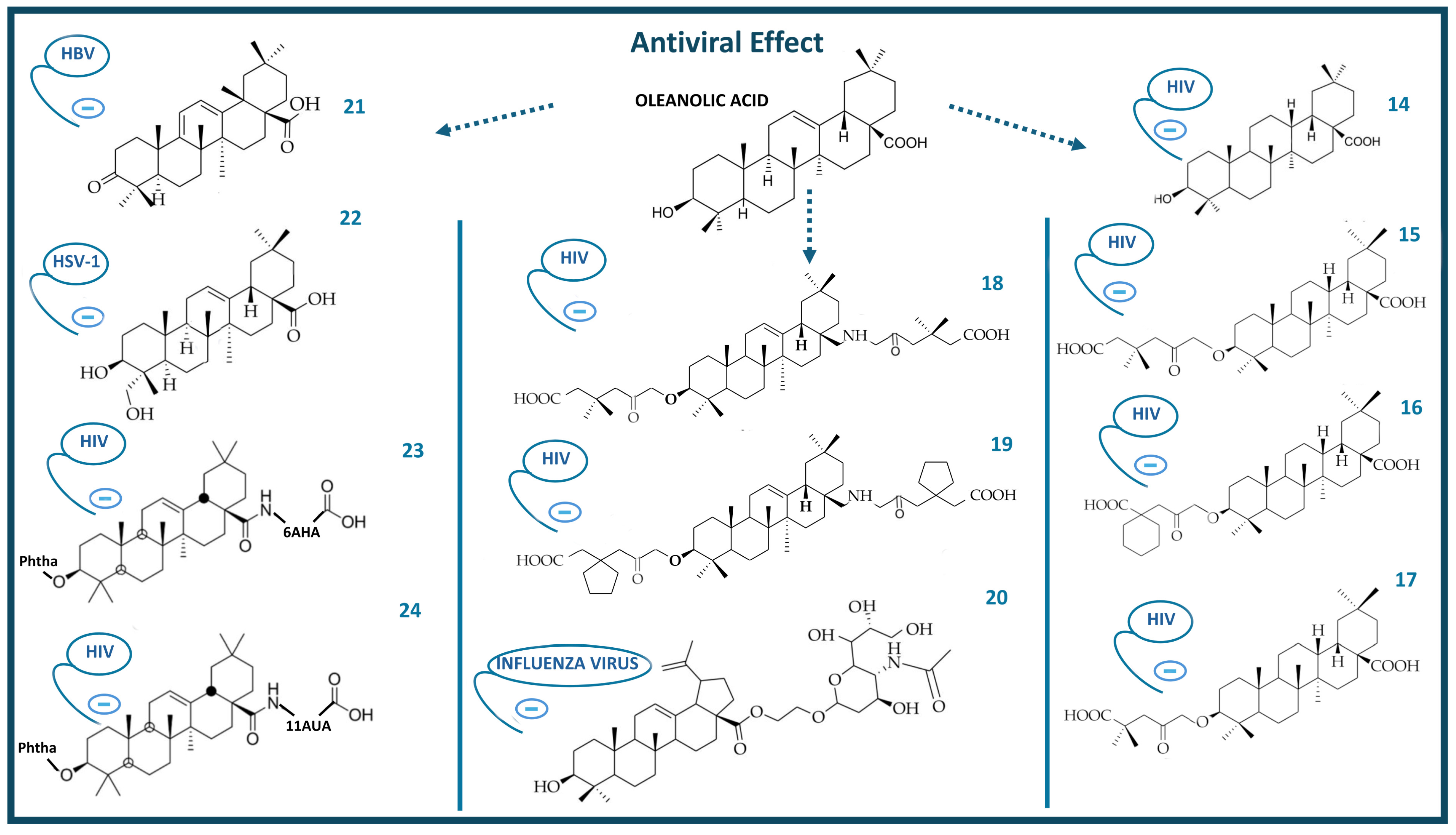
4. Antiviral Activity of Oleanolic Acid
5. Antidiabetic Activity of Oleanolic Acid
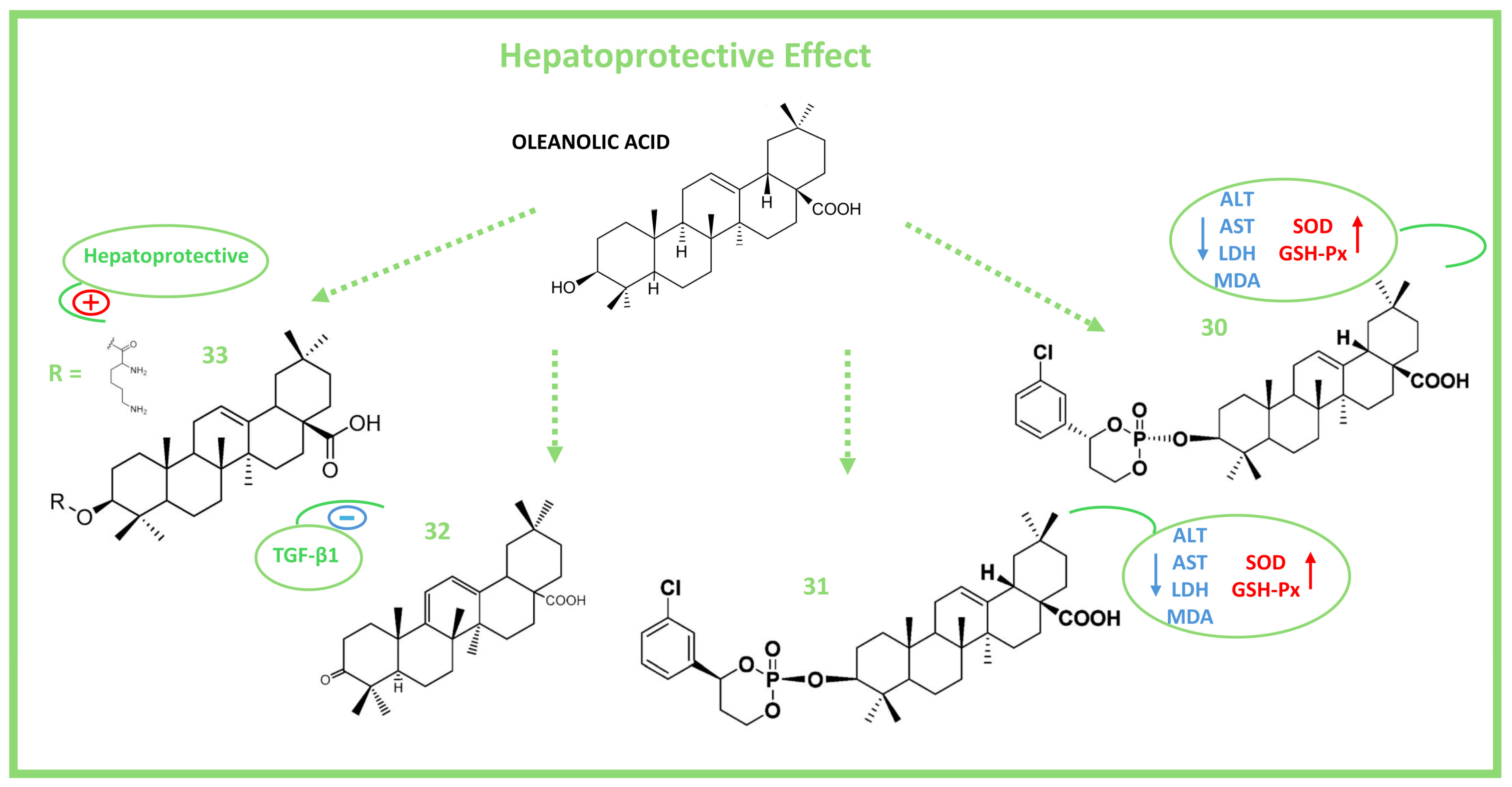
6. Hepatoprotective Activity of Oleanolic Acid
7. Neuroprotective Activity of Oleanolic Acid
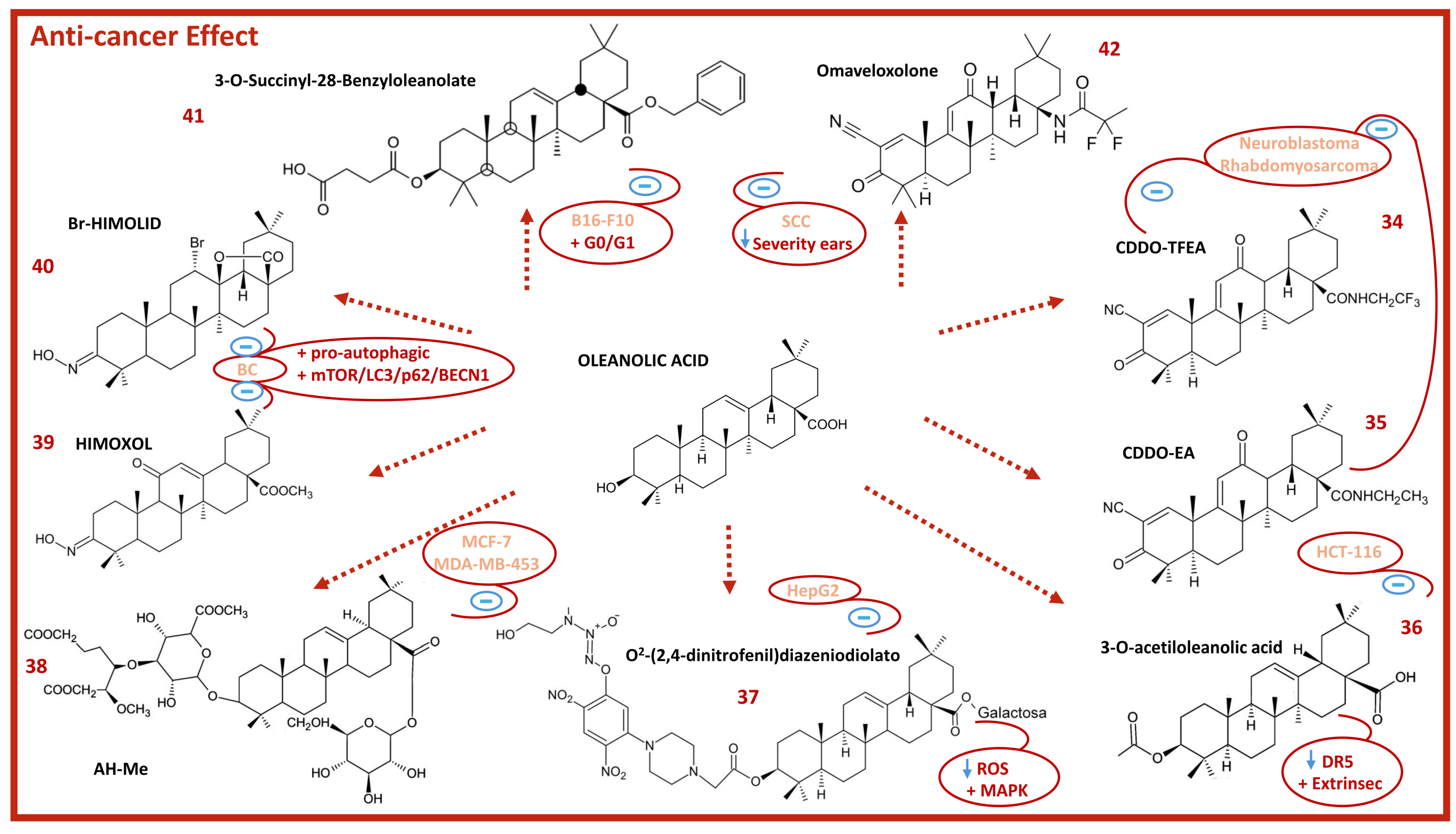
8. Anticancer Activity of Oleanolic Acid
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-A,28-MOA | 3-Acetoxy, 28-methylester oleanolic acid |
| 3-AOA | 3-Acetoxyoleanolic acid |
| 5-LOX | 5-Lipoxygenase |
| 6-OHDA | 6-Hydroxydopamine |
| AH-Me | Methyl ester achyranthoside H |
| AKT | Alpha serine/threonine-protein kinase |
| ALD | Alcoholic liver disease |
| ALT | Alanine aminotransferase |
| AMP | Antimicrobial peptides |
| AMR | Antimicrobial resistance |
| AST | Aspartate aminotransferase |
| ATP | Adenosine triphosphate |
| Bax | Bcl-2 associated X protein |
| Bak | Bcl-2 homologous antagonist killer |
| BC | Breast cancer |
| Bcl2 | B-cell lymphoma 2 |
| BECN1 | Beclin-1 |
| BBB | Blood–brain barrier |
| Br-HIMOLID | 12α-Bromo-3-hydroxyimonoolean-28→13-olide |
| cAMP | Cyclic adenosine monophosphate |
| CAT | Chloramphenicol acetyltransferase |
| CD206 | Cluster of differentiation 206 |
| CDDO | 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic |
| CDDO-Im | CDDO imidazolide |
| CDDO-EA | CDDO ethyl amide |
| CDDO-Me | CDDO methyl ester |
| CDDO-TFEA | CDDO-trifluoethyl amide |
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| CYP | Cytochrome |
| DCX | Doublecortin |
| DKS26 | DihydroOA methyl ester |
| DR5 | Death receptor 5 |
| EAE | Experimental autoimmune encephalomyelitis |
| EAM | Experimental autoimmune myositis |
| EGFR | Epidermal growth factor receptor |
| EPO | Erythropoietin |
| Erb-B2 | Receptor tyrosine kinase 2 |
| Erk1/2 | Extracellular-signal-regulated kinase 1/2 |
| EtOAc | Ethyl acetate |
| FA | Friedreich ataxia |
| FAK | Focal adhesion kinase |
| FDA | Food and drug administration |
| FOXO-1 | Forkhead-box-O1 |
| FOXP3 | Forkhead box P3 |
| G6Pase | Glucose 6-phosphatase |
| GC-LC | Glutamate cysteine ligase proteins |
| GC-LM | Glutamate-cysteine ligase |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT | Glucose transporter |
| GPX-1 | Glutathione peroxidase 1 |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSH-PX/GPx | Glutathione peroxidase. |
| GSK-3β | Glycogen synthase kinase-3 beta |
| Gtase | Glucosyltransferase |
| HAS | Human serum albumin |
| HAT1 | Histone acetyltransferase 1 |
| HbA1c | Hemoglobin A1c |
| HBeAg | Hepatitis B viral protein |
| HBsAg | Hepatitis B surface antigen |
| HCC | Hepatocellular carcinoma |
| HDAC | Histone deacetylase |
| HER2 | Human epidermal growth factor receptor 2 |
| HFD | High-fat diet |
| HIMOXOL | Methyl 3-hydroxyimino-11-oxoolean-12-en-28-oate |
| HIV | Human immunodeficiency virus |
| HO-1 | Heme oxygenase-1 |
| HSV | Herpes simplex virus |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IκBα | NF-κB inhibitor α |
| IKK | IkappaB kinase |
| JAK | Janus kinase |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| MA | Maslinic acid |
| MAP-2 | Microtubule-associated protein-2 |
| MAPK | Mitogen-activated protein kinases |
| MCAO | Middle cerebral artery occlusion |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| MDCK | Madin–Darby canine kidney |
| MIC | Minimum inhibitory concentration |
| MIC50 | MIC value at which growth was inhibited in 50% |
| MIP-1α | Macrophage inflammatory protein-1alpha |
| MMP | Mitochondrial membrane potential |
| MRSA | Methicillin-resistant staphylococcus aureus |
| MVD | Microvessel density |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| NAFLD | Non-alcoholic fatty liver disease |
| NDD | Neurodegenerative disease |
| NF-ĸB | Nuclear factor-kappa B |
| nH JEB | Non-Herlitz, junctional epidermolysis bullosa |
| NLRP3 | NOD-like receptor family, pyrin domain containing 3 |
| NOX-4 | NADPH oxidase 4 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSCs | Neural stem cells |
| OA | Oleanolic acid |
| OAA | Oleanolic acid acetate |
| OADP | Diamine-PEGylated oleanolic acid |
| OA-HDA | Oleanolic acid-hexane-1,6-diamine |
| OAO | Oleanolic acid oximes |
| p21 | Cyclin-dependent kinase inhibitory protein-1 |
| p53 | Tumor suppressor protein |
| p62/SQSTM1 | Sequestosome-1 |
| PMBn | Polymyxin B nonapeptide |
| PPAR-γ | Peroxisome proliferator activated receptor γ |
| PTP-1B | Protein tyrosine phosphatase 1B |
| RBCs | Red blood cells |
| RORγt | Retinoic-acid-related orphan receptors gamma |
| ROS | Reactive oxygen species |
| SALP | Src-like adaptor protein |
| SAR | Structure–activity relationship |
| SCC | Squamous cell carcinoma |
| SGK1 | Serine/threonine protein kinase 1 |
| SGOT | Serum glutamic-oxaloacetic transaminase |
| SGPT | Serum glutamic pyruvic transaminase |
| SOD | Superoxide dismutase type |
| Sox-2 | SRY-Box transcription factor 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
| TGF-β1 | Transforming growth factor β1 |
| TGR5 | Takeda G-protein-coupled receptor |
| THLE-2 | Transformed human liver epithelial-2 |
| TIM | Tumor-inducing macrophages |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
| TOR | Target of rapamycin |
| TRL | Triglyceride-rich lipoprotein |
References
- Petrovska, B.B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Boy, H.I.A.; Rutilla, A.J.H.; Santos, K.A.; Ty, A.M.T.; Yu, A.I.; Mahboob, T.; Tangpoong, J.; Nissapatorn, V. Recommended Medicinal Plants as Source of Natural Products: A Review. Digit. Chin. Med. 2018, 1, 131–142. [Google Scholar] [CrossRef]
- Díaz-Torres, R.D.C.; Alonso-Castro, A.J.; Carrillo-Inungaray, M.L.; Carranza-Alvarez, C. Chapter 6—Bioactive Compounds Obtained from Plants, Their Pharmacological Applications and Encapsulation. In Phytomedicine; Bhat, R.A., Hakeem, K.R., Dervash, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 181–205. ISBN 978-0-12-824109-7. [Google Scholar]
- Guclu, G.; Kelebek, H.; Selli, S. Chapter 26—Antioxidant Activity in Olive Oils. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 313–325. ISBN 978-0-12-819528-4. [Google Scholar]
- Papadaki, E.; Tsimidou, M.Z. Edible Oils from Olive Drupes as a Source of Bioactive Pentacyclic Triterpenes. Is There a Prospect for a Health Claim Authorization? Food Chem. 2022, 381, 132286. [Google Scholar] [CrossRef] [PubMed]
- García-González, A.; Espinosa-Cabello, J.M.; Cerrillo, I.; Montero-Romero, E.; Rivas-Melo, J.J.; Romero-Báez, A.; Jiménez-Andreu, M.D.; Ruíz-Trillo, C.A.; Rodríguez-Rodríguez, A.; Martínez-Ortega, A.J.; et al. Bioavailability and Systemic Transport of Oleanolic Acid in Humans, Formulated as a Functional Olive Oil. Food Funct. 2023, 14, 9681–9694. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evid. Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Cucu, A.-A.; Baci, G.-M.; Cucu, A.-B.; Dezsi, Ş.; Lujerdean, C.; Hegeduş, I.C.; Bobiş, O.; Moise, A.R.; Dezmirean, D.S. Calluna Vulgaris as a Valuable Source of Bioactive Compounds: Exploring Its Phytochemical Profile, Biological Activities and Apitherapeutic Potential. Plants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Vyas, N.; Argal, A. Isolation and Characterization of Oleanolic Acid from Roots of Lantana Camara. Asian J. Pharm. Clin. Res. 2014, 7, 189–191. [Google Scholar]
- Xia, E.-Q.; Wang, B.-W.; Xu, X.-R.; Zhu, L.; Song, Y.; Li, H.-B. Microwave-Assisted Extraction of Oleanolic Acid and Ursolic Acid from Ligustrum Lucidum Ait. Int. J. Mol. Sci. 2011, 12, 5319–5329. [Google Scholar] [CrossRef]
- Errichiello, F.; D’Amato, M.; Gambuti, A.; Moio, L.; Pastore, A.; AL-Hmadi, H.; Stornaiuolo, M.; Serino, E.; Taglialatela-Scafati, O.; Forino, M. Oleanolic Acid: A Promising Antidiabetic Metabolite Detected in Aglianico Grape Pomace. J. Funct. Foods 2023, 104, 105548. [Google Scholar] [CrossRef]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.; Catteau, L.; Boukricha, L.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.-P. Effect of Ursolic and Oleanolic Acids on Lipid Membranes: Studies on MRSA and Models of Membranes. Antibiotics 2021, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Ye, J.; Jia, J.; Wang, Z.; Jiang, Y.; Wang, Y.; Wang, Y.; Zheng, K.; Ren, Z. Viral UL8 Is Involved in the Antiviral Activity of Oleanolic Acid Against HSV-1 Infection. Front. Microbiol. 2021, 12, 689607. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y.-F.; Wu, Q.; Xu, S.-F.; Shi, F.-G.; Klaassen, C.D. Oleanolic Acid Reprograms the Liver to Protect against Hepatotoxicants, but Is Hepatotoxic at High Doses. Liver Int. 2019, 39, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Stępnik, K.; Kukula-Koch, W.; Plazinski, W.; Rybicka, M.; Gawel, K. Neuroprotective Properties of Oleanolic Acid—Computational-Driven Molecular Research Combined with In Vitro and In Vivo Experiments. Pharmaceuticals 2023, 16, 1234. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Leśków, A.; Szczuka, I.; Zaprutko, L.; Diakowska, D. The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines. Pharmaceuticals 2023, 16, 746. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, Y.; Lv, X.; Chen, W.; Yang, D.; Tuo, Q. The Effect and Mechanism of Oleanolic Acid in the Treatment of Metabolic Syndrome and Related Cardiovascular Diseases. Molecules 2024, 29, 758. [Google Scholar] [CrossRef] [PubMed]
- Jannus, F. Caracterización de la Capacidad Anticancerígena y Antiinflamatoria del OADP, Derivado Aminopegilado Semisintetico del Ácido Oleanólico y Aproximación a estas Actividades en n-Derivados del Diclofenaco. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2022. [Google Scholar]
- Baer-Dubowska, W.; Narożna, M.; Krajka-Kuźniak, V. Anti-Cancer Potential of Synthetic Oleanolic Acid Derivatives and Their Conjugates with NSAIDs. Molecules 2021, 26, 4957. [Google Scholar] [CrossRef] [PubMed]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Cano-Muñoz, M.; Martinez, A.; Lupiañez, J.A.; Parra, A. Oleanolic Acid Derivatives as Potential Inhibitors of HIV-1 Protease. J. Nat. Prod. 2019, 82, 2886–2896. [Google Scholar] [CrossRef]
- Yang, H.; Deng, M.; Jia, H.; Zhang, K.; Liu, Y.; Cheng, M.; Xiao, W. A Review of Structural Modification and Biological Activities of Oleanolic Acid. Chin. J. Nat. Med. 2024, 22, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; et al. Discovery of Pentacyclic Triterpenoids as Potential Entry Inhibitors of Influenza Viruses. J. Med. Chem. 2014, 57, 10058–10071. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Meng, L.; Sun, J.; Li, W.; Shao, L.; Chen, K.; Zhou, D.; Yang, F.; Yu, F. Design, Synthesis of Oleanolic Acid-Saccharide Conjugates Using Click Chemistry Methodology and Study of Their Anti-Influenza Activity. Eur. J. Med. Chem. 2019, 182, 111622. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.-W.; Dou, T.-Y.; Wang, P.; Lei, W.; Weng, Z.-M.; Hou, J.; Wang, D.-D.; Fan, Y.-M.; Zhang, W.-D.; Ge, G.-B.; et al. Structure-Activity Relationships of Pentacyclic Triterpenoids as Potent and Selective Inhibitors against Human Carboxylesterase 1. Front. Pharmacol. 2017, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Yang, S.; Sun, Y.; Zhang, L.; Bo, F.; Li, L. Development and Evaluation of Oleanolic Acid Dosage Forms and Its Derivatives. BioMed Res. Int. 2020, 2020, 1308749. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Ruiz-Gutiérrez, V.; Guinda, Á. Determination of Triterpenic Acids in Human Serum by High-Performance Liquid Chromatography: Triterpenoid Interaction with Serum Protein. J. Agric. Food Chem. 2011, 59, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Dopierała, K.; Krajewska, M.; Weiss, M. Physicochemical Characterization of Oleanolic Acid-Human Serum Albumin Complexes for Pharmaceutical and Biosensing Applications. Langmuir 2020, 36, 3611–3623. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ding, F.; Jiang, Y.-T.; Peng, Y.-K. Bioavailability and Activity of Natural Food Additive Triterpenoids as Influenced by Protein. J. Agric. Food Chem. 2014, 62, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Martin-Fonseca, S.; Rivas, F.; Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Martinez, A.; Garcia-Granados, A.; Lupiañez, J.A.; Albericio, F. Semi-Synthesis of Acylated Triterpenes from Olive-Oil Industry Wastes for the Development of Anticancer and Anti-HIV Agents. Eur. J. Med. Chem. 2014, 74, 278–301. [Google Scholar] [CrossRef]
- Creelan, B.C.; Gabrilovich, D.I.; Gray, J.E.; Williams, C.C.; Tanvetyanon, T.; Haura, E.B.; Weber, J.S.; Gibney, G.T.; Markowitz, J.; Proksch, J.W.; et al. Safety, Pharmacokinetics, and Pharmacodynamics of Oral Omaveloxolone (RTA 408), a Synthetic Triterpenoid, in a First-in-Human Trial of Patients with Advanced Solid Tumors. Onco Targets Ther. 2017, 10, 4239–4250. [Google Scholar] [CrossRef]
- Pilotto, F.; Chellapandi, D.M.; Puccio, H. Omaveloxolone: A Groundbreaking Milestone as the First FDA-Approved Drug for Friedreich Ataxia. Trends Mol. Med. 2024, 30, 117–125. [Google Scholar] [CrossRef]
- Kang, G.-D.; Lim, S.; Kim, D.-H. Oleanolic Acid Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mice by Restoring the Balance of Th17/Treg Cells and Inhibiting NF-κB Signaling Pathway. Int. Immunopharmacol. 2015, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive Oxygen Species (ROS) Scavenging Biomaterials for Anti-Inflammatory Diseases: From Mechanism to Therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Navas, A.; Jannus, F.; Fernández, B.; Cepeda, J.; Medina O’Donnell, M.; Díaz-Ruiz, L.; Sánchez-González, C.; Llopis, J.; Seco, J.M.; Rufino-Palomares, E.; et al. Designing Single-Molecule Magnets as Drugs with Dual Anti-Inflammatory and Anti-Diabetic Effects. Int. J. Mol. Sci. 2020, 21, 3146. [Google Scholar] [CrossRef]
- García-Valdivia, A.A.; García-García, A.; Jannus, F.; Zabala-Lekuona, A.; Méndez-Arriaga, J.M.; Fernández, B.; Medina-O’donnell, M.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Pastrana-Martínez, L.M.; et al. Antiparasitic, Anti-Inflammatory and Cytotoxic Activities of 2D Coordination Polymers Based on 1H-Indazole-5-Carboxylic Acid. J. Inorg. Biochem. 2020, 208, 111098. [Google Scholar] [CrossRef]
- García-Valdivia, A.A.; Jannus, F.; García-García, A.; Choquesillo-Lazarte, D.; Fernández, B.; Medina-O’donnell, M.; Lupiáñez, J.A.; Cepeda, J.; Reyes-Zurita, F.J.; Rodríguez-Diéguez, A. Anti-Cancer and Anti-Inflammatory Activities of a New Family of Coordination Compounds Based on Divalent Transition Metal Ions and Indazole-3-Carboxylic Acid. J. Inorg. Biochem. 2021, 215, 111308. [Google Scholar] [CrossRef]
- Galisteo, A.; Jannus, F.; García-García, A.; Aheget, H.; Rojas, S.; Lupiañez, J.A.; Rodríguez-Diéguez, A.; Reyes-Zurita, F.J.; Quílez del Moral, J.F. Diclofenac N-Derivatives as Therapeutic Agents with Anti-Inflammatory and Anti-Cancer Effect. Int. J. Mol. Sci. 2021, 22, 5067. [Google Scholar] [CrossRef]
- Jannus, F.; Medina-O’Donnell, M.; Neubrand, V.E.; Marín, M.; Saez-Lara, M.J.; Sepulveda, M.R.; Rufino-Palomares, E.E.; Martinez, A.; Lupiañez, J.A.; Parra, A.; et al. Efficient In Vitro and In Vivo Anti-Inflammatory Activity of a Diamine-PEGylated Oleanolic Acid Derivative. Int. J. Mol. Sci. 2021, 22, 8158. [Google Scholar] [CrossRef]
- Zentar, H.; Jannus, F.; Gutierrez, P.; Medina-O’Donnell, M.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Alvarez-Manzaneda, E.; Chahboun, R. Semisynthesis and Evaluation of Anti-Inflammatory Activity of the Cassane-Type Diterpenoid Taepeenin F and of Some Synthetic Intermediates. J. Nat. Prod. 2022, 85, 2372–2384. [Google Scholar] [CrossRef]
- Zentar, H.; Jannus, F.; Medina-O’Donnell, M.; Lupiáñez, J.A.; Justicia, J.; Alvarez-Manzaneda, R.; Reyes-Zurita, F.J.; Alvarez-Manzaneda, E.; Chahboun, R. Synthesis and Biological Evaluation of Cassane Diterpene (5α)-Vuacapane-8(14), 9(11)-Diene and of Some Related Compounds. Molecules 2022, 27, 5705. [Google Scholar] [CrossRef] [PubMed]
- Zentar, H.; Jannus, F.; Medina-O’Donnell, M.; El Mansouri, A.; Fernández, A.; Justicia, J.; Alvarez-Manzaneda, E.; Reyes-Zurita, F.J.; Chahboun, R. Synthesis of Tricyclic Pterolobirin H Analogue: Evaluation of Anticancer and Anti-Inflammatory Activities and Molecular Docking Investigations. Molecules 2023, 28, 6208. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; He, H.; Zhang, X.; Wu, R.; Gan, L.; Li, D.; Lu, Y.; Wu, P.; Wong, W.-L.; Zhang, K. The In Vitro and In Vivo Study of Oleanolic Acid Indole Derivatives as Novel Anti-Inflammatory Agents: Synthesis, Biological Evaluation, and Mechanistic Analysis. Bioorg. Chem. 2021, 113, 104981. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Xue, C.; Zhang, L.; Zhang, T.; Wang, C.; Bi, C.; Shan, A. Oleanolic Acid Enhances Tight Junctions and Ameliorates Inflammation in Salmonella Typhimurium-Induced Diarrhea in Mice via the TLR4/NF-κB and MAPK Pathway. Food Funct. 2020, 11, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Cordova, C.; San Román, J.A.; Gutierrez, B.; Cachofeiro, V.; Nieto, M.L. Oleanolic Acid Modulates the Immune-Inflammatory Response in Mice with Experimental Autoimmune Myocarditis and Protects from Cardiac Injury. Therapeutic Implications for the Human Disease. J. Mol. Cell. Cardiol. 2014, 72, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, S.; Lim, H.; Lee, J.; Park, J.-Y.; Kwon, H.-J.; Lee, I.-C.; Ryu, Y.-B.; Kim, J.; Shin, T.; et al. Oleanolic Acid Acetate Alleviates Symptoms of Experimental Autoimmune Encephalomyelitis in Mice by Regulating Toll-Like Receptor 2 Signaling. Front. Pharmacol. 2020, 11, 556391. [Google Scholar] [CrossRef]
- Dinh, C.H.L.; Yu, Y.; Szabo, A.; Zhang, Q.; Zhang, P.; Huang, X.-F. Bardoxolone Methyl Prevents High-Fat Diet-Induced Colon Inflammation in Mice. J. Histochem. Cytochem. 2016, 64, 237–255. [Google Scholar] [CrossRef]
- Nkeh-Chungag, B.N.; Oyedeji, O.O.; Oyedeji, A.O.; Ndebia, E.J. Anti-Inflammatory and Membrane-Stabilizing Properties of Two Semisynthetic Derivatives of Oleanolic Acid. Inflammation 2015, 38, 61–69. [Google Scholar] [CrossRef]
- Narożna, M.; Krajka-Kuźniak, V.; Bednarczyk-Cwynar, B.; Kucińska, M.; Kleszcz, R.; Kujawski, J.; Piotrowska-Kempisty, H.; Plewiński, A.; Murias, M.; Baer-Dubowska, W. Conjugation of Diclofenac with Novel Oleanolic Acid Derivatives Modulate Nrf2 and NF-κB Activity in Hepatic Cancer Cells and Normal Hepatocytes Leading to Enhancement of Its Therapeutic and Chemopreventive Potential. Pharmaceuticals 2021, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Kozai, K.; Miyake, Y.; Kohda, H.; Kametaka, S.; Yamasaki, K.; Suginaka, H.; Nagasaka, N. Inhibition of Glucosyltransferase from Streptococcus Mutans by Oleanolic Acid and Ursolic Acid. Caries Res. 1987, 21, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arellanes, A.; Meckes, M.; Torres, J.; Luna-Herrera, J. Antimycobacterial Triterpenoids from Lantana Hispida (Verbenaceae). J. Ethnopharmacol. 2007, 111, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, G.M.; Franzblau, S.G.; Zhang, F.; Wang, Y.; Timmermann, B.N. Inhibitory Effect of Sterols from Ruprechtia Triflora and Diterpenes from Calceolaria Pinnifolia on the Growth of Mycobacterium Tuberculosis. Planta Med. 2003, 69, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Cunha, W.R.; de Matos, G.X.; Souza, M.G.M.; Tozatti, M.G.; Andrade e Silva, M.L.; Martins, C.H.G.; da Silva, R.; Da Silva Filho, A.A. Evaluation of the Antibacterial Activity of the Methylene Chloride Extract of Miconia Ligustroides, Isolated Triterpene Acids, and Ursolic Acid Derivatives. Pharm. Biol. 2010, 48, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, Oleanolic and Betulinic Acids: Antibacterial Spectra and Selectivity Indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef]
- Martins, A.; Vasas, A.; Viveiros, M.; Molnár, J.; Hohmann, J.; Amaral, L. Antibacterial Properties of Compounds Isolated from Carpobrotus Edulis. Int. J. Antimicrob. Agents 2011, 37, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Grudniak, A.M.; Kurek, A.; Szarlak, J.; Wolska, K.I. Oleanolic and Ursolic Acids Influence Affect the Expression of the Cysteine Regulon and the Stress Response in Escherichia coli. Curr. Microbiol. 2011, 62, 1331–1336. [Google Scholar] [CrossRef]
- Kurek, A.; Grudniak, A.M.; Szwed, M.; Klicka, A.; Samluk, L.; Wolska, K.I.; Janiszowska, W.; Popowska, M. Oleanolic Acid and Ursolic Acid Affect Peptidoglycan Metabolism in Listeria Monocytogenes. Antonie Van Leeuwenhoek 2010, 97, 61–68. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lee, S.; Yoon, Y.; Choi, K.-H. Antimicrobial Action of Oleanolic Acid on Listeria Monocytogenes, Enterococcus Faecium, and Enterococcus Faecalis. PLoS ONE 2015, 10, e0118800. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Sinou, V.; Babkov, D.A.; Kazakova, O.; Brunel, J.M. Development of New Antimicrobial Oleanonic Acid Polyamine Conjugates. Antibiotics 2022, 11, 94. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Vega-Granados, K.; Moya-Andérico, L.; Vukomanovic, M.; Parra, A.; Álvarez de Cienfuegos, L.; Torrents, E. Novel Oleanolic and Maslinic Acid Derivatives as a Promising Treatment against Bacterial Biofilm in Nosocomial Infections: An In Vitro and In Vivo Study. ACS Infect. Dis. 2019, 5, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, Y.; Sun, X.; Ding, R.; Wang, Y.; Niu, X.; Wang, J.; Deng, X. Application of Oleanolic Acid and Its Analogues in Combating Pathogenic Bacteria In Vitro/Vivo by a Two-Pronged Strategy of β-Lactamases and Hemolysins. ACS Omega 2020, 5, 11424–11438. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Shen, J.K.; Wang, H.K.; Cosentino, L.M.; Lee, K.H. Synthesis and Anti-HIV Activity of Oleanolic Acid Derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 3115–3118. [Google Scholar] [CrossRef]
- Yu, D.; Sakurai, Y.; Chen, C.-H.; Chang, F.-R.; Huang, L.; Kashiwada, Y.; Lee, K.-H. Anti-AIDS Agents 69. Moronic Acid and Other Triterpene Derivatives as Novel Potent Anti-HIV Agents. J. Med. Chem. 2006, 49, 5462–5469. [Google Scholar] [CrossRef]
- Han, X.; Shi, Y.; Si, L.; Fan, Z.; Wang, H.; Xu, R.; Jiao, P.; Meng, K.; Tian, Z.; Zhou, X.; et al. Design, Synthesis and Biological Activity Evaluation of Novel Conjugated Sialic Acid and Pentacyclic Triterpene Derivatives as Anti-Influenza Entry Inhibitors. Med. Chem. Commun. 2016, 7, 1932–1945. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, C.; Li, B.; Xu, X.; Liang, M.; Gu, S.; Chu, F.; Xu, B.; Ren, J.; Wang, P.; et al. A Series of Oleanolic Acid Derivatives as Anti-Hepatitis B Virus Agents: Design, Synthesis, and In Vitro and In Vivo Biological Evaluation. Molecules 2016, 21, 402. [Google Scholar] [CrossRef]
- Ikeda, T.; Yokomizo, K.; Okawa, M.; Tsuchihashi, R.; Kinjo, J.; Nohara, T.; Uyeda, M. Anti-Herpes Virus Type 1 Activity of Oleanane-Type Triterpenoids. Biol. Pharm. Bull. 2005, 28, 1779–1781. [Google Scholar] [CrossRef]
- Malchoff, C.D. Diagnosis and Classification of Diabetes Mellitus. Conn. Med. 1991, 55, 625–629. [Google Scholar]
- Taylor, R. Insulin Resistance and Type 2 Diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chem. Int. Ed. 2023, 62, e202217809. [Google Scholar] [CrossRef] [PubMed]
- Compain, P. Iminosugar C-Glycosides: Synthesis and Biological Activity. In Iminosugars; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 63–86. ISBN 978-0-470-51743-7. [Google Scholar]
- Baloyi, C.M.; Khathi, A.; Sibiya, N.H.; Ngubane, P.S. The Haematological Effects of Oleanolic Acid in Streptozotocin-Induced Diabetic Rats: Effects on Selected Markers. J. Diabetes Res. 2019, 2019, 6753541. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Histone Modifications and Skeletal Muscle Metabolic Gene Expression. Clin. Exp. Pharmacol. Physiol. 2010, 37, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zeng, X.-Y.; Wang, H.; Li, S.; Jo, E.; Xue, C.C.L.; Tan, M.; Molero, J.C.; Ye, J.-M. Hepatic FoxO1 Acetylation Is Involved in Oleanolic Acid-Induced Memory of Glycemic Control: Novel Findings from Study 2. PLoS ONE 2014, 9, e107231. [Google Scholar] [CrossRef] [PubMed]
- Luvuno, M.; Mbongwa, H.P.; Khathi, A. The effects of Syzygium Aromaticum-derived triterpenes on gastrointestinal ghrelin expression in streptozotocin-induced diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wu, G.; Cheng, X.; Fan, J.; Peng, J.; Su, H.; Xu, Z.; Cao, M.; Long, Z.; Hao, Y.; et al. Oleanolic Acid Attenuates PCBs-Induced Adiposity and Insulin Resistance via HNF1b-Mediated Regulation of Redox and PPARγ Signaling. Free Radic. Biol. Med. 2018, 124, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-F.; Wang, J.-T.; Zhang, L.-X.; Xing, S.-F.; Wang, Y.-X.; Wang, K.; Deng, S.-L.; Zhang, J.-Q.; Tang, L.; Wu, H.-S. Oleanolic Acid Derivative DKS26 Exerts Antidiabetic and Hepatoprotective Effects in Diabetic Mice and Promotes Glucagon-like Peptide-1 Secretion and Expression in Intestinal Cells. Br. J. Pharmacol. 2017, 174, 2912–2928. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P. Hypolipidemic and Hypoglycemic Activities of a Oleanolic Acid Derivative from Malva Parviflora on Streptozotocin-Induced Diabetic Mice. Arch. Pharm. Res. 2017, 40, 550–562. [Google Scholar] [CrossRef]
- Yang, L.; Chen, F.; Gao, C.; Chen, J.; Li, J.; Liu, S.; Zhang, Y.; Wang, Z.; Qian, S. Design and Synthesis of Tricyclic Terpenoid Derivatives as Novel PTP1B Inhibitors with Improved Pharmacological Property and In Vivo Antihyperglycaemic Efficacy. J. Enzym. Inhib. Med. Chem. 2020, 35, 152–164. [Google Scholar] [CrossRef]
- Liu, Q.-C.; Guo, T.-T.; Zhang, L.; Yu, Y.; Wang, P.; Yang, J.-F.; Li, Y.-X. Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as PTP1B Inhibitors. Eur. J. Med. Chem. 2013, 63, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Lee, J.-S.; Park, H.-J.; Kim, J.-W.; Kim, C.-J.; Shim, I.-S.; Kim, N.-J.; Han, S.-M.; Lim, S. Inhibition of Cytochrome P450 Activities by Oleanolic Acid and Ursolic Acid in Human Liver Microsomes. Life Sci. 2004, 74, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Liu, R.; Liu, Y.; Zhang, T.; Fu, H.; Hai, C. Oleanolic Acid Co-Administration Alleviates Ethanol-Induced Hepatic Injury via Nrf-2 and Ethanol-Metabolizing Modulating in Rats. Chem. Biol. Interact. 2014, 221, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, Y.; Lv, H.; Zhang, L.; Bi, C.; Dong, N.; Shan, A.; Wang, J. Oleanolic Acid Targets the Gut–Liver Axis to Alleviate Metabolic Disorders and Hepatic Steatosis. J. Agric. Food Chem. 2021, 69, 7884–7897. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, W.; Peng, W.; Yu, R.; Li, G.; Jiang, T. Pharmacokinetics In Vitro and In Vivo of Two Novel Prodrugs of Oleanolic Acid in Rats and Its Hepatoprotective Effects against Liver Injury Induced by CCl4. Mol. Pharm. 2016, 13, 1699–1710. [Google Scholar] [CrossRef]
- Xiang, H.; Han, Y.; Zhang, Y.; Yan, W.; Xu, B.; Chu, F.; Xie, T.; Jia, M.; Yan, M.; Zhao, R.; et al. A New Oleanolic Acid Derivative against CCl4-Induced Hepatic Fibrosis in Rats. Int. J. Mol. Sci. 2017, 18, 553. [Google Scholar] [CrossRef]
- Chu, F.; Zhang, W.; Guo, W.; Wang, Z.; Yang, Y.; Zhang, X.; Fang, K.; Yan, M.; Wang, P.; Lei, H. Oleanolic Acid-Amino Acids Derivatives: Design, Synthesis, and Hepatoprotective Evaluation In Vitro and In Vivo. Molecules 2018, 23, 322. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, L.; Ji, X.; Shi, R.; Xu, F.; Gu, J. Neuroprotective Effects of Oleanolic Acid against Cerebral Ischemia-Reperfusion Injury in Mice. Exp. Neurol. 2021, 343, 113785. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Lin, K.L.; Law, C.Y.; Liu, B.; Fu, X.Q.; Tse, W.S.; Wong, S.S.M.; Sze, S.C.W.; Yung, K.K.L. Oleanolic Acid Enhances Neural Stem Cell Migration, Proliferation, and Differentiation In Vitro by Inhibiting GSK3β Activity. Cell Death Discov. 2018, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Zhou, Z.; Han, W.-W.; Zhang, L.-L.; Song, W.-S.; Huang, J.-H.; Liu, S. Oleanolic Acid Inhibiting the Differentiation of Neural Stem Cells into Astrocyte by Down-Regulating JAK/STAT Signaling Pathway. Am. J. Chin. Med. 2016, 44, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Huang, J.; Kalionis, B.; Bian, Q.; Dong, J.; Wu, J.; Tai, X.; Xia, S.; Shen, Z. Oleanolic Acid Induces Differentiation of Neural Stem Cells to Neurons: An Involvement of Transcription Factor Nkx-2.5. Stem Cells Int. 2015, 2015, 672312. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.-R.; Wang, S.E.; Kim, Y.-S.; Lee, C.H.; Son, H. Oleanolic Acid Promotes Neuronal Differentiation and Histone Deacetylase 5 Phosphorylation in Rat Hippocampal Neurons. Mol. Cells 2017, 40, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Pal, S.; Das, N.; Dinda, B. Ameliorative Effects of Oleanolic Acid on Fluoride Induced Metabolic and Oxidative Dysfunctions in Rat Brain: Experimental and Biochemical Studies. Food Chem. Toxicol. 2014, 66, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, B.C.; Daniels, W.M.U.; Mabandla, M.V. Amelioration of L-Dopa-Associated Dyskinesias with Triterpenoic Acid in a Parkinsonian Rat Model. Neurotox. Res. 2016, 29, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.-Q.; Wang, S.-S.; Zhu, J.-X.; Mu, R.-H.; Li, C.-F.; Geng, D.; Liu, Q.; Yi, L.-T. Oleanolic Acid Decreases SGK1 in the Hippocampus in Corticosterone-Induced Mice. Steroids 2019, 149, 108419. [Google Scholar] [CrossRef]
- Sapkota, A.; Choi, J.W. Oleanolic Acid Provides Neuroprotection against Ischemic Stroke through the Inhibition of Microglial Activation and NLRP3 Inflammasome Activation. Biomol. Ther. 2022, 30, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yuzhalin, A.E. Redefining Cancer Research for Therapeutic Breakthroughs. Br. J. Cancer 2024, 130, 1078–1082. [Google Scholar] [CrossRef]
- Vitale, I.; Pietrocola, F.; Guilbaud, E.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostini, M.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; et al. Apoptotic Cell Death in Disease—Current Understanding of the NCCD 2023. Cell Death Differ. 2023, 30, 1097–1154. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, P.; Sun, Y.; Jiang, J.; Du, H.; Wang, Z.; Duan, Z.; Lei, H.; Li, H. Induction of Apoptosis by an Oleanolic Acid Derivative in SMMC-7721 Human Hepatocellular Carcinoma Cells Is Associated with Mitochondrial Dysfunction. Oncol. Lett. 2018, 15, 2821–2828. [Google Scholar] [CrossRef]
- Kang, X.; Hu, J.; Gao, Z.; Ju, Y.; Xu, C. Synthesis, Anti-Proliferative and Proapoptotic Activity of Novel Oleanolic Acid Azaheterocyclic Derivatives. Med. Chem. Commun. 2012, 3, 1245–1249. [Google Scholar] [CrossRef]
- Yan, S.; Huang, C.; Wu, S.; Yin, M. Oleanolic Acid and Ursolic Acid Induce Apoptosis in Four Human Liver Cancer Cell Lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, Z.; Jiang, X.; Zhang, J.; Guo, X. Oleanolic Acid Inhibits Cell Proliferation Migration and Invasion and Induces SW579 Thyroid Cancer Cell Line Apoptosis by Targeting Forkhead Transcription Factor A. Anticancer Drugs 2019, 30, 812–820. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted Inhibition of Tumor Proliferation, Survival, and Metastasis by Pentacyclic Triterpenoids: Potential Role in Prevention and Therapy of Cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic Acid and Its Synthetic Derivatives for the Prevention and Therapy of Cancer: Preclinical and Clinical Evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef]
- Yang, J.; Liao, D.; Chen, C.; Liu, Y.; Chuang, T.-H.; Xiang, R.; Markowitz, D.; Reisfeld, R.A.; Luo, Y. Tumor-Associated Macrophages Regulate Murine Breast Cancer Stem Cells Through a Novel Paracrine EGFR/Stat3/Sox-2 Signaling Pathway. Stem Cells 2013, 31, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Borella, R.; Forti, L.; Gibellini, L.; De Gaetano, A.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Synthesis and Anticancer Activity of CDDO and CDDO-Me, Two Derivatives of Natural Triterpenoids. Molecules 2019, 24, 4097. [Google Scholar] [CrossRef]
- Alabran, J.; Cheuk, A.; Liby, K.; Sporn, M.; Khan, J.; Letterio, J.; S Leskov, K. Human Neuroblastoma Cells Rapidly Enter Cell Cycle Arrest and Apoptosis Following Exposure to C-28 Derivatives of the Synthetic Triterpenoid CDDO. Cancer Biol. Ther. 2008, 7, 709–717. [Google Scholar] [CrossRef]
- Yoo, K.H.; Park, J.; Cui, E.J.; Kim, K.I.; Kim, J.Y.; Kim, J.; Hong, S.G.; Baek, N.I.; Chung, I.S. 3-O-Acetyloleanolic Acid Induces Apoptosis in Human Colon Carcinoma Hct-116 Cells. Phytother. Res. 2012, 26, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, L.; Huang, Z.; Lai, Y.; Ji, H.; Peng, S.; Tian, J.; Zhang, Y. Hybrid Molecule from O2-(2,4-Dinitrophenyl)Diazeniumdiolate and Oleanolic Acid: A Glutathione S-Transferase π-Activated Nitric Oxide Prodrug with Selective Anti-Human Hepatocellular Carcinoma Activity and Improved Stability. J. Med. Chem. 2013, 56, 4641–4655. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, M.; Ando, H.; Hirai, Y.; Toriizuka, K.; Ida, Y.; Kuchino, Y. Achyranthoside H Methyl Ester, a Novel Oleanolic Acid Saponin Derivative from Achyranthes Fauriei Roots, Induces Apoptosis in Human Breast Cancer MCF-7 and MDA-MB-453 Cells via a Caspase Activation Pathway. J. Nat. Med. 2009, 63, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jannus, F.; Medina-O’Donnell, M.; Rivas, F.; Díaz-Ruiz, L.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Parra, A.; Reyes-Zurita, F.J. A Diamine-PEGylated Oleanolic Acid Derivative Induced Efficient Apoptosis through a Death Receptor and Mitochondrial Apoptotic Pathway in HepG2 Human Hepatoma Cells. Biomolecules 2020, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Lisiak, N.M.; Lewicka, I.; Kaczmarek, M.; Kujawski, J.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Rubis, B. Oleanolic Acid’s Semisynthetic Derivatives HIMOXOL and Br-HIMOLID Show Proautophagic Potential and Inhibit Migration of HER2-Positive Breast Cancer Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 11273. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Ferrer-Martin, R.M.; Rufino-Palomares, E.E.; Martin-Fonseca, S.; Rivas, F.; Martínez, A.; García-Granados, A.; Pérez-Jiménez, A.; García-Salguero, L. The Oleanolic Acid Derivative, 3-O-Succinyl-28-O-Benzyl Oleanolate, Induces Apoptosis in B16–F10 Melanoma Cells via the Mitochondrial Apoptotic Pathway. RSC Adv. 2016, 6, 93590–93601. [Google Scholar] [CrossRef]
- Cohen-Nowak, A.J.; Cohen, A.J.; Correia, E.D.; Portocarrero, C.P.; South, A.P.; Nikbakht, N. Omaveloxolone Attenuates Squamous Cell Carcinoma Growth and Disease Severity in an Epidermolysis Bullosa Mouse Model. Exp. Dermatol. 2022, 31, 1083–1088. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannus, F.; Sainz, J.; Reyes-Zurita, F.J. Principal Bioactive Properties of Oleanolic Acid, Its Derivatives, and Analogues. Molecules 2024, 29, 3291. https://doi.org/10.3390/molecules29143291
Jannus F, Sainz J, Reyes-Zurita FJ. Principal Bioactive Properties of Oleanolic Acid, Its Derivatives, and Analogues. Molecules. 2024; 29(14):3291. https://doi.org/10.3390/molecules29143291
Chicago/Turabian StyleJannus, Fatin, Juan Sainz, and Fernando J. Reyes-Zurita. 2024. "Principal Bioactive Properties of Oleanolic Acid, Its Derivatives, and Analogues" Molecules 29, no. 14: 3291. https://doi.org/10.3390/molecules29143291
APA StyleJannus, F., Sainz, J., & Reyes-Zurita, F. J. (2024). Principal Bioactive Properties of Oleanolic Acid, Its Derivatives, and Analogues. Molecules, 29(14), 3291. https://doi.org/10.3390/molecules29143291









