A Comparative Study of Surfactant Solutions Used for Enhanced Oil Recovery in Shale and Tight Formations: Experimental Evaluation and Numerical Analysis
Abstract
1. Introduction
1.1. How Does Chemicals’ EOR Start in Conventional Reservoirs?
1.2. Why Chemical EOR Is Needed in Tight and Shale Oil?
1.3. What Kinds of Solutions Are Used in Chemical EOR?
1.4. Why a Comparison Analysis Is Necessary
- = capillary pressure,
- = contact angle of liquid on the rock surface,
- = interfacial tension of water with oil, and
- r = radius of the porous media.
1.5. What Is the Research Novelty of This Comparison Study?
2. Materials and Methods
2.1. Tests of Chemical Morphology and Properties
2.1.1. Interfacial Tension and Wettability Measurement
2.1.2. Hydrodynamic Radius Measurement and Micro-Morphology Observation
2.1.3. The Adsorption Rate and Core Damage Assessment
2.2. Analysis of Micro-Oil-Displacing Performance
2.2.1. Threshold Pressure Gradient Determination
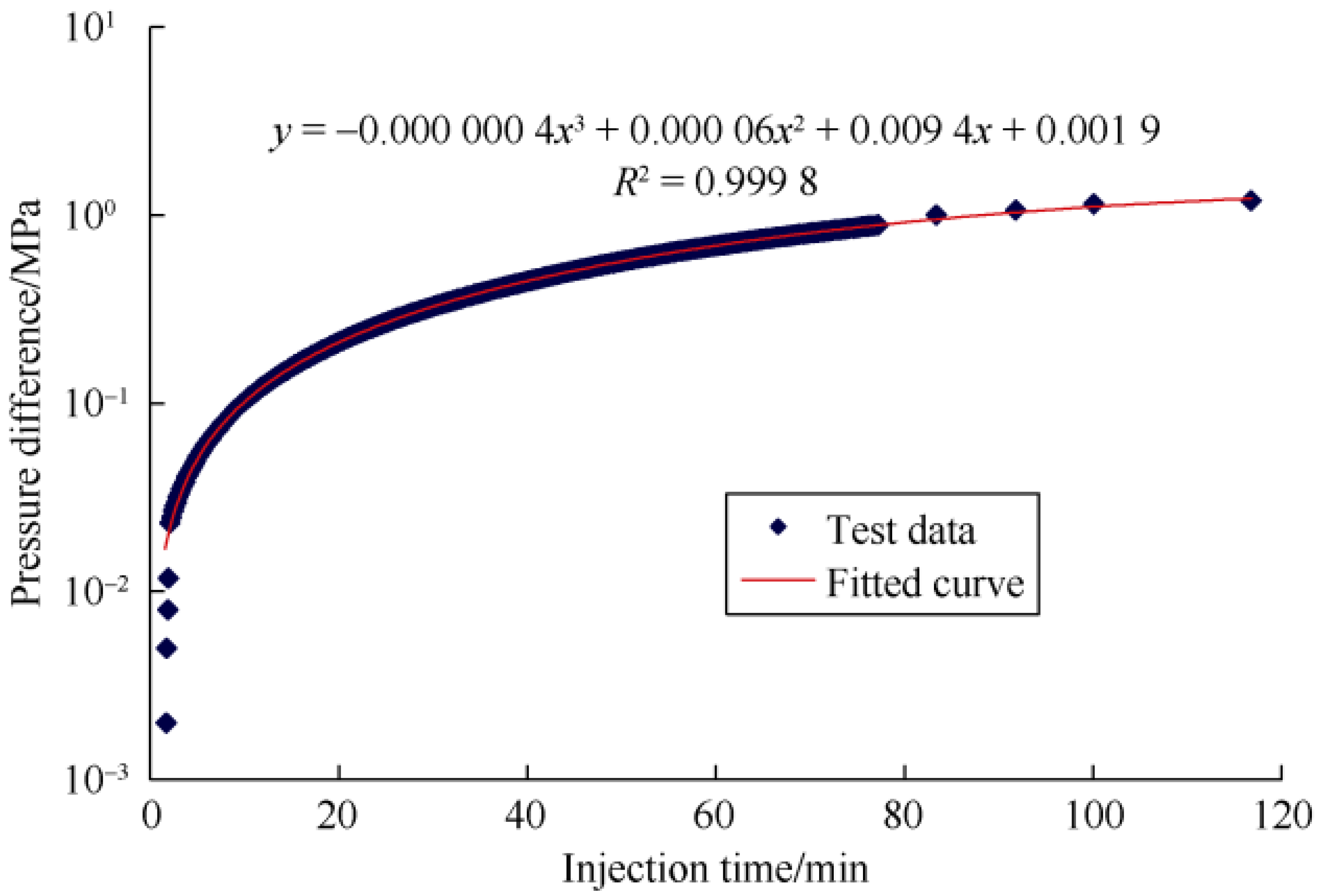
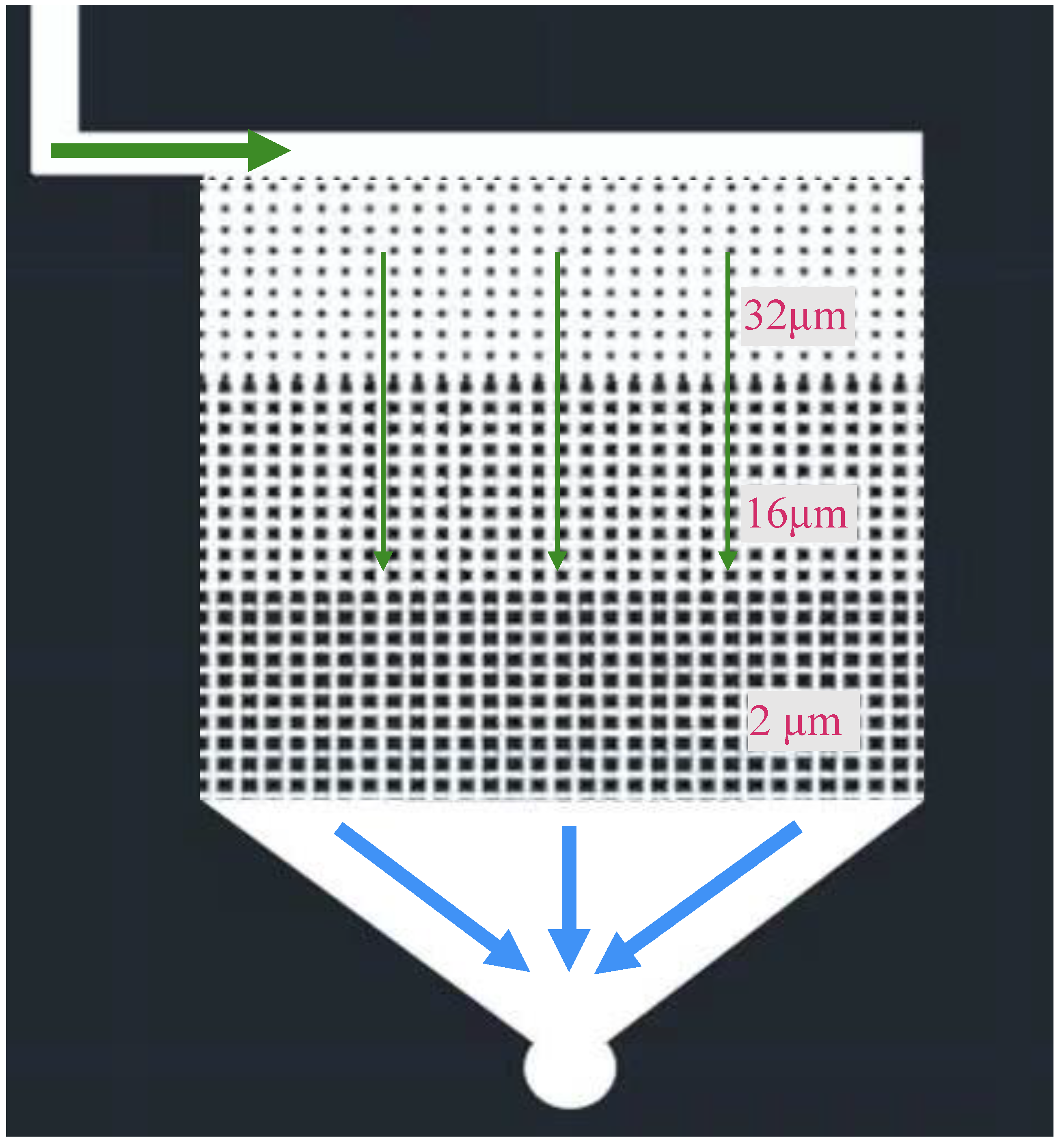
2.2.2. Micro-Fluidic Modeling
2.2.3. Core Injection with Low-Field NMR
2.3. Simulation of Macro-Oil-Increasing Effect
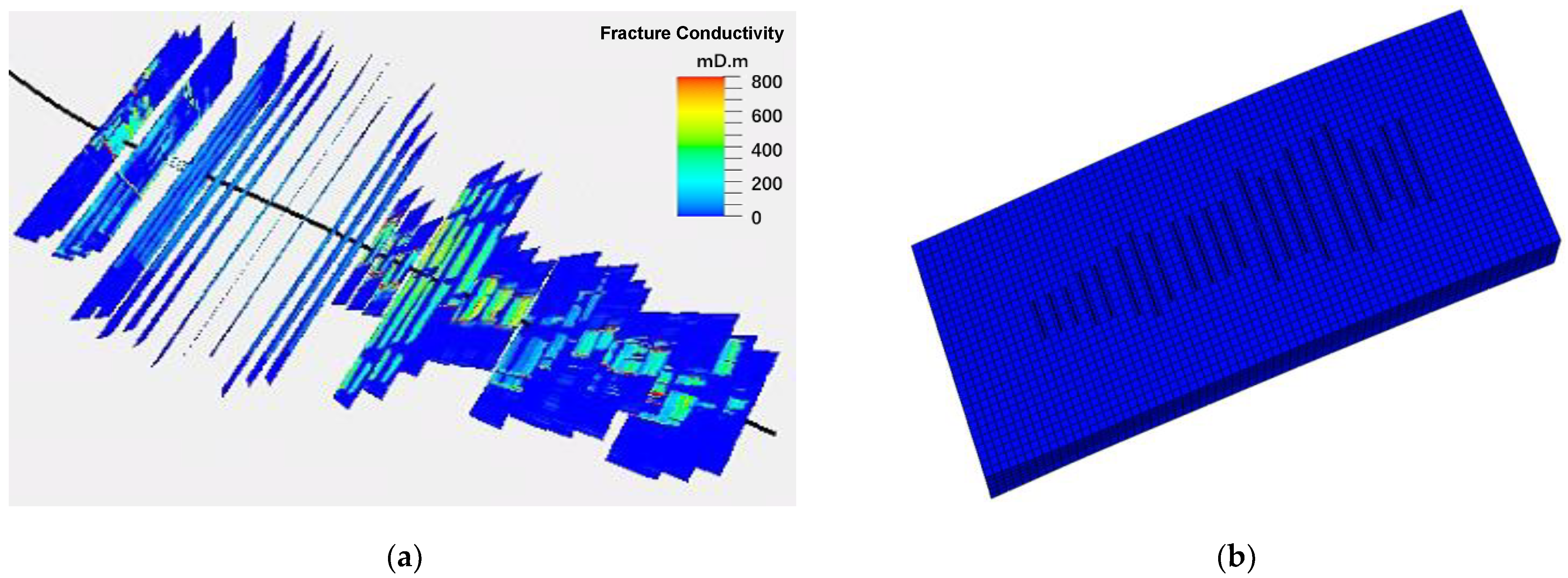
2.3.1. Fractured Geological Model
2.3.2. Mechanisms of Chemicals EOR
2.3.3. The Threshold Pressure Gradient in Simulation
3. Results and Discussions
3.1. Chemical Morphology and Properties
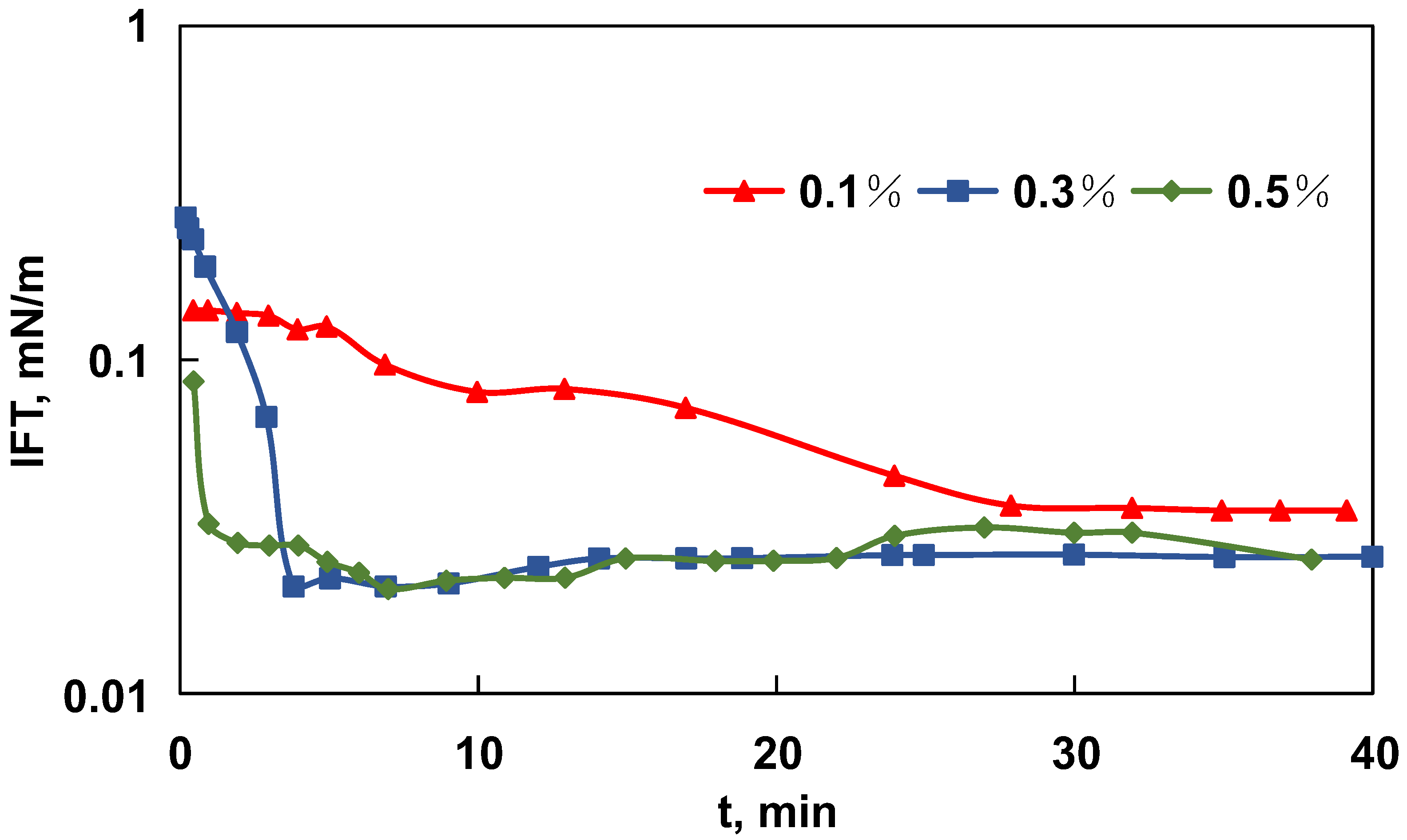
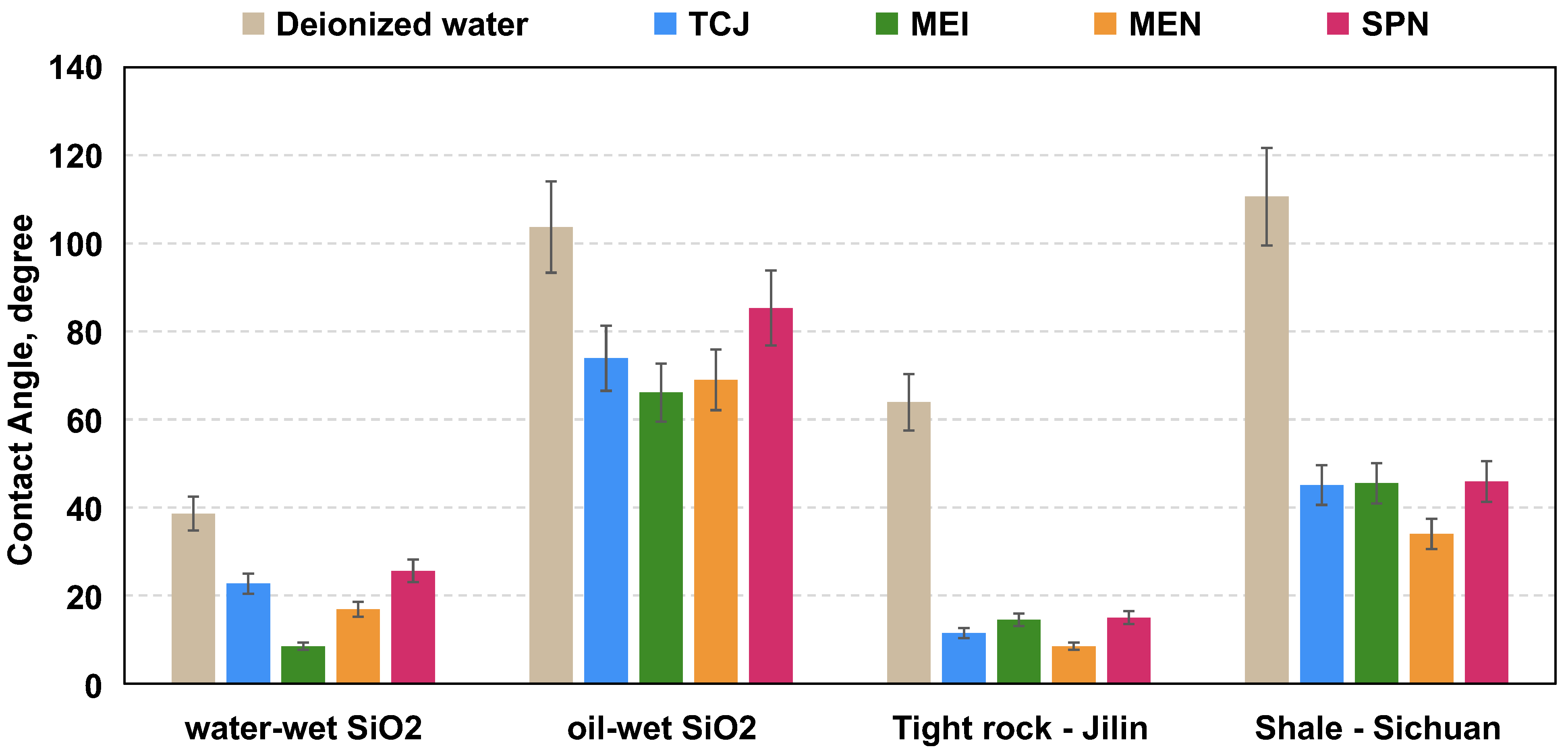
3.1.1. IFT Reduction and Wettability Alteration Effects
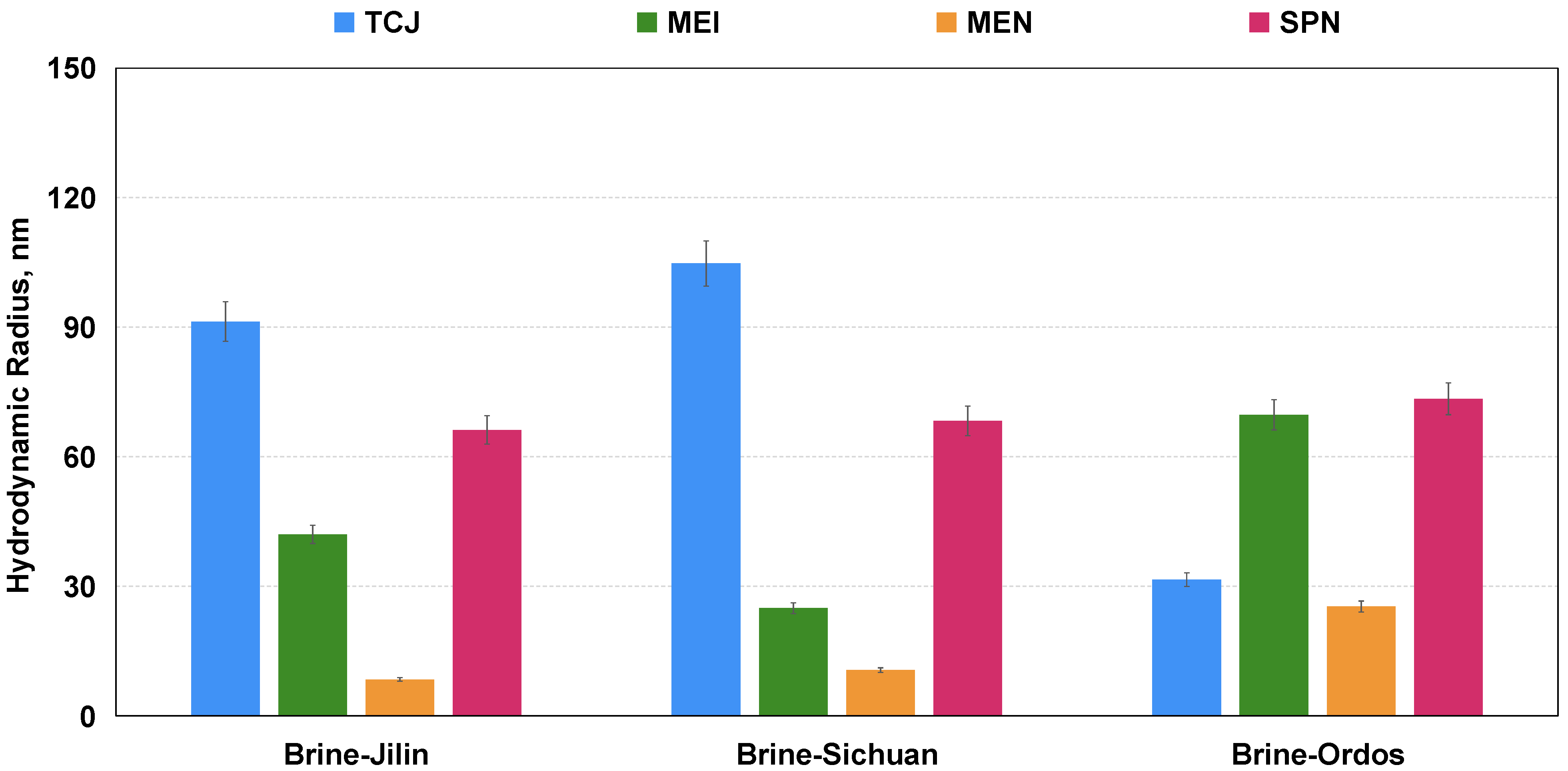
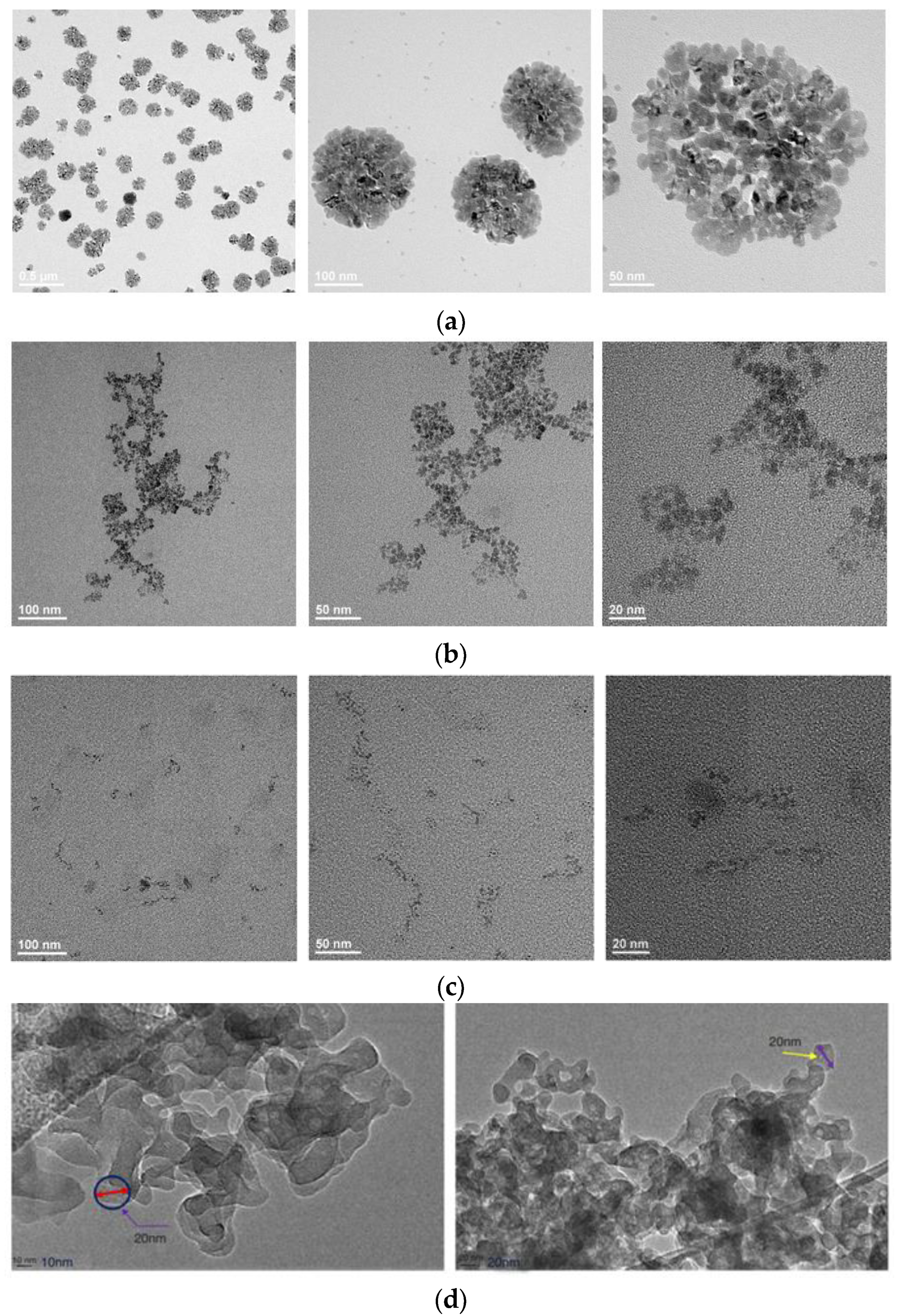
3.1.2. Hydrodynamic Radius and Micro-Morphology
3.1.3. Adsorption and the Core Damage Rate
3.2. Micro-Oil-Displacing Performance
3.2.1. The Threshold Pressure Gradient
3.2.2. Micro-Fluidic Model
3.2.3. Core Injection and Fluid Penetration
3.3. Macro-Oil-Increasing Effect
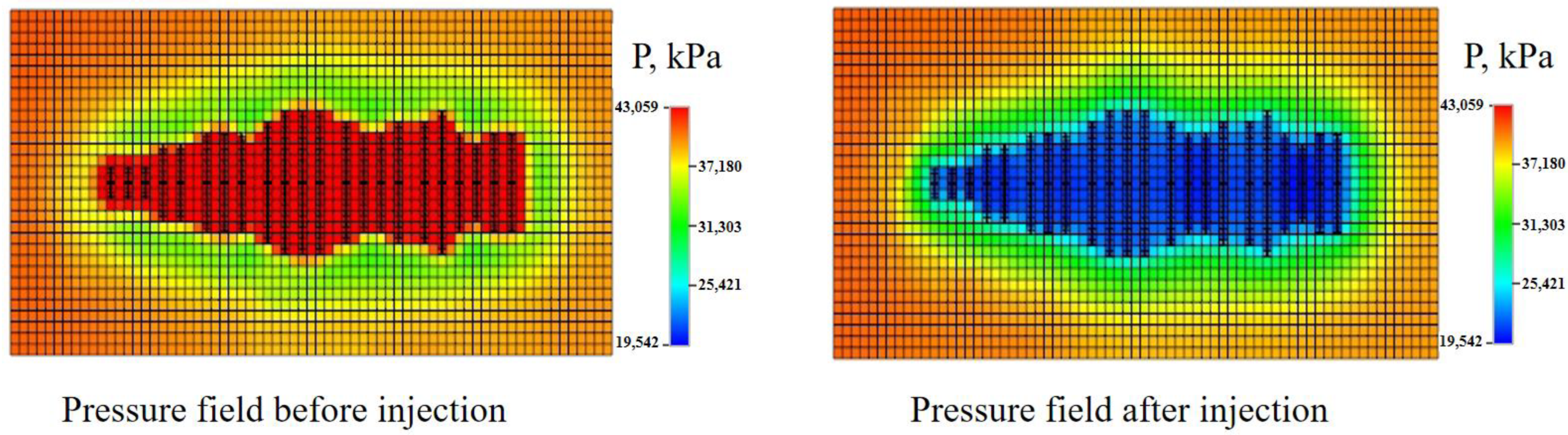
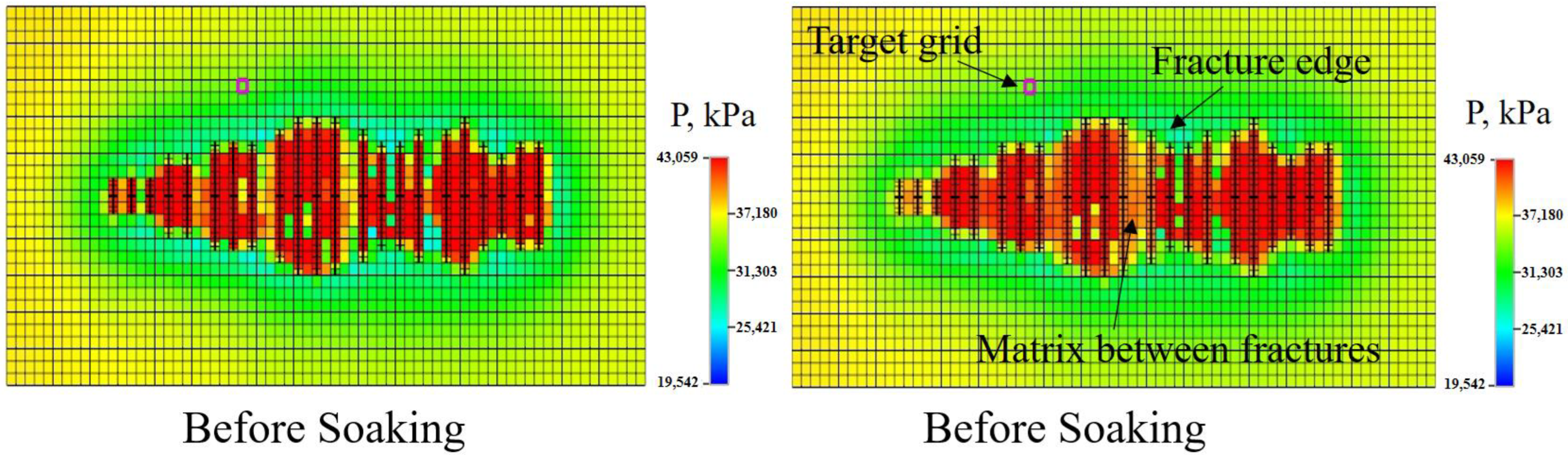
3.3.1. Pressure Field
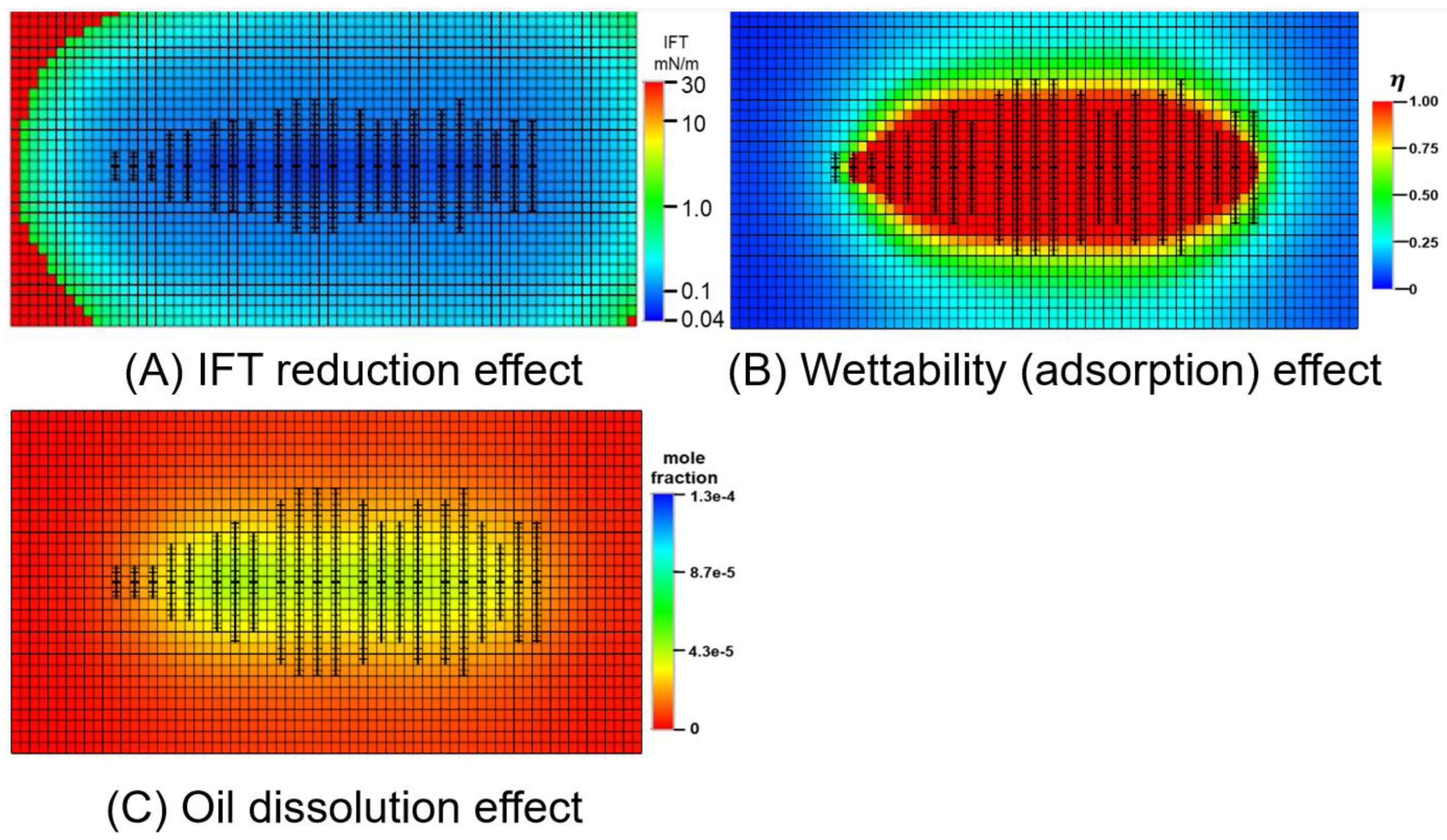
3.3.2. Oil-Increasing Mechanism
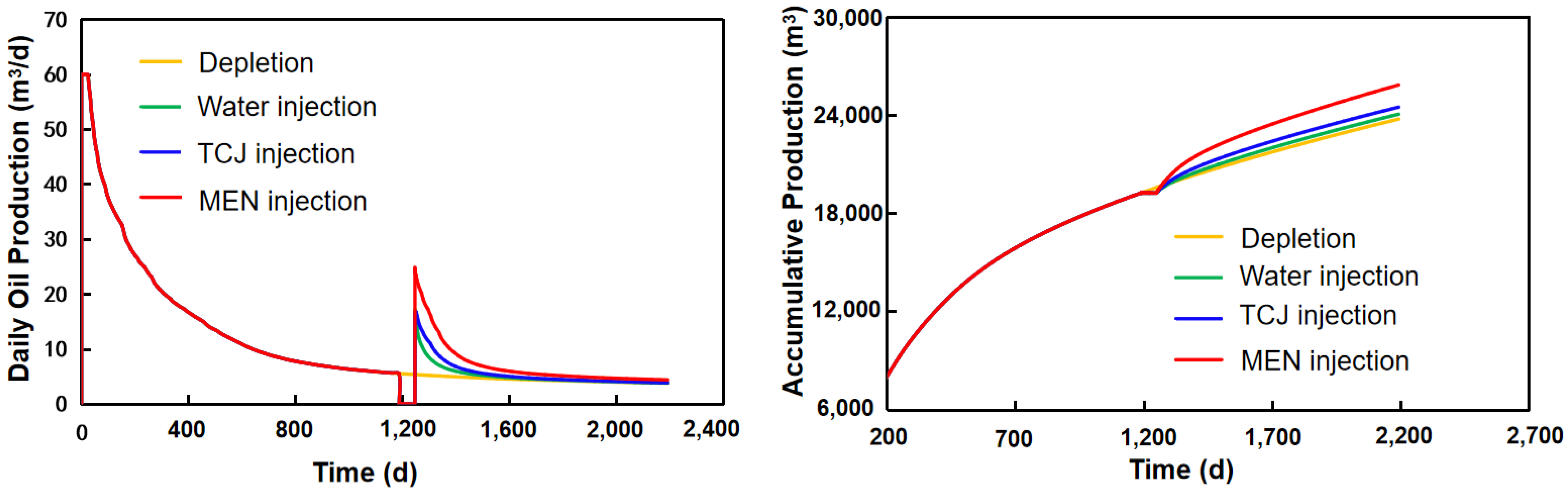
3.3.3. Incremental Oil Production
4. Conclusions
- (1)
- Consistent with the traditional understandings, solutions have the ability to reduce oil–water IFT to a certain level and alter wettability to water-wet whether it is a traditional surfactant or in the form of micro-emulsion and nano-fluids. Moreover, the appropriate adsorption rate and low core damage are the indispensable requirements for chemicals applied in EOR.
- (2)
- Unlike conventional reservoirs, water flooding does not work in tight reservoirs. Considering the size effect caused by nanoscale pores and throats in shale and tight reservoirs, this study suggests that small molecule sizes should be given priority when evaluating and selecting chemical systems.
- (3)
- The comparison analysis results demonstrate that the micro/nano-emulsion with the lowest hydrodynamic radius is the optimal selection. This is attributed to the properties of the nano-emulsion matching well with the physical properties of shale and tight reservoirs.
- (4)
- The aggregation behavior of the surfactant molecules and nanoparticles increases the apparent size of the aqueous system. Although both have their own advantages, it is still necessary to solve the problem of aggregation in order to effectively apply them in unconventional EOR.
- (1)
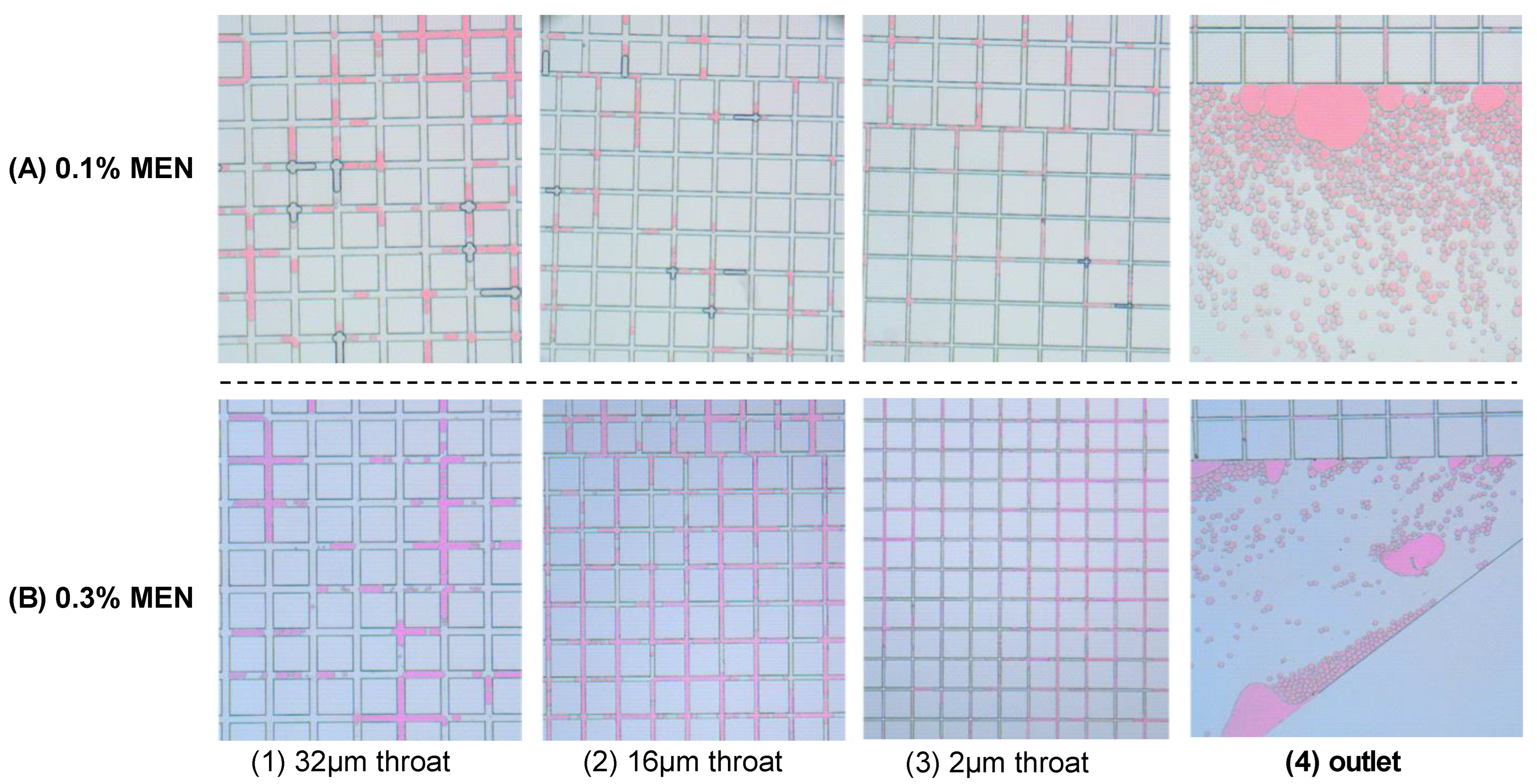
- A small-size oil effect of the chemicals was observed in the microfluidic model. Due to the injection of chemicals, the shape of crude oil changed from a continuous aggregate to small droplets. The heterogeneous model with different sizes of throats indicates that the size of droplets can adapt to the size of the throat. As the throat narrows, the size of the oil droplets also decreases. This small-size oil effect of chemical additives should be given more importance in projects recovering shale and tight oil.
- (2)
- The pressure field of the formation is transformed by injection of the aqueous solutions. Compared with water injection, the pressure propagation range of surfactant and micro-emulsion injection is wider due to reduced TPG. Oil production can be increased by different mechanisms, and the efficiency of those mechanisms has certain zones. Prediction of daily and accumulative oil production showed the application prospects of chemical EOR. Numerical modeling points out the direction for future research focus as well.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Celino, K.N.; de Souza, E.A.; de Carvalho Balaban, R. Emulsions of glycerol in olefin: A critical evaluation for application in oil well drilling fluids. Fuel 2022, 308, 121959. [Google Scholar] [CrossRef]
- Khan, R.A.; Murtaza, M.; Mustafa, A.; Abdulraheem, A.; Mahmoud, M.; Kamal, M.S. Ionic liquids as clay stabilizer additive in fracturing fluid. Fuel 2023, 334, 126154. [Google Scholar] [CrossRef]
- Javadi, A.H.; Fatemi, M. Impact of salinity on fluid/fluid and rock/fluid interactions in enhanced oil recovery by hybrid low salinity water and surfactant flooding from fractured porous media. Fuel 2022, 329, 125426. [Google Scholar] [CrossRef]
- Borchardt, J.K. Chemicals Used in Oil-Field Operations. In Oil-Field Chemistry; ACS Publications: Washington, DC, USA, 1989; pp. 3–54. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Bera, A.; Shah, S.; Shah, M.; Agarwal, J.; Vij, R.K. Mechanistic study on silica nanoparticles-assisted guar gum polymer flooding for enhanced oil recovery in sandstone reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124833. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, A. Critical investigation of zwitterionic surfactant for enhanced oil recovery from both sandstone and carbonate reservoirs: Adsorption, wettability alteration and imbibition studies. Chem. Eng. Sci. 2019, 209, 115222. [Google Scholar] [CrossRef]
- Xu, X.; Saeedi, A.; Liu, K. An experimental study of combined foam/surfactant polymer (SP) flooding for carbone dioxide-enhanced oil recovery (CO2-EOR). J. Pet. Sci. Eng. 2017, 149, 603–611. [Google Scholar] [CrossRef]
- O’brien, B.M. Enhanced oil recovery chemical needs. J. Am. Oil Chem. Soc. 1982, 59, 839A–852A. [Google Scholar] [CrossRef]
- Jie, C.; Guang, L.; Zhen, Y. Pilot Test of Asp Flooding in Daqing Oilfield. Pet. Geol. Oilfield Dev. Daqing 2001, 20, 46–49. [Google Scholar]
- Li, Y.; Zhao, Q.; Lyu, Q.; Xue, Z.; Cao, X.; Liu, Z. Evaluation technology and practice of continental shale oil development in China. Pet. Explor. Dev. 2022, 49, 1098–1109. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, W.; Hou, L.; Yang, Z.; Zhu, R.; Wu, S.; Bai, B.; Jin, X. Development potential and technical strategy of continental shale oil in China. Pet. Explor. Dev. 2020, 47, 877–887. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Hu, Y.; Ren, L.; Zhao, C. Shut-in time optimization after fracturing in shale oil reservoirs. Pet. Explor. Dev. 2022, 49, 671–683. [Google Scholar] [CrossRef]
- Ding, B.; Xiong, C.; Geng, X.; Guan, B.; Pan, J.; Xu, J.; Dong, J.; Zhang, C. Characteristics and EOR mechanisms of nanofluids permeation flooding for tight oil. Pet. Explor. Dev. 2020, 47, 810–819. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, J.J.; Wang, X.; Ge, H.; Yao, E. Experimental study of wettability alteration and spontaneous imbibition in Chinese shale oil reservoirs using anionic and nonionic surfactants. J. Pet. Sci. Eng. 2019, 175, 624–633. [Google Scholar] [CrossRef]
- Liu, X.; Kang, Y.; Yan, L.; Tian, J.; Li, J.; You, L. Implication of interfacial tension reduction and wettability alteration by surfactant on enhanced oil recovery in tight oil reservoirs. Energy Rep. 2022, 8, 13672–13681. [Google Scholar] [CrossRef]
- Qu, M.; Liang, T.; Xiao, L.; Hou, J.; Qi, P.; Zhao, Y.; Song, C.; Li, J. Mechanism study of spontaneous imbibition with lower-phase nano-emulsion in tight reservoirs. J. Pet. Sci. Eng. 2022, 211, 110220. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Zhong, X.; Zhang, S.; Pu, H.; Zhao, J.X. Development of silicon quantum dots based nano-fluid for enhanced oil recovery in tight Bakken cores. Fuel 2020, 277, 118203. [Google Scholar] [CrossRef]
- Mariyate, J.; Bera, A. Recent progresses of microemulsions-based nanofluids as a potential tool for enhanced oil recovery. Fuel 2021, 306, 121640. [Google Scholar] [CrossRef]
- Nazar, M.F.; Shah, S.S.; Khosa, M.A. Microemulsions in Enhanced Oil Recovery: A Review. Pet. Sci. Technol. 2011, 29, 1353–1365. [Google Scholar] [CrossRef]
- Suleimanov, B.A.; Ismailov, F.S.; Veliyev, E.F. Nanofluid for enhanced oil recovery. J. Pet. Sci. Eng. 2011, 78, 431–437. [Google Scholar] [CrossRef]
- Wijaya, N.; Sheng, J. Effect of compaction and imbibition on benefits of drawdown management in shale oil production: Uncertainty in recovery driving mechanisms. J. Pet. Sci. Eng. 2022, 210, 110014. [Google Scholar] [CrossRef]
- Viades-Trejo, J.; Gracia-Fadrique, J. Spinning drop method: From Young–Laplace to Vonnegut. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 549–552. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Anthony, L.S.; Perumal, V.; Mohamed, N.M.; Balakrishnan, S.R.; Gopinath, S.C.B. Characterization of synthesized nanoparticles for medical devices: Current techniques and recent advances. In Nanoparticles in Analytical and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2021; pp. 223–245. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, X.; Wu, Y.; Dai, C.; Gao, M.; Yan, R.; Cheng, Y.; Li, Y.; Song, X.; Wang, X.; et al. Development, performance evaluation and enhanced oil recovery regulations of a zwitterionic viscoelastic surfactant fracturing-flooding system. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127568. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, J.J. Discussion of liquid threshold pressure gradient. Petroleum 2017, 3, 232–236. [Google Scholar] [CrossRef]
- Ding, B.; Luo, J.; Geng, X.; Jia, C.; He, L.P.; Wang, P.M.; Peng, B.L. Visual evaluation method for fluids in cores based on low field nuclear magnetic resonance technology. Oilfield Chem. 2018, 35, 170175. [Google Scholar]
- Fan, J.; Qu, X.; Wang, C.; Lei, Q.; Cheng, L.; Yang, Z. Natural fracture distribution and a new method predicting effective fractures in tight oil reservoirs in Ordos Basin, NW China. Pet. Explor. Dev. 2016, 43, 806–814. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Yang, H.; Liu, Q.; Tian, G. Experimental study on relationship between fracture propagation and pumping parameters under constant pressure injection conditions. Fuel 2022, 307, 121789. [Google Scholar] [CrossRef]
- Lu, C.; Jiang, H.; Yang, J.; Yang, H.; Cheng, B.; Zhang, M.; He, J.; Li, J. Simulation and optimization of hydraulic fracturing in shale reservoirs: A case study in the Permian Lucaogou formation, China. Energy Rep. 2022, 8, 2558–2573. [Google Scholar] [CrossRef]
- Lu, C.; Jiang, H.; Qu, S.; Zhang, M.; He, J.; Xiao, K.; Yang, H.; Yang, J.; Li, J. Hydraulic fracturing design for shale oils based on sweet spot mapping: A case study of the Jimusar formation in China. J. Pet. Sci. Eng. 2022, 214, 110568. [Google Scholar] [CrossRef]
- Huh, C. Interfacial tensions and solubilizing ability of a microemulsion phase that coexists with oil and brine. J. Colloid Interface Sci. 1979, 71, 408–426. [Google Scholar] [CrossRef]
- Wijaya, N.; Sheng, J.J. Mitigating near-fracture blockage and enhancing oil recovery in tight reservoirs by adding surfactants in hydraulic fracturing fluid. J. Pet. Sci. Eng. 2020, 185, 106611. [Google Scholar] [CrossRef]
- Paternina, C.A.; Quintero, H.; Mercado, R. Improving the interfacial performance and the adsorption inhibition of an extended-surfactant mixture for enhanced oil recovery using different hydrophobicity nanoparticles. Fuel 2023, 350, 128760. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, L.; Luan, H.; Ren, J.; Xu, C.; Li, G.; Xiao, H.; Zhou, Z.; Zhang, L. Synergism for lowering interfacial tensions between betaines and extended surfactants: The role of self-regulating molecular size. J. Mol. Liq. 2023, 378, 121605. [Google Scholar] [CrossRef]
- Chen, Y.; Narayan, S.; Dutcher, C.S. Phase-Dependent Surfactant Transport on the Microscale: Interfacial Tension and Droplet Coalescence. Langmuir 2020, 36, 14904–14923. [Google Scholar] [CrossRef]
- Hou, X.; Sheng, J.J. Experimental study on the imbibition mechanism of the Winsor type I surfactant system with ultra-low IFT in oil-wet shale oil reservoirs by NMR. J. Pet. Sci. Eng. 2022, 216, 110785. [Google Scholar] [CrossRef]
- Mirchi, V.; Saraji, S.; Goual, L.; Piri, M. Dynamic interfacial tension and wettability of shale in the presence of surfactants at reservoir conditions. Fuel 2015, 148, 127–138. [Google Scholar] [CrossRef]
- Alvarez, J.O.; Saputra, I.W.R.; Schechter, D.S. Potential of Improving Oil Recovery with Surfactant Additives to Completion Fluids for the Bakken. Energy Fuels 2017, 31, 5982–5994. [Google Scholar] [CrossRef]
- Qi, Z.; Han, M.; Chen, S.; Wang, J. Surfactant enhanced imbibition in carbonate reservoirs: Effect of IFT reduction and surfactant partitioning. JCIS Open 2022, 5, 100045. [Google Scholar] [CrossRef]
- Ren, X.; Li, A.; Liu, P.; He, B. Experiments and Modeling on the Influence of Interfacial Tension on Imbibition Height of Low-Permeability Reservoir. Geofluids 2020, 2020, 8837897. [Google Scholar] [CrossRef]
- Shen, A.; Liu, Y.; Bai, M.; Liang, S.; Wang, F.; Cai, B.; Gao, Y. Surfactant Effects of Wettability Alteration and Low IFT on Countercurrent Imbibition for Tight Oil Formation. Energy Fuels 2018, 32, 12365–12372. [Google Scholar] [CrossRef]
- Wu, J.; Mei, P.; Lai, L. Microemulsion and interfacial properties of anionic/nonionic surfactant mixtures based on sulfonate surfactants: The influence of alcohol. J. Mol. Liq. 2023, 371, 120814. [Google Scholar] [CrossRef]
- Morrow, N.R.; Mason, G. Recovery of oil by spontaneous imbibition. Curr. Opin. Colloid Interface Sci. 2001, 6, 321–337. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Huseynov, E.; Garibov, A.; Mehdiyeva, R. TEM and SEM study of nano SiO2 particles exposed to influence of neutron flux. J. Mater. Res. Technol. 2016, 5, 213–218. [Google Scholar] [CrossRef]
- Hua, X.; Bevan, M.; Frechette, J. Competitive Adsorption between Nanoparticles and Surface Active Ions for the Oil-Water Interface. Langmuir 2018, 34, 4830–4842. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Yue, X.; Shi, X.; Ling, S.; Zhang, B.; Li, X. Effect of dynamic pseudo threshold pressure gradient on well production performance in low-permeability and tight oil reservoirs. J. Pet. Sci. Eng. 2019, 173, 69–76. [Google Scholar] [CrossRef]
- Lu, C.; Qin, X.; Ma, C.; Yu, L.; Geng, L.; Bian, H.; Zhang, K.; Zhou, Y. Investigation of the impact of threshold pressure gradient on gas production from hydrate deposits. Fuel 2022, 319, 123569. [Google Scholar] [CrossRef]
- Xu, K.; Liang, T.; Zhu, P.; Qi, P.; Lu, J.; Huh, C.; Balhoff, M. A 2.5-D glass micromodel for investigation of multi-phase flow in porous media. Lab Chip 2017, 17, 640–646. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Guo, C.; Su, H.; Yu, F.; Ma, M.; Wang, L.; Jiang, H. Mechanisms of water block removal by surfactant micellar solutions in low permeability reservoirs. Pet. Explor. Dev. 2022, 49, 394–405. [Google Scholar] [CrossRef]
- Yaich, E.; Williams, S.; Bowser, A.; Goddard, P.; de Souza, O.C.D.; Foster, R.A. A Case Study: The Impact of Soaking on Well Performance in the Marcellus. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, San Antonio, TX, USA, 20–22 July 2015. [Google Scholar] [CrossRef]





| Solutions | Characteristic | Target Basin | Conclusions Drawn | Source |
|---|---|---|---|---|
| Surfactants (anionic and non-ionic) | Wettability alteration and IFT reduction on the spontaneous imbibition process | Sichuan, China | The anionic surfactants produced oil more than the non-ionic surfactants because it changed oil-wet shale to more water-wet. | Liu et al., 2019 [15] |
| Surfactants (fluoride and gemini) | Fluoride changed the wettability to oil-wet, while the gemini surfactant has strong interfacial activity | Ordos, China | Reducing the oil–water IFT and altering the wettability can achieve pressure reduction and increase fluid injection. Fluorine had more obvious pressure reduction. | Liu et al., 2022 [16] |
| Micro-emulsion (Nano-emulsion) | Changing wettability and reducing IFT to 0.0038 mN/m at 0.2 wt%, | ShengLi Oilfield, China | In addition to IFT reduction and wettability alteration, the emulsification and solubilization effects are revealed to be the dominant mechanisms. | Qu et al., 2022 [17] |
| Nano-fluids (consisting of nanoparticles) | Average hydrodynamic diameter was 2.8 ± 0.4 nm | Bakken, United States | Both the disjoining pressure and synergistic effect between the surfactant and nanoparticles benefit oil recovery. | Zhou et al., 2020 [18] |
| micro-emulsion-based nano-fluids | Ultra-low IFT between oil and water, bring down the IFT to the value of 0.001 mN/m | Not Given | The implementation of nanoparticles nano-fluid stabilizes the surface-active materials. It requires lesser quantity of surfactant. | Mariyate et al., 2021 [19] |
| Chemical | Surface Tension (mN/m) | Concentration (%) | Absorption Loss Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | First Round | Second Round | Third Round | Initial | First Round | Second Round | Third Round | ||
| TCJ | 30.730 | 31.462 | 32.068 | 32.957 | 0.2 | 0.124 | 0.094 | 0.063 | 62.59 |
| MEI | 39.164 | 39.326 | 40.915 | 42.843 | 0.3 | 0.261 | 0.141 | 0.067 | 77.78 |
| MEN | 32.093 | 35.946 | 36.958 | 37.389 | 0.6 | 0.552 | 0.343 | 0.280 | 53.36 |
| SPN | 25.443 | 27.548 | 28.129 | 29.876 | 0.3 | 0.145 | 0.114 | 0.054 | 82.0 |
| Core | Chemical | Length (cm) | Diameter (cm) | Injection Pressure (MPa) | Initial Permeability (mD) | Permeability after Injection (mD) | Permeability Recovery Rate (%) |
|---|---|---|---|---|---|---|---|
| 1# | TCJ | 10.015 | 2.499 | 0.260 | 26.4530 | 14.2010 | 53.68 |
| 4# | MEI | 10.106 | 2.506 | 0.218 | 31.7302 | 11.0875 | 34.94 |
| 2# | MEN | 10.024 | 2.503 | 0.232 | 29.9381 | 17.5948 | 58.77 |
| 3# | SPN | 10.094 | 2.492 | 0.214 | 32.4305 | 13.2333 | 40.81 |
| Solution | Type | IFT Reduction | Wet | Droplet Radius | Cluster | Adsorption | Core Damage Rate | Threshold Pressure Gradient | “Small-Size Oil” Effect | General |
|---|---|---|---|---|---|---|---|---|---|---|
| TCJ | Traditional surfactant | I | II | III | III | I | I | II | II | II |
| MEI | Micro-emulsion | II | I | II | II | II | III | II | II | II |
| MEN | Micro-emulsion | II | II | I | I | I | I | I | I | I |
| SPN | Nano-fluid with nano particles | II | II | II | III | III | II | III | Not tested | III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Geng, X.; Ding, B.; Liu, W.; Jiang, K.; Xu, Q.; Guan, B.; Peng, L.; Peng, H. A Comparative Study of Surfactant Solutions Used for Enhanced Oil Recovery in Shale and Tight Formations: Experimental Evaluation and Numerical Analysis. Molecules 2024, 29, 3293. https://doi.org/10.3390/molecules29143293
Chen W, Geng X, Ding B, Liu W, Jiang K, Xu Q, Guan B, Peng L, Peng H. A Comparative Study of Surfactant Solutions Used for Enhanced Oil Recovery in Shale and Tight Formations: Experimental Evaluation and Numerical Analysis. Molecules. 2024; 29(14):3293. https://doi.org/10.3390/molecules29143293
Chicago/Turabian StyleChen, Weidong, Xiangfei Geng, Bin Ding, Weidong Liu, Ke Jiang, Qinglong Xu, Baoshan Guan, Lin Peng, and Huan Peng. 2024. "A Comparative Study of Surfactant Solutions Used for Enhanced Oil Recovery in Shale and Tight Formations: Experimental Evaluation and Numerical Analysis" Molecules 29, no. 14: 3293. https://doi.org/10.3390/molecules29143293
APA StyleChen, W., Geng, X., Ding, B., Liu, W., Jiang, K., Xu, Q., Guan, B., Peng, L., & Peng, H. (2024). A Comparative Study of Surfactant Solutions Used for Enhanced Oil Recovery in Shale and Tight Formations: Experimental Evaluation and Numerical Analysis. Molecules, 29(14), 3293. https://doi.org/10.3390/molecules29143293





