Synthesis of a Borrelia burgdorferi-Derived Muropeptide Standard Fragment Library
Abstract
:1. Introduction
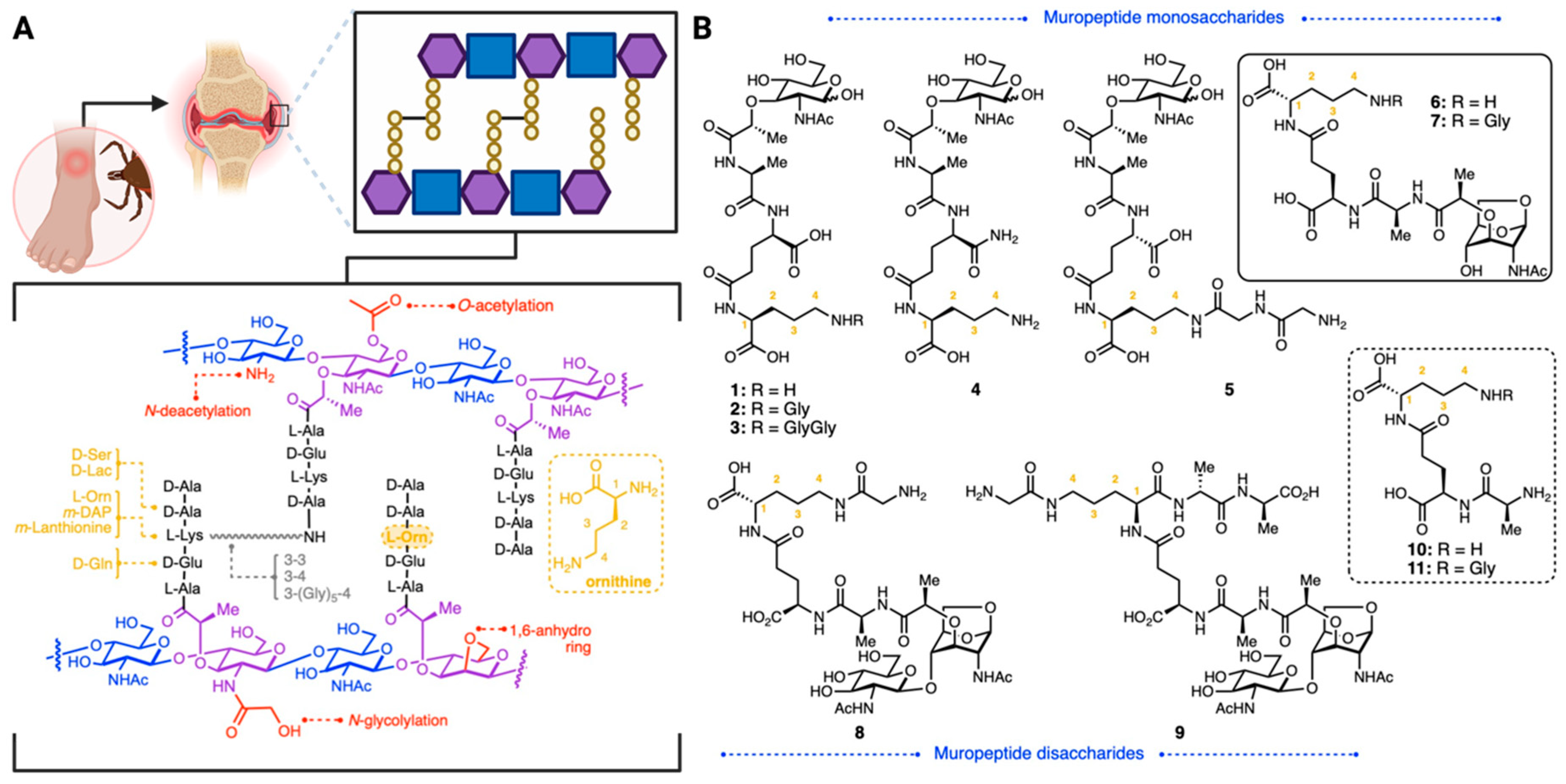
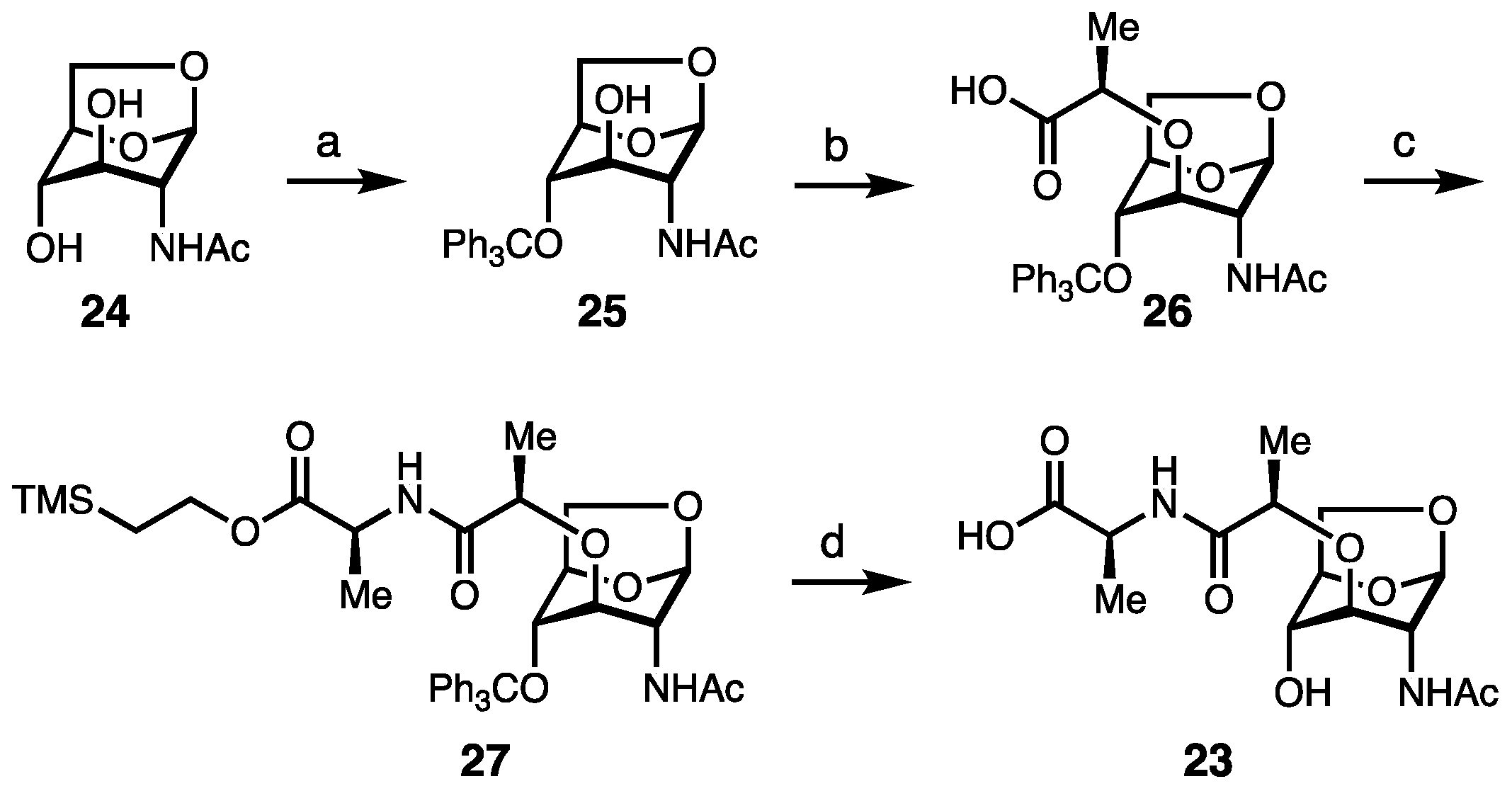
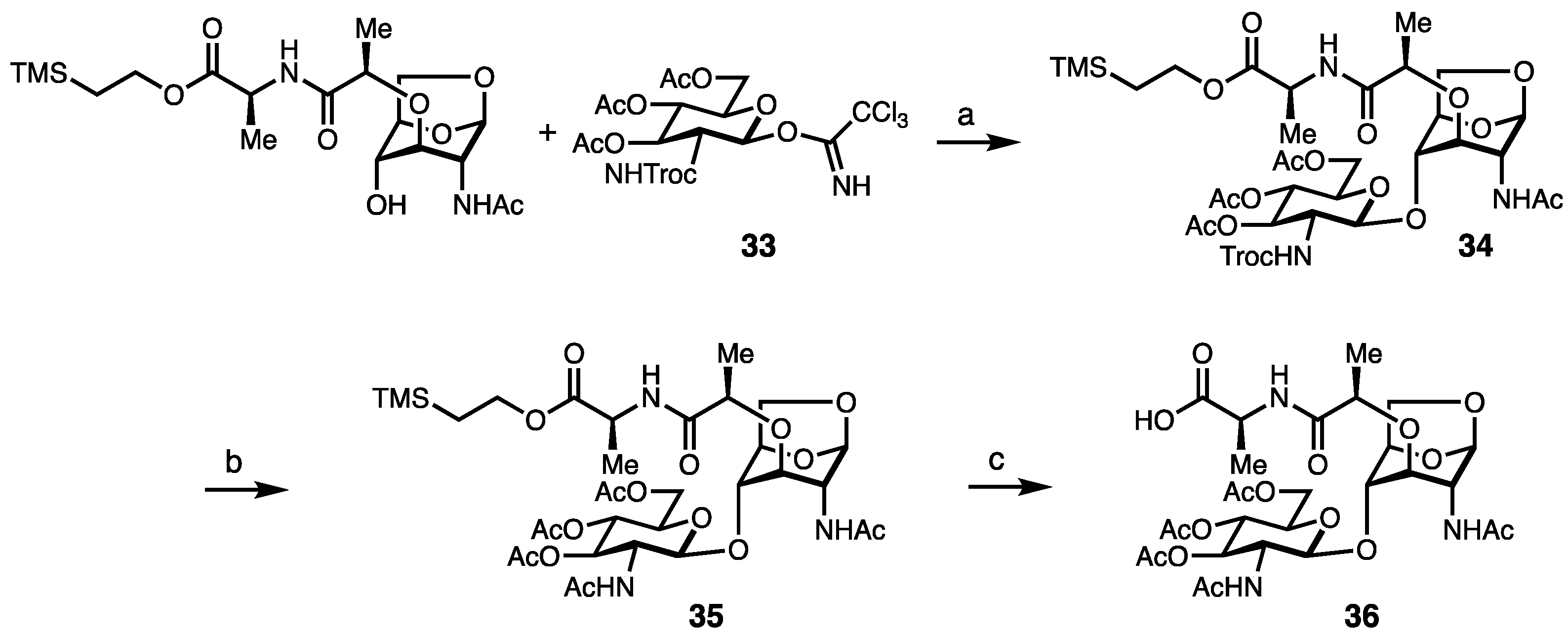
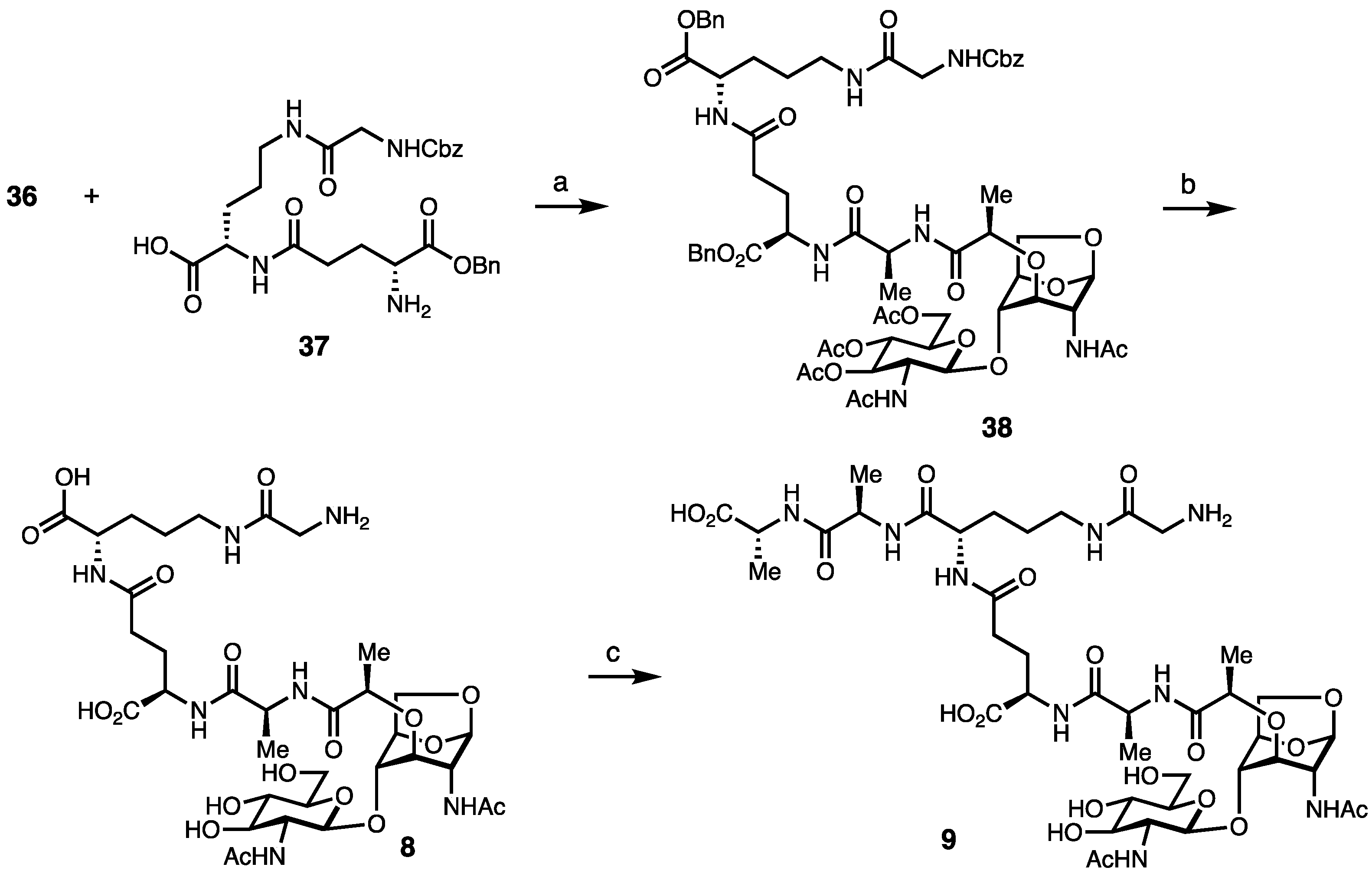
2. Results and Discussion
Synthesis of Borrelia-Inspired Muropeptides
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Instrumentation
4.3. General Information and Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mead, P. Epidemiology of Lyme Disease. Infect. Dis. Clin. N. Am. 2022, 36, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Hatchette, T.F.; Davis, I.; Johnston, B.L. Lyme disease: Clinical diagnosis and treatment. Can. Commun. Dis. Rep. 2014, 40, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Arvikar, S.L.; Steere, A.C. Diagnosis and treatment of Lyme arthritis. Infect. Dis. Clin. N. Am. 2015, 29, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.; Hernandez, J.; Bloom, B.J.; Coburn, J.; Aversa, J.M.; Steere, A.C. Lack of Borrelia burgdorferi DNA in synovial samples from patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 1999, 42, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; McHugh, G.A.; Damle, N.; Sikand, V.K.; Glickstein, L.; Steere, A.C. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 2011, 63, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Jutras, B.L.; Lochhead, R.B.; Kloos, Z.A.; Biboy, J.; Strle, K.; Booth, C.J.; Govers, S.K.; Gray, J.; Schumann, P.; Vollmer, W.; et al. Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc. Natl. Acad. Sci. USA 2019, 116, 13498–13507. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.T.; Drouin, E.E.; Pianta, A.; Strle, K.; Wang, Q.; Costello, C.E.; Steere, A.C. A Highly Expressed Human Protein, Apolipoprotein B-100, Serves as an Autoantigen in a Subgroup of Patients with Lyme Disease. J. Infect. Dis. 2015, 212, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.T.; Strle, K.; Drouin, E.E.; Pianta, A.; Arvikar, S.L.; Wang, Q.; Costello, C.E.; Steere, A.C. Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J. Autoimmun. 2016, 69, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Pianta, A.; Drouin, E.E.; Crowley, J.T.; Arvikar, S.; Strle, K.; Costello, C.E.; Steere, A.C. Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin. Immunol. 2015, 160, 336–341. [Google Scholar] [CrossRef]
- Strle, K.; Sulka, K.B.; Pianta, A.; Crowley, J.T.; Arvikar, S.L.; Anselmo, A.; Sadreyev, R.; Steere, A.C. T-Helper 17 Cell Cytokine Responses in Lyme Disease Correlate with Borrelia burgdorferi Antibodies During Early Infection and with Autoantibodies Late in the Illness in Patients with Antibiotic-Refractory Lyme Arthritis. Clin. Infect. Dis. 2017, 64, 930–938. [Google Scholar] [CrossRef]
- Drouin, E.E.; Seward, R.J.; Strle, K.; McHugh, G.; Katchar, K.; Londoño, D.; Yao, C.; Costello, C.E.; Steere, A.C. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2013, 65, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Girschick, H.J. Lyme borreliosis: From infection to autoimmunity. Clin. Microbiol. Infect. 2004, 10, 598–614. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T.; Uehara, T. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol. Mol. Biol. Rev. 2008, 72, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Arora, G.; Rosen, C.E.; Kloos, Z.; Cao, Y.; Cerny, J.; Sajid, A.; Hoornstra, D.; Golovchenko, M.; Rudenko, N.; et al. A human secretome library screen reveals a role for Peptidoglycan Recognition Protein 1 in Lyme borreliosis. PLOS Pathog. 2020, 16, e1009030. [Google Scholar] [CrossRef]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; Medzhitov, R. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 2009, 227, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C.A., Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002, 296, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Höltje, J.V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 1998, 62, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.J. Peptidoglycans (mucopeptides): Structure, function, and variations. Ann. N. Y Acad. Sci. 1974, 235, 29–51. [Google Scholar] [CrossRef]
- Grimes, C.L.; Ariyananda Lde, Z.; Melnyk, J.E.; O’Shea, E.K. The Innate Immune Protein Nod2 Binds Directly to MDP, a Bacterial Cell Wall Fragment. J. Am. Chem. Soc. 2012, 134, 13535–13537. [Google Scholar] [CrossRef]
- Mo, J.; Boyle, J.P.; Howard, C.B.; Monie, T.P.; Davis, B.K.; Duncan, J.A. Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP. J. Biol. Chem. 2012, 287, 23057–23067. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Yan, Y.; Narui, Y.; Ingersoll, S.A.; Ayyadurai, S.; Charania, M.A.; Zhou, F.; Wang, B.; Salaita, K.; Sitaraman, S.V.; et al. L-Ala-gamma-D-Glu-meso-diaminopimelic acid (DAP) interacts directly with leucine-rich region domain of nucleotide-binding oligomerization domain 1, increasing phosphorylation activity of receptor-interacting serine/threonine-protein kinase 2 and its interaction with nucleotide-binding oligomerization domain 1. J. Biol. Chem. 2011, 286, 31003–31013. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.C. Anatomy and chemistry of spirochetes. Microbiol. Rev. 1978, 42, 114–160. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.; Benach, J.L.; Habicht, G.S. Isolation, preliminary chemical characterization, and biological activity of Borrelia burgdorferi peptidoglycan. Biochem. Biophys. Res. Commun. 1990, 167, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W. Structural variation in the glycan strands of bacterial peptidoglycan. FEMS Microbiol. Rev. 2008, 32, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Scheurwater, E.M.; Clarke, A.J. The C-terminal domain of Escherichia coli YfhD functions as a lytic transglycosylase. J. Biol. Chem. 2008, 283, 8363–8373. [Google Scholar] [CrossRef] [PubMed]
- Brott, A.S.; Clarke, A.J. Peptidoglycan O-Acetylation as a Virulence Factor: Its Effect on Lysozyme in the Innate Immune System. Antibiotics 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Hesek, D.; Lee, M.; Zhang, W.; Noll, B.C.; Mobashery, S. Total Synthesis of N-Acetylglucosamine-1,6-anhydro-N-acetylmuramylpentapeptide and Evaluation of Its Turnover by AmpD from Escherichia coli. J. Am. Chem. Soc. 2009, 131, 5187–5193. [Google Scholar] [CrossRef] [PubMed]
- Goodell, E.W. Recycling of murein by Escherichia coli. J. Bacteriol. 1985, 163, 305–310. [Google Scholar] [CrossRef]
- Cookson, B.T.; Cho, H.L.; Herwaldt, L.A.; Goldman, W.E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect. Immun. 1989, 57, 2223–2229. [Google Scholar] [CrossRef]
- Rosenthal, R.S.; Nogami, W.; Cookson, B.T.; Goldman, W.E.; Folkening, W.J. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect. Immun. 1987, 55, 2117–2120. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K.; Rosenthal, R.S. Release of soluble peptidoglycan from growing conococci: Demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 1980, 29, 914–925. [Google Scholar] [CrossRef]
- Paik, D.; Monahan, A.; Caffrey, D.R.; Elling, R.; Goldman, W.E.; Silverman, N. SLC46 Family Transporters Facilitate Cytosolic Innate Immune Recognition of Monomeric Peptidoglycans. J. Immunol. 2017, 199, 263–270. [Google Scholar] [CrossRef]
- Melnyk, J.E.; Mohanan, V.; Schaefer, A.K.; Hou, C.-W.; Grimes, C.L. Peptidoglycan Modifications Tune the Stability and Function of the Innate Immune Receptor Nod2. J. Am. Chem. Soc. 2015, 137, 6987–6990. [Google Scholar] [CrossRef]
- Gigg, R.O.Y.; Carroll, P.M. A Convenient Synthesis of Muramic Acid and Other 3-O-Ethers of D-Glucosamine. Nature 1961, 191, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh, S.; Bersch, K.L.; Ramsey, J.; Harmon, T.; Prather, B.; Genova, L.A.; Grimes, C.L. Synthesis of Bacterial-Derived Peptidoglycan Cross-Linked Fragments. J. Org. Chem. 2020, 85, 16243–16253. [Google Scholar] [CrossRef]
- Lafont, D.; Boullanger, P.; Cadas, O.; Descotes, G. A Mild Procedure for the Preparation of 1,6-Anhydro-β-D-hexopyranoses and Derivatives. Synthesis 1989, 1989, 191–194. [Google Scholar] [CrossRef]
- Calvert, M.B.; Mayer, C.; Titz, A. An efficient synthesis of 1,6-anhydro-N-acetylmuramic acid from N-acetylglucosamine. Beilstein J. Org. Chem. 2017, 13, 2631–2636. [Google Scholar] [CrossRef]
- Bersch, K.L.; DeMeester, K.E.; Zagani, R.; Chen, S.; Wodzanowski, K.A.; Liu, S.; Mashayekh, S.; Reinecker, H.-C.; Grimes, C.L. Bacterial Peptidoglycan Fragments Differentially Regulate Innate Immune Signaling. ACS Cent. Sci. 2021, 7, 688–696. [Google Scholar] [CrossRef]
- Crich, D. Mechanism of a Chemical Glycosylation Reaction. Acc. Chem. Res. 2010, 43, 1144–1153. [Google Scholar] [CrossRef]
- Chen, S.; Putnik, R.; Li, X.; Liu, S.; Zhou, J.; Guo, L.; Xu, L.; Temme, S.; Bersch, K.; Gildersleeve, J.C.; et al. PGLYRP-1: Intracellular Receptor for GMTP that Controls Innate Immunity and Mucosal Recovery. Immun. Sneak Peak 2023. Available online: https://ssrn.com/abstract=4663948 (accessed on 1 April 2024). [CrossRef]
- D’Ambrosio, E.A.; Bersch, K.L.; Lauro, M.L.; Grimes, C.L. Differential Peptidoglycan Recognition Assay Using Varied Surface Presentations. J. Am. Chem. Soc. 2020, 142, 10926–10930. [Google Scholar] [CrossRef] [PubMed]
- Lioux, T.; Busson, R.H.C.; Rozenski, J.; Nguyen-Distèche, M.; Frère, J.M.; Herdewijn, P. Synthesis of Peptidoglycan Units with UDP at the Anomeric Position. Collect. Czechoslov. Chem. Commun. 2005, 70, 1615–1641. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putnik, R.; Zhou, J.; Irnov, I.; Garner, E.; Liu, M.; Bersch, K.L.; Jacobs-Wagner, C.; Grimes, C.L. Synthesis of a Borrelia burgdorferi-Derived Muropeptide Standard Fragment Library. Molecules 2024, 29, 3297. https://doi.org/10.3390/molecules29143297
Putnik R, Zhou J, Irnov I, Garner E, Liu M, Bersch KL, Jacobs-Wagner C, Grimes CL. Synthesis of a Borrelia burgdorferi-Derived Muropeptide Standard Fragment Library. Molecules. 2024; 29(14):3297. https://doi.org/10.3390/molecules29143297
Chicago/Turabian StylePutnik, Rachel, Junhui Zhou, Irnov Irnov, Elise Garner, Min Liu, Klare L. Bersch, Christine Jacobs-Wagner, and Catherine Leimkuhler Grimes. 2024. "Synthesis of a Borrelia burgdorferi-Derived Muropeptide Standard Fragment Library" Molecules 29, no. 14: 3297. https://doi.org/10.3390/molecules29143297





