User-Friendly and Responsive Electrochemical Detection Approach for Triclosan by Nano-Metal–Organic Framework
Abstract
1. Introduction
2. Results
2.1. Structure and Morphology of Nano-Cu-BTC
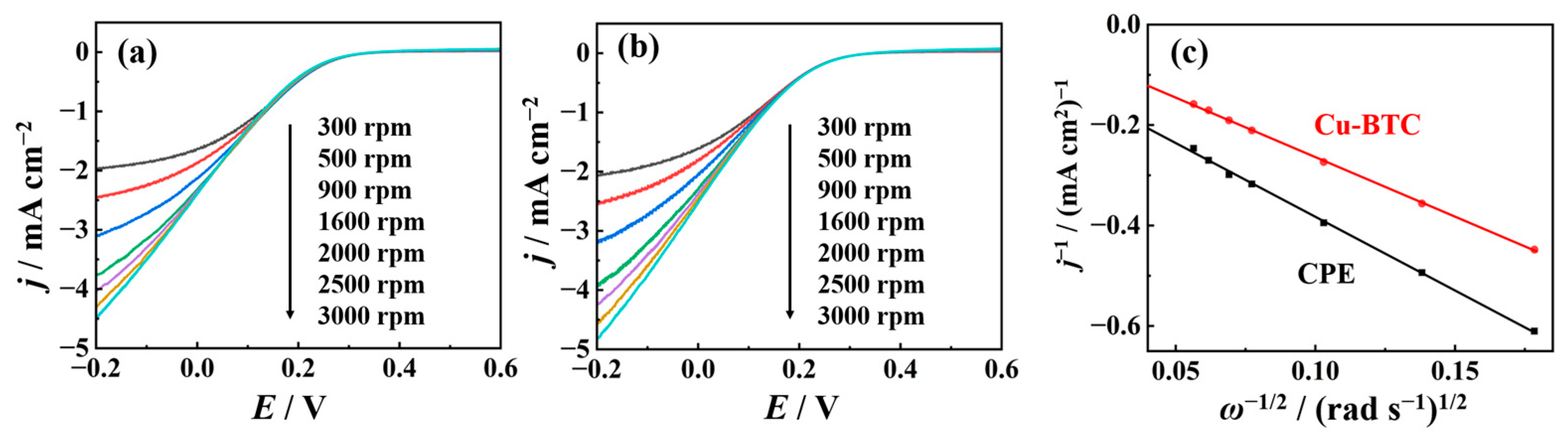
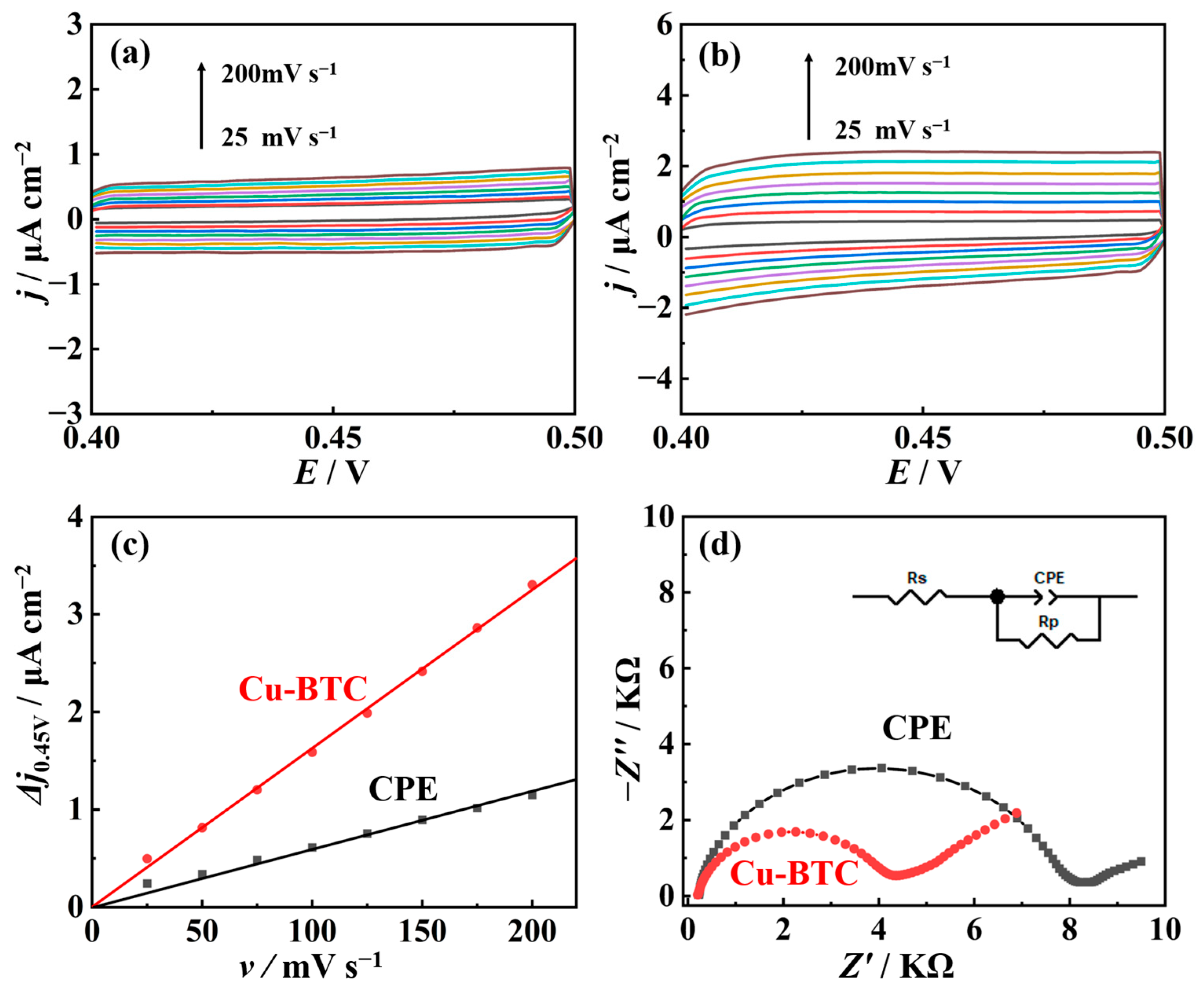
2.2. Electrochemical Performance of Nano-Cu-BTC
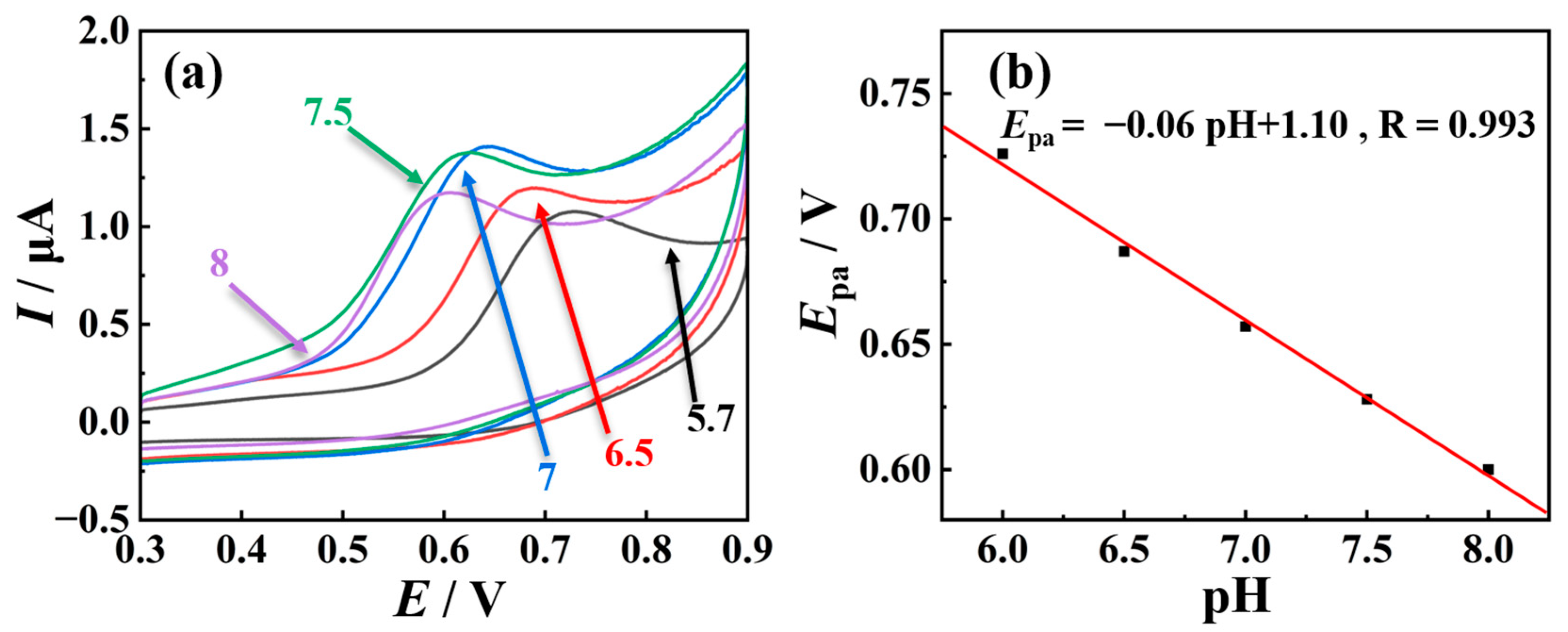
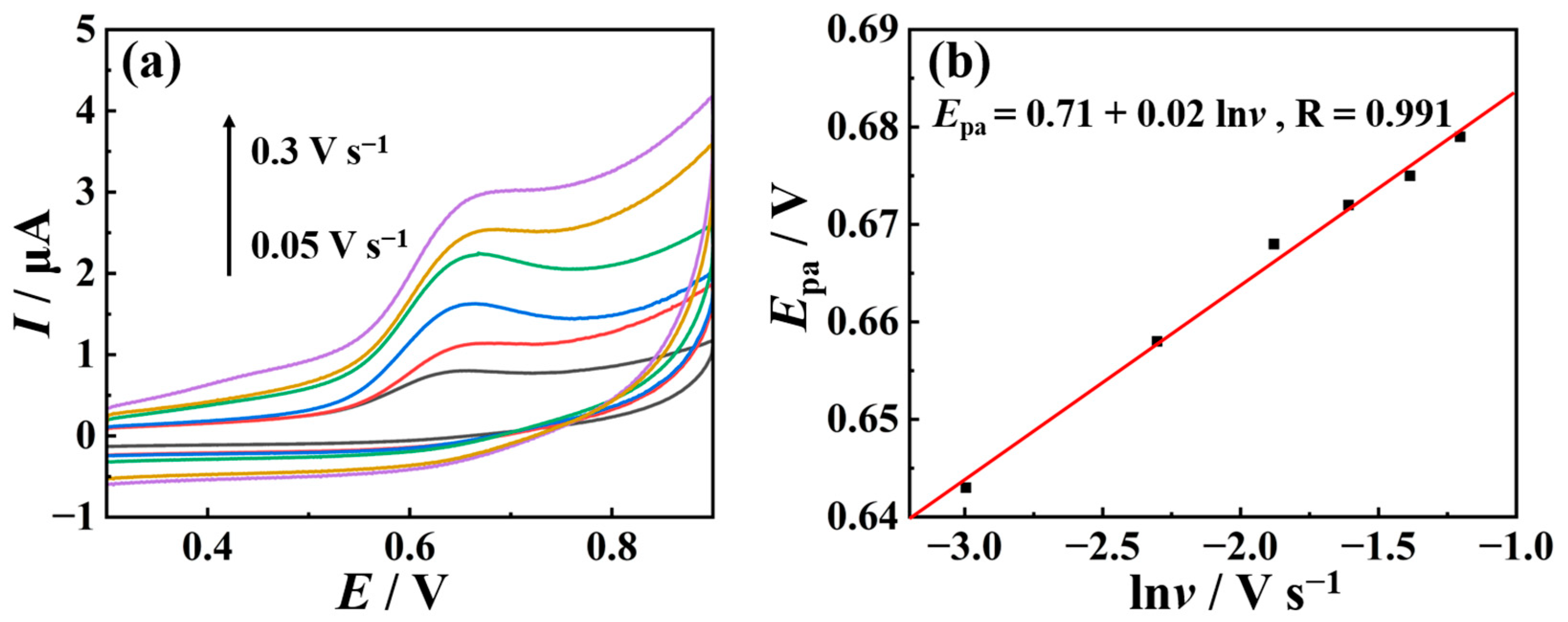
2.3. Mechanistic Study of TSC Oxidation Reaction
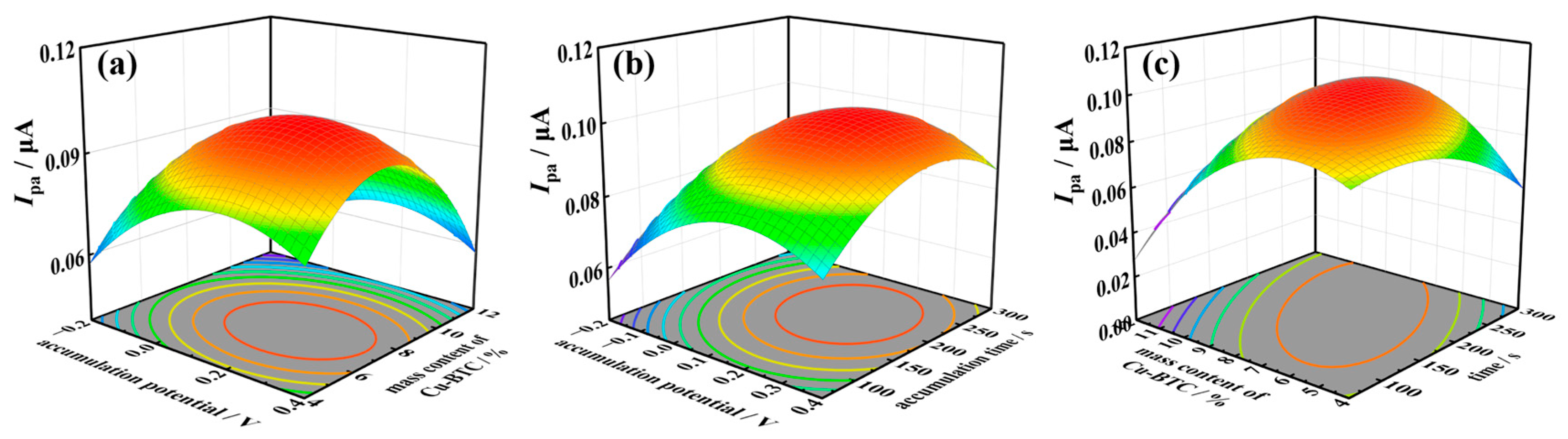
2.4. TSC Detection Method Based on Cu-BTC
3. Materials and Methods
3.1. Chemicals and Instrument
3.2. Synthesis of Nano-Cu-BTC
3.3. Preparation of Nano-Cu-BTC Modified CPE and CPE
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, R.D.; Coast, J. Antimicrobial Resistance: A Global Response. Bull. World Health Organ. 2002, 80, 126–133. Available online: https://www.scielosp.org/article/ssm/content/raw/?resource_ssm_path=/media/assets/bwho/v80n2/a08v80n2.pdf (accessed on 1 March 2024). [PubMed]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. Mechanisms of Antimicrobial Resistance in Bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Rodricks, J.V.; Swenberg, J.A.; Borzelleca, J.F.; Maronpot, R.R.; Shipp, A.M. Triclosan: A Critical Review of the Experimental Data and Development of Margins of Safety for Consumer Products. Crit. Rev. Toxicol. 2010, 40, 422–484. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current Status, Occurrence, Environmental Risks and Bioaccumulation Potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in US Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. On the Need and Speed of Regulating Triclosan and Triclocarban in the United States; ACS Publications: Washington, DC, USA, 2014; pp. 3603–3611. [Google Scholar]
- Rule, K.L.; Ebbett, V.R.; Vikesland, P.J. Formation of Chloroform and Chlorinated Organics by Free-Chlorine-Mediated Oxidation of Triclosan. Environ. Sci. Technol. 2005, 39, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, N.; Skirrow, R.C.; Osachoff, H.; Wigmore, H.; Clapson, D.J.; Gunderson, M.P.; Van Aggelen, G.; Helbing, C.C. The Bactericidal Agent Triclosan Modulates Thyroid Hormone-Associated Gene Expression and Disrupts Postembryonic Anuran Development. Aquat. Toxicol. 2006, 80, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Fiss, E.M.; Rule, K.L.; Vikesland, P.J. Formation of Chloroform and Other Chlorinated Byproducts by Chlorination of Triclosan-Containing Antibacterial Products. Environ. Sci. Technol. 2007, 41, 2387–2394. [Google Scholar] [CrossRef]
- Kim, D.; Han, J.; Choi, Y. On-Line Solid-Phase Microextraction of Triclosan, Bisphenol A, Chlorophenols, and Selected Pharmaceuticals in Environmental Water Samples by High-Performance Liquid Chromatography–Ultraviolet Detection. Anal. Bioanal. Chem. 2013, 405, 377–387. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Thomaidis, N.S.; Kannan, K. Widespread Occurrence of Bisphenol A Diglycidyl Ethers, p-Hydroxybenzoic Acid Esters (Parabens), Benzophenone Type-UV Filters, Triclosan, and Triclocarban in Human Urine from Athens, Greece. Sci. Total Environ. 2014, 470, 1243–1249. [Google Scholar] [CrossRef]
- Vitku, J.; Horackova, L.; Kolatorova, L.; Duskova, M.; Skodova, T.; Simkova, M. Derivatized versus Non-Derivatized LC-MS/MS Techniques for the Analysis of Estrogens and Estrogen-like Endocrine Disruptors in Human Plasma. Ecotoxicol. Environ. Saf. 2023, 260, 115083. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Becerril, E.; Garcia-Jares, C.; Llompart, M. Trace Analysis of Parabens, Triclosan and Related Chlorophenols in Water by Headspace Solid-Phase Microextraction with In Situ Derivatization and Gas Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2009, 1216, 4693–4702. [Google Scholar] [CrossRef] [PubMed]
- Arismendi, D.; Díaz, K.; Aguilera-Marabolí, N.; Sepúlveda, B.; Richter, P. Rotating-Disk Sorptive Extraction for the Determination of Sex Hormones and Triclosan in Urine by Gas Chromatography-Mass Spectrometry: Clean-up Integrated Steps and Improved Derivatization. Microchem. J. 2020, 158, 105149. [Google Scholar] [CrossRef]
- Song, S.; Song, Q.J.; Chen, Z. Online Phototransformation–Flow Injection Chemiluminescence Determination of Triclosan. Anal. Bioanal. Chem. 2007, 387, 2917–2922. [Google Scholar] [CrossRef]
- Montaseri, H.; Forbes, P.B.C. A Review of Monitoring Methods for Triclosan and Its Occurrence in Aquatic Environments. TrAC Trends Anal. Chem. 2016, 85, 221–231. [Google Scholar] [CrossRef]
- Sri, V.R.; Shwetharani, R.; Mohammed, J.; Mabkhoot, A.; Balakrishna, R.G.; Harraz, F.A. Review on Electrochemical Sensing of Triclosan Using Nanostructured Semiconductor Materials. ChemElectroChem 2022, 9, e202101664. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Li, Y.; Lan, J.; Zhao, Z.; Shi, R. Copper-Zinc Nanoparticle-Decorated Nitrogen-Doped Carbon Composite for Electrochemical Determination of Triclosan. Microchim. Acta 2024, 191, 155. [Google Scholar] [CrossRef]
- Cetiner, R.; Sarilmaz, A.; Ozel, F.; Bas, S.Z.; Ozmen, M. A New Bluetooth-Assisted Sensor Based on Lab-Made Screen-Printed Electrode Modified with Ni-Ag2S for Electrochemical Detection of Triclosan. Electrochim. Acta 2024, 489, 144223. [Google Scholar] [CrossRef]
- Dutta, K.; Subramaniam, P. An O-Phenylenediamine and MWCNT-Based Electrochemical Sensor for the Detection of Triclosan by Cyclic Voltammetry. J. Electrochem. Soc. 2024, 171, 057502. [Google Scholar] [CrossRef]
- Wu, T.; Li, T.; Liu, Z.; Guo, Y.; Dong, C. Electrochemical Sensor for Sensitive Detection of Triclosan Based on Graphene/Palladium Nanoparticles Hybrids. Talanta 2017, 164, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lu, Q. A Facial Electrochemical Method for Efficient Triclosan Detection Constructed on Dodecanethiol Monolayers Functioned Au Nanoparticles-ErGO. Microchem. J. 2022, 175, 107144. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.; Pollet, B.G.; Kalanur, S.S.; Shetti, N.P. Preparation and Performance of WO3/rGO Modified Carbon Sensor for Enhanced Electrochemical Detection of Triclosan. Electrochim. Acta 2022, 429, 141010. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Mullangi, D.; Evans, H.A.; Yildirim, T.; Wang, Y.; Deng, Z.; Zhang, Z.; Mai, T.T.; Wei, F.; Wang, J.; Hight Walker, A.R. Noncryogenic Air Separation Using Aluminum Formate Al (HCOO) 3 (ALF). J. Am. Chem. Soc. 2023, 145, 9850–9856. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Mazloum-Ardakani, M.; Alizadeh, S.; Nematollahi, D. Template-Free Electrodeposition of Sponge-like Porous Polymer Interwoven with the Bi-Metallic Metal-Organic Framework and Reduced Graphene Oxide and Application in Energy Storage Device. J. Energy Storage 2022, 55, 105381. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Z.; Evans, H.A.; Mullangi, D.; Kang, C.; Peh, S.B.; Wang, Y.; Brown, C.M.; Wang, J.; Canepa, P. Exclusive Recognition of CO2 from Hydrocarbons by Aluminum Formate with Hydrogen-Confined Pore Cavities. J. Am. Chem. Soc. 2023, 145, 11643–11649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, Y.; Xu, K.; Zhong, Y.; He, C.; Jiang, L.; Sun, J.; Rao, Z.; Zhu, J.; Huang, J.; et al. Natural Oxidase-Mimicking Copper-Organic Frameworks for Targeted Identification of Ascorbate in Sensitive Sweat Sensing. Nat. Commun. 2023, 14, 69. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, Q.; Hu, P.; Ji, L. Tunable Non-Enzymatic Glucose Electrochemical Sensing Based on the Ni/Co Bimetallic MOFs. Molecules 2023, 28, 5649. [Google Scholar] [CrossRef]
- Kumar, L.; Tetala, K.K.R. Fabrication of a Bi-metallic Metal Organic Framework Nanocomposite for Selective and Sensitive Detection of Triclosan. Anal. Methods 2023, 15, 2408–2416. [Google Scholar] [CrossRef]
- Sahoo, S.J.; Barik, B.; Maji, B.; Nayak, P.S.; Behera, N.; Dash, P. A redox accessible Cu-BTC metal organic framework-based nanocomposite for selective and sensitive electrochemical sensing of Triclosan in real sample. J. Electroanal. Chem. 2023, 943, 117589. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Wu, K.; Yang, N. Tunable electrochemistry of electrosynthesized copper metal–organic frameworks. Adv. Funct. Mater. 2018, 28, 1706961. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Wu, C.; Wu, K. Strategy for Highly Sensitive Electrochemical Sensing: In Situ Coupling of a Metal–Organic Framework with Ball-Mill-Exfoliated Graphene. Anal. Chem. 2019, 91, 6043–6050. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, L.; Xu, F.; Zhang, J.; Jiao, C.; Li, F.; Li, Z.; Wang, S.; Wang, Z.; Jiang, X. Nanosized Cu-MOFs Induced by Graphene Oxide and Enhanced Gas Storage Capacity. Energy Environ. Sci. 2013, 6, 818–823. [Google Scholar] [CrossRef]
- Randles, J.E.B. A Cathode Ray Polarograph. Trans. Faraday Soc. 1948, 44, 322–327. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Wu, C.; Wu, K. Ball-Mill-Exfoliated Graphene: Tunable Electrochemistry and Phenol Sensing. Small 2019, 15, 1805567. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C Nanoparticles Dispersed on Hierarchical Carbon Microflowers for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar] [CrossRef]
- Anson, F.C. Innovations in the Study of Adsorbed Reactants by Chronocoulometry. Anal. Chem. 1966, 38, 54–57. [Google Scholar] [CrossRef]
- Li, C.; Hao, J.; Wu, K. Triethylamine-controlled Cu-BTC Frameworks for Electrochemical Sensing Fish Freshness. Anal. Chim. Acta 2019, 1085, 68–74. [Google Scholar] [CrossRef]
- Safwat, N.; Mahmoud, A.M.; Abdel-Ghany, M.F.; Ayad, M.F. In Situ Monitoring of Triclosan in Environmental Water with Subnanomolar Detection Limits Using Eco-Friendly Electrochemical Sensors Modified with Cyclodextrins. Environ. Sci. Process. Impacts 2021, 23, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Safwat, N.; Mahmoud, A.M.; Abdel-Ghany, M.F.; Ayad, M.F. Eco-Friendly Monitoring of Triclosan as an Emerging Antimicrobial Environmental Contaminant Utilizing Electrochemical Sensors Modified with CNTs Nanocomposite Transducer Layer. BMC Chem. 2023, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Luyen, N.D.; Toan, T.T.T.; Trang, H.T.; Nguyen, V.T.; Son LV, T.; Thanh, T.S.; Thanh, N.M.; Quy, P.T.; Khieu, D.Q. Electrochemical Determination of Triclosan Using ZIF-11/Activated Carbon Derived from the Rice Husk Modified Electrode. J. Nanomater. 2021, 2021, 8486962. [Google Scholar] [CrossRef]
- Santana, E.R.; Martins, E.C.; Spinelli, A. Electrode Modified with Nitrogen-Doped Graphene Quantum Dots Supported in Chitosan for Triclocarban Monitoring. Microchem. J. 2021, 167, 106297. [Google Scholar] [CrossRef]
- Santana, E.R.; Spinelli, A. Electrode Modified with Graphene Quantum Dots Supported in Chitosan for Electrochemical Methods and Non-Linear Deconvolution of Spectra for Spectrometric Methods: Approaches for Simultaneous Determination of Triclosan and Methylparaben. Microchim. Acta 2020, 187, 250. [Google Scholar] [CrossRef]
- Pınar, P.T.; Allahverdiyeva, S.; Yardım, Y.; Şentürk, Z. Voltammetric Sensing of Dinitrophenolic Herbicide Dinoterb on Cathodically Pretreated Boron-Doped Diamond Electrode in the Presence of Cationic Surfactant. Microchem. J. 2020, 155, 104772. [Google Scholar] [CrossRef]





| No. | Enrichment Time (s) | Nano-Cu-BTC Modification Amount (%) | Enrichment Potential (V) | Ipa (μA) |
|---|---|---|---|---|
| 1 | 300 | 8 | 0.4 | 0.099 |
| 2 | 180 | 8 | 0.1 | 0.100 |
| 3 | 300 | 4 | 0.1 | 0.042 |
| 4 | 180 | 4 | −0.2 | 0.063 |
| 5 | 180 | 8 | 0.1 | 0.100 |
| 6 | 60 | 8 | 0.4 | 0.066 |
| 7 | 300 | 12 | 0.1 | 0.072 |
| 8 | 180 | 12 | −0.2 | 0.044 |
| 9 | 60 | 8 | −0.2 | 0.043 |
| 10 | 300 | 8 | −0.2 | 0.081 |
| 11 | 180 | 8 | 0.1 | 0.100 |
| 12 | 180 | 8 | 0.1 | 0.100 |
| 13 | 60 | 4 | 0.1 | 0.090 |
| 14 | 180 | 8 | 0.1 | 0.100 |
| 15 | 180 | 4 | 0.4 | 0.076 |
| 16 | 180 | 12 | 0.4 | 0.052 |
| 17 | 60 | 12 | 0.1 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, G.; Zuhra, Z.; Wang, S. User-Friendly and Responsive Electrochemical Detection Approach for Triclosan by Nano-Metal–Organic Framework. Molecules 2024, 29, 3298. https://doi.org/10.3390/molecules29143298
Li X, Zhang G, Zuhra Z, Wang S. User-Friendly and Responsive Electrochemical Detection Approach for Triclosan by Nano-Metal–Organic Framework. Molecules. 2024; 29(14):3298. https://doi.org/10.3390/molecules29143298
Chicago/Turabian StyleLi, Xiaoyu, Gaocheng Zhang, Zareen Zuhra, and Shengxiang Wang. 2024. "User-Friendly and Responsive Electrochemical Detection Approach for Triclosan by Nano-Metal–Organic Framework" Molecules 29, no. 14: 3298. https://doi.org/10.3390/molecules29143298
APA StyleLi, X., Zhang, G., Zuhra, Z., & Wang, S. (2024). User-Friendly and Responsive Electrochemical Detection Approach for Triclosan by Nano-Metal–Organic Framework. Molecules, 29(14), 3298. https://doi.org/10.3390/molecules29143298





