Effects of Selective and Mixed-Action Kappa and Delta Opioid Receptor Agonists on Pain-Related Behavioral Depression in Mice
Abstract
:1. Introduction
2. Results
3. Discussion
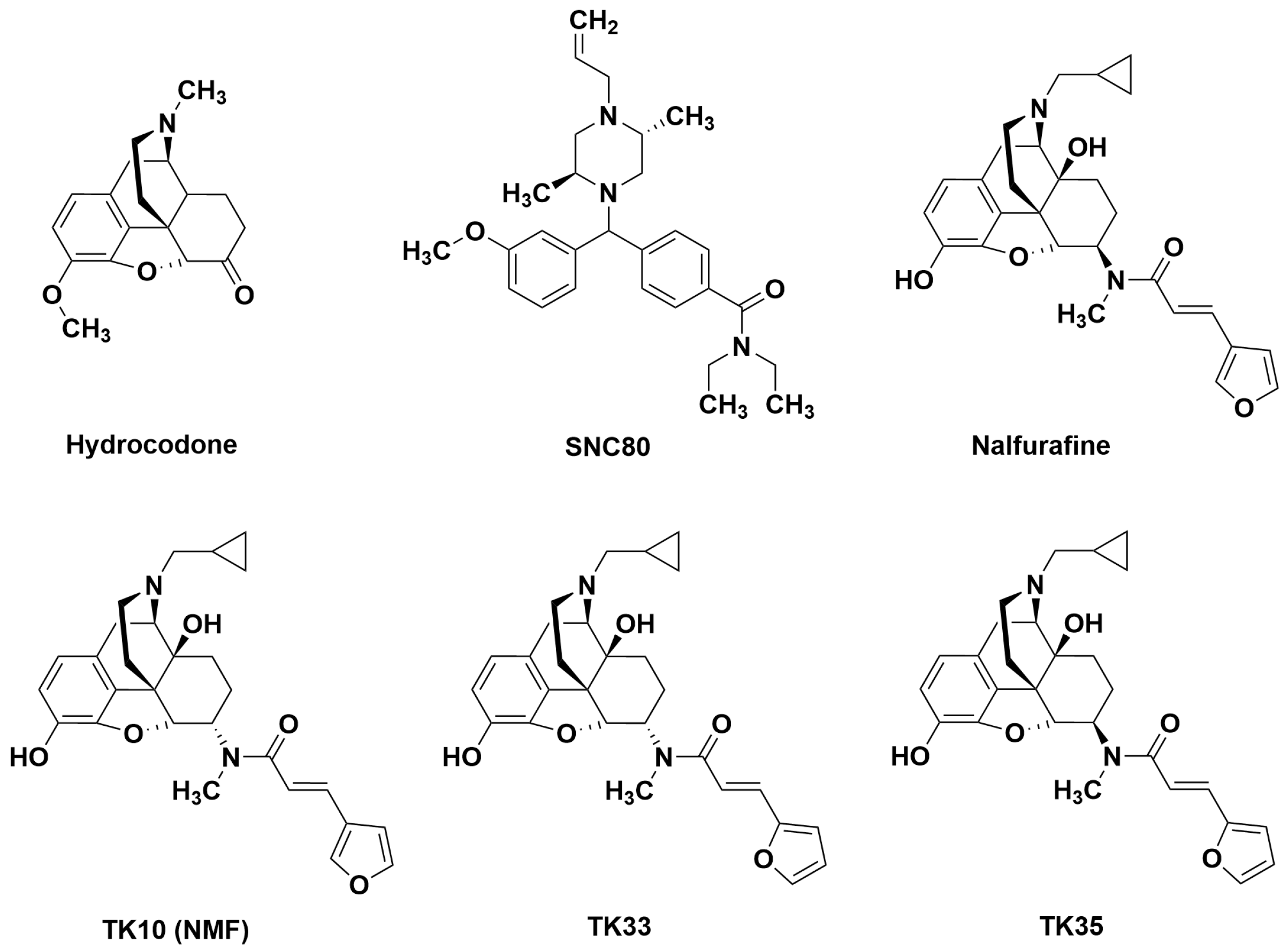
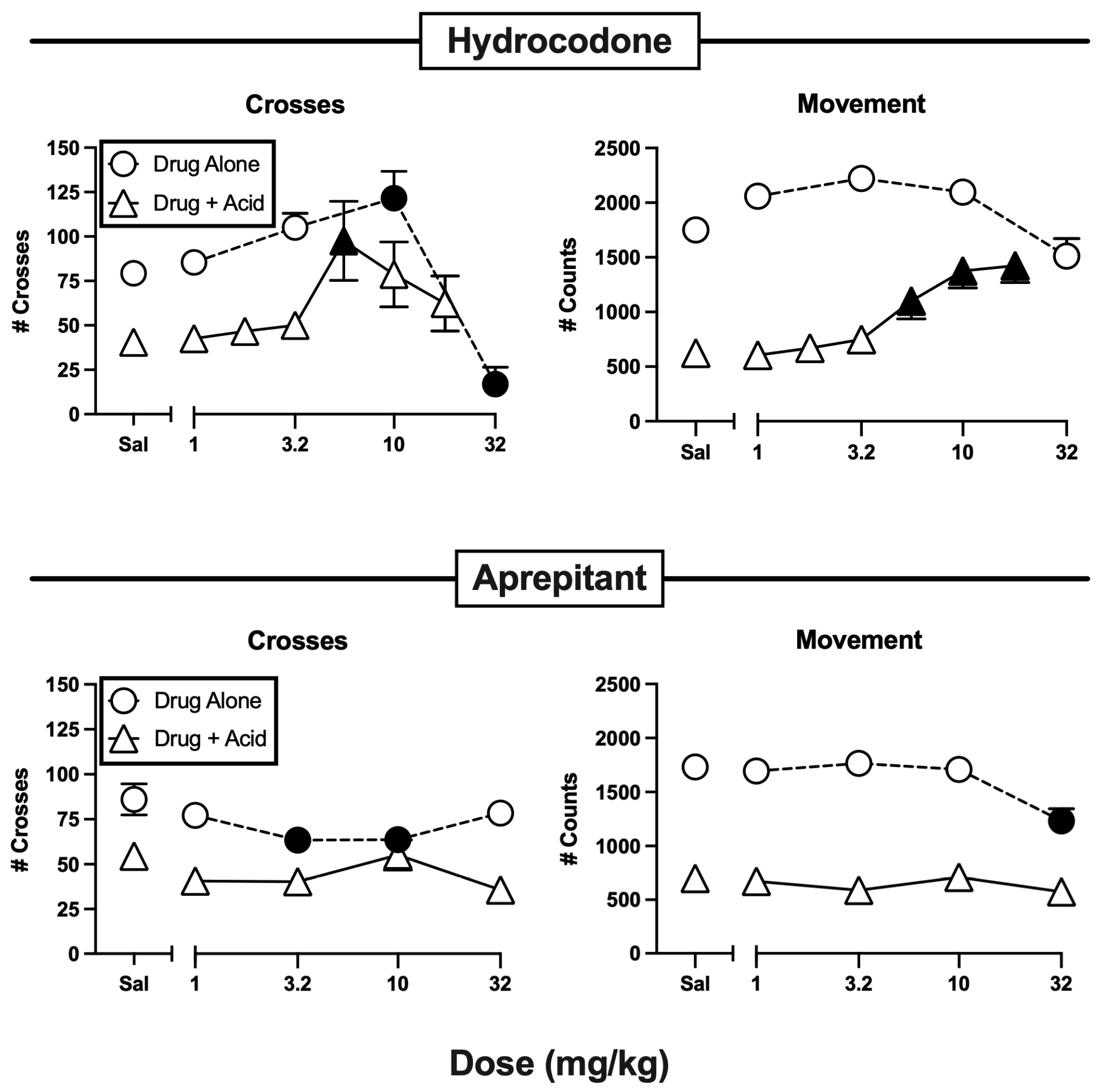
3.1. Effects of Hydrocodone and Aprepitant
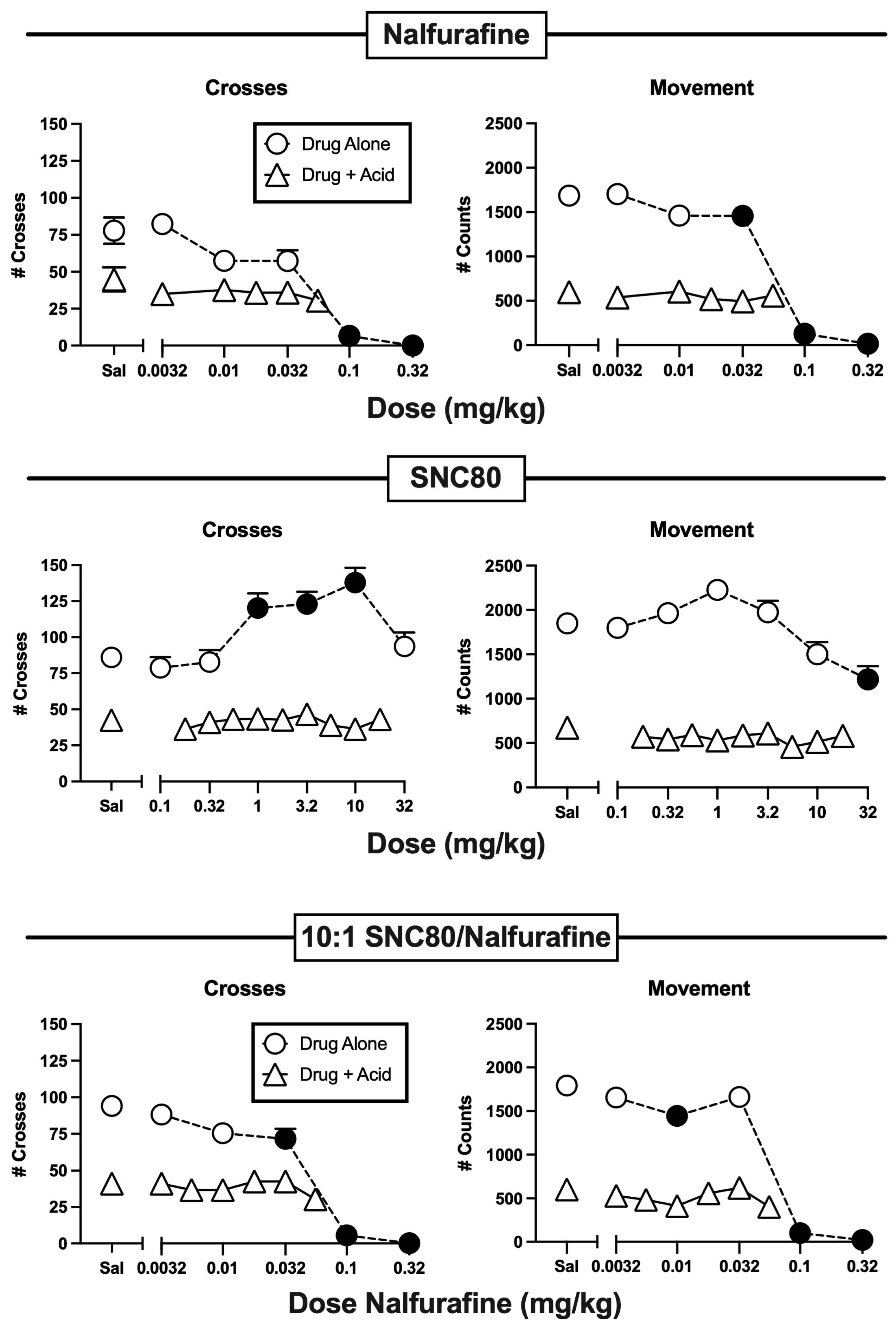
3.2. Effects of Nalfurafine and SNC80 Administered Alone or in Combination
3.3. Effects of Novel Mixed-Action Kappa Opioid Receptor/Delta Opioid Receptor Agonists
3.4. Sex as a Determinant of Test-Drug Effects
4. Materials and Methods
4.1. Subjects
4.2. Drugs
4.3. Behavioral Testing
4.3.1. General Experiment Design
4.3.2. Apparatus
4.3.3. Procedure
4.3.4. Dependent Measures
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, R.G.; Koroshetz, W.J.; Volkow, N.D. The Helping to End Addiction Long-term (HEAL) Initiative of the National Institutes of Health. JAMA 2021, 326, 1005–1006. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Stevens, D.L.; Arriaga, M.; Townsend, E.A.; Mendez, R.E.; Blajkevch, N.A.; Selley, D.E.; Banks, M.L.; Negus, S.S.; Dewey, W.L.; et al. Characterization of a Potential KOR/DOR Dual Agonist with No Apparent Abuse Liability via a Complementary Structure-Activity Relationship Study on Nalfurafine Analogues. ACS Chem. Neurosci. 2022, 13, 3608–3628. [Google Scholar] [CrossRef] [PubMed]
- Negus, S.S. Expression and treatment of pain-related behavioral depression. Lab. Anim. 2013, 42, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Negus, S.S. Addressing the Opioid Crisis: The Importance of Choosing Translational Endpoints in Analgesic Drug Discovery. Trends Pharmacol. Sci. 2018, 39, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Negus, S.S. Core Outcome Measures in Preclinical Assessment of Candidate Analgesics. Pharmacol. Rev. 2019, 71, 225–266. [Google Scholar] [CrossRef] [PubMed]
- Cobos, E.J.; Ghasemlou, N.; Araldi, D.; Segal, D.; Duong, K.; Woolf, C.J. Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain 2012, 153, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.B.; Marshall, L.S.; Boyer, N.M.; Alapatt, V.; Miller, L.L. Effects of Ketoprofen and Morphine on Pain-Related Depression of Nestlet Shredding in Male and Female Mice. Front. Pain Res. 2021, 2, 673940. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.I.; Eisenach, J.C.; Kawatani, M.; Martin, T.J. Peripheral nerve injury in rats induces alternations in choice behavior associated with food reinforcement. J. Physiol. Sci. 2019, 69, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, R.; Morgan, M.M. ‘Reinventing the wheel’ to advance the development of pain therapeutics. Behav. Pharmacol. 2021, 32, 142–152. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Bilsky, E.J.; Negus, S.S. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: Effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J. Pain 2006, 7, 408–416. [Google Scholar] [CrossRef]
- Wilkerson, J.L.; Curry, Z.A.; Kinlow, P.D.; Mason, B.L.; Hsu, K.L.; van der Stelt, M.; Cravatt, B.F.; Lichtman, A.H. Evaluation of different drug classes on transient sciatic nerve injury-depressed marble burying in mice. Pain 2018, 159, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lecker, I.; Collymore, C.; Dokova, A.; Pham, M.C.; Rosen, S.F.; Crawhall-Duk, H.; Zain, M.; Valencia, M.; Filippini, H.F.; et al. Cage-lid hanging behavior as a translationally relevant measure of pain in mice. Pain 2021, 162, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad Med. Singap. 1994, 23, 129–138. [Google Scholar] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Katz, N.P.; Kerns, R.D.; Stucki, G.; Allen, R.R.; Bellamy, N.; et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005, 113, 9–19. [Google Scholar] [CrossRef] [PubMed]
- St Onge, C.M.; Pagare, P.P.; Zheng, Y.; Arriaga, M.; Stevens, D.L.; Mendez, R.E.; Poklis, J.L.; Halquist, M.S.; Selley, D.E.; Dewey, W.L.; et al. Systematic Structure-Activity Relationship Study of Nalfurafine Analogues toward Development of Potentially Nonaddictive Pain Management Treatments. J. Med. Chem. 2024, 67, 9552–9574. [Google Scholar] [CrossRef] [PubMed]
- Negus, S.S.; Akbarali, H.I.; Kang, M.; Lee, Y.K.; Marsh, S.A.; Santos, E.J.; Zhang, Y. Role of mu opioid receptor (MOR) agonist efficacy as a determinant of opioid antinociception in a novel assay of pain-depressed behavior in female and male mice. Front. Pain Res. 2023, 4, 1281698. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.J.; Akbarali, H.I.; Bow, E.W.; Chambers, D.R.; Gutman, E.S.; Jacobson, A.E.; Kang, M.; Lee, Y.K.; Lutz, J.A.; Rice, K.C.; et al. Low-Efficacy Mu Opioid Agonists as Candidate Analgesics: Effects of Novel C-9 Substituted Phenylmorphans on Pain-Depressed Behavior in Mice. J. Pharmacol. Exp. Ther. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Lazenka, M.L.; Moerke, M.J.; Townsend, E.A.; Freeman, K.B.; Carroll, F.I.; Negus, S.S. Dissociable effects of the kappa opioid receptor agonist nalfurafine on pain/itch-stimulated and pain/itch-depressed behaviors in male rats. Psychopharmacology 2018, 235, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Hayakawa, J.; Kawamura, K.; Kawai, K.; Takezawa, Y.; Matsuura, H.; Tajima, C.; Endo, T. Discovery of a structurally novel opioid kappa-agonist derived from 4,5-epoxymorphinan. Chem. Pharm. Bull. 1998, 46, 366–369. [Google Scholar] [CrossRef]
- Calderon, S.N.; Rothman, R.B.; Porreca, F.; Flippen-Anderson, J.L.; McNutt, R.W.; Xu, H.; Smith, L.E.; Bilsky, E.J.; Davis, P.; Rice, K.C. Probes for narcotic receptor mediated phenomena. 19. Synthesis of (+)-4-[(alpha R)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC 80): A highly selective, nonpeptide delta opioid receptor agonist. J. Med. Chem. 1994, 37, 2125–2128. [Google Scholar] [CrossRef]
- Negus, S.S.; Rosenberg, M.B.; Altarifi, A.A.; O’Connell, R.H.; Folk, J.E.; Rice, K.C. Effects of the delta opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J. Pain 2012, 13, 317–327. [Google Scholar] [CrossRef]
- Endoh, T.; Matsuura, H.; Tajima, A.; Izumimoto, N.; Tajima, C.; Suzuki, T.; Saitoh, A.; Suzuki, T.; Narita, M.; Tseng, L.; et al. Potent antinociceptive effects of TRK-820, a novel kappa-opioid receptor agonist. Life Sci. 1999, 65, 1685–1694. [Google Scholar] [CrossRef]
- Endoh, T.; Tajima, A.; Izumimoto, N.; Suzuki, T.; Saitoh, A.; Suzuki, T.; Narita, M.; Kamei, J.; Tseng, L.F.; Mizoguchi, H.; et al. TRK-820, a selective kappa-opioid agonist, produces potent antinociception in cynomolgus monkeys. Jpn. J. Pharmacol. 2001, 85, 282–290. [Google Scholar] [CrossRef]
- Endoh, T.; Tajima, A.; Suzuki, T.; Kamei, J.; Narita, M.; Tseng, L.; Nagase, H. Characterization of the antinociceptive effects of TRK-820 in the rat. Eur. J. Pharmacol. 2000, 387, 133–140. [Google Scholar] [CrossRef]
- Fraser, G.L.; Gaudreau, G.A.; Clarke, P.B.; Menard, D.P.; Perkins, M.N. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. Br. J. Pharmacol. 2000, 129, 1668–1672. [Google Scholar] [CrossRef]
- Gallantine, E.L.; Meert, T.F. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin. Pharmacol. Toxicol. 2005, 97, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Sora, I.; Li, X.F.; Funada, M.; Kinsey, S.; Uhl, G.R. Visceral chemical nociception in mice lacking mu-opioid receptors: Effects of morphine, SNC80 and U-50,488. Eur. J. Pharmacol. 1999, 366, R3–R5. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.; Wallace, W. Opioids, analgesia, and pain management. In Goodman and Gilman’s: The Pharmacological Basis of Therapeutics, 13e; Brunton, L.L., Hilal-Dandan, R., Knollman, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Patel, L.; Lindley, C. Aprepitant—A novel NK1-receptor antagonist. Expert Opin. Pharmacother. 2003, 4, 2279–2296. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, B.Y.; Saghetlians, A.; Zhang, X.; Okida, T.; Kim, S.Y. Anti-nociceptive effects of dual neuropeptide antagonist therapy in mouse model of neuropathic and inflammatory pain. Korean J. Pain 2022, 35, 173–182. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Zheng, W.; Qian, T.; Wang, H.; Hou, X. Antagonism of NK-1R using aprepitant suppresses inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1628–1634. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, W.; Xu, X.; Ge, X.; Wang, F.; Zhang, G.Q.; Miao, L.; Deng, X. Aprepitant Inhibits JNK and p38/MAPK to Attenuate Inflammation and Suppresses Inflammatory Pain. Front. Pharmacol. 2021, 12, 811584. [Google Scholar] [CrossRef] [PubMed]
- Hill, R. NK1 (substance P) receptor antagonists—Why are they not analgesic in humans? Trends Pharmacol. Sci. 2000, 21, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Seguin, L.; Le Marouille-Girardon, S.; Millan, M.J. Antinociceptive profiles of non-peptidergic neurokinin1 and neurokinin2 receptor antagonists: A comparison to other classes of antinociceptive agent. Pain 1995, 61, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Yezierski, R.P.; Hansson, P. Inflammatory and Neuropathic Pain From Bench to Bedside: What Went Wrong? J. Pain 2018, 19, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.; Hill, R.G. Substance OP (NK1) Receptor Antagonists-Analgesics or Not? In Tachykinins. Handbook of Experimental Pharmacology; Holzer, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 164, pp. 441–457. [Google Scholar]
- Zhou, Y.; Freeman, K.; Setola, V.; Cao, D.; Kaski, S.; Kreek, M.J.; Liu-Chen, L.Y. Preclinical Studies on Nalfurafine (TRK-820), a Clinically Used KOR Agonist. Handb. Exp. Pharmacol. 2022, 271, 137–162. [Google Scholar] [PubMed]
- Lazenka, M.F. Antinociceptive Effects of Kappa-Opioid Receptor Agonists. Handb. Exp. Pharmacol. 2022, 271, 293–313. [Google Scholar] [PubMed]
- Negus, S.S.; Morrissey, E.M.; Rosenberg, M.; Cheng, K.; Rice, K.C. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology 2010, 210, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Negus, S.S.; Neddenriep, B.; Altarifi, A.A.; Carroll, F.I.; Leitl, M.D.; Miller, L.L. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain 2015, 156, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.R.; Furness, M.S.; Mello, N.K.; Rice, K.C.; Negus, S.S. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: Effects on chemically induced thermal hypersensitivity. J. Pharmacol. Exp. Ther. 2001, 296, 939–946. [Google Scholar]
- Broom, D.C.; Nitsche, J.F.; Pintar, J.E.; Rice, K.C.; Woods, J.H.; Traynor, J.R. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J. Pharmacol. Exp. Ther. 2002, 303, 723–729. [Google Scholar] [CrossRef]
- Narita, M.; Suzuki, T. Delta opioid receptor-mediated antinociception/analgesia. In The Delta Receptor; Chang, K.J., Porreca, F., Woods, J.H., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 331–354. [Google Scholar]
- Ossipov, M.H.; Lai, J.; Vanderah, T.W.; Porreca, F. The delta opioid receptor subtypes and pain modulation. In The Delta Receptor; Chang, K.J., Porreca, F., Woods, J.H., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 297–330. [Google Scholar]
- Spahn, V.; Stein, C. Targeting delta opioid receptors for pain treatment: Drugs in phase I and II clinical development. Expert Opin. Investig. Drugs 2017, 26, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Dripps, I.J.; Wang, Q.; Neubig, R.R.; Rice, K.C.; Traynor, J.R.; Jutkiewicz, E.M. The role of regulator of G protein signaling 4 in delta-opioid receptor-mediated behaviors. Psychopharmacology 2017, 234, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Mori, T.; Sawaguchi, T. Dopamine-independent psychostimulant activity of a delta-agonist. Behav. Pharmacol. 2008, 19, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, O.S.; Dripps, I.J.; Ramani, S.; Chang, C.; Han, J.L.; Rice, K.C.; Jutkiewicz, E.M. Automated touch screen device for recording complex rodent behaviors. J. Neurosci. Methods 2014, 233, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Diester, C.M.; Banks, M.L.; Neigh, G.N.; Negus, S.S. Experimental design and analysis for consideration of sex as a biological variable. Neuropsychopharmacology 2019, 44, 2159–2162. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]

| Test Drug | Crosses | Movement |
|---|---|---|
| Hydrocodone | t(22) = 4.76, p < 0.001 | t(22) = 10.73, p < 0.001 |
| Aprepitant | t(22) = 2.92, p = 0.0040 | t(22) = 13.11, p = <0.001 |
| Nalfurafine | t(22) = 2.73, p = 0.0061 | t(22) = 11.16, p < 0.001 |
| SNC80 | t(22) = 6.52, p < 0.001 | t(22) = 10.85, p < 0.001 |
| 10:1 SNC80/Nalfurafine | t(22) = 6.56, p < 0.001 | t(22) = 12.08, p < 0.001 |
| TK10 | t(22) = 4.33, p = 0.0003 | t(22) = 9.56, p < 0.001 |
| TK33 | t(22) = 3.60, p = 0.0008 | t(22) = 10.54, p < 0.001 |
| TK35 | t(22) = 3.81, p = 0.0010 | t(22) = 8.24, p < 0.001 |
| Test Drug | Crosses | Movement | |
|---|---|---|---|
| Hydrocodone | Alone | F(4,55) = 17.92, p < 0.0001-m | F(4,55) = 7.21, p < 0.0001 |
| + IP lactic acid | F(6,77) = 2.55, p = 0.0264 | F(6,77) = 9.08, p < 0.0001 | |
| Aprepitant | Alone | F(4,55) = 2.77, p = 0.0359-F | F(4,55) = 8.16, p < 0.0001 |
| + IP lactic acid | F(4,55) = 1.75, p = 0.1525-m | F(4,55) = 0.88, p = 0.4846 | |
| Nalfurafine | Alone | F(5,66) = 34.34, p < 0.0001-m | F(5,66) = 156.60, p < 0.0001 |
| + IP lactic acid | F(5,66) = 0.88, p = 0.4991-m | F(5,66) = 0.51, p = 0.7679-f | |
| SNC80 | Alone | F(6,77) = 7.34, p < 0.0001 | F(6,77) = 8.38, p < 0.0001-f |
| + IP lactic acid | F(9,110) = 0.45, p < 0.9061-FM | F(9,110) = 1.25, p = 0.2722-F | |
| 10:1 SNC/Nal | Alone | F(5,66) = 56.84, p < 0.0001 | F(5,66) = 123.00, p < 0.0001 |
| + IP lactic acid | F(6,77) = 1.19, p = 0.32 | F(6,77) = 1.63, p = 0.1513 | |
| TK10 | Alone | F(4,55) = 62.16, p < 0.0001 | F(4,55) = 89.24, p < 0.0001-f |
| + IP lactic acid | F(4,55) = 0.94, p = 0.4480 | F(4,55) = 0.41, p = 0.8002 | |
| TK33 | Alone | F(4,55) = 33.38, p < 0.0001 | F(4,55) = 110.80, p < 0.0001 |
| + IP lactic acid | F(4,55) = 1.00, p = 0.4142-m | F(4,55) = 0.88, p = 0.4848 | |
| TK35 | Alone | F(4,49) = 43.57, p < 0.0001 | F(4,49) = 93.17, p < 0.0001-f |
| + IP lactic acid | F(4,55) = 1.22, p = 0.3144 | (4,55) = 1.81, p = 0.1398-F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negus, S.S.; St. Onge, C.M.; Lee, Y.K.; Li, M.; Rice, K.C.; Zhang, Y. Effects of Selective and Mixed-Action Kappa and Delta Opioid Receptor Agonists on Pain-Related Behavioral Depression in Mice. Molecules 2024, 29, 3331. https://doi.org/10.3390/molecules29143331
Negus SS, St. Onge CM, Lee YK, Li M, Rice KC, Zhang Y. Effects of Selective and Mixed-Action Kappa and Delta Opioid Receptor Agonists on Pain-Related Behavioral Depression in Mice. Molecules. 2024; 29(14):3331. https://doi.org/10.3390/molecules29143331
Chicago/Turabian StyleNegus, S. Stevens, Celsey M. St. Onge, Young K. Lee, Mengchu Li, Kenner C. Rice, and Yan Zhang. 2024. "Effects of Selective and Mixed-Action Kappa and Delta Opioid Receptor Agonists on Pain-Related Behavioral Depression in Mice" Molecules 29, no. 14: 3331. https://doi.org/10.3390/molecules29143331





