LDH-Indomethacin Nanoparticles Antitumoral Action: A Possible Coadjuvant Drug for Cancer Therapy
Abstract
:1. Introduction
2. Results
2.1. X-ray Diffraction
2.2. FT-IR Vibrational Spectroscopy, Scanning Electron Microscopy (SEM) and Thermogravimetric Analyzes (TGA/DTA)
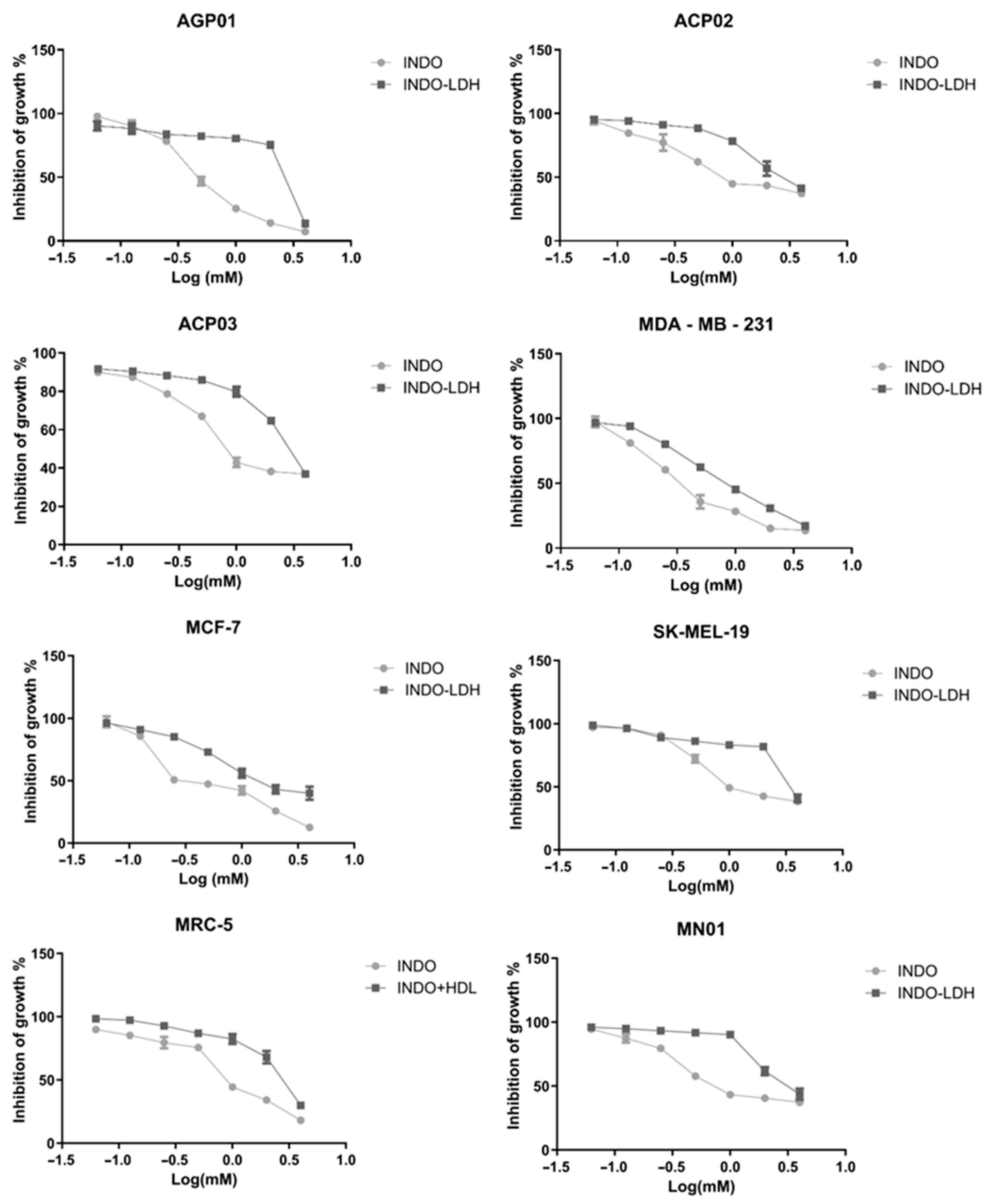
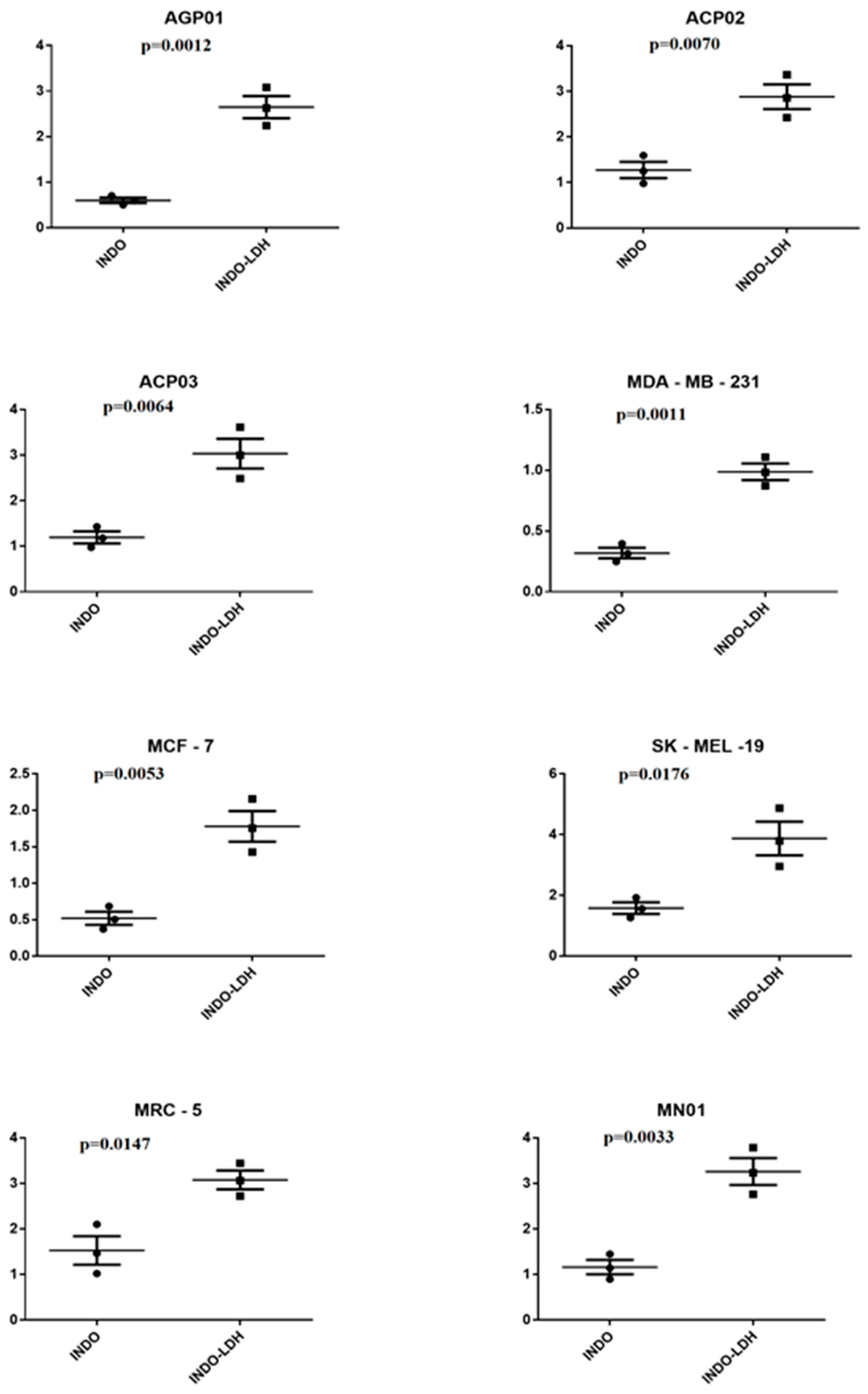
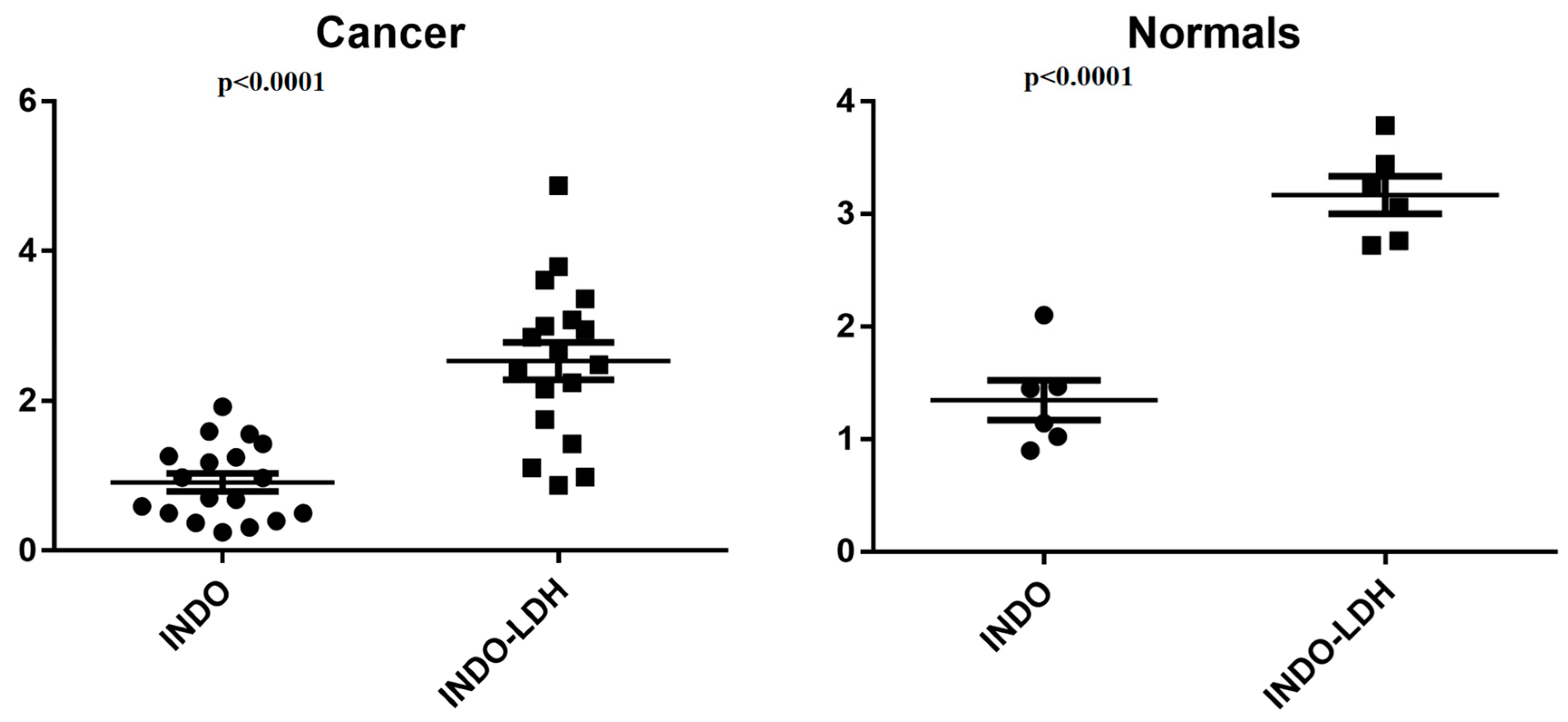
2.3. Cell Viability Assay
3. Discussion
4. Materials and Methods
4.1. Syntheses
4.2. Samples Characterization
4.3. Anticancer Activity
4.3.1. Cellular Lines
4.3.2. Cell Culture
4.3.3. MTT Cytotoxicity Assay
4.3.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santos, M.; de Lima, F.C.D.S.; Martins, L.F.L.; Oliveira, J.F.P.; de Almeida, L.M.; de Camargo Cancela, M. Estimativa de Incidência de Câncer No Brasil, 2023–2025. Rev. Bras. Cancerol. 2023, 69. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Z.P.; Lu, J.; Tang, Z.Y.; Zhao, H.J.; Good, D.A.; Wei, M.Q. Potential for Layered Double Hydroxides-Based, Innovative Drug Delivery Systems. Int. J. Mol. Sci. 2014, 15, 7409–7428. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Hussein, M.Z.; Fakurazi, S.; Zainal, Z. Development of Drug Delivery Systems Based on Layered Hydroxides for Nanomedicine. Int. J. Mol. Sci. 2014, 15, 7750–7786. [Google Scholar] [CrossRef] [PubMed]
- Rives, V.; del Arco, M.; Martín, C. Intercalation of Drugs in Layered Double Hydroxides and Their Controlled Release: A Review. Appl. Clay Sci. 2014, 88–89, 239–269. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, G.J.; Kang, J.H.; Kim, H.J.; Kim, T.I.; Oh, J.M. Anticancer Drug-Incorporated Layered Double Hydroxide Nanohybrids and Their Enhanced Anticancer Therapeutic Efficacy in Combination Cancer Treatment. BioMed Res. Int. 2014, 2014, 193401. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.C.; Edwards, A.D.; Wyatt, J.S.; Potter, A.; Cope, M.; Delpy, D.T.; Reynolds, E.O.R. Effect of Indomethacin on Cerebral Oxidized Cytochrome-Aa3 Concentration in Preterm Infants. Pediatr. Res. 1990, 28, 290. [Google Scholar] [CrossRef]
- Chennamaneni, S.; Zhong, B.; Lama, R.; Su, B. COX Inhibitors Indomethacin and Sulindac Derivatives as Antiproliferative Agents: Synthesis, Biological Evaluation, and Mechanism Investigation. Eur. J. Med. Chem. 2012, 56, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Jie, C.; Bhujwalla, Z.M. Mechanisms of Indomethacin-Induced Alterations in the Choline Phospholipid Metabolism of Breast Cancer Cells. Neoplasia 2006, 8, 758–771. [Google Scholar] [CrossRef]
- Agrawal, A.; Fentiman, I.S. NSAIDs and Breast Cancer: A Possible Prevention and Treatment Strategy. Int. J. Clin. Pract. 2008, 62, 444–449. [Google Scholar] [CrossRef]
- Boland, G.P.; Butt, I.S.; Prasad, R.; Knox, W.F.; Bundred, N.J. COX-2 Expression Is Associated with an Aggressive Phenotype in Ductal Carcinoma in Situ. Br. J. Cancer 2004, 90, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, D.; Gillmore, R.; Waxman, J. COX and Cancer. QJM 2005, 98, 711–718. [Google Scholar] [CrossRef]
- Jacobs, J.W.; Bijlsma, J.W. NSAIDs: A Critical Appraisal. Neth. J. Med. 1997, 51, 198–204. [Google Scholar] [CrossRef]
- Noreen, F.; Aman, T.; Mumtaz, A.; Nazir, S. Spectrophotometric Determination of Indomethacin Using Partial Least Square Method. Proc. Pak. Acad. Sci. 2007, 44, 173–179. [Google Scholar]
- Tomisato, W.; Tanaka, K.I.; Katsu, T.; Kakuta, H.; Sasaki, K.; Tsutsumi, S.; Hoshino, T.; Aburaya, M.; Li, D.; Tsuchiya, T.; et al. Membrane Permeabilization by Non-Steroidal Anti-Inflammatory Drugs. Biochem. Biophys. Res. Commun. 2004, 323, 1032–1039. [Google Scholar] [CrossRef]
- Touhey, S.; O’Connor, R.; Plunkett, S.; Maguire, A.; Clynes, M. Structure-Activity Relationship of Indomethacin Analogues for MRP-1, COX-1 and COX-2 Inhibition: Identification of Novel Chemotherapeutic Drug Resistance Modulators. Eur. J. Cancer 2002, 38, 1661–1670. [Google Scholar] [CrossRef]
- Ajmone-Cat, M.A.; Bernardo, A.; Greco, A.; Minghetti, L. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals 2010, 3, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Ammoury, N.; Fessi, H.; Devissaguet, J.P.; Dubrasquet, M.; Benita, S. Jejunal Absorption, Pharmacological Activi-ty, and Pharmacokinetic Evaluation of Indomethacin-Loaded Poly(d,l-Lactide) and Poly(Isobutyl-Cyanoacrylate) Nanocapsules in Rats. Pharm. Res. 1991, 8, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Srinath, P.; Diwan, P.V. Pharmacodynamic and Pharmacokinetic Evaluation of Lipid Microspheres of Indomethacin. Pharm. Acta Helv. 1998, 73, 199–203. [Google Scholar] [CrossRef]
- Srinath, P.; Vyas, S.P.; Diwan, P.V. Preparation and Pharmacodynamic Evaluation of Liposomes of Indomethacin. Drug Dev. Ind. Pharm. 2000, 26, 313–321. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, H.C. Abrupt Structural Transformation in Hydrotalcite-like Compounds Mg1−xAlx(OH)2(NO3)x·nH2O as a Continuous Function of Nitrate Anions. J. Phys. Chem. B 2001, 105, 1743–1749. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, H.C. Decomposition Pathways of Hydrotalcite-like Compounds Mg1−xAlx(OH)2(NO3)x·nH2O as a Continuous Function of Nitrate Anions. Chem. Mater. 2001, 2, 4564–4572. [Google Scholar] [CrossRef]
- Mohanambe, L.; Vasudevan, S. Anionic Clays Containing Anti-Inflammatory Drug Molecules: Comparison of Molecular Dynamics Simulation and Measurements. J. Phys. Chem. B 2005, 109, 15651–15658. [Google Scholar] [CrossRef] [PubMed]
- Rives, V.; Del Arco, M.; Martín, C. Layered Double Hydroxides as Drug Carriers and for Controlled Release of Non-Steroidal Antiinflammatory Drugs (NSAIDs): A Review. J. Control. Release 2013, 169, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, M.; Cebadera, E.; Gutiérrez, S.; Martín, C.; Montero, M.J.; Rives, V.; Rocha, J.; Sevilla, M.A. Mg,Al Layered Double Hydroxides with Intercalated Indomethacin: Synthesis, Characterization, and Pharmacological Study. J. Pharm. Sci. 2004, 93, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Aisawa, S.; Takahashi, S.; Ogasawara, W.; Umetsu, Y.; Narita, E. Direct Intercalation of Amino Acids into Layered Double Hydroxides by Coprecipitation. J. Solid State Chem. 2001, 162, 52–62. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Mendieta, S.; Nuñez, P.R.; Oliva, M.; Pérez, C.; Fernández, J.; Crivello, M. Intercalation of Anti-Inflammatory Drugs Sodium Indomethacin into Nanocomposites of Mg-Al. Structural Characterization. Procedia Mater. Sci. 2012, 1, 580–587. [Google Scholar] [CrossRef]
- Henrist, C.; Mathieu, J.P.; Vogels, C.; Rulmont, A.; Cloots, R. Morphological Study of Magnesium Hydroxide Nanoparticles Precipitated in Dilute Aqueous Solution. J. Cryst. Growth 2003, 249, 321–330. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Thermogravimetric Analysis of Selected Layered Double Hydroxides. J. Therm. Anal. Calorim. 2013, 112, 649–657. [Google Scholar] [CrossRef]
- Natarajan, K.; Mori, N.; Artemov, D.; Bhujwalla, Z.M. Exposure of Human Breast Cancer Cells to the Anti-Inflammatory Agent Indomethacin Alters Choline Phospholipid Metabolites and Nm23 Expression. Neoplasia 2002, 4, 409–416. [Google Scholar] [CrossRef]
- Ackerstaff, E.; Gimi, B.; Artemov, D.; Bhujwalla, Z.M. Anti-Inflammatory Agent Indomethacin Reduces Invasion and Alters Metabolism in a Human Breast Cancer Cell Line. Neoplasia 2007, 9, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Bronger, H.; Kraeft, S.; Schwarz-Boeger, U.; Cerny, C.; Stöckel, A.; Avril, S.; Kiechle, M.; Schmitt, M. Modulation of CXCR3 Ligand Secretion by Prostaglandin E2 and Cyclooxygenase Inhibitors in Human Breast Cancer. Breast Cancer Res. 2012, 14, R30. [Google Scholar] [CrossRef]
- Terry, M.B.; Gammon, M.D.; Teitelbaum, S.L.; Britton, J.A.; Neugut, A.I. Association of Frequency and Duration with Breast Cancer Risk. JAMA 2004, 291, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Chang, C.M.; Hsu, W.L.; Chiu, S.J.; Tsai, Y.T.; Chou, Y.H.; Hou, M.F.; Wang, J.Y.; Lee, M.H.; Tsai, K.L.; et al. Indomethacin Inhibits Cancer Cell Migration via Attenuation of Cellular Calcium Mobilization. Molecules 2013, 18, 6584–6596. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.K.; Hoa, N.; Hodges, A.; Ge, L.; Jadus, M.R. Indomethacin Promotes Apoptosis in Gastric Cancer Cells through Concomitant Degradation of Survivin and Aurora B Kinase Proteins. Apoptosis 2014, 19, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.K.W.; Cao, H.H.; Cheng, C.Y.; Kwan, H.Y.; Yu, H.; Fong, W.F.; Yu, Z.L. Indomethacin Sensitizes TRAIL-Resistant Melanoma Cells to TRAIL-Induced Apoptosis through Ros-Mediated Upregulation of Death Receptor 5 and downregulation of Survivin. J. Investig. Dermatol. 2014, 134, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Costantino, U.; Ambrogi, V.; Nocchetti, M.; Perioli, L. Hydrotalcite-like Compounds: Versatile Layered Hosts of Molecular Anions with Biological Activity. Microporous Mesoporous Mater. 2008, 107, 149–160. [Google Scholar] [CrossRef]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Leal, M.F.; Martins do Nascimento, J.L.; da Silva, C.E.A.; Vita Lamarão, M.F.; Calcagno, D.Q.; Khayat, A.S.; Assumpção, P.P.; Cabral, I.R.; de Arruda Cardoso Smith, M.; Burbano, R.R. Establishment and Conventional Cytogenetic Characterization of Three Gastric Cancer Cell Lines. Cancer Genet. Cytogenet. 2009, 195, 85–91. [Google Scholar] [CrossRef]
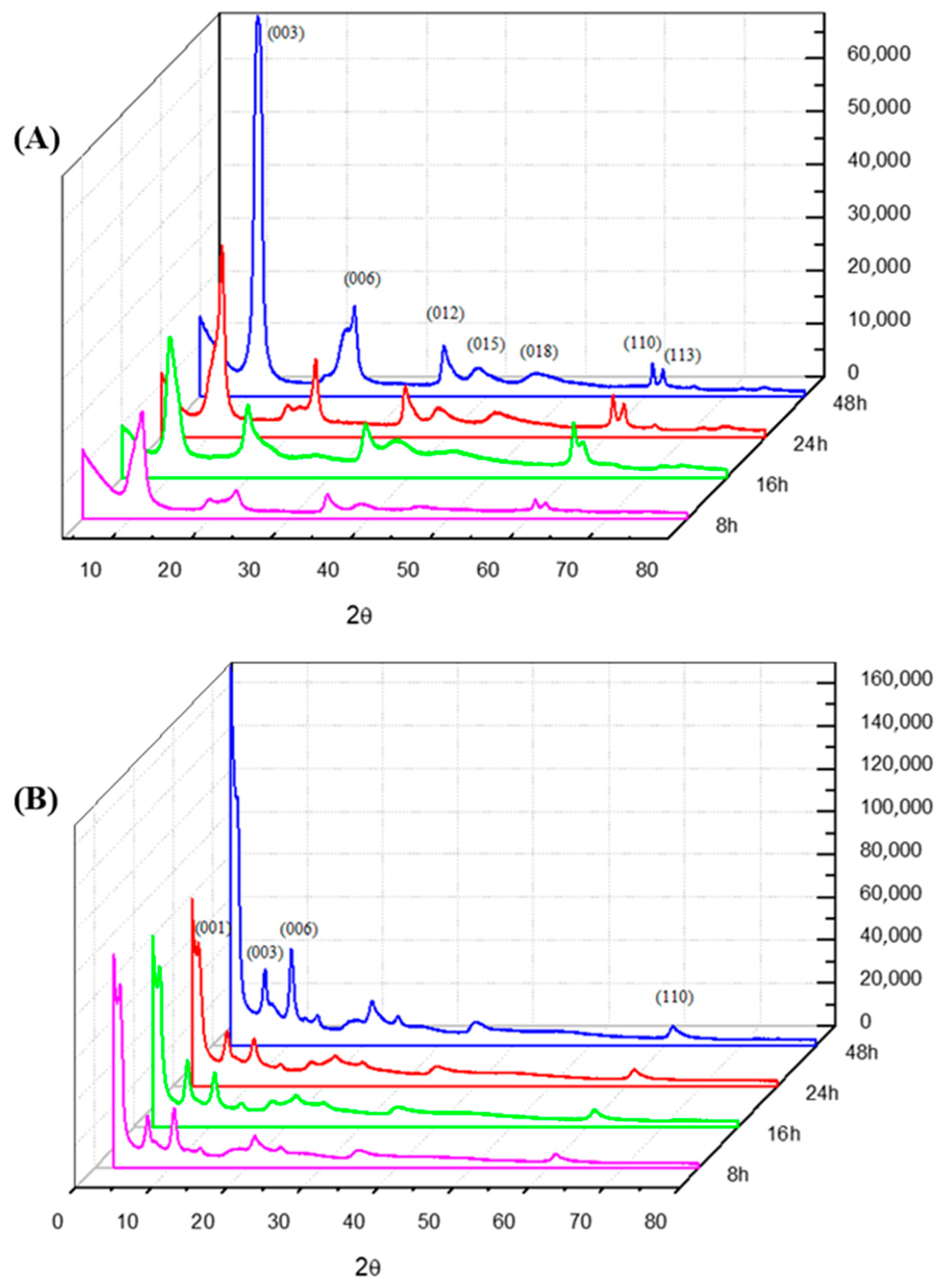
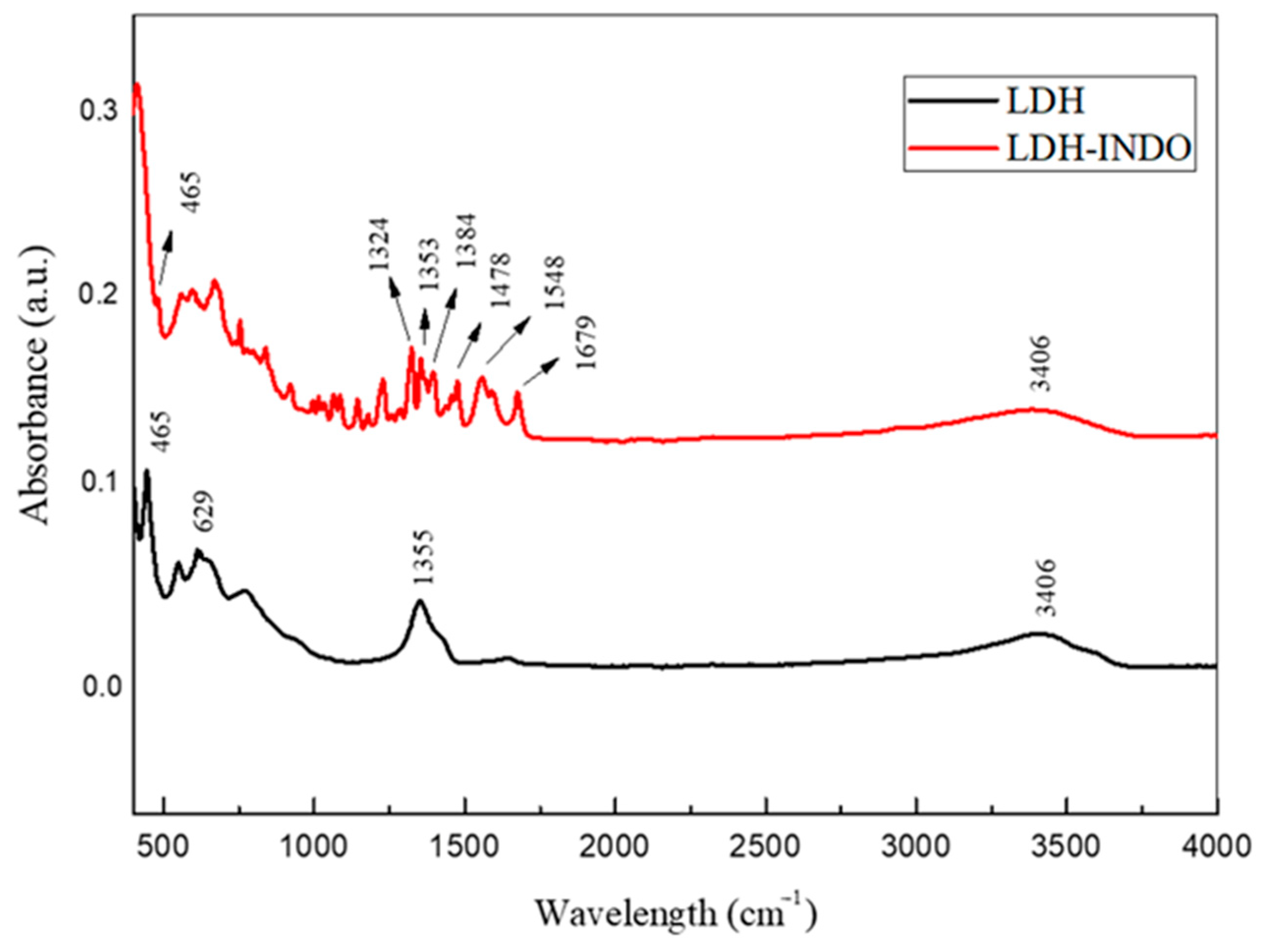
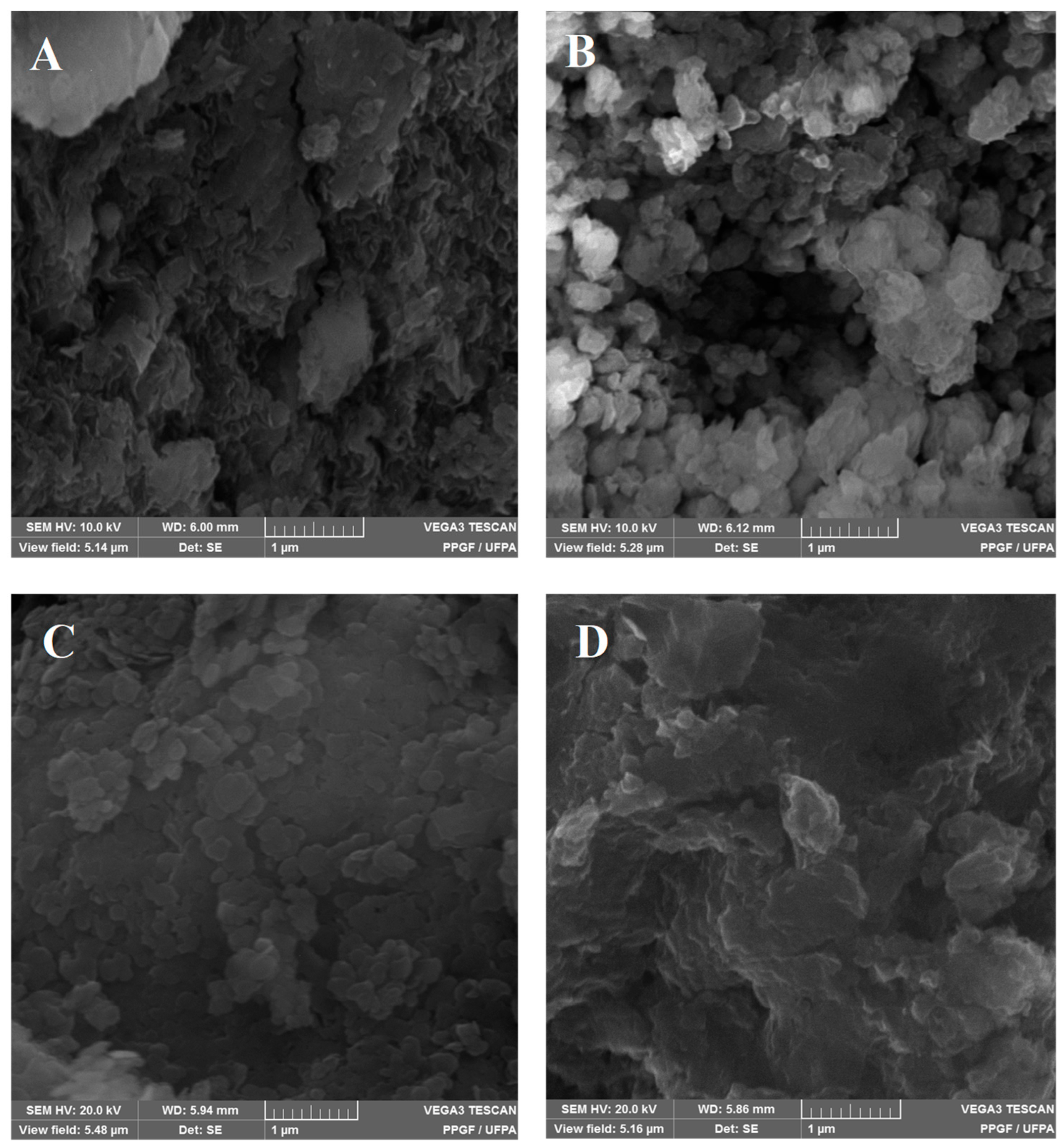

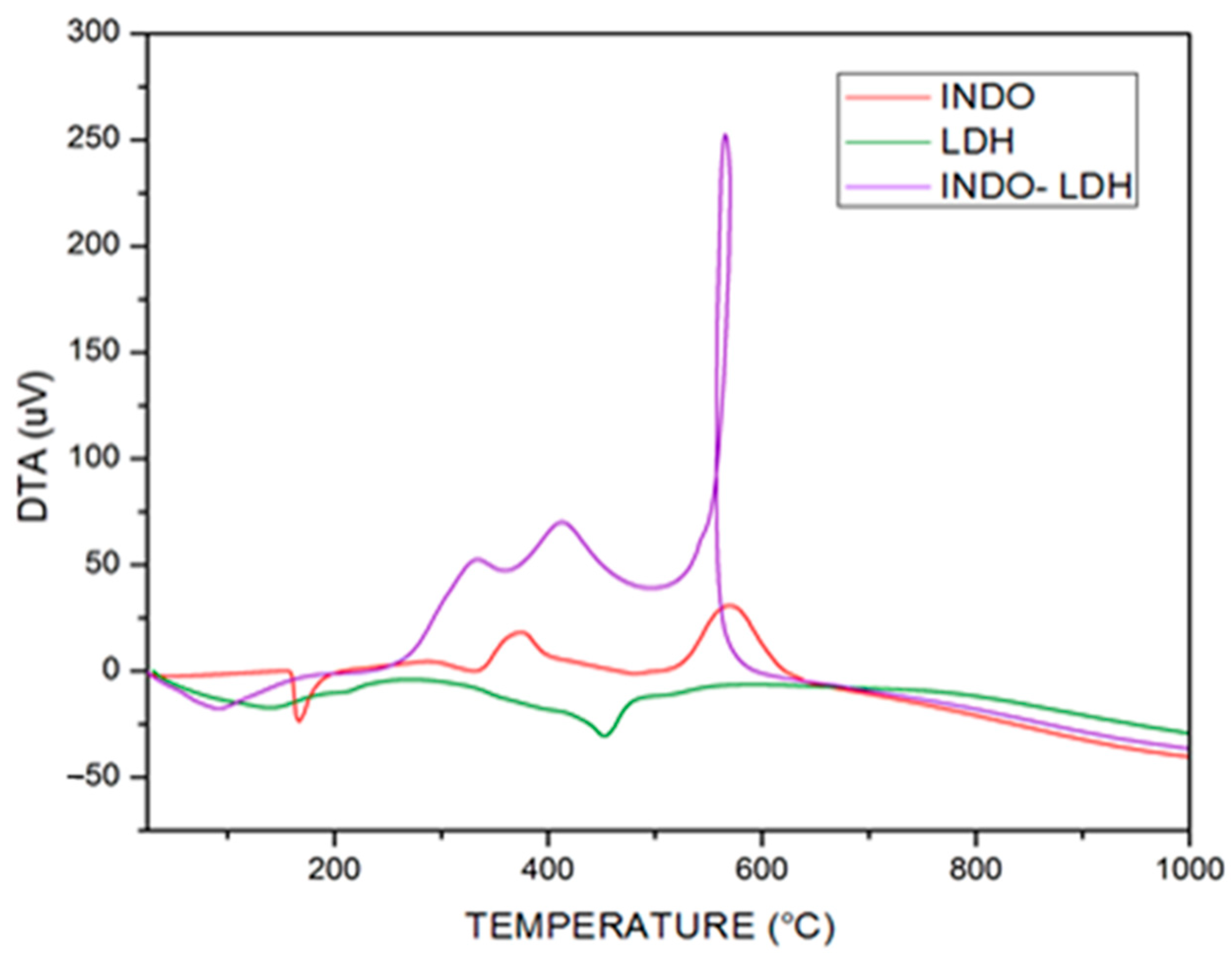

| Samples | Temperature (°C) | d(003) (Å) | d(110) (Å) | 2θ (Å) | Particle Size T(hkl) [Å] | N° Stacking |
|---|---|---|---|---|---|---|
| LDH-8 h | 50 °C | 8.5404 | 1.5148 | 10.3495 | 79.2333 | 9.2773 |
| LDH-8 h | 70 °C | 8.5166 | 1.5146 | 10.4156 | 96.4288 | 11.3224 |
| LDH-8 h | 90 °C | 7.8816 | 1.5177 | 11.2173 | 186.4979 | 23.6622 |
| LDH-16 h | 50 °C | 8.8025 | 1.5229 | 10.0406 | 196.9760 | 22.3771 |
| LDH-16 h | 70 °C | 8.8027 | 1.5223 | 10.0404 | 206.9817 | 23.5134 |
| LDH-16 h | 90 °C | 8.7963 | 1.5239 | 10.0477 | 177.4458 | 20.1726 |
| LDH-24 h | 50 °C | 8.3150 | 1.5199 | 10.6309 | 93.6364 | 11.2611 |
| LDH-24 h | 70 °C | 7.6017 | 1.5222 | 11.6317 | 596.7659 | 78.5037 |
| LDH-24 h | 90 °C | 7.6507 | 1.5141 | 11.5571 | 392.5261 | 51.3059 |
| LDH-48 h | 50 °C | 7.8247 | 1.5123 | 11.2992 | 169.0668 | 21.6067 |
| LDH-48 h | 70 °C | 8.2095 | 1.5088 | 10.7679 | 538.4711 | 65.5906 |
| LDH-48 h | 90 °C | 8.7205 | 1.5239 | 11.4521 | 597.7298 | 77.4205 |
| Samples | Temperature (°C) | d(001) (Å) | d(003) (Å) | d(110) (Å) | 2θ (Å) | Particle Size T(hkl) [Å] | N° Stacking |
|---|---|---|---|---|---|---|---|
| LDH-INDO-8 h | 50 °C | 12.6614 | 8.4211 | 1.5154 | 10.4966 | 213.0154 | 25.2952 |
| LDH-INDO-8 h | 70 °C | 25.2083 | 12.2255 | 1.5158 | 7.2249 | 300.9974 | 24.6204 |
| LDH-INDO-8 h | 90 °C | 42.5754 | 12.0456 | 1.5085 | 7.3329 | 198.9354 | 16.5151 |
| LDH-INDO-16 h | 50 °C | 42.5323 | 12.3747 | 1.5134 | 7.1377 | 135.1622 | 10.9224 |
| LDH-INDO-16 h | 70 °C | 41.6789 | 11.2367 | 1.5154 | 7.2190 | 170.1067 | 4.0813 |
| LDH-INDO-16 h | 90 °C | 40.8845 | 11.3459 | 1.5142 | 7.1276 | 162.8568 | 3.9833 |
| LDH-INDO-24 h | 50 °C | 32.9748 | 12.2700 | 1.5115 | 7.1986 | 225.1180 | 18.3469 |
| LDH-INDO-24 h | 70 °C | 20.4234 | 9.8009 | 1.5085 | 9.0156 | 210.8176 | 21.5100 |
| LDH-INDO-24 h | 90 °C | 23.1638 | 13.5995 | 1.5120 | 6.4941 | 132.0095 | 9.7068 |
| LDH-INDO-48 h | 50 °C | 26.1137 | 12.6181 | 1.5102 | 6.9998 | 836.2663 | 66.2746 |
| LDH-INDO-48 h | 70 °C | 21.8045 | 10.8109 | 1.5116 | 8.1718 | 158.8192 | 14.6905 |
| LDH-INDO-48 h | 90 °C | 25.7321 | 18.1079 | 1.5168 | 4.8761 | 175.9703 | 9.7178 |
| Samples | Temperature (°C) | Network Parameters | |
|---|---|---|---|
| a (Å) | c (nm) | ||
| LDH-8 h | 50 °C | 3.9896 | 0.2562 |
| LDH-8 h | 70 °C | 3.0293 | 0.2554 |
| LDH-8 h | 90 °C | 3.5354 | 0.2364 |
| LDH-16 h | 50 °C | 3.0437 | 0.2653 |
| LDH-16 h | 70 °C | 3.0447 | 0.2640 |
| LDH-16 h | 90 °C | 3.0478 | 0.2638 |
| LDH-24 h | 50 °C | 3.0398 | 0.2494 |
| LDH-24 h | 70 °C | 3.0444 | 0.2280 |
| LDH-24 h | 90 °C | 3.8282 | 0.2295 |
| LDH-48 h | 50 °C | 3.8247 | 0.2347 |
| LDH-48 h | 70 °C | 3.6177 | 0.2462 |
| LDH-48 h | 90 °C | 3.0478 | 0.2316 |
| LDH-INDO-8 h | 50 °C | 3.0309 | 0.3798 |
| LDH-INDO-8 h | 70 °C | 3.0317 | 0.3667 |
| LDH-INDO-8 h | 90 °C | 3.0170 | 0.3613 |
| LDH-INDO-16 h | 50 °C | 3.8904 | 0.3713 |
| LDH-INDO-16 h | 70 °C | 3.0309 | 0.3371 |
| LDH-INDO-16 h | 90 °C | 3.0285 | 0.3403 |
| LDH-INDO-24 h | 50 °C | 3.0844 | 0.3681 |
| LDH-INDO-24 h | 70 °C | 3.0171 | 0.2940 |
| LDH-INDO-24 h | 90 °C | 3.0240 | 0.2922 |
| LDH-INDO-48 h | 50 °C | 3.0204 | 0.2518 |
| LDH-INDO-48 h | 70 °C | 3.0232 | 0.3243 |
| LDH-INDO-48 h | 90 °C | 3.0337 | 0.2540 |
| Cell Lineage | INDO | LDH-INDO | ||||
|---|---|---|---|---|---|---|
| IC50 (mM) | CI (95%) | R2 | IC50 (mM) * | CI (95%) | R2 | |
| AGP01 | 0.51 | 0.46–0.56 | 0.98 | 2.51 | 2.05–3.07 | 0.80 |
| ACP02 | 1.24 | 0.97–1.59 | 0.90 | 2.85 | 2.42–3.36 | 0.93 |
| ACP03 | 1.18 | 0.97–1.42 | 0.93 | 2.99 | 2.48–3.61 | 0.92 |
| MDA-MB-231 | 0.39 | 0.33–0.46 | 0.95 | 0.89 | 0.81–0.96 | 0.98 |
| MCF-7 | 0.52 | 0.40–0.66 | 0.90 | 1.72 | 1.42–2.09 | 0.93 |
| SK-MEL-19 | 1.55 | 1.26–1.92 | 0.91 | 3.79 | 2.95–4.87 | 0.84 |
| MRC-5 | 0.99 | 0.85–1.15 | 0.95 | 2.68 | 2.34–3.08 | 0.93 |
| MN01 | 1.14 | 0.89–1.44 | 0.90 | 3.23 | 2.76–3.78 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, K.C.; Costa, C.E.F.d.; Remédios, C.M.R.; Calcagno, D.Q.; Lima, M.d.O.; Silva, J.R.A.; Alves, C.N. LDH-Indomethacin Nanoparticles Antitumoral Action: A Possible Coadjuvant Drug for Cancer Therapy. Molecules 2024, 29, 3353. https://doi.org/10.3390/molecules29143353
Alves KC, Costa CEFd, Remédios CMR, Calcagno DQ, Lima MdO, Silva JRA, Alves CN. LDH-Indomethacin Nanoparticles Antitumoral Action: A Possible Coadjuvant Drug for Cancer Therapy. Molecules. 2024; 29(14):3353. https://doi.org/10.3390/molecules29143353
Chicago/Turabian StyleAlves, Kelly Costa, Carlos Emmerson Ferreira da Costa, Cláudio Márcio Rocha Remédios, Danielle Queiroz Calcagno, Marcelo de Oliveira Lima, José Rogério A. Silva, and Cláudio Nahum Alves. 2024. "LDH-Indomethacin Nanoparticles Antitumoral Action: A Possible Coadjuvant Drug for Cancer Therapy" Molecules 29, no. 14: 3353. https://doi.org/10.3390/molecules29143353
APA StyleAlves, K. C., Costa, C. E. F. d., Remédios, C. M. R., Calcagno, D. Q., Lima, M. d. O., Silva, J. R. A., & Alves, C. N. (2024). LDH-Indomethacin Nanoparticles Antitumoral Action: A Possible Coadjuvant Drug for Cancer Therapy. Molecules, 29(14), 3353. https://doi.org/10.3390/molecules29143353







