Assembly of Homochiral Magneto-Optical Dy6 Triangular Clusters by Fixing Carbon Dioxide in the Air
Abstract
:1. Introduction
2. Results and Discussion
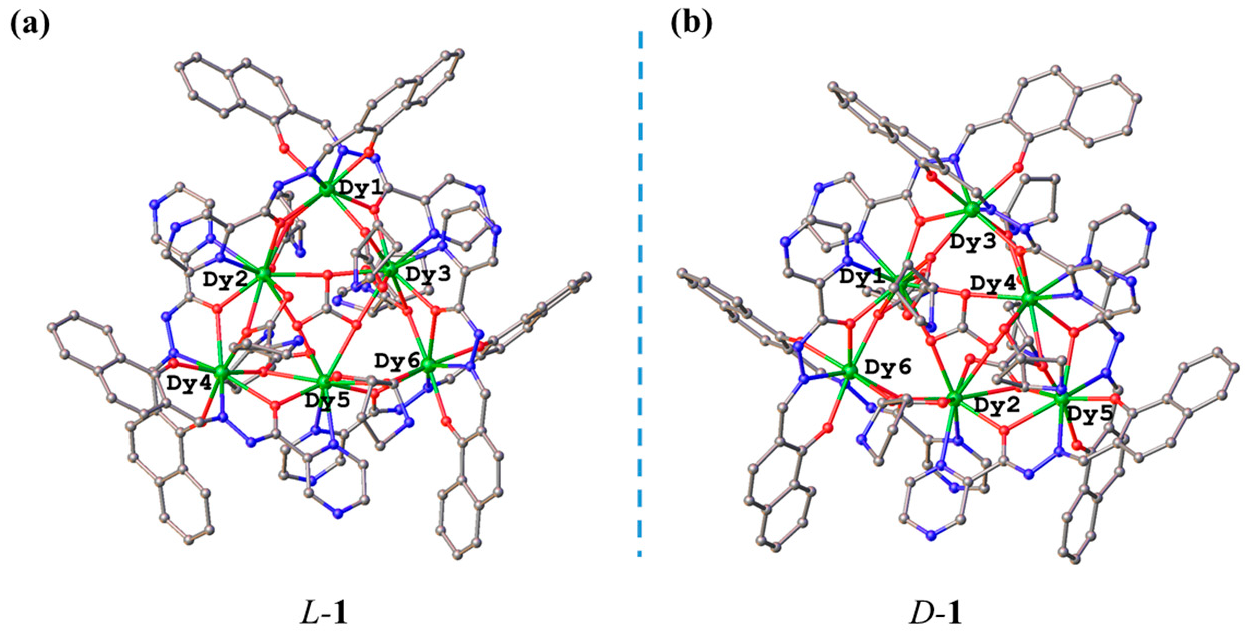
2.1. Synthesis and Crystal Structures
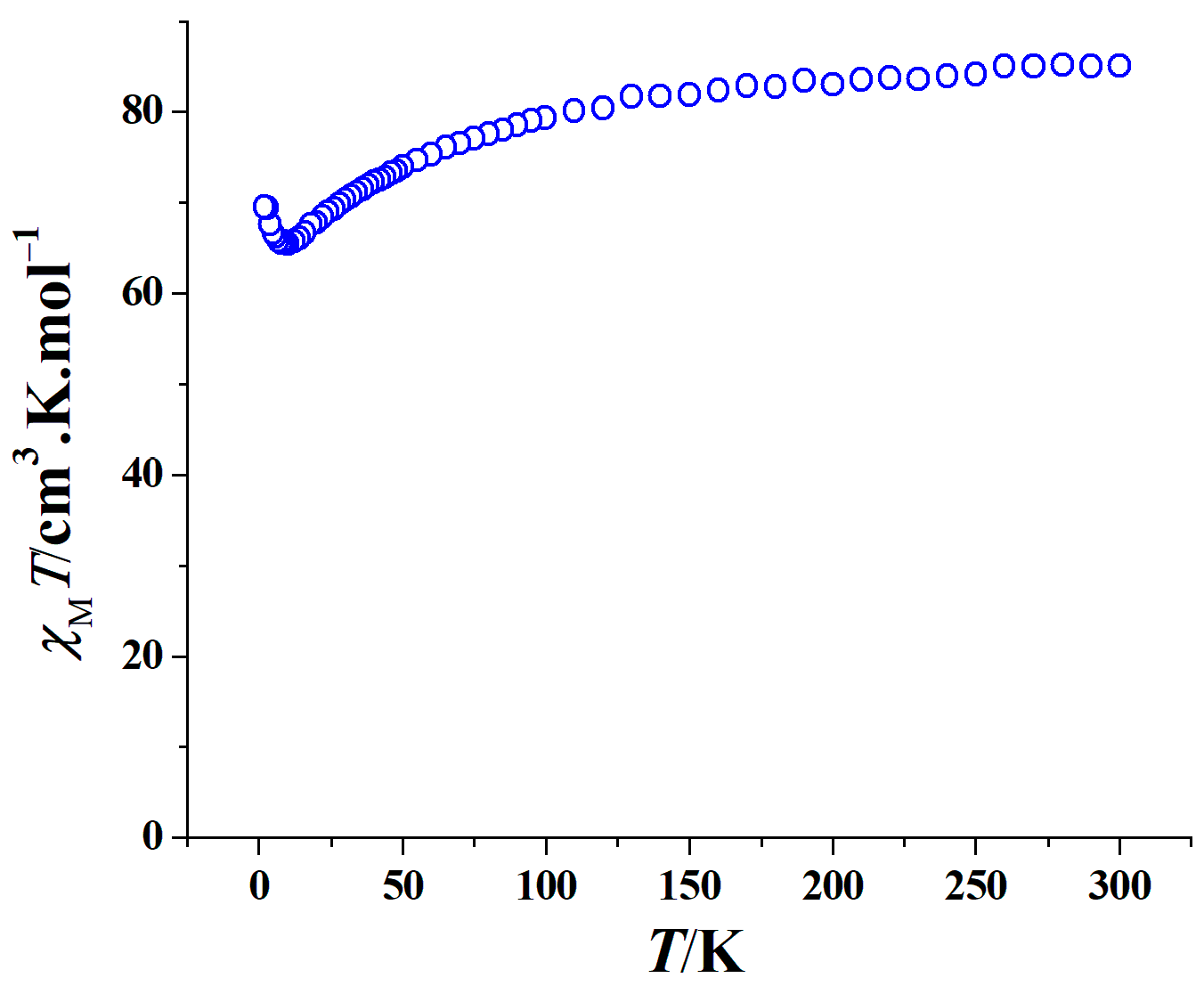
2.2. Magnetic Properties
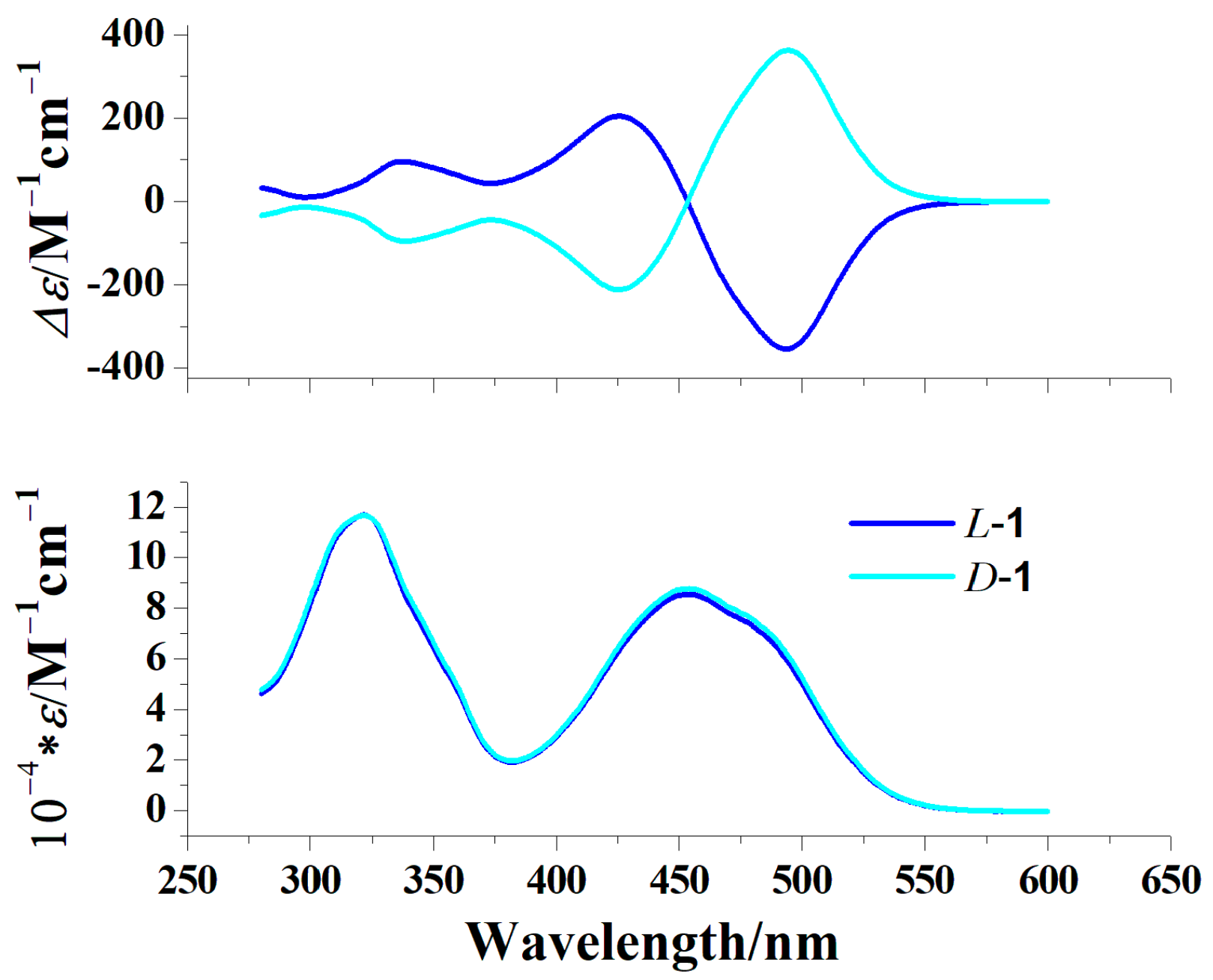
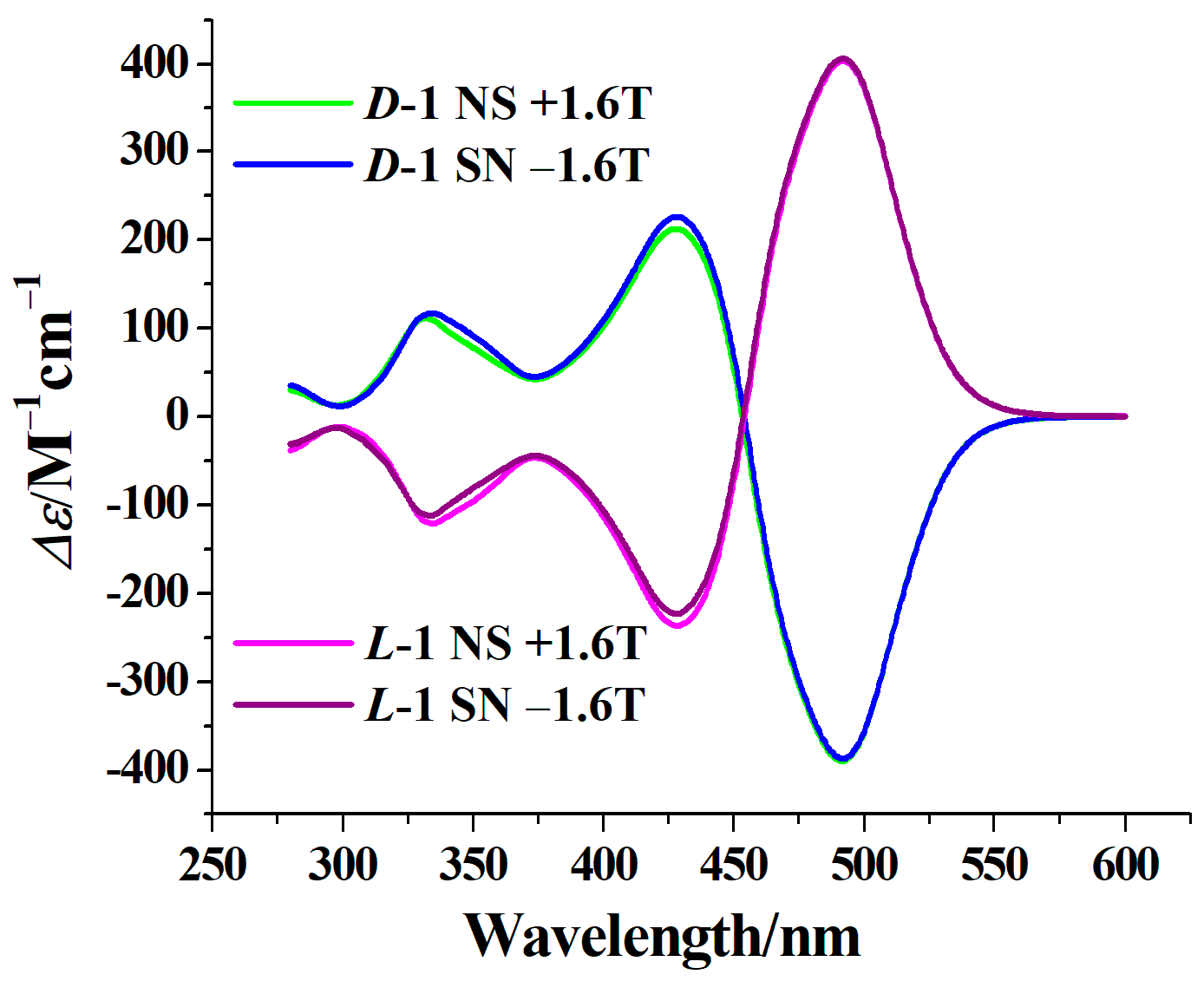
2.3. Circular Dichroism Spectra (CD) and Magnetic Circular Dichroism Spectra (MCD)
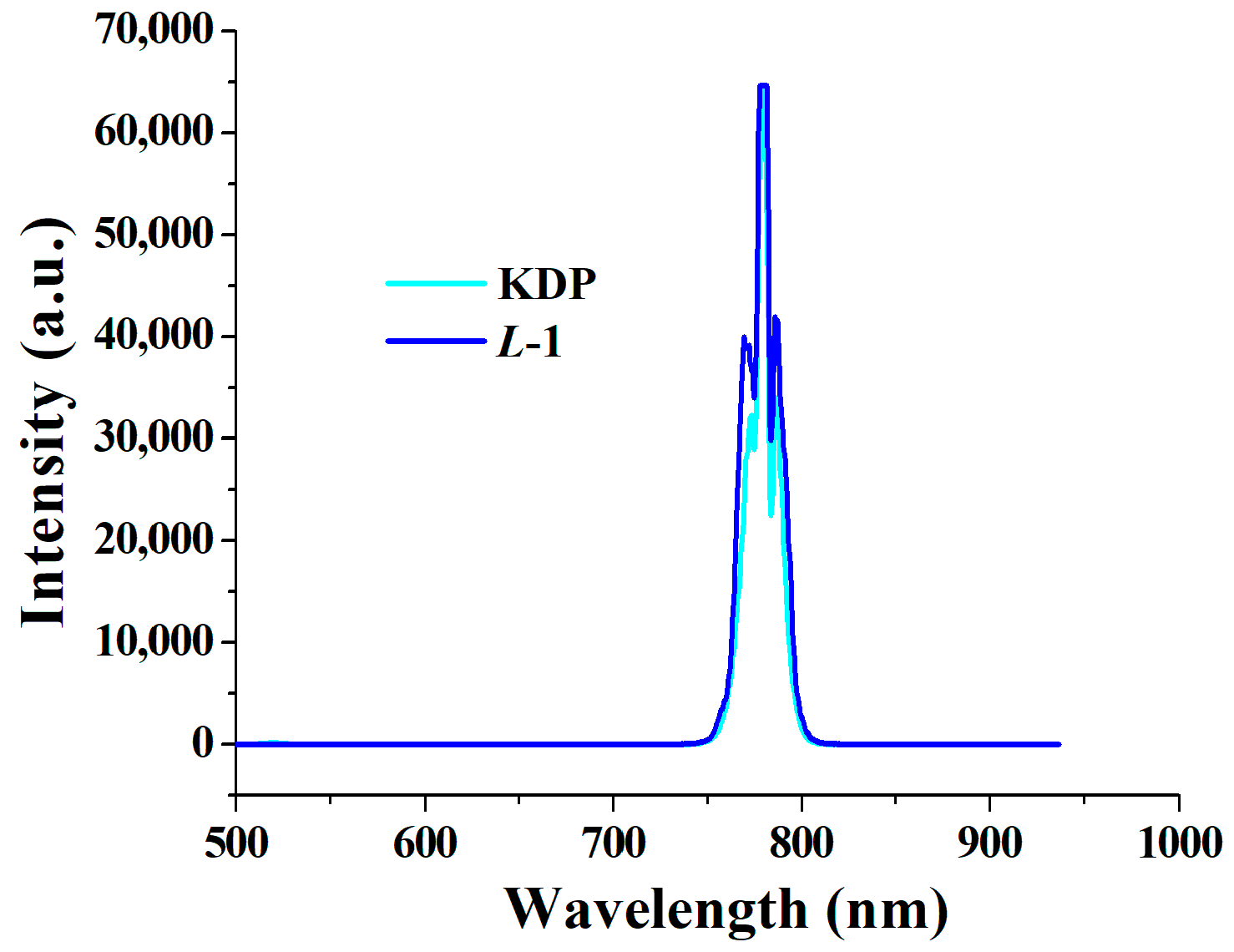
2.4. Nonlinear Optical Properties
3. Experimental Section
3.1. Materials and Instruments
3.2. Nonlinear Optical (NLO) Response Measurements
3.3. Synthesis of H2LSchiff
3.4. Syntheses of L-1 and D-1
3.5. X-ray Single-Crystal Structure Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- English, N.J.; El-Hendawy, M.M.; Mooney, D.A.; MacElroy, J.M.D. Perspectives on atmospheric CO2 fixation in inorganic and biomimetic structures. Coord. Chem. Rev. 2014, 269, 85–95. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Chen, X.-H.; Xue, S.; Tang, J. Molecular Assembly and Magnetic Dynamics of Two Novel Dy6 and Dy8 Aggregates. Inorg. Chem. 2012, 51, 4035–4042. [Google Scholar] [CrossRef]
- Tang, X.-L.; Wang, W.-H.; Dou, W.; Jiang, J.; Liu, W.-S.; Qin, W.-W.; Zhang, G.-L.; Zhang, H.-R.; Yu, K.-B.; Zheng, L.-M. Olive-Shaped Chiral Supramolecules: Simultaneous Self-Assembly of Heptameric Lanthanum Clusters and Carbon Dioxide Fixation. Angew. Chem. Int. Ed. 2009, 48, 3499–3502. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Y.-N.; Zhao, L.; Tang, J.; Liu, Z. Hexanuclear Dysprosium(III) Compound Incorporating Vertex- and Edge-Sharing Dy3 Triangles Exhibiting Single-Molecule-Magnet Behavior. Inorg. Chem. 2011, 50, 8688–8690. [Google Scholar] [CrossRef] [PubMed]
- Gass, I.A.; Moubaraki, B.; Langley, S.K.; Battena, S.R.; Murray, K.S. A π-π 3D network of tetranuclear μ2/μ3-carbonato Dy(iii) bis-pyrazolylpyridine clusters showing single molecule magnetism features. Chem. Commun. 2012, 48, 2089–2091. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhao, L.; Guo, Y.-N.; Guo, Y.; Tang, J.; Liu, Z. Quadruple-CO32− bridged octanuclear dysprosium(iii) compound showing single-molecule magnet behavior. Chem. Commun. 2012, 48, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.K.; Gass, I.A.; Moubaraki, B.; Murray, K.S. Magnetic Properties of Hexanuclear Lanthanide (III) Clusters Incorporating a Central μ6-Carbonate Ligand Derived from Atmospheric CO2 Fixation. Inorg. Chem. 2012, 51, 3947–3949. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, M.; Zhao, L.; Guo, Y.-N.; Guo, Y.; Tang, J.; Liu, Z. A Discrete Dysprosium Trigonal Prism Showing Single-Molecule Magnet Behaviour. Chem. Eur. J. 2012, 18, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, R.J.; Kuo, C.-J.; Gabidullin, B.; Wang, C.-W.; Clérac, R.; Murugesu, M.; Lin, P.-H. A propeller-shaped μ4-carbonate hexanuclear dysprosium complex with a high energetic barrier to magnetisation relaxation. Dalton Trans. 2016, 45, 16769–16773. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-M.; Wu, Z.-L.; Zhang, Y.-X.; Wei, H.-Y.; Gao, H.-L.; Cui, J.-Z. Self-assembly of tetra-nuclear lanthanide clusters via atmospheric CO2 fixation: Interesting solvent-induced structures and magnetic relaxation conversions. Inorg. Chem. Front. 2018, 5, 2346–2354. [Google Scholar] [CrossRef]
- Kumar, P.; Swain, A.; Acharya, J.; Li, Y.; Kumar, V.; Rajaraman, G.; Colacio, E.; Chandrasekhar, V. Synthesis, Structure, and Zero-Field SMM Behavior of Homometallic Dy2, Dy4, and Dy6 Complexes. Inorg. Chem. 2022, 61, 11600–11621. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Hao, X. Anion-dependent dysprosium(iii) cluster single-molecule magnets. New J. Chem. 2023, 47, 18849–18855. [Google Scholar] [CrossRef]
- Qin, L.; Zhou, G.-J.; Yu, Y.-Z.; Nojiri, H.; Schröder, C.; Winpenny, R.E.P.; Zheng, Y.-Z. Topological Self-Assembly of Highly Symmetric Lanthanide Clusters: A Magnetic Study of Exchange-Coupling “Fingerprints” in Giant Gadolinium(III) Cages. J. Am. Chem. Soc. 2017, 139, 16405–16411. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, N.; Fujisawa, K.; Koda, T.; Hikichi, S.; Moro-oka, Y. Fixation of atmospheric CO2 by a copper(II) complex. J. Chem. Soc. Chem. Commun. 1990, 19, 1357–1358. [Google Scholar] [CrossRef]
- Tanase, T.; Nitta, S.; Yoshikawa, S.; Kobayashi, K.; Sakurai, T.; Yano, S. Spontaneous fixation of carbon dioxide in air by a nickel diamine complex: Synthesis and characterization of a trinuclear nickel(II) complex with a novel hydrogen bonding system around a carbonate ligand. Inorg. Chem. 1992, 31, 1058–1062. [Google Scholar] [CrossRef]
- El-Hendawy, M.M.; English, N.J.; Mooney, D.A. Mechanism of Atmospheric CO2 Fixation in the Cavities of a Dinuclear Cryptate. Inorg. Chem. 2012, 51, 5282–5288. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wen, Y.; Su, J.; Ma, H.; Wang, H.-Y.; Kurmoo, M.; Zuo, J.-L. Carbon Dioxide (CO2) Fixation: Linearly Bridged Zn2 Paddlewheel Nodes by CO2 in a Metal-Organic Framework. Inorg. Chem. 2019, 58, 16040–16046. [Google Scholar] [CrossRef]
- Tian, X.-F.; Ji, B.-Q.; Feng, L.; Sheng, K.; Su, Y.-M.; Jagodič, M.; Jagličić, Z.; Tung, C.-H.; Sun, D. Self-assembly of a nonanuclear NiII cluster via atmospheric CO2 fixation: Synthesis, structure, collision-induced dissociation mass spectrometry and magnetic property. Dalton Trans. 2020, 49, 10977–10982. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Wang, R.; Tang, X.; Jagodič, M.; Jagličić, Z.; Pang, L.; Dou, J.-M.; Gao, Z.-Y.; Feng, H.-Y.; Tung, C.-H.; et al. Carbonate-Templated Decanuclear Mn Nanocage with Two Different Silsesquioxane Ligands. Inorg. Chem. 2021, 60, 14866–14871. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-J.; Ren, Y.-P.; Long, L.-S.; Zheng, Z.; Huang, R.-B.; Zheng, L.-S. A Keplerate Magnetic Cluster Featuring an Icosidodecahedron of Ni(II) Ions Encapsulating a Dodecahedron of La(III) Ions. J. Am. Chem. Soc. 2007, 129, 701–7017. [Google Scholar] [CrossRef] [PubMed]
- Titos-Padilla, S.; Ruiz, J.; Herrera, J.M.; Brechin, E.K.; Wersndorfer, W.; Lloret, F.; Colacio, E. Dilution-Triggered SMM Behavior under Zero Field in a Luminescent Zn2Dy2 Tetranuclear Complex Incorporating Carbonato-Bridging Ligands Derived from Atmospheric CO2 Fixation. Inorg. Chem. 2013, 52, 9620–9626. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Fujinami, K.; Nishi, N.; Matsumoto, N.; Mochida, T.; Ishida, Y.; Sunatsuki, N.; Re, N. Carbonato-Bridged NiII2LnIII2 (LnIII = GdIII, TbIII, DyIII) Complexes Generated by Atmospheric CO2 Fixation and Their Single-Molecule-Magnet Behavior: [(μ4-CO3)2{NiII(3-MeOsaltn)(MeOH or H2O)LnIII(NO3)}2]·solvent [3-MeOsaltn = N,N′-Bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato]. Inorg. Chem. 2013, 52, 7218–7229. [Google Scholar] [PubMed]
- Zhang, P.; Zhang, L.; Lin, S.-Y.; Tang, J. Tetranuclear [MDy]2 Compounds and Their Dinuclear [MDy] (M = Zn/Cu) Building Units: Their Assembly, Structures, and Magnetic Properties. Inorg. Chem. 2013, 52, 6595–6602. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Lorusso, G.; Evangelisti, M.; Brechin, E.K.; Pope, S.J.A.; Colacio, E. Closely-Related ZnII2LnIII2 Complexes (LnIII = Gd, Yb) with Either Magnetic Refrigerant or Luminescent Single-Molecule Magnet Properties. Inorg. Chem. 2014, 53, 3586–3594. [Google Scholar] [CrossRef]
- Upadhyay, A.; Das, C.; Langley, S.K.; Murray, K.S.; Srivastava, A.K.; Shanmugam, M. Heteronuclear Ni(ii)-Ln(iii) (Ln = La, Pr, Tb, Dy) complexes: Synthesis and single-molecule magnet behavior. Dalton Trans. 2016, 45, 3616–3626. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.-R.; Hu, J.-J.; Yang, K.; Zhang, J.-L.; Liu, S.-J.; Liao, J.-S.; Liu, C.-M. Family of Chiral ZnII-LnIII (Ln = Dy and Tb) Heterometallic Complexes Derived from the Amine-Phenol Ligand Showing Multifunctional Properties. Inorg. Chem. 2020, 59, 2811–2824. [Google Scholar] [CrossRef]
- Liu, C.-M.; Zhang, D.-Q.; Hao, X.; Zhu, D.-B. Zn2Ln2 complexes with carbonate bridges formed by the fixation of carbon dioxide in the atmosphere: Single-molecule magnet behaviour and magnetocaloric effect. Dalton Trans. 2020, 49, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Hao, X.; Zhang, D.-Q. Effects of substituents on bridging ligands on the single-molecule magnet properties of Zn2Dy2 cluster complexes. Appl. Organometal. Chem. 2021, 35, e6048. [Google Scholar] [CrossRef]
- Sheikh, J.A.; Jena, H.S.; Konar, S. Co3Gd4 Cage as Magnetic Refrigerant and Co3Dy3 Cage Showing Slow Relaxation of Magnetisation. Molecules 2022, 27, 1130. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R. Quantum Tunneling of Magnetization and Related Phenomena in Molecular Materials. Angew. Chem. Int. Ed. 2003, 42, 268–297. [Google Scholar] [CrossRef]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.C.; Liu, J.L.; Vieru, V.; Ungur, L.; Jia, J.H.; Chibotaru, L.F.; Lan, Y.H.; Wernsdorfer, W.; Gao, S.; et al. A Stable Pentagonal-Bipyramidal Dy(III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.S.; Jiang, S.D.; Wang, B.W.; Gao, S. Understanding the Magnetic Anisotropy toward Single-Ion Magnets. Acc. Chem. Res. 2016, 49, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.S.; Chilton, N.F.; Winpenny, R.E.; Zheng, Y.Z. On Approaching the Limit of Molecular Magnetic Anisotropy: A Near-Perfect Pentagonal Bipyramidal Dysprosium(III) Single-Molecule Magnet. Angew. Chem. Int. Ed. 2016, 55, 16071–16074. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zhang, D.Q.; Hao, X.; Zhu, D.B. Assembly of chiral 3d-4f wheel-like cluster complexes with achiral ligands: Single-molecule magnetic behavior and magnetocaloric effect. Inorg. Chem. Front. 2020, 7, 3340–3351. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhu, S.D.; Lu, Y.B.; Hao, X.; Wen, H.R. Homochiral Cu6Dy3 single-molecule magnets displaying proton conduction and a strong magneto-optical Faraday effect. Inorg. Chem. Front. 2023, 10, 3714–3722. [Google Scholar] [CrossRef]
- Liu, C.M.; Sun, R.; Wang, B.W.; Wu, F.; Hao, X.; Shen, Z. Homochiral Ferromagnetic Coupling Dy2 Single-Molecule Magnetswith Strong Magneto-Optical Faraday Effects at Room Temperature. Inorg. Chem. 2021, 60, 12039–12048. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Sun, R.; Hao, X.; Wang, B.-W. Two Pairs of Homochiral Parallelogram-like Dy4 Cluster Complexes with Strong Magneto-Optical Properties. Inorg. Chem. 2023, 62, 20184–20193. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Sun, R.; Hao, X.; Li, X.-L.; Wang, B.-W. Homochiral Dy2 single-molecule magnets with strong magneto-optical Faraday effects and strong third-harmonic generation. Inorg. Chem. Front. 2024, 11, 3296–3308. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, S.; Wang, H.; Dou, J.; Jiang, J. Magneto-chiral dichroism in chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker SMMs. Inorg. Chem. Front. 2014, 1, 167–171. [Google Scholar] [CrossRef]
- Wang, X.; Du, M.-H.; Xu, H.; Long, L.-S.; Kong, X.-J.; Zheng, L.-S. Cocrystallization of Chiral 3d-4f Clusters {Mn10Ln6} and {Mn6Ln2}. Inorg. Chem. 2021, 60, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-D.; Zhou, Y.-L.; Liu, F.; Lei, Y.; Liu, S.-J.; Wen, H.-R.; Shi, B.; Zhang, S.-Y.; Liu, C.-M.; Lu, Y.-B. A Pair of Multifunctional Cu(II)-Dy(III) Enantiomers with Zero-Field Single-Molecule Magnet Behaviors, Proton Conduction Properties and Magneto–Optical Faraday Effects. Molecules 2023, 28, 7506. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sun, R.; Wu, X.-F.; Liu, Y.; Zhan, J.-Z.; Wang, B.-W.; Gao, S. Circularly polarized luminescence and magneto-optic effects from chiral Dy(iii) single molecule magnets. Dalton Trans. 2023, 52, 7646–7651. [Google Scholar] [CrossRef] [PubMed]
- Dhbaibi, K.; Grasser, M.; Douib, H.; Dorcet, V.; Cador, O.; Vanthuyne, N.; Riobé, F.; Maury, O.; Guy, S.; Bensalah-Ledoux, A.; et al. Multifunctional Helicene-Based Ytterbium Coordination Polymer Displaying Circularly Polarized Luminescence, Slow Magnetic Relaxation and Room Temperature Magneto-Chiral Dichroism. Angew. Chem. Int. Ed. 2023, 62, e202215558. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Xiong, R.-G.; Zhang, D.-Q.; Zhu, D.-B. Nanoscale Homochiral C3-Symmetric Mixed-Valence Manganese Cluster Complexes with Both Ferromagnetic and Ferroelectric Properties. J. Am. Chem. Soc. 2010, 132, 4044–4045. [Google Scholar] [CrossRef] [PubMed]
- Thielemann, D.T.; Wagner, A.T.; Lan, Y.; Anson, C.E.; Gamer, M.T.; Powell, A.K.; Roesky, P.W. Slow magnetic relaxation in four square-based pyramidal dysprosium hydroxo clusters ligated by chiral amino acid anions-a comparative study. Dalton Trans. 2013, 42, 14794–14800. [Google Scholar] [CrossRef] [PubMed]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem.-Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Hao, X.; Zhu, D.-M.; Zhang, Y.-Q. Effect of coordinated anions on ferromagnetically coupled Dy2 zero-field single-molecule magnets. Dalton Trans. 2024, 53, 6120–6127. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, J.; Filoti, G.; Kuncser, V.; Schinteie, G.; Mereacre, V.; Anson, C.E.; Powell, A.K.; Prodius, D.; Turta, C. Magnetostructural correlations in the tetranuclear series of {Fe3LnO2} butterfly core clusters: Magnetic and Mössbauer spectroscopic study. Phys. Rev. B. 2009, 80, 014430–014446. [Google Scholar] [CrossRef]
- Pantelis, K.N.; Perlepe, P.S.; Grammatikopoulos, S.; Lampropoulos, C.; Tang, J.; Stamatatos, T.C. 4f-Metal Clusters Exhibiting Slow Relaxation of Magnetization: A {Dy7} Complex with An Hourglass-like Metal Topology. Molecules 2020, 25, 2191. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Bag, R.; Satpathi, S.; Pati, S.K.; Butcher, R.J.; Tang, J.; Goswami, S. Structure and Magnetism of LnIII2 (Ln = Gd, Tb, Dy, and Ho) Assemblies Constructed from a Bis(Hydrazone) Compartmental Ligand: Slow Magnetic Relaxation in the DyIII2 Analogue. Cryst. Growth Des. 2023, 23, 7459–7471. [Google Scholar] [CrossRef]
- Ewing, G.W.; Steck, E.A. Absorption spectra of heterocyclic compounds. I. Quinolinols and isoquinolinols. J. Am. Chem. Soc. 1946, 68, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Rikken, G.L.J.A.; Raupach, E. Enantioselective magnetochiral photochemistry. Nature 2000, 405, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Sun, R.; Wang, B.-W.; Hao, X.; Li, X.-L. Effects of Counterions, Coordination Anions, and Coordination Solvent Molecules on Single-Molecule Magnetic Behaviors and Nonlinear Optical Properties of Chiral Zn2Dy Schiff Base Complexes. Inorg. Chem. 2022, 61, 18510–18523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Wang, A.; Cui, M.; Gao, C.; Yu, X.; Su, B.; Zhou, L.; Liu, C.-M.; Xiao, H.-P.; Zhang, Y.-Q. Modulating Two Pairs of Chiral DyIII Enantiomers by Distinct β-Diketone Ligands to Show Giant Differences in Single-Ion Magnet Performance and Nonlinear Optical Response. Inorg. Chem. 2022, 61, 9283–9294. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zhang, D.-Q.; Xiong, R.-G.; Hao, X.; Zhu, D.-B. A homochiral Zn-Dy heterometallic left-handed helical chain complex without chiral ligands: Anion-induced assembly and multifunctional integration. Chem. Commun. 2018, 54, 13379–13382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-D.; Hu, J.-J.; Dong, L.; Wen, H.-R.; Liu, S.-J.; Lu, Y.-B.; Liu, C.-M. Multifunctional Zn(II)-Yb(III) complex enantiomers showing second-harmonic generation, near-infrared luminescence, single-molecule magnet behaviour and proton conduction. J. Mater. Chem. C 2020, 8, 16032–16041. [Google Scholar] [CrossRef]


| L-1 (Squeeze) | D-1 (Squeeze) | |
| formula | C127H104Dy6N30O27 | C127H104Dy6N30O27 |
| FW | 3457.40 | 3457.40 |
| crystal system | monoclinic | monoclinic |
| space group | C2 | C2 |
| a [Å] | 33.8797(5) | 33.8283(9) |
| b [Å] | 17.3214(2) | 17.3710(4) |
| c [Å] | 26.3394(3) | 26.3514(6) |
| β [°] | 102.5770(10) | 102.613(2) |
| V [Å3] | 15,086.2(3) | 15,111.2(6) |
| Z | 4 | 4 |
| ρcalc [g·cm−3] | 1.522 | 1.520 |
| μ [mm−1] | 3.006 | 3.002 |
| T [K] | 170 | 170 |
| λ [Å] | 0.71073 | 0.71073 |
| reflections collected | 142,419 | 132,511 |
| unique reflections | 41,244 | 39,636 |
| observed reflections | 33,159 | 29,637 |
| parameters | 1987 | 1981 |
| GoF [I ≥ 2σ (I)] | 1.048 | 1.063 |
| R1 [I ≥ 2σ (I)] | 0.0568 | 0.0602 |
| WR2 [I ≥ 2σ (I)] | 0.1380 | 0.1439 |
| Flack parameter | 0.086(12) | 0.005(7) |
| CCDC number | 2,363,753 | 2,363,754 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-M.; Hao, X.; Li, X.-L. Assembly of Homochiral Magneto-Optical Dy6 Triangular Clusters by Fixing Carbon Dioxide in the Air. Molecules 2024, 29, 3402. https://doi.org/10.3390/molecules29143402
Liu C-M, Hao X, Li X-L. Assembly of Homochiral Magneto-Optical Dy6 Triangular Clusters by Fixing Carbon Dioxide in the Air. Molecules. 2024; 29(14):3402. https://doi.org/10.3390/molecules29143402
Chicago/Turabian StyleLiu, Cai-Ming, Xiang Hao, and Xi-Li Li. 2024. "Assembly of Homochiral Magneto-Optical Dy6 Triangular Clusters by Fixing Carbon Dioxide in the Air" Molecules 29, no. 14: 3402. https://doi.org/10.3390/molecules29143402





