Electrocatalytic Properties of Quasi-2D Oxides LaSrMn0.5M0.5O4 (M = Co, Ni, Cu, and Zn) for Hydrogen and Oxygen Evolution Reactions
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structure
2.2. Oxidation State and Oxygen Content
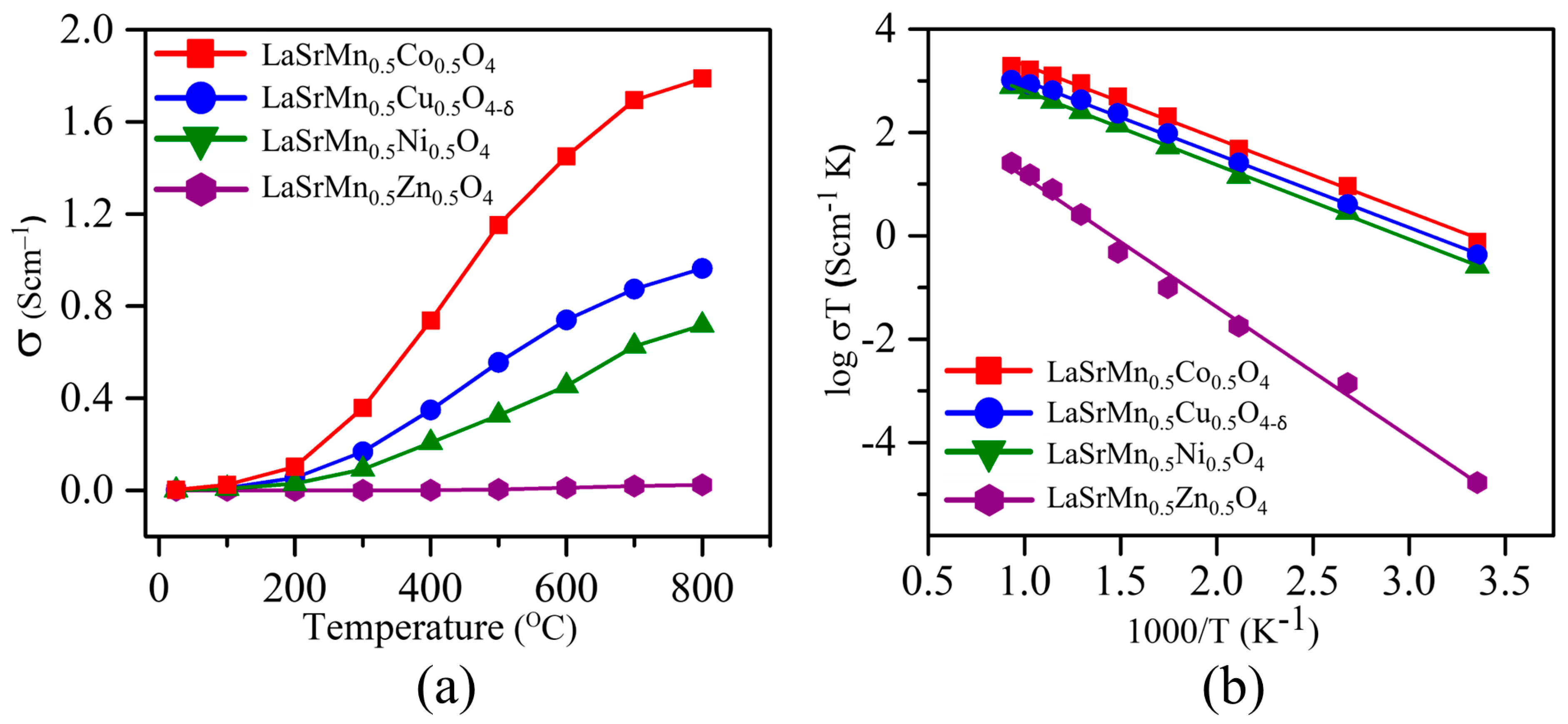
2.3. Electrical Conductivity
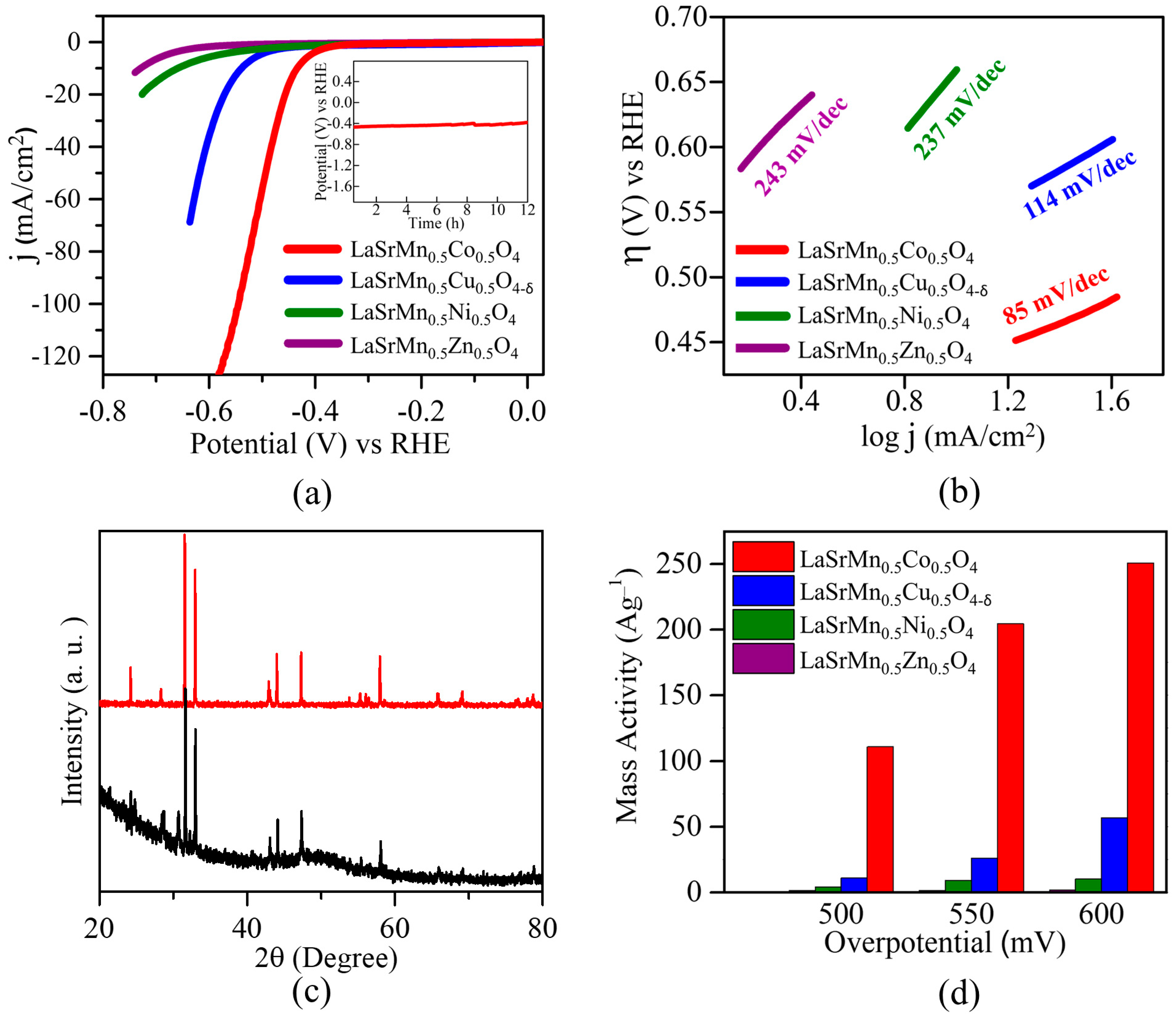
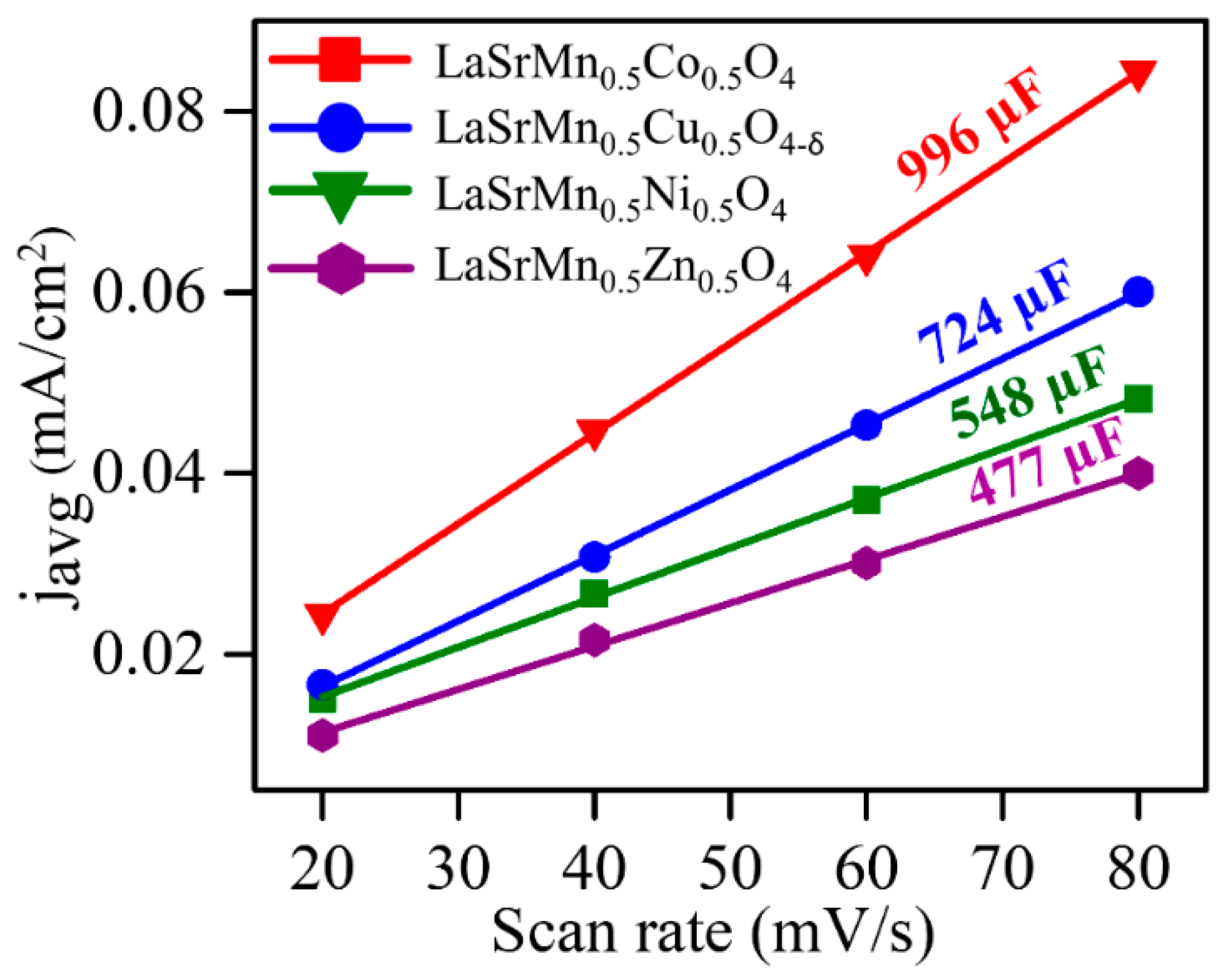
2.4. Hydrogen Evolution Reaction
- Volmer reaction (acidic): H3O+ + M + e− ⇌ M − H * + H2O
- Volmer reaction (alkaline): H2O + M + e− ⇌ M − H * + OH
- Heyrovsky reaction (acidic): M − H * + H3O+ + e− ⇌ M + H2 + H2O
- Heyrovsky reaction (alkaline): M − H * + H2O + e− ⇌ M + H2 + OH−
- Tafel reaction (acidic and alkaline): 2M − H * ⇌ 2M + H2
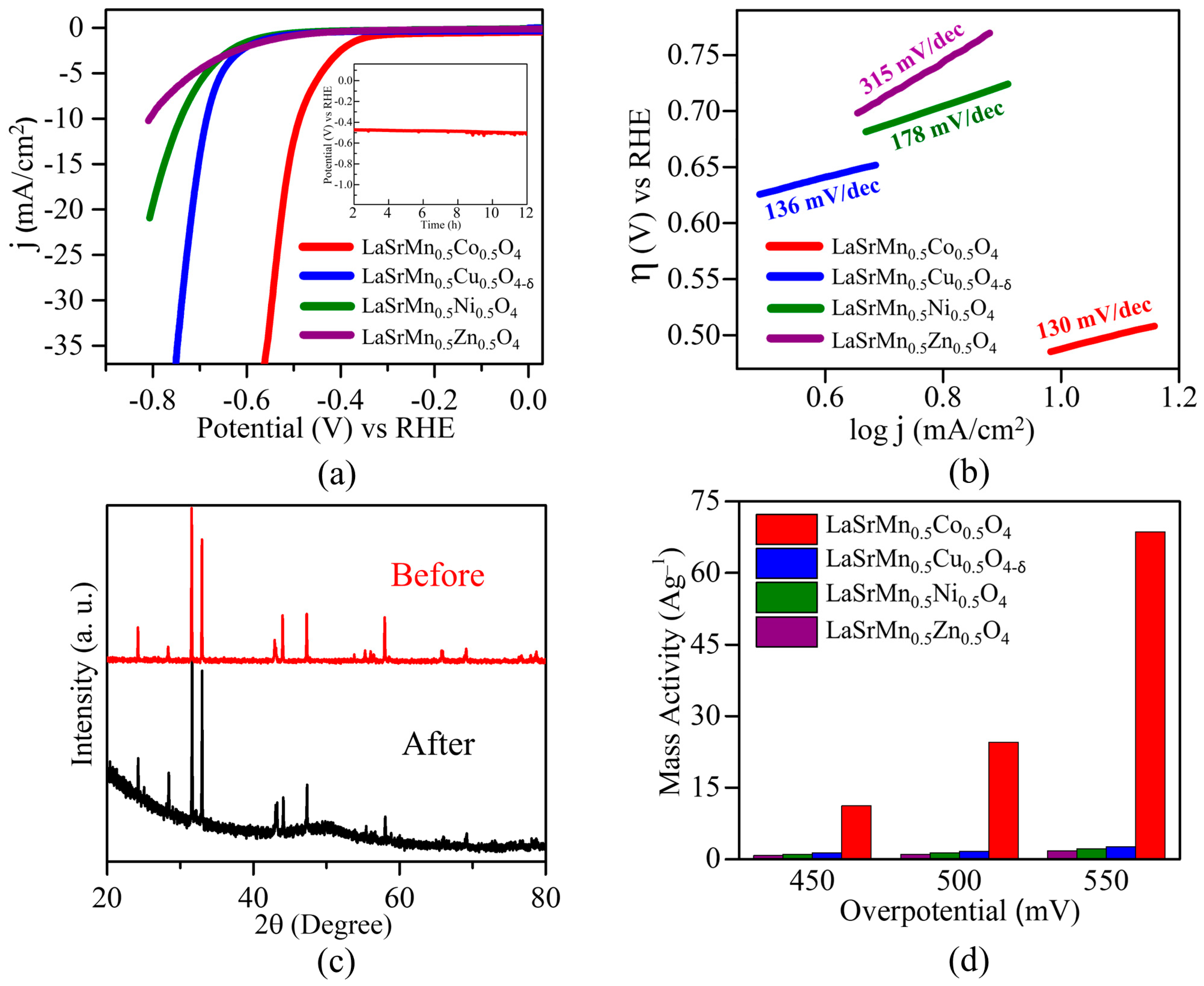
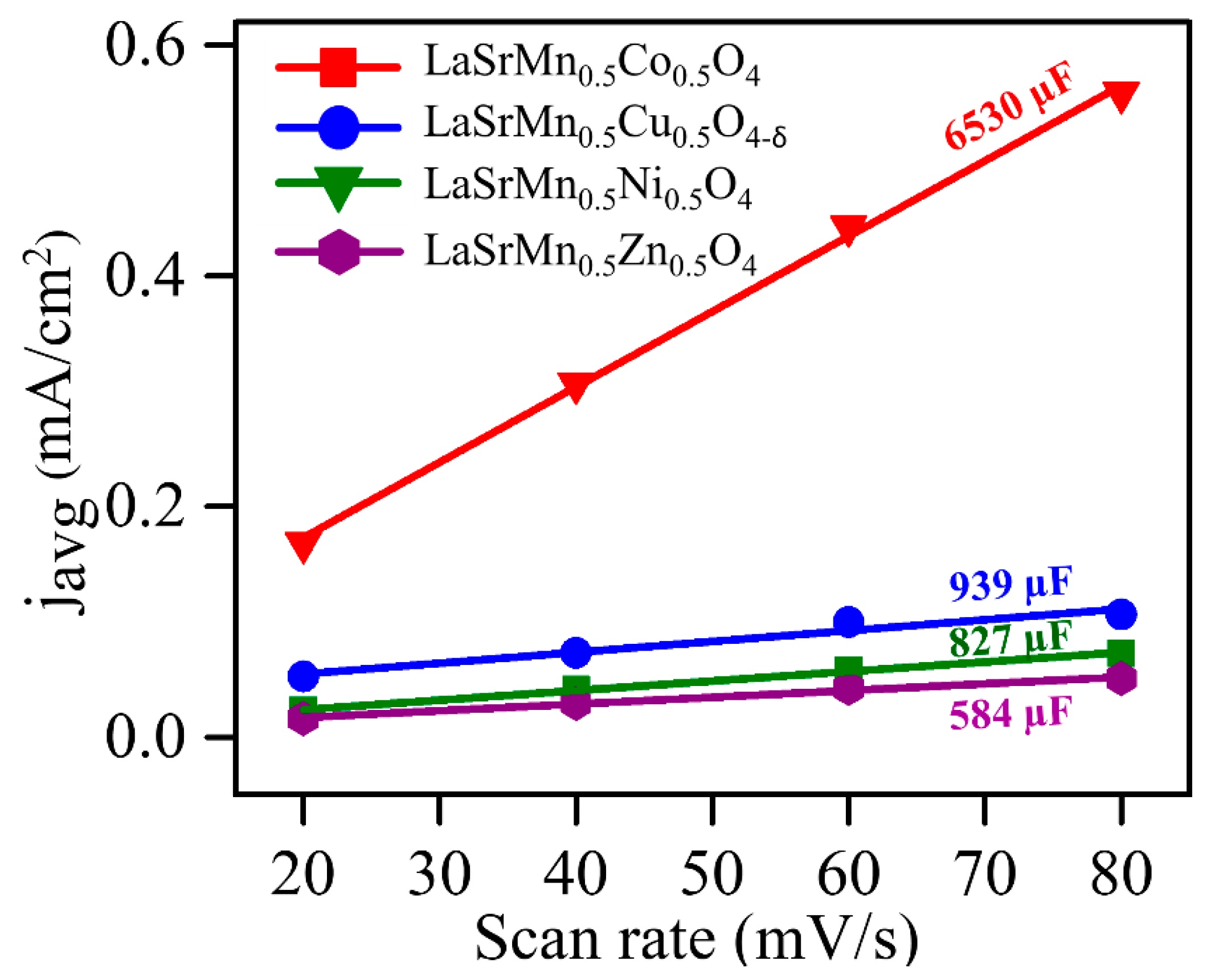
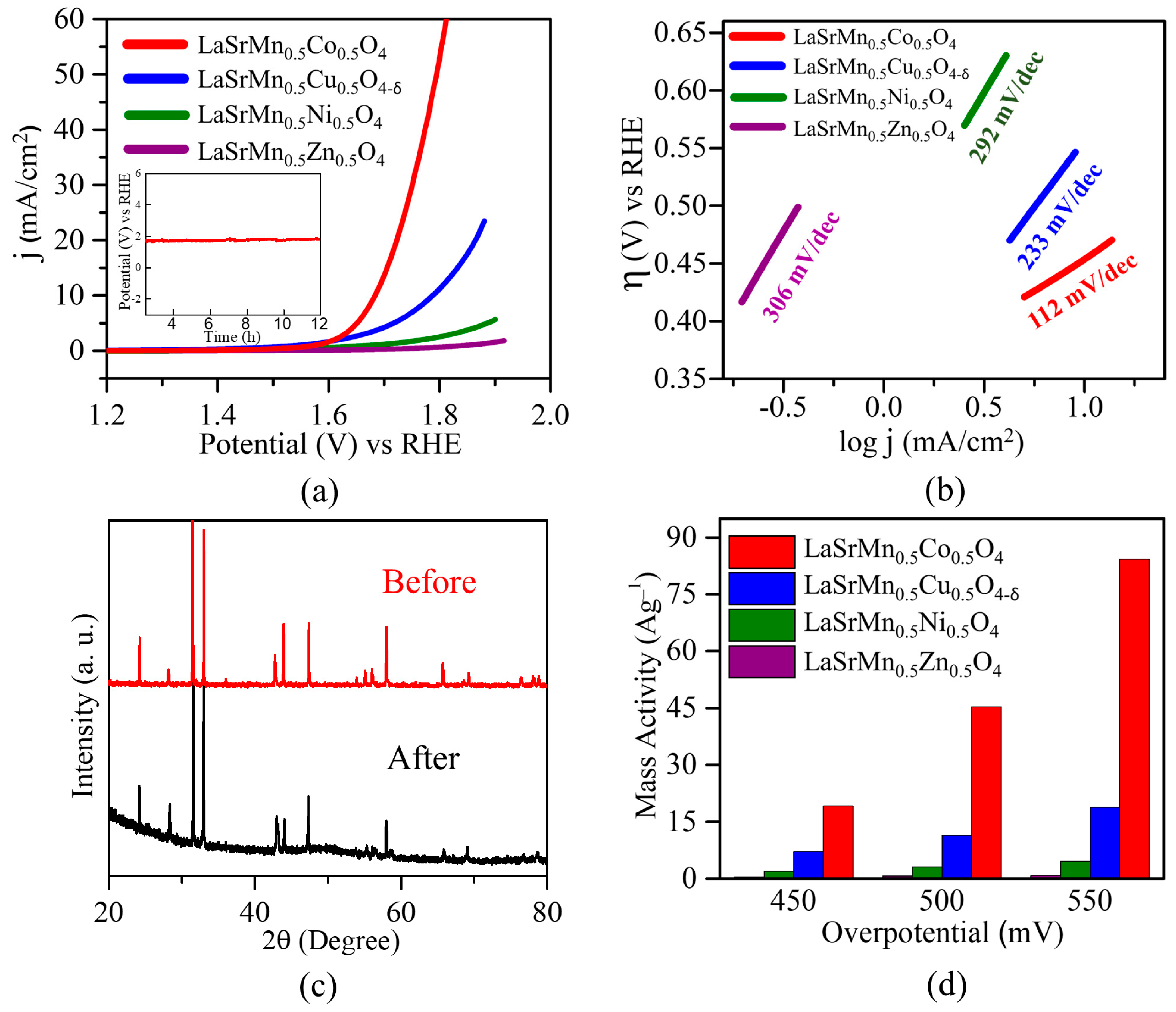
2.5. Oxygen Evolution Reaction
3. Experimental Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, J.G.; Hwang, J.; Hwang, H.J.; Jeon, O.S.; Jang, J.; Kwon, O.; Lee, Y.; Han, B.; Shul, Y.-G. A new family of perovskite catalysts for oxygen-evolution reaction in alkaline media: BaNiO3 and BaNi0. 83O2. 5. J. Am. Chem. Soc. 2016, 138, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2019, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wu, W.; Tang, Z.; Wu, Y.; Burns, R.; Tichnell, B.; Liu, Z.; Chen, S. Oxygen reduction reaction and hydrogen evolution reaction catalyzed by Pd–Ru nanoparticles encapsulated in porous carbon nanosheets. Catalysts 2018, 8, 329. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Wickramaratne, K.M.; Ramezanipour, F. Impact of oxygen-vacancies on electrical conductivity and electrocatalytic activity of La3-xCaxFe2GaO9-δ (x= 0, 2; δ= 0, 1). Solid State Sci. 2023, 141, 107208. [Google Scholar] [CrossRef]
- Karki, S.B.; Hona, R.K.; Yu, M.; Ramezanipour, F. Enhancement of Electrocatalytic Activity as a Function of Structural Order in Perovskite Oxides. ACS Catal. 2022, 12, 10333–10337. [Google Scholar] [CrossRef]
- Hona, R.K.; Karki, S.B.; Cao, T.; Mishra, R.; Sterbinsky, G.E.; Ramezanipour, F. Sustainable oxide electrocatalyst for hydrogen-and oxygen-evolution reactions. ACS Catal. 2021, 11, 14605–14614. [Google Scholar] [CrossRef]
- Choi, M.-J.; Kim, T.L.; Kim, J.K.; Lee, T.H.; Lee, S.A.; Kim, C.; Hong, K.; Bark, C.W.; Ko, K.-T.; Jang, H.W. Enhanced Oxygen Evolution Electrocatalysis in Strained A-Site Cation Deficient LaNiO3 Perovskite Thin Films. Nano Lett. 2020, 20, 8040–8045. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Chen, D.; Liu, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Water splitting with an enhanced bifunctional double perovskite. ACS Catal. 2018, 8, 364–371. [Google Scholar] [CrossRef]
- Tian, S.; He, J.; Huang, H.; Song, T.-S.; Wu, X.; Xie, J.; Zhou, W. Perovskite-based multifunctional cathode with simultaneous supplementation of substrates and electrons for enhanced microbial electrosynthesis of organics. ACS Appl. Mater. Interfaces 2020, 12, 30449–30456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, W.; Zhong, Y.; Bu, Y.; Chen, X.; Zhong, Q.; Liu, M.; Shao, Z. A perovskite nanorod as bifunctional electrocatalyst for overall water splitting. Adv. Energy Mater. 2017, 7, 1602122. [Google Scholar] [CrossRef]
- Alom, M.S.; Ramezanipour, F. Layered Oxides SrLaFe1-xCoxO4-δ (x = 0–1) as Bifunctional Electrocatalysts for Water-Splitting. ChemCatChem 2021, 13, 3510–3516. [Google Scholar] [CrossRef]
- El Shinawi, H.; Greaves, C. Structural and Magnetic Characterisation of La1+xSr1–xCo0. 5M0. 5O4±δ (M = Cr, Mn). Z. Anorg. Allg. Chem. 2009, 635, 1856–1862. [Google Scholar] [CrossRef][Green Version]
- El Shinawi, H.; Marco, J.; Berry, F.; Greaves, C. Synthesis and characterization of La0. 8Sr1. 2Co0. 5M0. 5O4−δ (M = Fe, Mn). J. Solid State Chem. 2009, 182, 2261–2268. [Google Scholar] [CrossRef]
- McCabe, E.; Greaves, C. Synthesis and Structural and Magnetic Characterization of Mixed Manganese−Copper n = 1 Ruddlesden−Popper Phases. Chem. Mater. 2006, 18, 5774–5781. [Google Scholar] [CrossRef]
- Burley, J.C.; Battle, P.D.; Gaskell, P.J.; Rosseinsky, M.J. Structural and magnetic chemistry of La2Sr2BMnO8 (B = Mg, Zn). J. Solid State Chem. 2002, 168, 202–207. [Google Scholar] [CrossRef]
- El Shinawi, H.; Greaves, C. Synthesis and characterization of La1. 5+xSr0.5−xCo0.5Ni0.5O4±δ (x = 0, 0.2). J. Mater. Chem. 2010, 20, 504–511. [Google Scholar] [CrossRef]
- Yin, W.-J.; Weng, B.; Ge, J.; Sun, Q.; Li, Z.; Yan, Y. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2019, 12, 442–462. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Liu, C.; Xu, S.; Min, S.; Wang, W.; Mitsuzaki, N.; Chen, Z. A/B-Site Management Strategy to Boost Electrocatalytic Overall Water Splitting on Perovskite Oxides in an Alkaline Medium. Inorg. Chem. 2023, 62, 12590–12599. [Google Scholar] [CrossRef]
- Wickramaratne, K.M.K.; Karki, S.B.; Ramezanipour, F. Electrocatalytic Properties of Oxygen-Deficient Perovskites Ca3Fe3-xMnxO8 (x = 1–2) for Hydrogen Evolution Reaction. Inorg. Chem. 2023, 62, 20961–20969. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Mohanta, M.K.; Qureshi, M. Transcription methodology for rationally designed morphological complex metal oxides: A versatile strategy for improved electrocatalysis. Sustain. Energy Fuels. 2021, 5, 6392–6405. [Google Scholar] [CrossRef]
- Bhowmick, S.; Dhankhar, A.; Sahu, T.K.; Jena, R.; Gogoi, D.; Peela, N.R.; Ardo, S.; Qureshi, M. Low overpotential and stable electrocatalytic oxygen evolution reaction utilizing doped perovskite oxide, La0.7Sr0.3MnO3, modified by cobalt phosphate. ACS Appl. Energy Mater. 2020, 3, 1279–1285. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Benqlilou, H.; Vinatier, P.; Levasseur, A. XPS Analysis of New Lithium Cobalt Oxide Thin-films Before and After Lithium Deintercalation. Thin Solid Films 2001, 384, 23–32. [Google Scholar] [CrossRef]
- Davison, N.; McWhinnie, W.R.; Hooper, A. X-Ray Photoelectron Spectroscopic Study of Cobalt(II) and Nickel(II) Sorbed on Hectorite and Montmorillonite. Clays Clay Miner. 1991, 39, 22–27. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Chastain, J., Ed.; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Zhao, Q.; Fang, C.; Tie, F.; Luo, W.; Peng, Y.; Huang, F.; Ku, Z.; Cheng, Y.-B. Regulating the Ni3+/Ni2+ ratio of NiOx by plasma treatment for fully vacuum-deposited perovskite solar cells. Mater. Sci. Semicond. Process. 2022, 148, 106839. [Google Scholar] [CrossRef]
- Fan, L.; Liu, P.F.; Yan, X.; Gu, L.; Yang, Z.Z.; Yang, H.G.; Qiu, S.; Yao, X. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat. Commun. 2016, 7, 10667. [Google Scholar] [CrossRef]
- Cheng, M.; Fan, H.; Song, Y.; Cui, Y.; Wang, R. Interconnected hierarchical NiCo2O4 microspheres as high-performance electrode materials for supercapacitors. Dalton Trans. 2017, 46, 9201–9209. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Han, C.; Ma, X.; Tong, Z.; Tan, B. Cu2O/CuO heterojunction formed by thermal oxidation and decorated with Pt co-catalyst as an efficient photocathode for photoelectrochemical water splitting. J. Nanoparticle Res. 2021, 23, 268. [Google Scholar] [CrossRef]
- Dan, Z.; Yang, Y.; Qin, F.; Wang, H.; Chang, H. Facile Fabrication of Cu2O Nanobelts in Ethanol on Nanoporous Cu and Their Photodegradation of Methyl Orange. Materials 2018, 11, 446. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, C.; Qi, K.; Cui, X. Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci. Rep. 2017, 7, 39695. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Fan, D.; Shen, W. Catalyst-free direct vapor-phase growth of Zn1−xCuxO micro-cross structures and their optical properties. Nanoscale Res. Lett. 2013, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Andoulsi, R.; Horchani-Naifer, K.; Ferid, M. Electrical conductivity of La1−xCaxFeO3−δ solid solutions. Ceram. Int. 2013, 39, 6527–6531. [Google Scholar] [CrossRef]
- Karki, S.B.; Ramezanipour, F. Pseudocapacitive Energy Storage and Electrocatalytic Hydrogen-Evolution Activity of Defect-Ordered Perovskites SrxCa3–xGaMn2O8 (x = 0 and 1). ACS Appl. Energy Mater. 2020, 3, 10983–10992. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Lokuhewa, I.N.; Misture, S.T.; Edwards, D.D. p-Type thermoelectric properties of the oxygen-deficient perovskite Ca2Fe2O5 in the brownmillerite structure. J. Solid State Chem. 2010, 183, 1670–1677. [Google Scholar] [CrossRef]
- Kozhevnikov, V.; Leonidov, I.; Mitberg, E.; Patrakeev, M.; Petrov, A.; Poeppelmeier, K. Conductivity and carrier traps in La1− xSrxCo1−zMnzO3−δ (x = 0.3; z = 0 and 0.25). J. Solid State Chem. 2003, 172, 296–304. [Google Scholar] [CrossRef]
- Sudha, L.; Sukumar, R.; Uma Rao, K. Evaluation of activation energy (Ea) profiles of nanostructured alumina polycarbonate composite insulation materials. Int. J. Mater. Mech. Manuf. 2014, 2, 96–100. [Google Scholar]
- Alom, M.S.; Kananke-Gamage, C.C.; Ramezanipour, F. Perovskite Oxides as Electrocatalysts for Hydrogen Evolution Reaction. ACS Omega 2022, 7, 7444–7451. [Google Scholar] [CrossRef]
- Si, C.; Zhang, W.; Lu, Q.; Guo, E.; Yang, Z.; Chen, J.; He, X.; Luo, J. Recent Advances in Perovskite Catalysts for Efficient Overall Water Splitting. Catalysts 2022, 12, 601. [Google Scholar] [CrossRef]
- Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; Van Der Vliet, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Mehran, M.T.; Naqvi, S.R.; Khoja, A.H.; Mahmood, K.; Shahzad, F.; Hussain, S. Role of perovskites as a bi-functional catalyst for electrochemical water splitting: A review. Int. J. Energy Res. 2020, 44, 9714–9747. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; Zhang, W.; Yao, S. Urea-assisted synthesis of amorphous molybdenum sulfide on P-doped carbon nanotubes for enhanced hydrogen evolution. J. Mater. Sci. Mater. 2018, 53, 8951–8962. [Google Scholar] [CrossRef]
- Han, H.; Nayak, A.K.; Choi, H.; Ali, G.; Kwon, J.; Choi, S.; Paik, U.; Song, T. Partial dehydration in hydrated tungsten oxide nanoplates leads to excellent and robust bifunctional oxygen reduction and hydrogen evolution reactions in acidic media. ACS Sustain. Chem. Eng. 2020, 8, 9507–9518. [Google Scholar] [CrossRef]
- Karki, S.B.; Andriotis, A.N.; Menon, M.; Ramezanipour, F. Bifunctional Water-Splitting Electrocatalysis Achieved by Defect Order in LaA2Fe3O8 (A = Ca, Sr). ACS Appl. Energy Mater. 2021, 4, 12063–12066. [Google Scholar] [CrossRef]
- Galal, A.; Hassan, H.K.; Atta, N.F.; Jacob, T. An Efficient and Durable Electrocatalyst for Hydrogen Production Based on Earth-Abundant Oxide-Graphene Composite. ChemistrySelect 2017, 2, 10261–10270. [Google Scholar] [CrossRef]
- Kananke-Gamage, C.C.; Ramezanipour, F. Variation of the electrocatalytic activity of isostructural oxides Sr2LaFeMnO7 and Sr2LaCoMnO7 for hydrogen and oxygen-evolution reactions. Dalton Trans. 2021, 50, 14196–14206. [Google Scholar] [CrossRef]
- Murthy, A.P.; Theerthagiri, J.; Madhavan, J. Insights on Tafel constant in the analysis of hydrogen evolution reaction. J. Phys. Chem. C 2018, 122, 23943–23949. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Ling, C.; Ouyang, Y.; Shi, L.; Yuan, S.; Chen, Q.; Wang, J. Template-grown MoS2 nanowires catalyze the hydrogen evolution reaction: Ultralow kinetic barriers with high active site density. ACS Catal. 2017, 7, 5097–5102. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Remarkable Oxygen-Evolution Activity of a Perovskite Oxide from the Ca2−xSrxFe2O6−δ Series. Angew. Chem. 2019, 131, 2082–2085. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.; Li, X.; Liu, Y.; Liu, C. Nanostructured nickel sulfides: Phase evolution, characterization and electrocatalytic properties for the hydrogen evolution reaction. RSC Adv. 2015, 5, 104740–104749. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, D.U.; Park, M.G.; Ahmed, R.; Seo, M.H.; Nazar, L.F.; Chen, Z. Perovskite–nitrogen-doped carbon nanotube composite as bifunctional catalysts for rechargeable lithium–air batteries. ChemSusChem 2015, 8, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.Y.; Jeon, J.S.; Lee, J.J.; Kim, P.; Nahm, K.S. The bifunctional electrocatalytic activity of perovskite La0.6Sr0.4CoO3−δ for oxygen reduction and evolution reactions. RSC Adv. 2015, 5, 19190–19198. [Google Scholar] [CrossRef]
- Moritomo, Y.; Higashi, K.; Matsuda, K.; Nakamura, A. Spin-state transition in layered perovskite cobalt oxides: La2−xSrxCoO4 (0.4≤ x ≤ 1.0). Phys. Rev. B 1997, 55, R14725. [Google Scholar] [CrossRef]
- Alom, M.S.; Ramezanipour, F. Vacancy effect on the electrocatalytic activity of LaMn1/2Co1/2O3−δ for hydrogen and oxygen evolution reactions. Chem. Commun. 2023, 59, 5870–5873. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Sun, Y.; Scherer, G.N.G.; Fisher, A.C.; Sherburne, M.; Ager, J.W.; Xu, Z.J. Surface composition dependent ligand effect in tuning the activity of nickel–copper bimetallic electrocatalysts toward hydrogen evolution in alkaline. J. Am. Chem. Soc. 2020, 142, 7765–7775. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Kundu, A.; Chakraborty, B. Recent Progress on Copper-Based Electrode Materials for Overall Water-Splitting. ChemElectroChem 2021, 8, 1698–1722. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR; Los Alamos National Laboratory: Los Alamos, NM, USA, 2004; pp. 86–748.
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Hona, R.K.; Karki, S.B.; Ramezanipour, F. Oxide Electrocatalysts Based on Earth-Abundant Metals for Both Hydrogen-and Oxygen-Evolution Reactions. ACS Sustain. Chem. Eng. 2020, 8, 11549–11557. [Google Scholar] [CrossRef]
- Li, Q.; Thangadurai, V. A comparative 2 and 4-probe DC and 2-probe AC electrical conductivity of novel co-doped Ce0.9−xRExMo0.1O2.1–0.5x (RE = Y, Sm, Gd; x = 0.2, 0.3). J. Mater. Chem. 2010, 20, 7970–7983. [Google Scholar] [CrossRef]
- Niu, S.; Li, S.; Du, Y.; Han, X.; Xu, P. How to reliably report the overpotential of an electrocatalyst. ACS Energy Lett. 2020, 5, 1083–1087. [Google Scholar] [CrossRef]
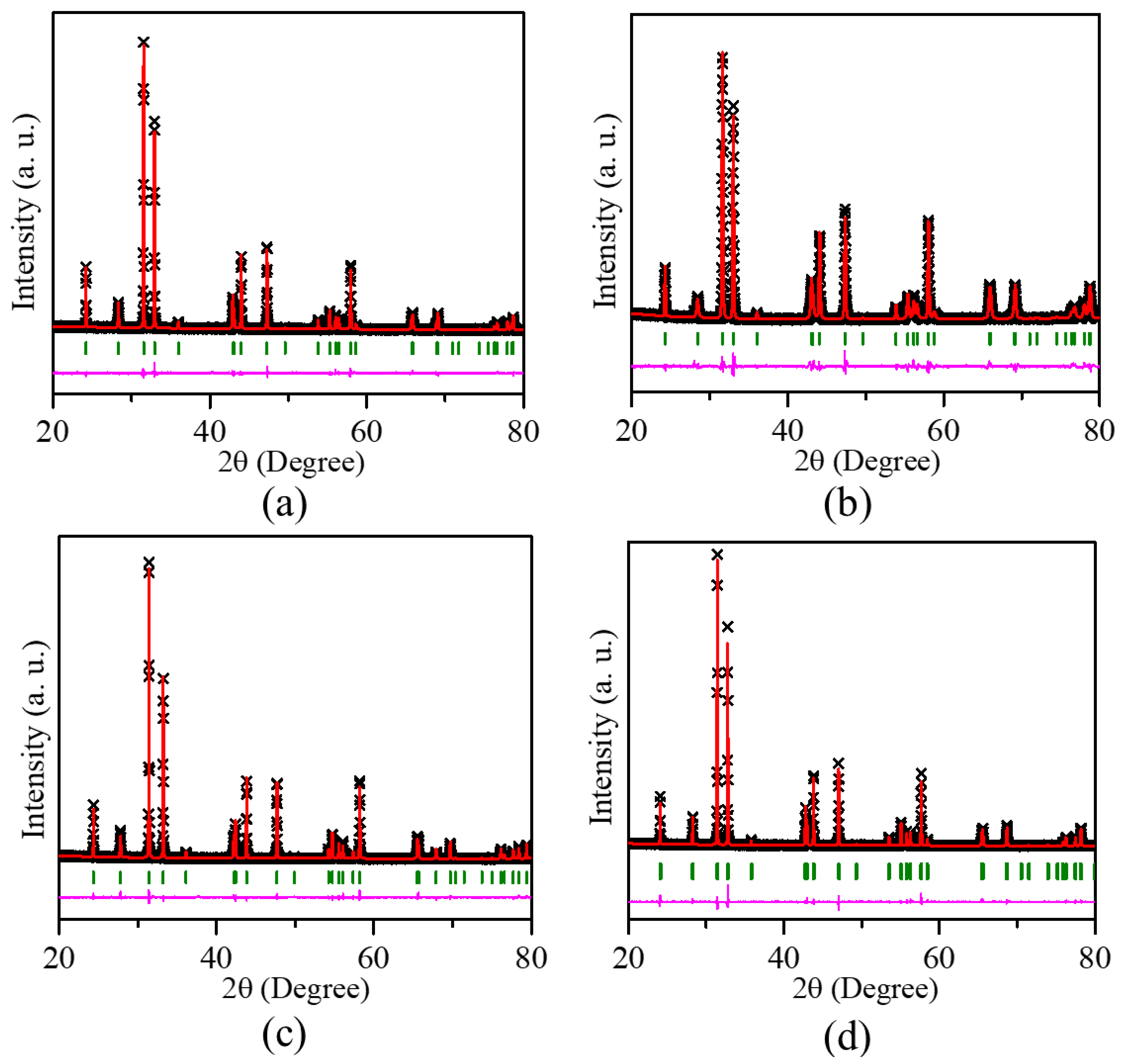
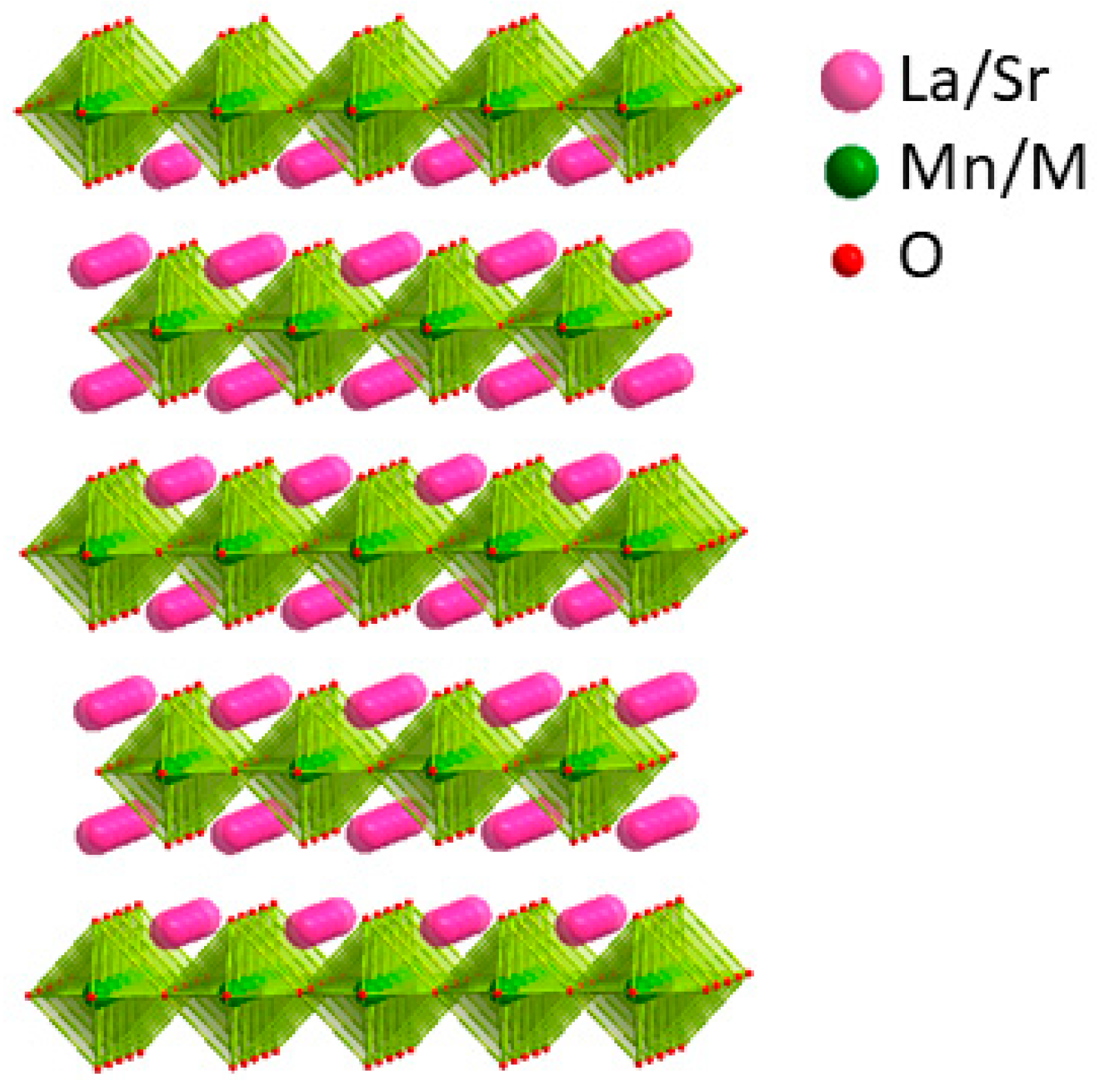
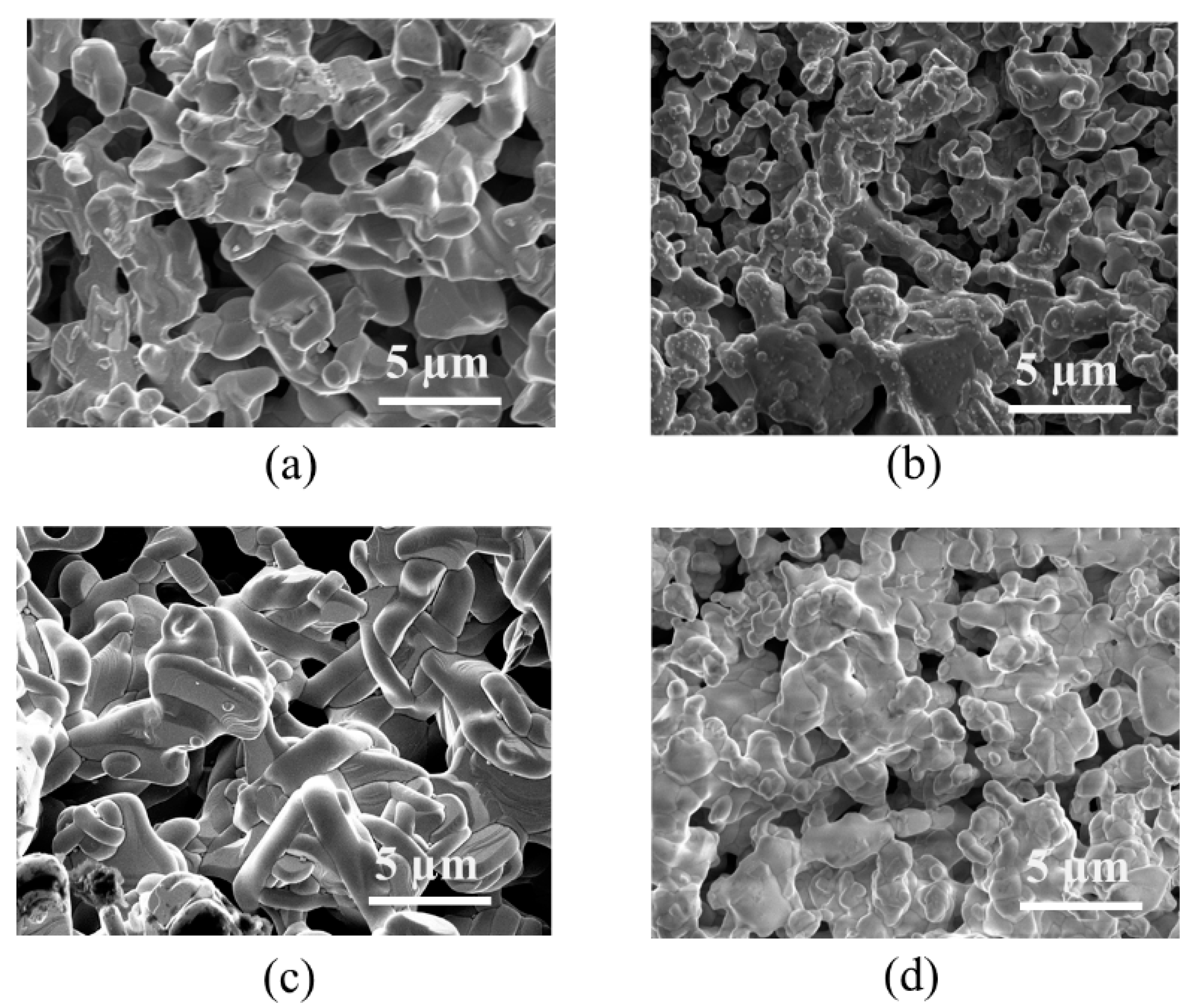
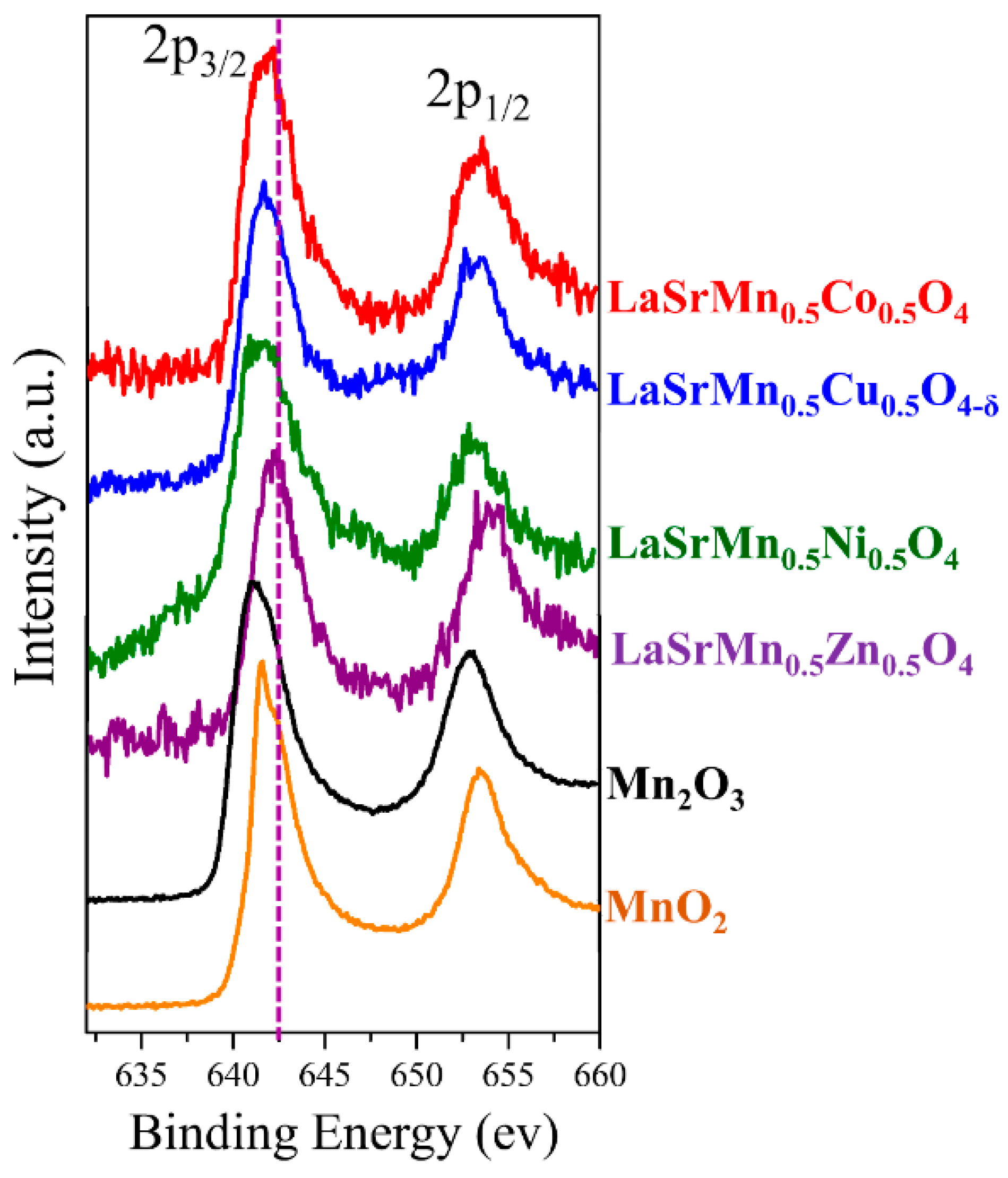

| Atom | x | y | z | Occupancy | Multiplicity | Uiso (Å2) |
|---|---|---|---|---|---|---|
| La | 0 | 0 | 0.35985 (8) | 0.5 | 4 | 0.0192 (5) |
| Sr | 0 | 0 | 0.35985 (8) | 0.5 | 4 | 0.0192 (5) |
| Mn | 0 | 0 | 0 | 0.5 | 2 | 0.0131 (9) |
| Co | 0 | 0 | 0 | 0.5 | 2 | 0.0131 (9) |
| O1 | 0.5 | 0 | 0 | 1 | 4 | 0.031 (2) |
| O2 | 0 | 0 | 0.1642 (6) | 1 | 4 | 0.038 (2) |
| Electrical Conductivity (S/cm) | Activation Energy (eV) | |
|---|---|---|
| LaSrMn0.5Co0.5O4 | 0.0026 | 0.2673 (2) |
| LaSrMn0.5Ni0.5O4 | 0.0014 | 0.2746 (2) |
| LaSrMn0.5Cu0.5O4-δ | 8.5961 × 10−4 | 0.2676 (3) |
| LaSrMn0.5Zn0.5O4 | 7.0755 × 10−4 | 0.4727 (7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickramaratne, K.M.K.; Ramezanipour, F. Electrocatalytic Properties of Quasi-2D Oxides LaSrMn0.5M0.5O4 (M = Co, Ni, Cu, and Zn) for Hydrogen and Oxygen Evolution Reactions. Molecules 2024, 29, 3107. https://doi.org/10.3390/molecules29133107
Wickramaratne KMK, Ramezanipour F. Electrocatalytic Properties of Quasi-2D Oxides LaSrMn0.5M0.5O4 (M = Co, Ni, Cu, and Zn) for Hydrogen and Oxygen Evolution Reactions. Molecules. 2024; 29(13):3107. https://doi.org/10.3390/molecules29133107
Chicago/Turabian StyleWickramaratne, Kinithi M. K., and Farshid Ramezanipour. 2024. "Electrocatalytic Properties of Quasi-2D Oxides LaSrMn0.5M0.5O4 (M = Co, Ni, Cu, and Zn) for Hydrogen and Oxygen Evolution Reactions" Molecules 29, no. 13: 3107. https://doi.org/10.3390/molecules29133107
APA StyleWickramaratne, K. M. K., & Ramezanipour, F. (2024). Electrocatalytic Properties of Quasi-2D Oxides LaSrMn0.5M0.5O4 (M = Co, Ni, Cu, and Zn) for Hydrogen and Oxygen Evolution Reactions. Molecules, 29(13), 3107. https://doi.org/10.3390/molecules29133107








