Analysis of Chemical Composition, Antioxidant Activity, and Toxicity of Essential Oil from Virola sebifera Aubl (Myristicaceae)
Abstract
:1. Introduction
2. Results
2.1. Yield of 2 EOs
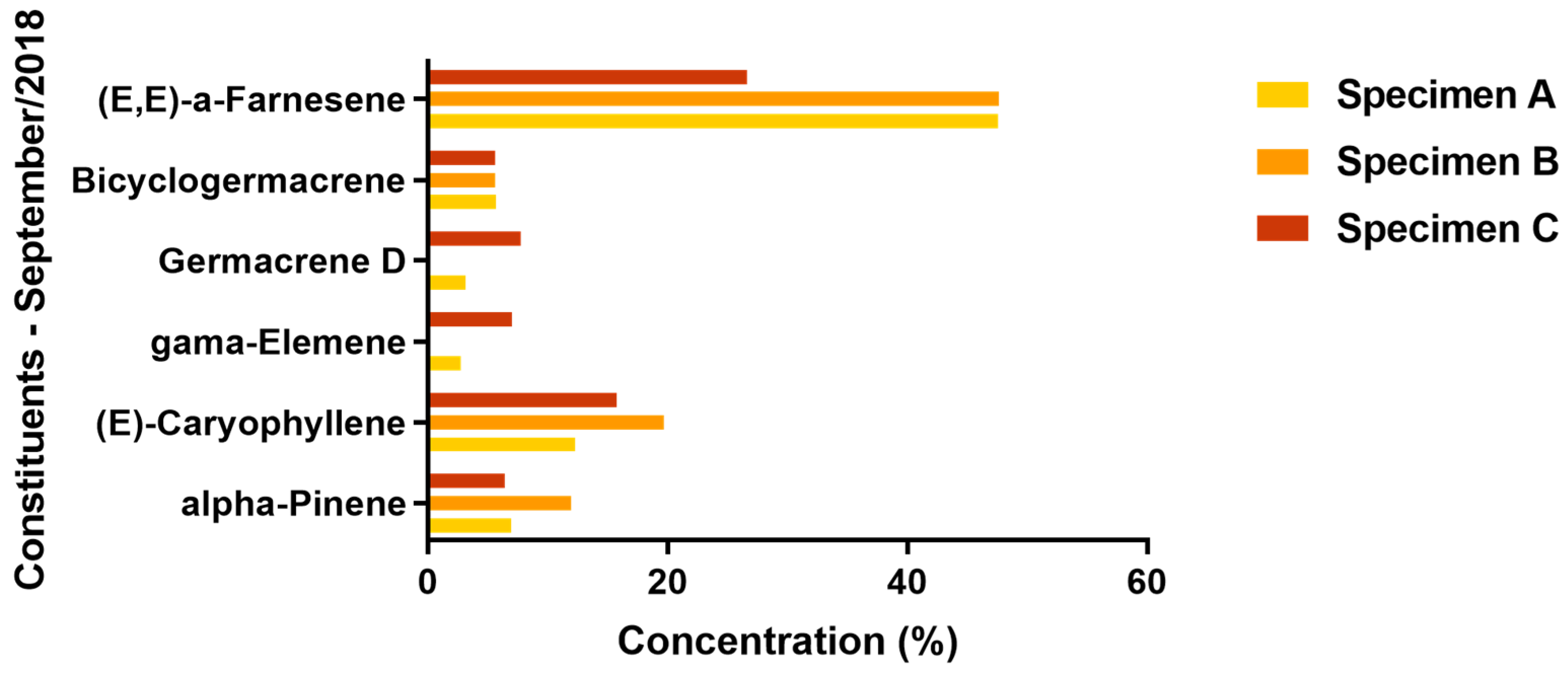
2.2. Chemical Composition
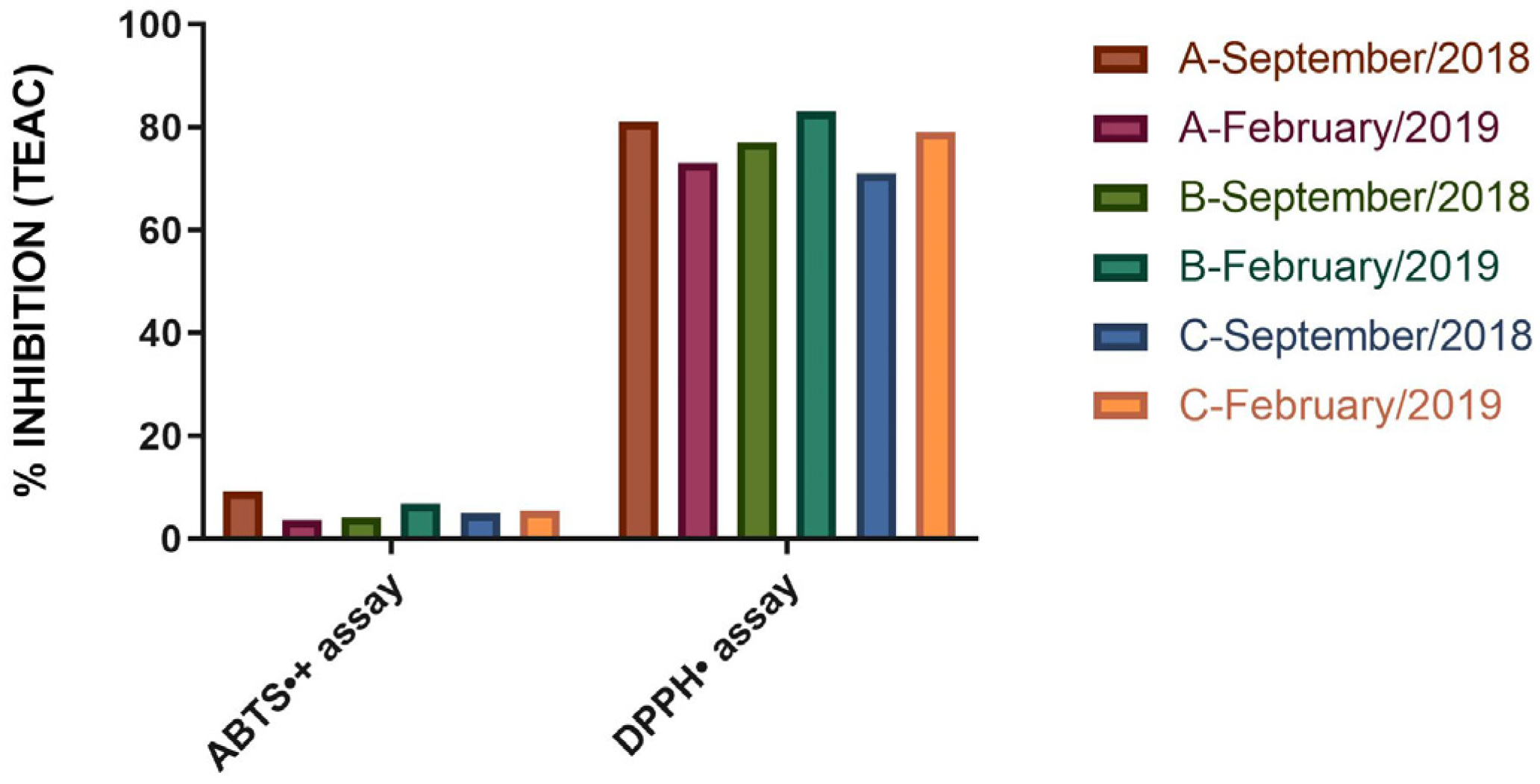
2.3. Antioxidant Capacity Equivalent to Trolox of V. sibifera Essential Oil
2.4. Toxicity Bioassay in Artemia salina
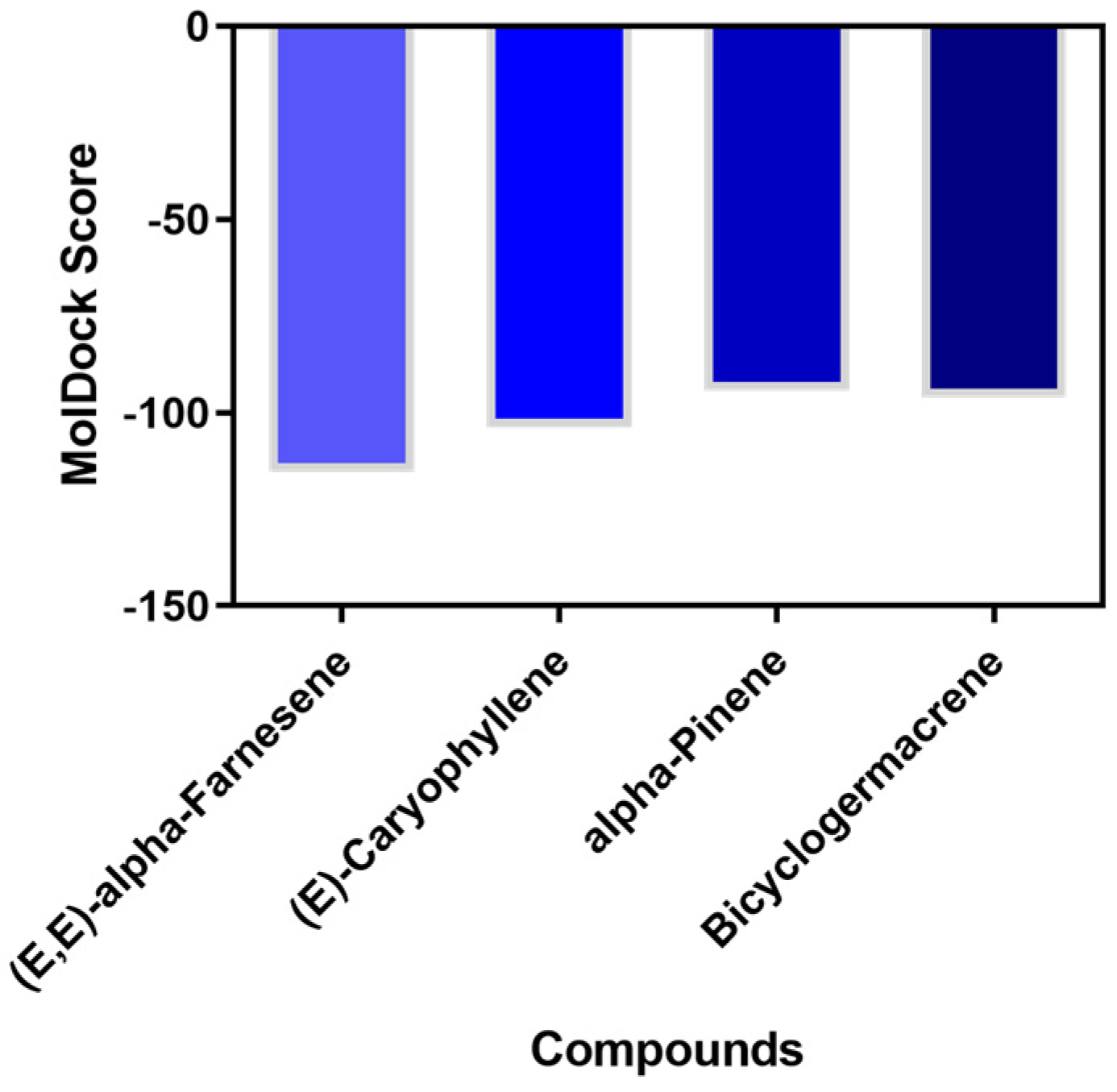
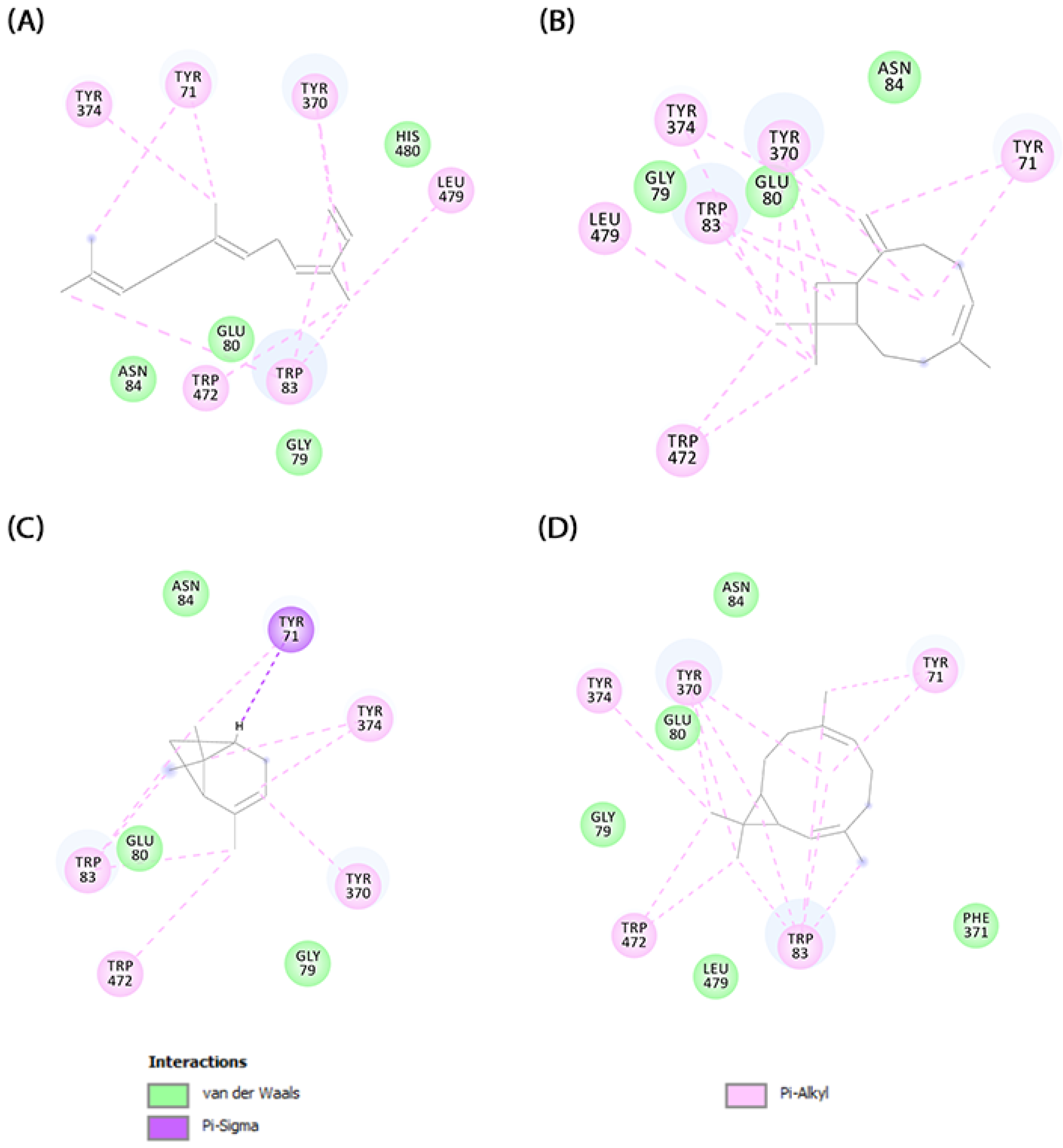
2.5. Molecular Docking
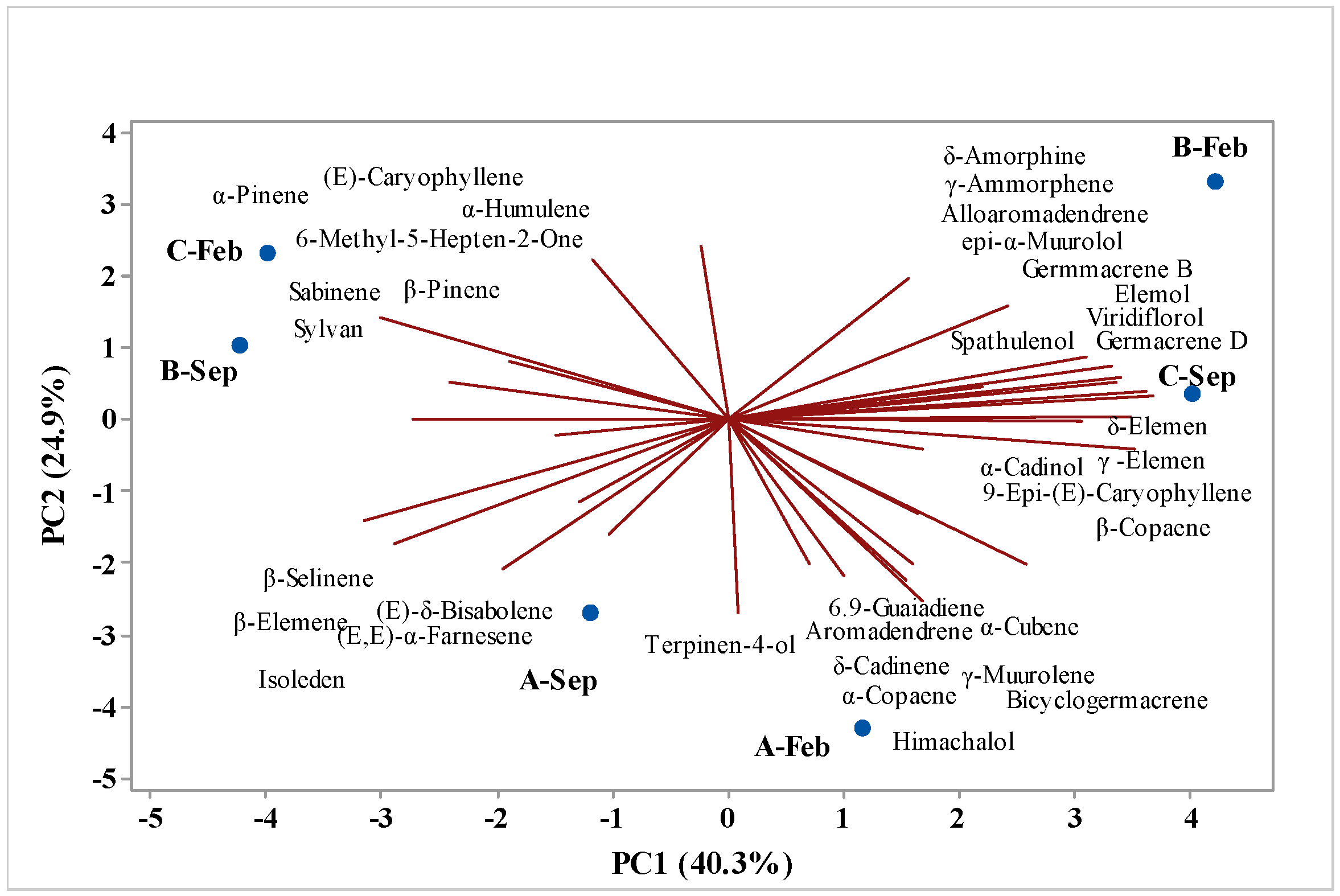
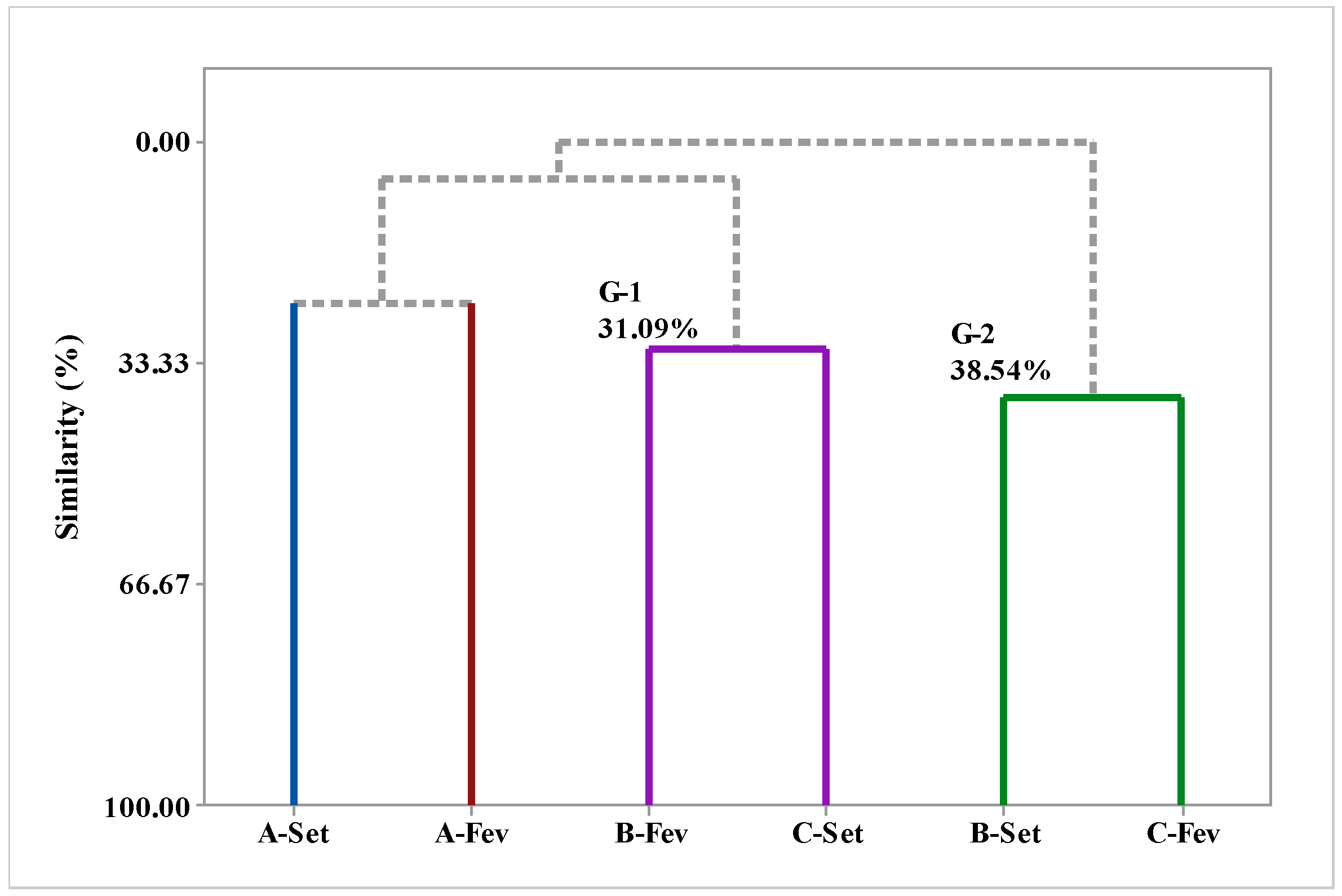
2.6. Multivariate Analysis
3. Materials and Methods
3.1. Botanical Material
3.2. Preparation and Characterization of Botanical Material
3.3. Extraction of EOs
3.4. Calculation of Yield (%) of Extracted Oil
3.5. Chemical Composition Analysis
3.6. Trolox Equivalent Antioxidant Capacity (TEAC)
3.6.1. ABTS•+ Assay
3.6.2. DPPH• Radical Scavenging Assay
3.7. Preliminary Toxicity Bioassay with Artemia salina Leach
3.8. In Silico Evaluation of MAJOR EO Components
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Oliveira Brito, W.R.; De Sales Dambros, C.; Cardoso, D.; Scudeller, V.V.; Zartman, C.E. Divergent Patterns of Intraspecific Trait Variation among Floral and Vegetative Characters in the Hyperdominant Dioecious Neotropical Tree Virola sebifera (Myristicaceae). Bot. J. Linn. Soc. 2023, 202, 233–248. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Fazekas, A.J.; Steeves, R.A.D.; Janovec, J. Testing Candidate Plant Barcode Regions in the Myristicaceae. Mol. Ecol. Resour. 2008, 8, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Denny, C.; Zacharias, M.E.; Ruiz, A.L.T.G.; Amaral, M.D.C.E.D.; Bittrich, V.; Kohn, L.K.; Sousa, I.M.D.O.; Rodrigues, R.A.F.; Carvalho, J.E.D.; Foglio, M.A. Antiproliferative Properties of Polyketides Isolated from Virola sebifera Leaves. Phyther. Res. 2007, 22, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Denny, C.; Zacharias, M.E.; Kohn, L.K.; Foglio, M.A.; Carvalho, J.E.D. Atividade Antiproliferativa Dos Extratos e Da Fração Orgânica Obtidos Das Folhas de Virola sebifera Aubl. (Myristicaceae). Rev. Bras. Farmacogn. 2007, 17, 598–603. [Google Scholar] [CrossRef]
- Funasaki, M.; Barroso, H.D.S.; Fernandes, V.L.A.; Menezes, I.S. Amazon rainforest cosmetics: Chemical approach for quality control. Quim. Nova 2016, 39, 194–209. [Google Scholar] [CrossRef]
- Rezende, K.R.; Davino, S.C.; Barros, S.B.M.; Kato, M.J. Antioxidant Activity of Aryltetralone Lignans and Derivatives from Virola sebifera (Aubl.). Nat. Prod. Res. 2005, 19, 661–666. [Google Scholar] [CrossRef]
- Singh, P.; Prakash, O.; Chandra, M.; Patil, A.R.; Pant, A.K.; Isidorov, V.A. Reinvestigation of Essential Oil of Rabdosia melissoides: Chemical Composition, Antioxidant, Anti-Inflammatory, Analgesic, Antipyretic, Antifungal and Antibacterial Activities. J. Essent. Oil Bear. Plants 2016, 19, 1859–1872. [Google Scholar] [CrossRef]
- Kazemi, M. Phytochemical Composition, Antioxidant, Anti-Inflammatory and Antimicrobial Activity of Nigella sativa L. Essential Oil. J. Essent. Oil Bear. Plants 2014, 17, 1002–1011. [Google Scholar] [CrossRef]
- Dosoky, N.; Setzer, W. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical Composition, Antioxidant, Anti-Inflammatory and Anti-Proliferative Activities of Essential Oils of Plants from Burkina Faso. PLoS One 2014, 9, e92122. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Sites, D.S.; Johnson, N.T.; Miller, J.A.; Torbush, P.H.; Hardin, J.S.; Knowles, S.S.; Nance, J.; Fox, T.H.; Tart, R.C. Controlled Breathing With or Without Peppermint Aromatherapy for Postoperative Nausea and/or Vomiting Symptom Relief: A Randomized Controlled Trial. J. PeriAnesthesia Nurs. 2014, 29, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.; K John, S.; Kamath, V.; Kumar R, S.; Jadav, R.; Swamy, S.; Adoor, G.; Shaji, A.; Nadig, R.; Badachi, S.; et al. Essential Oil Related Seizures (EORS): A Multi-Center Prospective Study on Essential Oils and Seizures in Adults. Epilepsy Res. 2021, 173, 106626. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Unal, İ.; Egri, S. Biosynthesis of Silver Nanoparticles Using the Aqueous Extract of Rheum ribes, Characterization and the Evaluation of Its Toxicity on HUVECs and Artemia salina. Inorg. Nano-Metal Chem. 2022, 1–14. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, X.L.; Meng, C.; Zhou, J.Q.; Sheng, J.J.; Wu, C.K.; Xu, S.W. The Toxicity Assay of Artemia salina as a Biological Model for the Preliminary Toxic Evaluation of Chemical Pollutants. Adv. Mater. Res. 2013, 726–731, 230–233. [Google Scholar] [CrossRef]
- Ribeiro, I.A.T.A.; Sá, J.L.F.; Lima, M.V.; Veras, S.T.S.; Aguiar, J.C.R.O.F.; Aires, A.L.; Albuquerque, M.C.P.A.; da Silva, M.V.; Melo, A.M.M.A.; Navarro, D.M.A.F.; et al. Toxic Effect of Croton rudolphianus Leaf Essential Oil against Biomphalaria glabrata, Schistosoma mansoni Cercariae and Artemia salina. Acta Trop. 2021, 223, 106102. [Google Scholar] [CrossRef] [PubMed]
- Mitić, Z.S.; Stojanović-Radić, Z.Z.; Jovanović, S.Č.; Cvetković, V.J.; Nikolić, J.S.; Ickovski, J.D.; Mitrović, T.L.; Nikolić, B.M.; Zlatković, B.K.; Stojanović, G.S. Essential Oils of Three Balkan Abies Species: Chemical Profiles, Antimicrobial Activity and Toxicity toward Artemia salina and Drosophila melanogaster. Chem. Biodivers. 2022, 19, e202200235. [Google Scholar] [CrossRef]
- Cabral, R.S.C.; Fernandes, C.C.; Dias, A.L.B.; Batista, H.R.F.; Magalhães, L.G.; Pagotti, M.C.; Miranda, M.L.D. Essential Oils from Protium heptaphyllum Fresh Young and Adult Leaves (Burseraceae): Chemical Composition, In Vitro Leishmanicidal and Cytotoxic Effects. J. Essent. Oil Res. 2021, 33, 276–282. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2019, 33, 95–118. [Google Scholar] [CrossRef]
- Lanzerstorfer, P.; Sandner, G.; Pitsch, J.; Mascher, B.; Aumiller, T.; Weghuber, J. Acute, Reproductive, and Developmental Toxicity of Essential Oils Assessed with Alternative in Vitro and in Vivo Systems. Arch. Toxicol. 2020, 95, 673–691. [Google Scholar] [CrossRef]
- Lima, L.R.; Andrade, F.K.; Alves, D.R.; de Morais, S.M.; Vieira, R.S. Anti-Acetylcholinesterase and Toxicity against Artemia salina of Chitosan Microparticles Loaded with Essential Oils of Cymbopogon flexuosus, Pelargonium x ssp and Copaifera officinalis. Int. J. Biol. Macromol. 2021, 167, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Ragusa, S.; Monforte, M.T.; D’angelo, V.; Circosta, C. Phytochemical Analysis and Evaluation of Antioxidant and Anti-Acetylcholinesterase Activities of Euphorbia Dendroides L. (Euphorbiaceae) Latex. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018, 153, 498–505. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Shivakumar, M.S. Stability of Insecticidal Molecule Aucubin and Their Toxicity on Anopheles stephensi, Aedes aegypti, Culex quinquefasciatus and Artemia salina. Int. J. Trop. Insect Sci. 2022, 42, 3403–3417. [Google Scholar] [CrossRef]
- Makarian, M.; Gonzalez, M.; Salvador, S.M.; Lorzadeh, S.; Hudson, P.K.; Pecic, S. Synthesis, Kinetic Evaluation and Molecular Docking Studies of Donepezil-Based Acetylcholinesterase Inhibitors. J. Mol. Struct. 2022, 1247, 131425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kumar, V.; Prashar, V.; Saini, S.; Dwivedi, A.R.; Bajaj, B.; Mehta, D.; Parkash, J.; Kumar, V. Dipropargyl Substituted Diphenylpyrimidines as Dual Inhibitors of Monoamine Oxidase and Acetylcholinesterase. Eur. J. Med. Chem. 2019, 177, 221–234. [Google Scholar] [CrossRef]
- Saxena, M.; Dubey, R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef]
- Baek, I.; Choi, H.J.; Rhee, J.S. Inhibitory Effects of Biocides on Hatching and Acetylcholinesterase Activity in the Brine Shrimp Artemia salina. Toxicol. Environ. Health Sci. 2015, 7, 303–308. [Google Scholar] [CrossRef]
- Mesquita, R.D.S.; de Oliveira, A.C.; Sá, I.S.C.; Sales, M.L.F.; Bastos, L.M.; Koolen, H.H.F.; Tadei, W.P.; da Silva, F.M.A.; Nunomura, R.C.S. Essential Oils from Leaves of Virola calophylla, Virola multinervia, and Virola pavonis (Myristicaceae): Chemical Composition and Larvicidal Activity against Aedes aegypti. J. Essent. Oil Bear. Plants 2020, 23, 453–463. [Google Scholar] [CrossRef]
- Leite-Filho, A.T.; Costa, M.H.; Fu, R. The Southern Amazon Rainy Season: The Role of Deforestation and Its Interactions with Large-scale Mechanisms. Int. J. Climatol. 2019, 40, 2328–2341. [Google Scholar] [CrossRef]
- Nunes, A.M.P.; Silva Dias, M.A.F.; Anselmo, E.M.; Morales, C.A. Severe Convection Features in the Amazon Basin: A TRMM-Based 15-Year Evaluation. Front. Earth Sci. 2016, 4, 37. [Google Scholar] [CrossRef]
- Wright, J.S.; Fu, R.; Worden, J.R.; Chakraborty, S.; Clinton, N.E.; Risi, C.; Sun, Y.; Yin, L. Rainforest-Initiated Wet Season Onset over the Southern Amazon. Proc. Natl. Acad. Sci. USA 2017, 114, 8481–8486. [Google Scholar] [CrossRef] [PubMed]
- Souleyre, E.J.F.; Bowen, J.K.; Matich, A.J.; Tomes, S.; Chen, X.; Hunt, M.B.; Wang, M.Y.; Ileperuma, N.R.; Richards, K.; Rowan, D.D.; et al. Genetic Control of A-farnesene Production in Apple Fruit and Its Role in Fungal Pathogenesis. Plant J. 2019, 100, 1148–1162. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Klein, R.A.; Quinn, M.T. Neutrophil Immunomodulatory Activity of Farnesene, a Component of Artemisia dracunculus Essential Oils. Pharmaceuticals 2022, 15, 642. [Google Scholar] [CrossRef] [PubMed]
- Nararak, J.; Di Giorgio, C.; Sukkanon, C.; Mahiou-Leddet, V.; Ollivier, E.; Manguin, S.; Chareonviriyaphap, T. Excito-Repellency and Biological Safety of β-Caryophyllene Oxide against Aedes albopictus and Anopheles dirus (Diptera: Culicidae). Acta Trop. 2020, 210, 105556. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.D.O.; Souza, E.L.D.; Diniz, M.D.F.F.M.; Trajano, V.N.; Medeiros, I.A.D. Inhibitory Effect of Beta-Pinene, Alpha-Pinene and Eugenol on the Growth of Potential Infectious Endocarditis Causing Gram-Positive Bacteria. Rev. Bras. Ciências Farm. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Potential Protective Effects of Alpha-Pinene against Cytotoxicity Caused by Aspirin in the IEC-6 Cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-Tumor Effect of α-Pinene on Human Hepatoma Cell Lines through Inducing G2/M Cell Cycle Arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef]
- Franco, C.D.J.P.; Ferreira, O.O.; Antônio Barbosa de Moraes, Â.; Varela, E.L.P.; Nascimento, L.D.D.; Percário, S.; de Oliveira, M.S.; Andrade, E.H.D.A. Chemical Composition and Antioxidant Activity of Essential Oils from Eugenia patrisii Vahl, E. punicifolia (Kunth) DC., and Myrcia tomentosa (Aubl.) DC., Leaf of Family Myrtaceae. Molecules 2021, 26, 3292. [Google Scholar] [CrossRef] [PubMed]
- Del-Vechio-Vieira, G.; Sousa, O.V.D.; Miranda, M.A.; Senna-Valle, L.; Kaplan, M.A.C. Analgesic and Anti-Inflammatory Properties of Essential Oil from Ageratum fastigiatum. Brazilian Arch. Biol. Technol. 2009, 52, 1115–1121. [Google Scholar] [CrossRef]
- Ferraz, R.P.C.; Cardoso, G.M.B.; da Silva, T.B.; Fontes, J.E.D.N.; Prata, A.P.D.N.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumour Properties of the Leaf Essential Oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem. 2013, 141, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Govindarajan, M.; AlSalhi, M.S.; Devanesan, S.; Maggi, F. High Toxicity of Camphene and γ-Elemene from Wedelia prostrata Essential Oil against Larvae of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2017, 25, 10383–10391. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Bhattacharyya, A.; Benelli, G. Eugenol, α-Pinene and β-Caryophyllene from Plectranthus barbatus Essential Oil as Eco-Friendly Larvicides against Malaria, Dengue and Japanese Encephalitis Mosquito Vectors. Parasitol. Res. 2015, 115, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Zhong, S.; Li, X.; Wang, Y.; Xun, M.-M.; Bai, Y.; Zhu, K. Total Synthesis, Structural Revision and Biological Evaluation of γ-Elemene-Type Sesquiterpenes. Org. Biomol. Chem. 2018, 16, 7843–7850. [Google Scholar] [CrossRef] [PubMed]
- Souza da Silva Júnior, O.; Franco, C.D.J.P.; de Moraes, Â.A.B.; Pastore, M.; Cascaes, M.M.; Diniz do Nascimento, L.; de Oliveira, M.S.; Andrade, E.H.D.A. Chemical Variability of Volatile Concentrate from Two Ipomoea L. Species within a Seasonal Gradient. Nat. Prod. Res. 2022, 37, 3344–3351. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Silva, S.G.; Cruz, J.N.; Santana de Oliveira, M.; Oliveira, J.; Moraes, A.A.B.D.; Costa, F.A.M.D.; da Costa, K.S.; Diniz do Nascimento, L.; Helena de Aguiar Andrade, E. First Report on the Annona exsucca DC. Essential Oil and in Silico Identification of Potential Biological Targets of Its Major Compounds. Nat. Prod. Res. 2021, 36, 4009–4012. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Dong, Y. Effect of 1-Methylcyclopropene on Superficial Scald Associated with Ethylene Production, α-Farnesene Catabolism, and Antioxidant System of Over-Mature ‘d’Anjou’ Pears After Long-Term Storage. Food Bioprocess Technol. 2018, 11, 1775–1786. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Shen, X.; Wang, Y. Effect of Sunlight-Exposure on Antioxidants and Antioxidant Enzyme Activities in ‘d’Anjou’ Pear in Relation to Superficial Scald Development. Food Chem. 2016, 210, 18–25. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of Oxidative Stress and Mitochondrial Dysfunction by β-Caryophyllene: A Focus on the Nervous System. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, S.; Bruno, M.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Fiorini, D.; Maggi, F. Kundmannia sicula (L.) DC: A Rich Source of Germacrene D. J. Essent. Oil Res. 2017, 29, 437–442. [Google Scholar] [CrossRef]
- Giorgi, A.; De Marinis, P.; Granelli, G.; Chiesa, L.M.; Panseri, S. Secondary Metabolite Profile, Antioxidant Capacity, and Mosquito Repellent Activity of Bixa orellana from Brazilian Amazon Region. J. Chem. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Sahebzadeh, N.; Shamakhi, L. Effects of α-Pinene, Trans-Anethole, and Thymol as the Essential Oil Constituents on Antioxidant System and Acetylcholine Esterase of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2018, 150, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Jeribi, C.; Karoui, I.J.; Hassine, D.B.; Abderrabba, M. Comparative Study of Bioactive Compounds and Antioxidant Activity of Schinus terebinthifolius RADDI Fruits and Leaves Essential Oils. Int. J. Sci. Res. 2014, 3, 452–458. [Google Scholar]
- Siebert, D.A.; Tenfen, A.; Yamanaka, C.N.; de Cordova, C.M.M.; Scharf, D.R.; Simionatto, E.L.; Alberton, M.D. Evaluation of Seasonal Chemical Composition, Antibacterial, Antioxidant and Anticholinesterase Activity of Essential Oil from Eugenia brasiliensis Lam. Nat. Prod. Res. 2014, 29, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.D.D.; Silva, S.G.; Cascaes, M.M.; Costa, K.S.D.; Figueiredo, P.L.B.; Costa, C.M.L.; Andrade, E.H.D.A.; de Faria, L.J.G. Drying Effects on Chemical Composition and Antioxidant Activity of Lippia thymoides Essential Oil, a Natural Source of Thymol. Molecules 2021, 26, 2621. [Google Scholar] [CrossRef]
- Fazelan, Z.; Hoseini, S.M.; Yousefi, M.; Khalili, M.; Hoseinifar, S.H.; Van Doan, H. Effects of Dietary Eucalyptol Administration on Antioxidant and Inflammatory Genes in Common Carp (Cyprinus carpio) Exposed to Ambient Copper. Aquaculture 2020, 520, 734988. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant Properties of Coriander Essential Oil and Linalool and Their Potential to Control Campylobacter Spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Silva, L.D.L.; Geihs, M.A.; Baldisserotto, B. Is Monoterpene Terpinen-4-Ol the Compound Responsible for the Anesthetic and Antioxidant Activity of Melaleuca alternifolia Essential Oil (Tea Tree Oil) in Silver Catfish? Aquaculture 2018, 486, 217–223. [Google Scholar] [CrossRef]
- Aydin, E.; Türkez, H.; Taşdemir, Ş. Anticancer and Antioxidant Properties of Terpinolene in Rat Brain Cells. Arch. Ind. Hyg. Toxicol. 2013, 64, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Maggiolino, A.; Faccia, M.; Holman, B.W.B.; Hopkins, D.L.; Bragaglio, A.; Natrella, G.; Mazzone, A.; De Palo, P. The Effect of Oral or Respiratory Exposure to Limonene on Goat Kid Performance and Meat Quality. Meat Sci. 2022, 191, 108865. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.R.; Junior, L.M.; de Souza, W.F.C.; Sato, H.H.; Alves, R.M.V.; Vieira, R.P. O-ATRP Synthesized Poly(β-Pinene) Blended with Chitosan for Antimicrobial and Antioxidant Bio-Based Films Production. Int. J. Biol. Macromol. 2021, 193, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Dhami, A.; Singh, A.; Palariya, D.; Kumar, R.; Prakash, O.; Rawat, D.S.; Pant, A.K. α-Pinene Rich Bark Essential Oils of Zanthoxylum armatum DC. from Three Different Altitudes of Uttarakhand, India and Their Antioxidant, in Vitro Anti-Inflammatory and Antibacterial Activity. J. Essent. Oil Bear. Plants 2019, 22, 660–674. [Google Scholar] [CrossRef]
- Ramos, S.C.S.; Oliveira, J.C.S.D.; da Câmara, C.A.G.; Castelar, I.; Carvalho, A.F.F.U.; Lima-Filho, J.V. Antibacterial and Cytotoxic Properties of Some Plant Crude Extracts Used in Northeastern Folk Medicine. Rev. Bras. Farmacogn. 2009, 19, 376–381. [Google Scholar] [CrossRef]
- da Anunciação, T.A.; Costa, R.G.A.; de Lima, E.J.S.P.; Silva, V.R.; Santos, L.D.S.; Soares, M.B.P.; Dias, R.B.; Rocha, C.A.G.; Costa, E.V.; Silva, F.M.A.D.; et al. In Vitro and in Vivo Inhibition of HCT116 Cells by Essential Oils from Bark and Leaves of Virola surinamensis (Rol. Ex Rottb.) Warb. (Myristicaceae). J. Ethnopharmacol. 2020, 262, 113166. [Google Scholar] [CrossRef]
- Satyal, P.; Shrestha, S.; Setzer, W.N. Composition and Bioactivities of an (E)-β-Farnesene Chemotype of Chamomile (Matricaria chamomilla) Essential Oil from Nepal. Nat. Prod. Commun. 2015, 10, 1453–1457. [Google Scholar] [CrossRef]
- Machado, R.R.P.; Valente, W.; Lesche, B.; Coimbra, E.S.; de Souza, N.B.; Abramo, C.; Soares, G.L.G.; Kaplan, M.A.C. Essential Oil from Leaves of Lantana Camara: A Potential Source of Medicine against Leishmaniasis. Rev. Bras. Farmacogn. 2012, 22, 1011–1017. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Paudel, P.; Satyal, P.; Dosoky, N.S.; Maharjan, S.; Setzer, W.N. Juglans Regia and J. Nigra, Two Trees Important in Traditional Medicine: A Comparison of Leaf Essential Oil Compositions and Biological Activities. Nat. Prod. Commun. 2013, 8, 1934578X1300801. [Google Scholar] [CrossRef]
- Judžentienė, A.; Būdienė, J. Mugwort (Artemisia vulgaris L.) Essential Oils Rich in Germacrene D, and Their Toxic Activity. J. Essent. Oil Res. 2021, 33, 256–264. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Sánchez-Aldana, D.; Chacón-Vargas, K.F.; Rivera-Chavira, B.E.; Sánchez-Torres, L.E.; Camacho, A.D.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Oviposition Deterrent and Larvicidal and Pupaecidal Activity of Seven Essential Oils and Their Major Components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism–Antagonism Effects. Insects 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Zárybnický, T.; Boušová, I.; Ambrož, M.; Skálová, L. Hepatotoxicity of Monoterpenes and Sesquiterpenes. Arch. Toxicol. 2017, 92, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paventi, G.; de Acutis, L.; De Cristofaro, A.; Pistillo, M.; Germinara, G.S.; Rotundo, G. Biological Activity of Humulus lupulus (L.) Essential Oil and Its Main Components against Sitophilus granarius (L.). Biomolecules 2020, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.R.M.; Abdelgaleil, S.A.M.; Shawir, M.S.; El-bakary, A.S.; Zhu, K.Y.; Phillips, T.W. Terpenoids, DEET and Short Chain Fatty Acids as Toxicants and Repellents for Rhyzopertha dominica (Coleoptera: Bostrichidae) and Lasioderma serricorne (Coleoptera: Ptinidae). J. Stored Prod. Res. 2020, 87, 101610. [Google Scholar] [CrossRef]
- Pajaro-Castro, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Neurotoxic Effects of Linalool and β-Pinene on Tribolium castaneum Herbst. Molecules 2017, 22, 2052. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.E.; Türkez, H.; Mardinoğlu, A. In Vitro Neuroprotective Effects of Farnesene Sesquiterpene on Alzheimer’s Disease Model of Differentiated Neuroblastoma Cell Line. Int. J. Neurosci. 2020, 131, 745–754. [Google Scholar] [CrossRef]
- Said-Al, H.A.H.; Said-Al Ahl, H.A.H.; Hikal, W.M.; Tkachenko, K.G. Essential Oils with Potential as Insecticidal Agents: A Review. Int. J. Environ. Plan. Manag. 2017, 3, 23–33. [Google Scholar]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S. Ocimum tenuiflorum Oil, a Potential Insecticide against Rice Weevil with Anti-Acetylcholinesterase Activity. Ind. Crops Prod. 2018, 126, 434–439. [Google Scholar] [CrossRef]
- Smeriglio, A.; Trombetta, D.; Alloisio, S.; Cornara, L.; Denaro, M.; Garbati, P.; Grassi, G.; Circosta, C. Promising in Vitro Antioxidant, Anti-acetylcholinesterase and Neuroactive Effects of Essential Oil from Two Non-psychotropic Cannabis Sativa L. Biotypes. Phyther. Res. 2020, 34, 2287–2302. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef] [PubMed]
- Calva, J.; Castillo, J.M.; Bec, N.; Ramirez, J.; Andrade, J.M.; Larroque, C.; Armijos, C. Chemical Composition, Enantiomeric Distribution and AChE-BChE Activities of the Essential Oil of Myrteola phylicoides (Benth) Landrum from Ecuador. Rec. Nat. Prod. 2019, 13, 355–362. [Google Scholar] [CrossRef]
- da Silva, J.K.R.; Pinto, L.C.; Burbano, R.M.R.; Montenegro, R.C.; Guimarães, E.F.; Andrade, E.H.A.; Maia, J.G.S. Essential Oils of Amazon Piper Species and Their Cytotoxic, Antifungal, Antioxidant and Anti-Cholinesterase Activities. Ind. Crops Prod. 2014, 58, 55–60. [Google Scholar] [CrossRef]
- Wang, S. A Review of Medicinal Plant Species with Elemene in China. African J. Pharm. Pharmacol. 2012, 6, 3032–3040. [Google Scholar] [CrossRef]
- Hussein, B.A.; Karimi, I.; Yousofvand, N. Computational Insight to Putative Anti-Acetylcholinesterase Activity of Commiphora myrrha (Nees), Engler, Burseraceae: A Lessen of Archaeopharmacology from Mesopotamian Medicine I. Silico Pharmacol. 2019, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.S.; Cruz, J.N.; de Farias Ramos, I.N.; do Nascimento Brandão, D.L.; Queiroz, R.N.; da Silva, G.V.G.V.; da Silva, G.V.G.V.; Dolabela, M.F.; da Costa, M.L.; Khayat, A.S.; et al. Evaluation of Antimicrobial Activity and Cytotoxicity Effects of Extracts of Piper nigrum L. and Piperine. Separations 2022, 10, 21. [Google Scholar] [CrossRef]
- Muzammil, S.; Neves Cruz, J.; Mumtaz, R.; Rasul, I.; Hayat, S.; Khan, M.A.; Khan, A.M.; Ijaz, M.U.; Lima, R.R.; Zubair, M. Effects of Drying Temperature and Solvents on In Vitro Diabetic Wound Healing Potential of Moringa oleifera Leaf Extracts. Molecules 2023, 28, 710. [Google Scholar] [CrossRef]
- Ferreira, O.O.; da Cruz, J.N.; Franco, C.D.J.P.; Silva, S.G.; da Costa, W.A.; de Oliveira, M.S.; Andrade, E.H.D.A. First Report on Yield and Chemical Composition of Essential Oil Extracted from Myrcia eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules 2020, 25, 783. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Spectrometry, M.; Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Adams, R.P., Ed.; Allured Pub Corp: New York, NY, USA, 2017; ISBN 9781932633214. [Google Scholar]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; Wiley Blackwell: Hoboken, NJ, USA, 2015; Volume 10. [Google Scholar]
- Miller, M.; Rao, J.K.M.; Wlodawer, A.; Gribskov, M.R. A Left-Handed Crossover Involved in Amidohydrolase Catalysis. FEBS Lett. 1993, 328, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Rajabi, S.; Ramazani, A.; Hamidi, M.; Naji, T. Artemia salina as a Model Organism in Toxicity Assessment of Nanoparticles. DARU J. Pharm. Sci. 2015, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Kryger, G.; Rosenberry, T.L.; Mallender, W.D.; Lewis, T.; Fletcher, R.J.; Guss, J.M.; Silman, I.; Sussman, J.L. Three-dimensional Structures of Drosophila melanogaster Acetylcholinesterase and of Its Complexes with Two Potent Inhibitors. Protein Sci. 2000, 9, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009; pp. 2–3. [Google Scholar]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]

| Specimen A | Specimen B | Specimen C | ||||
|---|---|---|---|---|---|---|
| September/2018 | February/2019 | September/2018 | February/2019 | September/2018 | February/2019 | |
| Moisture (%) | 9.44 | 10.72 | 10.5 | 11.39 | 8.95 | 10.70 |
| Yield (%) | 0.69 | 0.8 | 0.55 | 0.7 | 0.54 | 0.69 |
| IRL | IRC | Constituents | Specimen A | Specimen B | Specimen C | |||
|---|---|---|---|---|---|---|---|---|
| September/2018 | February/2019 | September/2018 | February/2019 | September/2018 | February/2019 | |||
| 924 | 924 | α-Thujene | 0.16 | 0.18 | 0.17 | 0.24 | ||
| 932 | 932 | α-pinene | 6.93 | 3.15 | 11.95 | 5.03 | 6.37 | 16.91 |
| 969 | 971 | Sabinene | 0.47 | 0.22 | 0.34 | 0.4 | 1.2 | |
| 974 | 976 | β-Pinene | 0.7 | 1.14 | 0.52 | |||
| 981 | 984 | 6-Methyl-5-hepten-2-one | 0.34 | 1.97 | ||||
| 988 | 989 | Myrcene | 0.26 | 0.13 | 0.43 | |||
| 1002 | 1005 | α-Phellandrene | 0.11 | |||||
| 1014 | 1016 | α-Terpinene | 0.1 | 0.07 | ||||
| 1016 | δ-2-Carene | 0.1 | ||||||
| 1025 | 1028 | Sylvestrene | 0.6 | 0.59 | 0.28 | 0.4 | ||
| 1044 | 1045 | (E)-β-Ocimene | 0.03 | 0.07 | ||||
| 1054 | 1057 | γ-Terpinene | 0.21 | 0.23 | 0.15 | |||
| 1086 | 1088 | Terpinolene | 0.07 | 0.06 | 0.04 | |||
| 1174 | 1177 | Terpinen-4-ol | 0.44 | 0.68 | 0.29 | 0.25 | 0.31 | 0.32 |
| 1186 | 1190 | α-Terpineol | 0.05 | 0.04 | ||||
| 1284 | 1286 | Bornyl acetate | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | |
| 1324 | 1326 | Myrtenyl acetate | 0.05 | 0.05 | 0.09 | 0.12 | ||
| 1335 | 1342 | δ-Elemene | 3.56 | 3.27 | 1.94 | 4.3 | 4.34 | 1.47 |
| 1345 | 1352 | α-Cubebene | 0.14 | 0.17 | 0.1 | 0.12 | ||
| 1374 | 1376 | Isoledene | 0.06 | 0.02 | ||||
| 1374 | 1379 | α-Copaene | 0.47 | 0.53 | 0.09 | 0.41 | ||
| 1387 | 1389 | ß-Bourbonene | 0.13 | |||||
| 1389 | 1396 | ß-Elemene | 1.97 | 2.23 | 1.74 | 1.75 | 1.79 | 2.15 |
| 1417 | 1430 | (E)-caryophyllene | 12.26 | 16.02 | 19.67 | 19.34 | 15.7 | 21.4 |
| 1430 | 1436 | ß-Copaene | 0.44 | 0.51 | 0.75 | |||
| 1434 | 1439 | γ-elemene | 2.7 | 2.92 | 6.81 | 7.01 | ||
| 1439 | 1445 | Aromadendrene | 0.64 | 0.84 | 0.67 | 0.63 | 0.7 | 0.61 |
| 1442 | 1448 | 6.9-Guaiadiene | 0.8 | 0.35 | 0.48 | 0.25 | ||
| 1449 | 1451 | Spirolepechinene | 0.29 | |||||
| 1458 | 1456 | Alloaromadendrene | 0.11 | 0.86 | ||||
| 1452 | 1460 | α-humulene | 2.14 | 2.71 | 3.04 | 3.4 | 2.88 | 3.58 |
| 1460 | 1462 | Dehydro aromadendrane | 0.08 | 0.04 | ||||
| 1464 | 1466 | 9-epi-(E)-caryophyllene | 0.54 | 0.64 | 0.43 | 0.66 | 0.64 | 0.58 |
| 1478 | 1478 | γ-Muurolene | 0.75 | 0.05 | ||||
| 1481 | 1479 | γ-Himachalene | 0.06 | 0.08 | ||||
| 1484 | 1487 | Germacrene D | 3.14 | 2.81 | 7.33 | 7.72 | ||
| 1489 | 1491 | β-selinene | 0.42 | 0.45 | 0.41 | 0.46 | ||
| 1483 | 1492 | α-Amorphene | 0.16 | |||||
| 1437 | 1494 | α-Guaiene | 0.38 | |||||
| 1495 | 1497 | γ-Amorphene | 0.32 | 3.55 | 0.59 | |||
| 1500 | 1505 | Bicyclogermacrene | 5.69 | 8.85 | 5.6 | 7.29 | 5.63 | 5.5 |
| 1505 | 1522 | (E.E)-α-Farnesene | 47.57 | 42.82 | 47.65 | 23.57 | 26.65 | 37.43 |
| 1511 | 1528 | δ-Amorphene | 1.87 | 0.5 | ||||
| 1522 | 1537 | δ-Cadinene | 1.08 | 1.2 | 0.29 | 1.68 | ||
| 1529 | 1541 | (E)-γ-Bisabolene | 0.37 | 0.32 | 0.12 | 0.25 | ||
| 1533 | 1543 | trans-Cadina-1.4-diene | 0.06 | 0.19 | ||||
| 1537 | 1547 | α-Cadinene | 0.15 | |||||
| 1548 | 1556 | Elemol | 0.16 | 0.13 | 0.47 | 0.67 | ||
| 1559 | 1566 | Germacrene B | 0.61 | 1.35 | 3.02 | 1.39 | ||
| 1577 | 1584 | Spathulenol | 0.38 | 0.93 | 0.22 | 1 | 0.73 | 0.85 |
| 1592 | 1591 | Viridiflorol | 1.48 | 1.79 | 1.04 | 2.31 | 2.28 | 1.7 |
| 1600 | 1608 | Rosifoliol | 0.24 | 0.24 | 0.18 | 0.25 | 0.25 | 0.23 |
| 1608 | 1613 | Humulene epoxide II | 0.05 | 0.06 | ||||
| 1618 | 1621 | 1.10-di-epi-Cubenol | 0.08 | |||||
| 1632 | 1632 | α-Acorenol | 0.44 | |||||
| 1627 | 1634 | 1-epi-Cubenol | 0.13 | 0.43 | ||||
| 1630 | 1638 | γ-Eudesmol | 0.28 | |||||
| 1639 | 1642 | Alloaromadendrene epoxide | 0.2 | 0.04 | 0.22 | 0.17 | ||
| 1645 | Naphth-1-ol | 0.12 | ||||||
| 1640 | 1647 | epi-α-Muurolol | 0.4 | 0.78 | 0.9 | |||
| 1645 | 1649 | Cubenol | 0.06 | 0.15 | ||||
| 1644 | 1652 | α-Muurolol | 0.22 | |||||
| 1652 | 1656 | Himachalol | 0.11 | 0.58 | 0.32 | |||
| 1651 | 1658 | Pogostol | 0.27 | |||||
| 1659 | Selin-11-en-4α-ol | 0.17 | ||||||
| 1652 | 1660 | α-Cadinol | 0.48 | 1.06 | ||||
| 1671 | 1680 | Tetradecanol | 0.06 | |||||
| 1685 | 1691 | Germacra-4(15).5.10(14)-trien-1-α-ol | 0.04 | |||||
| 1700 | 1700 | Eudesm-7(11)-en-4-ol | 0.03 | 0.09 | ||||
| 1740 | 1744 | Mint sulfide | 0.04 | |||||
| 1755 | 1767 | α-Sinensal | 0.02 | |||||
| 1942 | 2114 | Phytol | 0.05 | |||||
| Monoterpene hydrocarbons | 9.64 | 3.37 | 14.72 | 4.58 | 8.5 | 18.75 | ||
| Monoterpene oxygenated | 0.49 | 0.68 | 0.29 | 0.25 | 0.35 | 0.32 | ||
| Sesquiterpene hydrocarbons | 84.63 | 89.04 | 81.79 | 84.97 | 79.81 | 74.01 | ||
| Sesquiterpene oxygenated | 3.41 | 3.98 | 1.65 | 5.71 | 7.28 | 3.28 | ||
| Others | 0.09 | 0.43 | 0.25 | 4.04 | 0.19 | 2.14 | ||
| Total | 98.26 | 97.5 | 98.7 | 95.96 | 96.13 | 98.5 | ||
| Especies | Concentrations (μg·mL−1) | Mortality (%) | R2 | CL50 (μg·mL−1) |
|---|---|---|---|---|
| V. sebifera (Specimen A) | 200 | 76.67 | 0.6037 | 70.92 ± 2.22 |
| 150 | 46.67 | |||
| 100 | 46.67 | |||
| 50 | 40.00 | |||
| 25 | 36.67 | |||
| V. sebifera (Specimen B) | 200 | 93.32 | 0.8619 | 72.17 ± 5.43 |
| 150 | 63.33 | |||
| 100 | 60.00 | |||
| 50 | 40.00 | |||
| 25 | 16.67 | |||
| V. sebifera (Specimen C) | 200 | 93.33 | 0.73 | 51.17 ± 3.95 |
| 150 | 76,67 | |||
| 100 | 63.33 | |||
| 50 | 46.67 | |||
| 25 | 36.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.N.; de Oliveira, M.S.; Ferreira, O.O.; Gomes, A.R.Q.; Mali, S.N.; Pereira, S.F.M.; Ansar, S.; Santos, C.B.R.d.; Lima, R.R.; de Andrade, E.H.A. Analysis of Chemical Composition, Antioxidant Activity, and Toxicity of Essential Oil from Virola sebifera Aubl (Myristicaceae). Molecules 2024, 29, 3431. https://doi.org/10.3390/molecules29143431
Cruz JN, de Oliveira MS, Ferreira OO, Gomes ARQ, Mali SN, Pereira SFM, Ansar S, Santos CBRd, Lima RR, de Andrade EHA. Analysis of Chemical Composition, Antioxidant Activity, and Toxicity of Essential Oil from Virola sebifera Aubl (Myristicaceae). Molecules. 2024; 29(14):3431. https://doi.org/10.3390/molecules29143431
Chicago/Turabian StyleCruz, Jorddy Neves, Mozaniel Santana de Oliveira, Oberdan Oliveira Ferreira, Antonio Rafael Quadros Gomes, Suraj N. Mali, Soluan Felipe Melo Pereira, Sabah Ansar, Cleydson Breno Rodrigues dos Santos, Rafael Rodrigues Lima, and Eloisa Helena Aguiar de Andrade. 2024. "Analysis of Chemical Composition, Antioxidant Activity, and Toxicity of Essential Oil from Virola sebifera Aubl (Myristicaceae)" Molecules 29, no. 14: 3431. https://doi.org/10.3390/molecules29143431
APA StyleCruz, J. N., de Oliveira, M. S., Ferreira, O. O., Gomes, A. R. Q., Mali, S. N., Pereira, S. F. M., Ansar, S., Santos, C. B. R. d., Lima, R. R., & de Andrade, E. H. A. (2024). Analysis of Chemical Composition, Antioxidant Activity, and Toxicity of Essential Oil from Virola sebifera Aubl (Myristicaceae). Molecules, 29(14), 3431. https://doi.org/10.3390/molecules29143431














