Abstract
The present study provides a comprehensive analysis of the chemical composition of essential oils from species of the Myrcia genus and their applications. The compiled results highlight the chemical diversity and biological activities of these oils, emphasizing their potential importance for various therapeutic and industrial applications. The findings reveal that Myrcia essential oils present a variety of bioactive compounds, such as monoterpenes and sesquiterpenes, which demonstrate antimicrobial activities against a range of microorganisms, including Gram-positive and Gram-negative bacteria, as well as yeasts. Furthermore, this study highlights the phytotoxic activity of these oils, indicating their potential for weed control. The results also point to the insecticidal potential of Myrcia essential oils against a range of pests, showing their viability as an alternative to synthetic pesticides. Additionally, species of the genus Myrcia have demonstrated promising hypoglycemic effects, suggesting their potential in diabetes treatment. This comprehensive synthesis represents a significant advancement in understanding Myrcia essential oils, highlighting their chemical diversity and wide range of biological activities. However, the need for further research is emphasized to fully explore the therapeutic and industrial potential of these oils, including the identification of new compounds, understanding of their mechanisms of action, and evaluation of safety and efficacy in different contexts.
1. Introduction
Myrcia, a genus within the Myrtaceae family, offers a wide range of species valued for their medicinal and culinary uses. With leaves and fruits as the primary components, Myrcia species are extensively employed in traditional medicine and as flavor enhancers in culinary practices. In Brazil, where over 400 species thrive across diverse regions, Myrcia serves as a crucial reservoir of biodiversity, enriching the nation’s natural heritage. A distinctive feature of Myrcia species is their abundant essential oil content, primarily composed of mono- and sesquiterpenes, alongside phenolics and others. These bioactive compounds confer a broad spectrum of biological properties to Myrcia extracts, including antioxidant, anti-inflammatory, antimicrobial, and antiviral effects. The prevalence of terpenoids underscores the therapeutic potential of Myrcia essential oils, offering benefits against oxidative stress, inflammation, and microbial infections [1,2,3].
The extraction technique can be one of the factors that influence the chemical composition of essential oils. Studies on obtaining essential oils from species of the Myrcia genus have reported that they are obtained using techniques such as hydrodistillation (HD) and steam distillation (SD). These studies indicate that the HD technique is the one with the higher mass yields when compared to SD. The maximum yield observed for HD extraction of Myrcia multiflora was 0.36% (m/m), while for SD extraction, the yield observed was 0.08% (m/m) for the same sample [4]; however, the sampling period of the species can be a determining factor that causes variation in the chemical composition of the essential oils. These variations can directly impact their biological activities, as well as their industrial and pharmaceutical applications. Research carried out by Ferreira et al. [5] analyzed the influence on the chemical composition of essential oils obtained from the species Myrcia eximia DC, collected in 2017 and 2018; the results obtained by the authors revealed that the time of collection can have a significant influence both qualitatively and quantitatively on the chemical composition of the essential oils of this species.
Among the applications are properties such as antioxidant, antimicrobial, insecticidal, hypoglycemic effects, and phytotoxic. In molecular biology, degenerative processes are associated with the occurrence of excess free radicals, which promote oxidative processes that are harmful to the human body. Plant species are capable of producing compounds such as terpenes, carotenoids, phenols, flavonoids, and anthocyanins, with antioxidant properties capable of neutralizing free radicals, which have a negative impact on the biological cells of living organisms [6]. Some species of the Myrtaceae family reported in the literature have exhibited strong antioxidant properties, which can be utilized in the pharmaceutical and cosmetic industries due to their antioxidant potential [3,7,8].
The genus Myrcia is widely acknowledged for its hypoglycemic properties, with numerous species being prominently utilized in traditional medicinal practices for managing diabetes. Research indicates that extracts derived from these plants possess inhibitory effects against the α-glucosidase enzyme, a pivotal player in carbohydrate metabolism. This suggests promising potential in regulating blood glucose levels, which is crucial in diabetes management [9].
Moreover, essential oils (EOs) obtained from species within the Myrtaceae family exhibit a spectrum of activities, including cytotoxicity. The essential oil of M. floribunda showed moderate phytotoxicity, in addition, when it came to assessing the toxic effect on the crustacean Artemia salina, and the essential oil of M. floribunda was classified as moderately toxic to A. salina (LC50 = 82.96 µg.mL−1), while the essential oil of Myrcia sylvatica was classified as highly toxic (LC50 = 2.74 µg.mL−1) [10]. According to Ramos et al. [11], an essential oil is classified as toxic when the LC50 is less than 80 µg.mL−1; cytotoxicity is considered moderate when the LC50 is between 80 and 250 µg.mL−1, and it is considered non-toxic or mildly toxic when the LC50 is greater than 250 µg.mL−1. The essential oil of M. floribunda stood out due to its ability to neutralize the DPPH radical, showing a higher potential than that observed for the Trolox standard (p < 0.0001). This result indicated that the essential oil of M. floribunda has antioxidant potential, and its activity, according to the author, can be attributed to the chemical composition of the essential oil, which is rich in monoterpenes with antioxidant activity. These findings contribute significantly to our understanding of the genus; however, there remains a considerable need for further exploration to uncover the additional biological properties of Myrcia essential oils [10,12,13,14].
This study serves as a comprehensive compilation of the latest insights into the chemical composition and diverse biological activities associated with the essential oils derived from Myrcia species. By synthesizing this knowledge, it provides invaluable guidance for future research endeavors aimed at unraveling the full therapeutic potential of Myrcia essential oils.
2. Methods
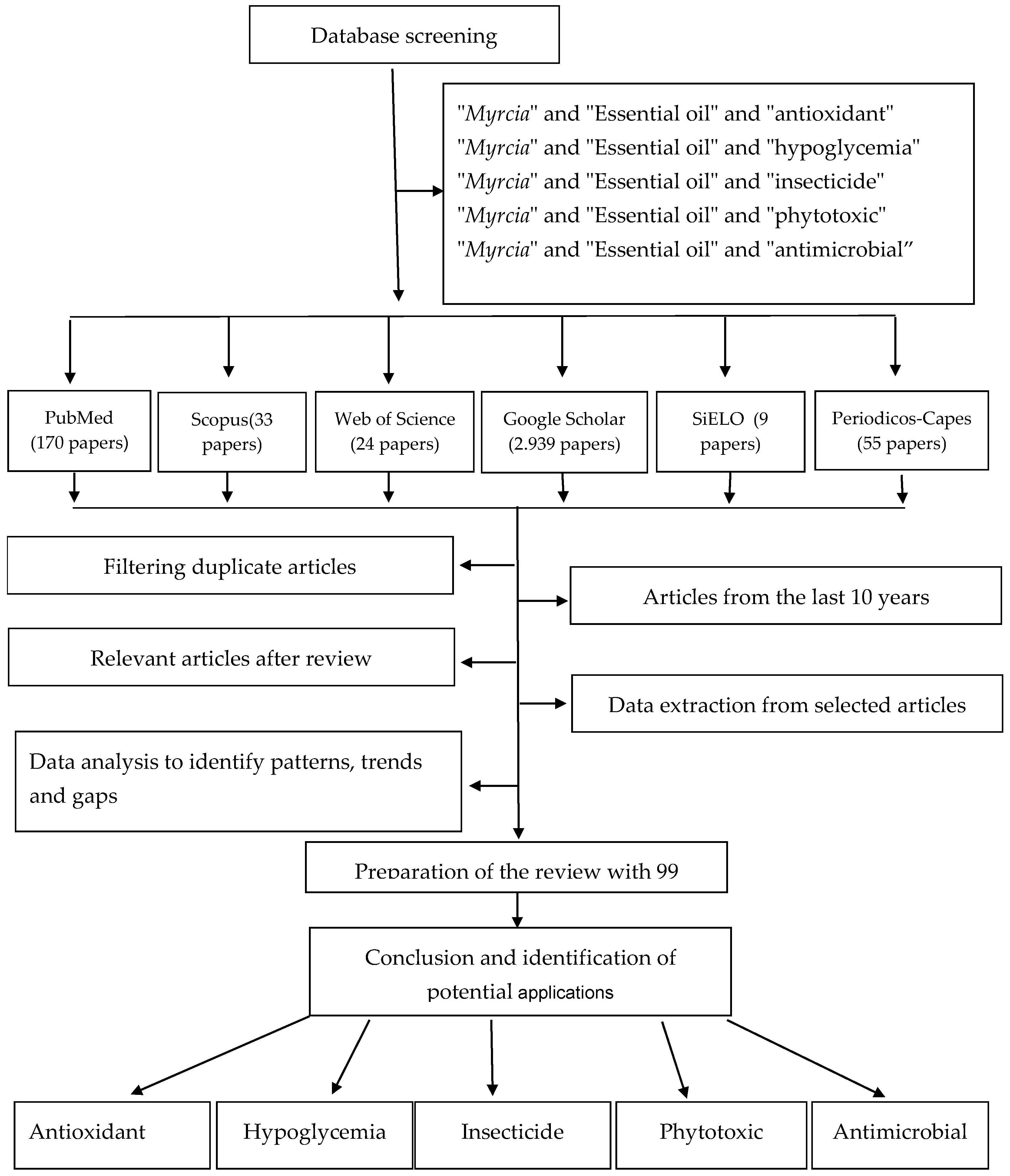
Figure 1 shows the search and selection methodology for the articles cited in this paper. Different databases were used, and the selection was based on the relevance of the work and recommendations related to the topics covered in this paper.
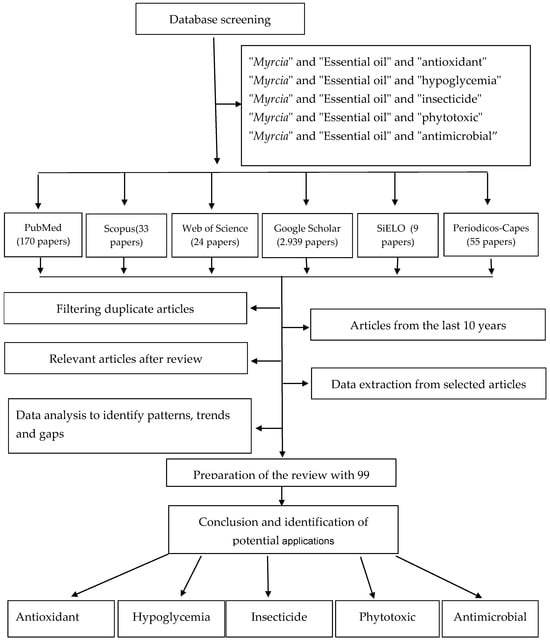
Figure 1.
Flow chart of article selection process in the present review.
3. Botanical Characteristics
The Myrtaceae family is one of the most important in humid tropics and is widely distributed in South America, Australia, and tropical Asia [2]. Some authors claim that besides the genus Myrcia, three other genera of Myrtaceae, namely Calyptranthes, Marlierea, and Gomidesia, are synonyms of this genus [15].
In Brazil, the genus Myrcia is represented by over 400 species, found in a variety of environments ranging from dense forests to drier areas. And 304 of these species are exclusive to Brazil, meaning they are not found anywhere else in the world [16]. This highlights not only the richness of Myrcia in the country but also the importance of the genus’ diversity to the complexity of Brazilian ecosystems, for example, Myrcia elevata, Myrcia cuprea, Myrcia intons, Myrcia fasciculata, Myrcia neospeciosa, Myrcia magna, Myrcia uaupensis, Myrcia cuspidata, Myrcia lepida, Myrcia laruotteana Myrcia revolutifolia, Myrcia clusiifolia, Myrcia saxatilis, Myrcia bicarinata, Myrcia neosuaveolens, Myrcia bicarinata, Myrcia cf. obversa, Myrcia hexasticha, Myrcia isaiana, Myrcia plusiantha, Myrcia tomentosa, Myrcia hatschbachii, and Myrcia pileata [17,18,19].
The botanical genus Myrcia is monophyletic, meaning it shares a common ancestor and includes a large number of species. Its distribution is comprehensive, extensively occupying the American tropical territory and extending across almost all phytogeographical domains [20]. Diversity is a prominent characteristic of this genus, with approximately 800 species identified in a broad geographic area, from Central America to tropical regions [15,21].
Each species of the genus Myrcia displays significant variations in its phenological patterns, influenced by intrinsic factors such as genetic characteristics and extrinsic factors including climatic and microenvironmental conditions. Seasonal responses, such as flowering, fruiting, budding, and leaf fall, often adjust to climatic changes, revealing a temporal synchrony that can be interpreted as an adaptive strategy. Micro environmental conditions, such as cardinal orientation and surrounding vegetation structure, play a crucial role in these phenological patterns [22].
The genus Myrcia comprises shrubs or small- to medium-sized trees, whose branching follows a dichotomous pattern. Young branches exhibit varied characteristics, such as being flattened, ridged, winged, or pubescent. Their leaves are opposite or simple, with well-defined veins and entire margins, and can be leathery or membranous, without stipules. Trichomes are generally simple, although some species may have bifurcations. Flowers are often arranged in panicles. Before flowering, the calyx of the flowers is closed, opening during flowering, with petals usually absent or small. The reproductive organs have numerous stamens and an ovary with two cells, each containing two ovules. The fruits are berries with 1 to 4 seeds, covered by the basal portion of the calyx, and the seeds tend to be subglobose, with large, thin, and often twisted cotyledons [17,23,24].
Myrcia presents its inflorescence in the form of a panicle, which refers to an arrangement of flowers where multiple branches radiate from a central point, presenting a structure resembling a panicle. This structural characteristic facilitates flower distribution, contributing to pollination and, consequently, promoting a high plant reproduction rate. Additionally, the panicle-type inflorescence can exhibit a variety of sizes and shapes, contributing to morphological diversity within the genus [25,26].
Species of the genus Myrcia exhibit aromatic characteristics due to the production of essential oils as part of their secondary metabolism. These essential oils are composed of volatile substances that impart fragrance to the plants and are stored in specialized structures called secretory cavities. These cavities are found in various parts of the plant, such as leaves, flowers, fruits, stems, or roots, and are responsible for the production, storage, and release of these aromatic substances. Secretory cavities are essential for the adaptation and survival of Myrcia species in their natural habitats [27,28,29,30,31]. The genus Myrcia is recognized for the presence of several species that produce volatile compounds [32], such as M. palustres, M. guianensis, M. sylvatica, M. rostrata, among others [33,34,35,36].
4. Chemical Composition of Essential Oils
The essential oils of the Myrcia genus exhibit chemovariability, which may be associated with factors such as climatic data, foliar nutrients, aerial parts, habitat, collection time, and also the different types of extraction techniques for these volatile oils [37]. Regarding this diversity in chemical profile, it is possible to observe in Table 1 the major compounds found in the essential oils of some species of the genus, with the presence of monoterpenes, sesquiterpenes, and other chemical classes.
Monoterpenes are natural organic compounds that represent a large group in the field of chemistry [38]. In this study, it was possible to observe that this chemical class characterized the profile of the essential oils of Myrcia lundiana (MLUN19), M. lundiana (MLUN23), M. mollis, M. ovata, and M. Paivae [7,13,39,40].
Sesquiterpenes are classes of compounds found in nature, which are present in both aromatic plants and marine life [41]. In this research, these organic compounds were found to be predominant in the essential oils of various species such as Myrcia eximia (Specimen B) [5], M. hatschbachii [42], M. loranthifolia [43], M. multiflora (Specimen A, B and C) [4], M. oblongata [44], M. sylvatica [10,45], M. rostrata [46], M. splendens [47,48], M. vitoriana [49], and finally, the samples of M. tomentosa [3,50].
Table 1 presents a comprehensive view of the diversity of plant species of the genus Myrcia, collected in various regions, along with the main chemical components extracted and their percentages. Among the species listed, M. eximia (Specimen A and B) from Magalhães Barata, Pará, Brazil, stands out, with hexanal (26.09%) and (E)-caryophyllene (20.3%) as major compounds, respectively. M. guianensis, collected in Rio Verde, Goiás, Brazil, presents methyl salicylate (11.13%) and Geraniol (8.03%) as predominant components. Another notable species is M. hatschbachii, from Paraná, Curitiba, Brazil, with trans-calamenene (19.10%) and (E)-caryophyllene (10.96%) as main compounds. Furthermore, M. loranthifolia, from Igarassu, Pernambuco, Brazil, is marked by (E)-caryophyllene (47.54%) and α-Humulene (9.22%) as major components. This chemical diversity reflects the influence of environmental and geographic factors on the chemical composition of plants, highlighting the importance of an integrated approach to understanding their biology and potential application.
Other species include M. lundiana, collected in Itabaiana, Sergipe, Brazil, presenting Isopulegol (40.29%) and Iso isopulegol (15.49%) as the main compounds in the chemotype isopulegol (MLUN19) and Citral (23.43%) and 1,8-Cineole (16.43%) in the citral chemotype (MLUN23). M. mollis, from Gonzanamá, Loja Province, Ecuador, has (Z)-caryophyllene (16.6–16.8%) and 1,8-Cineole (10.4–11.6%) as highlighted components. M. multiflora, collected in Magalhães Barata, Pará, Brazil, presents different specimens, with (E)-nerolidol (44.4%) and (E)-γ-Bisabolene (10.64%) in Specimen B and (E)-nerolidol (92.21%) in Specimen C, among other compounds. This variety of chemical composition highlights the richness and complexity of plants of the Myrcia genus, offering vast potential for applications in various areas, from the pharmaceutical industry to cosmetology and aromatherapy.

Table 1.
Main major compounds of Myrcia essential oils. The analyses of the essential oils presented in the table were carried out using Gas Chromatography Coupled with Mass Spectrometry (GC/MS).
Table 1.
Main major compounds of Myrcia essential oils. The analyses of the essential oils presented in the table were carried out using Gas Chromatography Coupled with Mass Spectrometry (GC/MS).
| Species | Collection Site | Plant Part | Extraction Type | Major Components | References |
|---|---|---|---|---|---|
| Myrcia eximia (Specimen A) | Magalhães Barata, Pará, Brazil | Leaves | HD | hexanal (26.09%), (E)-Caryophyllene (20.3%), and (2E)-hexenal (6.63%). | [5] |
| M. eximia (Specimen B) | Magalhães Barata, Pará, Brazil | Leaves | SD | Caryophyllene oxide (22.16%), α-Copaene (10.98%), and 14-Hydroxy-9-epi-(E)-caryophyllene (7.84%) | [5] |
| M. guianensis | Rio Verde, Goiás, Brazil. | Flores | HD | methyl salicylate (11.13%), geraniol (8.03%), and eugenol (8.12%) | [51] |
| M. hatschbachii | Parana, Curitiba, Brazil | Leaves | HD | trans-calamenene (19.10%), (E)-caryophyllene (10.96%), and spathulenol (5.03%). | [42] |
| M. ovata | Japaratuba, in the state of Sergipe, Brazil | Leaves | HD | nerolic acid (50.56–60.63%), geraniol (73.64–77.07%) neral (18.21–28.39%), geranial (36.96–48.48%), and (E)-nerolidol (26.97–58.27%) | [43] |
| M. lundiana chemotypes isopulegol (MLUN19) | Itabaiana, Sergipe, Brazil. | Freshly leaves | HD | Isopulegol (40.29%), Iso isopulegol (15.49%), and 1,8-cineol (14.16%) | [13] |
| M. lundiana chemotype citral (MLUN23) | Itabaiana, Sergipe, Brazil. | Freshly leaves | HD | Citral (23.43%), 1,8-cineol (16.43%), and Nerolic acid (16.42%) | [13] |
| M. mollis, | Gonzanamá, province of Loja, Equador | Leaves | SD | limonene (5.3–5.2%), 1,8-cineole (10.4–11.6%), and (Z)-caryophyllene (16.6–16.8%) | [39] |
| M. multiflora (Specimen A) | Magalhães Barata, Pará, Brazil | Leaves | HD | α-bulnesene (26.79%), pogostol (21.27%), and δ-amorphene (6.76%) | [4] |
| M. multiflora (Specimen B) | Magalhães Barata, Pará, Brazil | Leaves | HD | (E)-nerolidol (44.4%), (E)-γ-bisabolene (10.64%), and (E,E)-α-farnesene (8.19%) | [4] |
| M. multiflora (Specimen C) | Magalhães Barata, Pará, Brazil | Leaves | HD | (E)-nerolidol (92.21%), (E,E)-α-farnesene (3.28%), and E-caryophyllene (1.11%) | [4] |
| M. oblongata | Cascavel, Paraná, Brazil. | Leaves | HD | caryophyllene oxide (22.03%), and trans-verbenol (11.94%). | [44] |
| M. ovata | Japaratuba, Sergipe, Brazil | Leaves | HD | Geranial (40.10%), Neral (28.39%), and Citronellal (9.19%) | [40] |
| Guaramiranga, Ceará, Brazil | Leaves | HD | Geranial (52.60%) and neral (37.14%) | [52] | |
| M. paivae | Peixe-Boi, Pará, Brazil | Leaves | HD | terpinolene (14.70%), α-phellandrene (14.69%), and γ-terpinene (9.64%) | [7] |
| M. sylvatica | Santarém, Pará, Brazil | Leaves | HD | β-selinene (9.96%), cadalene (9.36%), α-calacorene (9.17%) | [45] |
| Bujaru, Pará, Brazil | Leaves | HD | (Z)-α-trans-bergamotene(24.57%), α-sinensal (13.44%), and (Z)-α-bisabolene (8.33%) | [10] | |
| M. rostrata | Alagoinhas, Bahia, Brazil | Leaves | HD | carotol (17.68%), germacreno B (7.28%), and (E)-caryophyllene (6.45%) | [46] |
| M. splendens | Manaus, Amazonas, Brazil | Leaves | HD | trans-nerolidol (67.81%), α-bisabolol (17.51%), and β-caryophyllene (4.21% | [48] |
| Sergipe, Brazil | Fresh leaves | HD | bicyclogermacrene (15.4%), germacrene D (8.9%), and E-caryophyllene (10.1%) | [47] | |
| M. vittoriana | community in southeastern Brazil | Fresh leaves | HD | germacrene D (21.90%), germacrene B (17.30%), and bicyclogermacrene (11.90%) | [49] |
| M. tomentosa (specimen A) | Magalhães Barata, Pará, Brazil | Leaves | HD | γ-elemene (12.52%), germacrene D (11.45%), and (E)-caryophyllene (10.22%) | [3] |
| M. tomentosa (specimen B) | Magalhães Barata, Pará, Brazil | Leaves | HD | spathulenol (40.70%), α-zingiberene (9.58%), and γ-elemene (6.89%). | [3] |
| M. tomentosa | Belo Horizonte, Minas Gerais | Fresh leaves | SD | germacrene D (17.62%), (E)-caryophyllene (15.05%), and δ-cadinene (6.42%) | [50] |
HD: Hydrodistillation; SD: Steam distillation.
Other chemical classes were also observed, such as aldehydes characterizing the essential oils of Myrcia eximia (Specimen A) [5] and phenylpropanoids in the oils of M. guianensis [51]. In summary, this research highlights the relevance of the volatile oils of this genus and their main implications in future studies, particularly in biological applications such as antimicrobial [53], antileishmanial [52], and insecticidal [54].
5. Antioxidant Activity
Antioxidant activity refers to the ability of certain substances to inhibit the damaging effects of free radicals and oxidative stress in biological systems. Free radicals are highly reactive molecules that can cause damage to cells, proteins, DNA, and other biomolecules, leading to various diseases and aging processes. This activity helps protect cells and tissues from oxidative damage and is associated with various health benefits, including reducing the risk of chronic diseases such as cardiovascular disease, cancer, and neurodegenerative disorders.
The antioxidant potential of the investigated substances was assessed by comparing them to Trolox, a water-soluble synthetic analog of vitamin E. This evaluation was based on their ability to inhibit the radical cation ABTS+•, which is formed by the reaction between 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt and potassium persulfate. The antioxidants reduce the ABTS+• to ABTS, and the extent of this reduction depends on factors such as antioxidant capacity, concentration, and reaction time. Both TEAC and DPPH assays were employed to evaluate the antioxidant capacities of the essential oils. The DPPH method assesses the ability of EOs to neutralize 1,1-diphenyl-2-picrylhydrazyl. The EO of M. tomentosa exhibited significant inhibition of both ABTS+• and DPPH• radicals by 53.6% and 213%, respectively. This study represents the first report on the antioxidant activity of EOs extracted from Myrcia tomentosa reported by Franco et al. [3].
De Moraes et al. [7] reported the antioxidant activity of Myrcia floribunda and Myrcia sylvatica essential oil via ABTS•+ and DPPH• methods. The results of their study indicated that the essential oil (EO) of M. floribunda exhibited an ABTS•+ inhibition rate of 53.27 ± 8.27%, comparable to Trolox (45.74 ± 4.16%) in terms of antioxidant potential. In the DPPH assay, the EO showed an AIR of 81.91 ± 3.46%, surpassing Trolox (50.53 ± 0.52%) significantly (p < 0.0001), suggesting excellent antioxidant activity attributed to its high levels of antioxidant monoterpenes. Conversely, M. sylvatica EO had a lower ABTS•+ AIR of 7.20 ± 0.72% compared to M. floribunda and Trolox. However, in the DPPH assay, M. sylvatica EO showed comparable AIR (80.55 ± 2.00%) to M. floribunda and superior to Trolox (p < 0.0001), possibly due to the presence of reactive oxygenated sesquiterpenes. Table 2 summarizes the antioxidant efficacy of various Myrcia species using different methods.

Table 2.
Antioxidant activity of Myrcia genus.
6. Biological Activities
6.1. Antimicrobial (Fungi and Bacteria)
Essential oils are associated with various biological activities, as seen in species of Myrcia (Myrtaceae family), which have been reported to possess antioxidant, anticancer, antiparasitic, anti-inflammatory, antiproliferative, cytotoxic, insecticidal, anticholinesterase, sedative, anesthetic, and antimicrobial properties [40,58,59]. It is important to highlight that these activities may be related to genetic or environmental (edaphoclimatic) variations, such as ambient temperature, light incidence, rainfall, and soil characteristics, which influence the plant’s metabolism and, consequently, the concentration of its secondary metabolites [59].
Essential oils from various Myrcia species have been investigated for their use against pathogenic microorganisms affecting humans, animals, and plants. Nine essential oils from M. ovata Cambessedes were studied and showed inhibitory and bactericidal effects against Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, and Pseudomonas aeruginosa at concentrations ranging from 3.13 to 25 μL/mL. Gram-positive bacteria were more susceptible to the essential oil than Gram-negative ones. The study emphasized the inhibition and elimination effects of most essential oils against P. aeruginosa, a bacterium known for its high resistance to other essential oils and even synthetic antibiotics [40].
The antibacterial activity of the essential oil from M. hatschbachii D. Legrand leaves was studied using the Minimum Inhibitory Concentration (MIC) test. The essential oil showed moderate activity against E. faecalis (500 µg/mL) and weak activity against S. aureus (1000 µg/mL) [42].
Three specimens of Myrcia multiflora (A, B, C) had their essential oils evaluated for antifungal activity against Candida albicans, C. tropicalis, C. famata, C. krusei, and C. auris. In other studies, it was found that specimen B showed greater sensitivity against the studied yeasts, with inhibition halos ranging from 9 to 11 mm. Specimen A showed an MIC of 0.78 µL/mL against C. famata growth and a minimal fungicidal concentration (MFC) of 12.5 against C. auris, while specimen C showed a minimal inhibitory concentration of 5 µL/mL against C. auris growth and an MFC greater than or equal to 50 against the studied yeasts [4].
The essential oil of M. oblongata was studied for its MIC and Minimum Bactericidal Concentration (MBC) against ten Salmonella spp. serotypes (S. Albany, S. braenderup, S. gafsa, S. heidelberg, S. idikan, S. lexington, S. livingstone, S. montevideo, S. saintpaul, and S. senftenberg). In the study, Santana et al. [55] showed that the essential oil exhibited an MIC and MBC ranging from 437.5 to 7000 µg/mL for the ten serotypes, with better activities against S. Lexington (MIC = 437.5 and MBC = 3500) and S. Saintpaul (MIC = 875 and MBC = 1750).
The essential oil of M. ovata was first evaluated in 2010 for its antimicrobial activity against Enterococcus faecalis, Escherichia coli, Helicobacter pylori, Pseudomonas aeruginosa, Salmonella choleraesuis, Staphylococcus aureus, Streptococcus pneumoniae, and Candida parapsilosis, as well as biofilm formation by Enterococcus faecalis. The authors reported that the essential oil exhibited antimicrobial activity against all tested microorganisms (diameter of inhibition zone = 16 to 36 mm), except for Helicobacter pylori (0 mm), and was effective against biofilm formation compared to the control (sterile saline) [60].
The antibacterial activity of the essential oil of M. splendens was evaluated against Gram-positive and Gram-negative bacteria, with the MIC determined by the serial microdilution method. The oil showed more satisfactory effects against phytopathogen strains, with greater sensitivity and, consequently, lower MIC value (125 μg/mL) for the Gram-positive C. michiganensis subsp. Nebraskensis; all Gram-negative bacteria showed resistance to the essential oil, except for P. syringae pv. seringas, which showed an MIC value of 250 μg/mL. These effects were attributed to the major compounds, the sesquiterpenes trans-nerolidol (67.81%) and α-bisabolol (17.51%) [48].
The antimicrobial activity of essential oils from Myrcia species, as well as from other essential oils, is mainly related to the presence of their volatile compounds such as terpenes (mono-, sesqui-, and diterpenes), alcohols, acids, esters, epoxides, aldehydes, ketones, amines, and sulfides [3,5,61].
The antifungal activity is attributed to the lipophilic nature and low molecular weight of these compounds, which can lead to the inhibition of sporulation, germination, and cell death [4,62]. Meanwhile, the antibacterial activity is associated with the ability of essential oils to penetrate the lipid membrane of bacteria, leading to leakage of intracellular components, inhibiting their functional properties and, eventually, leading to cell death [40,61].
It is important to highlight that the antifungal and antibacterial activities of essential oils are also attributed to the synergistic action of minor concentration compounds with the major compounds. Table 3 presents the antifungal and antibacterial activities of essential oils from Myrcia species, along with the main chemical compounds found in these oils.

Table 3.
Synthesis of antifungal and antibacterial activities and the main constituents of essential oils from Myrcia species.
6.2. Phytotoxic Activity of Myrcia Genus
Allelochemicals, compounds produced by plants and other species, induce allelopathic (phytotoxic) activity by affecting the growth and development of nearby plants. Allelochemicals can intervene in physiological processes in different ways and exhibit diversified mechanisms of action. Some substances may inhibit seed germination by interfering with water absorption or cell division. Other compounds affect root growth, so plants are unable to absorb nutrients from the soil, or photosynthesis, reducing energy production. Allelochemicals can also affect the metabolism of the host plant, hindering the production of essential enzymes or hormones. Studies also indicate that some of these compounds limit the production of reactive oxygen species, creating conditions of oxidative stress. The effectiveness of allelochemicals varies based on environmental conditions or the concentrations in which they are found [63,64].
Phytotoxic activity refers to the ability of certain substances, such as plant extracts, essential oils, etc. to inhibit the growth of other plants. This activity is often studied in the context of weed control or understanding the interactions between different plant species in ecosystems. Phytotoxic compounds can affect various processes in plants, including germination, root growth, shoot development, and overall plant health. Studying phytotoxic activity helps in identifying natural or synthetic compounds that could potentially be used for weed management or understanding ecological relationships between plants. Vasconcelos et al. [49] reported a macroscopic analysis that involved assessing the germination of Lactuca sativa and Allium cepa, followed by evaluating germination percentage and root growth. Notably, the essential oil of Myrcia vittoriana decreased the germination rate of A. cepa. All essential oil concentrations except 375 µg.mL−1 reduced the germination speed index of A. cepa, with the highest concentration showing the most substantial reduction. The essential oil strongly inhibited aerial growth in L. sativa across all concentrations compared to water, with a dose–response relationship evident. At the highest concentration, it caused a 54.8% inhibition in aerial growth, accompanied by yellowish seedling coloration and leaf necrosis. The growth of the aerial part of Lactuca sativa was strongly inhibited by the essential oil of M. vittoriana.
The phytotoxic activity was assessed by examining the impact of Myrcia hatschbachii essential oil on the germination and growth of Lactuca sativa seeds. Results revealed that the highest concentration (1%) of the EO exhibited the most pronounced effects on both hypocotyl and radicle growth. All concentrations of the EO hindered hypocotyl growth in L. sativa. For the radicle, the EO showed significant growth inhibition at the 1% concentration, while concentrations of 0.001% and 0.01% also displayed adverse effects compared to the control groups (water and 1% polysorbate in water), as presented by Gatto et al. [42]. The study investigated the allelopathic effects of extracts, fractions, essential oils, and isolated chemical compounds (gallic acid and protocatechuic acid) on the germination, radicle, and hypocotyl growth of weed species Mimosa pudica and Senna obtusifolia. Extracts and fractions were examined at a concentration of 1%, while essential oils and isolated chemical compounds were tested at 15, 30, 45, and 60 ppm. Interestingly, the essential oil inhibited the germination of M. pudica [65].
6.3. Insecticide
Several plants possess defense mechanisms against insects that can harm their growth and development [66]. Among them is the production of chemical compounds with insecticidal properties that are capable of repelling, incapacitating, or even killing insects [67,68]. These compounds can be found in various parts of the plant, such as leaves, flowers, stems, and roots, and play a crucial role in protecting plants against herbivorous insects, helping them survive and successfully reproduce in their natural environment [69,70,71,72].
Many of these natural insecticidal compounds, in addition to protecting the plant itself, can also be used for pest control in agriculture, gardening, and forestry management and can be applied directly to plants as natural extracts, essential oils, or plant-based products [73,74]. Thus, studies of plant insecticidal compounds have been developed to obtain safer and more sustainable pesticides, reducing dependence on environmentally harmful synthetic chemicals and human health risks.
Among these studies are some conducted with the genus Myrcia, which demonstrate the insecticidal potential of the species. In a study conducted with extracts and essential oil from the leaves of Myrcia oblongata DC, for example, the insecticidal effect on the beetle Alphitobius diaperinus was evaluated. In the study, the hexane extract, composed of steroids, triterpenoids, alkaloids, tannins, and flavonoids, and the essential oil showed the highest mortality rates (<95%) against A. diaperinus larvae, while the other extracts showed mortality rates above 50%, and the aqueous extract showed no activity. The highest lethality against adult A. diaperinus was observed for the essential oil (92.5%), while the extracts had mortality values below 20% [55].
In another study conducted with the methanolic extract of Myrcia splendens leaves, insecticidal action against the Ceratitis capitata fly species was observed. The activity was observed in extract fractions obtained by HPLC, which had their compounds isolated and identified as myricetin-3-O-(6″-O-galloyl)-β-galactopyranoside, myricitrin, and quercitrin. Among these, the compound myricetin-3-O-(6″-O-galloyl)-β galactopyranoside showed moderate activity at 2500 ppm [75].
In another study on the insecticidal potential of the Myrcia genus, the insecticidal action of the essential oil from the leaves of two chemotypes of Myrcia lundiana and their major compounds, isopulegol and citral, against the Acromyrmex balzani ant species was evaluated. Other essential oils from M. lundiana showed activity. The oil from the isopulegol chemotype presented LC50 values of 2.29 μL/L (CI 2.08–2.49) and LC95 of 6.28 μL/L (CI 5.32–7.97), and its major compound, isopulegol (40.29%), presented LC50 and LC95 values equal to 0.16 μL/L (CI 0.14–0.19) and 1.08 μL/L (0.81–1.58), respectively, while the citral chemotype presented LC50 values of 2.35 μL/L (CI 2.13–2.61) and LC95 of 9.14 μL/L (CI 7.25–12.61), and its major compound, citral (23.43%), presented LC50 and LC95 values equal to 0.59 μL/L (CI 0.48–0.69) and 3.83 μL/L (2.87–5.77), respectively [13].
Another species from the genus that had its insecticidal potential evaluated was Myrcia dictyophylla. A study conducted with the species’ essential oil against Aedes aegypti observed insecticidal action against the arbovirus vector, where an LC50 value of 17.6 µg/mL for mosquito larvae after 24 h of exposure and 92.5% knockdown was observed in adult mosquitoes after 4 h of exposure, in addition to repellent action. The studied oil presented three major sesquiterpenes: α-amorphene (19.8%), δ-cadinene (12.5%), and germacrene-D (10.3%) [76].
Although incipient, these studies demonstrate the insecticidal potential of the genus Myrcia, especially its essential oils, not only in pest control for agriculture but also in controlling disease vector insects. These studies reveal the genus as a promising sustainable strategy in insect control and as a natural alternative to synthetic compounds in the biological control of parasites, highlighting the need for further studies to explore and better elucidate this potential.
6.4. Hypoglycemic Potential of Myrcia Species
Diabetes refers to a group of diseases characterized by high blood glucose levels. Generally, it results from a deficiency in the production or action of insulin, resulting in metabolic disorders. Type 2 diabetes mellitus (DM) is the most common form of diabetes, and its main characteristics are insulin resistance, chronic hyperglycemia, and inefficient insulin secretion and action. According to the World Health Organization (2017), diabetes is responsible for the deaths of around 1.6 million people worldwide [77].
The main drugs used to treat DM2 include insulin sensitizers, glucosidase inhibitors, incretin-based therapies, and sodium-glucose co-transporter 2 inhibitors. Some species of plants, fruits, and teas, for example, may emerge as an alternative to traditional pharmacological therapy. So-called dietary supplements can be recommended to prevent and also improve disorders caused by DM2. The treatment of DM2, in an advanced phase, with synthetic drugs and compounds of natural origin cause synergistic interactions resulting in a more efficient treatment [78].
Therefore, knowledge about the properties, chemical composition, and beneficial characteristics of Myrcia species, popularly used to treat diabetes, can help offer a complementary natural alternative to the use of synthetic drugs [79].
Among the species of Myrcia, plants known as “pedra-hume-caá”, “pedra-ume-caá”, or “insulin plant” are noteworthy. They are known by these designations due to their use by indigenous communities in Brazil to lower blood sugar levels and are commonly used in the treatment of diabetes. In the literature, several species are related to the term “pedra-hume-caá”, including: Myrcia guianensis (Aubl.) DC., Myrcia punicifolia (Kunth) DC., Myrcia citrifolia (Aubl.) Urb., Myrcia uniflora DC., Myrcia multiflora (Lam.) DC., Myrcia salicifolia DC., Myrcia speciosa (Amsh.) Mc Vaugh, Myrcia rubella Cambess., Myrcia virgata Cambess., Myrcia paivae O. Berg., Myrcia laruotteana Camb., and Myrcia sylvatica (G. Mey) DC [37,80,81,82].
A study has shown that extracts obtained from the leaves of M. guianensis exhibit inhibition against α-glucosidase, which is the enzyme responsible for the metabolism of carbohydrates and a known target for type 2 diabetes mellitus (DM2), alongside demonstrating nephroprotective and hepatoprotective action. The ethyl acetate extract of M. guianensis showed the greatest potential for inhibiting both alpha-glucosidase and PTP1B (protein tyrosine phosphatase). The extract showed IC50 values for alpha-glucosidase of 7.80 µg/mL, while for the enzyme PTP1B, the value observed was 3.26 µg/mL [81]. This same chemical extract has been shown to be active against the protein tyrosine phosphatase (PTP1B), which is a recent target for DM2 and is involved in insulin signaling [9].
Another species of the genus, also known as the insulin plant, is M. multiflora (Lam.) DC., a shrubby species, also known as “pedra-ume-caá”, typical of the Brazilian Amazon region, occurring in the states of Amazonas, Pará, and Amapá [9]. This species is also found in regions of the Brazilian Cerrado, characteristic of mountainous terrain, poor soils, and margins of sandy or non-floodable fields [4]. M. multiflora produces a globose fruit, with succulent and edible pulp, highly appreciated by children [83]. The fruits range in color from dark red to purple when ripe, containing one, two, or three seeds, with a bitter taste [84].
Studies involving ripe fruits of M. multiflora have shown that they have a high antioxidant potential, supporting the idea that their consumption may result in health benefits for humans. Samples obtained from the fruits were analyzed for non-enzymatic antioxidant content and showed significant amounts of vitamin C, alongside being rich in anthocyanins, flavonoids, and polyphenols [4,84]. Another study has indicated the presence of a substance beneficial for preventing obesity, capable of reducing intestinal lipid absorption [83].
Research involving the use of substances known as myrciacitrins obtained from the leaves of M. multiflora has shown, in rats, activity against the enzyme aldose reductase, which is responsible for catalyzing and reducing glucose. The dry extract obtained from the infusion of M. multiflora leaves showed a glycemic effect in animals induced for 28 days. In the group of animals whose glucose levels were measured at the beginning of and during treatment with 50 mg/kg (body weight of dry extract), there was a 74.5% reduction in glucose levels when compared to the results of the levels before the start of treatment. At the end of the treatment, after 28 days, the author reported that there was an 11% reduction in glucose levels compared to the beginning of the tests. According to him, this result indicated that the use of the dry extract of M. multiflora is capable of maintaining the hypoglycemic effect [85,86].
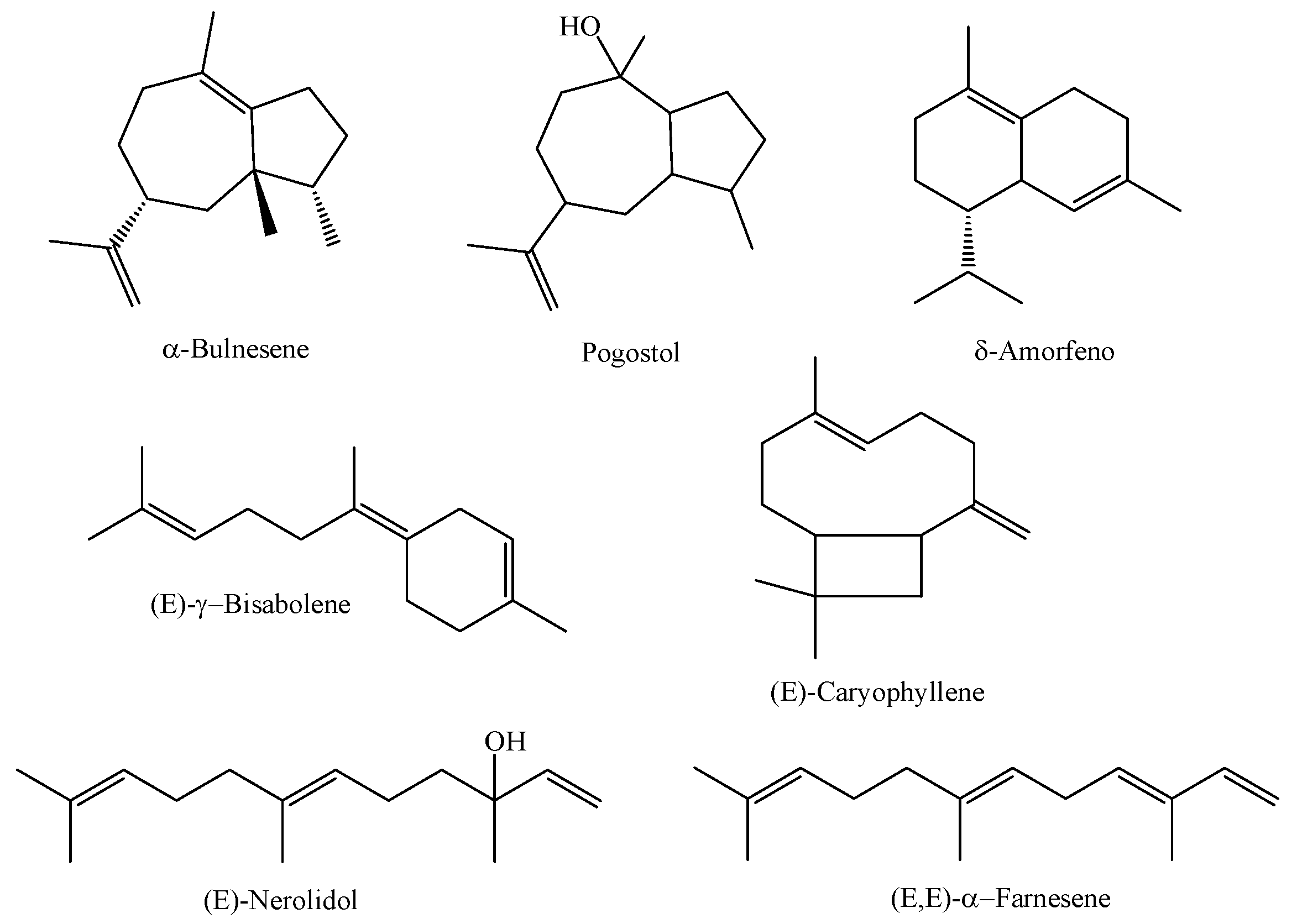
In a study involving the essential oil obtained from the leaves of three species of M. multiflora (referred to as A, B, and C) collected in different areas of a secondary forest in the coastal region of the state of Pará in the city of Magalhães Barata, the essential oils obtained from the species referred to as A (collected in 2017 in an area of the secondary forest -capoeira-), B (collected in 2017 in areas of swidden planting exposed to sunlight), and C (collected in 2019 in an area of the secondary forest -capoeira-) were characterized as having α-bulnesene (26.79%), pogostol (21.27%), and δ-amorphene (6.76%) as major compounds for chemical type A, (E)-nerolidol (44.4%), (E)-γ-bisabolene (10.64%), and (E,E)-α-farnesene (8.19%) for chemical type B, and the substances (E)-nerolidol (92.21%), (E,E)-α-farnesene (3.28%), and (E)-caryophyllene (1.11%) were the major constituents identified in the essential oil of M. multiflora—chemical type C. The research also reported a significant difference between the chemical profiles of the M. multiflora species studied [4]. The chemical structures of the main constituents of M. multiflora (A, B, and C) are shown in Figure 2.
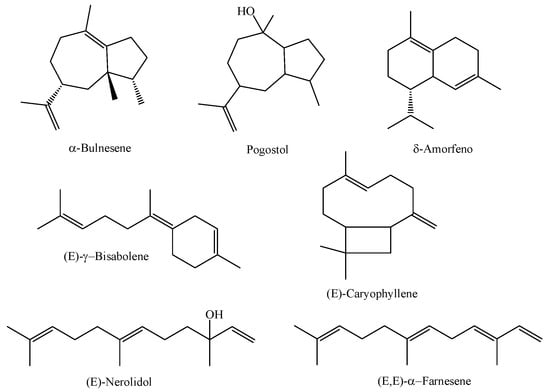
Figure 2.
Major constituents of essential oils from M. multiflora species collected in the state of Pará.
The essential oils of M. multiflora are rich in (E)-nerolidol, which is the main component of the essential oil of two chemotypes (B and C) collected in the state of Pará [41]. A study showed that nerolidol significantly improved insulin levels and body weight when administered to obese and diabetic animals at a dose of 25 mg/kg (body weight/day) for 28 days. As a result, high glucose and glycated hemoglobin levels were reduced. In addition, the study reported that during the use of nerolidol, liver integrity was preserved, and the regulation of insulin-dependent glucose transport was improved in skeletal muscles due to the activation of GLU4 (Glucose transporter type 4) [87,88]. It is also worth mentioning that another substance with reported antihyperglycemic activity in the literature is caryophyllene, also identified in the essential oil of M. multiflora, which showed significant antidiabetic effects on glucose homeostasis in diabetic rats at a dose of 200 mg/kg (body weight) [89].
M. bella Cambess., also popularly known as “mercurinho”, is a common species in savanna fragments and easily found in the Brazilian Cerrado [86]. This species is widely used in traditional medicine due to its use as a phytotherapeutic medication for the treatment of gastrointestinal disorders and diabetes mellitus [90]. The species is a source of phenolic compounds and flavonoids, with fractions rich in flavonoids showing cytotoxic effects against osteosarcoma cells [91].
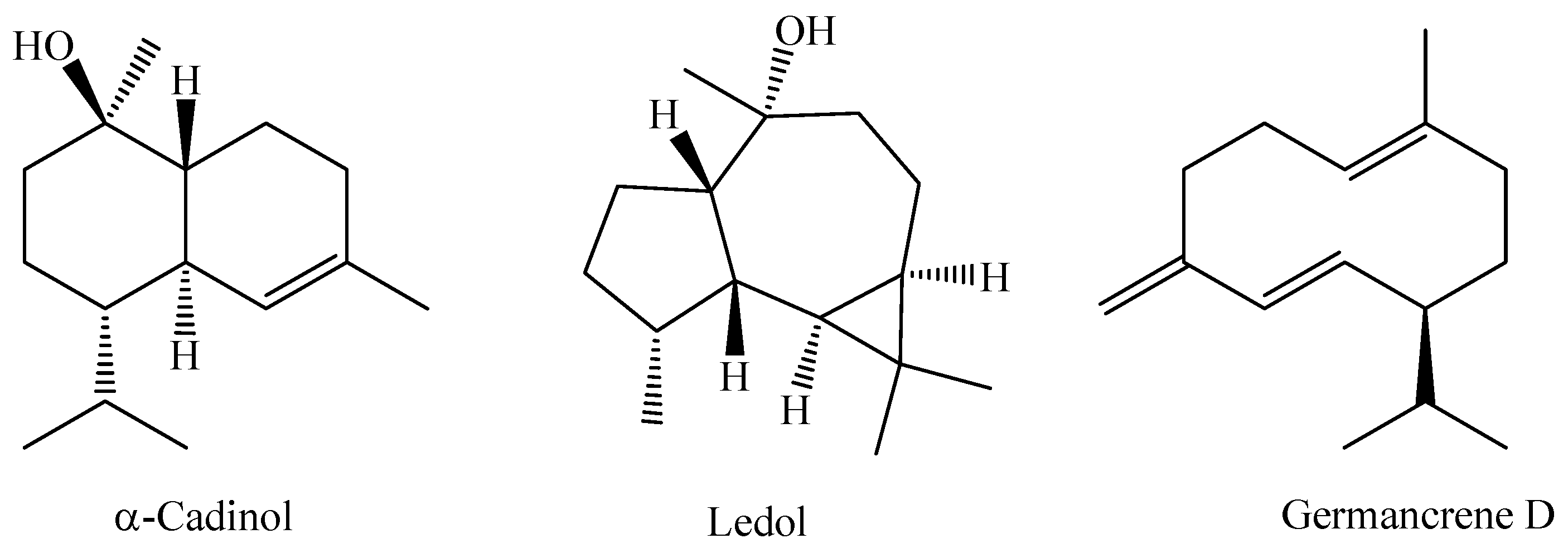
In a recent study, the chemical composition of the essential oil obtained from the leaves of an M. bella specimen collected during the rainy season in the Assis State Forest, São Paulo State, Brazil, was analyzed. The research indicated that the essential oil of M. bella is mainly composed of oxygenated sesquiterpenes α-cadinol (14.4%), ledol (12.2%), and the sesquiterpene hydrocarbon germacrene D (11.8%) [92]. The chemical structures of α-Cadinol, Ledol, and Germancrene D from M. bella are shown in Figure 3.
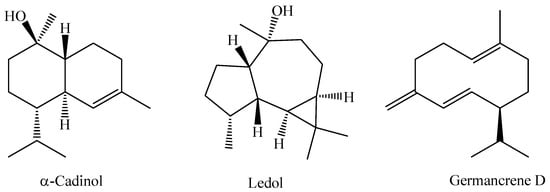
Figure 3.
Major constituents of essential oil of M. bella leaves.
The species M. laruotteana Camb. is commonly known as “cambuí” in the southern and southeastern regions of Brazil. In the Amazon, it is also recognized by popular names such as “murta” and “smooth-skinned guava”. The species is a shrub or small tree that not only occurs in the southern, southeastern, and central-western regions of Brazil but also has a wide distribution in the states of Pará, Tocantins, Maranhão, Mato Grosso, Goiás, Distrito Federal, Mato Grosso do Sul, Minas Gerais, Espírito Santo, São Paulo, Paraná, and Santa Catarina [18].
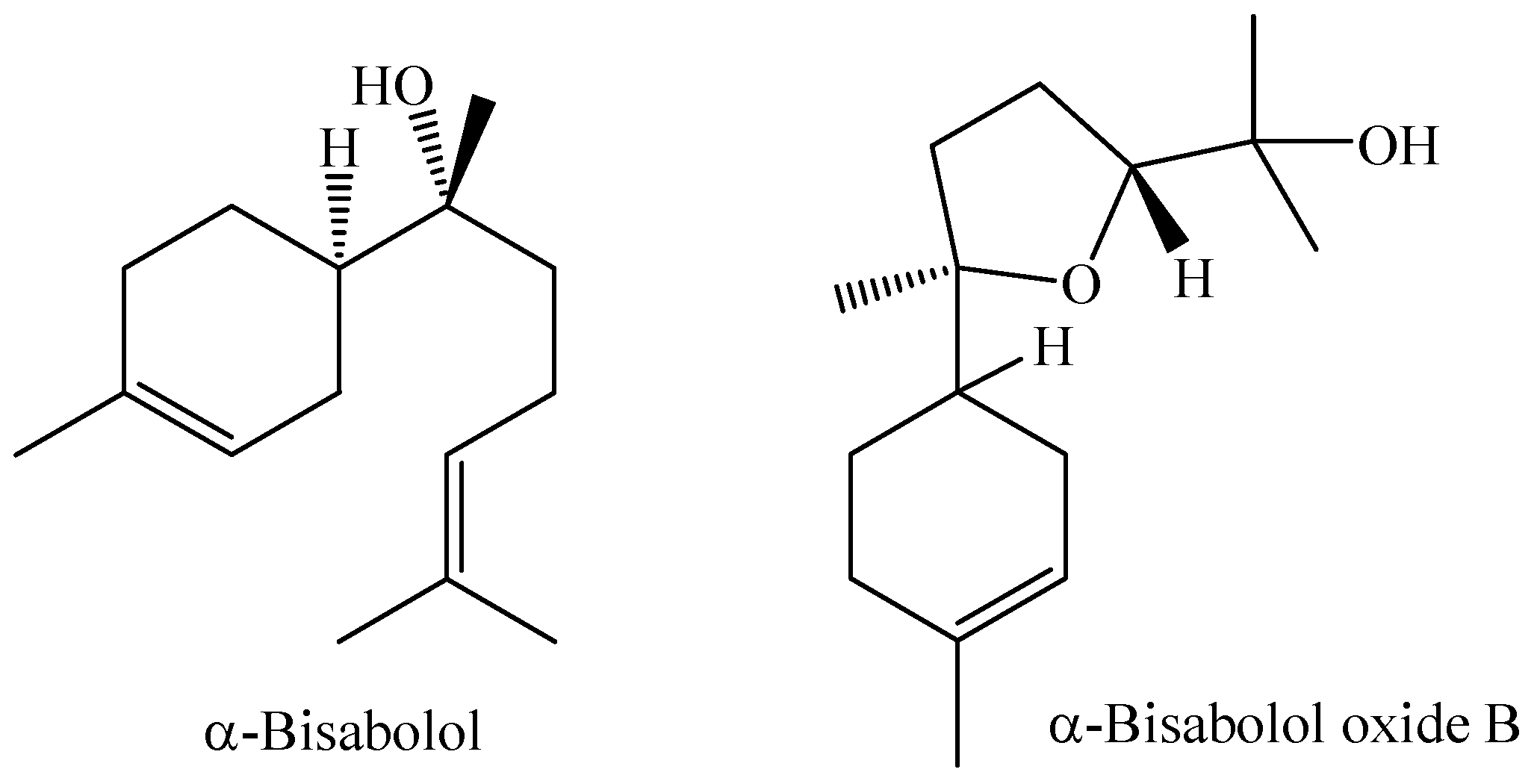
The flowers of M. laruotteana are white and strongly aromatic, while the green fruits contain essential oil, which decreases as they ripen, giving rise to small edible fruits with a purple color. This species occurs in upland areas and floodplain forests, and morphological variations associated with different environments where individuals occur are observed. The study of the essential oil obtained by hydrodistillation from the green fruits of the species M. laruotteana Camb. showed that the major constituents are α-bisabolol (23.6%) and α-bisabolol oxide B (11.5%) [93]. Chemical structures of the compounds α-Bisabolol and α-Bisabolol oxide B are shown in Figure 4.
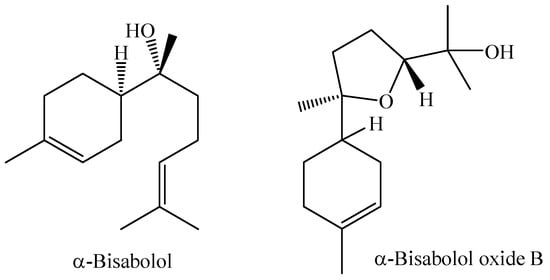
Figure 4.
Main chemical constituents of the essential oil from M. laruotteana fruits.
α-Bisabolol is the main constituent of the M. laruotteana essential oil and is also reported to have hypoglycemic properties. A study reports that α-bisabolol has antidiabetic properties related to reducing insulin resistance, glucose intolerance, plasma triacylglycerol, and LDL/VLDL cholesterol. Other factors such as the modulation of molecular pathways involved in the expression of target genes such as PPARs (Peroxisome proliferator-activated receptors) are among the properties described for this molecule [94].
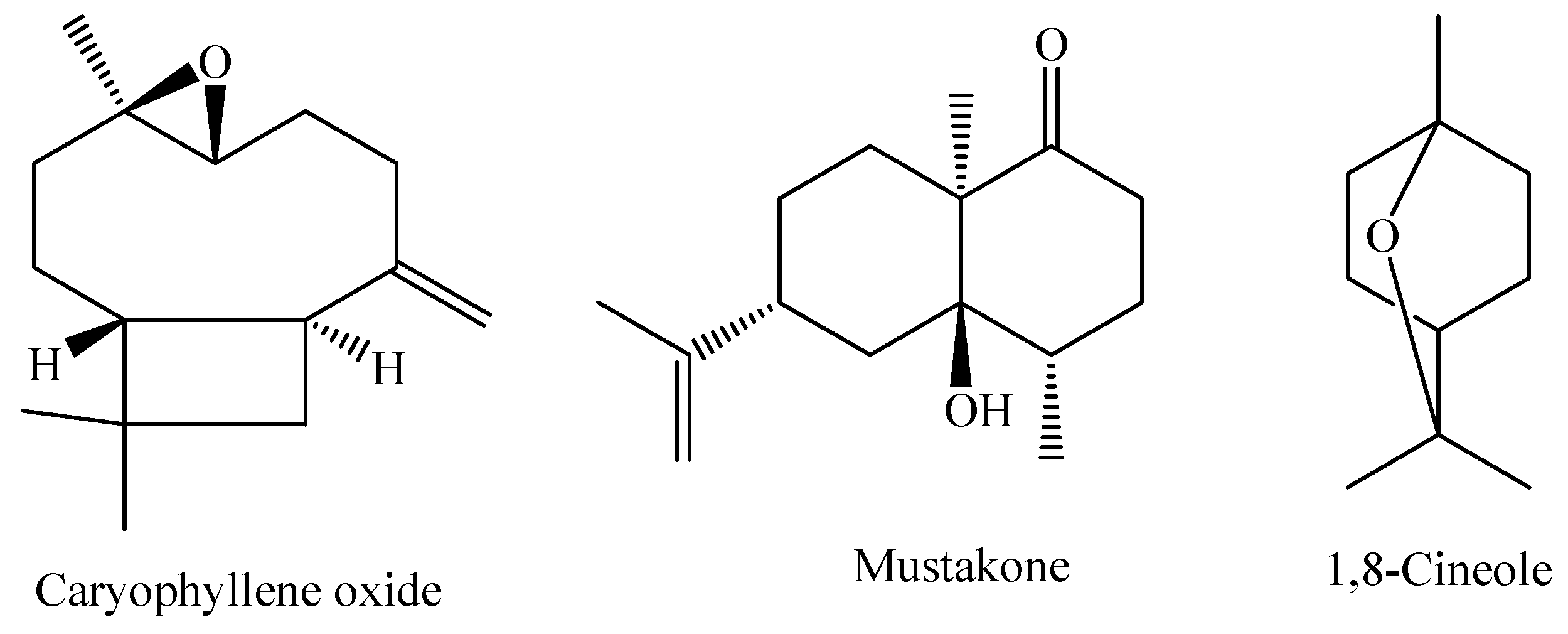
The species M. pubiflora DC. is widely found in South America, especially in the region ranging from Brazil to Paraguay. It is a shrub that predominantly occurs in tropical and humid biomes [86]. The essential oil obtained from the fresh leaves of M. pubiflora shows the oxygenated sesquiterpene caryophyllene oxide as the major component (22.2%), with other components present in smaller amounts, such as mustakone (11.3%), 1,8-cineole (5.4%), and tryciclene (5.3%) [95]. The chemical structures of the main constituents of the essential oil from M. pubiflora leaves are shown in Figure 5.
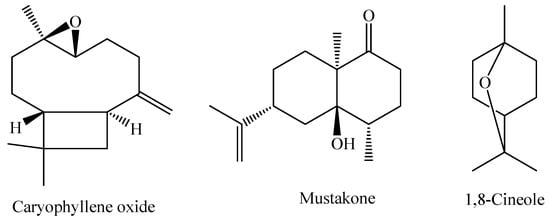
Figure 5.
Major constituents of the essential oil of M. pubiflora.
The main constituent of the M. publiflora essential oil is caryophyllene oxide (caryophyllene-type structure). The antihyperglycemic activity of compounds with a caryophyllene-type structure has been reported in the literature. One study evaluated the activity of the compound β-caryophyllene in streptozotocin (STZ)-induced diabetic rats. The results showed that administration of β-caryophyllene at a dose of 200 mg/kg (body weight) had significant antidiabetic effects, demonstrating beneficial effects on glucose homeostasis in diabetic rats. Therefore, the hypoglycemic activity may be associated with the presence of a compound with a similar structure to this one but not only this one [89].
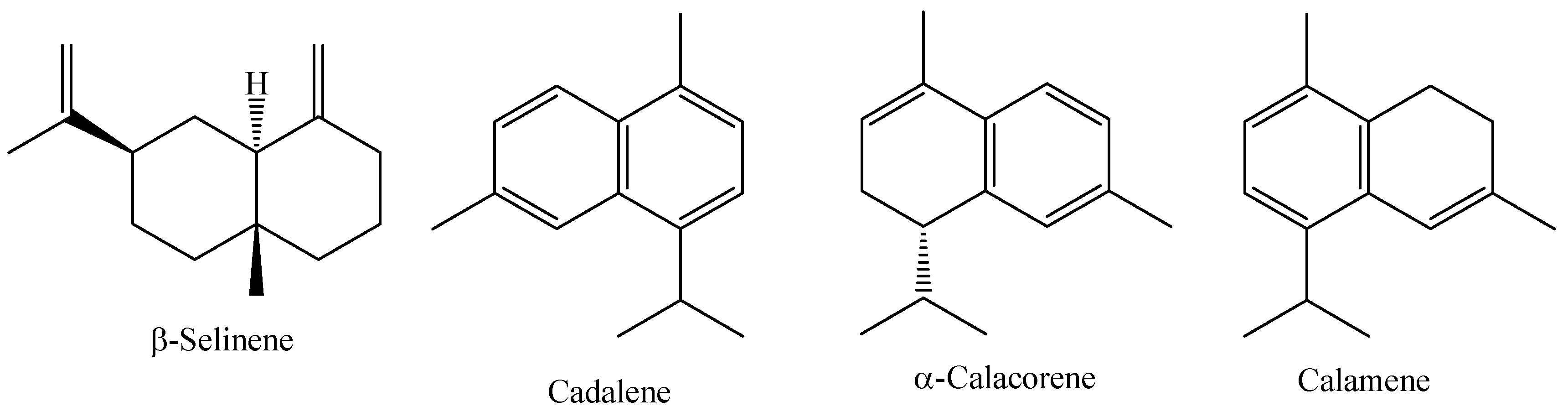
Myrcia sylvatica (G. Mey) DC. is a species widely distributed in South America, from the Guianas to Brazil. This species is popularly known in the Amazon region as “murta”, “kumate-folha-miúda”, or “murtinha”, and it is also known as the “insulin plant” because local communities empirically use infusions of various parts of these plants to treat diabetes [82]. Res Research carried out with Myrcia loranthifolia grown in Atlantic Forest and Dry Forest forests in Brazil, showed that the essential oil of this species has an average yield of 0.23 to 0.33%, with the major compounds identified being (E)–caryophyllene (47.80%), Cis-calamenene (11.40%), and germacrene D (10.07%) [96]. The chemical structure of the main constituents of the essential oil of fresh M. sylvatica leaves are shown in Figure 6.
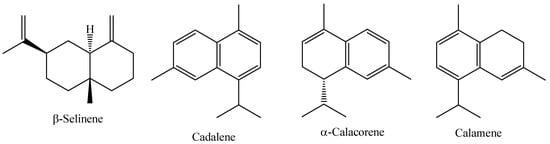
Figure 6.
Major constituents of the essential oil of M. sylvatica fresh leaves.
Another species known for its traditional use as a hypoglycemic agent is Mycia. It is a tree species that occurs in the Amazon biome in the northern regions, especially in the states of Amazon [9]. In the central-western region, it is commonly found in the state of Mato Grosso, in both upland forest and floodplain areas. This species is considered a medicinal plant in the Amazon region, and infusions of its leaves are used to prepare teas that are beneficial for consumption during pregnancy and for treating diabetes [9].
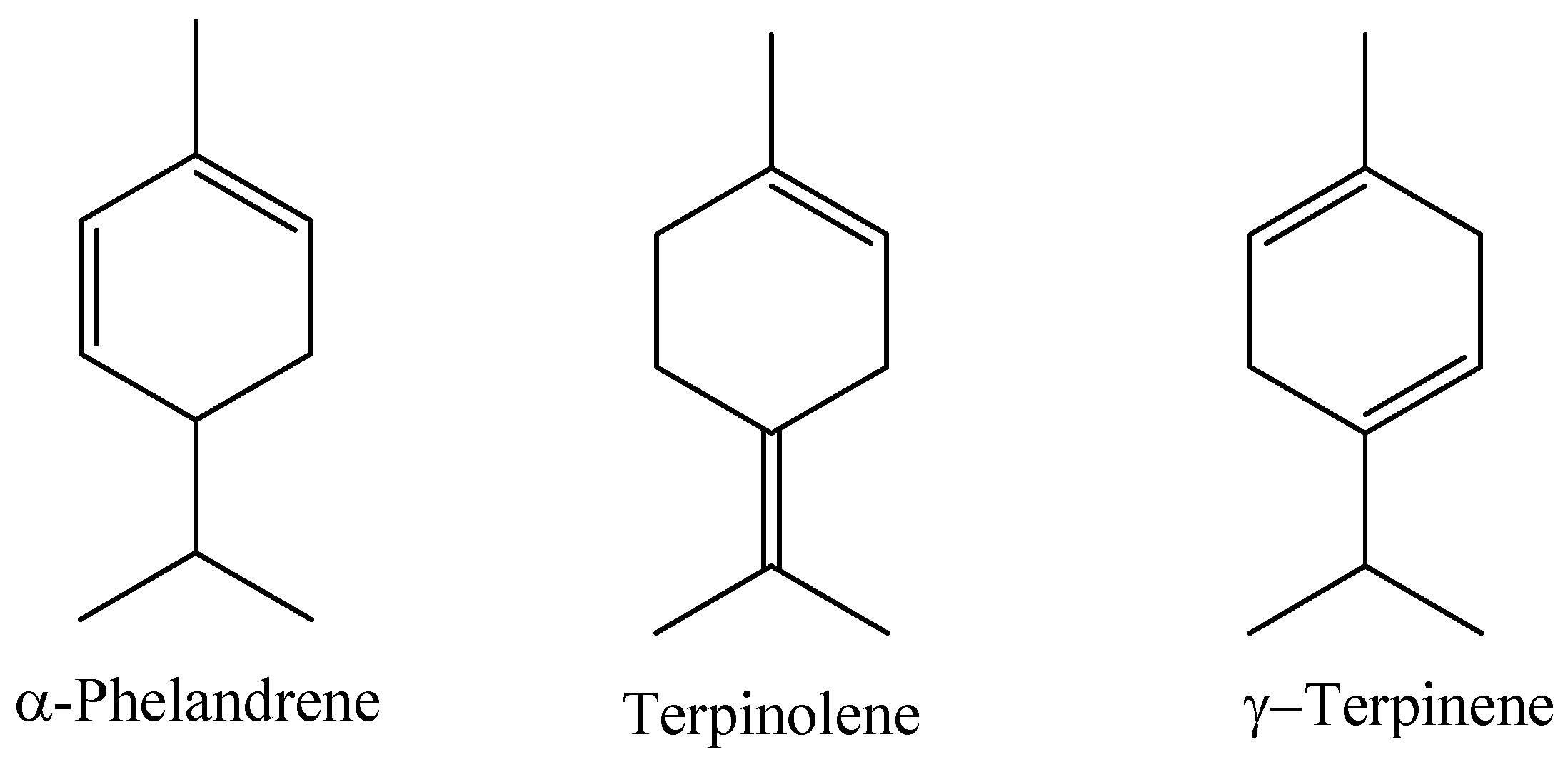
A study evaluating the chemical composition of the essential oil obtained by hydrodistillation of the leaves of a Myrcia paivae specimen collected in the municipality of Peixe-boi, Pará State, Brazil, identified the main constituents as compounds of the class of monoterpene hydrocarbons (77.93%), followed by sesquiterpene hydrocarbons (10.16%) and oxygenated monoterpenes (7.97%). In this study, the compounds α-phellandrene (14.69%), terpinolene (14.70%), and γ-terpinene (9.64%) were identified as the major ones [7]; the majority compounds can be seen in Figure 7.
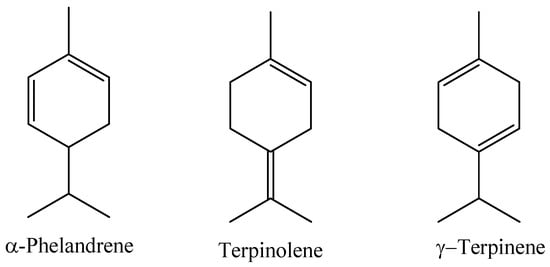
Figure 7.
Major constituents of the essential oil of M. paivae leaves.
In a study investigating the effect of α-Phelandrene, a major constituent in the essential oil of M. paivae, on the function of adipocytes under conditions of insulin resistance, the reduction in glucose uptake by insulin-resistant 3T3-L1 adipocyte cells was evaluated. The 3T3-L1 adipocytes were exposed to 25 µM glucose and insulin (0.6 nM) for 24 h. The author reported that α-Phelandrene contributed to benefiting the functions of adipocytes under conditions of insulin resistance. Cells treated with alpha-Phelandrene (65 µM) for 24 h showed a significant improvement in glucose uptake in the adipocytes of 3T3-L1 cells, with values of 11.31 mm/L, a result similar to that presented by the group treated with rosiglitazone [97]. Terpinolene, which is the second main constituent of the Myrcia paivae essential oil, is a substance whose activity in reducing blood glucose levels (mg/dL) has been described in the literature. The author reported that an extremely significant reduction in blood glucose levels was observed in diabetic female rats treated with 12.5 and 25.0 mg/kg of the terpene terpinolene for 4 weeks. In the group treated with 12.5 mg/kg of the substance, the animals had a blood sugar level of 580 mg/dL, and after treatment, the value was reduced to approximately 290 mg/dL. A similar result was observed for the group treated with 25 mg/kg of the terpene; the value before treatment was approximately 500 mg/dL and after treatment, 200 mg/dL [98]. In in vitro research involving various types of terpenes commonly found in essential oils, such as myrcene, eugenol, eucalyptol, β-pinene, citrol and terpineol, the effect and potential benefits in the prevention and treatment of diabetes were evaluated based on the action of these compounds against the α-amylase enzyme (enzyme responsible for hydrolyzing oligosaccharides into monosaccharides, increasing blood sugar levels). In this study, solutions were prepared containing different concentrations (µM.cm−3) of the selected terpenes. The results indicated that terpenes, which are the primary components of essential oils, have the potential to inhibit α-amylase to a significant degree, with the most efficient terpene concentrations ranging from 1.16 μmol.cm−3 for β-pinene to 5.50 μmol.cm−3 for eugenol [99].
The species mentioned above are particularly noteworthy for their popular uses by traditional indigenous communities and for their hypoglycemic activity. Studies highlighting the chemical composition of the leaves, flowers, and fruits, as well as the essential oils of these species, provide the scientific community with knowledge about this natural wealth. Most of these Myrcia species, as the text highlights, occur in Brazilian biomes, being predominantly distributed in the Cerrado, Caatinga, and the Amazon, and constitute a natural wealth that is still little known.
The data cited in the previous text reveal interesting activities for the essential oils obtained from Myrcia species as natural pharmacological agents, which could be important for the development of phyto-formulations against diseases such as DM2. The essential oils from the leaves of these Myrcia species, popularly used as hypoglycemic agents, can be considered a potential source of pharmacological agents, which could be used in formulations for the treatment of diabetes, Alzheimer’s, and diseases linked to oxidative stress and applied as active ingredients in phyto-formulations as an alternative to the use of synthetic drugs.
7. Conclusions
The Myrcia genus is a promising source of essential oils, whose chemical composition is characterized by great complexity, which depends on climate location and seasonal factors, among others. The results obtained by the consulted studies indicate that Myrcia essential oils can constitute a promising source of bioactive compounds such as monoterpenes and sesquiterpenes with different biological activities including antioxidant, antimicrobial, phytotoxic, insecticidal, and hypoglycemic. All these effects can attract the attention of several industrial sectors, such as the pharmaceutical and cosmetic industries, which open up a vast field of therapeutic possibilities. The M. eximia sample A shows a high quantity in percentage of hexanal (26.09%) and (E)-caryophyllene (20.3%), and sample B, caryophyllene oxide (22.16%) and α-copaene. Furthermore, M. hatschbachii exhibits trans-calamenene (19.10%) and (E)-caryophyllene (10.96%), whilst M guianensis is notable for its methyl salicylate (11.13%) and Geraniol (8.03%). Among the variety of Myrcia species, (E)-caryophyllene and caryophyllene oxide are substances most commonly found in the various Myrcia species. These complex chemical compositions are present in several Myrcia plant species and sections, demonstrating the genetics and environmental complexity of plants, as well as the impact of geographical and climatic variables. Understanding these intricacies is vital for pragmatic implementations in the fields of medicine, cosmetics, and other domains. Promoting the sustainable use of these vital resources all depends on protecting these species and their habitats.
Author Contributions
Conceptualization, E.d.J.B.d.S., L.R.R.d.S., F.W.F.B., M.P.d.S., O.O.F., L.H.d.S.M., A.M.d.J.C.-N., A.d.S.B., R.K., P.B., K.d.S.d.S.V., E.H.d.A.A. and M.S.d.O.; methodology, E.d.J.B.d.S.; validation, E.H.d.A.A. and M.S.d.O.; formal analysis, E.d.J.B.d.S., F.W.F.B., M.P.d.S., O.O.F., L.H.d.S.M., A.M.d.J.C.-N., A.d.S.B., R.K., P.B., K.d.S.d.S.V. and E.H.d.A.A.; investigation, E.d.J.B.d.S., L.R.R.d.S., F.W.F.B., M.P.d.S., O.O.F., L.H.d.S.M., A.M.d.J.C.-N., A.d.S.B., R.K., P.B., K.d.S.d.S.V., E.H.d.A.A. and M.S.d.O.; resources, E.d.J.B.d.S.; data curation, E.d.J.B.d.S.; writing—original draft preparation, E.d.J.B.d.S.; writing—review and editing, E.H.d.A.A. and M.S.d.O.; visualization, E.H.d.A.A. and M.S.d.O.; supervision, M.S.d.O.; project administration, M.S.d.O.; funding acquisition, M.S.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
CAPES process number 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The author Mozaniel Santana de Oliveira thanks PDPG-POSDOC—Programa de Desenvolvimento da Pós-Graduação (PDPG) Pós-Doutorado Estratégico, as well as CAPES for the scholarship (process number: (88887.852405/2023-00). The authors thank CAPES process number 001.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Santos, M.F.; Amorim, B.S.; Burton, G.P.; Fernandes, T.; Gaem, P.H.; Lourenço, A.R.L.; Lima, D.F.; Rosa, P.O.; Santos, L.L.D.; Staggemeier, V.G.; et al. Myrcia in Flora Do Brasil. Jardim Botânico Do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB10660 (accessed on 6 February 2024).
- dos Santos, C.; Galaverna, R.S.; Angolini, C.F.F.; Nunes, V.V.A.; de Almeida, L.F.R.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Duarte, R.M.T.; Duarte, M.C.T.; Eberlin, M.N. Antioxidative, Antiproliferative and Antimicrobial Activities of Phenolic Compounds from Three Myrcia Species. Molecules 2018, 23, 986. [Google Scholar] [CrossRef] [PubMed]
- de Franco, C.J.P.; Ferreira, O.O.; Antônio Barbosa de Moraes, Â.; Varela, E.L.P.; do Nascimento, L.D.; Percário, S.; de Oliveira, M.S.; de Andrade, E.H.A. Chemical Composition and Antioxidant Activity of Essential Oils from Eugenia patrisii Vahl, E. Punicifolia (Kunth) DC., and Myrcia tomentosa (Aubl.) DC., Leaf of Family Myrtaceae. Molecules 2021, 26, 3292. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.O.; da Silva, S.H.M.; de Oliveira, M.S.; de Andrade, E.H.A. Chemical Composition and Antifungal Activity of Myrcia multiflora and Eugenia florida Essential Oils. Molecules 2021, 26, 7259. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.O.; da Cruz, J.N.; de Franco, C.J.P.; Silva, S.G.; da Costa, W.A.; de Oliveira, M.S.; de Andrade, E.H.A. First Report on Yield and Chemical Composition of Essential Oil Extracted from Myrcia eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules 2020, 25, 783. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, Â.A.B.; de Jesus Pereira Franco, C.; Ferreira, O.O.; Varela, E.L.P.; do Nascimento, L.D.; Cascaes, M.M.; da Silva, D.R.P.; Percário, S.; de Oliveira, M.S.; de Aguiar Andrade, E.H. Myrcia paivae O.Berg (Myrtaceae) Essential Oil, First Study of the Chemical Composition and Antioxidant Potential. Molecules 2022, 27, 5460. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, L.M.S.; Riba, I.T.; Montanher, P.F.; Antonelo, F.A. Chemical Composition, Antioxidant and Antimicrobial Activities of Essential Oil Extracted from Myrceugenia euosma (O. Berg) D. Legrand (Myrtaceae). S. Afr. J. Bot. 2023, 159, 35–42. [Google Scholar] [CrossRef]
- Oliveira, E.S.C.; Acho, L.D.R.; Morales-Gamba, R.D.; do Rosário, A.S.; Barcellos, J.F.M.; Lima, E.S.; Machado, M.B. Hypoglycemic Effect of the Dry Leaf Extract of Myrcia multiflora in Streptozotocin-Induced Diabetic Mice. J. Ethnopharmacol. 2023, 307, 116241. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, Â.A.B.; Ferreira, O.O.; da Costa, L.S.; Almeida, L.Q.; Varela, E.L.P.; Cascaes, M.M.; de Jesus Pereira Franco, C.; Percário, S.; do Nascimento, L.D.; de Oliveira, M.S.; et al. Phytochemical Profile, Preliminary Toxicity, and Antioxidant Capacity of the Essential Oils of Myrciaria floribunda (H. West Ex Willd.) O. Berg. and Myrcia sylvatica (G. Mey) DC. (Myrtaceae). Antioxidants 2022, 11, 2076. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.C.S.; De Oliveira, J.C.S.; Da Câmara, C.A.G.; Castelar, I.; Carvalho, A.F.F.U.; Lima-Filho, J.V. Antibacterial and Cytotoxic Properties of Some Plant Crude Extracts Used in Northeastern Folk Medicine. Rev. Bras. Farmacogn. 2009, 19, 376–381. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Porte, A.; de Oliveira Godoy, R.L.; da Costa Souza, M.; Pacheco, S.; de Araujo Santiago, M.C.P.; Gouvêa, A.C.M.S.; da Silva de Mattos do Nascimento, L.; Borguini, R.G. Chemical Characterization of Myrciaria floribunda (H. West Ex Willd) Fruit. Food Chem. 2018, 248, 247–252. [Google Scholar] [CrossRef]
- Melo, C.R.; Blank, A.F.; Oliveira, B.M.S.; Santos, A.C.C.; Cristaldo, P.F.; Araújo, A.P.A.; Bacci, L. Formicidal Activity of Essential Oils of Myrcia lundiana Chemotypes on Acromyrmex balzani. Crop Prot. 2021, 139, 105343. [Google Scholar] [CrossRef]
- da Barbosa, D.C.S.; Holanda, V.N.; Ghosh, A.; Maia, R.T.; da Silva, W.V.; de Lima, V.L.M.; da Silva, M.V.; dos Santos Correia, M.T.; de Figueiredo, R.C.B.Q. Leishmanicidal and Cytotoxic Activity of Essential Oil from the Fruit Peel of Myrciaria floribunda (H. West Ex Willd.) O. Berg: Molecular Docking and Molecular Dynamics Simulations of Its Major Constituent onto Leishmania Enzyme Targets. J. Biomol. Struct. Dyn. 2022, 40, 13001–13016. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.J.; Amorim, B.S.; Lima, D.F.; Lima-Lourenço, A.R.; Nic Lughadha, E.M.; Proença, C.E.B.; Rosa, P.O.; Rosário, A.S.; Santos, L.L.; Santos, M.F.; et al. A New Infra-Generic Classification of the Species-Rich Neotropical Genus Myrcia s.l. Kew Bull. 2018, 73, 9. [Google Scholar] [CrossRef]
- Santos, M.F.; Amorim, B.S.; Burton, G.P.; Fernandes, T.; Gaem, P.H.; Lourenço, A.R.L.; Lima, D.F.; Rosa, P.O.; Santos, L.L.D.; Staggemeier, V.G.; et al. Myrcia in Flora do Brasil Research Institute Rio de Janeiro Botanical Garden. Available online: https://floradobrasil.jbrj.gov.br/FB10660 (accessed on 6 April 2024).
- Gaem, P.H.; Lucas, E.; Andrade, A.; Vicentini, A.; Mazine, F.F. A Taxonomic Account of Myrcia (Myrtaceae) at the Sites of the Biological Dynamics of Forest Fragments Project, Amazonas, Brazil. Rodriguésia 2022, 73, e00712020. [Google Scholar] [CrossRef]
- Do Rosário, A.S.; Baumgratz, J.F.A.; De Secco, R.S. Taxonomic Studies of Myrcia (Myrciinae, Myrtaceae) in Brazil: Morphological Novelties, Circumscriptions, and New Records for the Amazon. Iheringia Série Botânica 2017, 72, 165–172. [Google Scholar] [CrossRef]
- Faria, J.E.Q.; Lucas, E.J.; Sobral, M. Two New Species of Myrcia (Myrtaceae) from Brazil. Phytotaxa 2017, 319, 159–166. [Google Scholar] [CrossRef]
- Lucas, E.J.; Holst, B.; Sobral, M.; Mazine, F.F.; Nic Lughadha, E.M.; Barnes Proença, C.E.; Ribeiro da Costa, I.; Vasconcelos, T.N.C. A New Subtribal Classification of Tribe Myrteae (Myrtaceae). Syst. Bot. 2019, 44, 560–569. [Google Scholar] [CrossRef]
- Lucas, E.J.; Matsumoto, K.; Harris, S.A.; Nic Lughadha, E.M.; Benardini, B.; Chase, M.W. Phylogenetics, Morphology, and Evolution of the Large Genus Myrcia s.l. (Myrtaceae). Int. J. Plant Sci. 2011, 172, 915–934. [Google Scholar] [CrossRef]
- Vogado, N.O.; de Camargo, M.G.G.; Locosselli, G.M.; Morellato, L.P.C. Les Effets de Bord Sur La Phénologie de La Guamirim, Myrcia guianensis (Myrtaceae), Un Arbre de Cerrado. Trop. Conserv. Sci. 2016, 9, 291–312. [Google Scholar] [CrossRef]
- Scaravelli, F.S.; Gaem, P.H.; Valdemarin, K.S.; Lucas, E.; Mazine, F.F. Myrcia (Myrtaceae) in the Vale Natural Reserve, Linhares, Espírito Santo, Brazil. Rodriguésia 2022, 73, e02352020. [Google Scholar] [CrossRef]
- Gonçalves, E.G.; Lorenzini, H. Morfologia Vegetal: Organografia e Dicionário Ilustrado de Morfologia das Plantas Vasculares; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brasil, 2007; pp. 39–52. [Google Scholar]
- Pellis, V.F.; Ferreira, R.B.; Caddah, M.K. Sinopse de Myrtaceae Juss. No Monumento Natural Municipal Da Lagoa Do Peri, Florianópolis, SC, Brasil. Hoehnea 2021, 48, e202021. [Google Scholar] [CrossRef]
- do de Miranda, S.C.; De-Carvalho, P.S.; Ribon, A.A. Tópicos em Conservação e Manejo do Cerrado: Biodiversidade, Solos e Uso Sustentável; Editora Kelps: Goiânia, Brazil, 2019; ISBN 9788540026971. [Google Scholar]
- Stefanello, M.É.A.; Cervi, A.C.; Wisniewski, A.; Simionatto, E.L. Essential Oil Composition of Myrcia laruotteana Camb. J. Essent. Oil Res. 2007, 19, 466–467. [Google Scholar] [CrossRef]
- Alves, M.F.; de Castro Nizio, D.A.; Sampaio, T.S.; do Nascimento Junior, A.F.; de Andrade Brito, F.; de Melo, J.O.; de Fátima Arrigoni-Blank, M.; Gagliardi, P.R.; Machado, S.M.F. Myrcia Lundiana Kiaersk Native Populations Have Different Essential Oil Composition and Antifungal Activity against Lasiodiplodia theobromae. Ind. Crops Prod. 2016, 85, 266–273. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.C.; Souza-Silva, É.A.; Alvarenga, D.R.; Saboia, G.; Soares, G.L.G.; Zini, C.A.; Cavalleri, A.; Isaias, R.M.S. Structural and Chemical Profiles of Myrcia splendens (Myrtaceae) Leaves Under the Influence of the Galling Nexothrips Sp. (Thysanoptera). Front. Plant Sci. 2018, 9, 402567. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.D.O.T.; de Lucena, E.M.P.; Bonilla, O.H.; Edson-Chaves, B.; Freitas, M.A. Anatomia Ecológica Foliar de Myrcia guianensis (Aubl.) DC. Na Restinga Cearense. Ciência Florest. 2020, 30, 307–322. [Google Scholar] [CrossRef]
- da Silva, L.A.; Raposo, J.D.A.; Campos, L.P.G.; da Conceição, E.C.; de Oliveira, R.B.; Mourão, R.H.V. Atividade Antioxidante Do Óleo Essencial de Myrcia sylvatica (G. Mey.) DC. Por Diferentes Métodos de Análises Antioxidantes (ABTS, DPPH, FRAP, β-Caroteno/Ácido Linoleico). Rev. Fitos 2018, 12, 117–126. [Google Scholar] [CrossRef]
- Do Nascimento Silva, A.; Bomfim, H.F.; Magalhães, A.O.; Da Rocha, M.L.; Lucchese, A.M. Chemical Composition and Antinociceptive Activity of Essential Oil from Myrcia rostrata Dc. (Myrtaceae) in Animal Models. Quim. Nova 2018, 41, 982–988. [Google Scholar]
- Raposo, J.D.A.; Figueiredo, P.L.B.; Santana, R.L.; da Silva Junior, A.Q.; Suemitsu, C.; da Silva, R.; Mourão, R.H.V.; Maia, J.G.S. Seasonal and Circadian Study of the Essential Oil of Myrcia Sylvatica (G. Mey) DC., a Valuable Aromatic Species Occurring in the Lower Amazon River Region. Biochem. Syst. Ecol. 2018, 79, 21–29. [Google Scholar] [CrossRef]
- de Menezes Filho, A.C.P.; de Sousa, W.C.; de Castro, C.F.S. Composição Química, Físico-Química e Atividade Antifúngica Dos Óleos Essenciais Da Flor e Do Fruto de Myrcia guianensis (Aubl.) DC. Rev. Principia Divulg. Cient. Tecnol. IFPB 2020, 1, 92–104. [Google Scholar] [CrossRef]
- dos Santos, C.V.; Mallmann, A.P.; Toledo, A.G.; Bandeira, D.M.; da Costa, W.F.; Marins, D.M.Á.; Corrêa, J.M.; da Pinto, F.G.S. Composição Química, Atividade Antimicrobiana e Antioxidante Do Óleo Essencial de Folhas Myrcia palustris DC. (MYRTACEAE). Res. Soc. Dev. 2021, 10, e20510313303. [Google Scholar] [CrossRef]
- Cascaes, M.; Guilhon, G.; Andrade, E.; Zoghbi, M.; Santos, L. Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef] [PubMed]
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a Renewable Source of Biologically Active Compounds. Pure Appl. Chem. 2017, 89, 1105–1117. [Google Scholar] [CrossRef]
- Montalván, M.; Peñafiel, M.A.; Ramírez, J.; Cumbicus, N.; Bec, N.; Larroque, C.; Bicchi, C.; Gilardoni, G. Chemical Composition, Enantiomeric Distribution, and Sensory Evaluation of the Essential Oils Distilled from the Ecuadorian Species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) Dc. (Myrtaceae). Plants 2019, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, I.C.; Santos Frazão, G.G.; Blank, A.F.; de Aquino Santana, L.C.L. Myrcia ovata Cambessedes Essential Oils: A Proposal for a Novel Natural Antimicrobial against Foodborne Bacteria. Microb. Pathog. 2016, 99, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and Their Derivatives-Natural Anticancer Compounds: An Update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.J.; Fabri, N.T.; de Souza, A.M.; da Fonseca, N.S.T.; Furusho, A.D.S.; Miguel, O.G.; de Dias, J.F.G.; Zanin, S.M.W.; Miguel, M.D. Chemical Composition, Phytotoxic Potential, Biological Activities and Antioxidant Properties of Myrcia hatschbachii D. Legrand Essential Oil. Braz. J. Pharm. Sci. 2020, 56, e18402. [Google Scholar] [CrossRef]
- Sampaio, T.S.; de Castro Nizio, D.A.; White, L.A.S.; Melo, J.d.O.; Almeida, C.S.; Alves, M.F.; Gagliardi, P.R.; de Fátima Arrigoni-Blank, M.; Wisniewski Junior, A.; Sobral, M.E.G.; et al. Chemical Diversity of a Wild Population of Myrcia Ovata Cambessedes and Antifungal Activity against Fusarium Solani. Ind. Crops Prod. 2016, 86, 196–209. [Google Scholar] [CrossRef]
- Santana, C.B.; de Souza, J.G.L.; Coracini, M.D.A.; Walerius, A.H.; Soares, V.D.; da Costa, W.F.; da Pinto, F.G.S. Chemical Composition of Essential Oil from Myrcia oblongata DC and Potencial Antimicrobial, Antioxidant and Acaricidal Activity against Dermanyssus gallinae (Degeer, 1778). Biosci. J. 2018, 34, 996–1009. [Google Scholar] [CrossRef]
- Saccol, E.M.H.; Toni, C.; Pês, T.S.; Ourique, G.M.; Gressler, L.T.; Silva, L.V.F.; Mourão, R.H.V.; Oliveira, R.B.; Baldisserotto, B.; Pavanato, M.A. Anaesthetic and Antioxidant Effects of Myrcia sylvatica (G. Mey.) DC. and Curcuma longa L. Essential Oils on Tambaqui (Colossoma macropomum). Aquac. Res. 2017, 48, 2012–2031. [Google Scholar] [CrossRef]
- Silva, A.; Bomfim, H.; Magalhães, A.; Rocha, M.; Lucchese, A. Composição Química e Atividade Antinociceptiva em Modelo Animal do Óleo Essencial de Myrcia rostrata DC. (MYRTACEAE). Quim. Nova 2018, 41, 982–988. [Google Scholar] [CrossRef]
- Montalvão, M.M.; Felix, F.B.; Propheta dos Santos, E.W.; Santos, J.F.; de Lucca Júnior, W.; Farias, A.S.; de Souza Ribeiro, A.; Cavaleiro, C.; Machado, S.M.F.; Scher, R.; et al. Cytotoxic Activity of Essential Oil from Leaves of Myrcia splendens against A549 Lung Cancer Cells. BMC Complement. Med. Ther. 2023, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.; Sacchetti, G.; Guerrini, A. Myrcia splendens (Sw.) DC. (Syn. M. Fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile. Molecules 2017, 22, 1163. [Google Scholar] [CrossRef]
- Vasconcelos, L.C.; Carrijo, T.T.; Venancio, A.N.; Alves, T.A.; Tuler, A.C.; Hollunder, R.K.; Garbin, M.L.; Menini, L.; Praca-Fontes, M.M. Phytochemical Screening and Phytocytotoxic Effects of the Tropical Myrcia vittoriana (Myrtaceae). An. Acad. Bras. Cienc. 2022, 94, e20210820. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.A.C.; Garcia, I.P.; Corrêa, E.J.A.; de Lima, L.H.F.; de Santos, H.L.; de Assis, R.M.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Larvicidal Susceptibility of Essential Oils from Cinnamodendron dinisii, Callistemon viminalis and Myrcia tomentosa against Culex quinquefasciatus (Say) (Diptera: Culicidae). S. Afr. J. Bot. 2023, 163, 95–104. [Google Scholar] [CrossRef]
- de Menezes Filho, A.C.P.; Sousa, W.C.; de Souza Castro, C.F.; de Souza, L.F. Chemical Composition of Essential Oil from Flowers of Myrcia guianensis (Aubl.) DC. Rev. Cuba. Plantas Med. 2019, 24, 1–12. [Google Scholar]
- Amorim Gomes, G.; Martins-Cardoso, K.; dos Santos, F.R.; Florencio, M.; Rosa, D.; Araujo Zuma, A.; Pinheiro Santiago, G.M.; Motta, M.C.M.; de Carvalho, M.G.; Fampa, P. Antileishmanial Activity of the Essential Oils of Myrcia ovata Cambess. and Eremanthus erythropappus (DC) McLeisch Leads to Parasite Mitochondrial Damage. Nat. Prod. Res. 2021, 35, 6117–6121. [Google Scholar] [CrossRef]
- Silva, L.; Sarrazin, S.; Oliveira, R.; Suemitsu, C.; Maia, J.; Mourão, R. Composition and Antimicrobial Activity of Leaf Essential Oils of Myrcia sylvatica (G. Mey.) DC. Eur. J. Med. Plants 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Rosa, C.S.; Veras, K.S.; Silva, P.R.; Lopes Neto, J.J.; Cardoso, H.L.M.; Alves, L.P.L.; Brito, M.C.A.; Amaral, F.M.M.; Maia, J.G.S.; Monteiro, O.S.; et al. Composição Química e Toxicidade Frente Aedes aegypti L. e Artemia salina Leach Do Óleo Essencial Das Folhas de Myrcia sylvatica (G. Mey.) DC. Rev. Bras. Plantas Med. 2016, 18, 19–26. [Google Scholar] [CrossRef]
- Santana, C.B.; Souza, J.G.L.; Toledo, A.G.; Alves, L.F.A.; Alves, D.S.; Corrêa, J.M.; Pinto, F.G.S. Antimicrobial and Insecticidal Effects of Essential Oil and Plant Extracts of Myrcia Oblongata DC in Pathogenic Bacteria and Alphitobius diaperinus. Braz. J. Biol. 2022, 82, e233425. [Google Scholar] [CrossRef] [PubMed]
- Antonelo, F.A.; Rodrigues, M.S.; Cruz, L.C.; Pagnoncelli, M.G.; da Cunha, M.A.A.; Bonatto, S.J.R.; Busso, C.; Wagner Júnior, A.; Montanher, P.F. Bioactive Compounds Derived from Brazilian Myrtaceae Species: Chemical Composition and Antioxidant, Antimicrobial and Cytotoxic Activities. Biocatal. Agric. Biotechnol. 2023, 48, 102629. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; da Costa, J.S.; Pinto, L.C.; Montenegro, R.C.; Setzer, W.N.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Antioxidant and Cytotoxic Activities of Myrtaceae Essential Oils Rich in Terpenoids From Brazil. Nat. Prod. Commun. 2021, 16, 1934578X21996156. [Google Scholar] [CrossRef]
- Ferreira Macedo, J.G.; Linhares Rangel, J.M.; de Oliveira Santos, M.; Camilo, C.J.; Martins da Costa, J.G.; Maria de Almeida Souza, M. Therapeutic Indications, Chemical Composition and Biological Activity of Native Brazilian Species from Psidium genus (Myrtaceae): A Review. J. Ethnopharmacol. 2021, 278, 114248. [Google Scholar] [CrossRef] [PubMed]
- Anjos da Silva, L.; Santos da Silva, R.; Rodrigues de Oliveira, M.; Guimarães, A.C.; Takeara, R. Chemical Composition and Biological Activities of Essential Oils from Myrtaceae Species Growing in Amazon: An Updated Review. J. Essent. Oil Res. 2023, 35, 103–116. [Google Scholar] [CrossRef]
- Cândido, C.S.; Portella, C.S.A.; Laranjeira, B.J.; da Silva, S.S.; Arriaga, A.M.C.; Santiago, G.M.P.; Gomes, G.A.; Almeida, P.C.; Carvalho, C.B.M. Effects of Myrcia ovata Cambess. Essential Oil on Planktonic Growth of Gastrointestinal Microorganisms and Biofilm Formation of Enterococcus Faecalis. Braz. J. Microbiol. 2010, 41, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in Foods by Using Essential Oils: A Review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cheng, Z. Research Progress on the Use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tan, J.; Yu, Y.; Dong, J.; Cao, L.; Yao, L.; Zhang, Y.; Yan, Z. Phytotoxic Effects and Potential Allelochemicals from Water Extracts of Paulownia tomentosa Flower Litter. Agronomy 2024, 14, 367. [Google Scholar] [CrossRef]
- Souza Filho, A.P.S.; Santos, R.A.; Santos, L.S.; Guilhon, G.M.P.; Santos, A.S.; Arruda, M.S.P.; Muller, A.H.; Arruda, A.C. Potencial Alelopático de Myrcia guianensis. Planta Daninha 2006, 24, 649–656. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M. Indirect Plant Defense against Insect Herbivores: A Review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.; Chomistek, N.; Dayment, H.; Goerzen, W.; Baines, D. Insecticidal Activity of Plant Powders against the Parasitoid, Pteromalus Venustus, and Its Host, the Alfalfa Leafcutting Bee. Insects 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.M.; Serrão, J.E.; dos Santos, M.M.; da Silva, R.S.; de Carvalho, A.G.; Pires, E.M.; Zanuncio, J.C.; Soares, M.A. Insecticidal Plants as Trade Opportunities and Use by Small Vegetable Producers: An Example Using Essential Oils to Control Diaphania hyalinata (Lepidoptera: Crambidae). An. Acad. Bras. Cienc. 2023, 95, e20191305. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.F.M.; Matos, A.P.; Volante, A.C.; Cunha, G.O.S.; Gualtieri, S.C.J. Insecticidal Activity from Leaves and Sesquiterpene Lactones of Tithonia diversifolia (Helms.) A. Gray (Asteraceae) on Spodoptera frugiperda (Lepidoptera: Noctuidae). S. Afr. J. Bot. 2022, 144, 377–379. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Rakotosaona, R.; Nzekoue, F.K.; Canale, A.; Nicoletti, M.; Maggi, F. Insecticidal and Mosquito Repellent Efficacy of the Essential Oils from Stem Bark and Wood of Hazomalania voyronii. J. Ethnopharmacol. 2020, 248, 112333. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, J.; Ma, S.; Gao, L.; Chen, C.; Ji, Z.; Hu, Z.; Shi, B.; Wu, W. Identification and Mechanism of Insecticidal Periplocosides from the Root Bark of Periploca sepium Bunge. Pest Manag. Sci. 2021, 77, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Kerdudo, A.; Ellong, E.N.; Burger, P.; Gonnot, V.; Boyer, L.; Chandre, F.; Adenet, S.; Rochefort, K.; Michel, T.; Fernandez, X. Chemical Composition, Antimicrobial and Insecticidal Activities of Flowers Essential Oils of Alpinia zerumbet (Pers.) B.L.Burtt & R.M.Sm. from Martinique Island. Chem. Biodivers. 2017, 14, e1600344. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Maggi, F.; Nkuimi Wandjou, J.G.; Yvette Fofie, N.G.B.; Koné-Bamba, D.; Sagratini, G.; Vittori, S.; Caprioli, G. Insecticidal Activity of the Essential Oil and Polar Extracts from Ocimum gratissimum Grown in Ivory Coast: Efficacy on Insect Pests and Vectors and Impact on Non-Target Species. Ind. Crops Prod. 2019, 132, 377–385. [Google Scholar] [CrossRef]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal Activity of Essential Oil from Cephalotaxus sinensis and Its Main Components against Various Agricultural Pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Guldbrandsen, N. Screening of Panamanian Plant Extracts for Pesticidal Properties, and HPLC-Based Identification of Active Compounds. Sci. Pharm. 2015, 83, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.L.; Mello, T.R.B.; Sousa, J.P.B.; Albernaz, L.C.; Magalhães, N.M.G.; Morais, L.S.; Francisco, L.R.; Leal, W.S.; Espindola, L.S. Brazilian Cerrado Biome Essential Oils to Control the Arbovirus Vectors Aedes aegypti and Culex quinquefasciatus. Ind. Crops Prod. 2022, 178, 114568. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and Incidence of Type 1 Diabetes in the World: A Systematic Review and Meta-Analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.G.; Vesa, C.M.; Bustea, C.; Purza, A.L.; Tit, D.M.; Brisc, M.C.; Radu, A.-F. Antioxidant and Hypoglycemic Potential of Essential Oils in Diabetes Mellitus and Its Complications. Int. J. Mol. Sci. 2023, 24, 16501. [Google Scholar] [CrossRef] [PubMed]
- Vareda, P.M.P.; Saldanha, L.L.; de Camaforte, N.A.P.; Violato, N.M.; Dokkedal, A.L.; Bosqueiro, J.R. Myrcia bella Leaf Extract Presents Hypoglycemic Activity via PI3k/Akt Insulin Signaling Pathway. Evid. Based Complement. Altern. Med. 2014, 2014, 543606. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Lemos Lima, R.; Kato, L.; Kongstad, K.T.; Staerk, D. Brazilian Insulin Plant as a Bifunctional Food: Dual High-Resolution PTP1B and α-Glucosidase Inhibition Profiling Combined with HPLC-HRMS-SPE-NMR for Identification of Antidiabetic Compounds in Myrcia rubella Cambess. J. Funct. Foods 2018, 45, 444–451. [Google Scholar] [CrossRef]
- da Costa, J.S.; da Freitas, J.J.S.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Variability in the Chemical Composition of Myrcia sylvatica (G. Mey) DC. Essential Oils Growing in the Brazilian Amazon. Molecules 2022, 27, 8975. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, N.; Melo, J.C.S.; Batista, L.F.; Paula-Souza, J.; Fronza, P.; Brandão, M.G.L. Edible Fruits from Brazilian Biodiversity: A Review on Their Sensorial Characteristics versus Bioactivity as Tool to Select Research. Food Res. Int. 2019, 119, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Nazareno, L.S.Q.; de Miranda, M.R.A.; Pinto, M.K.N.A.; Lopes, M.M.A.; Rufino, M.D.S.M. Non-Enzymatic and Enzymatic Antioxidant Components of the Mature Cambuí Metabolism. Acta Agron. 2019, 68, 16–21. [Google Scholar] [CrossRef]
- Matsuda, H.; Nishida, N.; Yoshikawa, M. Antidiabetic Principles of Natural Medicines. V. Aldose Reductase Inhibitors from Myrcia multiflora DC. (2): Structures of Myrciacitrins III, IV, and V. Chem. Pharm. Bull. 2002, 50, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Serpeloni, J.M.; Leal Specian, A.F.; Ribeiro, D.L.; Tuttis, K.; Vilegas, W.; Martínez-López, W.; Dokkedal, A.L.; Saldanha, L.L.; de Syllos Cólus, I.M.; Varanda, E.A. Antimutagenicity and Induction of Antioxidant Defense by Flavonoid Rich Extract of Myrcia bella Cambess. in Normal and Tumor Gastric Cells. J. Ethnopharmacol. 2015, 176, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Ejiofor, E.; Oyedemi, S.; Egedigwe-Ekeleme, C.; Obike, C.; Aguwamba, C.; Nweje-Anyalowu, P.; Ejiofor, M.; Okwuonu, O. Essential Oil Components with Antidiabetic and Anti-Obesity Properties: A Review of Mechanisms of Action and Toxicity. J. Essent. Oil Res. 2023, 35, 335–371. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Y. Antidiabetic Effects of Nerolidol through Promoting Insulin Receptor Signaling in High-Fat Diet and Low Dose Streptozotocin-Induced Type 2 Diabetic Rats. Hum. Exp. Toxicol. 2022, 41, 09603271221126487. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.H.; Sankaranarayanan, C. A-Caryophyllene, a Natural Sesquiterpene, Modulates Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Acta Histochem. 2014, 116, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, L.; Vilegas, W.; Dokkedal, A. Characterization of Flavonoids and Phenolic Acids in Myrcia bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS Combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, V.S.; de Pessoa, A.S.; Tokuhara, C.K.; Pagnan, A.L.; de Oliveira, G.S.N.; Liessa, M.R.S.; Inacio, K.K.; de Melo, F.P.d.S.R.; Dokkedal, A.L.; de Oliveira, R.C.; et al. Evaluation of Myrcia bella in Murine Osteosarcoma Cells: Effect of the Extract and Enriched Fractions of Tannins and Flavonoids. Nat. Prod. Res. 2022, 36, 5823–5827. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.; de Melo, N.C.; Ruiz, A.L.T.G.; Floglio, M.A. Antiproliferative Activity from Five Myrtaceae Essential Oils. Res. Soc. Dev. 2023, 12, e14612340536. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Riva, D.; Simionatto, E.L.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Salvador, M.J. Chemical Composition and Cytotoxic Activity of Essential Oil from Myrcia laruotteana Fruits. J. Essent. Oil Res. 2011, 23, 7–10. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and Biological Effects of Alpha-Bisabolol: An Updated Review of the Molecular Mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.S.; Guimarães, A.G.; Santana, M.T.; Siqueira, R.S.; Passos, L.O.; Machado, S.M.F.; de Ribeiro, A.S.; Sobral, M.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Phytochemical Screening, Antinociceptive and Anti-Inflammatory Effects of the Essential Oil of Myrcia pubiflora in Mice. Rev. Bras. Farmacogn. 2012, 22, 181–188. [Google Scholar] [CrossRef]
- dos Santos, R.H.G.; de Moura Silva, M.H.; de Oliveira Farias de Aguiar, J.C.R.; do Amaral Ferraz Navarro, D.M.; de Oliveira, A.F.M.; dos Santos Correia, M.T. Chemical Composition of the Essential Oil of Myrcia Loranthifolia (Myrtaceae) Leaves Grown in Atlantic Forest and Dry Forest of Brazil. Braz. J. Bot. 2023, 46, 845–852. [Google Scholar] [CrossRef]
- Seethalakshmi, S.; Sarumathi, R.; Sankaranarayanan, C. Effect of Alpha-Phellandrene on Glucose Uptake and Adipogenesis in Insulin Resistant 3T3-L1 Adipocytes: An in Vitro and in Silico Approach. Asian J. Biol. Life Sci. 2023, 12, 603. [Google Scholar] [CrossRef]
- Sheeba, S.; Nahida, T. Effect of Some Naturally Occurring Monoterpenes Viz D-Limonene, p-Cymene and Terpinolene on the Glycemic and Hepatic Function in a Rat Model of Type 2 Diabetes Mellitus. Pharmacogn. Res. 2022, 14, 446–453. [Google Scholar]
- Panigrahy, S.K.; Bhatt, R.; Kumar, A. Targeting Type II Diabetes with Plant Terpenes: The New and Promising Antidiabetic Therapeutics. Biologia 2021, 76, 241–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).