Stationary External Electric Field—Mimicking the Solvent Effect on the Ground-State Tautomerism and Excited-State Proton Transfer in 8-(Benzo[d]thiazol-2-yl)quinolin-7-ol
Abstract
:1. Introduction
2. Results and Discussion
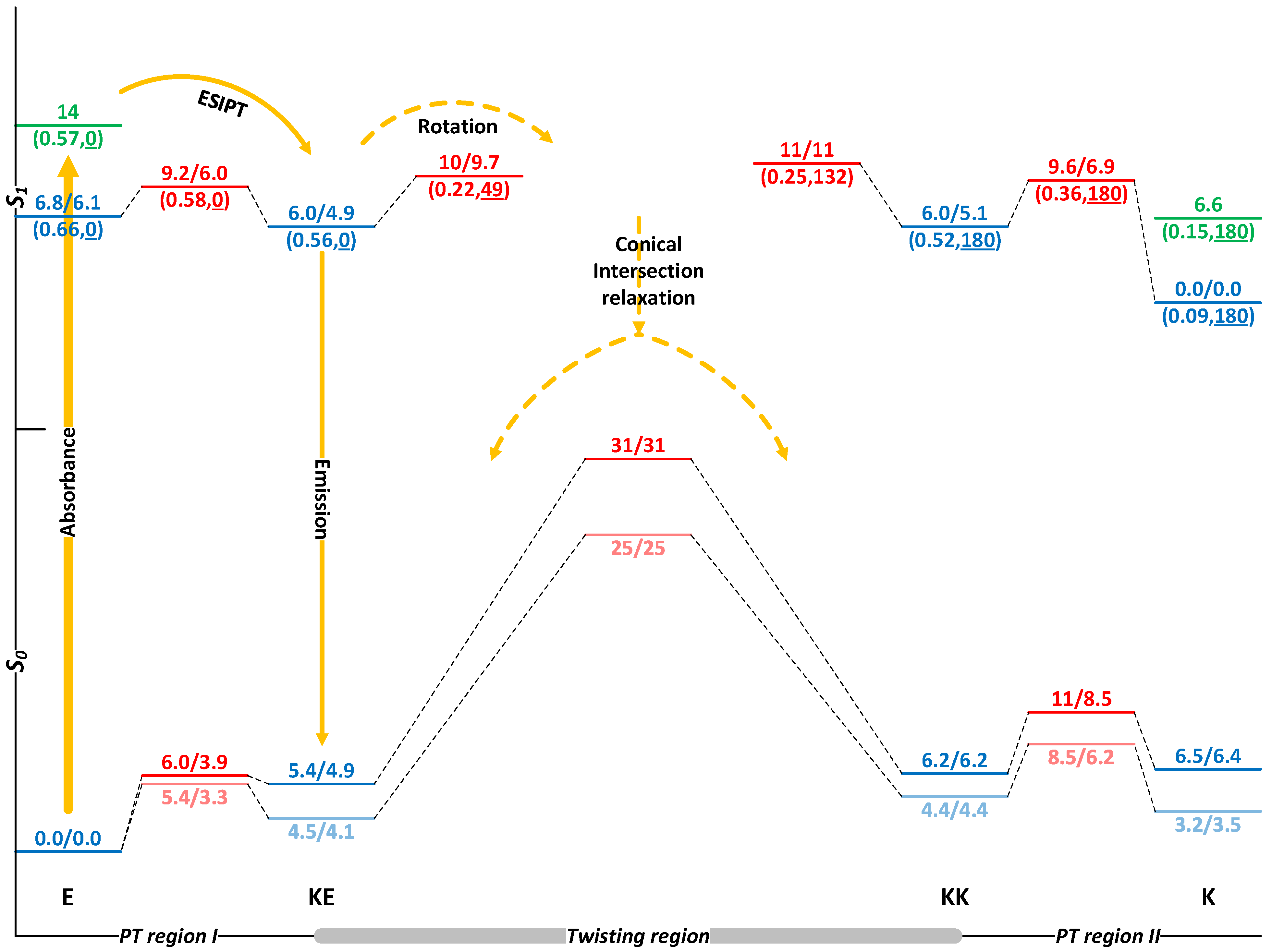
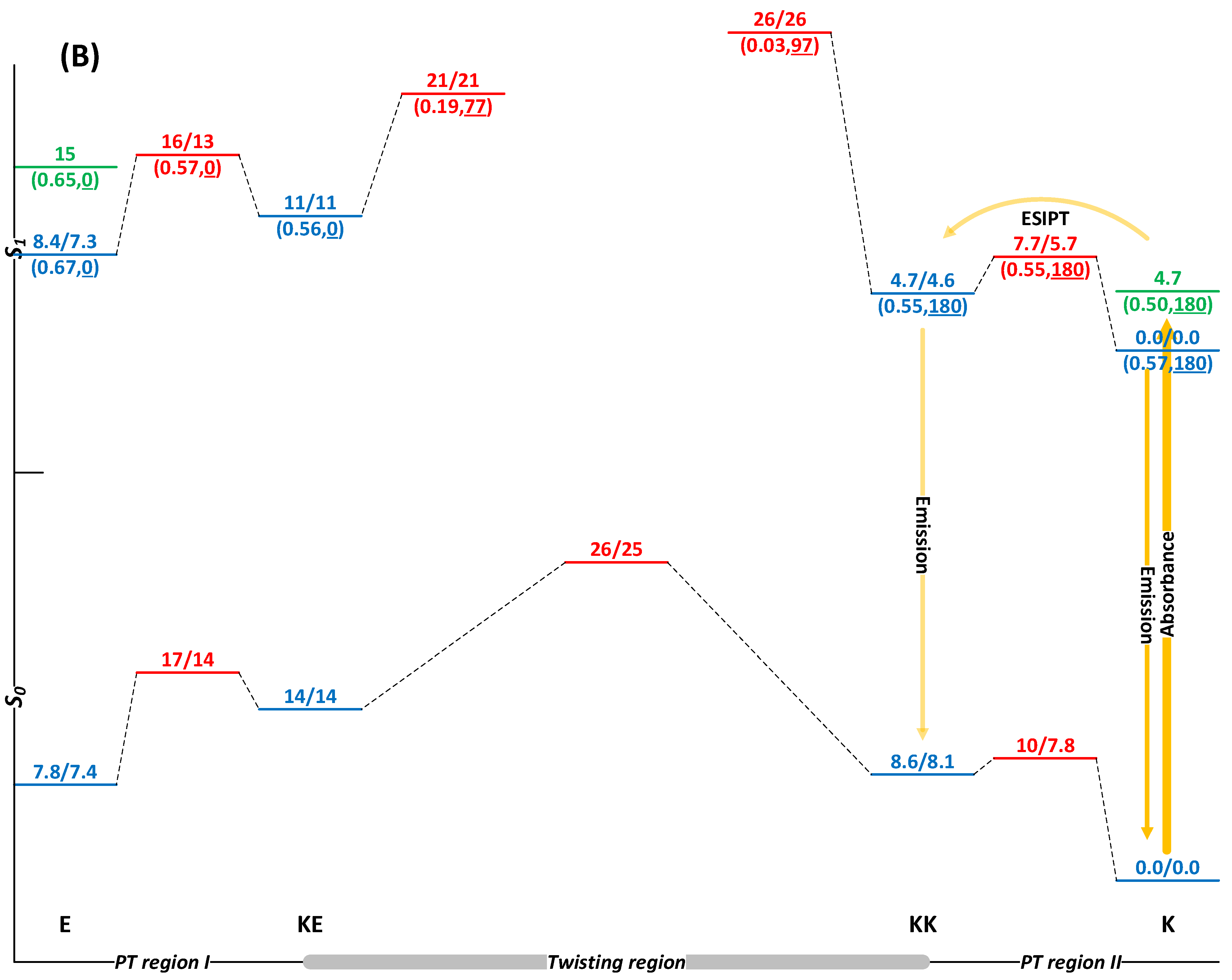
2.1. Switching of HQBT in the Absence of EEF
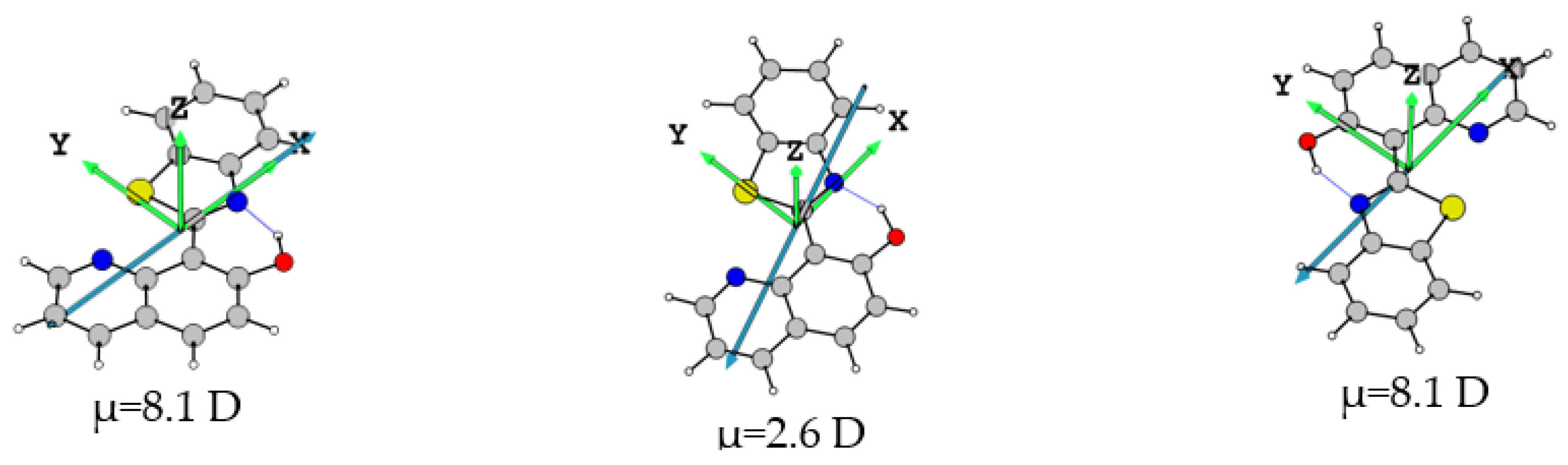
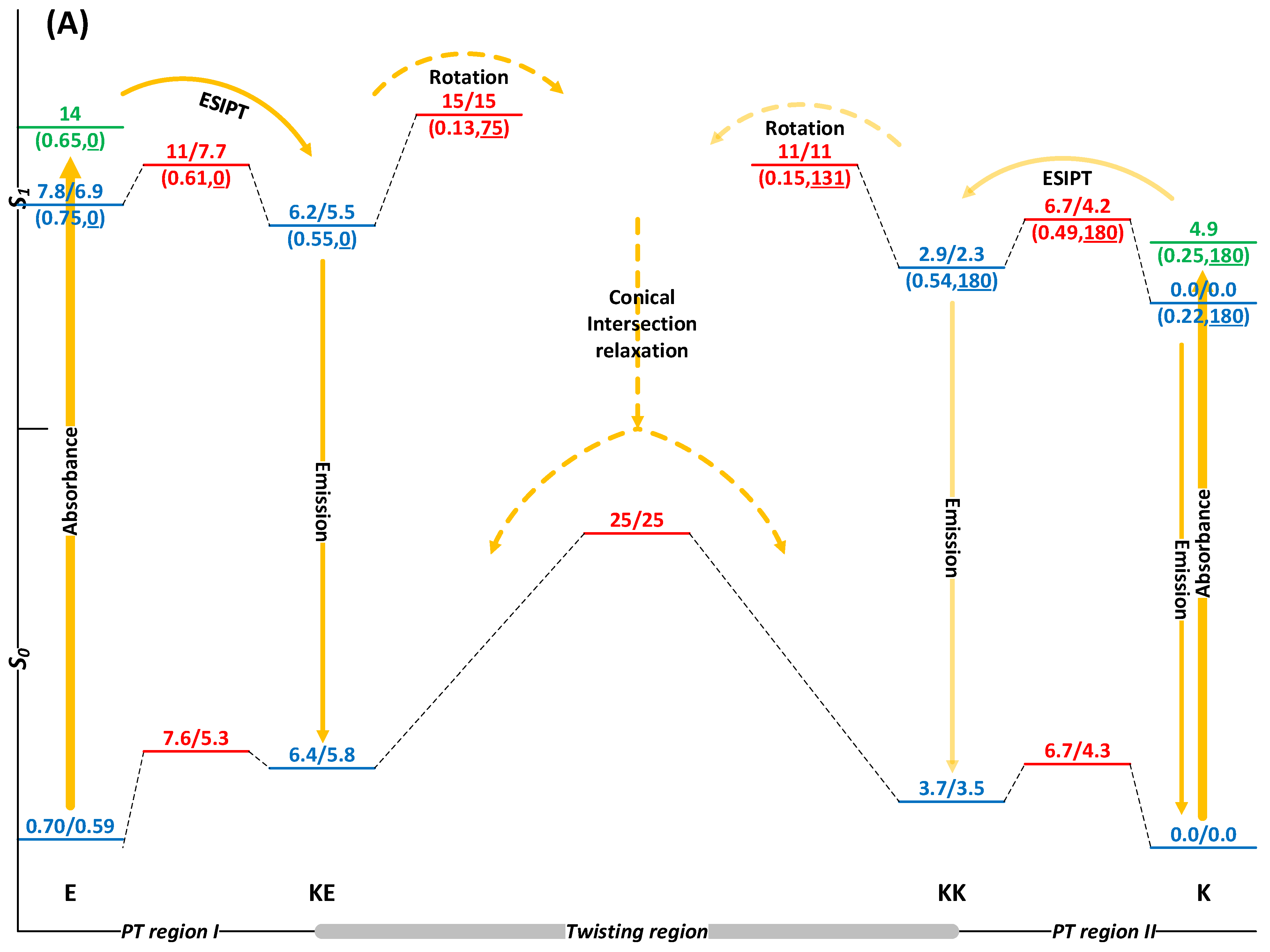
2.2. The Effect of EEF in Solution
2.3. Theoretical Methodology
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, P.J.; van der Zwan, G.; Antonov, L. Tautomerism: Introduction, History, and Recent Developments in Experimental and Theoretical Methods. In Tautomerism: Methods and Theories; Antonov, L., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 1–24. ISBN 978-3-527-65882-4. [Google Scholar]
- Claisen, L. Beitrage Zur Kenntniss Der 1,3-Diketone Und Verwandter Verbindungen. Annalen 1896, 291, 25–137. [Google Scholar] [CrossRef]
- Antonov, L. Absorption UV-Vis Spectroscopy and Chemometrics: From Qualitative Conclusions to Quantitative Analysis. In Tautomerism: Methods and Theories; Antonov, L., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 25–47. ISBN 978-3-527-65882-4. [Google Scholar]
- Powling, J.; Bernstein, H.J. The Effect of Solvents on Tautomeric Equilibria. J. Am. Chem. Soc. 1951, 73, 4353–4356. [Google Scholar] [CrossRef]
- Mitsuishi, M.; Kamimura, R.; Ieda, M.; Shinohara, K.; Ishii, N. Tautomerism of 4-phenylazo-1-naphthol in Organic Solvent-water Mixtures. Sen-i Gakkaishi 1976, 32, T382–T388. [Google Scholar] [CrossRef]
- Spencer, J.N.; Holmboe, E.S.; Kirshenbaum, M.R.; Firth, D.W.; Pinto, P.B. Solvent Effects on the Tautomeric Equilibrium of 2,4-Pentanedione. Can. J. Chem. 1982, 60, 1178–1182. [Google Scholar] [CrossRef]
- Ishida, T.; Hirata, F.; Kato, S. Thermodynamic Analysis of the Solvent Effect on Tautomerization of Acetylacetone: An Ab Initio Approach. J. Chem. Phys. 1999, 110, 3938–3945. [Google Scholar] [CrossRef]
- Antonov, L.; Kawauchi, S.; Satoh, M.; Komiyama, J. Ab Initio Modeling of the Solvent Influence on the Azo-Hydrazone Tautomerism. Dyes Pigm. 1999, 40, 163–170. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; ISBN 978-3-527-63222-0. [Google Scholar]
- Angelov, I.; Zaharieva, L.; Antonov, L. Effects and Influence of External Electric Fields on the Equilibrium Properties of Tautomeric Molecules. Molecules 2023, 28, 695. [Google Scholar] [CrossRef] [PubMed]
- Enchev, V.; Monev, V.; Markova, N.; Rogozherov, M.; Angelova, S.; Spassova, M. A Model System with Intramolecular Hydrogen Bonding: Effect of External Electric Field on the Tautomeric Conversion and Electronic Structures. Comput. Theor. Chem. 2013, 1006, 113–122. [Google Scholar] [CrossRef]
- Enchev, V.; Markova, N. Effect of External Electric Field on the Tautomeric Equilibrium and Structure of 2-carbamido-1,3-indandione. Int. J. Quantum Chem. 2021, 121, e26760. [Google Scholar] [CrossRef]
- Jankowska, J.; Sadlej, J.; Sobolewski, A.L. Electric Field Control of Proton-Transfer Molecular Switching: Molecular Dynamics Study on Salicylidene Aniline. Phys. Chem. Chem. Phys. 2015, 17, 14484–14488. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Su, Q.; Wang, B.; Guo, N.; Liu, F. Tuning of Energetics and Reaction Mechanism of Water-Assisted Intramolecular Proton Transfer of 7-Azaindole by External Electric Field Applied in Various Directions: A TD-DFT Study. Theor. Chem. Acc. 2017, 136, 27. [Google Scholar] [CrossRef]
- Arabi, A.A.; Matta, C.F. Effects of Intense Electric Fields on the Double Proton Transfer in the Watson–Crick Guanine–Cytosine Base Pair. J. Phys. Chem. B 2018, 122, 8631–8641. [Google Scholar] [CrossRef]
- Gheorghiu, A.; Coveney, P.V.; Arabi, A.A. The Influence of External Electric Fields on Proton Transfer Tautomerism in the Guanine–Cytosine Base Pair. Phys. Chem. Chem. Phys. 2021, 23, 6252–6265. [Google Scholar] [CrossRef] [PubMed]
- Rehhagen, C.; Argüello Cordero, M.A.; Kamounah, F.S.; Deneva, V.; Angelov, I.; Krupp, M.; Svenningsen, S.W.; Pittelkow, M.; Lochbrunner, S.; Antonov, L. Reversible Switching Based on Truly Intramolecular Long-Range Proton Transfer–Turning the Theoretical Concept into Experimental Reality. J. Am. Chem. Soc. 2024, 146, 2043–2053. [Google Scholar] [CrossRef]
- Dutta Dubey, K.; Stuyver, T.; Kalita, S.; Shaik, S. Solvent Organization and Rate Regulation of a Menshutkin Reaction by Oriented External Electric Fields Are Revealed by Combined MD and QM/MM Calculations. J. Am. Chem. Soc. 2020, 142, 9955–9965. [Google Scholar] [CrossRef]
- Ciampi, S.; Darwish, N.; Aitken, H.M.; Díez-Pérez, I.; Coote, M.L. Harnessing Electrostatic Catalysis in Single Molecule, Electrochemical and Chemical Systems: A Rapidly Growing Experimental Tool Box. Chem. Soc. Rev. 2018, 47, 5146–5164. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, F.; Cao, D. A Dynamic and Electrostatic Potential Prediction of the Prototropic Tautomerism between Imidazole 3-Oxide and 1-Hydroxyimidazole in External Electric Field. J. Mol. Model. 2019, 25, 330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, Y. Elaboration and Controlling Excited State Double Proton Transfer Mechanism of 2,5-Bis(Benzoxazol-2-Yl)Thiophene-3,4-Diol. Sci. Rep. 2017, 7, 44897. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Peng, C.; Ayala, P.Y.; Schlegel, H.B.; Frisch, M.J. Using Redundant Internal Coordinates to Optimize Equilibrium Geometries and Transition States. J. Comput. Chem. 1996, 17, 49–56. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, S.; Antonov, L. Description of the Tautomerism in Some Azonaphthols. J. Phys. Org. Chem. 2013, 26, 643–652. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K. A Comparative Examination of Density Functional Performance against the ISOL24/11 Isomerization Energy Benchmark. Comput. Theor. Chem. 2016, 1090, 147–152. [Google Scholar] [CrossRef]
- Antonov, L. Tautomerism in Azo and Azomethyne Dyes: When and If Theory Meets Experiment. Molecules 2019, 24, 2252. [Google Scholar] [CrossRef] [PubMed]
- Deneva, V.; Vassilev, N.G.; Hristova, S.; Yordanov, D.; Hayashi, Y.; Kawauchi, S.; Fennel, F.; Völzer, T.; Lochbrunner, S.; Antonov, L. Chercher de l’eau: The Switching Mechanism of the Rotary Switch Ethyl-2-(2-(Quinolin-8-Yl)Hydrazono)-2-(Pyridin-2-Yl)Acetate. Comput. Mater. Sci. 2020, 177, 109570. [Google Scholar] [CrossRef]
- Georgiev, A.; Yordanov, D.; Ivanova, N.; Deneva, V.; Vassilev, N.; Kamounah, F.S.; Pittelkow, M.; Crochet, A.; Fromm, K.M.; Antonov, L. 7-OH Quinoline Schiff Bases: Are They the Long Awaited Tautomeric Bistable Switches? Dyes Pigm. 2021, 195, 109739. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of Electronic Excitations within the Adiabatic Approximation of Time Dependent Density Functional Theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Improta, R. UV-Visible Absorption and Emission Energies in Condensed Phase by PCM/TD-DFT Methods. In Computational Strategies for Spectroscopy; Barone, V., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 37–75. ISBN 978-1-118-00872-0. [Google Scholar]
- Liu, J.; Liang, W. Analytical Approach for the Excited-State Hessian in Time-Dependent Density Functional Theory: Formalism, Implementation, and Performance. J. Chem. Phys. 2011, 135, 184111. [Google Scholar] [CrossRef]
- Guo, Y.; Riplinger, C.; Becker, U.; Liakos, D.G.; Minenkov, Y.; Cavallo, L.; Neese, F. Communication: An Improved Linear Scaling Perturbative Triples Correction for the Domain Based Local Pair-Natural Orbital Based Singles and Doubles Coupled Cluster Method [DLPNO-CCSD(T)]. J. Chem. Phys. 2018, 148, 011101. [Google Scholar] [CrossRef] [PubMed]
- Berraud-Pache, R.; Neese, F.; Bistoni, G.; Izsák, R. Unveiling the Photophysical Properties of Boron-Dipyrromethene Dyes Using a New Accurate Excited State Coupled Cluster Method. J. Chem. Theory Comput. 2020, 16, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Antonov, L.; Kawauchi, S.; Okuno, Y. Prediction of the Color of Dyes by Using Time-Dependent Density Functional Theory. Bulg. Chem. Commun. 2014, 46, 228–237. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Nedeltcheva-Antonova, D.; Antonov, L. Ground-State Tautomerism and Excited-State Proton Transfer in 7-Hydroxy-4-Methyl-8-((Phenylimino)Methyl)-2H-Chromen-2-One as a Potential Proton Crane. Physchem 2024, 4, 91–105. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharieva, L.; Angelov, I.; Antonov, L. Stationary External Electric Field—Mimicking the Solvent Effect on the Ground-State Tautomerism and Excited-State Proton Transfer in 8-(Benzo[d]thiazol-2-yl)quinolin-7-ol. Molecules 2024, 29, 3506. https://doi.org/10.3390/molecules29153506
Zaharieva L, Angelov I, Antonov L. Stationary External Electric Field—Mimicking the Solvent Effect on the Ground-State Tautomerism and Excited-State Proton Transfer in 8-(Benzo[d]thiazol-2-yl)quinolin-7-ol. Molecules. 2024; 29(15):3506. https://doi.org/10.3390/molecules29153506
Chicago/Turabian StyleZaharieva, Lidia, Ivan Angelov, and Liudmil Antonov. 2024. "Stationary External Electric Field—Mimicking the Solvent Effect on the Ground-State Tautomerism and Excited-State Proton Transfer in 8-(Benzo[d]thiazol-2-yl)quinolin-7-ol" Molecules 29, no. 15: 3506. https://doi.org/10.3390/molecules29153506
APA StyleZaharieva, L., Angelov, I., & Antonov, L. (2024). Stationary External Electric Field—Mimicking the Solvent Effect on the Ground-State Tautomerism and Excited-State Proton Transfer in 8-(Benzo[d]thiazol-2-yl)quinolin-7-ol. Molecules, 29(15), 3506. https://doi.org/10.3390/molecules29153506







