N-(2-Hydroxyphenyl)-2-Propylpentanamide (HO-AAVPA) Induces Apoptosis and Cell Cycle Arrest in Breast Cancer Cells, Decreasing GPER Expression
Abstract
:1. Introduction
2. Results
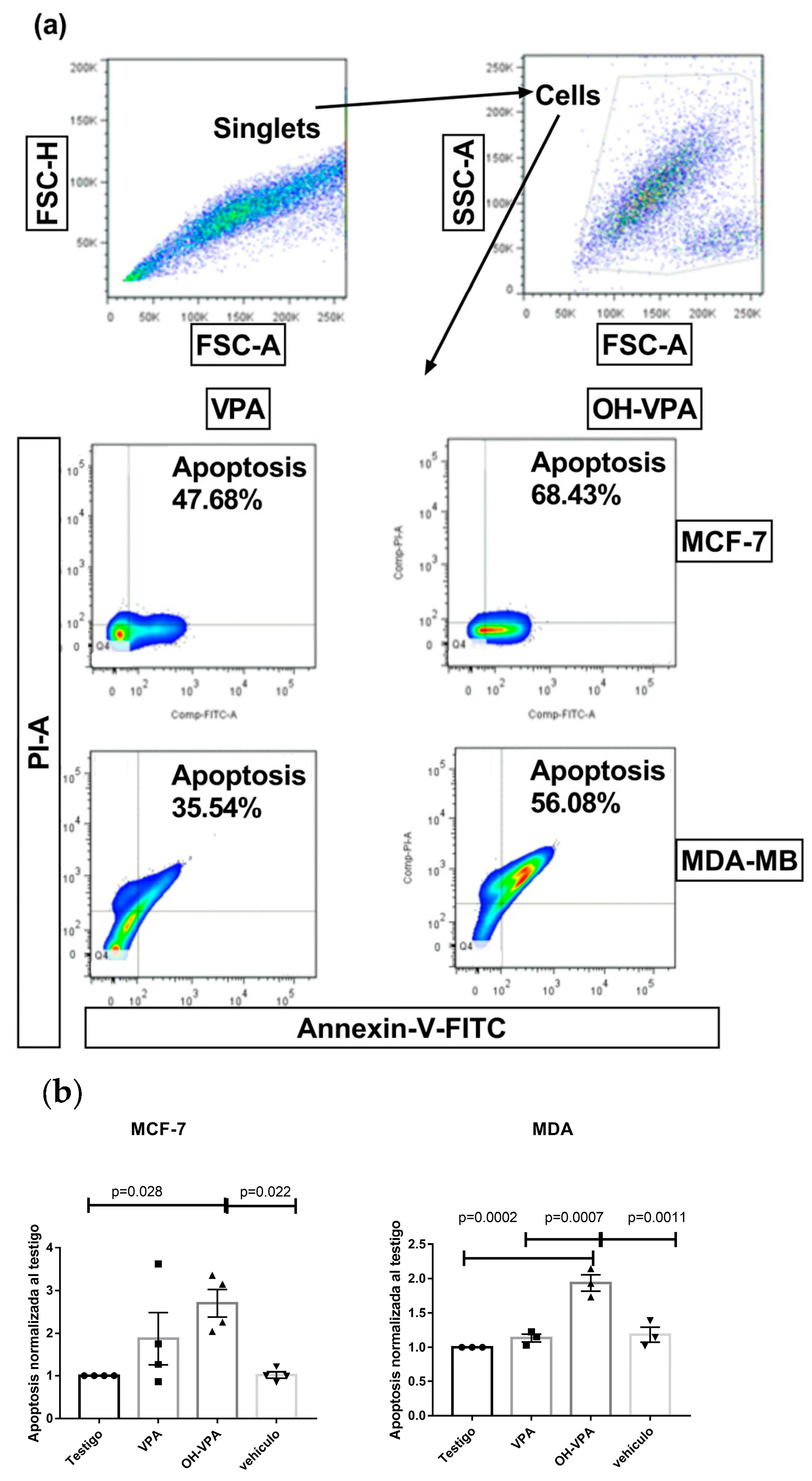
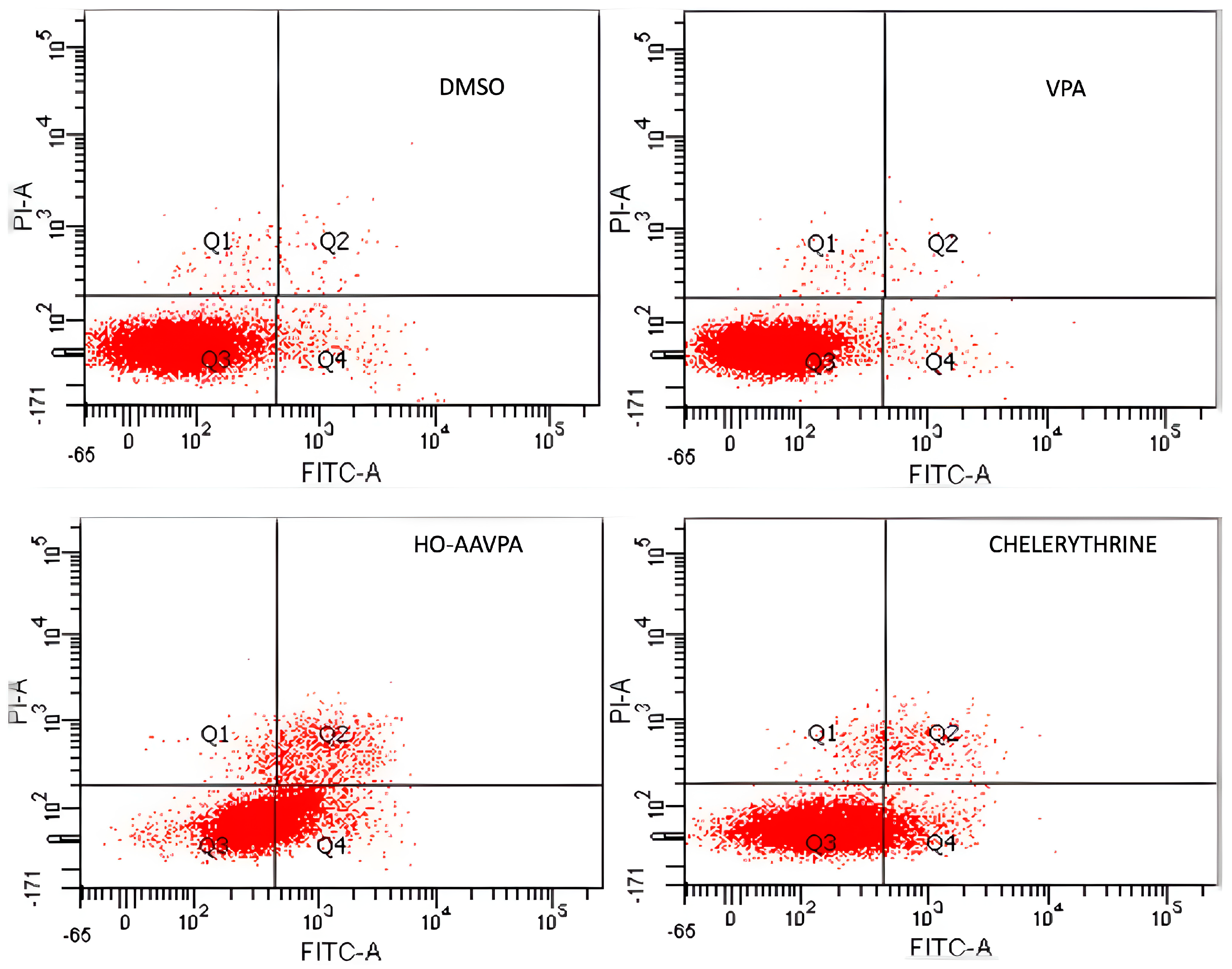
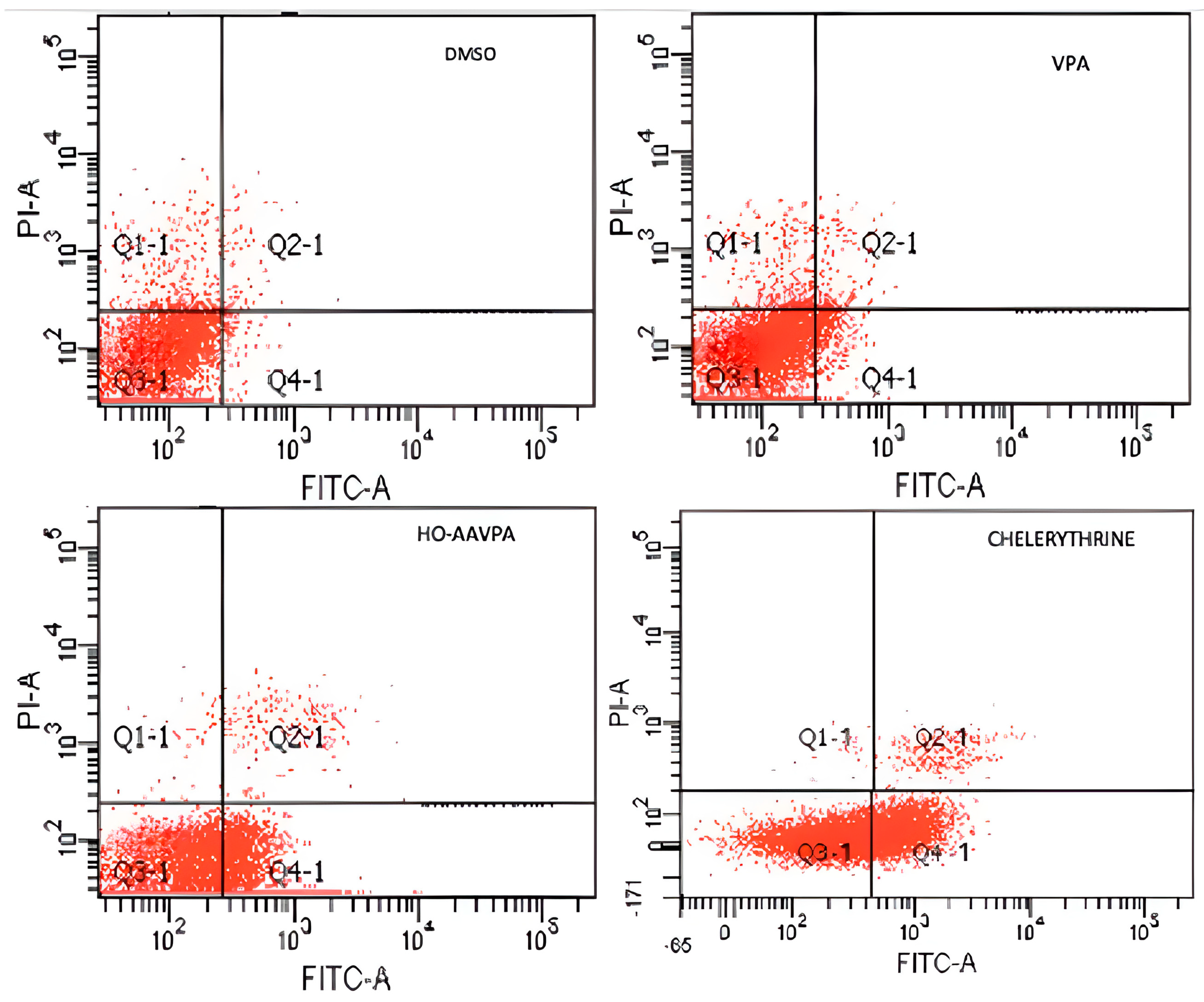
2.1. HO-AAVPA Induced Apoptosis in Breast Cancer Cell Lines
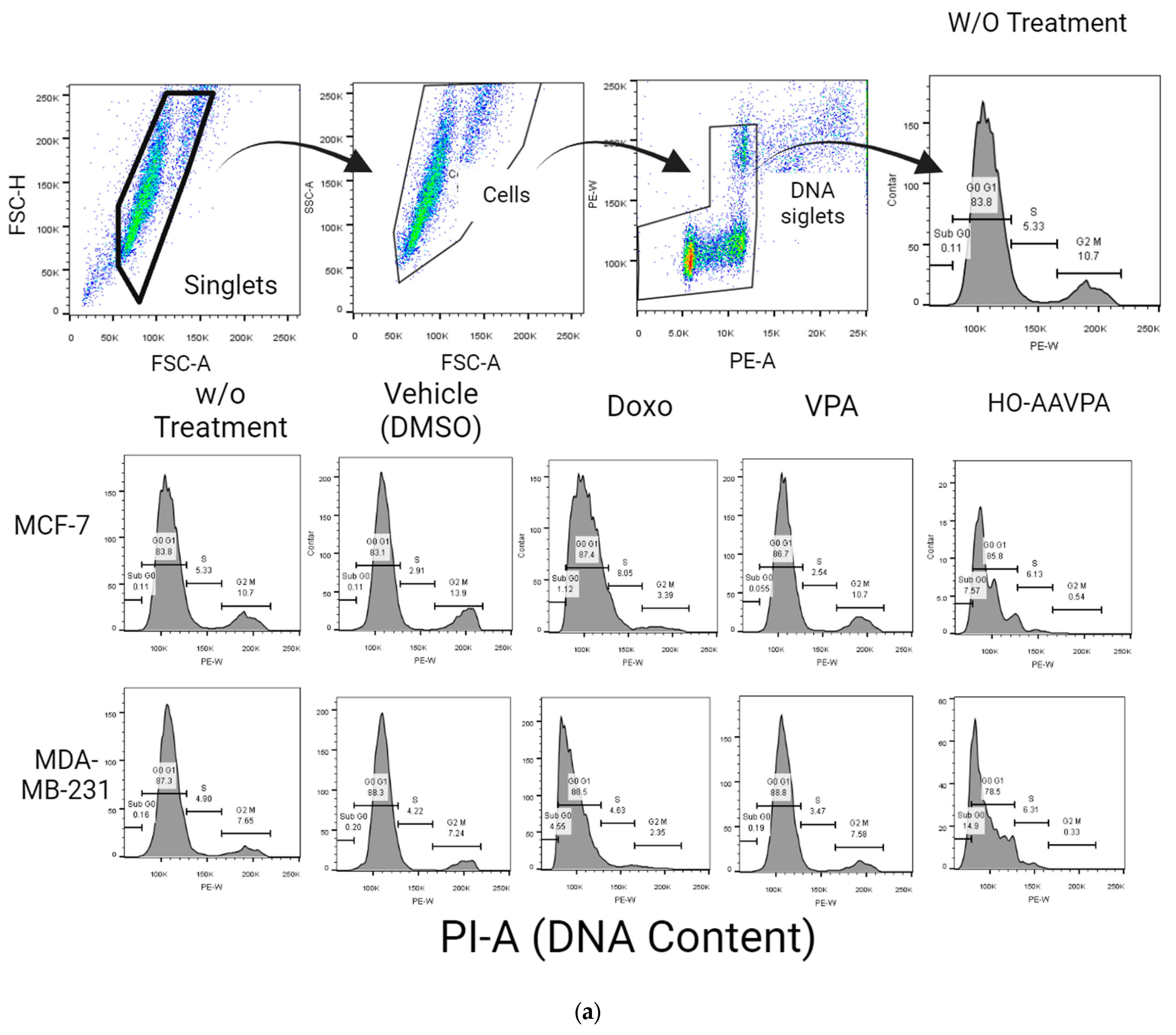
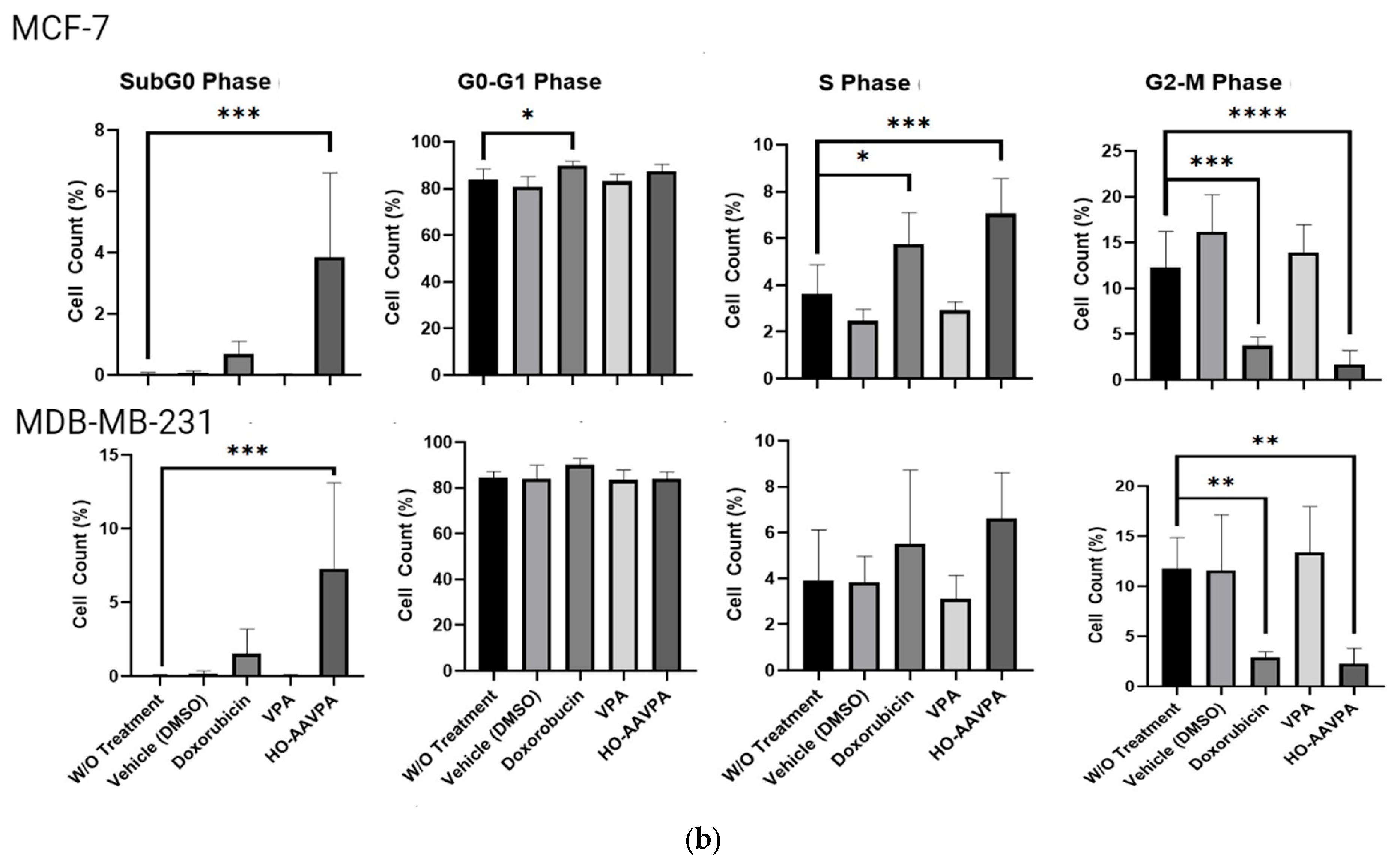
G2-M Levels in MDA-MB-231 and MCF-7 Cells Treated with HO-AAVPA
2.2. HO-AAVPA Inhibits GPER Expression
3. Discussion
4. Materials and Methods
4.1. Reagents
Cell Lines and Culture
4.2. Apoptosis via Flow Cytometry
Cell Cycle Assay
4.3. Protein Extraction and Western Blot
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.E.; Qian, Y.; Liu, G.; Chen, H.; Chen, X. p53, a target of estrogen receptor (ER) alpha, modulates DNA damage-induced growth suppression in ER-positive breast cancer cells. J. Biol. Chem. 2012, 287, 30117–30127. [Google Scholar] [CrossRef] [PubMed]
- Carmeci, C.; Thompson, D.A.; Ring, H.Z.; Francke, U.; Weigel, R.J. Identification of a gene (GPR30) with homology to the G-protein coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 1997, 45, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Kato, C.; Kondo, S.; Korenaga, R.; Ando, J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem. Biophys. Res. Commun. 1997, 240, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Bieliauskas, A.V.; Pflum, M.K.H. Isoform-selective histone deacetylase inhibitors. Chem. Soc. Rev. 2008, 37, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Rosano, C.; Santolla, M.F.; Pupo, M.; De Francesco, E.M.; De Marco, P.; Ponassi, M.; Spallarossa, A.; Ranise, A.; Maggiolini, M. Two novel GPER agonists induce gene expression changes and growth effects in cancer cells. Curr. Cancer Drug Targets 2012, 12, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Steiman, J.; Peralta, E.A.; Louis, S.; Kamel, O. Biology of the estrogen receptor, GPR30, in triple negative breast cancer. Am. J. Surg. 2013, 206, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Pisano, A.; Maggiolini, M. GPER function in breast cancer: An overview. Front. Endocrinol. 2014, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Filigheddu, N.; Sampietro, S.; Chianale, F.; Porporato, P.E.; Gaggianesi, M.; Gregnanin, I.; Rainero, E.; Ferrara, M.; Perego, B.; Riboni, F.; et al. Diacylglycerol kinase alpha mediates 17-beta-estradiol induced proliferation, motility, and anchorage-independent growth of Hec-1A endometrial cancer cell line through the G protein-coupled estrogen receptor GPR30. Cell Signal. 2011, 23, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, R.J. Historical review: A brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 2004, 25, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.J.; Liu, F.; Lin, Z.K.; Xie, Y.F.; Xu, J.L.; Tong, Q.C.; Shu, R. Genistein regulates the IL-1 beta induced activation of MAPKs in human periodontal ligament cells through G protein-coupled receptor 30. Arch. Biochem. Biophys. 2012, 522, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Maggiolini, M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol. Cell Endocrinol. 2009, 308, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.D.; Simoncini, T. Extra-nuclear signaling of estrogen receptors. IUBMB Life 2008, 60, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.; Bustamante, F.; Ortloff, A.; Ramos, I.; Ehrenfeld, P.; Figueroa, C.D. Continuous Exposure of Breast Cancer Cells to Tamoxifen Upregulates GPER-1 and Increases Cell Proliferation. Front. Endocrinol. 2020, 11, 563165. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, X.; He, C.; Hua, G.; Tsai, M.Y.; Davis, J.S. The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization. Cell Death Dis. 2013, 4, e869. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.K.Y.; Lam, H.M.; Ng, C.F.; Lee, A.Y.Y.; Chan, E.S.Y.; Ng, H.K.; Ho, S.M.; Lau, K.M. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G (2) cell-cycle arrest. Cell Death Differ. 2010, 17, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Xu, Y.; Wang, L.; Zhou, K.; He, J.; Lu, J.; Huang, Q.; Sun, P.; Wang, T. Estrogen-Related Receptor α (ERRα) and G Protein-Coupled Estrogen Receptor (GPER) Synergistically Indicate Poor Prognosis in Patients with Triple-Negative Breast Cancer. OncoTargets Ther. 2020, 13, 8887–8899. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Talia, M.; Santolla, M.F.; Pellegrino, M.; Scordamaglia, D.; Spinelli, A.; De Rosis, S.; Giordano, F.; Muglia, L.; Zicarelli, A.; et al. GPER deletion triggers inhibitory effects in triple negative breast cancer (TNBC) cells through the JNK/c-Jun/p53/Noxa transduction pathway. Cell Death Discov. 2023, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.H.; Chu, N.M.; Lin, Y.F.; Kao, S.H. G-Protein Coupled Estrogen Receptor in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 306. [Google Scholar] [CrossRef]
- Xu, T.; Ma, D.; Chen, S.; Tang, R.; Yang, J.; Meng, C.; Feng, Y.; Liu, L.; Wang, J.; Luo, H.; et al. High GPER expression in triple-negative breast cancer is linked to pro-metastatic pathways and predicts poor patient outcomes. NPJ Breast Cancer 2022, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.K.; Field, A.S.; Burai, R.; Ramesh, C.; Petrie, W.K.; Bologa, C.G.; Oprea, T.I.; Yamaguchi, Y.; Hayashi, S.-I.; Sklar, L.A.; et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J. Steroid Biochem. Mol. Biol. 2011, 127, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Girgert, R.; Emons, G.; Gründker, C. Inactivation of GPR30 reduces growth of triple-negative breast cancer cells: Possible application in targeted therapy. Breast Cancer Res. Treat. 2012, 134, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Rosano, C.; Pisano, A.; Santolla, M.F.; De Francesco, E.M.; De Marco, P.; Dolce, V.; Ponassi, M.; Felli, L.; Cafeo, G.; et al. A calixpyrrole derivative acts as an antagonist to GPER, a G-protein coupled receptor: Mechanisms and models. Dis. Model Mech. 2015, 8, 1237–1246. [Google Scholar] [PubMed]
- Ruan, S.Q.; Wang, S.W.; Wang, Z.H.; Zhang, S.Z. Regulation of HRG-β1-induced proliferation, migration and invasion of MCF-7 cells by upregulation of GPR30 expression. Mol. Med. Rep. 2012, 6, 131–138. [Google Scholar] [PubMed]
- Tao, S.; He, H.; Chen, Q.; Yue, W. GPER mediated estradiol reduces miRNA148 to promote HLA-G expression in breast cancer. Biochem. Biophys. Res. Commun. 2014, 451, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yao, Y. Mechanisms of GPER promoting proliferation, migration and invasion of triple-negative breast cancer cells through CAF. Am. J. Transl. Res. 2019, 11, 5858–5868. [Google Scholar] [PubMed]
- Pupo, M.; Pisano, A.; Lappano, R.; Santolla, M.F.; De Francesco, E.M.; Abonante, S.; Rosano, C.; Maggiolini, M. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 2012, 120, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Mishra, S.R.; Behera, B.P.; Jena, M.; Bhutia, S.K. Dysregulation of histone deacetylases in carcinogenesis and tumor progression: A possible link to apoptosis and autophagy. Cell Mol. Life Sci. 2019, 76, 3263–3282. [Google Scholar] [CrossRef]
- Brochier, C.; Dennis, G.; Rivieccio, M.A.; McLaughlin, K.; Coppola, G.; Ratan, R.R.; Langley, B. Specific acetylation of p53 by HDAC inhibition prevents DNA damage-induced apoptosis in neurons. J. Neurosci. 2013, 33, 8621–8632. [Google Scholar] [CrossRef]
- Telles, E.; Seto, E. Modulation of cell cycle regulators by HDACs. Front. Biosci. 2012, 4, 831–839. [Google Scholar] [CrossRef]
- Ropero, S.; Esteller, M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Vesci, L.; Battistuzzi, G.; Vignola, D.; Milazzo, F.M.; Guglielmi, M.B.; Barbarino, M.; Santaniello, M.; Fantò, N.; Mor, M.; et al. ST7612AA1, a thioacetate-ω(γ-lactam carboxamide) derivative selected from a novel generation of oral HDAC inhibitors. J. Med. Chem. 2014, 57, 8358–8377. [Google Scholar] [CrossRef]
- Yoon, S.; Eom, G.H. HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases. Chonnam Med. J. 2016, 52, 1–11. [Google Scholar] [CrossRef]
- Al-Yacoub, N.; Fecker, L.F.; Möbs, M.; Plötz, M.; Braun, F.K.; Sterry, W.; Eberle, J. Apoptosis induction by SAHA in cutaneous T-cell lymphoma cells is related to downregulation of c-FLIP and enhanced TRAIL signaling. J. Investig. Dermatol. 2012, 132, 2263–2274. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Jiang, G.; Zhou, Y.; Yang, X.; Huang, H.; Liu, H.; Du, J.; Wang, H. Epigenetic down regulation of G protein-coupled estrogen receptor (GPER) functions as a tumor suppressor in colorectal cancer. Mol. Cancer 2017, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Weng, J.R.; Hu, J.L.; Wang, D.; Sargeant, A.M.; Chiu, C.F. G15, a GPR30 antagonist, induces apoptosis and autophagy in human oral squamous carcinoma cells. Chem. Biol. Interact. 2013, 206, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Weißenborn, C.; Ignatov, T.; Poehlmann, A.; Wege, A.K.; Costa, S.D.; Zenclussen, A.C.; Ignatov, A. GPER functions as a tumor suppressor in MCF-7 and SK-BR-3 breast cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Imesch, P.; Samartzis, E.P.; Dedes, K.J.; Fink, D.; Fedier, A. Histone deacetylase inhibitors down-regulate G-protein-coupled estrogen receptor and the GPER-antagonist G-15 inhibits proliferation in endometriotic cells. Fertil. Steril. 2013, 100, 770–776. [Google Scholar] [CrossRef] [PubMed]
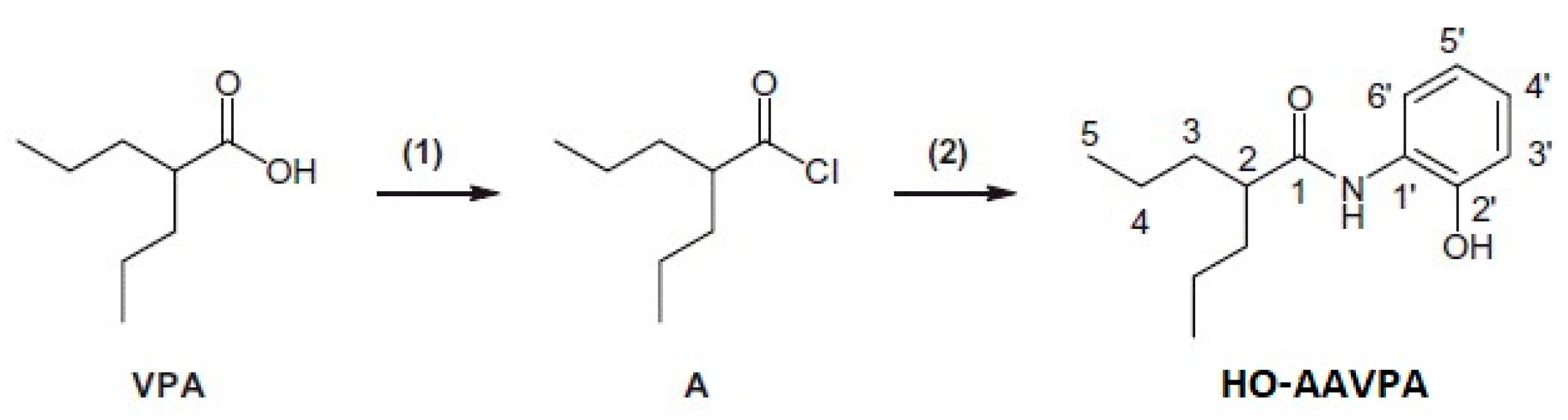
- Prestegui-Martel, B.; Bermúdez-Lugo, J.A.; Chávez-Blanco, A.; Dueñas-González, A.; García-Sánchez, J.R.; Pérez-González, O.A.; Padilla-Martínez, I.I.; Fragoso-Vázquez, M.J.; Mendieta-Wejebe, J.E.; Correa-Basurto, A.M.; et al. N-(2-hydroxyphenyl)-2-propylpentanamide, a valproic acid aryl derivative designed in silico with improved anti-proliferative activity in HeLa, rhabdomyosarcoma and breast cancer cells. J. Enzyme Inhib. Med. Chem. 2016, 31, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sixto-López, Y.; Rosales-Hernández, M.C.; Contis-Montes de Oca, A.; Fragoso-Morales, L.G.; Mendieta-Wejebe, J.E.; Correa-Basurto, A.M.; Abarca-Rojano, E.; Correa-Basurto, J. N-(2′-hydroxyphenyl)-2-propylpentanamide (HO-AAVPA) inhibits HDAC1 and increases the translocation of HMGB1 levels in human cervical cancer cells. Int. J. Mol. Sci. 2020, 21, 5873. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, T.; Ninomiya, I.; Inokuchi, M.; Harada, S.; Hayashi, H.; Oyama, K.; Makino, I.; Nakagawara, H.; Miyashita, T.; Tajima, H.; et al. Valproic acid inhibits proliferation of HER2-expressing breast cancer cells by inducing cell cycle arrest and apoptosis through Hsp70 acetylation. Int. J. Oncol. 2015, 47, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Luszczki, J.J.; Grabarska, A.; Gumbarewicz, E.; Dmoszynska-Graniczka, M.; Polberg, K.; Stepulak, A. Assessment of interactions between cisplatin and two histone deacetylase inhibitors in MCF7, T47D and MDA-MB-231 human breast cancer cell lines—An isobolographic analysis. PLoS ONE 2015, 10, e0143013. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, V.; Gozzini, A.; Cheloni, G.; Marzi, I.; Fabiani, E.; Santini, V.; Dello Sbarba, P.; Rovida, E. Time- and residue-specific differences in histone acetylation induced by VPA and SAHA in AML1/ETO-positive leukemia cells. Epigenetics 2013, 8, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, P.; Sanchez, S.C.; Chávez-Blanco, A.D.; Mendoza-Figueroa, H.L.; Correa-Basurto, J. Apoptotic effects of N-(2-hydroxyphenyl)-2-propylpentanamide on U87-MG and U-2 OS cells and antiangiogenic properties. Anti-Cancer Agents Med. Chem. 2021, 21, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Anirudh, B.V.M.; Ezhilarasan, D. Reactive Oxygen Species-Mediated Mitochondrial Dysfunction Triggers Sodium Valproate-Induced Cytotoxicity in Human Colorectal Adenocarcinoma Cells. J. Gastrointest. Cancer 2021, 52, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Wang, Y.S.; Gu, W.P.; Zhao, X. The role and possible molecular mechanism of valproic acid in the growth of MCF-7 breast cancer cells. Croat. Med. J. 2017, 58, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Yarmohamadi, A.; Asadi, J.; Gharaei, R.; Mir, M.; Khoshnazar, A.K. Valproic Acid, a Histone Deacetylase Inhibitor, Enhances Radiosensitivity in Breast Cancer Cell Line. J. Radiat. Cancer Res. 2018, 9, 86–92. [Google Scholar]
- Weißenborn, C.; Ignatov, T.; Ochel, H.J.; Costa, S.D.; Zenclussen, A.C.; Ignatova, Z.; Ignatov, A. GPER functions as a tumor suppressor in triple-negative breast cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.M.; Augereau, J.M.; Gleye, J.; Maffrand, J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990, 172, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.S.; Oon, C.E.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2016, 54, 1223–1236. [Google Scholar] [PubMed]


| DMSO 0.1% | VPA 283 µM | HO-AAVPA 283 µM | Chelerythrine 50 µM | |
|---|---|---|---|---|
| Q1 (necrosis) | 0.7 ± 0.04% | 0.6 ± 0.07% | 1.5 ± 0.3% | 1.4 ± 0.1% |
| Q2 (late apoptisis) | 1.0 ± 0.03% | 0.4 ± 0.07% | 11.7 ± 1.2% | 3.0 ± 0.2% |
| Q3 (lives) | 95.3 ± 2.3% | 97.6 ± 2.3% | 55.4 ± 3.0% | 85.0 ± 2.3% |
| Q4 (early apoptosis) | 3.0 ± 0.7% | 1.3 ± 0.1% | 31.4 ± 1.4% | 10.6 ± 1.1% |
| DMSO 0.1% | VPA 283 µM | HO-AAVPA 283 µM | Chelerythrine 50 µM | |
|---|---|---|---|---|
| Q1 (necrosis) | 5.5 ± 0.9% | 4.7 ± 0.7% | 6.6 ± 0.3% | 0.5 ± 0.05% |
| Q2 (late apoptisis) | 1.4 ± 0.4% | 2.2 ± 0.3% | 17.3 ± 0.2% | 2.7 ± 0.2% |
| Q3 (lives) | 91.1 ± 0.5% | 86.8 ± 0.7% | 38.5 ± 0.7% | 64.2 ± 1.2% |
| Q4 (early apoptosis) | 2.0 ± 0.3% | 6.3 ± 0.5% | 37.6 ± 0.3% | 32.6 ± 0.9% |
| Cells | MCF-7 | MDA-MB-231 | SKBR3 |
| IC50 (mM) | 0.192.4 | 0.283 | 0.142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prestegui Martel, B.; Chávez-Blanco, A.D.; Domínguez-Gómez, G.; Dueñas González, A.; Gaona-Aguas, P.; Flores-Mejía, R.; Somilleda-Ventura, S.A.; Rodríguez-Cortes, O.; Morales-Bárcena, R.; Martínez Muñoz, A.; et al. N-(2-Hydroxyphenyl)-2-Propylpentanamide (HO-AAVPA) Induces Apoptosis and Cell Cycle Arrest in Breast Cancer Cells, Decreasing GPER Expression. Molecules 2024, 29, 3509. https://doi.org/10.3390/molecules29153509
Prestegui Martel B, Chávez-Blanco AD, Domínguez-Gómez G, Dueñas González A, Gaona-Aguas P, Flores-Mejía R, Somilleda-Ventura SA, Rodríguez-Cortes O, Morales-Bárcena R, Martínez Muñoz A, et al. N-(2-Hydroxyphenyl)-2-Propylpentanamide (HO-AAVPA) Induces Apoptosis and Cell Cycle Arrest in Breast Cancer Cells, Decreasing GPER Expression. Molecules. 2024; 29(15):3509. https://doi.org/10.3390/molecules29153509
Chicago/Turabian StylePrestegui Martel, Berenice, Alma Delia Chávez-Blanco, Guadalupe Domínguez-Gómez, Alfonso Dueñas González, Patricia Gaona-Aguas, Raúl Flores-Mejía, Selma Alin Somilleda-Ventura, Octavio Rodríguez-Cortes, Rocío Morales-Bárcena, Alberto Martínez Muñoz, and et al. 2024. "N-(2-Hydroxyphenyl)-2-Propylpentanamide (HO-AAVPA) Induces Apoptosis and Cell Cycle Arrest in Breast Cancer Cells, Decreasing GPER Expression" Molecules 29, no. 15: 3509. https://doi.org/10.3390/molecules29153509
APA StylePrestegui Martel, B., Chávez-Blanco, A. D., Domínguez-Gómez, G., Dueñas González, A., Gaona-Aguas, P., Flores-Mejía, R., Somilleda-Ventura, S. A., Rodríguez-Cortes, O., Morales-Bárcena, R., Martínez Muñoz, A., Mejia Barradas, C. M., Mendieta Wejebe, J. E., & Correa Basurto, J. (2024). N-(2-Hydroxyphenyl)-2-Propylpentanamide (HO-AAVPA) Induces Apoptosis and Cell Cycle Arrest in Breast Cancer Cells, Decreasing GPER Expression. Molecules, 29(15), 3509. https://doi.org/10.3390/molecules29153509






