Mussel-Inspired Multiwalled Carbon Nanotube Nanocomposite for Methyl Orange Removal: Adsorption and Regeneration Behaviors
Abstract
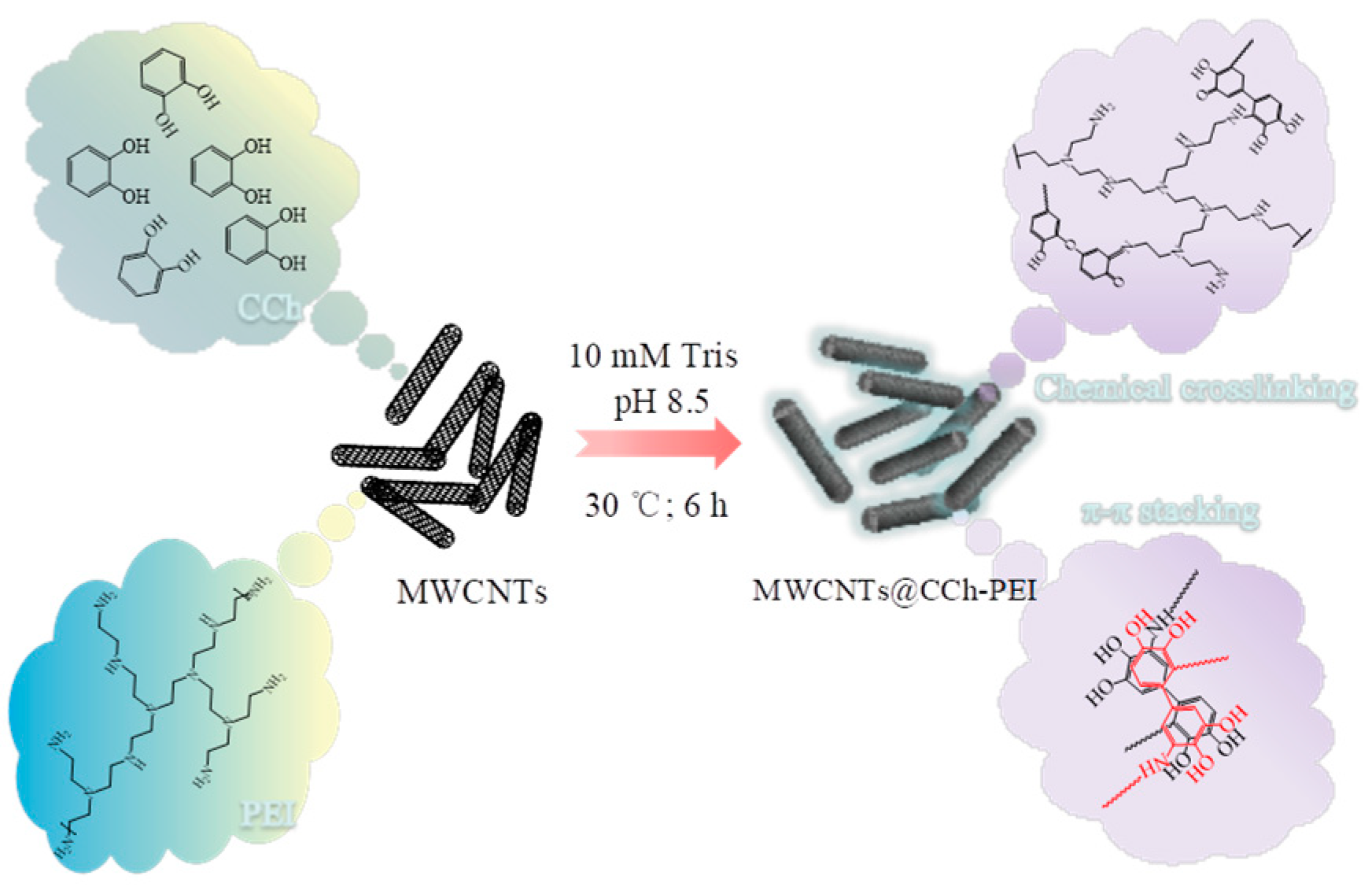
:1. Introduction
2. Results
2.1. Characterizations
2.2. Adsorption Properties of As-Synthesized MWCNTs@CCh-PEI
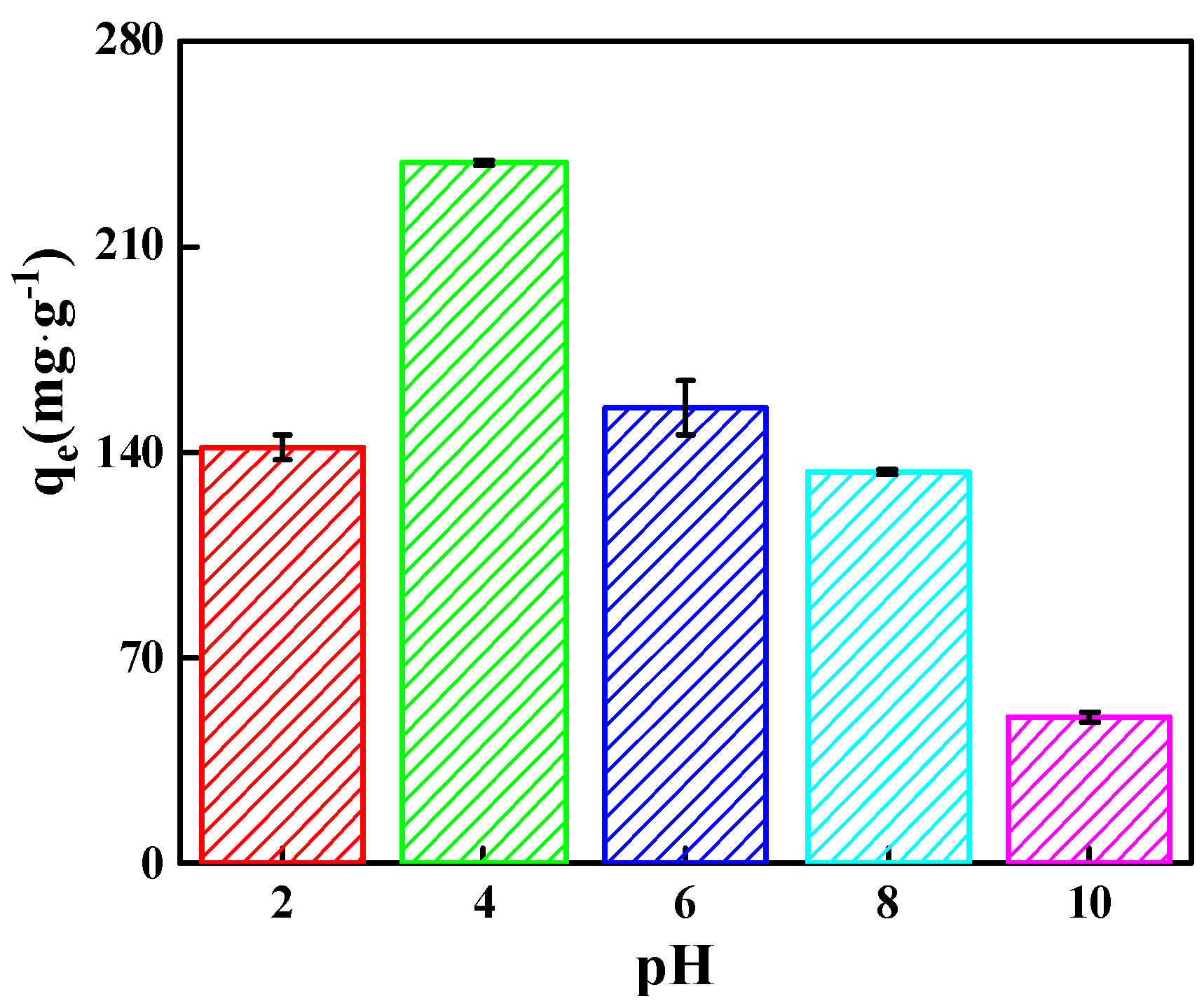
2.2.1. Effect of MO Solution pH on Adsorption Capacity
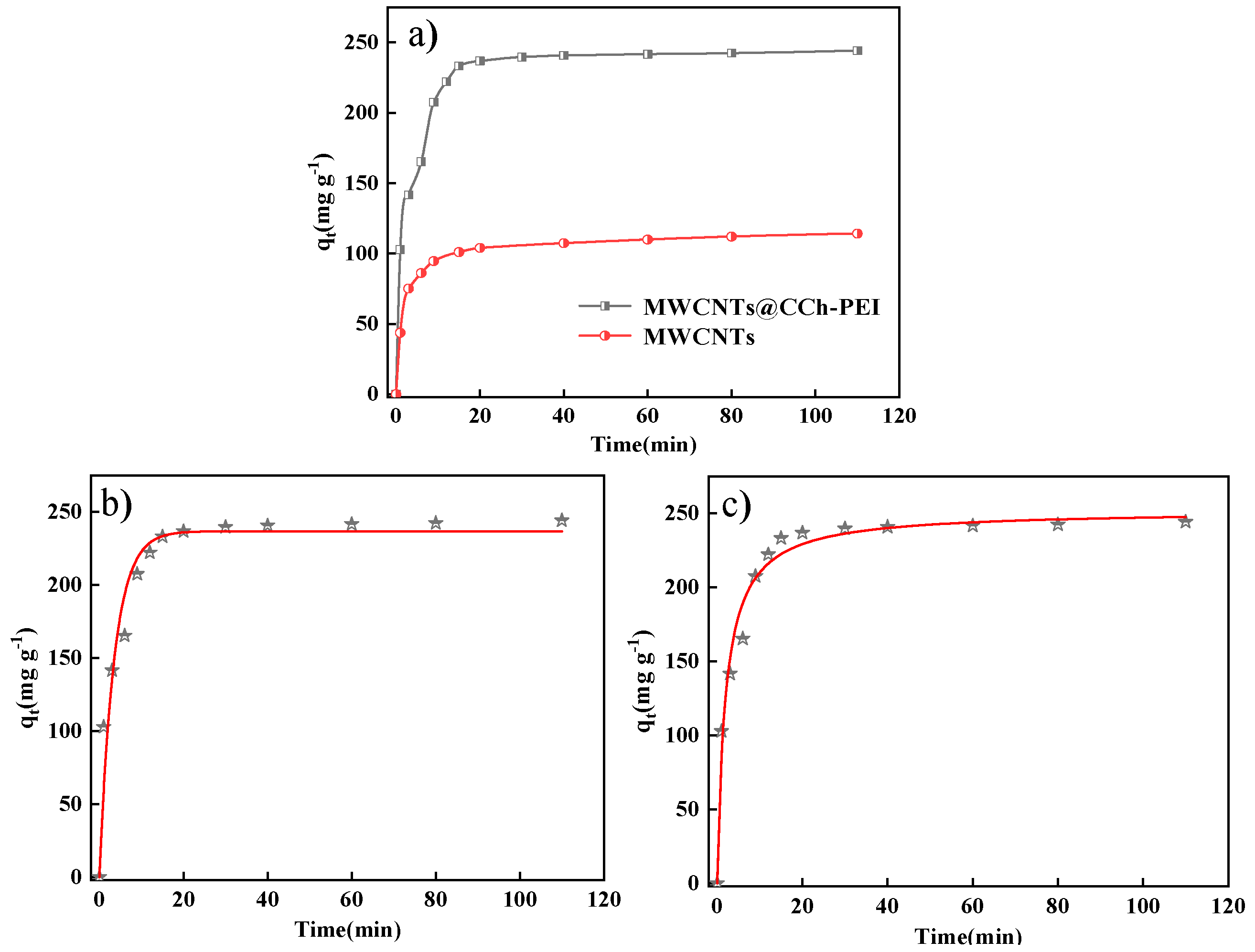
2.2.2. Effect of Contact Time on Adsorption Capacity and Adsorption Kinetics
2.2.3. Effect of MO Initial Concentration on Adsorption Capacity and Adsorption Isotherm
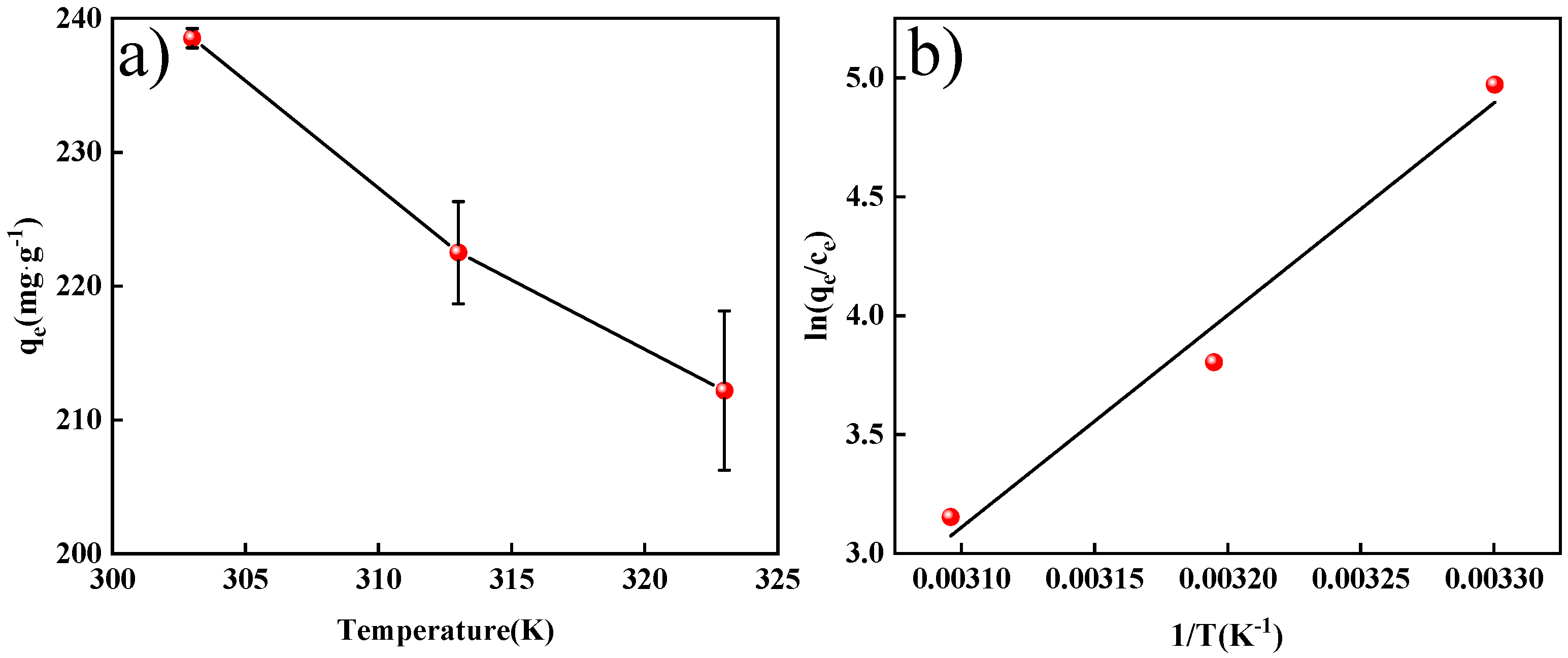
2.2.4. Effect of Temperature on Adsorption Capacity and Adsorption Thermodynamic
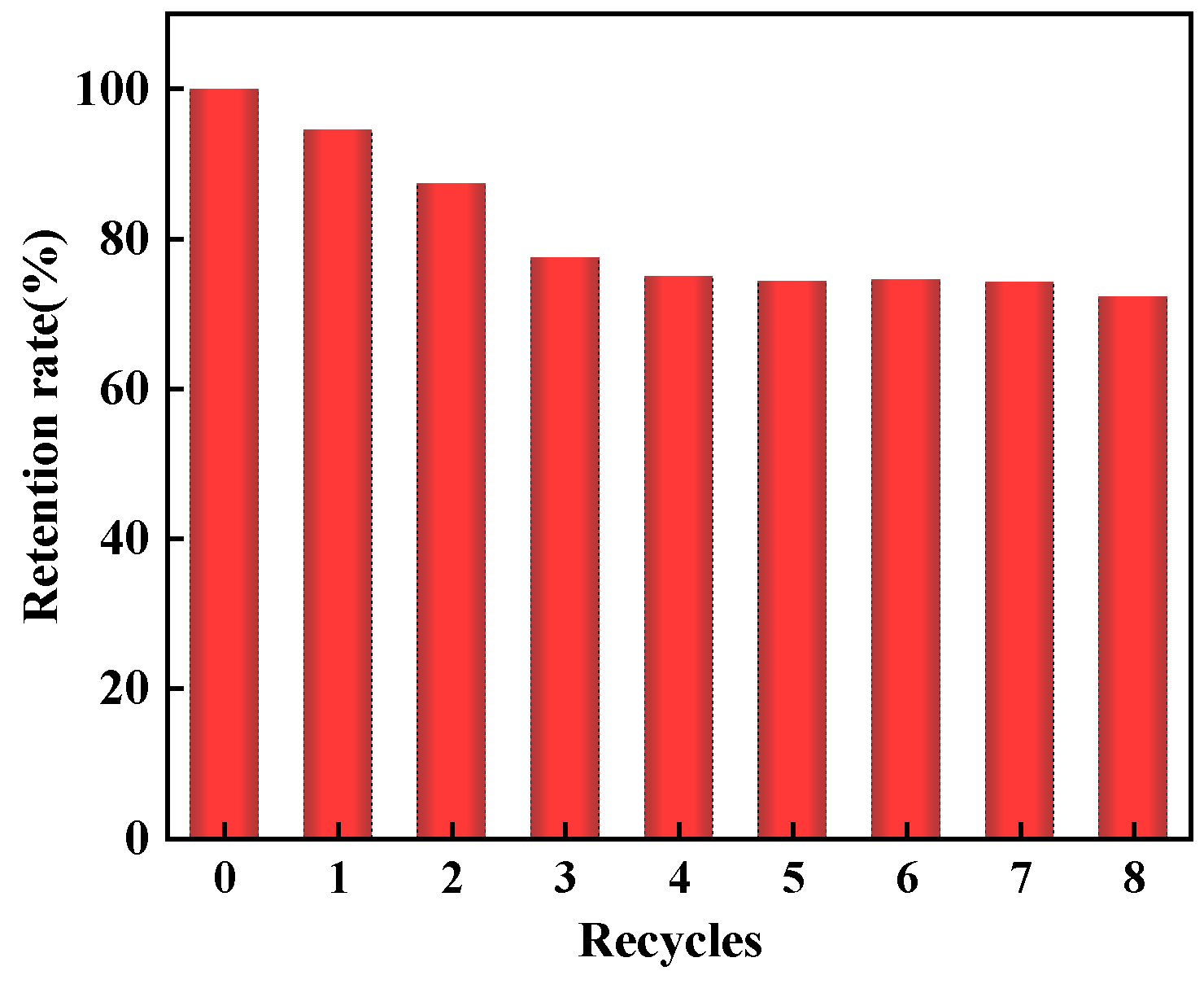
2.3. Regeneration
2.4. Adsorption Mechanisms
3. Materials and Methods
3.1. Materials
3.2. Preparation of MWCNTs@CCh-PEI
3.3. Characterizations
3.4. Adsorption and Reusability Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Josephy, P.D.; Allen-Vercoe, E. Reductive metabolism of azo dyes and drugs: Toxicological implications. Food Chem. Toxicol. 2023, 178, 113932. [Google Scholar] [CrossRef] [PubMed]
- El-Hout, S.I.; El-Sheikh, S.M.; Gaber, A.; Shawky, A.; Ahmed, A.I. Highly efficient sunlight-driven photocatalytic degradation of malachite green dye over reduced graphene oxide-supported CuS nanoparticles. J. Alloys Compd. 2020, 849, 156573. [Google Scholar] [CrossRef]
- Sun, E.; Jiang, Y.; Wang, B.; Wang, X.; Zhao, F. Synthesis of catechol-polyethyleneimine nano/submicro-particles via mussel-inspired chemistry for highly efficient removal of methyl orange. Powder Technol. 2022, 403, 117396. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, X.; Liu, M.; Huang, Q.; Xu, D.; Wan, Q.; Huang, H.; Deng, F.; Zhang, X.; Wei, Y. Facile preparation of carbon nanotubes based carboxymethyl chitosan nanocomposites through combination of mussel inspired chemistry and Michael addition reaction: Characterization and improved Cu2+ removal capability. J. Taiwan Inst. Chem. Eng. 2016, 68, 446–454. [Google Scholar] [CrossRef]
- Hong, Y.; Jin, H.-J.; Kwak, H.W. Nitrogen-Rich Magnetic Bio-Activated Carbon from Sericin: A Fast Removable and Easily Separable Superadsorbent for Anionic Dye Removal. Macromol. Res. 2020, 28, 986–996. [Google Scholar] [CrossRef]
- Marjani, A.; Soltani, R.; Shirazian, S.; Soltani, R. Hierarchical multi-shell hollow micro–meso–macroporous silica for Cr (VI) adsorption. Sci. Rep. 2020, 10, 9788. [Google Scholar]
- Jun, B.-M.; Kim, S.; Rho, H.; Park, C.M.; Yoon, Y. Ultrasound-assisted Ti3C2Tx MXene adsorption of dyes: Removal performance and mechanism analyses via dynamic light scattering. Chemosphere 2020, 254, 126827. [Google Scholar] [CrossRef]
- Sahu, B.; Sinha, P.; Mishra, B.; Tripathi, B.P.; Banerjee, S. Recyclable Alloy Nanocatalyst-Mediated Photo-Controlled Synthesis of Functional Polymers for Toxic Metal and Dye Removal. Acs Appl. Polym. Mater. 2024, 58, 104748. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Alzahrani, E.; Shaltout, A.A.; Moghazy, R.M. Development of biological macroalgae lignins using copper based metal-organic framework for selective adsorption of cationic dye from mixed dyes. Int. J. Biol. Macromol. 2020, 165, 2984–2993. [Google Scholar] [CrossRef]
- Wani, A.A.; Rather, R.A.; Shaari, N.; Khan, U.; Muhammad, T.; Hussain, S.M.; Seddek, L.F.; Abed, A.M. Aspects of superior photocatalytic dye degradation and adsorption efficiency of reduced graphene oxide multiwalled carbon nanotubes with modified ZnO-Al2O3 nanocomposites. J. Environ. Chem. Eng. 2024, 2, 112461. [Google Scholar] [CrossRef]
- Elghamry, I.; Gouda, M.; Al-Fayiz, Y.S.S. Synthesis of Chemically Modified Acid-Functionalized Multiwall Carbon Nanotubes with Benzimidazole for Removal of Lead and Cadmium Ions from Wastewater. Polymers 2023, 15, 1421. [Google Scholar] [CrossRef] [PubMed]
- Sianipar, M.; Kim, S.H.; Khoiruddin, K.; Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Ma, Q.; Jia, X.; Ma, P.-C. Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds. Carbon 2017, 118, 765–771. [Google Scholar] [CrossRef]
- Ceroni, L.; Benazzato, S.; Pressi, S.; Calvillo, L.; Marotta, E.; Menna, E. Enhanced Adsorption of Methylene Blue Dye on Functionalized Multi-Walled Carbon Nanotubes. Nanomaterials 2024, 14, 14060522. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, L.; Zhao, Z.; Han, Y.; Li, Y.; Peng, Q.; Zhang, Q. Fast and robust lead (II) removal from water by bioinspired amyloid lysozyme fibrils conjugated with polyethyleneimine (PEI). Chem. Eng. J. 2020, 390, 124667. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Chen, R.; Liu, Q.; Liu, J.; Wang, C.; Sun, Z.; Wang, J. Mussel-inspired antifouling magnetic activated carbon for uranium recovery from simulated seawater. J. Colloid. Interface Sci. 2019, 534, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhao, J.; Liu, M.; Li, Y.; Ruan, J.; Li, Q.; Tian, J.; Zhu, X.; Zhang, X.; Wei, Y. Synthesis of polyacrylamide immobilized molybdenum disulfide (MoS 2@PDA@PAM) composites via mussel-inspired chemistry and surface-initiated atom transfer radical polymerization for removal of copper (II) ions. J. Taiwan Inst. Chem. Eng. 2018, 86, 174–184. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, X.; Zhao, S.; Du, Q.; Pi, X.; Jing, Z.; Jin, Y. Polydopamine coating for enhanced electrostatic adsorption of methylene blue by multiwalled carbon nanotubes in alkaline environments. J. Colloid. Interface Sci. 2024, 675, 263–274. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, J.; Ma, J.; Shao, L. Highly regenerable alkali-resistant magnetic nanoparticles inspired by mussels for rapid selective dye removal offer high-efficiency environmental remediation. J. Mater. Chem. A 2015, 3, 19960–19968. [Google Scholar] [CrossRef]
- Dong, K.; Liu, Q.; Wei, G.; Hu, T.; Yao, J.; Zhang, X.; Gao, T. Mussel-inspired magnetic adsorbent: Adsorption/reduction treatment for the toxic Cr (VI) from simulated wastewater. J. Appl. Polym. Sci. 2018, 135, 46530. [Google Scholar] [CrossRef]
- Li, Q.; Liao, Z.; Fang, X.; Wang, D.; Xie, J.; Sun, X.; Wang, L.; Li, J. Tannic acid-polyethyleneimine crosslinked loose nanofiltration membrane for dye/salt mixture separation. J. Membr. Sci. 2019, 584, 324–332. [Google Scholar] [CrossRef]
- Wang, J.; He, R.; Han, X.; Jiao, D.; Zhu, J.; Lai, F.; Liu, X.; Liu, J.; Zhang, Y.; Van der Bruggen, B. High performance loose nanofiltration membranes obtained by a catechol-based route for efficient dye/salt separation. Chem. Eng. J. 2019, 375, 121982. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Y.; Zhao, F. Synthesis of mussel-inspired silica nanocomposites for efficient removal of methyl orange. Mater. Lett. 2022, 314, 131832. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Q.; Li, C.; Liu, S.; Xia, K.; Han, B.; Zhou, C. Effective coating of crosslinked polyethyleneimine on elastic spongy monolith for highly efficient batch and continuous flow adsorption of Pb (II) and acidic red 18. Chem. Eng. J. 2019, 391, 123610. [Google Scholar] [CrossRef]
- Zhao, J.; Fang, C.; Zhu, Y.; He, G.; Pan, F.; Jiang, Z.; Zhang, P.; Cao, X.; Wang, B. Manipulating the interfacial interactions of composite membranes via a mussel-inspired approach for enhanced separation selectivity. J. Mater. Chem. A 2015, 3, 19980–19988. [Google Scholar] [CrossRef]
- Jiang, X.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Shi, Z. Mussel-inspired surface modification of untreated wasted husks with stable polydopamine/polyethylenimine for efficient continuous Cr (VI) removal. Mater. Res. Bull. 2018, 102, 218–225. [Google Scholar] [CrossRef]
- Jiang, X.; Xi, M.; Bai, L.; Wang, W.; Yang, L.; Chen, n.H.; Niu, Y.; Cui, Y.; Yang, H.; Wei, D. Surface-initiated PET-ATRP and mussel-inspired chemistry for surface engineering of MWCNTs and application in self-healing nanocomposite hydrogels. J. Mat. Sci. Eng. C-Mater. 2020, 109, 110553. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Dou, J.; Huang, Q.; Yuan, L.; Chen, J.; Wen, Y.; Li, Y.; Zhang, X.; Wei, Y. Surface modification of carbon nanotubes with polyethyleneimine through “mussel inspired chemistry” and “Mannich reaction” for adsorptive removal of copper ions from aqueous solution. J. Environ. Chem. Eng. 2020, 8, 103721. [Google Scholar] [CrossRef]
- Long, Y.; Xiao, L.; Cao, Q. Co-polymerization of catechol and polyethylenimine on magnetic nanoparticles for efficient selective removal of anionic dyes from water. Powder Technol. 2017, 310, 24–34. [Google Scholar] [CrossRef]
- Qiu, W.-Z.; Yang, H.-C.; Wan, L.-S.; Xu, Z.-K. Co-deposition of catechol/polyethyleneimine on porous membranes for efficient decolorization of dye water. J. Mater. Chem. A 2015, 3, 14438–14444. [Google Scholar] [CrossRef]
- Kang, S.; Qin, L.; Zhao, Y.; Wang, W.; Zhang, T.; Yang, L.; Rao, F.; Song, S. Enhanced removal of methyl orange on exfoliated montmorillonite/chitosan gel in presence of methylene blue. Chemosphere 2020, 238, 124693. [Google Scholar] [CrossRef] [PubMed]
- Haitham, K.; Razak, S.; Nawi, M.A. Kinetics and isotherm studies of methyl orange adsorption by a highly recyclable immobilized polyaniline on a glass plate. Arab. J. Chem. 2019, 12, 1595–1606. [Google Scholar] [CrossRef]
- Gapusan, R.B.; Balela, M.D.L. Adsorption of anionic methyl orange dye and lead (II) heavy metal ion by polyaniline-kapok fiber nanocomposite. Mater. Chem. Phys. 2020, 243, 122682. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Atia, A.A.; Mahfouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Vincent, T.; Guibal, E. Dy (III) recovery from dilute solutions using magnetic-chitosan nano-based particles grafted with amino acids. J. Mater. Sci. 2015, 50, 2832–2848. [Google Scholar] [CrossRef]
- Hubbe, M.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.M.A.W. Hexavalent chromium adsorption on impregnated palm shell activated carbon with polyethyleneimine. Bioresour. Technol. 2010, 101, 5098–5103. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, M.; Chen, J.; Wan, Q.; Tian, J.; Huang, L.; Jiang, R.; Deng, F.; Wen, Y.; Zhang, X.; et al. Marrying the mussel inspired chemistry and Kabachnik–Fields reaction for preparation of SiO2 polymer composites and enhancement removal of methylene blue. Appl. Surf. Sci. 2017, 422, 17–27. [Google Scholar]
- Li, P.; Fu, T.; Gao, X.; Zhu, W.; Han, C.; Liu, N.; He, S.; Luo, Y.; Ma, W. Adsorption and reduction transformation behaviors of Cr (VI) on mesoporous polydopamine/titanium dioxide composite nanospheres. J. Chem. Eng. Data 2019, 64, 2686–2696. [Google Scholar] [CrossRef]
- Mohammad-Salim, H.A. Oxygen functionalized and pristine carbon nanotubes efficiency for adsorption of methyl orange dye. Int. Res. J. Pure Appl. Chem. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Zou, W.; Liu, L.; Li, H.; Han, X. Investigation of synergistic adsorption between methyl orange and Cd (II) from binary mixtures on magnesium hydroxide modified clinoptilolite. Korean J. Chem. Eng. 2016, 33, 2073–2083. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, B.; Liu, M.; Xie, Z.; Wang, D.; Feng, G. The adsorption properties of defect controlled metal-organic frameworks of UiO-66. Sep. Purif. Technol. 2021, 270, 118842. [Google Scholar] [CrossRef]
- Murakami, K.; Sugawara, R.; Nakamura, A. Synthesis of PNIPAM/magnetite/multiamine-functionalized mesoporous silica composite and investigation of temperature of its aggregation and adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 624, 126833. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.; Qian, X.; Dong, M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J. Colloid. Interface Sci. 2018, 509, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhai, S.; Zhu, B.; Liu, J.; Lu, A.; Jiang, H. S-Doped magnetic mesoporous carbon for efficient adsorption of methyl orange from aqueous solution. Clean. Soil. Air Water 2021, 49, 2000285. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.Z.; Wu, Z.T.; Wu, Y.; Gao, T.T.; Yao, J.S. Efficient removal of methyl orange and alizarin red S from pH-unregulated aqueous solution by the catechol-amine resin composite using. Acs Sustain. Chem. Eng. 2017, 5, 1871–1880. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Parameswaranpillai, J.; Siengchin, S. Efficient removal of methyl orange from aqueous solution using mesoporous ZSM-5 zeolite: Synthesis, kinetics and isotherm studies. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 611, 125852. [Google Scholar] [CrossRef]
- Wong, S.; Tumari, H.H.; Ngadi, N.; Mohamed, N.B.; Hassan, O.; Mat, R.; Amin, N.A.S. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean. Prod. 2019, 206, 394–406. [Google Scholar] [CrossRef]
- Su, Y.; Jiao, Y.; Dou, C.; Han, R. Biosorption of methyl orange from aqueous solutions using cationic surfactant-modified wheat straw in batch mode. Desalination Water Treat. 2014, 52, 6145–6155. [Google Scholar] [CrossRef]
- Gan, D.; Liu, M.; Huang, H.; Chen, J.; Dou, J.; Wen, Y.; Huang, Q.; Yang, Z.; Zhang, X.; Wei, Y. Facile preparation of functionalized carbon nanotubes with tannins through mussel-inspired chemistry and their application in removal of methylene blue. J. Mol. Liq. 2018, 271, 246–253. [Google Scholar] [CrossRef]
- Tran, H.N.; Wang, Y.-F.; You, S.-J.; Chao, H.-P. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of π-π interactions. Process. Saf. Environ. Prot. 2017, 107, 168–180. [Google Scholar] [CrossRef]
- Huang, H.; Sha, X.; Cui, Y.; Sun, S.; Huang, H.; He, Z.; Liu, M.; Zhou, N.; Zhang, X.; Wei, Y. Highly efficient removal of iodine ions using MXene-PDA-Ag2Ox composites synthesized by mussel-inspired chemistry. J. Colloid Interface Sci. 2020, 567, 190–201. [Google Scholar] [CrossRef]
- Theydan, S.K.; Ahmed, M.J. Adsorption of methylene blue onto biomass-based activated carbon by FeCl3 activation: Equilibrium, kinetics, and thermodynamic studies. J. Anal. Appl. Pyrolysis 2012, 97, 116–122. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Liman, G.; Arica, M.Y. Removal of metal complexed azo dyes from aqueous solution using tris (2-aminoethyl) amine ligand modified magnetic p (GMA-EGDMA) cationic resin: Adsorption, isotherm and kinetic studies. Chem. Eng. Res. Des. 2017, 124, 85–97. [Google Scholar] [CrossRef]
- Bai, L.; Li, Z.; Zhang, Y.; Wang, T.; Lu, R.; Zhou, W.; Gao, H.; Zhang, S. Synthesis of water-dispersible graphene-modified magnetic polypyrrole nanocomposite and its ability to efficiently adsorb methylene blue from aqueous solution. Chem. Eng. J. 2015, 279, 757–766. [Google Scholar] [CrossRef]
- Ma, H.; Pu, S.Y.; Hou, Y.Q.; Zhu, R.X.; Zinchenko, A.; Chu, W. A highly efficient magnetic chitosan “fluid “adsorbent with a high capacity and fast adsorption kinetics for dyeing wastewater purification. Chem. Eng. J. 2018, 345, 556–565. [Google Scholar]
- Derafa, G.; Zaghouane-Boudiaf, H. Urtica dioica leaves-calcium alginate as a natural, low cost and very effective bioadsorbent beads in elimination of dyes from aqueous medium: Equilibrium isotherms and thermodynamic studies. Int. J. Biol. Macromol. 2018, 124, 915–921. [Google Scholar] [CrossRef]
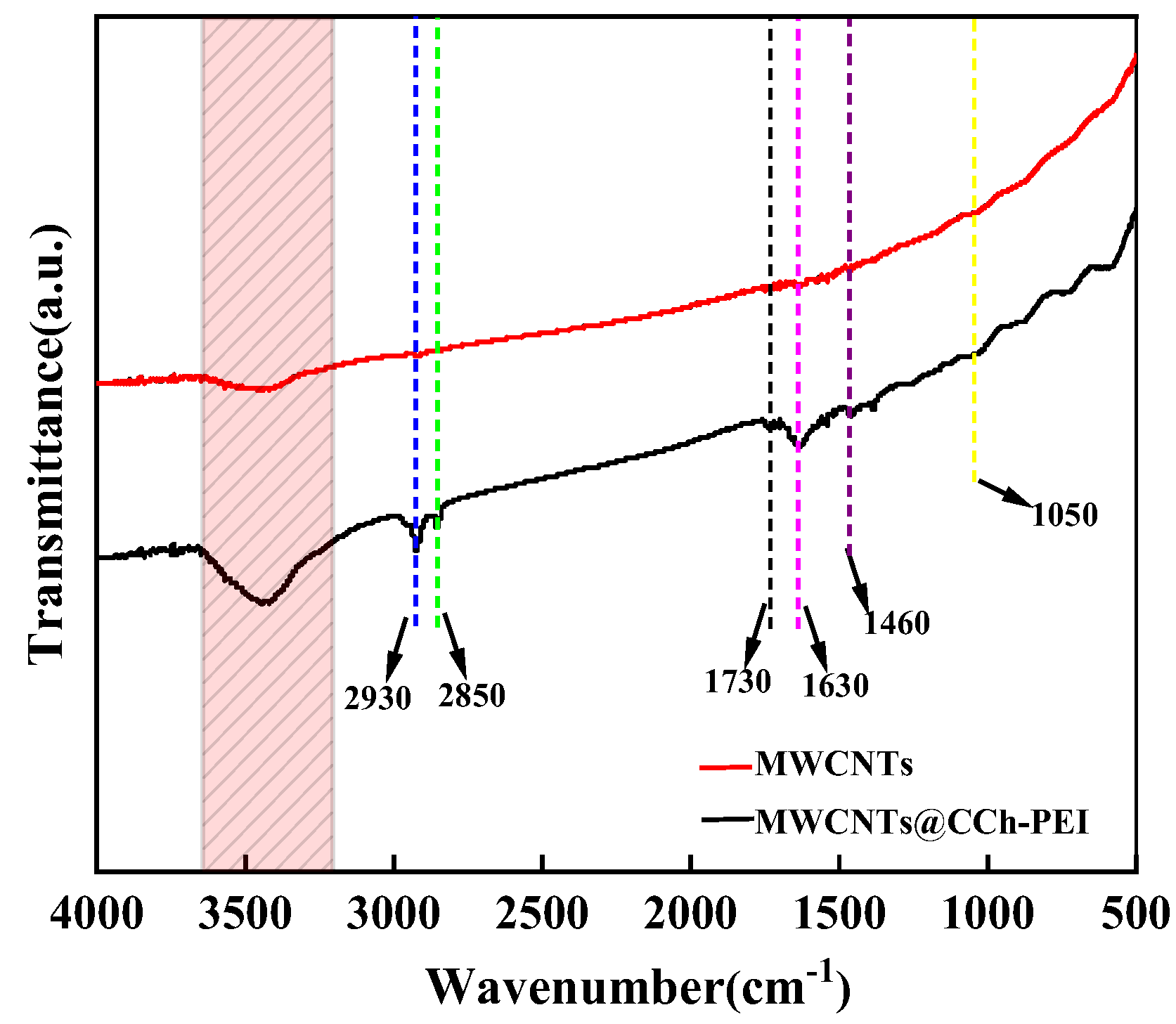
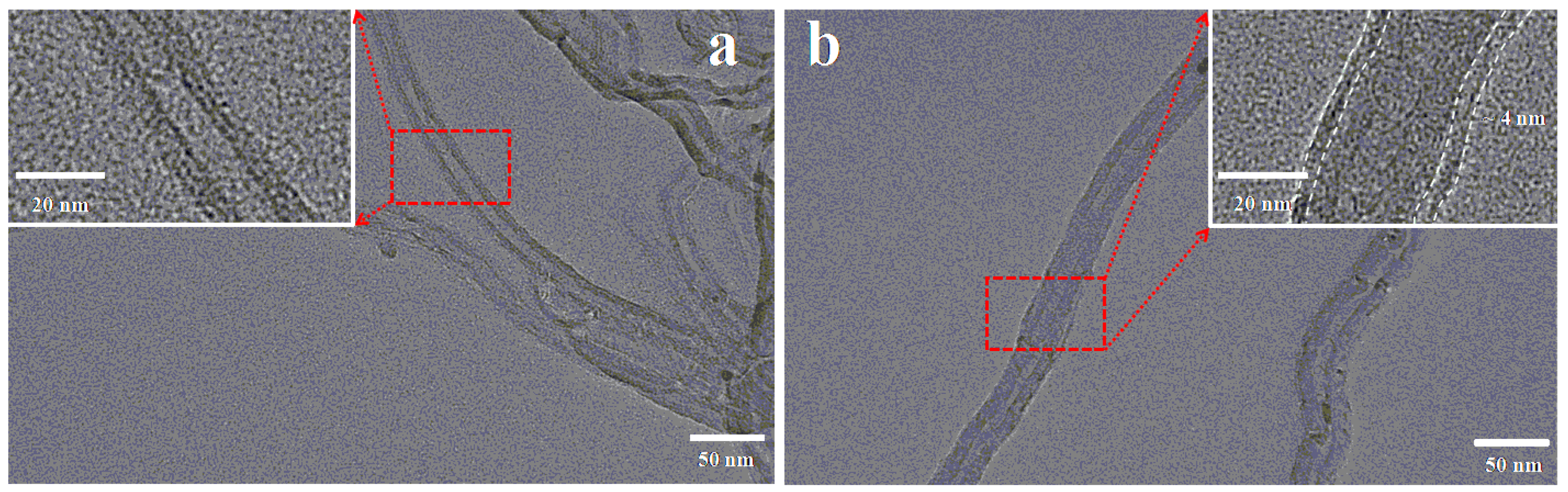
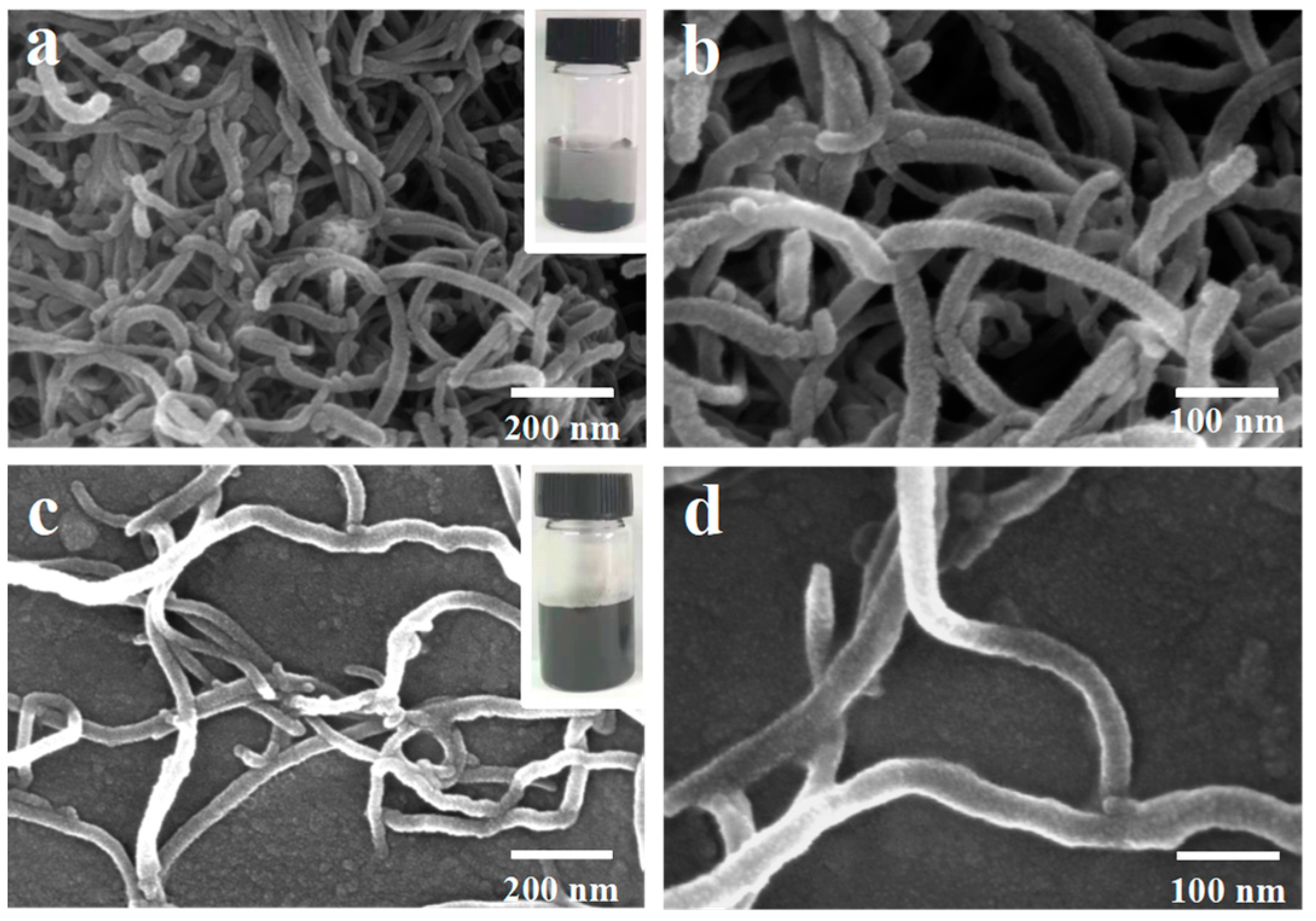

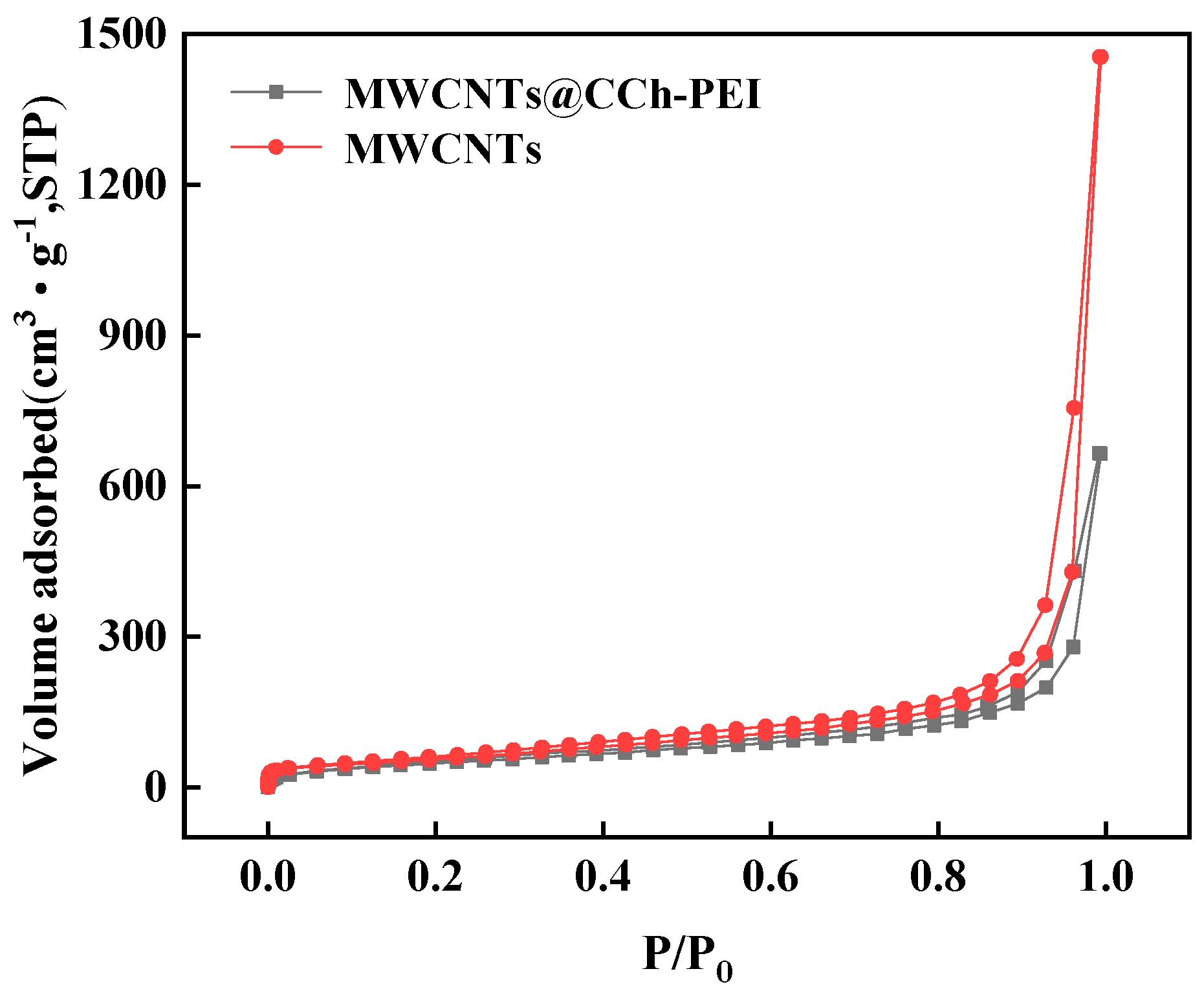


| Samples | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| MWCNTs | 215.9 | 2.3 | 4.1 |
| MWCNTs@CCh-PEI | 187.1 | 1.0 | 2.2 |
| Kinetic Model | Parameters | Initial MO Concentration (50 mg L−1) |
|---|---|---|
| qe (exp), (mg·g−1) | 238.53 | |
| Pseudo-first-order model | qe (cal), (mg g−1) | 236.82 |
| k1 (min−1) | 0.2832 | |
| R2 | 0.9481 | |
| Pseudo-second-order model | qe (cal), (mg g−1) | 252.00 |
| k2 (min−1) | 0.0020 | |
| R2 | 0.9788 | |
| Adsorption Isothermal Models | Parameters | Temperature (K) |
|---|---|---|
| 303 K | ||
| Langmuir | KL (L·mg−1) | 0.19 |
| qm (mg·g−1) | 400.00 | |
| R2 | 0.9873 | |
| RL | 0.0257–0.1742 | |
| Freundlich | KF [(mg·g−1)(L·mg−1)1/n] | 152.58 |
| n | 4.82 | |
| R2 | 0.8593 |
| Adsorbents | Maximum Adsorption Capacity (mg g−1) | Solution pH | Ref. |
|---|---|---|---|
| Oxygen functionalized CNTs | 185.18 | --- | [39] |
| Magnesium hydroxide-modified clinoptilolite | 99.84 | 7 | [40] |
| UiO-66 | 150.60 | Neutral conditions | [41] |
| PNIPAM/magnetite/multiamine-functionalized mesoporous silica composite | 225.86 | 3 | [42] |
| Ni/porous carbon CNTs | 271.00 | --- | [43] |
| S-doped magnetic mesoporous carbon | 114.20 | 4.5 | [44] |
| Poly(catechol-tetraethylenepentamine-cyanuric chloride)@hydrocellulose | 189.39 | No regulation | [45] |
| Mesoporous ZSM-5 zeolite | 25.00 | 1 | [46] |
| MWCNTs@CCh-PEI | 400.00 | 4 | This work |
| T (K) | ∆G (kJ mol−1) | ∆H (kJ mol−1) | ∆S (kJ mol−1 K−1) |
|---|---|---|---|
| 303 | −12.34 | −74.17 | −0.20 |
| 313 | −10.30 | ||
| 323 | −8.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Sun, E.; Zhao, F. Mussel-Inspired Multiwalled Carbon Nanotube Nanocomposite for Methyl Orange Removal: Adsorption and Regeneration Behaviors. Molecules 2024, 29, 3535. https://doi.org/10.3390/molecules29153535
Jiang Y, Sun E, Zhao F. Mussel-Inspired Multiwalled Carbon Nanotube Nanocomposite for Methyl Orange Removal: Adsorption and Regeneration Behaviors. Molecules. 2024; 29(15):3535. https://doi.org/10.3390/molecules29153535
Chicago/Turabian StyleJiang, Yongjian, Erqiang Sun, and Fengyang Zhao. 2024. "Mussel-Inspired Multiwalled Carbon Nanotube Nanocomposite for Methyl Orange Removal: Adsorption and Regeneration Behaviors" Molecules 29, no. 15: 3535. https://doi.org/10.3390/molecules29153535





