Infrared Matrix-Isolation and Theoretical Studies of the Reactions of Bis(benzene)chromium with Ozone
Abstract
:1. Introduction
2. Results and Discussion
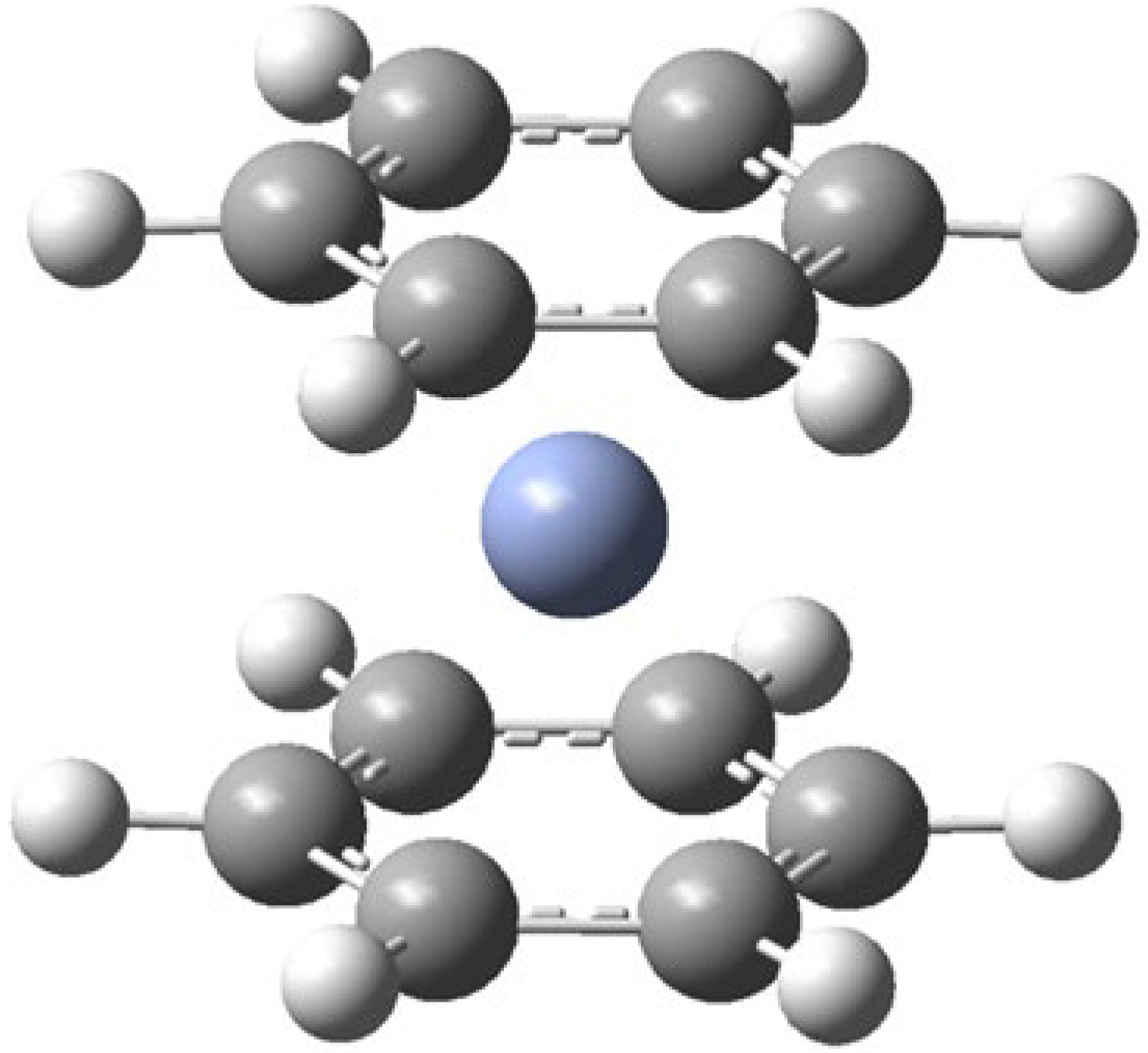
- Bis(benzene)chromium (Bz2Cr) blank
- 2.
- The reaction of Bz2Cr with O3 in an Ar matrix
3. Materials and Methods
4. Conclusions
- 1.
- Bis(benzene)chromium (Bz2Cr) was isolated in an Ar matrix from the vapor pressure of Bz2Cr(s) at 85 °C fed into a stream of argon gas.
- 2.
- The infrared spectrum of the resulting matrix-isolated Bz2Cr agrees with the infrared spectrum of matrix-isolated Bz2Cr produced by co-deposition of C6H6(g) and Cr(g) with Ar(g) [11].
- 3.
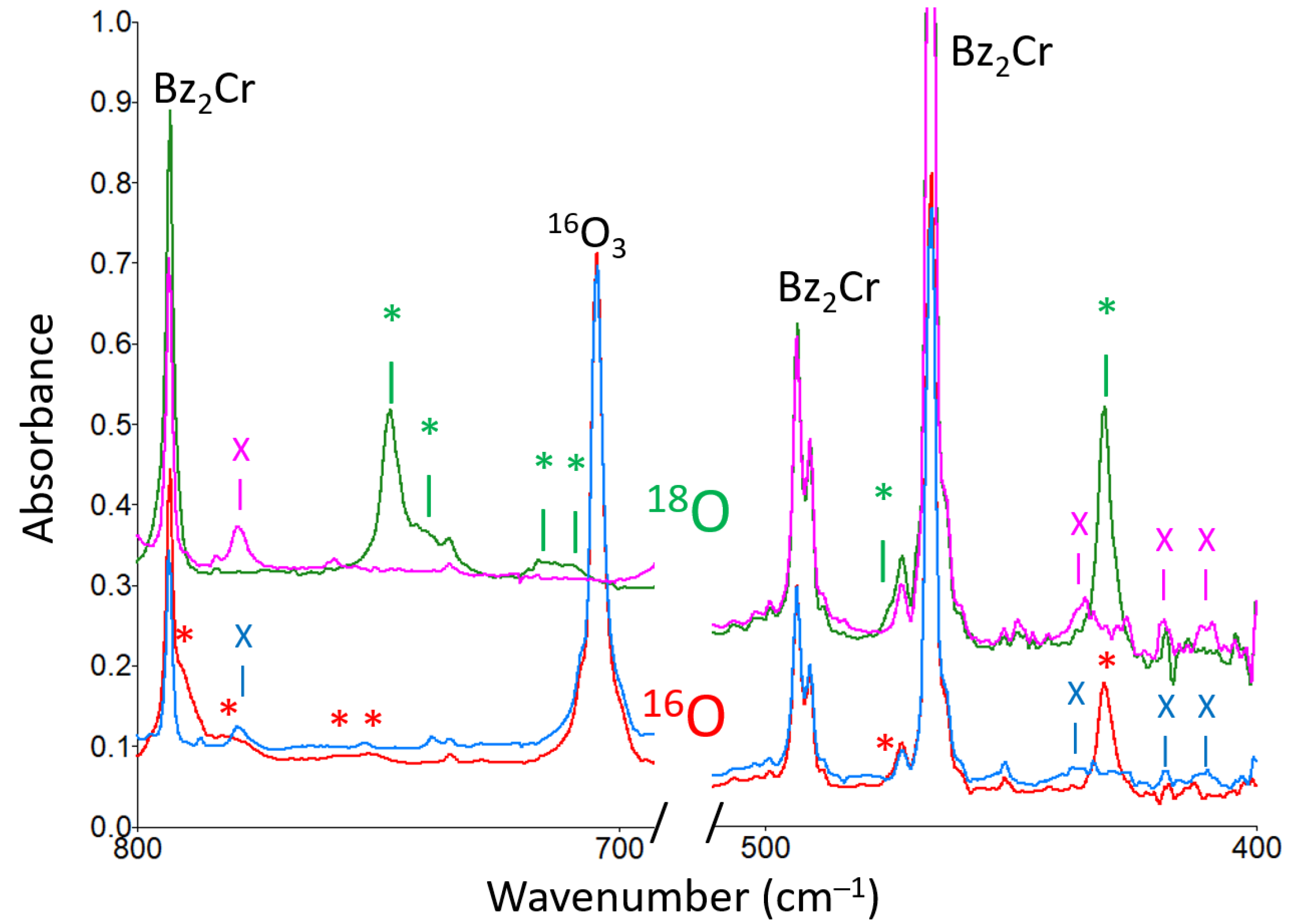
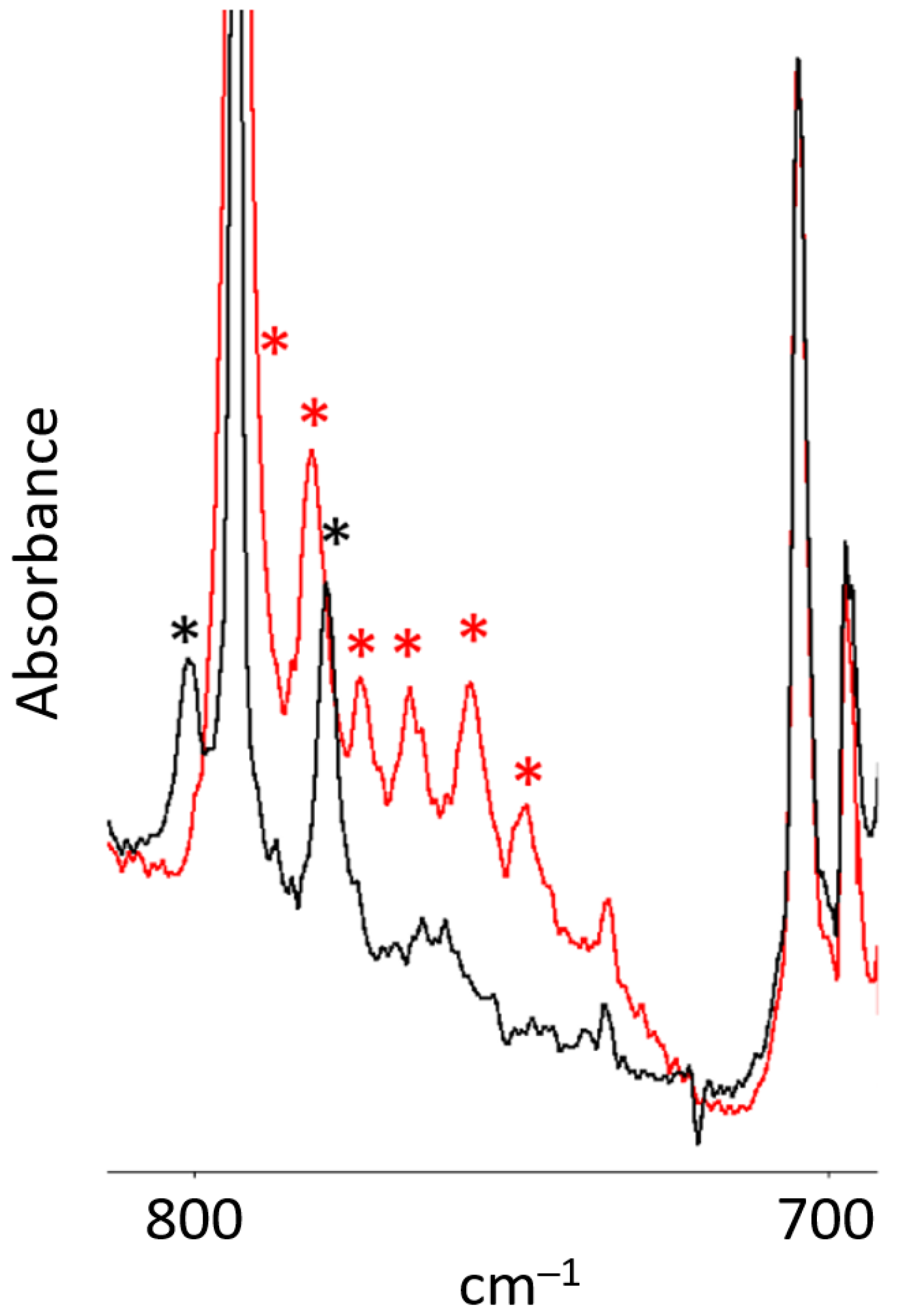
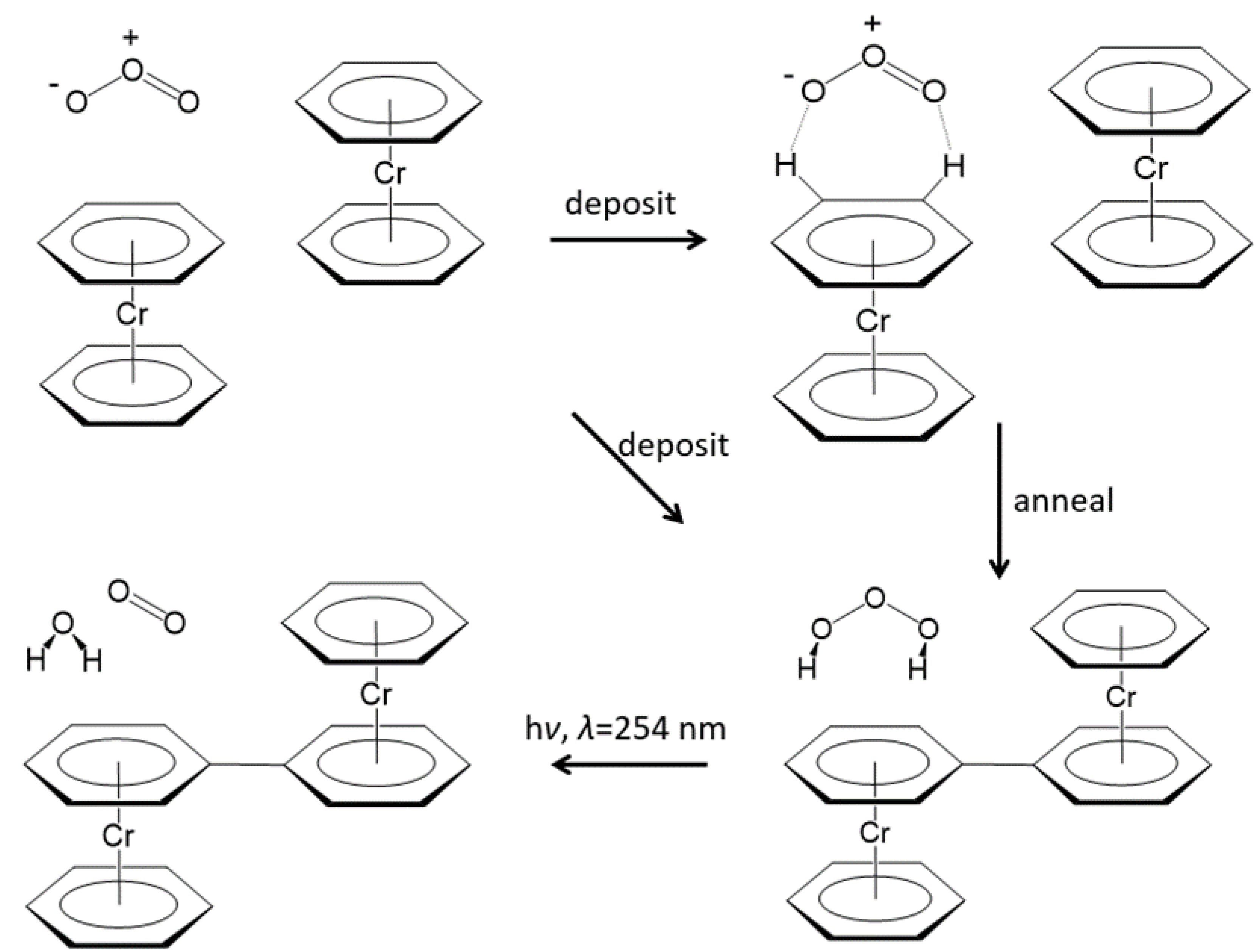
- When bis(benzene)chromium (Bz2Cr) was co-deposited in the dark with ozone (O3), a new product was formed upon deposition that increased upon annealing to 35 K, and was destroyed by UV irradiation at 254 nm.
- 4.
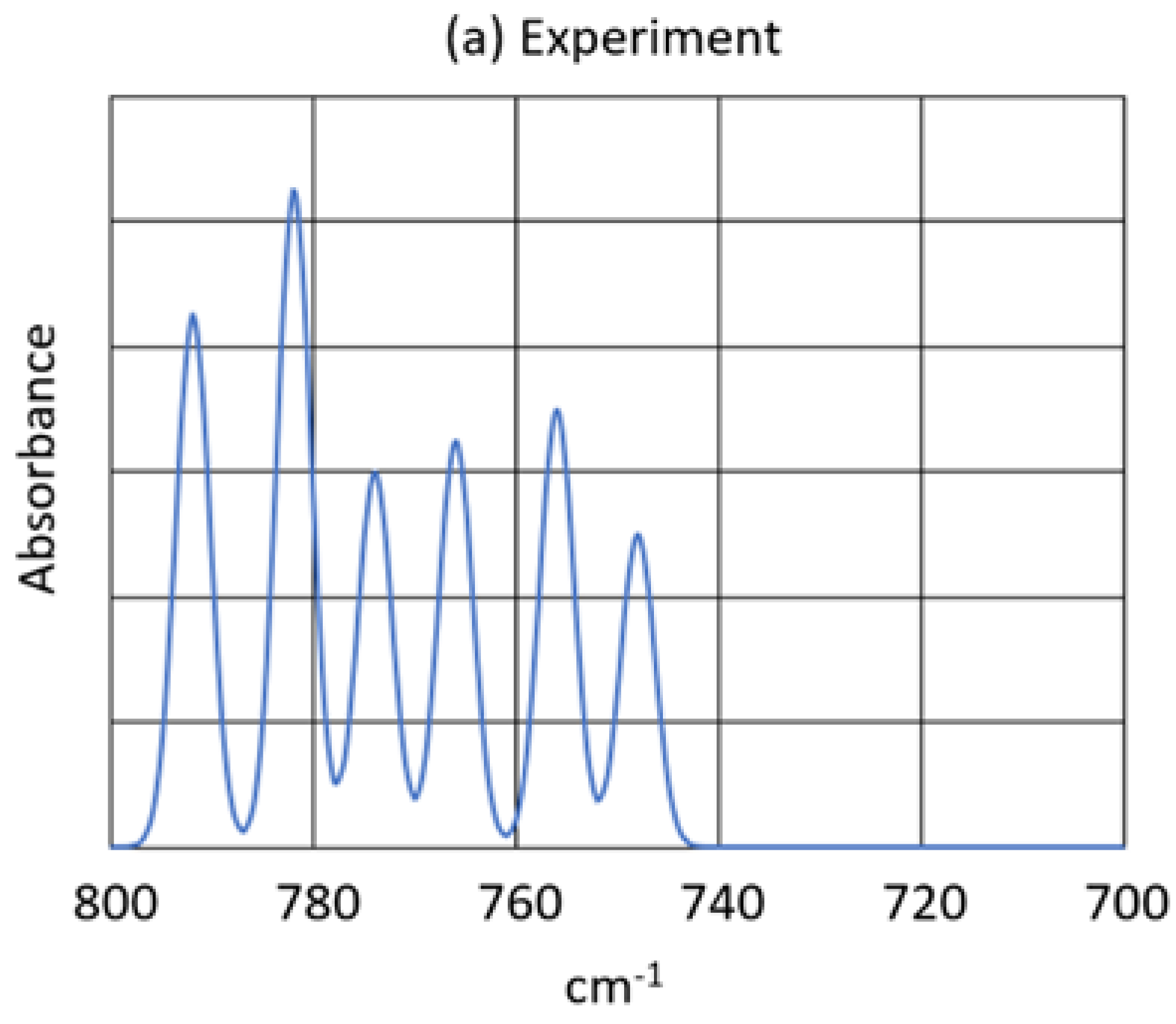
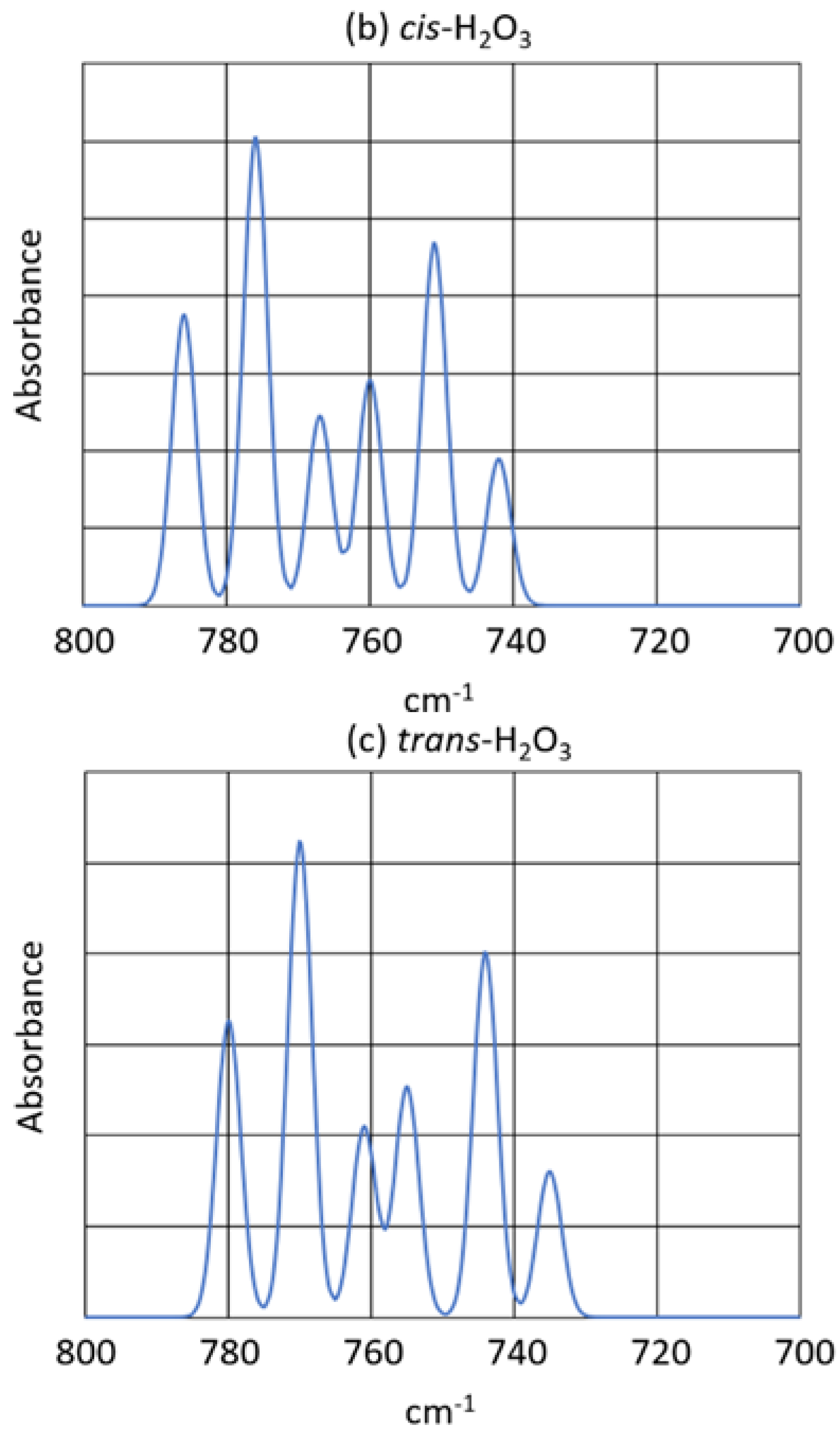
- The new product showed two strong bands in the infrared spectrum, at and at , as well as some minor peaks. The peak showed virtually no oxygen-18 isotope shift, but the peak showed an exceptionally large oxygen-18 isotope shift consistent with the antisymmetric O-O-O stretch of a symmetric ozonide product.
- 5.
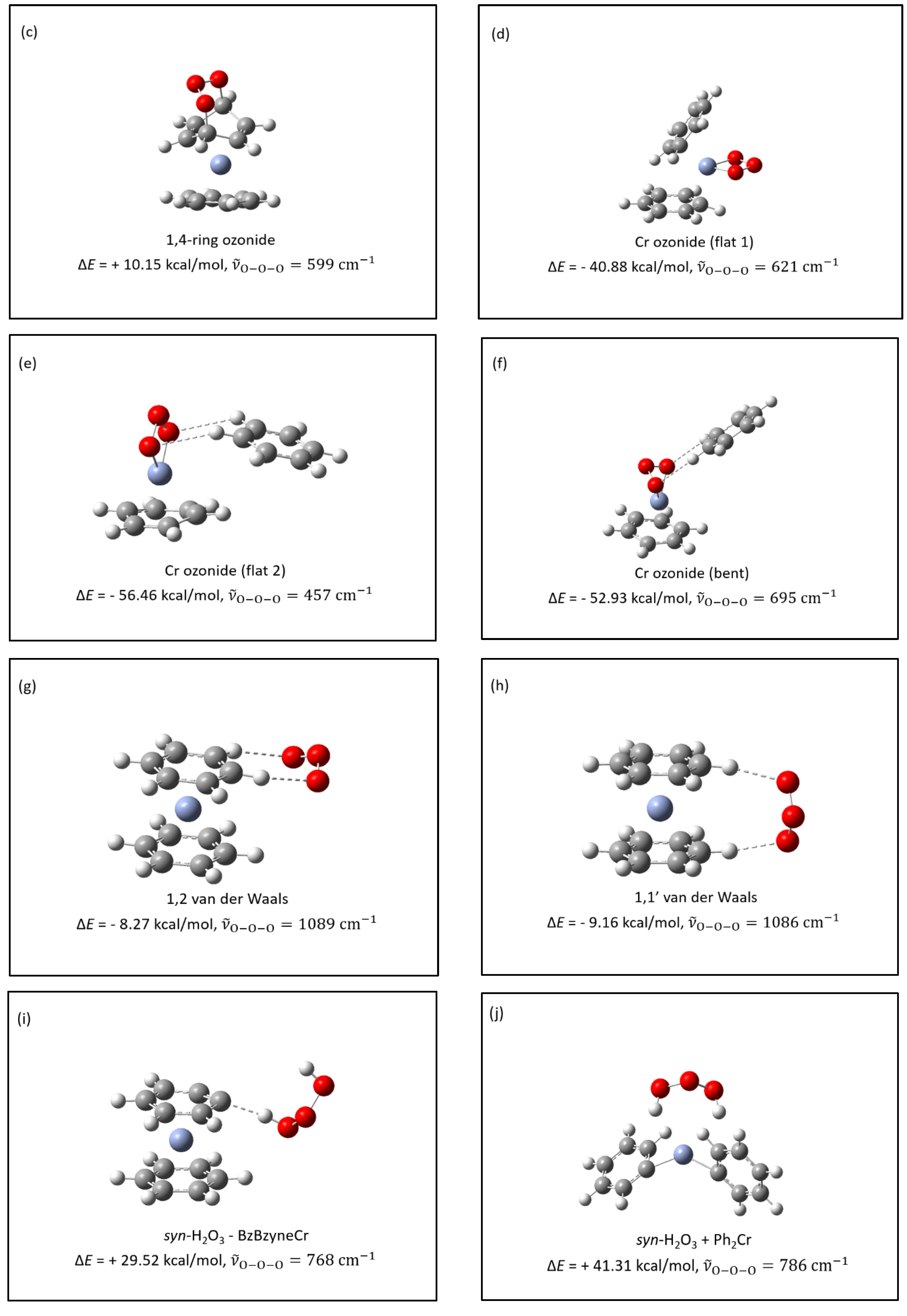
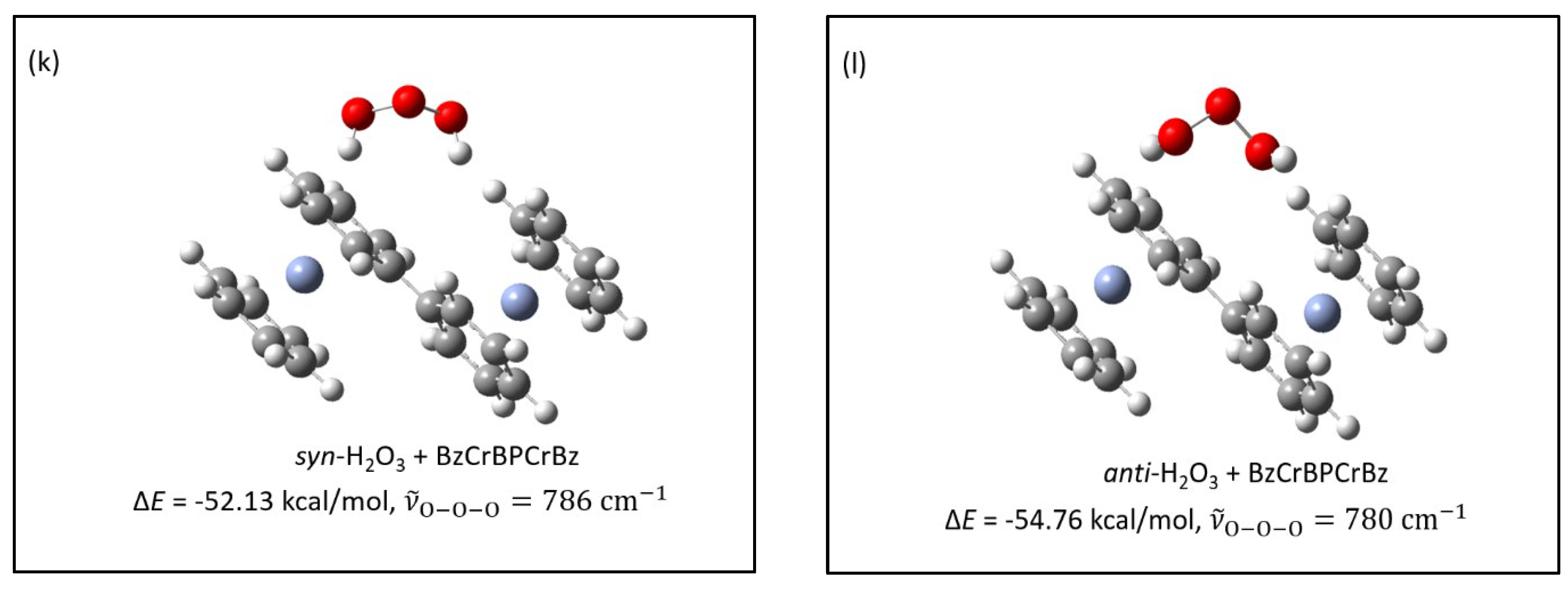
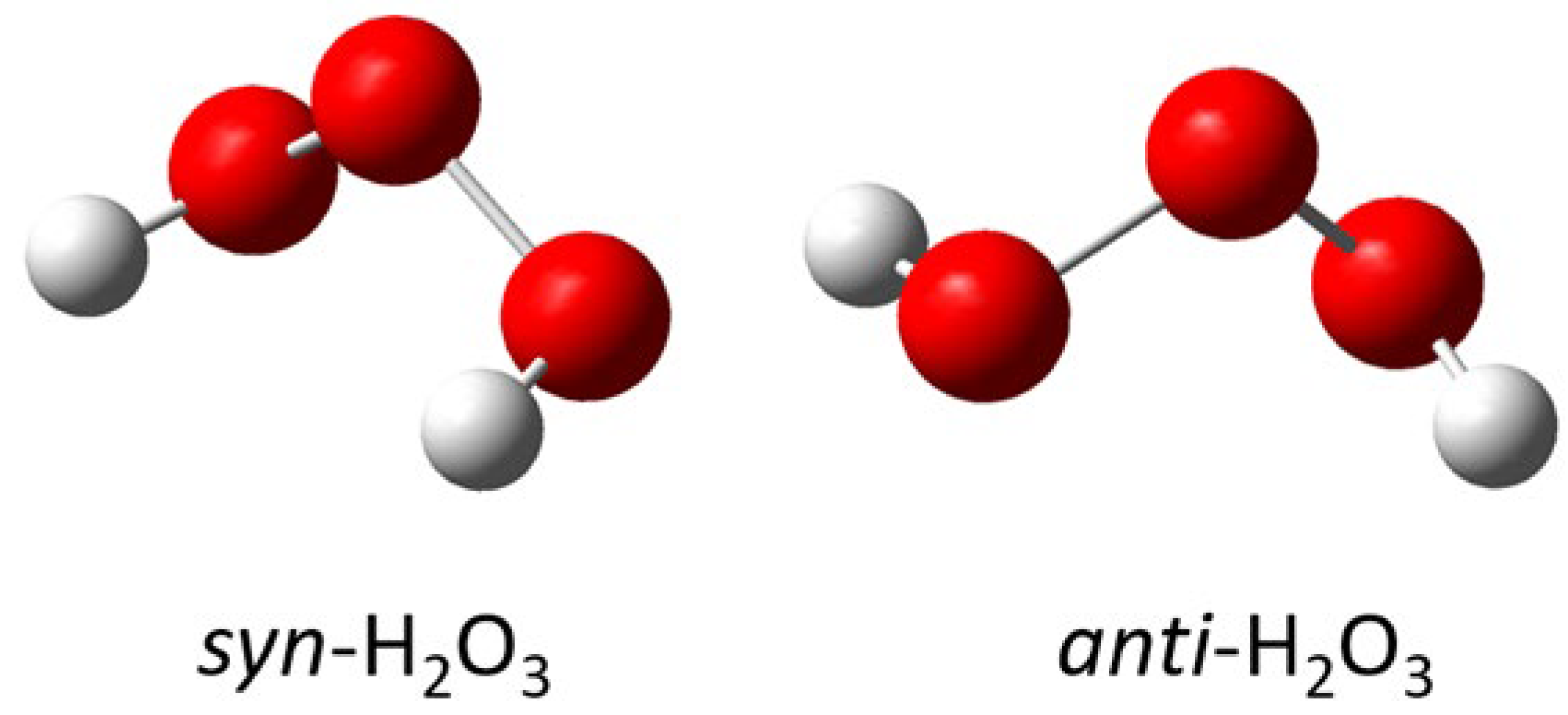
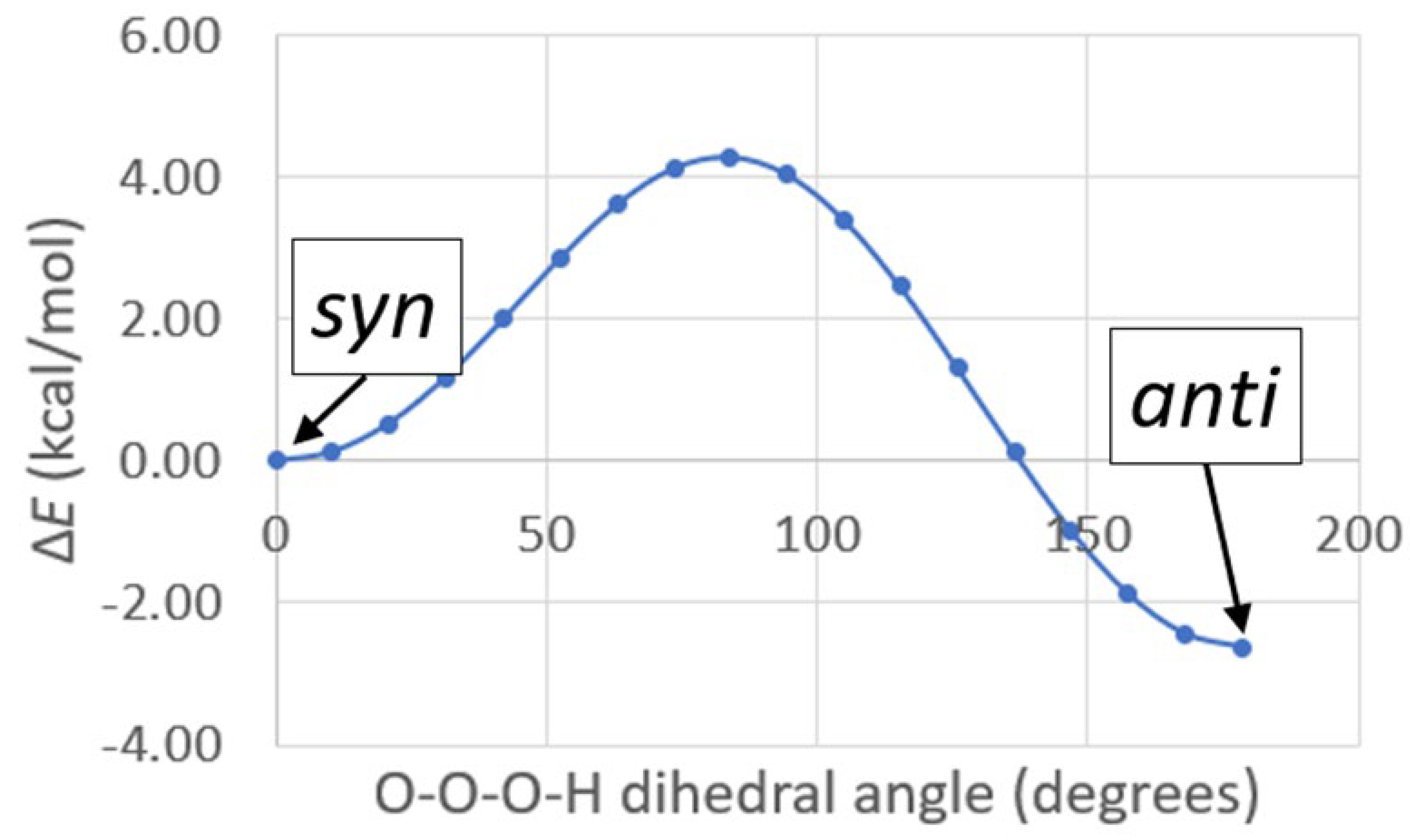
- The structures of several possible symmetric ozonide products of the reaction between O3 and Bz2Cr were calculated. The best fit of the antisymmetric stretch and its oxygen-18 isotope shifts was shown by hydrogen ozonide (H2O3) and its oxygen-18 isotopomers.
- 6.
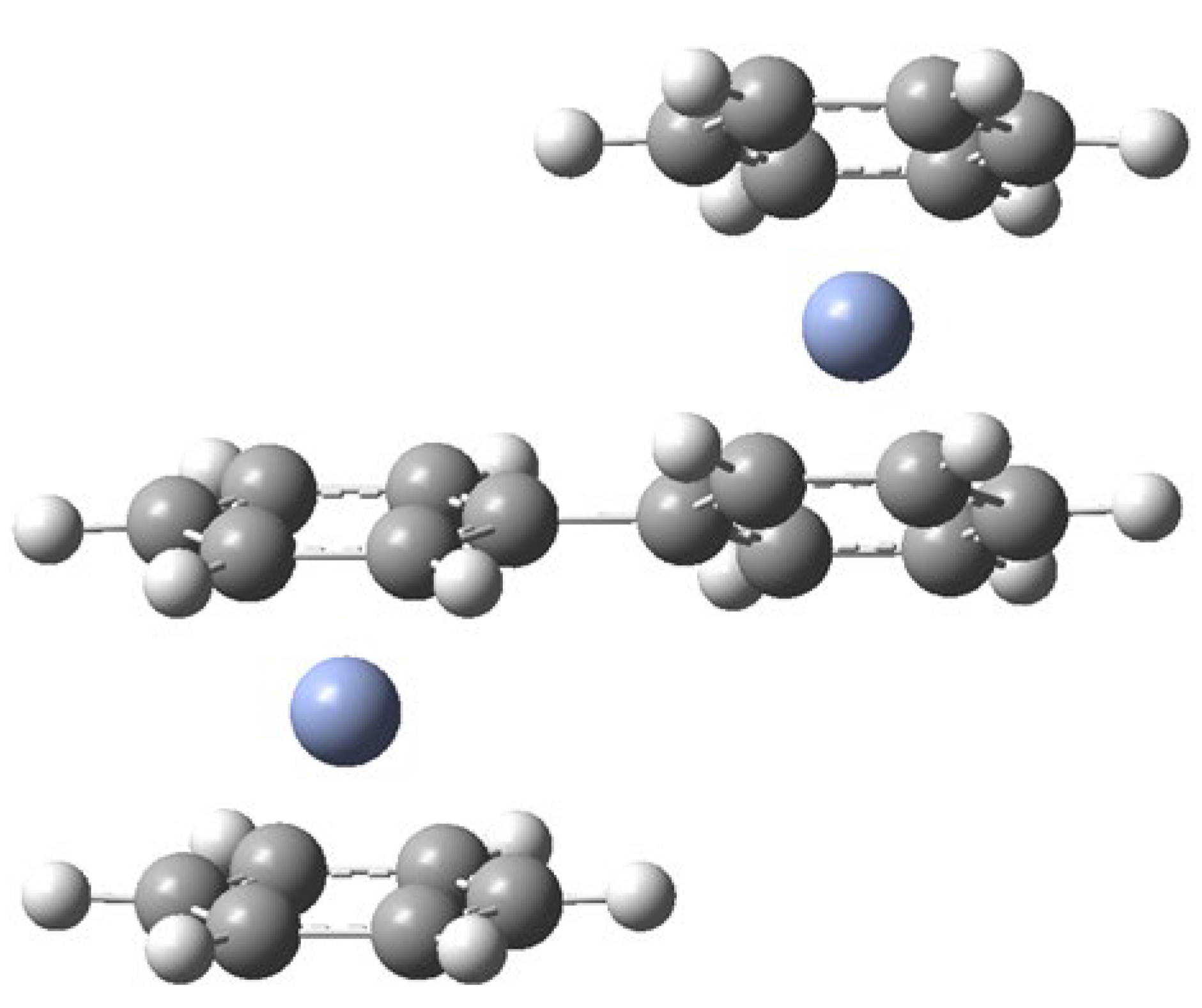
- The formation of H2O3 in this reaction is undoubtedly facilitated by the unusually strong intermolecular interactions of ozone with the Bz2Cr hydrogen atoms, which have calculated interaction energies of −8.27 to −9.16 kcal/mol, rivaling those of traditional hydrogen bonding.
- 7.
- H2O3 can be formed in an energetically favorable process if the hydrogen-deficient benzene-benzyne-Cr (BzByCr) couples with another Bz2Cr molecule and rearranges to benzene-Cr-biphenyl-Cr-benzene (BzCrBPCrBz), which was previously observed [21].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahir, A.A.; Wijayntha, K.G.U.; Saremi-Yarahmadi, S.; Mazhar, M.; McKee, V. Nanostructured α-Fe2O3 thin films for photoelectrochemical hydrogen generation. Chem. Mater. 2009, 21, 3763–3772. [Google Scholar] [CrossRef]
- Martinson, A.B.F.; DeVries, M.J.; Libera, J.A.; Christensen, S.T.; Hupp, J.T.; Pellin, M.J.; Elam, J.W. Atomic layer deposition of Fe2O3 using ferrocene and ozone. J. Phys. Chem. C 2011, 115, 4333–4339. [Google Scholar] [CrossRef]
- Aaltonen, T.; Ale’n, P.; Ritala, M.; Leskelä, M. Ruthenium thin films grown by atomic layer deposition. Chem. Vap. Depos. 2003, 9, 45–49. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Kim, Y.C.; Ahn, Y.H.; Lee, S.; Park, J.-Y. Large-area growth of high-quality graphene/MoS2 vertical heterostructures by chemical vapor deposition with nucleation control. Carbon 2020, 168, 580–587. [Google Scholar] [CrossRef]
- Topka, K.C.; Chliavoras, G.A.; Senocq, F.; Vergnes, H.; Samelor, D.; Sadowski, D.; Vahlas, C.; Caussat, B. Large temperature range model for the atmospheric pressure chemical vapor deposition of silicon dioxide films on thermosensitive substrates. Chem. Eng. Res. Des. 2020, 161, 146–158. [Google Scholar] [CrossRef]
- Maury, F.; Vahlas, C.; Abisset, S.; Gueroudji, L. Low temperature metalloorganic chemical vapor deposition routes to chromium metal thin folms using bis(benzene)chromium. J. Electrochem. Soc. 1999, 10, 3716–3723. [Google Scholar] [CrossRef]
- Kugel, R.W.; Pinelo, L.F.; Ault, B.S. Infrared matrix-isolation and theoretical studies of the reactions of ferrocene with ozone. J. Phys. Chem. A 2015, 119, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Kugel, R.W.; Ault, B.S. Infrared matrix-isolation and theoretical study of the reactions of ruthenocene with ozone. J. Phys. Chem. A 2019, 123, 5768–5780. [Google Scholar] [CrossRef] [PubMed]
- Hoops, M.D.; Ault, B.S. Matrix isolation study of the early intermediates in the ozonolysis of cyclopentene and cyclopentadiene: Observation of two Criegee intermediates. J. Am. Chem. Soc. 2009, 131, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Ault, B.S. Matrix Isolation Spectroscopic Studies: Thermal and Soft Photochemical Bimolecular Reactions. In Frontiers and Advances in Molecular Spectroscopy; Laane, J., Ed.; Elsevier Press: Amsterdam, The Netherlands, 2017; pp. 667–712. [Google Scholar]
- Boyd, J.W.; Lavoie, J.M.; Gruen, D.M. Direct synthesis and characterization of dibenzenechromium(0) in an argon matrix at 14°K. J. Chem. Phys. 1974, 60, 4088–4089. [Google Scholar] [CrossRef]
- Ngai, L.H.; Stafford, F.E.; Schafer, L. The symmetry of gaseous dibenzenechromium. J. Am. Chem. Soc. 1969, 91, 48–49. [Google Scholar] [CrossRef]
- Fritz, H.P.; Lüttke, W.; Stammreich, H.; Forneris, R. IR- und Raman-untersuchungen zur struktur des di-benzol-chroms, seines kations sowie verwandter verbindungen. Spectrochim. Acta 1961, 17, 1068–1091. [Google Scholar] [CrossRef]
- Engdahl, A.; Nelander, B. The vibrational spectrum of H2O3. Science 2002, 295, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, L.; Gudmundsdottir, A.D.; Ault, B.S. Matrix Isolation Study of the Ozonolysis of 1,3- and 1,4-Cyclohexadiene: Identification of Novel Reaction Pathways. J. Phys. Chem. A 2013, 117, 4174–4182. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Costa, A.L.; Grepioni, F.; Scaccianoce, L.; Tagliavini, E. OH–O and CH–O hydrogen bonding in hydrated crystals of paramagnetic [(η6-C6H6)2Cr]+. Organometallics 1996, 15, 1084–1086. [Google Scholar] [CrossRef]
- Miller, R.G.; Stiles, M. Reaction of Benzyne with Benzene and Naphthalene. J. Am. Chem. Soc. 1963, 85, 1798–1800. [Google Scholar] [CrossRef]
- Stiles, M.; Burckhardt, U.; Freund, G. The Reaction of Benzyne with Benzene. J. Org. Chem. 1967, 32, 3718–3719. [Google Scholar] [CrossRef]
- Seyferth, D. Bis(benzene)chromium. 1. Franz Hein at the University of Leipzig and Harold Zeiss and Minoru Tsutsui at Yale. Organometallics 2002, 21, 1520–1530. [Google Scholar] [CrossRef]
- Hein, F.; Eissner, W. Über das Tetraphenylchrom (C6H5)4Cr. (VI. Mitteilung über Chromorganische Verbindungen). Ber. Dtsch. Chem. Ges. 1926, 59, 362. [Google Scholar] [CrossRef]
- Seyferth, D. Bis(benzene)chromium. 2. Its discovery by E. O. Fischer and W. Hafner and subsequent work by the research groups of E. O. Fischer, H. H. Zeiss, F. Hein, C. Elschenbroich, and others. Organometallics 2002, 21, 2800–2820. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]

| Experiment a | Literature b | Calculations a | ||||
|---|---|---|---|---|---|---|
| Bz2Cr Vaporized at 85 C and Co-Deposited with Argon at 15 K | Bz2Cr Formed in the Ar Matrix on Deposition | DFT/B3LYP 6-311G++(d,2p) | ||||
| A (a.u.) c | A (a.u.) | d | Assignment | |||
| 467 | 0.65 | 466 | 0.81 | 435 | 177 d | |
| 491 494 | 0.16 0.20 | 492 | 0.23 | 478 478 | 42 42 | |
| 793 | 0.22 | 793 | 0.15 | 798 | 58 | |
| 861 | 0.04 | 862 | 0.02 | 876 876 | 7 7 | |
| 974 | 0.10 | 974 | 0.13 | 986 | 25 | |
| 1003 | 0.03 | 1001 | 0.03 | 1021 1021 | 9 9 | |
| 1433 | 0.07 | 1433 | 0.06 | 1466 1466 | 2 2 | |
| 3060 | 0.19 | 3062 | 0.11 | 3179 3179 | 83 83 | |
| 3185 | 18 | |||||
| New Peaks Observed upon Deposition a of Bz2Cr and O3 in Solid Ar at 15 K | ||
|---|---|---|
| Ab (a.u.) | ||
| 431 | 0.16 | 0 |
| 472 d | 0.03 | 0 |
| 622 | 0.02 | −35 |
| 752 | 0.02 | −42 |
| 758 | 0.02 | −41 |
| 782 | 0.03 | −42 |
| 792 | 0.12 | −44 |
| 865 d | 0.02 | −23 |
| 890 d | 0.03 | −21 |
| 1007 d | 0.03 | - |
| 1431 | 0.03 | −2 |
| Isotopomer Peaks for the 16,18O3 Scrambled Mixed Isotope Experiment | |
|---|---|
| A (a.u.) | |
| 748 | 0.040 |
| 756 | 0.056 |
| 766 | 0.052 |
| 774 | 0.048 |
| 782 | 0.084 |
| 792 | ? a |
| Symmetric Ozonide | |||
|---|---|---|---|
| (kcal/mol) | (cm−1) (km/mol) | ||
| (a) | 1,2-ring ozonide (exo) | −8.47 | 721 (14) |
| (b) | 1,2-ring ozonide (endo) | −8.62 | 687 (20) |
| (c) | 1,4-ring ozonide | +10.15 | 599 (14) |
| (d) | Cr-ozonide (flat 1) | −40.88 | 621 (26) |
| (e) | Cr-ozonide (flat 2) | −56.46 | 457 (6) |
| (f) | Cr-ozonide (bent) | −52.93 | 695 (33) |
| (g) | 1,2 van der Waals | −8.27 | 1089 (168) |
| (h) | 1,1′ van der Waals | −9.16 | 1086 (155) |
| (i) | syn-H2O3 + BzByCr | +29.52 | 786 (113) |
| (j) | syn-H2O3 + Ph2Cr | +41.31 | 786 (113) |
| 2 Bz2Cr + O3 | |||
| (k) | syn-H2O3 + BzCrBPCrBz | −52.13 | 786 (113) |
| 2 Bz2Cr + O3 | |||
| (l) | anti-H2O3 + BzCrBPCrBz | −54.76 | 780 (98) |
| (m) | Ozone, O3 a | --- | 1183 (239) |
| Experiment a | Literature b | Calculations a | ||||
|---|---|---|---|---|---|---|
| New Peaks Observed upon Deposition of Bz2Cr and O3 in Solid Ar at 15 K | DFT/B3LYP 6-311G++(d,2p) | |||||
| A (a.u.) | ||||||
| 431 | 0.14 | 0 | 387 | 444 | 118 | −3 |
| 622 | 0.02 | −35 | 509 | 514 | 9 | −29 |
| 792 | 0.10 | −44 | 776 | 786 | 113 | −44 |
| 1431 | 0.03 | −2 | 1359 | 1377 | 45 | −6 |
| - | - | - | 3530 | 3731 | 36 | −13 |
| Experiment | syn-H2O3 | anti-H2O3 | Assignment | |||
|---|---|---|---|---|---|---|
| 6 Represents 16O | ||||||
| 8 Represents 18O | ||||||
| 792 | 0 | 786 | 0 | 780 | 0 | 666 |
| 782 | 10 | 776 | 10 | 770 | 10 | 668 and 866 |
| 774 | 18 | 767 | 19 | 761 | 19 | 868 |
| 766 | 26 | 760 | 26 | 755 | 25 | 686 |
| 756 | 36 | 751 | 35 | 744 | 36 | 688 and 886 |
| 748 | 44 | 742 | 44 | 735 | 45 | 888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kugel, R.W.; Ault, B.S. Infrared Matrix-Isolation and Theoretical Studies of the Reactions of Bis(benzene)chromium with Ozone. Molecules 2024, 29, 3583. https://doi.org/10.3390/molecules29153583
Kugel RW, Ault BS. Infrared Matrix-Isolation and Theoretical Studies of the Reactions of Bis(benzene)chromium with Ozone. Molecules. 2024; 29(15):3583. https://doi.org/10.3390/molecules29153583
Chicago/Turabian StyleKugel, Roger W., and Bruce S. Ault. 2024. "Infrared Matrix-Isolation and Theoretical Studies of the Reactions of Bis(benzene)chromium with Ozone" Molecules 29, no. 15: 3583. https://doi.org/10.3390/molecules29153583





