Abstract
The equilibrium structures of silyl iodide, SiH3I, and silylene halides, SiHX (X = F, Cl, Br, I), were determined by using the mixed regression method, where approximate values of the rotational constants are supplemented by the structural parameters of a different origin. For this goal, it is shown that the r(Si-H) bond length can be determined by using the isolated SiH stretching frequency and that an accurate estimation of the bond angles is obtained by an MP2 calculation with a basis set of triple zeta quality. To check the accuracy of the experimental structures, they were also optimized by means of all electron CCSD(T) calculations using basis sets of quadruple zeta quality.
1. Introduction
Equilibrium structures are known for silyl fluoride, SiH3F [1], silyl chloride, SiH3Cl [2], silyl bromide, SiH3Br [3], SiH3CN [4], and silane, SiH4 [5]. It is not easy to determine an accurate equilibrium structure (corresponding to the minimum of the potential hypersurface) because, in addition to the ground state rotational constants, it requires the knowledge of at least the vibration–rotation interaction constants of all fundamental vibrational states or, in other words, the rotational constants of all fundamental vibrational states. This is often a difficult problem because some of these vibrational states are perturbed by nearby states (through anharmonic resonances or Coriolis interactions) and reliable analysis of the corresponding rovibrational spectra is not straightforward. This is particularly true for a molecule such as silyl iodide, SiH3I, whose rovibrational spectrum is complicated by the large quadrupole hyperfine structure due to the iodine nucleus. This is furthermore complicated by the small value of the B rotational constant, making the rovibrational spectrum very dense. For these reasons, approximate structures are often obtained by empirical methods. This is the case for SiH3I for which only empirical effective structures (r0) are available [6,7]. For this kind of empirical structure, all the rovibrational effects are neglected assuming that the equilibrium rotational constants are identical to the ground state rotational constants. These two r0-structures are significantly different and none of them is expected to be close to the equilibrium structure. This is unfortunate because no equilibrium value is known for the Si-I bond length. Actually, a r0 structure is available for monoiodosilylene, HSiI [8,9], but it is not expected to be a good approximation of the equilibrium structure.
The goal of this work is to present a set of consistent and accurate values for the Si-H bond length and to determine the equilibrium structure of SiH3I and of the silylene halides, HSiX (X = F, Cl, Br, I).
The Section 2 describes the used quantum chemical methods. The Section 3 tries to determine an accurate value for the Si-H bond length. The Section 4 estimates the accuracy of the ∠(HSiH) and ∠(HSiX) bond angles. The Section 5 is dedicated to the structure of silyl iodide and the Section 6 to the structure of halosilylenes. A general conclusion is provided in Section 7.
2. Quantum Chemical Calculations
The structure of the molecules was optimized using three different methods: (i) the second-order Møller–Plesset perturbation theory (MP2) [10], (ii) the hybrid density functional B3LYP [11,12], and (iii) the coupled-cluster method including single and double excitations (CCSD) [13] augmented with a perturbational estimate of the effects of connected triple excitations, CCSD(T) [14]. Several basis sets were used for frozen core calculations: Dunning’s correlation consistent polarized valence triple-zeta basis set: cc-pVTZ for first-row atoms [15], cc-pV(T+d)Z for second-row atoms [16], and for larger atoms, cc-pVTZ-PP with the corresponding small core relativistic pseudopotential [17]. The def2-SVP basis set [18] was also used as well as the 6-311G** basis set [19] for iodine and the 6-311+G(3df, 2pd) [20] for all other atoms.
CCSD(T) calculations correlating all electrons were also performed with the cc-pwCVQZ basis set for first- and second-row atoms [21] and with the cc-pwCVQZ-PP basis set and the corresponding small core pseudopotential [22] for larger atoms.
The CCSD(T) calculations were performed with Molpro [23,24] and the MP2 and B3LYP calculations with Gaussian16 [25].
Different Types of Structures
The equilibrium structure corresponding to the minimum of the potential hypersurface is obtained by high-level ab initio optimizations. It can also be determined experimentally from a fit of the equilibrium rotational constants (or the corresponding moments of inertia). The equilibrium rotational constants, Be, are obtained by correcting the ground state rotational constants, B0, from the rovibrational contribution: where αi are the vibration–rotation interaction constants and di the degeneracies of these states. The summation is over all the normal modes. The αi are derived from the rotational constants of the fundamental vibrational states. Alternatively, they can be calculated from an ab initio cubic force field giving the semi-experimental equilibrium structure.
As it is not always easy to determine an equilibrium structure, many empirical methods have been devised.
The simplest one is the effective structure, r0, which assumes that the equilibrium rotational constants are identical to the ground state rotational constants. Although the rovibrational correction is only a few percent of the rotational constants, it can be a poor approximation of the equilibrium structure.
A slightly better method is the substitution structure, rs, which assumes that the rovibrational correction is isotopically independent. In this case, the difference between the moment inertia of an isotopologue and the parent species is used.
The rz distance is the distance between the average nuclear positions in the vibrational ground state at 0 K. It has a clear physical meaning permitting comparisons between molecules. In this case, it is assumed that the anharmonic part of the rovibrational correction is zero. The rz bond length may be used to estimate the equilibrium using the “diatomic approximation” where it is assumed that only the stretching cubic force constant of the bond is different from zero (it is often assumed to be equal to the value of the corresponding diatomic molecule). The accuracy of the re-bond lengths derived from the rz structure is limited due to the diatomic approximation, and it cannot be better than the accuracy of the rz structure, which can be rather poor, in particular, for large molecules. Its accuracy is perhaps not better than a few thousandths of an Å.
3. Si-H Bond Length
It is well established that the MP2 method with a basis set of triple zeta quality gives a satisfactory approximation of the C-H bond length [26]. The same conclusion is valid for the Si-H bond, as shown in Table 1 where our ab initio values are compared to the equilibrium values [1,2,3,4,5,27,28,29,30,31,32,33,34,35,36].

Table 1.
Equilibrium values for the Si-H bond length (in Å).
The median of residuals (re–rMP2) is −0.0008 Å and the mean is −0.0004 Å. The corresponding MAD (mean absolute deviation) is 0.0005 Å corresponding to a standard deviation of 0.0008 Å. In other words, the MP2/cc-pV(T+d)Z level of theory gives a value that is slightly too large (by about 0.0008 Å) but with an excellent accuracy (standard deviation of 0.0008 Å). However, it has to be noted that there are large residuals for most silylene derivatives (up to 0.0095 Å for HSiBr). This discrepancy will be discussed later.
There is another way to estimate the Si-H bond length. It was noticed by Bernstein [43] and developed by McKean [44] that there is a correlation between the isolated C-H stretching frequency (i.e., when only one hydrogen atom is present in the molecule) and the C-H bond length. This correlation permits us to determine the C-H bond length with an accuracy of 0.001 Å [45]. McKean extended this method to the Si-H bond [46,47]; however, to establish the correlation, he used empirical (r0 or rs) bond lengths. Using instead equilibrium values, this correlation is confirmed, as shown in Table 1, where most νis(Si–H) values are taken from [6], see also Table 1 for specific references [27,28,32,34,37,38,39,40,41,42]. A fit of 11 data gives the following linear expression:
with a correlation coefficient ρ = 0.9997 and a standard deviation σ = 0.0004 Å.
re(Si–H) [Å] = 1.8823(27) − 1.871(12) × 10−4νis(Si–H) [cm−1]
Using Equation (1) and the ab initio results allows us to confirm that the published Si-H bond lengths of most silylene halides are probably inaccurate, see Table 1. This conclusion also applies to dichlorosilane, SiH2Cl2 [27]. The origin of this discrepancy is easy to explain: the re structures of these molecules were estimated from the rz structure and it is known that, in some cases, the method can be inaccurate when the difference rz–re is large. This is particularly true for the X-H bond lengths. Furthermore, the rz structure used for the extrapolation is already not accurate enough.
4. (HSiH) Bond Angles
The MP2 method with a basis set of at least triple zeta quality is able to predict the bond angles with an accuracy generally better than 0.4° [48]. This is well verified for the ∠(HSiX) (X = F, Cl, Br, I, CN, SiH3, =CH2) bond angles as shown in Table 2.

Table 2.
Equilibrium ∠(HSiH) and ∠(HSiX) bond angles in degrees (X = F. Cl. Br. I. …).
The median as well as the mean of residuals is −0.12°. The corresponding MAD is 0.11° corresponding to a standard deviation of 0.17°, which may be considered as small. Again, the silylene compounds show large deviations.
5. Structure of SiH3I
The method to determine the Si-I bond length is to use a well-conditioned least-squares fit. In such a case, the result is not sensitive to the accuracy of the rovibrational correction as shown for instance in the case of difluoroethane, CH2=CF2 [49]. One way to obtain a well-conditioned system is to use the method of mixed regression [50]. In this method, structures are fitted concurrently to the moments of inertia and bond parameters obtained from another method. The structures obtained in this work are determined by only three parameters: the Si-H and Si-X bond lengths, and the ∠(HSiX) (or ∠(HSiH)) bond angle. As shown in Section 3 and Section 4, two of these parameters can be determined accurately and can, therefore, be used as predicate observations in the least-squares fit. Everything happens as if there is only one parameter to determine. However, an approximate value of the rovibrational correction is required.
For SiH3I, it is relatively easy because it is known that the rovibrational correction ε = I0 − Ie is approximately proportional to the square root of the moment of inertia [51]. However, empirically, the exponent of I0 is often found to be different from ½. To determine it, the ε of two sets of molecules was used: CH3Br and CH3I, and SiH3Cl and SiH3Br. For both sets, an exponent of 0.777 was found. This allowed us to deduce an ε value of 0.561 μÅ2 for SiH3I from that of 0.452 μÅ2 for SiH3Br. A one-dimensional fit using Si-H = 1.471 Å and ∠(HSiH) = 110.54° gives Si-I = 2.4327 Å. When only one parameter is fitted, the least-squares system is well conditioned (condition number = 1). Therefore, there is no amplification of errors. In this case, ∂r(Si-I)/∂ε = 0.0082 μ−1Å−1 to be compared with the value of ε which is about 0.66 μÅ2. Assuming an error of 0.001 Å for Si-H, 0.3° for ∠(HSiH), and a rather large error of 0.1 μÅ2 for ε, gives an uncertainty of 0.0012 Å, see Table 3. Using the isotopologues 28Si and 30Si for the moments of inertia gives identical results. This value is significantly smaller than the r0 values: 2.437 Å or 2.438 Å. Finally, a mixed regression was performed using three predicate values: r(Si-I) = 2.435(5) Å, r(Si-H) = 1.470(2) Å; ∠(HSiI) = 108.51(30)° and the three estimated equilibrium Be rotational constants of 28SiH3I, 2çSiH3I, and 30SiH3I. The uncertainty used to weigh these rotational constants was 0.1 MHz. The fit is rather well-conditioned and the residuals are small. The used semi-experimental rotational constants and the residuals of the fit are given in Table S1 of the Supplementary Material. However, the number of data is small: three rotational constants and three predicate values. Therefore, the resulting standard deviations are probably too small.

Table 3.
Structure of SiH3I (bond lengths in Å, angles in degrees).
6. Structure of Halosilylenes
For the Si-H bond lengths, the equilibrium values for SiH2 [28] and SiHCl [29] are in good agreement with the ab initio optimizations and with the prediction using the isolated stretching frequency, see Table 1 and Table 2. However, for SiHF [30], SiHBr [31], and SiHI [8], there are significant discrepancies. It is important to determine whether these discrepancies are due either to the different bonding or to the poor accuracy of the equilibrium structures. The first information is obtained by comparing the ab initio optimizations and the prediction from the isolated stretching frequencies which are in perfect agreement with the Si-H bond length. However, a more stringent test is welcomed. For this reason, the structures of SiHF, SiHBr, and SiHI have been redetermined.
6.1. Structure of SiHF
The equilibrium structure of SiHF has been determined twice [30,52]. In each case, it has been derived from the average (rz) structure. First, it has to be noted that the Si-H bond length is extremely imprecise and quite different from the predictions of Table 1.
There is an obvious explanation for this poor result. As the molecule is planar, the number of independent rotational constants is only four for SiHF and SiDF whereas there are three parameters to determine. In other words, the system is ill-conditioned and quite sensitive to small errors in the data.
This a typical example where the mixed regression should improve the situation. To calculate a semi-experimental equilibrium structure, the rovibrational corrections were obtained from an ab initio anharmonic force field calculated at the MP2/6-311+G(3df,2pd) level of theory. The ground state rotational constants were taken from Refs. [30,52] and the semi-experimental rotational constants were corrected for a small electronic effect. The values of the diagonal elements of the rotational g-tensor were calculated at the B3LYP/6-311+G(3df,2pd) level of theory. The results are gaa = −4.29; gbb = −0.099; gcc = −1.70. The electronic correction is small for B and C, of the order of magnitude of one MHz, i.e., similar to the experimental accuracy. On the other hand, it is extremely large for the A rotational constants: 534 MHz for SiHF. However, this is not a problem because the A rotational constants were not used in the fit. Three predicate values were used: r(Si-H) = 1.523(2) Å, r(Si-F) = 1.602(5) Å, and ∠(HSiF) = 96.9(5)° (CCSD(T)/cc-pwCVQZ values, all electrons correlated). The estimates of the standard deviations are larger than their probable values to avoid a preponderant role of the predicate values. The predicate value of the Si-F bond length was estimated assuming that the error of the MP2 method is constant for a given bond. In other words:
re(Si-F[SiHF]) = rMP2(Si-F[SiHF) + re(Si-F[SiH3F]) − rMP2(Si-F[SiH3F])
The resulting structure is given in Table 4 and the semi-experimental rotational constants and their residuals in Table S2 of the Supplementary Material. From the residuals and the standard deviations of the fitted parameters, it appears that the predicate values are compatible with the semi-experimental rotational constants. In other words, the structure is expected to be reliable.

Table 4.
Structure of HSiF (bond lengths in Å, angle in degrees).
As the number of data is quite small, the standard deviations of the parameters are not expected to be a reliable estimate of their accuracy. To palliate this inconvenient, the standard deviations were also calculated using the law of propagation of errors assuming an error of 2 MHz for the rotational constants and for the predicates: 0.002 Å for r(Si-H), 0.005 Å for r(Si-F) and 0.5° for ∠HSiF).
It is interesting to note that the Si-F bond length at 1.603 Å is close to the value found for the radical SiF, 1.601 Å [53].
6.2. Structure of SiHCl
There are already equilibrium structures available for SiHCl. The equilibrium structure was first derived from the average (rz) structure [54]. There are also two high-level ab initio structures [29,55] optimized at the CCSD(T) level of theory. Finally, there are two semi-experimental structures [29] using two different force fields [29], see Table 5. However, there are small differences between these two semi-experimental structures. It might be explained by the fact that rotational constants are available for only three isotopologues, i.e., six independent data (as the molecule is planar). To improve the accuracy, we have repeated the semi-experimental determination by adding the three CCSD(T)_ae/cc-pwCVQZ parameters with conservative uncertainties as predicates, see Table 5. This new structure is in agreement with the previous ones, but it is believed to be slightly more accurate.

Table 5.
Structure of HSiCl (bond lengths in Å, angle in degrees).
6.3. Structure of SiHBr
The structure of SiHBr was first determined in 1964 by Herzberg and Varma [56]. They recorded the spectrum of SiHBr in the region 6000 to 4100 Å obtained in the flash photolysis of SiH3Br. More recently, Harjanto et al. [42] examined the ground and first excited singlet electronic states of SiHBr through analysis of the 500–400 nm band system, using pulsed discharge jet and laser-induced fluorescence techniques. Rotational analysis of the electronic ground state bands of SiHBr and SiDBr yielded average ground state rotational constants because the bromine isotope splittings could not be resolved. A structural fit using the average of the two bromine isotopologues yielded an effective (r0) structure. Hostutler et al. [31] determined the ground state harmonic frequencies of gas phase H/DSi79Br by exciting single vibronic bands of the 1A″–1A′ electronic transition and recorded the dispersed fluorescence. The derived harmonic force constants and the ground state rotational constants of Harjanto et al. [42] were used to calculate an average (rz) structure which permitted us to estimate an equilibrium (re) structure. Finally, Tackett et al. [57] measured the microwave spectrum by Fourier transform microwave spectroscopy and were able to determine the ground state values of = B + C for the 79Br and 81Br isotopologues. We used these values to calculate a semi-experimental equilibrium structure. The rovibrational corrections were obtained from an ab initio anharmonic force field calculated at the MP2/6-311+G(3df,2pd) level of theory. As a check, the calculation was repeated with the def2-TZVP basis set. Almost identical values were obtained. The two experimental data are not enough to determine a complete structure. A sensitivity analysis was first performed and it was found that is mainly sensitive to the value of the r(Si-Br) bond length: ∂/∂r(Si-Br) = 4168 MHz Å−1, ∂/∂r(Si-H) = 80 MHz Å−1 and ∂/∂(∠SiHBr) = −4 MHz degree−1. For this reason, the Si-H bond length derived from the isolated Si-H stretching frequencies (see Table 1) was fixed as well as the ∠(SiHBr) bond angle calculated at the MP2 level of theory (see Table 2). Only the SiBr bond length was fitted. To estimate the accuracy of the result, it was assumed that the standard deviation of r(Si-H) is 0.002 Å, that of (∠SiHBr) was 0.4° (in agreement with the results of Section 3 and Section 4), and that the accuracy of the rovibrational correction is 10% [58]. It gives re(Si-Br) = 2.2321(4) Å. The structures are given in Table 6.

Table 6.
Structure of HSiBr (bond lengths in Å, angle in degrees).
As a check the Si-Br bond length was estimated from the MP2 optimizations, see Equation (2). It gives r(Si-Br) 2.230 Å, in excellent agreement with the previous result.
6.4. Structure of SiHI
The analysis of single vibronic level emission spectra of jet-cooled SiHI and SiDI permitted us to determine a harmonic force field [8]. Using this force field and previously determined ground state rotational constants [32], Tackett and Clouthier were able to determine an average (rz) structure which was used to derive an approximate equilibrium structure. Later, the accuracy of the rotational constants was improved by measuring the Fourier transform microwave spectra of SiHI and SiDI and a new effective structure was determined [9].
To determine a semi-experimental structure, an anharmonic force field was calculated at the MP2/6-311G** level of theory. This permitted us to estimate the rovibrational corrections that were used to correct the ground state rotational constants of Kang et al. [9] and obtain semi-experimental equilibrium moments of inertia. These moments of inertia were fitted by the mixed regression method. Three predicate values were used: (i) the Si-H bond length derived from the isolated Si-H stretching frequencies (see Table 1), (ii) the ∠(HSiI) bond angle calculated at the MP2 level of theory (see Table 2), (iii) an estimate of the Si-I bond length which was estimated assuming that the error of the MP2 method is constant for a given bond, see Equation (2) where F is replaced by I.
As a check, the structure was calculated with anharmonic force fields computed at the MP2/def2-TZVP and B3LYP/def2-TZVP levels of theory. Nearly identical results were obtained. The results are given in Table 7.

Table 7.
Structure of HSiI (bond lengths in Å, angle in degrees).
7. Discussion
Accurate equilibrium structures were determined for SiH3I, SiHF, SiHBr, and SiHI. The method was to use well-conditioned least-squares fits, i.e., not sensitive to the uncertainties of the input data. For all these molecules, the structure is defined by three parameters: the r(Si-H) and r(Si-X) bond lengths, as well as the ∠(HSiX) bond angle (with X = F, Cl, Br, I). It was shown that it is possible to obtain an accurate value of the r(Si-H) bond length using either isolated stretching frequencies or MP2 calculations with a triple-zeta basis set. Furthermore, the ∠(HSiX) bond angle can be accurately estimated with MP2 calculations. Finally, using the method of mixed estimation, the third parameter, r(Si-X), is determined by the semi-experimental equilibrium moments of inertia. In such a case, it was confirmed that the results are not much sensitive to the accuracy of the rovibrational corrections.
There is another way to check the accuracy of the results by plotting the r(Si-X) bond of SiHX molecules as a function of the r(Si-X) bond of SiH3X molecules [59]. As shown in Figure 1, a nice linear correlation is obtained.
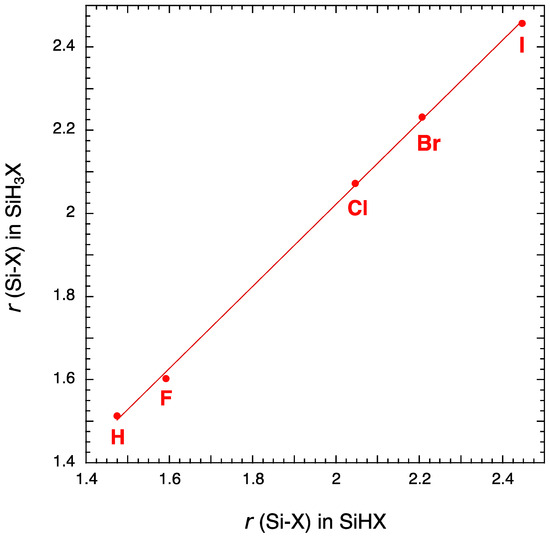
Figure 1.
Plot of r(Si-X) in SiH3X as a function of r(Si-X) in SiHX.
Our predictions are also found to be in good agreement with the results of high-level CCSD(T)/cc-pwCVQZ calculations correlating with all electrons (see Table 8). The mean absolute deviation is of 0.0006 and 0.0013 Å for the r(Si-H) and r(Si-X) bond lengths, respectively, and 0.13° for the HSiX bond angle.

Table 8.
Comparison of CCSD(T)a and equilibrium structures (bond lengths in Å, angles in degrees).
As already observed by Duncan et al. [6], the structures of the halides form a remarkably consistent set, Si-H bond length and the ∠(HSiX) bond angle remaining almost constant for the silyl halides. For the silylene halides, this conclusion remains true if we exclude SiHF. For the silylene halides, both the bond angle ∠(HSiX) and the bond length r(Si-H) increase with the electronegativity of the halogen X.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29133101/s1. Table S1. Semi-experimental values for the B rotational constants of SiH3I and residuals of the fit (MHz). Table S2. Semi-experimental values for the rotational constants of HSiF and residuals of the fit (MHz).
Author Contributions
Conceptualization, J.D. and J.L. Methodology, J.D. and J.L. Software, J.D. and J.L. Validation, J.D. and J.L. Formal Analysis, J.D. and J.L. Investigation, J.D. and J.L. Resources, J.L. Data Curation, J.D. and J.L. Writing—Original Draft Preparation, J.D. Writing—Review and Editing, J.L. Visualization, J.L. Supervision, J.D. Project Administration, J.D. Funding Acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and Supplementary Materials.
Acknowledgments
J.L. thanks the Fonds de la Recherche Scientifique-FNRS through IISN (Grant No. 4.4504.10) for financial support and the Consortium des Equipements de Calcul Intensif (CECI), funded by the Fonds de la Recherche Scientifique de Belgique (F.R.S.-FNRS) under grant number 2.5020.11 and by the Walloon Region, for computational resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boulaftali, N.; Ben Sari-Zizi, N.; Graner, G.; Demaison, J. The equilibrium structure of silyl fluoride. J. Mol. Spectrosc. 2002, 216, 284–291. [Google Scholar] [CrossRef]
- Demaison, J.; Šormova, H.; Bürger, H.; Margulès, L.; Constantin, F.L.; Ceausu-Velescu, A. Pure rotational spectrum, ab initio anharmonic force field, and equilibrium structure of silyl chloride. J. Mol. Spectrosc. 2005, 232, 323–330. [Google Scholar] [CrossRef]
- Demaison, J.; Cosléou, J.; Bürger, H.; Mkadmi, E.B. High-Resolution Infrared Spectrum of D3Si79Br in the ν1/ν4 Region: The Structure of Silyl Bromide. J. Mol. Spectrosc. 1997, 185, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Priem, D.; Cosléou, J.; Demaison, J.; Merke, I.; Stahl, W.; Jerzembeck, W.; Bürger, H. Analysis of the Rotational Spectra of SiH3CN and Its Isotopomers: Experimental and Ab Initio Determinations of the dipole moment and structure. J. Mol. Spectrosc. 1998, 191, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Matsuura, H.; Endo, Y.; Hirota, E. The microwave spectra of deuterated silanes, germanes, and stannanes. J. Mol. Spectrosc. 1986, 118, 1–17. [Google Scholar] [CrossRef]
- Duncan, J.L.; Harvie, J.L.; McKean, D.C.; Cradock, S. The ground state structures of disilane, methyl silane and the silyl halides, and an SiH bond length correlation with stretching frequency. J. Mol. Struct. 1986, 145, 225–242. [Google Scholar] [CrossRef]
- Kewley, R.; McKinney, P.M.; Robiette, A.G. The microwave spectra and molecular structures of the silyl halides. J. Mol. Spectrosc. 1970, 34, 390–398. [Google Scholar] [CrossRef]
- Tackett, B.S.; Clouthier, D.J. Structural and spectroscopic trends in the ground states of the monohalosilylenes: Emission spectroscopy of jet-cooled HSiI and DSiI. J. Chem. Phys. 2003, 118, 2612–2619. [Google Scholar] [CrossRef]
- Kang, L.; Gharaibeh, M.A.; Clouthier, D.J.; Novick, S.E. Fourier transform microwave spectroscopy of the reactive intermediate monoiodosilylene, HSiI and DSiI. J. Mol. Spectrosc. 2012, 271, 33–37. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry. III. The role of exact change. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Purvis III, G.D.; Bartlett, R.J. Full Coupled-Cluster Singles and Doubles Model: The Inclusion of Disconnected Triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M.A. Fifth-Order Perturbation Comparison of Electron Correlation Theories. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Peterson, K.A.; Wilson, A.K. Gaussian basis sets for use in correlated molecular calculations. X. The atoms aluminum through argon revisited. J. Chem. Phys. 2001, 114, 9244–9253. [Google Scholar] [CrossRef]
- Peterson, K.A.; Shepler, B.C.; Figgen, D.; Stoll, H. On the spectroscopic and thermochemical properties of ClO, BrO, IO, and their anions. J. Phys. Chem. A 2006, 110, 13877–13883. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split-valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Glukhovtsev, M.N.; Pross, A.; McGrath, M.P.; Radom, L. Extension of Gaussian-2 (G2) theory to bromine- and iodine-containing molecules: Use of effective core potentials. J. Chem. Phys. 1995, 103, 1878–1885. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods. 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Peterson, K.A.; Dunning, T.H., Jr. Accurate Correlation Consistent Basis Sets for Molecular Core–Valence Correlation Effects: The Second Row Atoms Al–Ar, and the First Row Atoms B–Ne Revisited. J. Chem. Phys. 2002, 117, 10548–10560. [Google Scholar] [CrossRef]
- Peterson, K.A.; Yousaf, K.E. Molecular core-valence correlation effects involving the post-d elements Ga-Rn: Benchmarks and new pseudopotential-based correlation consistent basis sets. J. Chem. Phys. 2010, 133, 174116. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, V.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. MOLPRO, version 2022.1; A Package of Ab Initio Programs. Available online: https://www.molpro.net (accessed on 1 January 2022).
- Werner, H.-J.; Knowles, P.J.; Manby, F.R.; Black, J.A.; Doll, K.; Heßelmann, A.; Kats, D.; Köhn, A.; Korona, T.; Kreplin, D.A.; et al. The Molpro quantum chemistry package. J. Chem. Phys. 2020, 152, 144107. [Google Scholar] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Demaison, J.; Craig, N.C. Semiexperimental equilibrium structure for cis, trans-1,4- difluorobutadiene by the mixed estimation method. J. Phys. Chem. A 2011, 115, 8049–8054. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.W.; Robiette, A.G.; Gerry, M.C.L. The harmonic force fields of methylene chloride and dichlorosilane from combined microwave and infrared data. J. Mol. Spectrosc. 1981, 85, 399–415. [Google Scholar] [CrossRef]
- Kokkin, D.L.; Ma, T.; Steimle, T.; Sears, T.J. Detection and characterization of singly deuterated silylene, SiHD, via optical spectroscopy. J. Chem. Phys. 2016, 144, 244304/1–244304/13. [Google Scholar] [CrossRef]
- Vázquez, R.; Stanton, J.F. Theoretical Investigation of the Structure and Vibrational Spectrum of the Electronic Ground State (1A′) of HSiCl. J. Phys. Chem. A 2002, 106, 4429–4434. [Google Scholar] [CrossRef]
- Hostutler, D.A.; Clouthier, D.J.; Judge, R.H. Single vibronic level emission spectroscopy of jet-cooled HSiF and DSiF. J. Chem. Phys. 2001, 114, 10728–10732. [Google Scholar] [CrossRef]
- Hostutler, D.A.; Ndiege, N.; Clouthier, D.J.; Pauls, S.W. Emission spectroscopy, harmonic vibrational frequencies, and improved ground state structures of jet-cooled monochloro- and monobromosilylene (HSiCl and HSiBr). J. Chem. Phys. 2001, 115, 5485–5491. [Google Scholar]
- Clouthier, D.J.; Harper, W.W.; Klusek, C.M.; Smith, T.C. The electronic spectrum of monoiodosilylene (HSiI) revisited. J. Chem. Phys. 1998, 109, 7827–7834. [Google Scholar] [CrossRef]
- D’Eu, J.-F.; Demaison, J.; Buerger, H. Millimeter-wave and high-resolution FTIR spectroscopy of SiH2F2: The ground and v4 = 1 states. J. Mol. Spectrosc. 2003, 218, 12–21. [Google Scholar]
- Demaison, J.; Margules, L.; Breidung, J.; Thiel, W.; Bürger, H. Ab initio anharmonic force field, spectroscopic parameters and equilibrium structure of trifluorosilane. Mol. Phys. 1999, 97, 1053–1067. [Google Scholar] [CrossRef]
- Puzzarini, C.; Taylor, P.R. An ab initio study of the structure, torsional potential energy function, and electric properties of disilane, ethane, and their deuterated isotopomers. J. Chem. Phys. 2005, 122, 054315. [Google Scholar] [CrossRef] [PubMed]
- Bailleux, S.; Bogey, M.; Demaison, J.; Bürger, H.; Senzlober, M.; Breidung, J.; Thiel, W.; Fajgar, R.; Pola, J. The equilibrium structure of silene H2C=SiH2 from millimeter wave spectra and from ab initio calculations. J. Chem. Phys. 1997, 106, 10016–10026. [Google Scholar] [CrossRef]
- Shimanouchi, T. Tables of Molecular Vibrational Frequencies Consolidated Volume I; National Bureau of Standards: Gaithersburg, MD, USA, 1972; pp. 1–160. [Google Scholar]
- McKean, D.C. On the assignment of SiH and SiD stretching frequencies: A reanalysis of the ν7 bands of Si2H6 and Si2D6 and a harmonic local mode force field for disilane. Spectrochim. Acta A 1992, 48, 1335–1345. [Google Scholar]
- Bunnell, J.; Ford, T.A. Intramolecular coupling of vibrational modes and the assignments of the partially deuterated dihalogenomethanes, -silanes, and -germanes. J. Mol. Spectrosc. 1983, 100, 215–233. [Google Scholar]
- Fredin, L.; Hauge, R.H.; Kafafi, Z.H.; Margrave, J.L. Matrix isolation studies of the reactions of silicon atoms with molecular hydrogen. The infrared spectrum of silylene. J. Chem. Phys. 1985, 82, 3542–3545. [Google Scholar] [CrossRef]
- Suzuki, T.; Hakuta, K.; Saito, S.; Hirota, E. Doppler limited dye laser excitation spectroscopy of the Ã1A″(000)–1A′(000) band of HSiF. J. Chem. Phys. 1985, 82, 3580–3583. [Google Scholar] [CrossRef]
- Harjanto, H.; Harper, W.W.; Clouthier, D.J. Resolution of anomalies in the geometry and vibrational frequencies of monobromosilylene (SiHBr) by pulsed discharge jet spectroscopy. J. Chem. Phys. 1996, 105, 10189–10200. [Google Scholar] [CrossRef]
- Bernstein, H.J. The average XH stretching frequency as a measure of XH bond properties. Spectrochim. Acta 1962, 18, 161–170. [Google Scholar] [CrossRef]
- McKean, D.C. Individual CH bond strengths in simple organic compounds: Effects of conformation and substitution. Chem. Soc. Rev. 1978, 7, 399–422. [Google Scholar] [CrossRef]
- Demaison, J.; Rudolph, H.D. Ab initio anharmonic force field and equilibrium structure of propene. J. Mol. Spectrosc. 2008, 248, 66–76. [Google Scholar] [CrossRef]
- McKean, D.C. New light on the stretching vibrations, lengths and strengths of CH, SiH and GeH bonds. J. Mol. Struct. 1981, 113, 251–266. [Google Scholar] [CrossRef]
- McKean, D.C.; Torto, I.; Boggs, J.E.; Fan, K. Infrared and ab initio studies of molecules containing SiH bonds. J. Mol. Struct. (Theochem.) 1992, 260, 27–46. [Google Scholar] [CrossRef]
- Margulès, L.; Demaison, J.; Boggs, J.E. Ab initio and equilibrium bond angles. Structures of HNO and H2O2. J. Mol. Struct. (Theochem.) 2000, 500, 245–258. [Google Scholar] [CrossRef]
- Vogt, N.; Demaison, J.; Vogt, J.; Rudolph, H.D. Why it is sometimes difficult to determine the accurate position of a hydrogen atom by the semiexperimental method: Structure of molecules containing the OH or the CH3 group. J. Comput. Chem. 2014, 35, 2333–2342. [Google Scholar] [CrossRef]
- Belsley, D.A. Conditioning Diagnostics. Collinearity and Weak Data in Regression; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Demaison, J.; Nemes, L.L. A correlation between the vibrational correction and the effective moment of inertia. J. Mol. Struct. 1979, 55, 295–299. [Google Scholar] [CrossRef]
- Harper, W.W.; Hostutler, D.A.; Clouthier, D.J. Pulsed discharge jet spectroscopy of DSiF and the equilibrium molecular structure of monofluorosilylene. J. Chem. Phys. 1997, 106, 4367–4375. [Google Scholar] [CrossRef]
- Tanaka, T.; Tamura, M.; Tanaka, K. Infrared diode laser spectroscopy of the SiF radical. Analysis of hot bands up to ν = 9–8. J. Mol. Struct. 1997, 413–414, 153–166. [Google Scholar] [CrossRef]
- Harper, W.W.; Clouthier, D.J. Reinvestigation of the HSiCl electronic spectrum: Experimental reevaluation of the geometry, rotational constants, and vibrational frequencies. J. Chem. Phys. 1997, 106, 9461–9473. [Google Scholar] [CrossRef]
- Coriani, S.; Marchesan, D.; Gauss, J.; Hättig, C.; Helgaker, T.; Jørgensen, P. The accuracy of ab initio molecular geometries for systems containing second-row atoms. J. Chem. Phys. 2005, 123, 184107. [Google Scholar] [CrossRef]
- Herzberg, G.; Verma, R.D. Spectra and structures of the free SiHCl and SiHBr radicals Can. J. Phys. 1964, 42, 395–432. [Google Scholar]
- Tackett, B.S.; Clouthier, D.J.; Landry, J.N.; Jaeger, W. Fourier transform microwave spectroscopy of SiHBr: Exploring the Si-Br bond through quadrupole hyperfine coupling. J. Chem. Phys. 2005, 122, 214314. [Google Scholar] [CrossRef]
- Vogt, N.; Vogt, J.; Demaison, J. Accuracy of the rotational constants. J. Mol. Struct. 2011, 988, 119–127. [Google Scholar] [CrossRef]
- Demaison, J.; Vogt, N. Accurate Structure Determination of Free Molecules; Springer: Cham, Switzerland, 2020; pp. 205–232. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).