Self-Assembly of Three-Dimensional Hyperbranched Magnetic Composites and Application in High-Turbidity Water Treatment
Abstract
:1. Introduction
2. Result and Discussion
2.1. Characterization of Fe3O4@HAAP
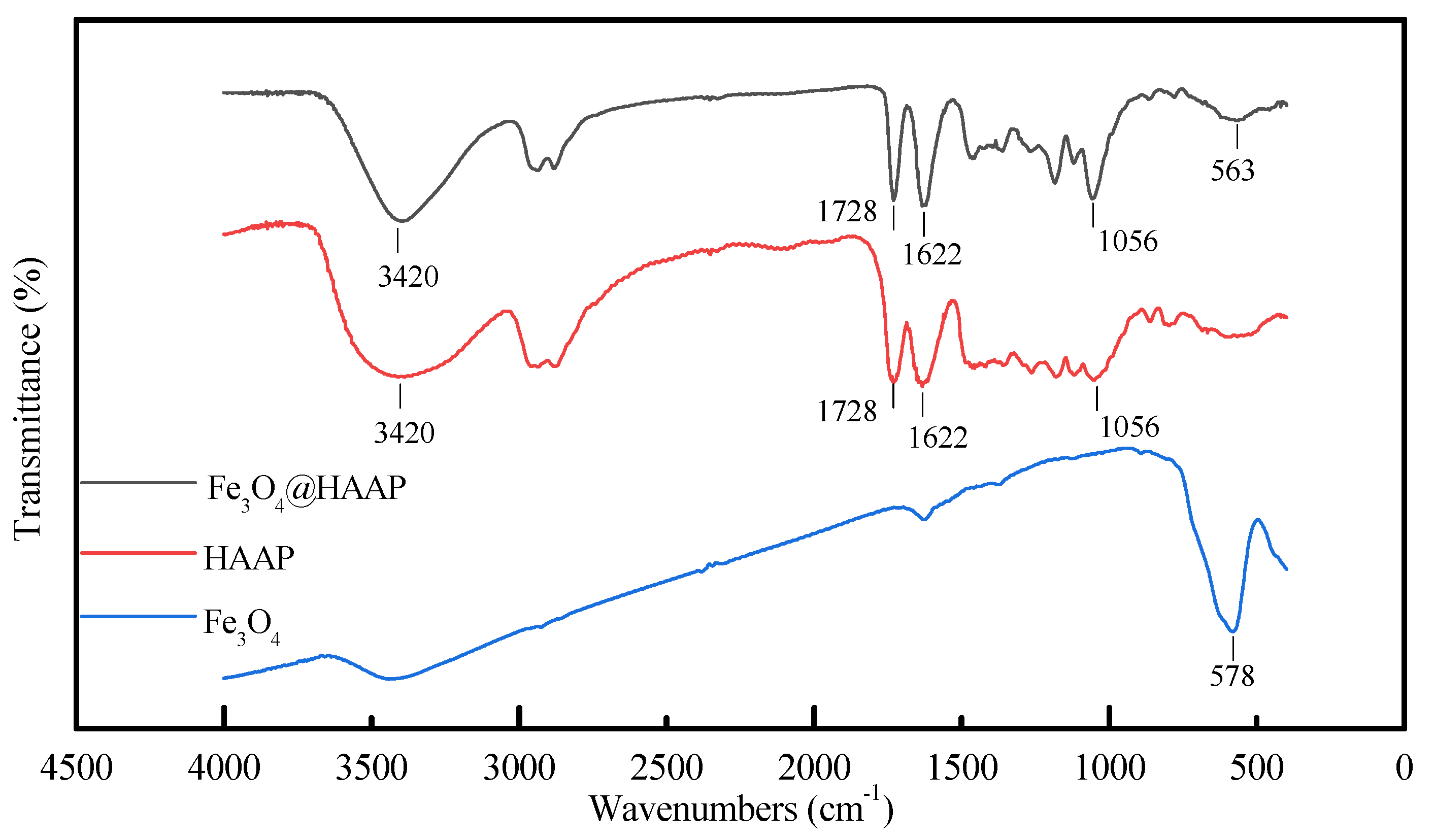
2.1.1. FTIR and XPS Spectra
2.1.2. SEM and EDS
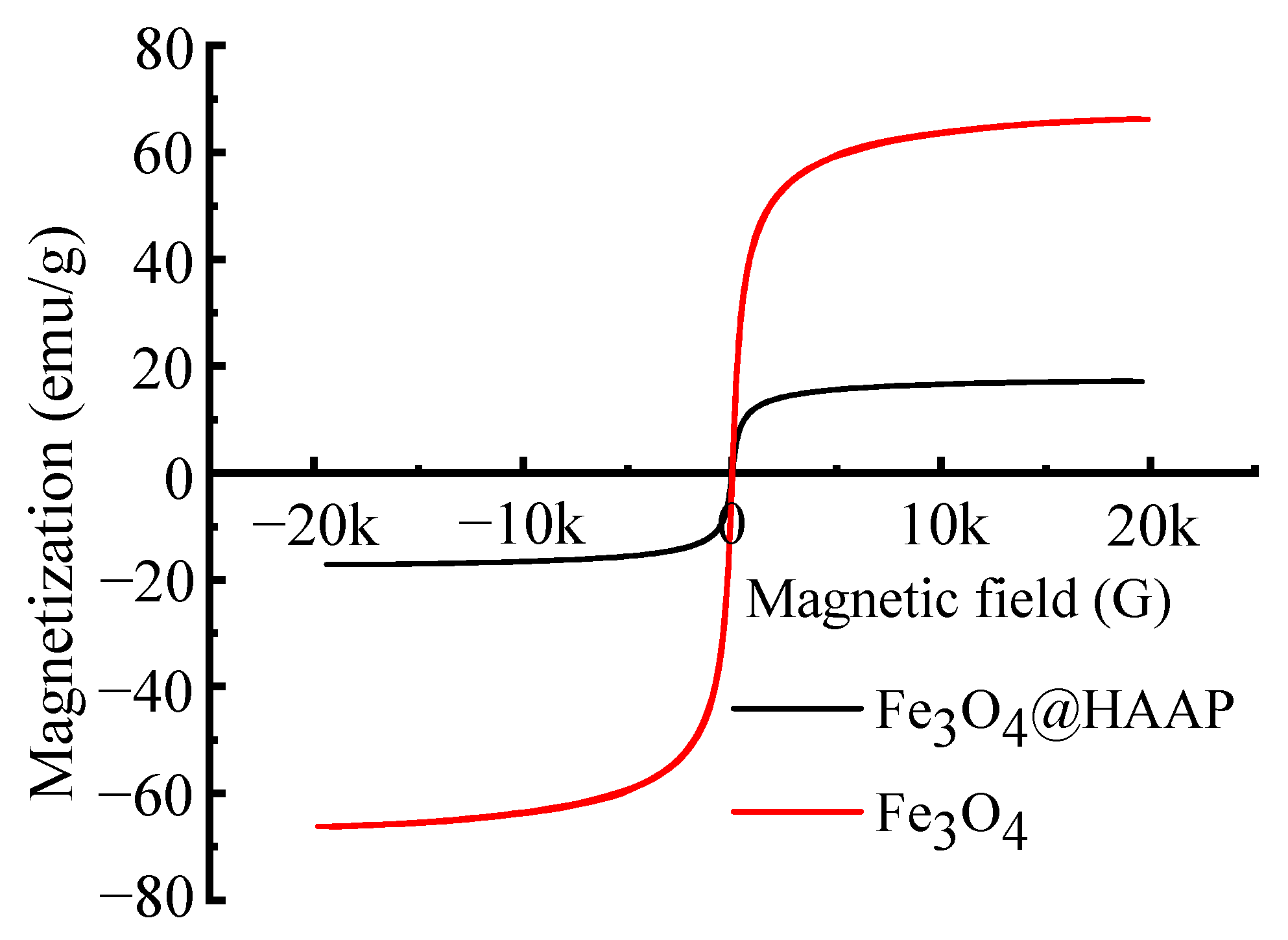
2.1.3. VSM and Zeta Potential
2.2. Application in Wastewater Treatment
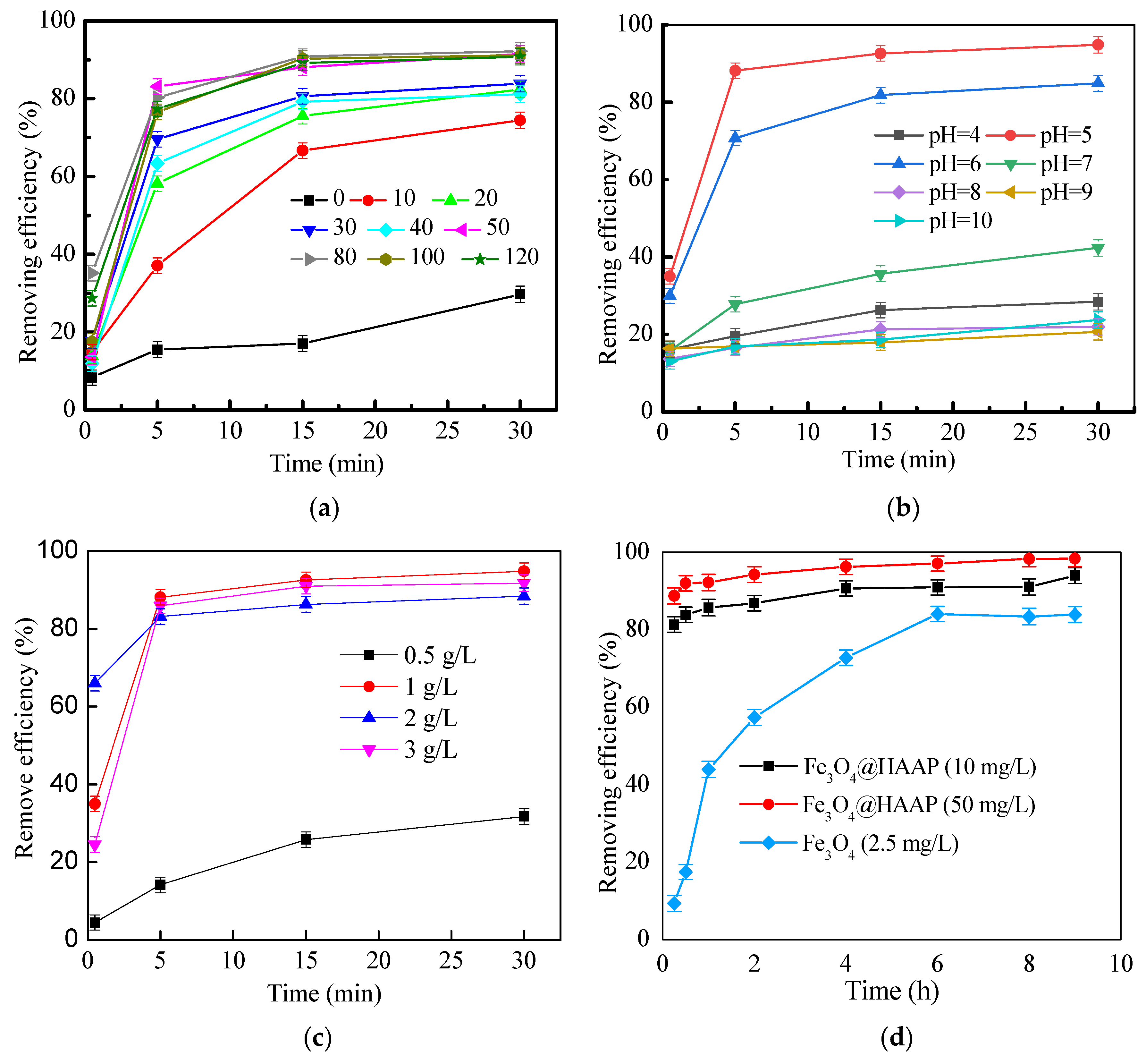
2.2.1. Simulated Wastewater
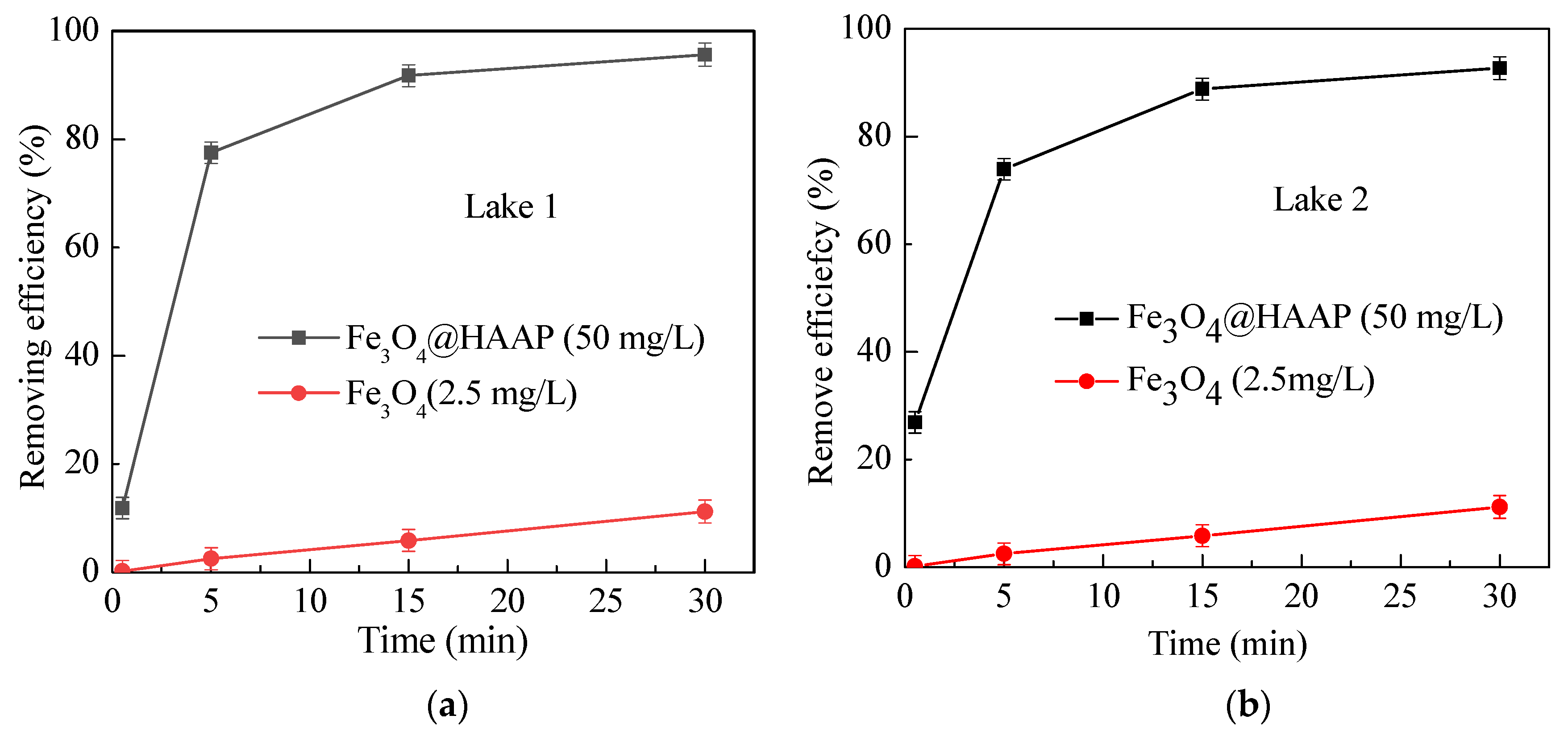
2.2.2. Actual Water
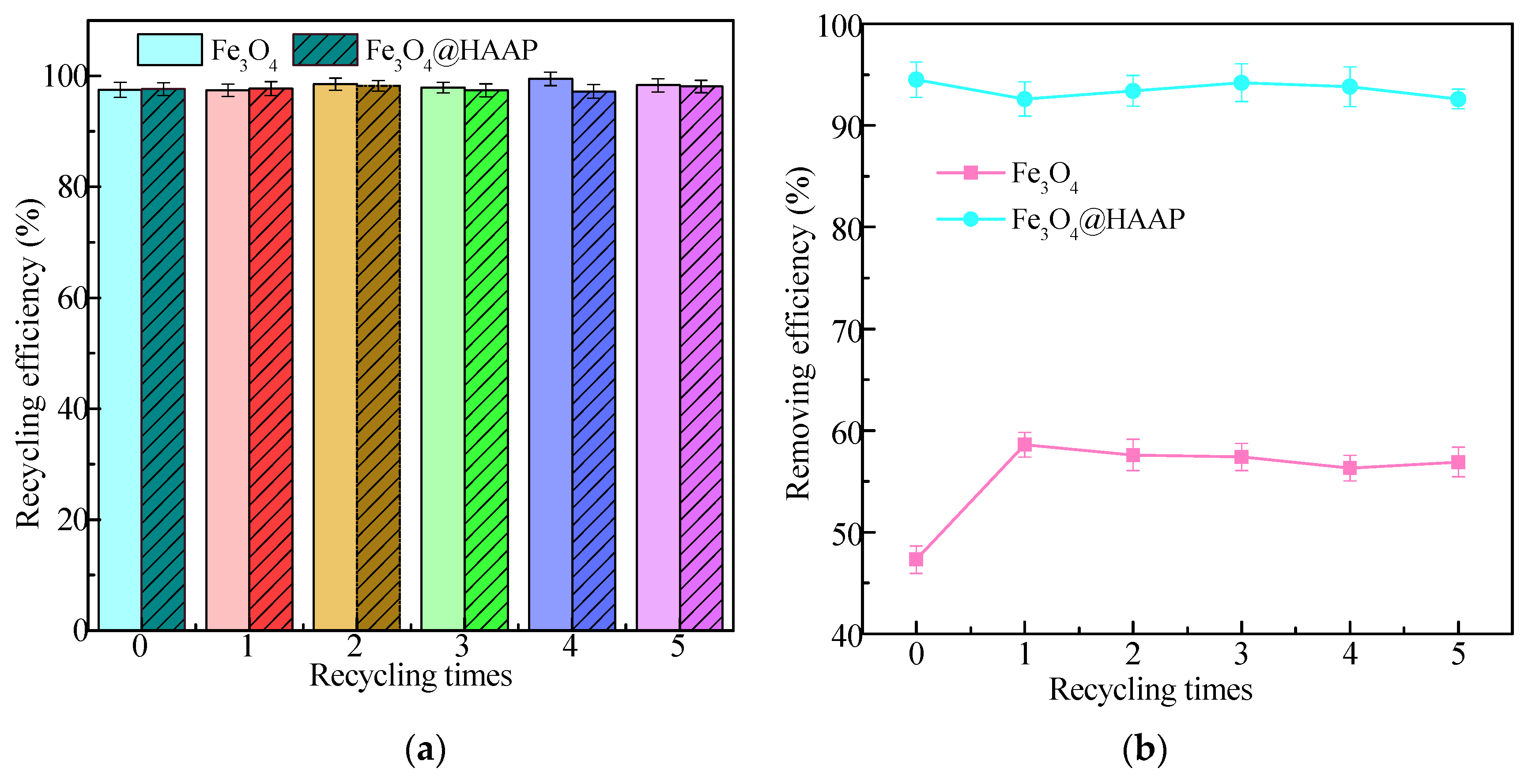
2.2.3. Recycling and Reuse
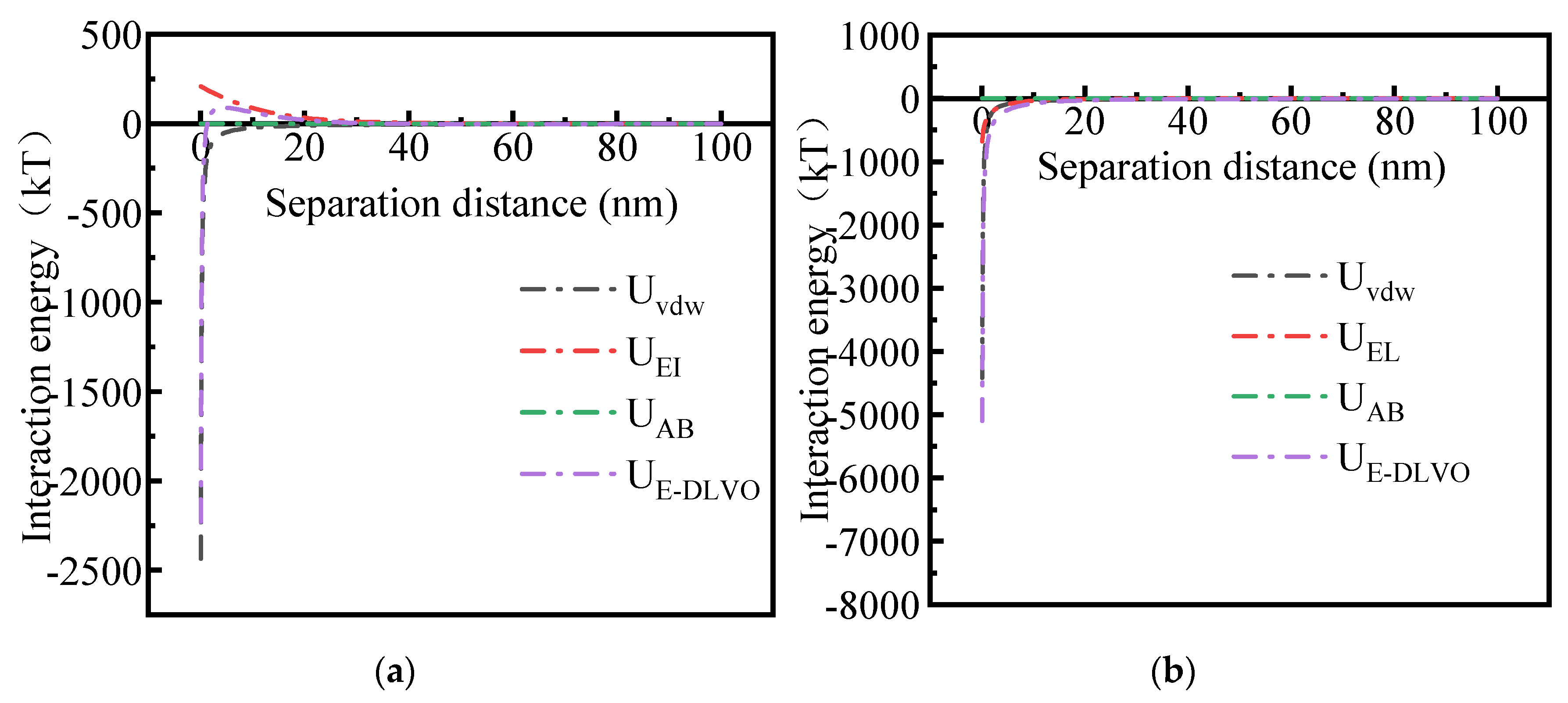
2.3. Interaction Energy Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fe3O4@HAAP
3.3. Characterization of Fe3O4@HAAP
3.4. Application in Wastewater Treatment
3.5. Interaction Energy Analysis of Fe3O4@HAAP and Kaolin
3.6. Analysis Methods
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, W.I.; Jeong, S.; Im, S.J.; Jang, A. High turbidity water treatment by ceramic microfiltration membrane: Fouling identification and process optimization. Environ. Technol. Inno. 2020, 17, 100578. [Google Scholar] [CrossRef]
- De Roos, A.J.; Gurian, P.L.; Robinson, L.F.; Rai, A.; Zakeri, I.; Kondo, M.C. Review of Epidemiological Studies of Drinking-Water Turbidity in Relation to Acute Gastrointestinal Illness. Environ. Health Persp. 2017, 125, 086003. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.S.; Manu, B.; Azhoni, A. Sustainable treatment of paint industry wastewater: Current techniques and challenges. J. Environ. Manag. 2021, 296, 113105. [Google Scholar] [CrossRef] [PubMed]
- Bayarkhuu, B.; Byun, J. Optimization of coagulation and sedimentation conditions by turbidity measurement for nano- and microplastic removal. Chemosphere 2022, 306, 135572. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.M.; Shao, G.Y.; Zhang, W.J.; Chen, J.J.; Qu, Y.X.; Zhang, F.; Tian, S.C.; Zhou, Z.Y.; Ren, Z.Q. The degradation of printing and dyeing wastewater by manganese-based catalysts. Sci. Total Environ. 2022, 828, 154390. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Nicu, R.; Latour, I.; Lupei, M.; Bobu, E.; Blanco, A. Efficiency of chitosans for the treatment of papermaking process water by dissolved air flotation. Chem. Eng. J. 2013, 231, 304–313. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Du, S.; Cheng, P.; Liang, W. Magnetic coagulation and flocculation of kaolin suspension using Fe3O4 with plant polyphenol self-assembled flocculants. Int. J. Biol. Macromol. 2023, 253, 126578. [Google Scholar] [CrossRef]
- Saxena, K.; Brighu, U.; Choudhary, A. Pilot-scale coagulation of organic and inorganic impurities: Mechanisms, role of particle concentration and scale effects. J. Environ. Chem. Eng. 2020, 8, 103990. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.Y.; Qin, L.L.; Li, H.J.; Liang, W.Y. Magnetic coagulation and flocculation of a kaolin suspension using Fe3O4 coated with SiO2. J. Environ. Chem. Eng. 2021, 9, 105980. [Google Scholar] [CrossRef]
- Abirhire, O.; Davies, J.M.; Guo, X.L.; Hudson, J. Understanding the factors associated with long-term reconstructed turbidity in Lake Diefenbaker from Landsat-imagery. Sci. Total. Environ. 2020, 724, 138222. [Google Scholar] [CrossRef]
- Ma, Z.H.; Ge, Z.H.; Liu, K.X.; Wang, C.; Wu, T.; Zhang, J.B. Application of calcium peroxide for efficient treatment of surface water turbidity: Mechanisms and microbial community responses. J. Environ. Chem. Eng. 2023, 11, 110905. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, W.B.; Zhu, Z.Q.; Peng, C.; Peng, Y. Study on the preparation of polysilicate ferric flocculant and its treatment of high turbidity tailings water. J. Water Process Eng. 2021, 44, 102457. [Google Scholar] [CrossRef]
- Chiavola, A.; Di Marcantonio, C.; D’Agostini, M.; Leoni, S.; Lazzazzara, M. A combined experimental-modeling approach for turbidity removal optimization in a coagulation-flocculation unit of a drinking water treatment plant. J. Process Contr. 2023, 130, 103068. [Google Scholar] [CrossRef]
- Ma, J.Y.; Fu, K.; Fu, X.; Guan, Q.Q.; Ding, L.; Shi, J.; Zhu, G.C.; Zhang, X.X.; Zhang, S.H.; Jiang, L.Y. Flocculation properties and kinetic investigation of polyacrylamide with different cationic monomer content for high turbid water purification. Sep. Purif. Technol. 2017, 182, 134–143. [Google Scholar] [CrossRef]
- Zhang, X.H.; He, X.W.; Wei, M.; Li, F.Q.; Hou, P.; Zhang, C.H. Magnetic Flocculation Treatment of Coal Mine Water and a Comparison of Water Quality Prediction Algorithms. Mine Water Environ. 2019, 38, 391–401. [Google Scholar] [CrossRef]
- Yang, Z.J.; Hou, J.; Pan, Z.G.; Wu, M.; Zhang, M.Z.; Yin, X.X.; Wu, J.; Miao, L.Z.; Liu, Q.D. A novel La(OH)3 decorated co-graft tannin and polyethyleneimine co-coating magnetic adsorbent for effective and selective phosphate removal from natural water and real wastewater. J. Clean. Prod. 2022, 369, 133345. [Google Scholar] [CrossRef]
- Fang, Y.; Yao, Y.X.; Wang, J.; Li, B.; Dou, L.P.; Wei, L.F.; Zhuo, B.; Zhang, W.; Hu, X.Y. Effective dewatering and resourceful utilization of high-viscosity waste slurry through magnetic flocculation. Constr. Build. Mater. 2024, 425, 136014. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, J.H.; Kim, I.; Jeon, H. Efficient separation performance of suspended soil and strontium from aqueous solution using magnetic flocculant. J. Environ. Chem. Eng. 2021, 9, 106810. [Google Scholar] [CrossRef]
- Lü, T.; Zhang, S.; Qi, D.M.; Zhang, D.; Zhao, H.T. Enhanced demulsification from aqueous media by using magnetic chitosan-based flocculant. J. Colloid Interf. Sci. 2018, 518, 76–83. [Google Scholar] [CrossRef]
- Ge, S.J.; Agbakpe, M.; Wu, Z.Y.; Kuang, L.Y.; Zhang, W.; Wang, X.Q. Influences of Surface Coating, UV Irradiation and Magnetic Field on the Algae Removal Using Magnetite Nanoparticles. Environ. Sci. Technol. 2015, 49, 1190–1196. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, Q.L.; Liu, Y.H.; Wang, S.W.; Guo, X.D.; Guo, L.J.; Zhu, M.C.; Wang, X. Preparation and Application of Amino-Terminated Hyperbranched Magnetic Composites in High-Turbidity Water Treatment. Molecules. 2023, 28, 6787. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Xu, Z.J.; Zhang, D.H. Synthesis and application of epoxy-ended hyperbranched polymers. Chem. Eng. J. 2018, 343, 283–302. [Google Scholar] [CrossRef]
- Liu, Z.K.; Xu, Q.Q.; Yan, C.; Li, J.; Zhou, W.F.; Gao, H.X.; Zhang, S.B.; Lu, R.H. Hyperbranched aromatic polyamide modified magnetic nanoparticles for the extraction of benzoylurea insecticides. J. Sep. Sci. 2021, 44, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Tang, R.; Li, Q.; Li, Z. Functional hyperbranched polymers with advanced optical, electrical and magnetic properties. Chem. Soc. Rev. 2015, 44, 3997–4022. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.H.; Wang, L.; Zhang, D.; Yan, P.; Nie, J.; Sharma, V.K.; Wang, C.Y. Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem. Eng. J. 2019, 358, 253–263. [Google Scholar] [CrossRef]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Synthesis and functionalization of hyperbranched polymers for targeted drug delivery. J. Control. Release 2020, 321, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qu, R.J.; Xu, T.; Zhang, Y.; Sun, C.M.; Ji, C.N.; Wang, Y. Fabrication of Triethylenetetramine Terminal Hyperbranched Dendrimer-Like Polymer Modified Silica Gel and Its Prominent Recovery Toward Au (III). Front. Chem. 2019, 7, 577. [Google Scholar] [CrossRef] [PubMed]
- Kuchkina, N.V.; Haskell, A.K.; Sorokina, S.A.; Torozova, A.S.; Nikoshvili, L.Z.; Sulman, E.M.; Stein, B.D.; Morgan, D.G.; Bronstein, L.M.; Shifrina, Z.B. Pd Catalyst Based on Hyperbranched Polypyridylphenylene Formed In Situ on Magnetic Silica Allows for Excellent Performance in Suzuki-Miyaura Reaction. ACS Appl. Mater. Interfaces 2020, 12, 22170–22178. [Google Scholar] [CrossRef] [PubMed]
- Gosecki, M.; Urbaniak, M.; Martinho, N.; Gosecka, M.; Zloh, M. Evaluation of Encapsulation Potential of Selected Star-Hyperbranched Polyglycidol Architectures: Predictive Molecular Dynamics Simulations and Experimental Validation. Molecules 2023, 28, 7308. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, J.S.; Liu, X.Y.; Chen, C.H.; Li, G.Y.; Meng, Y.F. Graphene oxides cross-linked with hyperbranched polyethylenimines: Preparation, characterization and their potential as recyclable and highly efficient adsorption materials for lead(II) ions. Chem. Eng. J. 2016, 285, 698–708. [Google Scholar] [CrossRef]
- Qiao, S.Y.; Liu, Q.W.; Fan, Z.Z.; Tong, Q.L.; Cai, L.; Fu, Y.F. Magnetic Hyperbranched Molecular Materials for Treatment of Oily Sewage Containing Polymer in Oilfield Compound Flooding. Front. Chem. 2022, 10, 865832. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Wang, Z.W.; Yue, R.R.; Gao, F.; Ren, R.L.; Wei, J.F.; Wane, X.L.; Kong, Z.Y. Functional group-rich hyperbranched magnetic material for simultaneous efficient removal of heavy metal ions from aqueous solution. J. Hazard. Mater. 2020, 384, 121288. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.Y.; Du, S.C.; Liang, W.Y. Synthesis of chitosan-based grafting magnetic flocculants for flocculation of kaolin suspensions. J. Environ. Sci. 2024, 139, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, X.J.; Nie, R.; Feng, L.; Li, X.H.; Liu, B.Z. Chitosan Modified Cationic Polyacrylamide Initiated by UV-H2O2 for Sludge Flocculation and New Insight on the Floc Characteristics Study. Polymers 2020, 12, 2738. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Fu, X.; Xia, W.; Zhang, R.; Fu, K.; Wu, G.Y.; Jia, B.T.; Li, S.; Li, J.C. Removal of emulsified oil from water by using recyclable chitosan based covalently bonded composite magnetic flocculant: Performance and mechanism. J. Hazard. Mater. 2021, 419, 126529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Liu, C.; Qin, L.L.; Liang, W.Y. Self-assembly of Fe3O4 with natural tannin as composites for microalgal harvesting. Fuel 2022, 321, 124038. [Google Scholar] [CrossRef]
- Demircan, D.; Zhang, B.Z. Facile synthesis of novel soluble cellulose-grafted hyperbranched polymers as potential natural antimicrobial materials. Carbohydr. Polym. 2017, 157, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Jin, Y.J.; Wang, B.; Tian, H.F.; Kang, K.; Men, S.; Weng, Y.X. High-toughening modification of polylactic acid by long-chain hyperbranched polymers. J. Appl. Polym. Sci. 2021, 138, 51295. [Google Scholar] [CrossRef]
- Majumder, S.; Sardar, M.; Satpati, B.; Kumar, S.; Banerjee, S. Magnetization Enhancement of Fe3O4 by Attaching onto Graphene Oxide: An Interfacial Effect. J. Phys. Chem. C 2018, 122, 21356–21365. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, H.F.; Shao, J.W.; Zhang, K.F.; Wu, J.; Yan, L.F. S4-Containing hyperbranched polymer modified graphene oxide as strong linker for both rubber and carbon black to enhance the crosslinking and mechanical properties of nitrile butadiene rubber. Chem. Eng. J. 2021, 417, 129336. [Google Scholar] [CrossRef]
- Abd El-Aal, M.; Said, A.A.; Goda, M.N.; Zeid, E.F.A.; Ibrahim, S.M. Fe3O4@CMC-Cu magnetic nanocomposite as an efficient catalyst for reduction of toxic pollutants in water. J. Mol. Liq. 2023, 385, 122317. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, Y.; Bian, M.; Guo, X.; Zhang, N. Synthesis of easily renewable and recoverable magnetic PEI-modified Fe3O4 nanoparticles and its application for adsorption and enrichment of tungsten from aqueous solutions. Environ. Pollut. 2023, 330, 121703. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.N.; Han, P.; Gu, Z. Grafting of a novel hyperbranched polymer onto carbon fiber for interfacial enhancement of carbon fiber reinforced epoxy composites. Mater. Design. 2021, 200, 109456. [Google Scholar] [CrossRef]
- Ma, L.C.; Song, G.J.; Zhang, X.C.; Zhou, S.F.; Liu, Y.Q.; Zhang, L.Y. Attaching SiO2 nanoparticles to GO sheets via amino-terminated hyperbranched polymer for epoxy composites: Extraordinary improvement in thermal and mechanical properties. Eur. Polym. J. 2021, 157, 110677. [Google Scholar] [CrossRef]
- Shen, H.Y.; Chen, Z.X.; Li, Z.H.; Hu, M.Q.; Dong, X.Y.; Xia, Q.H. Controlled synthesis of 2,4,6-trichlorophenol-imprinted amino-functionalized nano-Fe3O4-polymer magnetic composite for highly selective adsorption. Colloid Surf. A Physicochem. Eng. Asp. 2015, 481, 439–450. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Zou, Y.H.; Sun, Q.; Liu, S.; Sun, M.L.; Zheng, H.L.; Li, H. Copolymers functionalized with quaternary ammonium compounds under template chain exhibit simultaneously efficient bactericidal and flocculation properties: Characterization, performance and mechanism. J. Hazard. Mater. 2024, 465, 133476. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Ghorai, S.; Pal, S. Flocculation characteristics of polyacrylamide grafted hydroxypropyl methyl cellulose: An efficient biodegradable flocculant. Chem. Eng. J. 2013, 229, 144–152. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, X.T.; Xi, H.P.; Sun, J.X.; Liang, X.L.; Wei, J.; Xiao, X.; Liu, Z.G.; Li, S.W.; Liang, Z.S.; et al. Interpretation of adhesion behaviors between bacteria and modified basalt fiber by surface thermodynamics and extended DLVO theory. Colloid Surf. B. 2019, 177, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Tanudjaja, H.J.; Chew, J.W. Assessment of oil fouling by oil-membrane interaction energy analysis. J. Membrane. Sci. 2018, 560, 21–29. [Google Scholar] [CrossRef]
- Paula, F.L.O.; Castro, L.L.; Cassiano, T.S.A.; dos Santos, S.G.; Gomide, G.; Depeyrot, J.; Campos, A.F.C. pH-dependent phase transitions in ferrofluids: A Monte Carlo simulation study using an extended DLVO model. Colloid Surf. A Physicochem. Eng. Asp. 2023, 658, 130578. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.B.; Liu, J.X.; Ying, H.W.; Han, H.F.; Chen, X. Enhanced dispersing properties of kaolin due to high-strength kneading process. Appl. Clay. Sci. 2024, 247, 107218. [Google Scholar] [CrossRef]
- Dai, D.; Qv, M.; Liu, D.Y.; Tang, C.M.; Wang, W.; Wu, Q.R.; Yin, Z.H.; Zhu, L.D. Structural insights into mechanisms of rapid harvesting of microalgae with pH regulation by magnetic chitosan composites: A study based on E-DLVO model and component fluorescence analysis. Chem. Eng. J. 2023, 456, 141071. [Google Scholar] [CrossRef]
- Agbakpe, M.; Ge, S.; Zhang, W.; Zhang, X.; Kobylarz, P. Algae harvesting for biofuel production: Influences of UV irradiation and polyethylenimine (PEI) coating on bacterial biocoagulation. Bioresour. Technol. 2014, 166, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Kim, S.B. DLVO and XDLVO calculations for bacteriophage MS2 adhesion to iron oxide particles. J. Contam. Hydrol. 2015, 181, 131–140. [Google Scholar] [CrossRef] [PubMed]



| C | N | O | Si | Fe | |||||
|---|---|---|---|---|---|---|---|---|---|
| Wt% | At% | Wt% | At% | Wt% | At% | Wt% | At% | Wt% | At% |
| 52.78 | 64.33 | 6.90 | 7.21 | 26.10 | 23.88 | 3.26 | 1.70 | 10.96 | 2.874 |
| 38.58 | 53.81 | 4.58 | 5.48 | 29.76 | 31.16 | 4.83 | 2.88 | 22.25 | 6.68 |
| Symbols | Parameters |
|---|---|
| R | The radius of magnetic materials and kaolin |
| A132 | The effective Hamaker constant (J) for particles (1) interacting with particles (2) in the aqueous medium (3) |
| h0 | The minimum equilibrium distance due to the Born repulsion, 0.157 nm |
| D | The separation distance between the two interacting particles (nm) |
| λ | The correlation length of molecules in a liquid medium, 0.6 nm |
| κ | The inverse Debye length (m−1), 0.11 nm−1 |
| NA | Avogadro’s number, 6.02 × 1023 mol−1 |
| exp | Unit charge, 1.602 × 10−19 C |
| ε | the dielectric constant of the solution, (80 × 8.854 × 10−12 C2/J/m for aqueous) |
| K | Boltzmann constant, 1.38 × 10−23 J·K−1 |
| The absolute temperature taken as 298 K | |
| φ | The magnetic materials and kaolin surface potentials (V), depending on the zeta potential |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Fan, Q.; Liu, Y.; Liu, J.; Zhu, M.; Wang, X.; Shen, L. Self-Assembly of Three-Dimensional Hyperbranched Magnetic Composites and Application in High-Turbidity Water Treatment. Molecules 2024, 29, 3639. https://doi.org/10.3390/molecules29153639
Zhao Y, Fan Q, Liu Y, Liu J, Zhu M, Wang X, Shen L. Self-Assembly of Three-Dimensional Hyperbranched Magnetic Composites and Application in High-Turbidity Water Treatment. Molecules. 2024; 29(15):3639. https://doi.org/10.3390/molecules29153639
Chicago/Turabian StyleZhao, Yuan, Qianlong Fan, Yinhua Liu, Junhui Liu, Mengcheng Zhu, Xuan Wang, and Ling Shen. 2024. "Self-Assembly of Three-Dimensional Hyperbranched Magnetic Composites and Application in High-Turbidity Water Treatment" Molecules 29, no. 15: 3639. https://doi.org/10.3390/molecules29153639






