Cheminformatics-Guided Exploration of Synthetic Marine Natural Product-Inspired Brominated Indole-3-Glyoxylamides and Their Potentials for Drug Discovery
Abstract
1. Introduction
2. Results
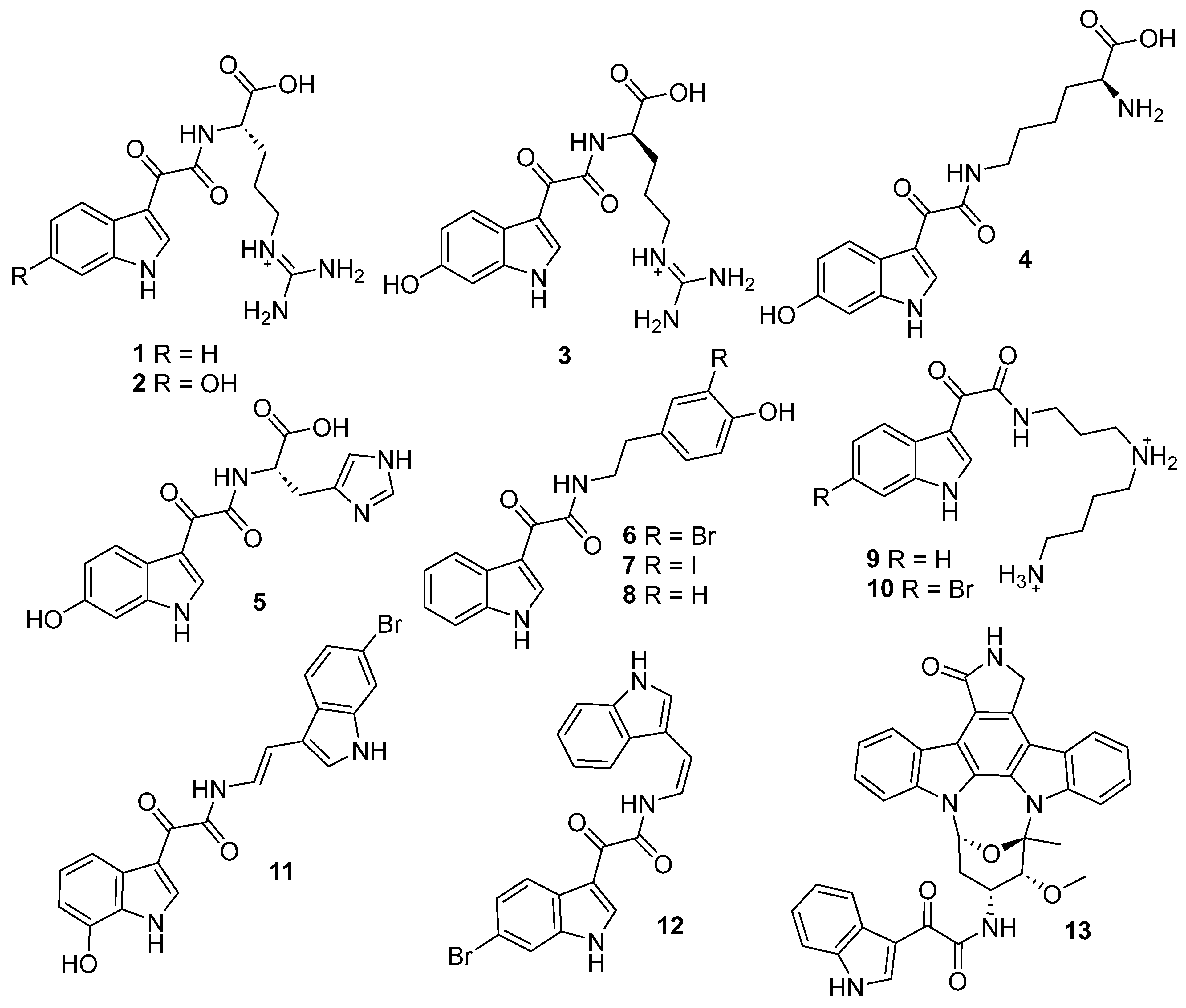
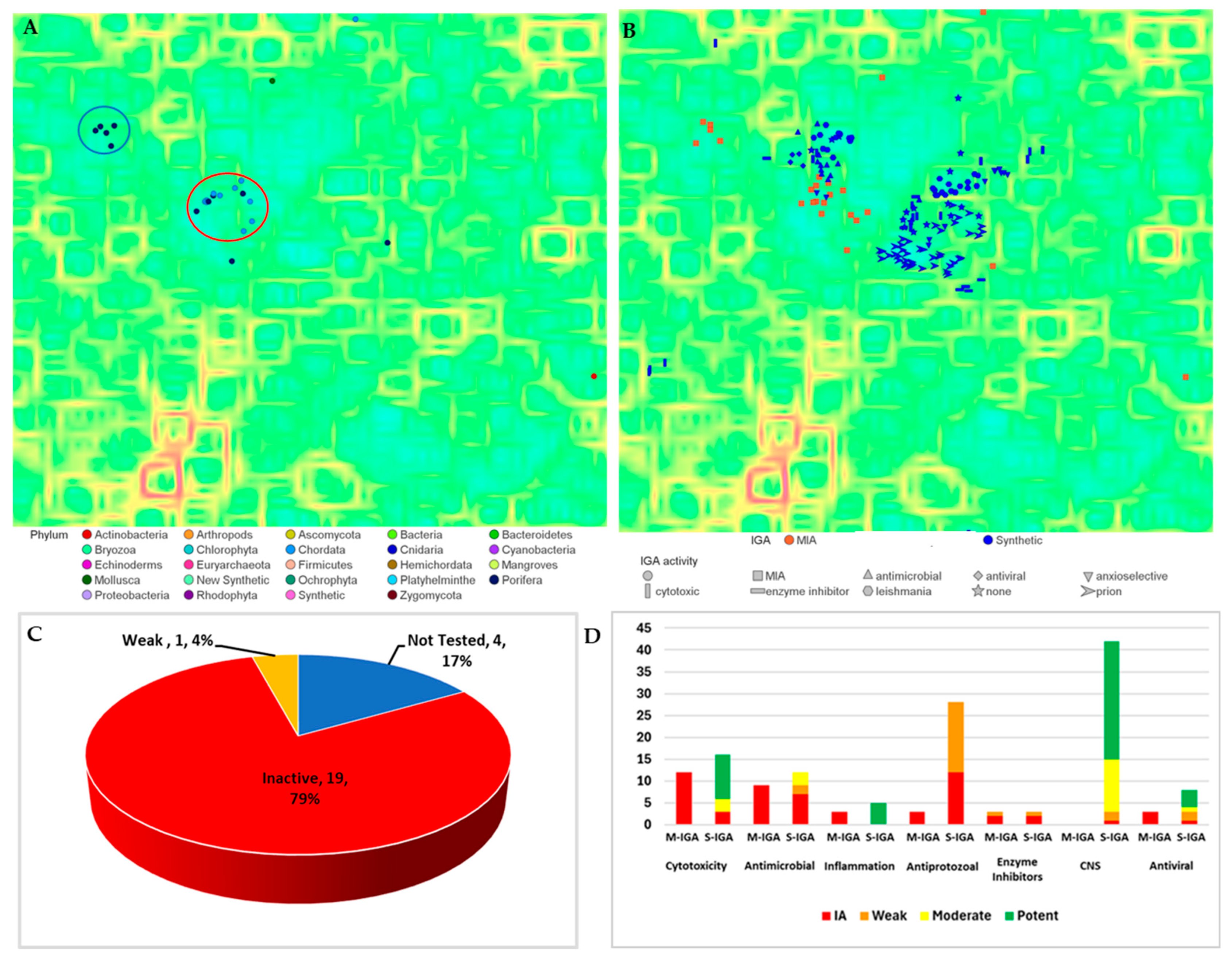
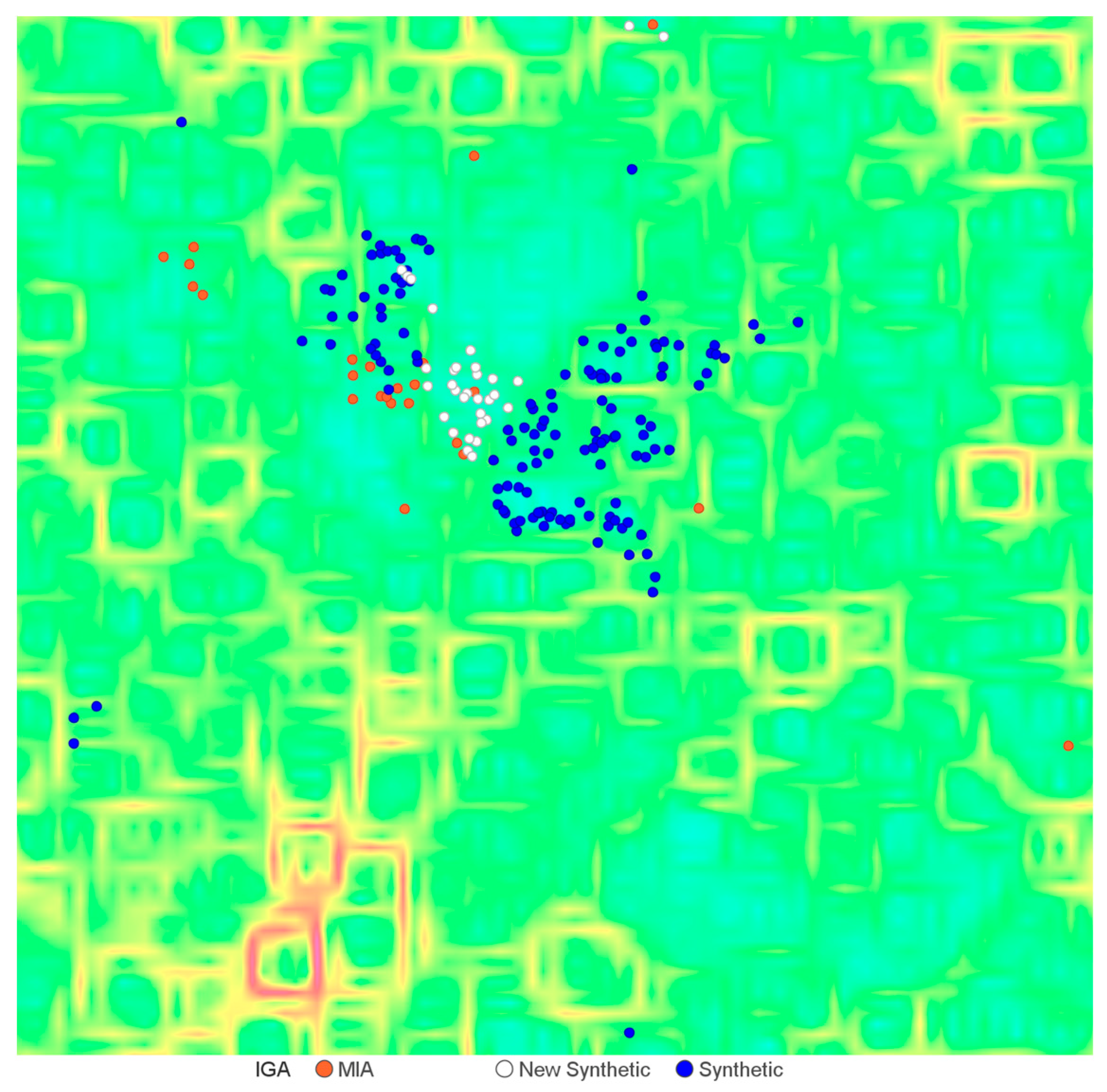
2.1. Cheminformatics Analyses of Marine Natural Product Indol-3-yl-Glyoxylamides
2.2. Synthesis of Indol-3-yl-Glyoxylamides 25–56
2.3. Exploration of Biological Chemical Space of Brominated Indol-3-yl-Glyoxylamides (25–56)
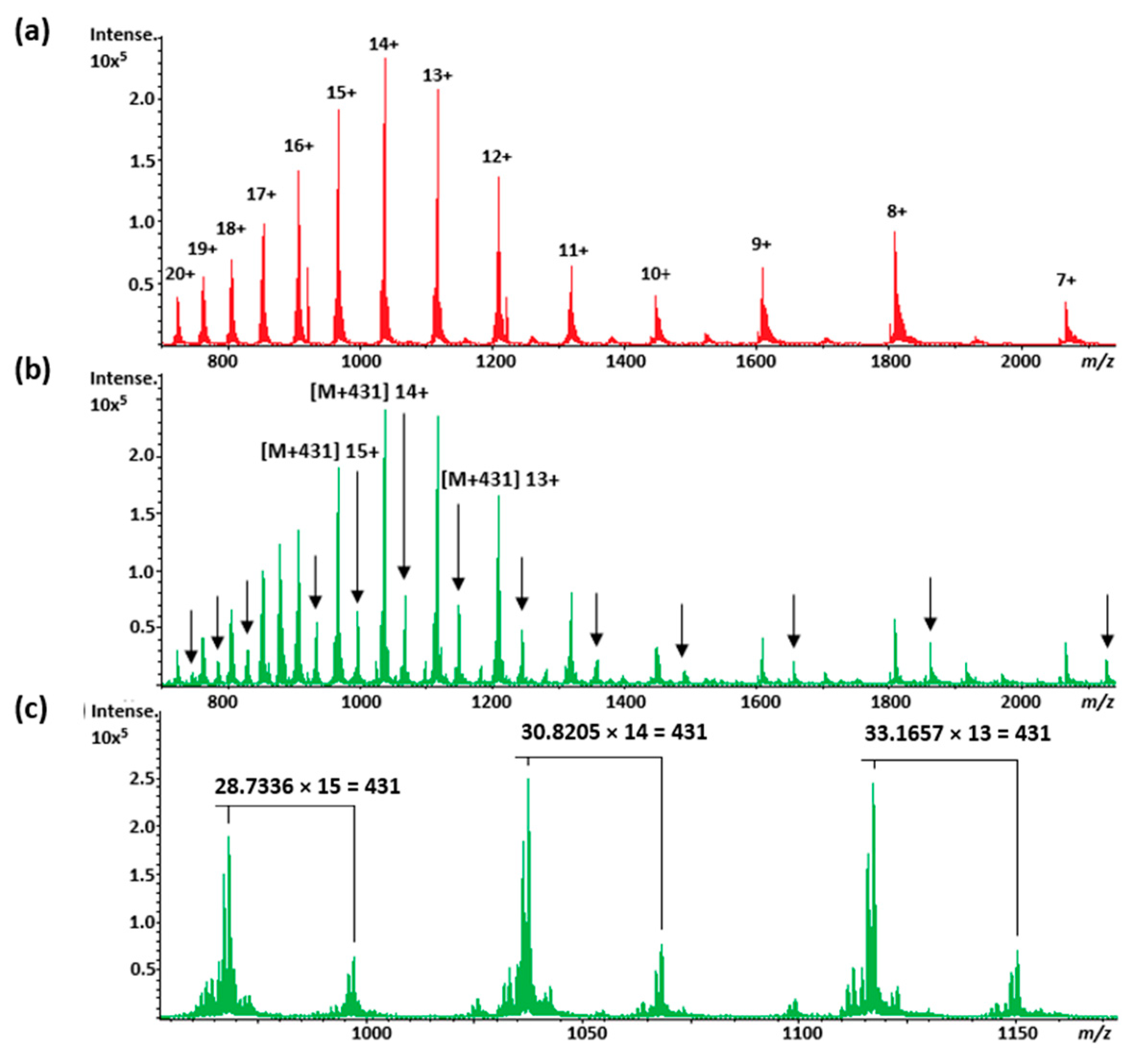
2.3.1. Parkinson’s Disease—Amyloid Protein Binding (α-syn)
2.3.2. Antiplasmodial Activity—Plasmodium falciparum
2.3.3. SARS-CoV-2 3CL Protease Activity
2.3.4. Mammalian Serine Protease (Chymotrypsin and Elastase) Activity
2.3.5. Cytotoxicity against Human Breast, Ovarian, and Colon Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Synthetic Procedures
4.2.1. Synthesis of Indol-3-yl-Glyoxlyamides 25–56
4.2.2. HPLC Purification of Analogues 25–56
4.3. Cheminformatics Analyses of Marine and Synthetic Indole Chemical Diversity
4.4. In Vitro α-Synuclein MS-Binding Assay
4.5. Antiplasmodial Image-Based Assay
4.6. SARS-CoV-2 3CLpro Inhibition Assays
4.7. Serine Protease Assays
4.8. Human Cancer Cell Cytotoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marine Pharmacology. Available online: https://www.marinepharmacology.org/approved (accessed on 11 May 2024).
- Boufridi, A.; Quinn, R.J. Harnessing the Properties of Natural Products. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 451–470. [Google Scholar] [CrossRef]
- Holland, D.C.; Carroll, A.R. Marine indole alkaloid diversity and bioactivity. What do we know and what are we missing? Nat. Prod. Rep. 2023, 40, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C. Organic Synthesis: The Art and Science of Replicating the Molecules of Living Nature and Creating Others like Them in the Laboratory. Proc. R. Soc. A 2014, 470, 20130690. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kirchmair, J. Cheminformatics in Natural Product-Based Drug Discovery. Mol. Inform. 2020, 39, 2000171. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; Saldívar-González, F.I. Cheminformatics to Characterize Pharmacologically Active Natural Products. Biomolecules 2020, 10, 1566. [Google Scholar] [CrossRef]
- Rodrigues, T. Harnessing the Potential of Natural Products in Drug Discovery from a Cheminformatics Vantage Point. Org. Biomol. Chem. 2017, 15, 9275–9282. [Google Scholar] [CrossRef]
- MarinLit. Available online: http://pubs.rsc.org/marinlit/ (accessed on 11 May 2024).
- SuperNatural 3.0. Available online: https://bioinf-applied.charite.de/supernatural_3/subpages/compounds.php (accessed on 2 May 2024).
- COCONUT. Available online: https://coconut.naturalproducts.net/ (accessed on 2 May 2024).
- Holland, D.C.; Hayton, J.B.; Kiefel, M.J.; Carroll, A.R. Synthesis and Cheminformatics-Directed Antibacterial Evaluation of Echinosulfonic Acid-Inspired Bis-Indole Alkaloids. Molecules 2024, 29, 2806. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Barresi, E.; Robello, M.; Baglini, E.; Poggetti, V.; Viviano, M.; Salerno, S.; da Settimo, F.; Taliani, S. Indol-3-ylglyoxylamide as Privileged Scaffold in Medicinal Chemistry. Pharmaceuticals 2023, 16, 997. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Wang, J.; Jin, W.; Zhou, X.; Li, J.; Xu, C.; Ma, Z.; Wang, J.; Qin, L.; Zhou, B.; Ding, W.; et al. Identification, Structure-Activity Relationships of Marine-Derived Indolocarbazoles, and a Dual PKCθ/ΔInhibitor with Potent Antipancreatic Cancer Efficacy. J. Med. Chem. 2020, 63, 12978–12991. [Google Scholar] [CrossRef]
- Carroll, A.R.; Avery, V.M. Leptoclinidamines A-C, Indole Alkaloids from the Australian Ascidian Leptoclinides durus. J. Nat. Prod. 2009, 72, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, R.; Pearce, A.N.; Page, M.J.; Kaiser, M.; Bourguet-Kondracki, M.L.; Harper, J.L.; Webb, V.L.; Copp, B.R. Didemnidines A and B, Indole Spermidine Alkaloids from the New Zealand Ascidian Didemnum sp. J. Nat. Prod. 2011, 74, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Bokesch, H.R.; Pannell, L.K.; McKee, T.C.; Boyd, M.R. Coscinamides A, B and C, Three New Bis Indole Alkaloids from the Marine Sponge Coscinoderma sp. Tetrahedron Lett. 2000, 41, 6305–6308. [Google Scholar] [CrossRef]
- Li, J.L.; Xiao, B.; Park, M.; Yoo, E.S.; Shin, S.; Hong, J.; Chung, H.Y.; Kim, H.S.; Jung, J.H. PPAR-γ Agonistic Metabolites from the Ascidian Herdmania momus. J. Nat. Prod. 2012, 75, 2082–2087. [Google Scholar] [CrossRef]
- Vasconcelos, S.N.S.; Meissner, K.A.; Ferraz, W.R.; Trossini, G.H.G.; Wrenger, C.; Stefani, H.A. Indole-3-Glyoxyl Tyrosine: Synthesis and Antimalarial Activity against Plasmodium falciparum. Future Med. Chem. 2019, 11, 525–538. [Google Scholar] [CrossRef]
- Stefani, H.A.; Vasconcelos, S.N.S.; Souza, F.B.; Manarin, F.; Zukerman-Schpector, J. One-Pot Three-Component Synthesis of Indole-3-Glyoxyl Derivatives and Indole-3-Glyoxyl Triazoles. Tetrahedron Lett. 2013, 54, 5821–5825. [Google Scholar] [CrossRef]
- Pujols, J.; Peña-Díaz, S.; Conde-Giménez, M.; Pinheiro, F.; Navarro, S.; Sancho, J.; Ventura, S. High-Throughput Screening Methodology to Identify Alpha-Synuclein Aggregation Inhibitors. Int. J. Mol. Sci. 2017, 18, 478. [Google Scholar] [CrossRef]
- Young, L.M.; Saunders, J.C.; Mahood, R.A.; Revill, C.H.; Foster, R.J.; Tu, L.H.; Raleigh, D.P.; Radford, S.E.; Ashcroft, A.E. Screening and Classifying Small-Molecule Inhibitors of Amyloid Formation Using Ion Mobility Spectrometry-Mass Spectrometry. Nat. Chem. 2015, 7, 73–81. [Google Scholar] [CrossRef]
- Velander, P.; Wu, L.; Henderson, F.; Zhang, S.; Bevan, D.R.; Xu, B. Natural Product-Based Amyloid Inhibitors. Biochem. Pharmacol. 2017, 139, 40–55. [Google Scholar] [CrossRef]
- Jennings, L.K.; Ahmed, I.; Munn, A.L.; Carroll, A.R. Yeast-based screening of natural product extracts results in the identification of prion inhibitors from a marine sponge. Prion 2018, 12, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Prebble, D.W.; Holland, D.C.; Hayton, J.B.; Ferretti, F.; Jennings, L.K.; Everson, J.; Xu, M.; Kiefel, M.J.; Mellick, G.D.; Carroll, A.R. α-Synuclein Aggregation Inhibitory Procerolides and Diphenylalkanes from the Ascidian Polycarpa procera. J. Nat. Prod. 2023, 86, 533–540. [Google Scholar] [CrossRef]
- Prebble, D.W.; Er, S.; Xu, M.; Hlushchuk, I.; Domanskyi, A.; Airavaara, M.; Ekins, M.G.; Mellick, G.D.; Carroll, A.R. α-Synuclein Aggregation Inhibitory Activity of the Bromotyrosine Derivatives Aerothionin and Aerophobin-2 from the Subtropical Marine Sponge Aplysinella sp. Results Chem. 2022, 4, 100472. [Google Scholar] [CrossRef]
- Hai, Y.; Cai, Z.M.; Li, P.J.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Trends of Antimalarial Marine Natural Products: Progresses, Challenges and Opportunities. Nat. Prod. Rep. 2022, 39, 969–990. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Pawlik, B.; Wang, X.; Jochmans, D.; Neyts, J.; Młynarski, W.; et al. SARS-CoV-2 Mpro Inhibitors and Activity-Based Probes for Patient-Sample Imaging. Nat. Chem. Biol. 2021, 17, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Ackerley, D.F.; Calcott, M.J. High-Throughput Screening for Inhibitors of the SARS-CoV-2 Protease Using a FRET-Biosensor. Molecules 2020, 25, 4666. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hu, Y.; Townsend, J.A.; Lagarias, P.I.; Marty, M.T.; Kolocouris, A.; Wang, J. Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin Are Nonspecific Promiscuous SARS-CoV-2 Main Protease Inhibitors. ACS Pharmacol. Transl. Sci. 2020, 3, 1265–1277. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, B.; Jiang, X.M.; Su, H.; Li, J.; Zhao, Y.; Xie, X.; Jin, Z.; Peng, J.; Liu, F.; et al. Structure-Based Design of Antiviral Drug Candidates Targeting the SARS-CoV-2 Main Protease. Science 2020, 368, 1331–1335. [Google Scholar] [CrossRef]
- Dorababu, A. Indole-a Promising Pharmacophore in Recent Antiviral Drug Discovery. RSC Med. Chem. 2020, 11, 1335–1353. [Google Scholar] [CrossRef]
- Wang, T.; Wallace, O.B.; Zhang, Z.; Fang, H.; Yang, Z.; Robinson, B.A.; Spicer, T.P.; Gong, Y.F.; Blair, W.S.; Shi, P.Y.; et al. A Survey of Core Replacements in Indole-Based HIV-1 Attachment Inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 1423–1429. [Google Scholar] [CrossRef]
- Holland, D.C.; Schroder, W.A.; Calcott, M.J.; Kaemmerer, E.; Avery, V.M.; Ekins, M.G.; Carroll, A.R. Cyclotheonellazoles D-I, Potent Elastase Inhibitory Thiazole-Containing Cyclic Peptides from Theonella sp. J. Nat. Prod. 2023, 86, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Clardy, J.; Hagihara, M.; Karmacharya, R.; Albers, M.W.; Schreiber, S.L. Atomic Structure of the Trypsin-Cyclotheonamide A Complex: Lessons for the Design of Serine Protease Inhibitors. J. Am. Chem. Soc. 1993, 115, 12619–12620. [Google Scholar] [CrossRef]
- Issac, M.; Aknin, M.; Gauvin-Bialecki, A.; De Voogd, N.; Ledoux, A.; Frederich, M.; Kashman, Y.; Carmeli, S. Cyclotheonellazoles A-C, Potent Protease Inhibitors from the Marine Sponge Theonella aff. swinhoei. J. Nat. Prod. 2017, 80, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Maryanoff, B.E.; Costanzo, M.J. Inhibitors of Proteases and Amide Hydrolases That Employ an α-Ketoheterocycle as a Key Enabling Functionality. Bioorganic Med. Chem. 2008, 16, 1562–1595. [Google Scholar] [CrossRef] [PubMed]
- Donarska, B.; Łaczkowski, K.Z. Recent Advances in the Development of Elastase Inhibitors. Future Med. Chem. 2020, 12, 1809–1813. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Xu, H.; Zhang, T.; Zhang, S.; Zhao, X.; Jiang, P.; Li, J.; Ye, B.; Sun, Y.; et al. Elastase Inhibitor Cyclotheonellazole A: Total Synthesis and in Vivo Biological Evaluation for Acute Lung Injury. J. Med. Chem. 2022, 65, 2971–2987. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, C.; Aguzzi, A. Prions, prionoids and protein misfolding disorders. Nat. Reviews. Genet. 2018, 19, 405–418. [Google Scholar] [CrossRef]
- Thompson, M.J.; Borsenberger, V.; Louth, J.C.; Judd, K.E.; Chen, B. Design, Synthesis, and Structure−Activity Relationship of Indole-3-glyoxylamide Libraries Possessing Highly Potent Activity in a Cell Line Model of Prion Disease. J. Med. Chem. 2009, 52, 7503–7511. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Gupta, L.; Mittal, M.; Vishwakarma, P.; Gupta, S.; Chauhan, P.M.S. Synthesis and biological evaluation of indolyl glyoxylamides as a new class of antileishmanial agents. Bioorganic Med. Chem. Lett. 2010, 20, 6191–6194. [Google Scholar] [CrossRef]
- Duffy, S.; Avery, V.M. Identification of Inhibitors of Plasmodium falciparum Gametocyte Development. Malar. J. 2013, 12, 408. [Google Scholar] [CrossRef]
- Feng, B.Y.; Shoichet, B.K. A Detergent-Based Assay for the Detection of Promiscuous Inhibitors. Nat. Protoc. 2006, 1, 550–553. [Google Scholar] [CrossRef] [PubMed]
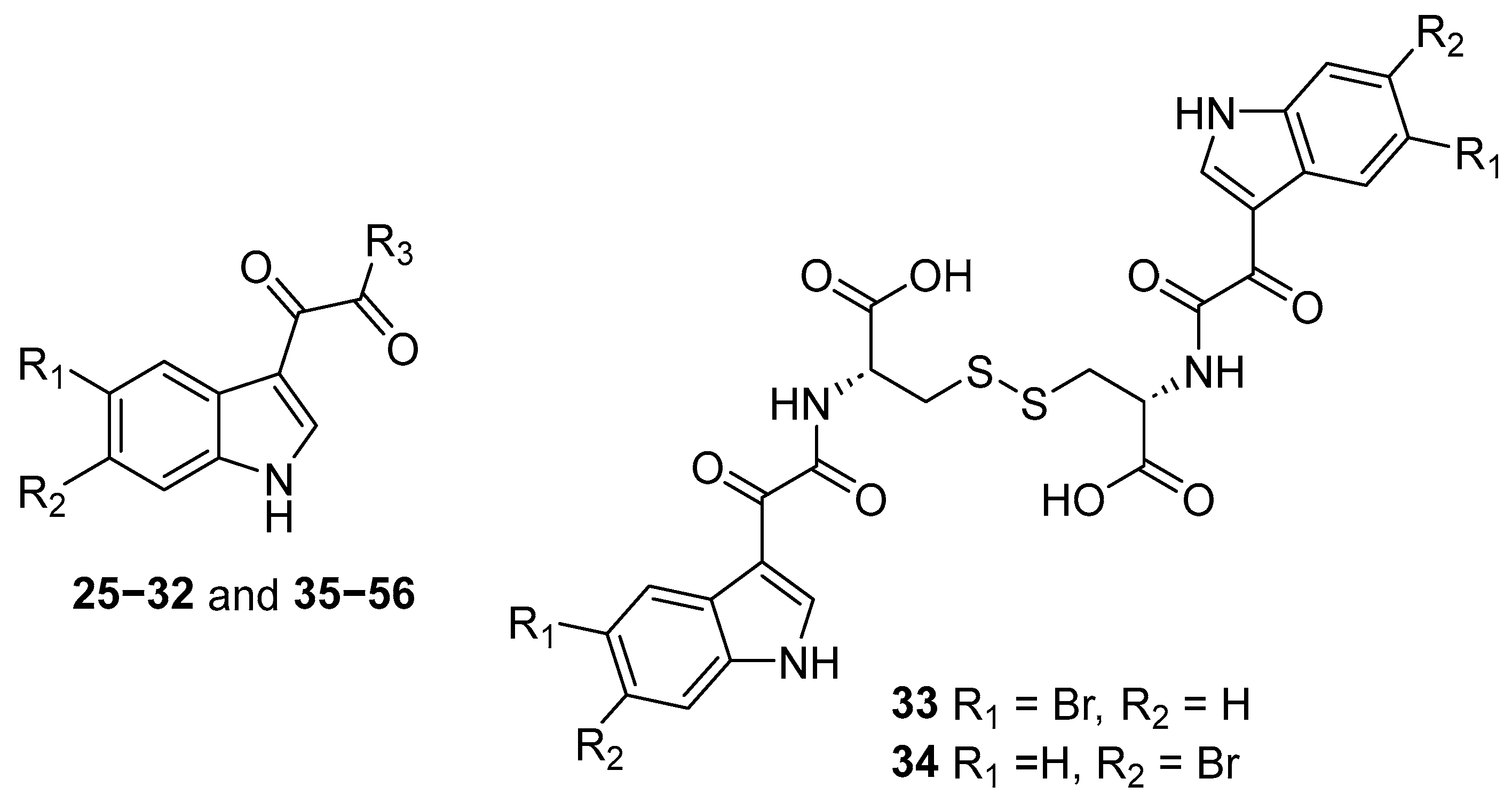

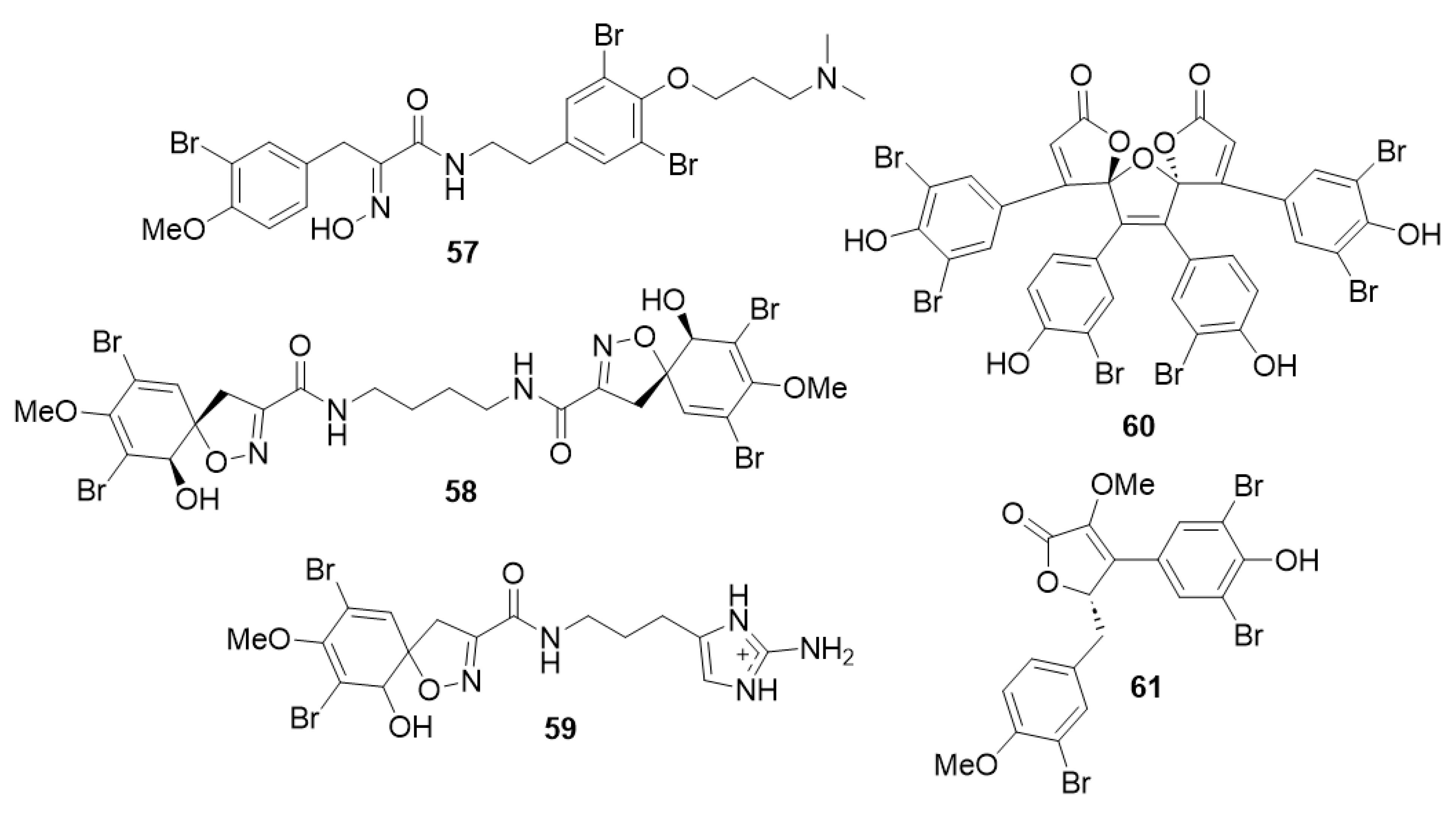
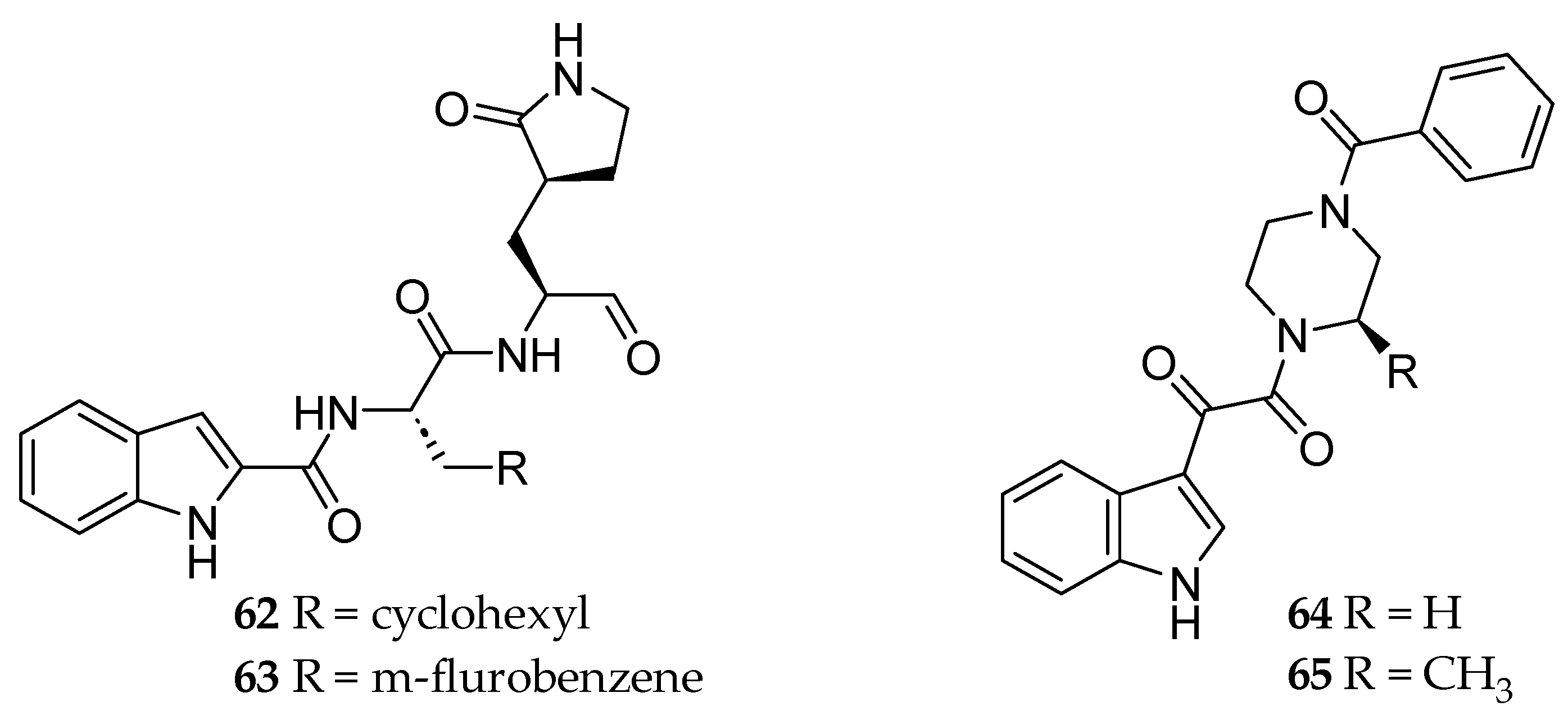
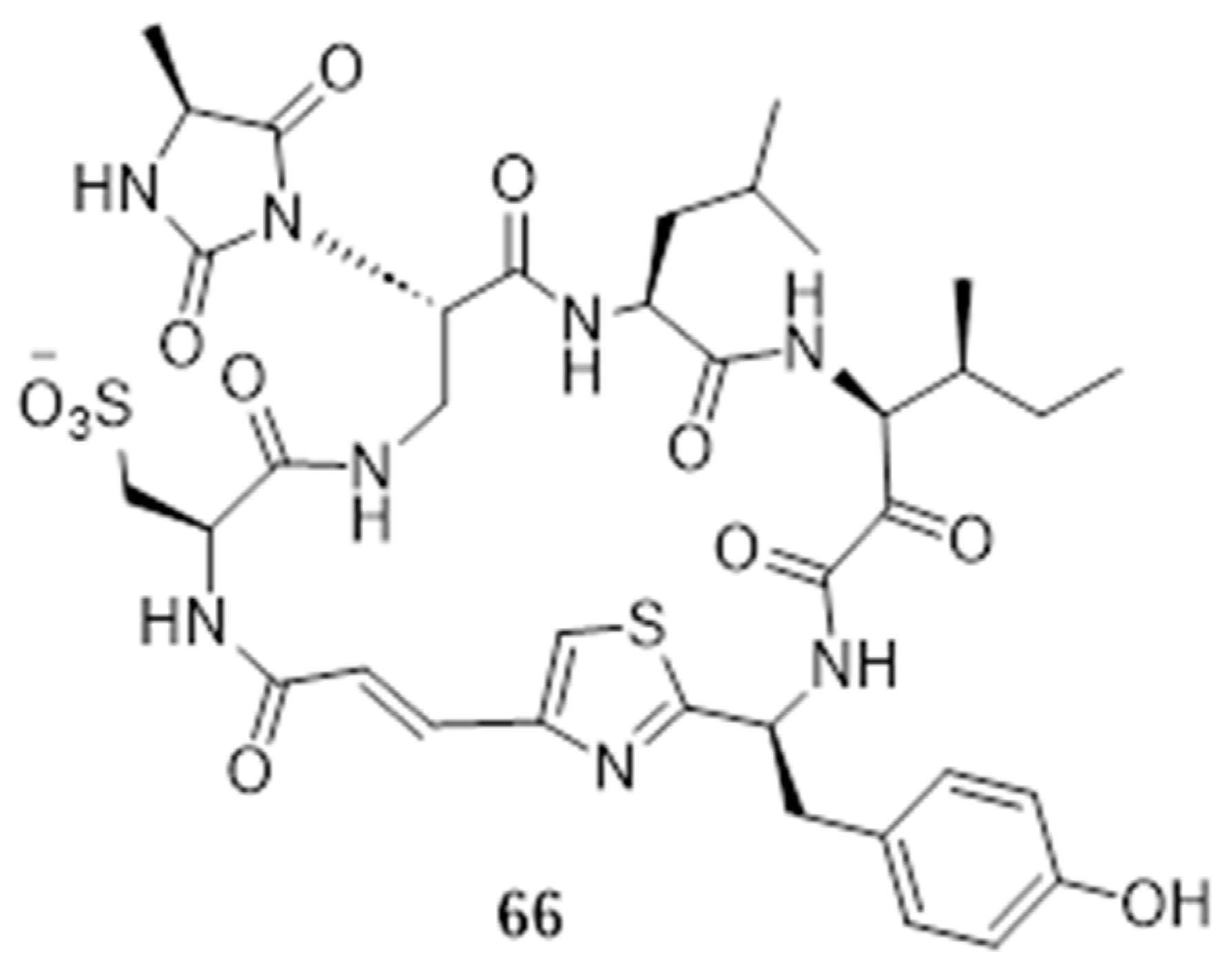

| Compound | R1 | R2 | R3 | Compound | R1 | R2 | R3 |
|---|---|---|---|---|---|---|---|
| 25 | Br | H | L-alanine | 41 | H | Br | L-glutamic acid |
| 26 | Br | H | D-alanine | 42 | H | Br | D-glutamic acid |
| 27 | H | Br | L-alanine | 43 | Br | H | L-serine |
| 28 | H | Br | D-alanine | 44 | H | Br | L-serine |
| 29 | Br | H | L-arginine | 45 | Br | H | L-tryptophan |
| 30 | Br | H | D-arginine | 46 | Br | H | D-tryptophan |
| 31 | H | Br | L-arginine | 47 | H | Br | L-tryptophan |
| 32 | H | Br | D-arginine | 48 | H | Br | D-tryptophan |
| 33 | Br | H | L-cysteine | 49 | Br | H | L-tyrosine |
| 34 | H | Br | L-cysteine | 50 | Br | H | D-tyrosine |
| 35 | Br | H | L-histidine | 51 | H | Br | L-tyrosine |
| 36 | H | Br | L-histidine | 52 | H | Br | D-tyrosine |
| 37 | Br | H | L-isoleucine | 53 | Br | H | L-valine |
| 38 | H | Br | L-isoleucine | 54 | Br | H | D-valine |
| 39 | Br | H | L-glutamic acid | 55 | H | Br | L-valine |
| 40 | Br | H | D-glutamic acid | 56 | H | Br | D-valine |
| % Inhibition @ 60 µM (IC50) | ||||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | 3D7 a | Dd2 b | HEK293 c |
| 25 | H | Br | L-Arg | 1.0 | 6.5 | 18.5 |
| 26 | H | Br | D-Arg | 56.0 | 33.0 | 14.5 |
| 36 | H | Br | L-His | 7.0 | 15.0 | 14.5 |
| 37 | Br | H | L-Ile | 11.5 | 9.0 | 15.0 |
| 38 | H | Br | L-Ile | 23.5 | 4.5 | 14.5 |
| 40 | Br | H | D-Glu | 2.0 | 2.5 | 18.5 |
| 41 | H | Br | L-Glu | 6.0 | 1.0 | 22.0 |
| 43 | Br | H | L-Ser | 1.0 | 1.0 | 21.0 |
| 45 | Br | H | L-Trp | 73.0 | 56.0 | 24.0 |
| 46 | Br | H | D-Trp | 100.0 (7.4 µM) | 97.5 (8.2 µM) | 21.5 (IA @ 60 µM) |
| 47 | H | Br | L-Trp | 14.0 | 20.0 | 14.0 |
| 48 | H | Br | D-Trp | 99.0 | 97.5 | 16.0 |
| 50 | Br | H | D-Tyr | 99.0 | 97.5 | 20.5 |
| 51 | H | Br | L-Tyr | 58.0 | 56.0 | 21.5 |
| Dihydroartemisinin | - | - | - | 100.0 (1.3 nM) | 100.0 (1.7 nM) | IA @ 2 µM |
| Puromycin | - | - | - | (79.2 nM) | (86.5 nM) | (673 nM) |
| Chloroquine | - | - | - | (77.8 nM) | (695.5 nM) | IA @ 10 µM |
| Pyrimethamine | - | - | - | −10.0 (14.1 nM) | IA @ 10 µM | IA @ 10 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holland, D.C.; Prebble, D.W.; Calcott, M.J.; Schroder, W.A.; Ferretti, F.; Lock, A.; Avery, V.M.; Kiefel, M.J.; Carroll, A.R. Cheminformatics-Guided Exploration of Synthetic Marine Natural Product-Inspired Brominated Indole-3-Glyoxylamides and Their Potentials for Drug Discovery. Molecules 2024, 29, 3648. https://doi.org/10.3390/molecules29153648
Holland DC, Prebble DW, Calcott MJ, Schroder WA, Ferretti F, Lock A, Avery VM, Kiefel MJ, Carroll AR. Cheminformatics-Guided Exploration of Synthetic Marine Natural Product-Inspired Brominated Indole-3-Glyoxylamides and Their Potentials for Drug Discovery. Molecules. 2024; 29(15):3648. https://doi.org/10.3390/molecules29153648
Chicago/Turabian StyleHolland, Darren C., Dale W. Prebble, Mark J. Calcott, Wayne A. Schroder, Francesca Ferretti, Aaron Lock, Vicky M. Avery, Milton J. Kiefel, and Anthony R. Carroll. 2024. "Cheminformatics-Guided Exploration of Synthetic Marine Natural Product-Inspired Brominated Indole-3-Glyoxylamides and Their Potentials for Drug Discovery" Molecules 29, no. 15: 3648. https://doi.org/10.3390/molecules29153648
APA StyleHolland, D. C., Prebble, D. W., Calcott, M. J., Schroder, W. A., Ferretti, F., Lock, A., Avery, V. M., Kiefel, M. J., & Carroll, A. R. (2024). Cheminformatics-Guided Exploration of Synthetic Marine Natural Product-Inspired Brominated Indole-3-Glyoxylamides and Their Potentials for Drug Discovery. Molecules, 29(15), 3648. https://doi.org/10.3390/molecules29153648








