Functionalizing Thiosemicarbazones for Covalent Conjugation
Abstract
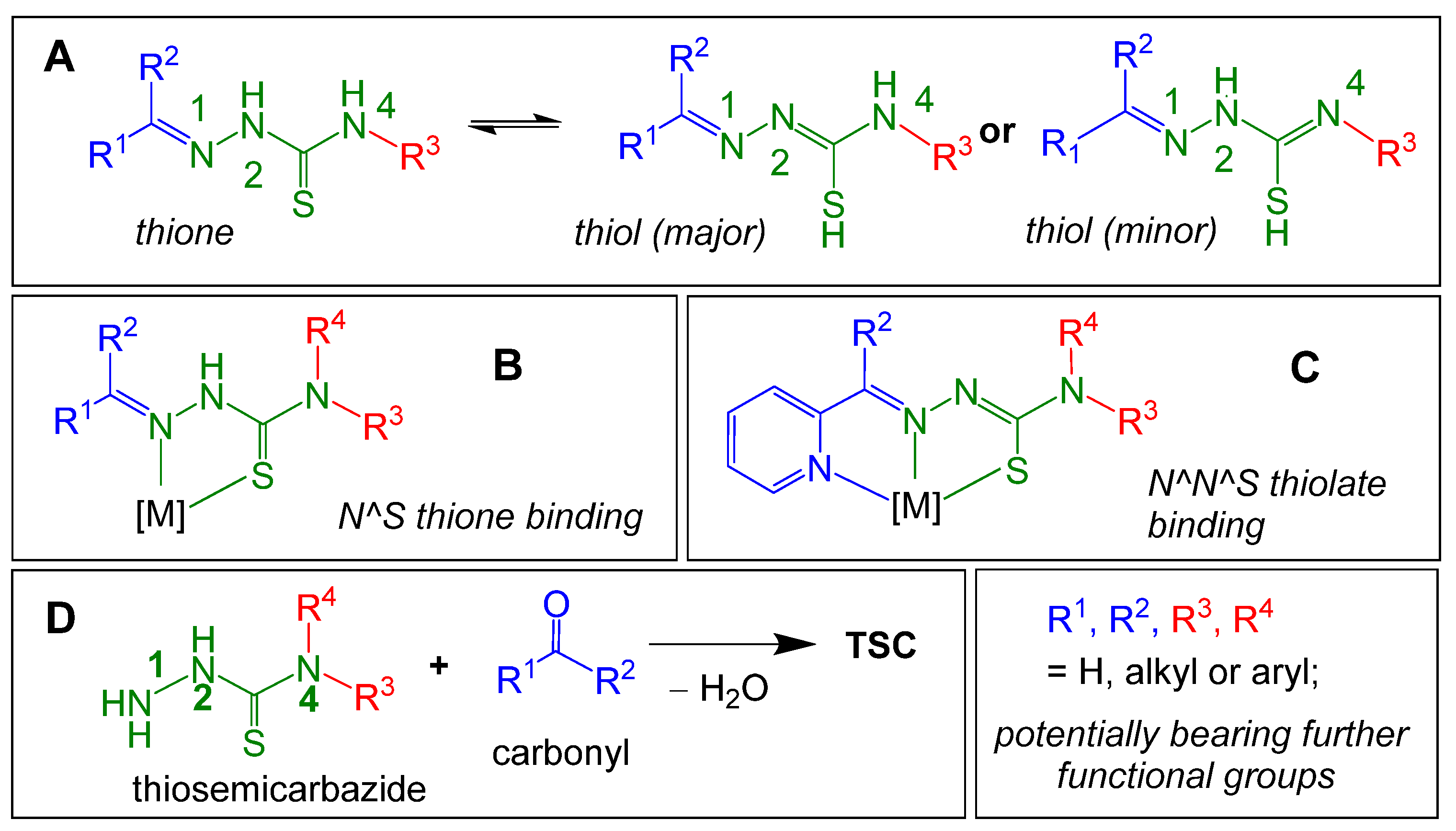
1. Introduction
2. Results and Discussion
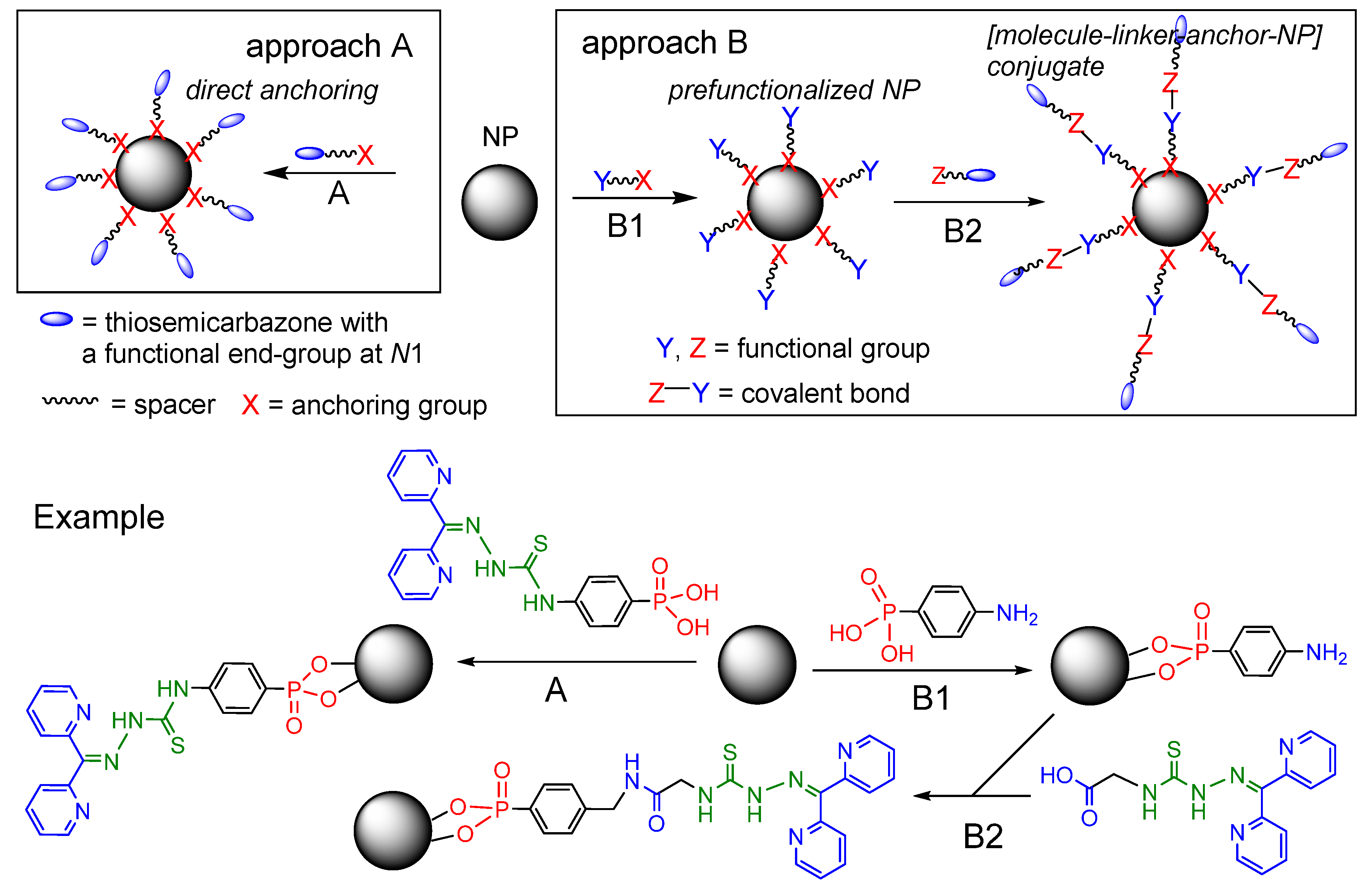
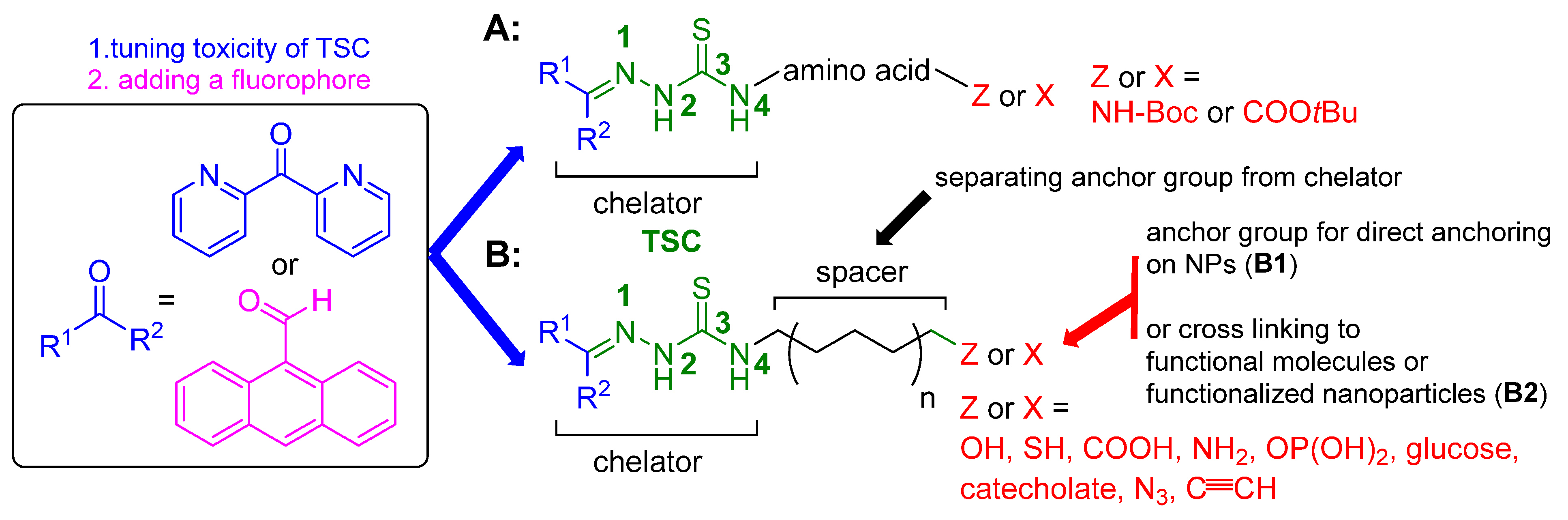
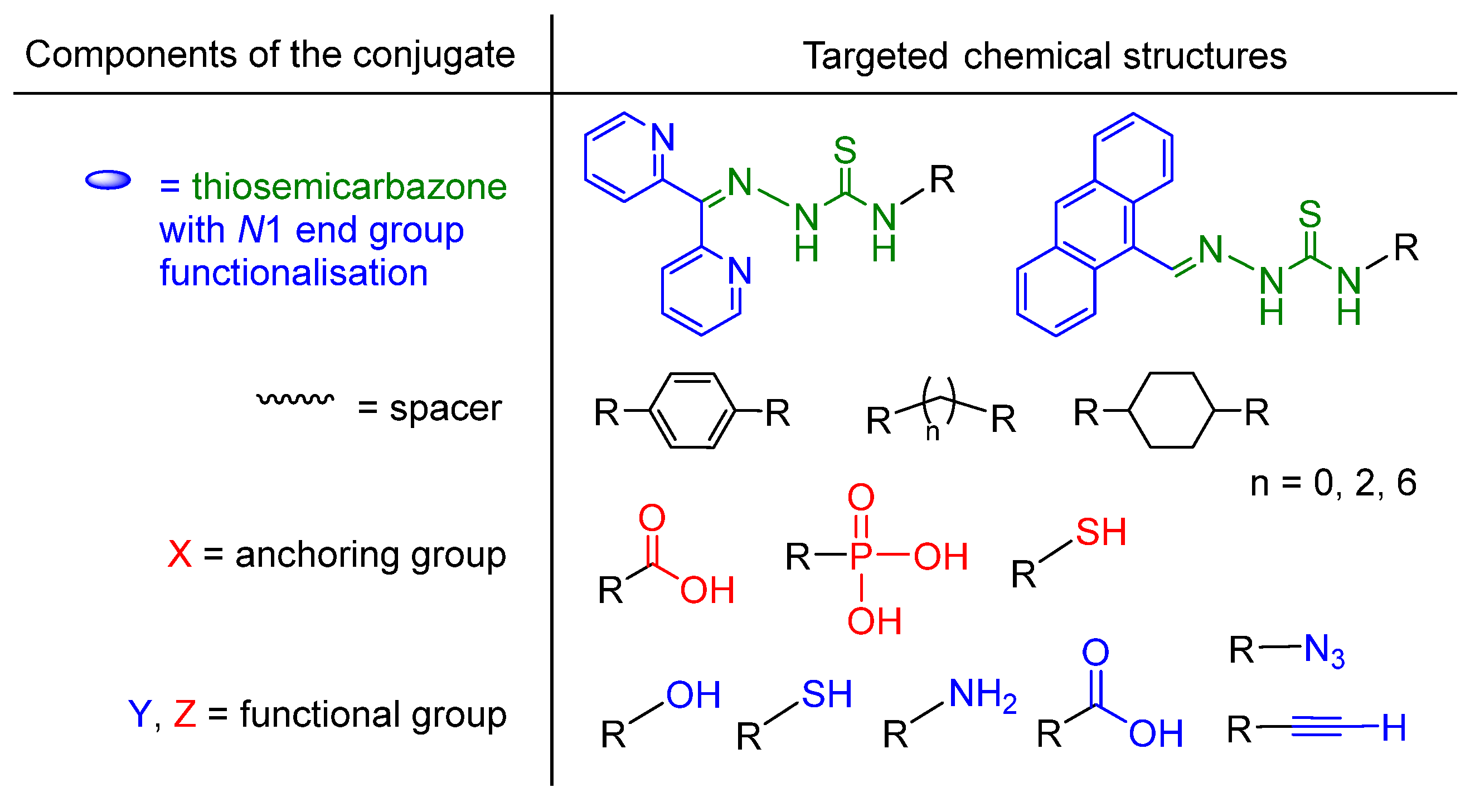
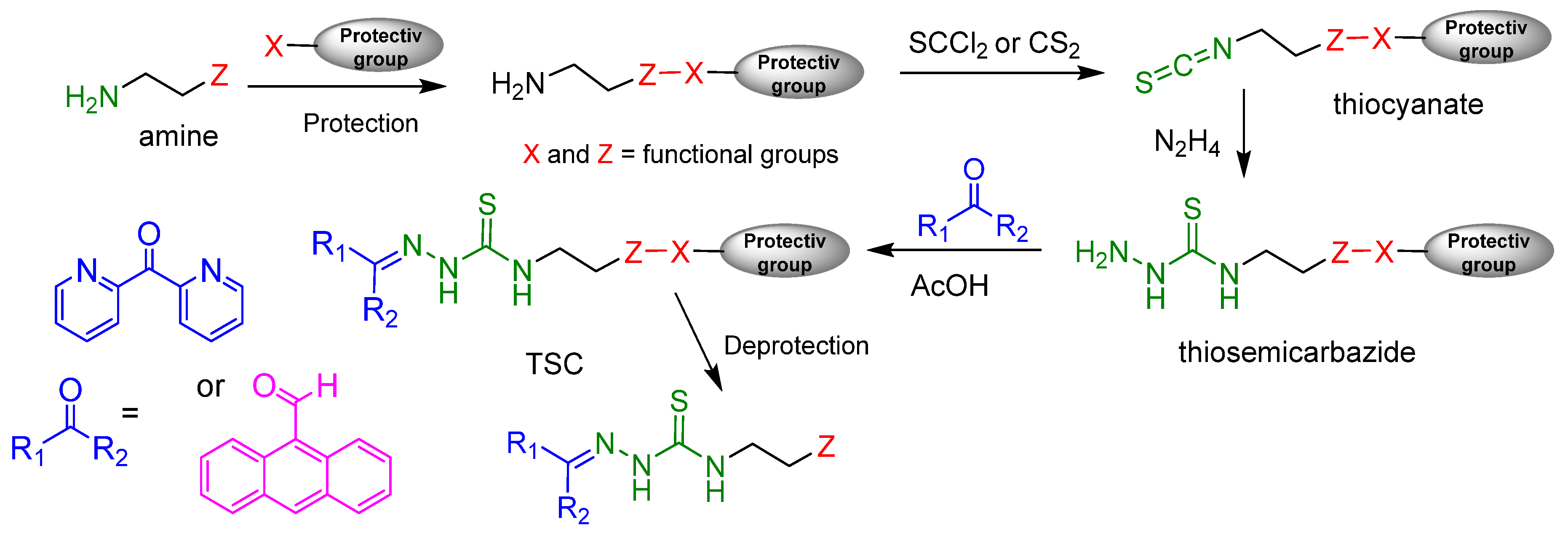
2.1. General Approaches and Targets
2.2. Syntheses of TSCs
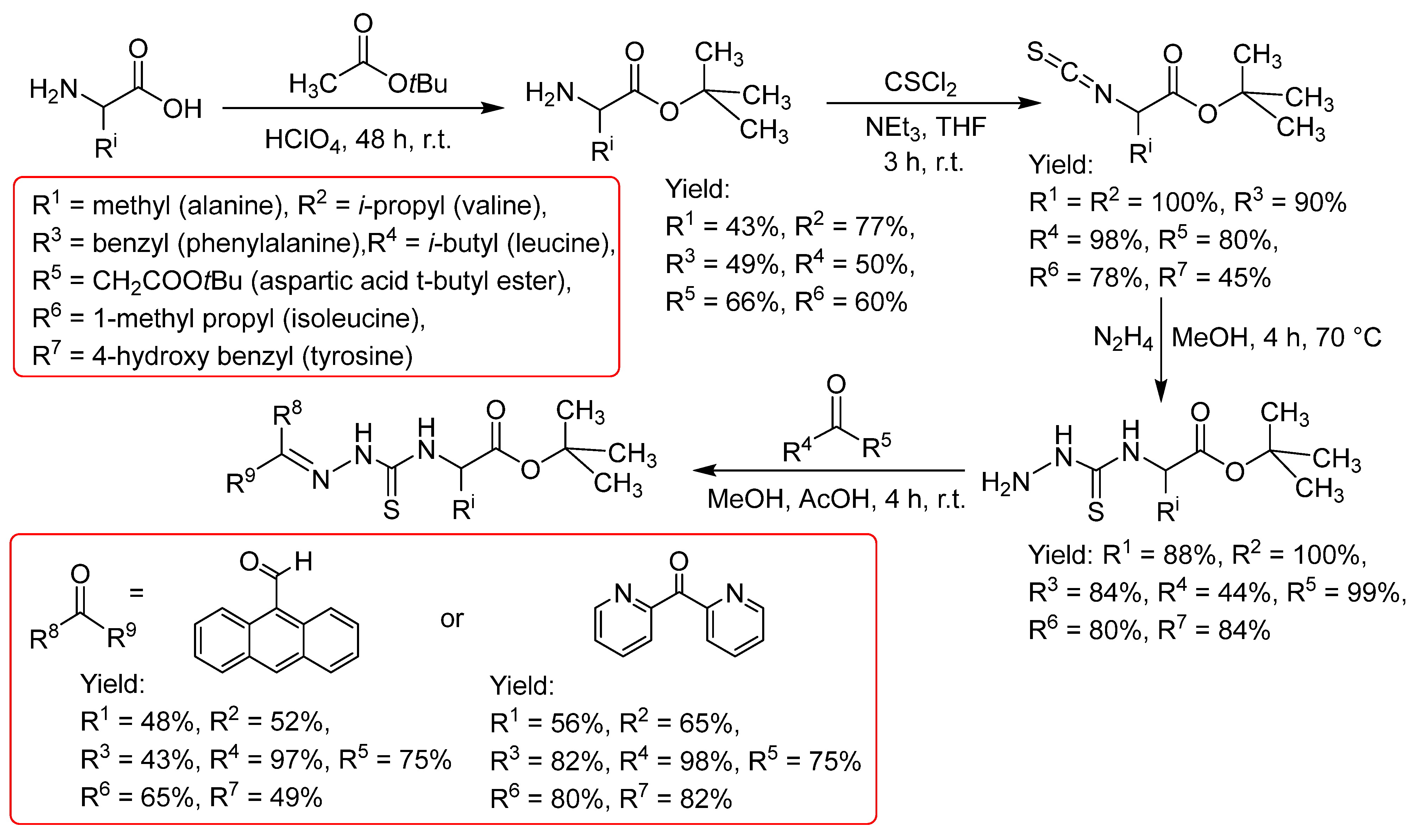
2.2.1. Thiosemicarbazones Derived from Amino Acids
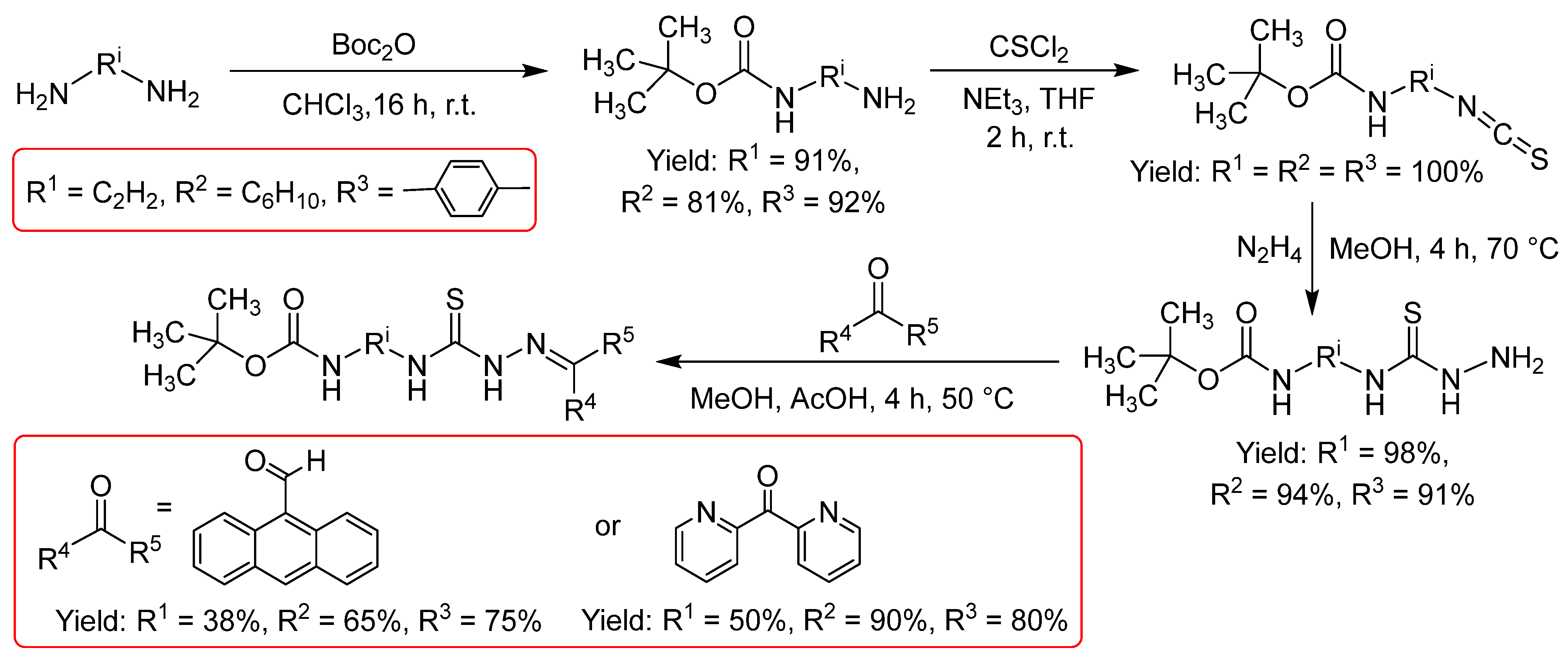
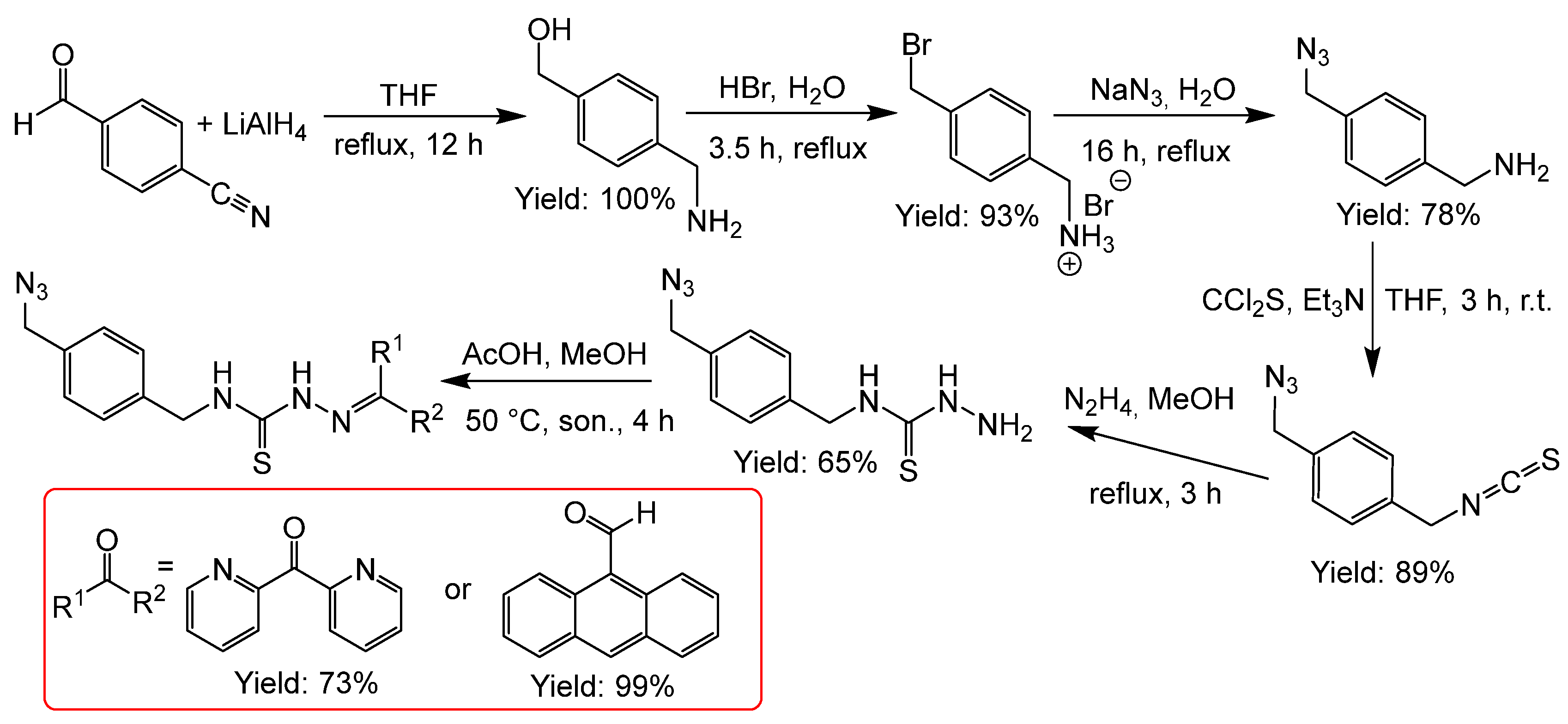
2.2.2. Thiosemicarbazones Derived from Diamines
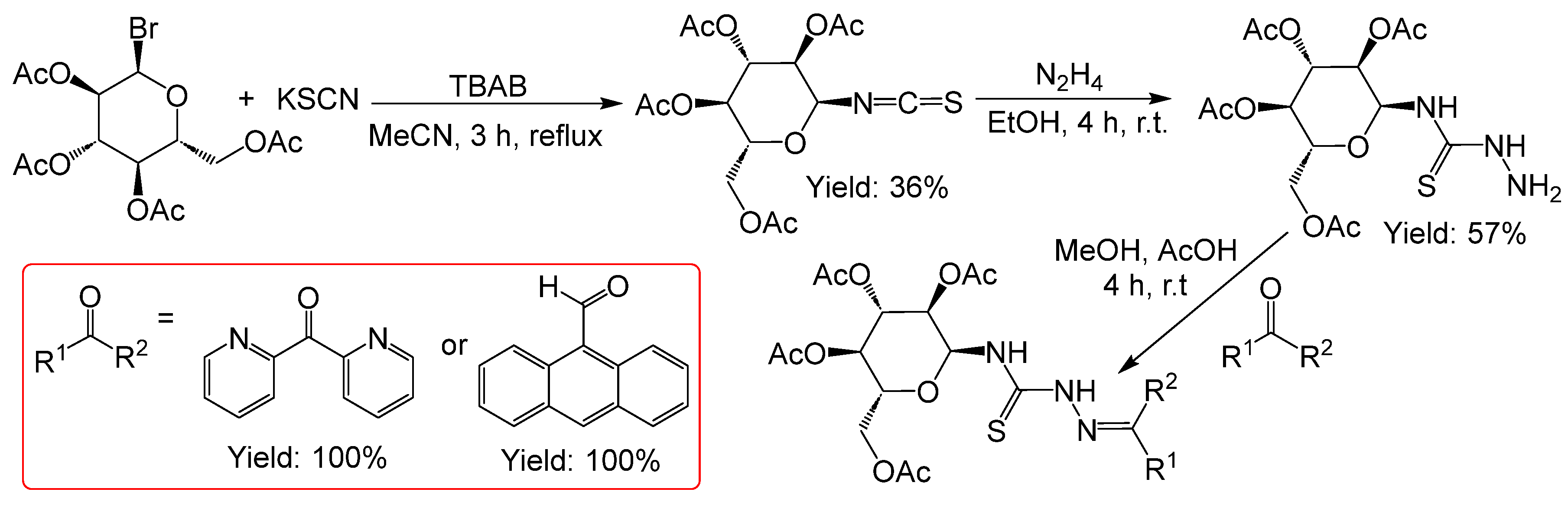
2.2.3. Thiosemicarbazones Derived from Acetobromo-α-D-Glucose
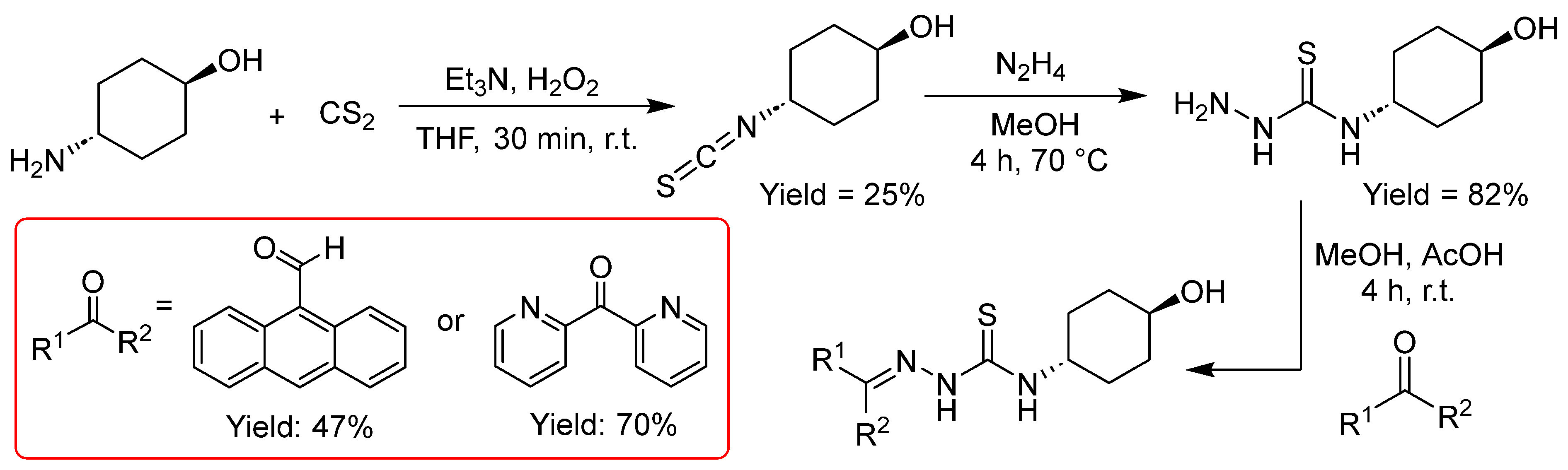
2.2.4. Thiosemicarbazones with a Hydroxy-Alkyl Function
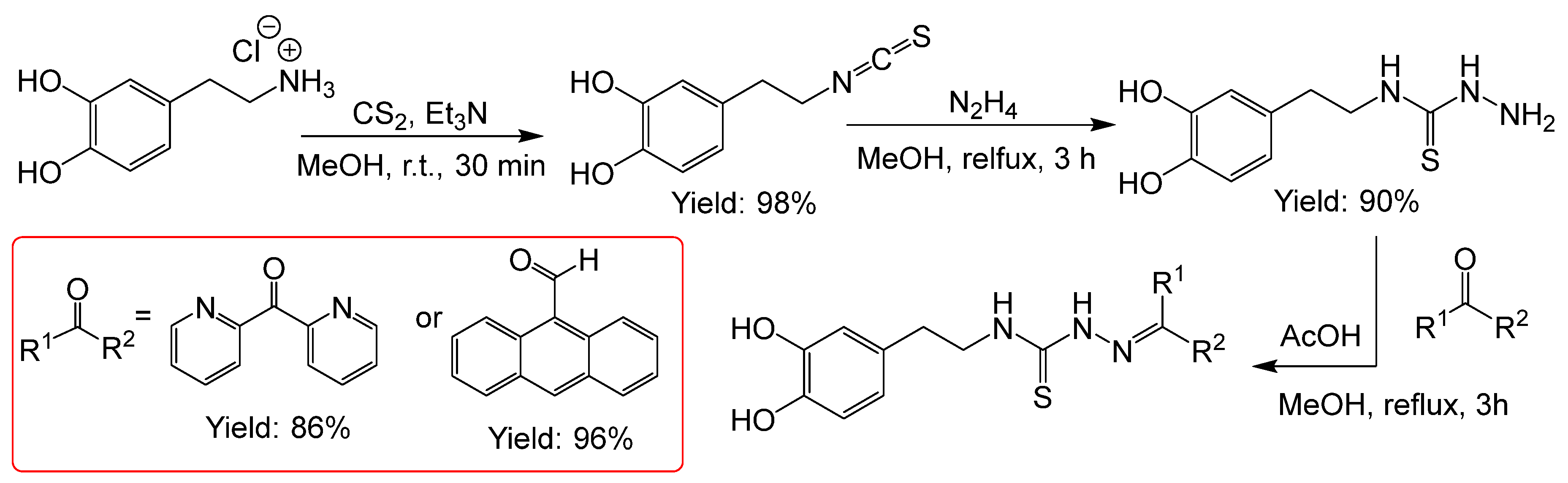
2.2.5. Thiosemicarbazones with a o-Hydroquinone Function
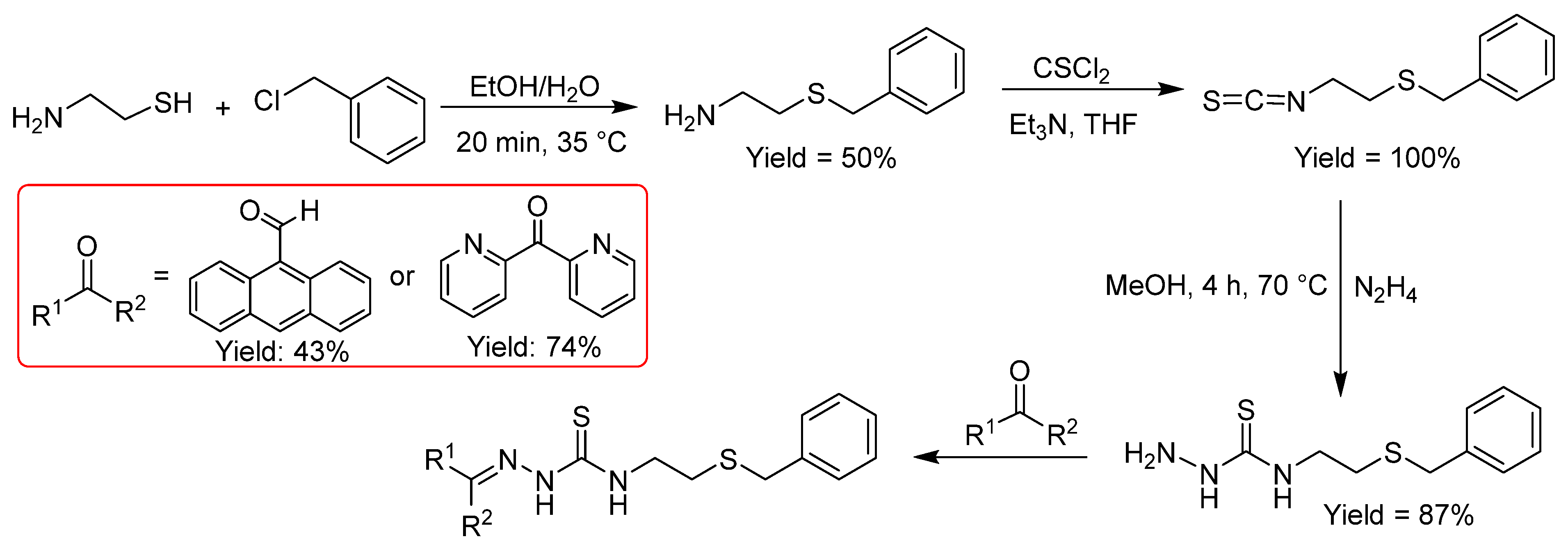
2.2.6. Thiosemicarbazones Derived from Amino Thiols
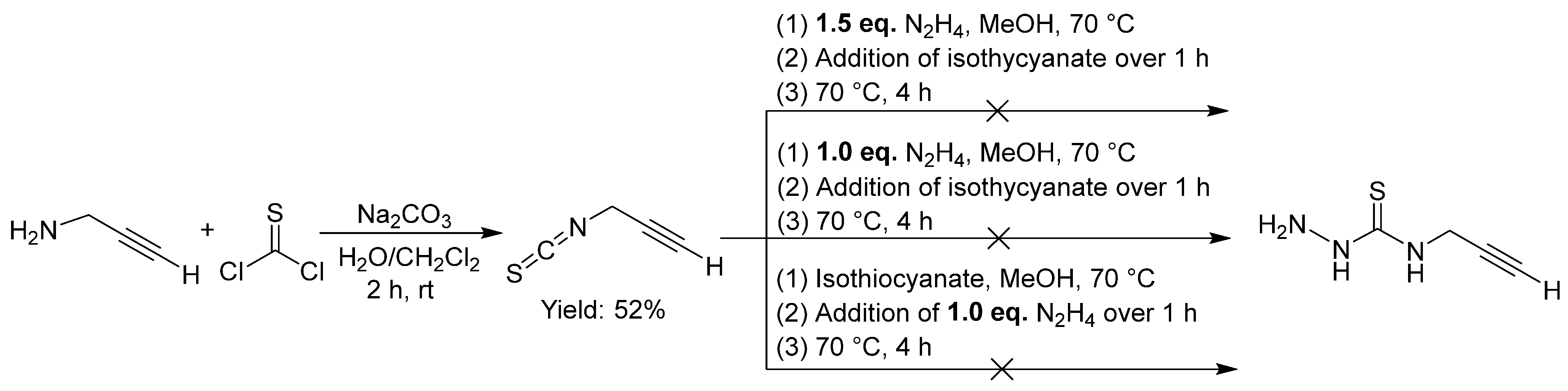
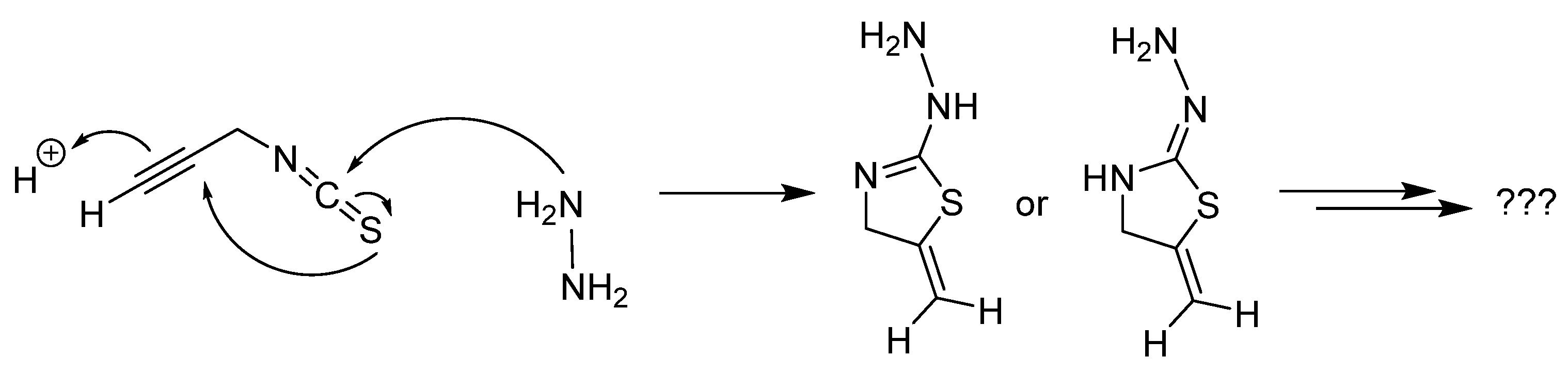
2.2.7. Thiosemicarbazones with an Alkyne Function
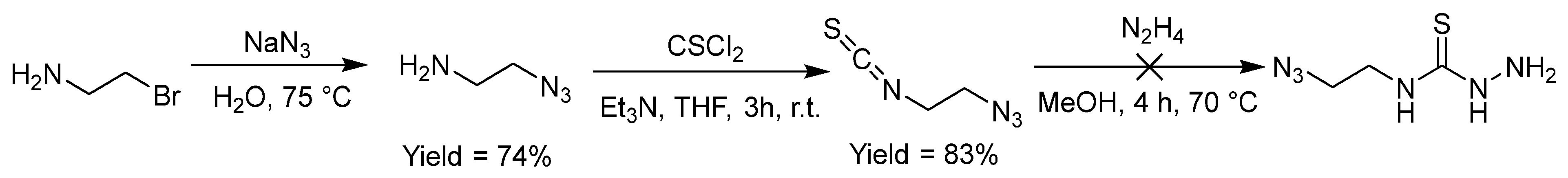
2.2.8. Thiosemicarbazones with an Azide Function
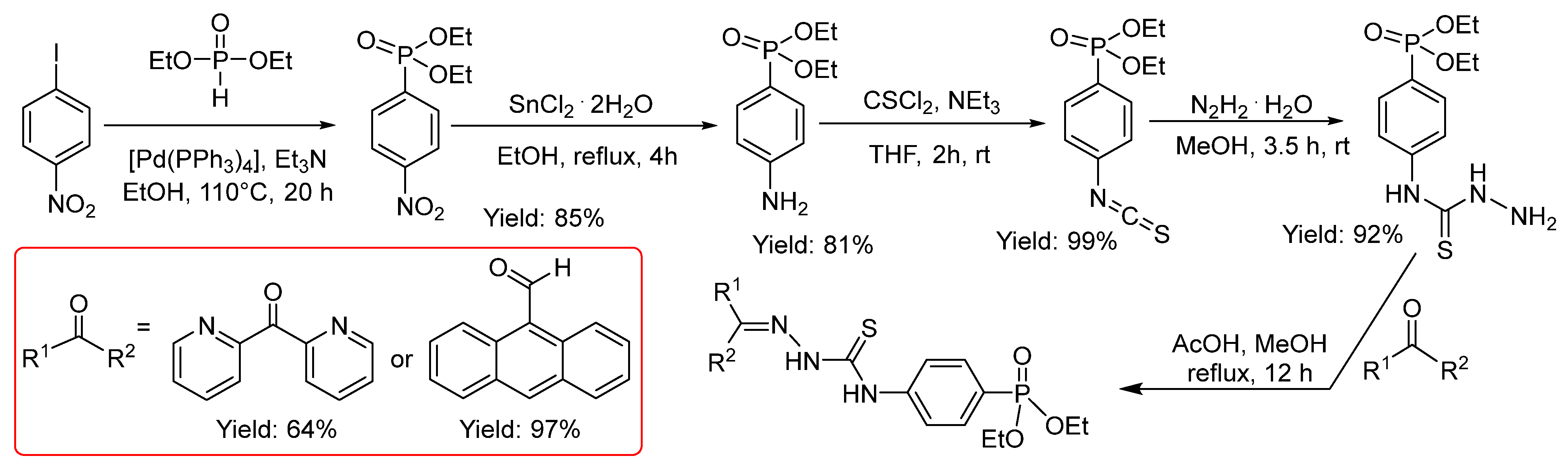
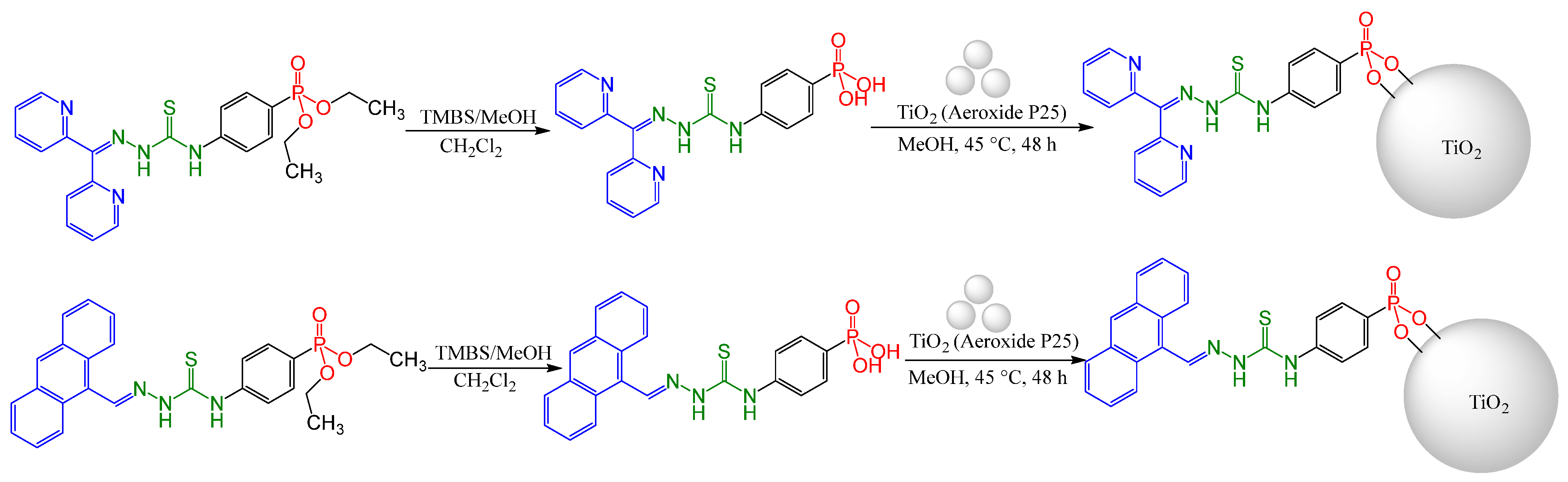
2.2.9. Thiosemicarbazones with a Phosphonate Group
2.3. Direct Anchoring of Phenyl Phosphonium Acid TSCs on TiO2
3. Materials and Methods
3.1. Materials and Synthesis
3.2. Instrumentation
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Summers, K.L. A Structural Chemistry Perspective on the Antimalarial Properties of Thiosemicarbazone Metal Complexes. Mini-Rev. Med. Chem. 2019, 19, 569–590. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.S.; Quach, P.; Richardson, D.R. Thiosemicarbazones: The new wave in cancer treatment. Fut. Med. Chem. 2009, 1, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.-J.; Wang, L.-W.; Hsu, T.-A.; Yueh, A.; Lee, C.C.; Lee, Y.-C.; Lee, C.-Y.; Chao, Y.-S.; Shih, S.-R.; Chern, J.-H. Isatin-β-thiosemicarbazones as potent herpes simplex virus inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, P.N.; Keter, F.K.; Meyer, D. New bis(thiosemicarbazonate) gold(III) complexes inhibit HIV replication at cytostatic concentrations: Potential for incorporation into virostatic cocktails. J. Inorg. Biochem. 2011, 105, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Bisceglie, F.; Bacci, C.; Vismarra, A.; Barilli, E.; Pioli, M.; Orsoni, N.; Pelosi, G. Antibacterial activity of metal complexes based on cinnamaldehyde thiosemicarbazone analogues. J. Inorg. Biochem. 2020, 203, 110888. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.F.; Marques, F.; Pinheiro, T.; Brito, M.J.V.; Scalese, G.; Pérez-Díaz, L.; Otero, L.; António, J.P.M.; Gambino, D.; Morais, T.S. Copper(I)-Thiosemicarbazone Complexes with Dual Anticancer and Antiparasitic Activity. ChemMedChem 2023, 18, e202300074. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, J.R.; Hueting, R. Metal complexes of thiosemicarbazones for imaging and therapy. Inorg. Chim. Acta 2012, 389, 3–15. [Google Scholar] [CrossRef]
- Jain, P.; Sharma, S.; Kuma, N.; Misra, N. Ni(II) and Cu(II) complexes of bidentate thiosemicarbazone ligand: Synthesis, structural, theoretical, biological studies and molecular modeling. Appl. Organomet. Chem. 2020, 34, e5736. [Google Scholar] [CrossRef]
- Mathuber, M.; Hager, S.; Keppler, B.K.; Heffeter, P.; Kowol, C.R. Liposomal formulations of anticancer copper(II) thiosemicarbazone complexes. Dalton Trans. 2021, 50, 16053–16066. [Google Scholar] [CrossRef]
- Fischer, B.; Kryeziu, K.; Kallus, S.; Heffeter, P.; Berger, W.; Kowol, C.R.; Keppler, B.K. Nanoformulations of anticancer thiosemicarbazones to reduce methemoglobin formation and improve anticancer activity. RSC Adv. 2016, 6, 55848–55859. [Google Scholar] [CrossRef]
- Toan, V.N.; Thanh, N.D.; Tri, N.M.; Huong, N.T.T. Synthesis and biological screening of thiosemicarbazones of substituted 3-acetylcoumarins having d-glucose moiety. Bioorg. Med. Chem. Lett. 2020, 30, 127664. [Google Scholar] [CrossRef]
- Pósa, V.; Stefanelli, A.; Nunes, J.H.B.; Hager, S.; Mathuber, M.; May, N.V.; Berger, W.; Keppler, B.K.; Kowol, C.R.; Enyedy, É.A.; et al. Thiosemicarbazone Derivatives Developed to Overcome COTI-2 Resistance. Cancers 2022, 14, 4455. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.D.; Duc, H.T.; Duyen, V.T.; Tuong, P.M.; Quoc, N.V. Synthesis and antibacterial and antifungal activities of N-(tetra-O-acetyl-β-d-glucopyranosyl) thiosemicarbazones of substituted 4-formylsydnones. Chem. Cent. J. 2015, 9, 60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thanh, N.D.; Giang, N.T.K.; Quyen, T.H.; Huong, D.T.; Toan, V.N. Synthesis and evaluation of in vivo antioxidant, in vitro antibacterial, MRSA and antifungal activity of novel substituted isatin N-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)thiosemicarbazones. Eur. J. Med. Chem. 2016, 123, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, H.; Zhang, H.; Li, M.; Huang, Z.; Wang, Q.; Li, X. Copper-thiosemicarbazone complexes conjugated-cellulose fibers: Biodegradable materials with antibacterial capacity. Carbohydr. Polym. 2022, 294, 119839. [Google Scholar] [CrossRef] [PubMed]
- Akam, E.A.; Tomat, E. Targeting Iron in Colon Cancer via Glycoconjugation of Thiosemicarbazone Prochelators. Bioconjugate Chem. 2016, 27, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Milunovic, M.N.M.; Enyedy, É.A.; Nagy, N.V.; Kiss, T.; Trondl, R.; Jakupec, M.A.; Keppler, B.K.; Krachler, R.; Novitchi, G.; Arion, V.B. l and d-Proline Thiosemicarbazone Conjugates: Coordination Behavior in Solution and the Effect of Copper(II) Coordination on Their Antiproliferative Activity. Inorg. Chem. 2012, 51, 9309–9321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paterson, B.M.; Karas, J.A.; Scanlon, D.B.; White, J.M.; Donnelly, P.S. Versatile New Bis(thiosemicarbazone) Bifunctional Chelators: Synthesis, Conjugation to Bombesin(7−14)-NH2, and Copper-64 Radiolabeling. Inorg. Chem. 2010, 49, 1884–1893. [Google Scholar] [CrossRef]
- Haseloer, A.; Lützenburg, T.; Strache, J.P.; Neudörfl, J.; Neundorf, I.; Klein, A. Building up PtII−Thiosemicarbazone−Lysine−sC18 Conjugates. ChemBioChem 2021, 22, 694–704. [Google Scholar] [CrossRef]
- Kallus, S.; Uhlik, L.; Schoonhoven, S.; Pelivan, K.; Berger, W.; Enyedy, E.A.; Hofmann, T.; Heffeter, P.; Kowol, C.R.; Keppler, B.K. Synthesis and biological evaluation of biotin-conjugated anticancer thiosemicarbazones and their iron(III) and copper(II) complexes. J. Inorg. Biochem. 2019, 190, 85–97. [Google Scholar] [CrossRef]
- Sung, Y.S.; Wu, W.; Ewbank, M.A.; Utterback, R.D.; Marty, M.T.; Tomat, E. Albumin Conjugates of Thiosemicarbazone and Imidazole-2-thione Prochelators: Iron Coordination and Antiproliferative Activity. ChemMedChem 2021, 16, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cui, J.; Ding, T.; Liu, Y.; Liang, H. Research Progress on the Biological Activities of Metal Complexes Bearing Polycyclic Aromatic Hydrazones. Molecules 2022, 27, 8393. [Google Scholar] [CrossRef]
- Franciscato, D.S.; Matias, T.A.; Shinohara, J.; Gonçalves, J.M.; Coelho, N.P.; Fernandes, C.S.; Basso, E.A.; Nakatani, H.S.; Araki, K.; Toma, H.E.; et al. Thiosemicarbazone@Gold nanoparticle hybrid as selective SERS substrate for Hg2+ ions. Spectrochim. Acta A Mol. Biomol. 2018, 204, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Isaac, I.O.; Ahmed, F.; Aslam, F.; Ali, S.; Imran, M.; Alharthy, R.D.; Shah, M.R.; Malik, M.I.; Hameed, A. Acridine-Thiosemicarbazones-Stabilized Silver Nanoparticles as a Selective Sensor for Copper(II)-Ion in Tap Water. ChemSelect 2019, 4, 8757–8763. [Google Scholar] [CrossRef]
- Kaviani, N.; Behrouz, S.; Jafari, A.A.; Rad, M.N.S. Functionalization of Fe3O4@SiO2 nanoparticles with Cu(I)-thiosemicarbazone complex as a robust and efficient heterogeneous nanocatalyst for N-arylation of N-heterocycles with aryl halides. RSC Adv. 2023, 13, 30293–30305. [Google Scholar] [CrossRef]
- Doğan, M.; Koçyiğit, Ü.M.; Gürdere, M.B.; Ceylan, M.; Budak, Y. Synthesis and biological evaluation of thiosemicarbazone derivatives. Med. Oncol. 2022, 39, 157. [Google Scholar] [CrossRef]
- Özbek, O.; Berkel, C. Sensor properties of thiosemicarbazones in different analytical methods. Polyhedron 2023, 238, 116426. [Google Scholar] [CrossRef]
- Britta, E.A.; da Silva, C.C.; Rubira, A.F.; Nakamura, C.V.; Borsali, R. Generating nanoparticles containing a new 4-nitrobenzaldehyde thiosemicarbazone compound with antileishmanial activity. Mater. Sci. Eng. C 2016, 69, 1159–1166. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Alsaif, N.; Algrain, N.; Wani, T.A.; Bhat, M.A. Enhanced Efficacy of Thiosemicarbazone Derivative-Encapsulated Fibrin Liposomes against Candidiasis in Murine Model. Pharmaceutics 2021, 13, 333. [Google Scholar] [CrossRef]
- Holley, C.K.; Sinquefield, B.; Majd, S. Optimization of the Single Emulsion Method for Encapsulation of a Cancer Drug in Nanoparticles. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1078–1081. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Dalmolin, L.F.; Silva, R.T.C.; Lopez, R.F.V.; Maia, P.I.S.; Moreto, J.A. PLGA-nanoparticles loaded with a thiosemicarbazone derived palladium(II) complex as a potential agent to new formulations for human ovarian carcinoma treatment. New J. Chem. 2020, 44, 14928–14935. [Google Scholar] [CrossRef]
- Spadola, G.; Sanna, V.; Bartoli, J.; Carcelli, M.; Pelosi, G.; Bisceglie, F.; Restivo, F.M.; Degola, D.; Rogolino, D. Thiosemicarbazone nano-formulation for the control of Aspergillus flavus. Environ. Sci. Pollut. Res. 2020, 27, 20125–20135. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017, 46, 6024–6045. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Bai, H.; Peng, B.; Fang, B.; Baell, J.; Li, L.; Huang, W.; Voelcker, N.H. Stimulus-cleavable chemistry in the field of controlled drug delivery. Chem. Soc. Rev. 2021, 50, 4872–4931. [Google Scholar] [CrossRef] [PubMed]
- Dal Corso, A.; Pignataro, L.; Belvisi, L.; Gennari, C. Innovative Linker Strategies for Tumor-Targeted Drug Conjugates. Chem.–Eur. J. 2019, 25, 14740–14757. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pan, Z.; Choudhury, M.R.; Yuan, Z.; Anifowose, A.; Yu, B.; Wang, W.; Wang, B. Making smart drugs smarter: The importance of linker chemistry in targeted drug delivery. Med. Res. Rev. 2020, 40, 2682–2713. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ding, H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xing, L.; Cao, Y.; Che, S. Coordination bonding based pH-responsive drug delivery systems. Coord. Chem. Rev. 2013, 257, 1933–1944. [Google Scholar] [CrossRef]
- Novio, F.; Simmchen, J.; Vázquez-Mera, N.; Amorín-Ferré, L.; Ruiz-Molina, D. Coordination polymer nanoparticles in medicine. Coord. Chem. Rev. 2013, 257, 2839–2847. [Google Scholar] [CrossRef]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef]
- Jeong, W.-j.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.S.; Hong, S. Peptide–nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 38. [Google Scholar] [CrossRef]
- Hernández, W.; Vaisberg, A.J.; Tobar, M.; Álvarez, M.; Manzur, J.; Echevarría, Y.; Spodine, E. In vitro antiproliferative activity of palladium(II) thiosemicarbazone complexes and the corresponding functionalized chitosan coated magnetite nanoparticles. New J. Chem. 2016, 40, 1853–1860. [Google Scholar] [CrossRef]
- Gholipour, N.; Akhlaghi, M.; Kheirabadi, A.M.; Geramifar, P.; Beiki, D. Development of Ga-68 labeled, biotinylated thiosemicarbazone dextran-coated iron oxide nanoparticles as multimodal PET/MRI probe. Int. J. Biol. Macromol. 2020, 148, 932–941. [Google Scholar] [CrossRef]
- Shafiei, I.; Tavassoli, S.P.; Rahmatollahi, H.R.; Ghasemian, R.; Salehzadeh, A. A Novel Copper Oxide Nanoparticle Conjugated by Thiosemicarbazone Promote Apoptosis in Human Breast Cancer Cell Line. J. Clust. Sci. 2022, 33, 2697–2706. [Google Scholar] [CrossRef]
- Badrooh, M.; Shokrollahi, F.; Javan, S.; Ghasemipour, T.; Mojdehi, S.R.; Farahnak, H.; Noveiri, M.J.S.; Hedayati, M.; Salehzadeh, A. Trigger of apoptosis in adenocarcinoma gastric cell line (AGS) by a complex of thiosemicarbazone and copper nanoparticles. Mol. Biol. Rep. 2022, 49, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Ataollah, S.; Shandi, S.; Salehzadeh, A.; Moradi-Shoeili, Z. Novel pyridinecarboxaldehyde thiosemicarbazone conjugated magnetite nanoparticulates (MNPs) promote apoptosis in human lung cancer A549 cells. Biol. Inorg. Chem. 2020, 25, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, M.R.; Salehzadeh, A.; Zaefizadeh, M.; Nikpasand, M. Functionalisation of Fe3O4 nanoparticles by 2-((pyrazol-4-yl)methylene)hydrazinecarbothioamide enhances the apoptosis of human breast cancer MCF-7 cells. IET Nanobiotechnol. 2020, 14, 508–518. [Google Scholar] [CrossRef]
- Shakya, B.; Yadav, P.N. Thiosemicarbazones as Potent Anticancer Agents and their Modes of Action. Mini-Rev. Med. Chem. 2020, 20, 638–661. [Google Scholar] [CrossRef]
- Richardson, D.R.; Sharpe, P.C.; Lovejoy, D.B.; Senaratne, D.; Kalinowski, D.S.; Islam, M.; Bernhardt, P.V. Dipyridyl Thiosemicarbazone Chelators with Potent and Selective Antitumor Activity Form Iron Complexes with Redox Activity. J. Med. Chem. 2006, 49, 6510–6521. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kalinowski, D.S.; Kovacevic, Z.; Siafakas, A.R.; Jansson, P.J.; Stefani, C.; Lovejoy, D.B.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Thiosemicarbazones from the Old to New: Iron Chelators That Are More Than Just Ribonucleotide Reductase Inhibitors. J. Med. Chem. 2009, 52, 5271–5294. [Google Scholar] [CrossRef]
- Jansson, P.J.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Novel Thiosemicarbazones of the ApT and DpT Series and Their Copper Complexes: Identification of Pronounced Redox Activity and Characterization of Their Antitumor Activity. J. Med. Chem. 2010, 53, 5759–5769. [Google Scholar] [CrossRef]
- Dharmasivam, M.; Kaya, B.; Wijesinghe, T.P.; Richardson, V.; Harmer, J.R.; Gonzalvez, M.A.; Lewis, W.; Azad, M.G.; Bernhardt, P.V.; Richardson, D.R. Differential transmetallation of complexes of the anti-cancer thiosemicarbazone, Dp4e4mT: Effects on anti-proliferative efficacy, redox activity, oxymyoglobin and oxy-hemoglobin oxidation. Chem. Sci. 2024, 15, 974–990. [Google Scholar] [CrossRef] [PubMed]
- Kate, A.N.; Kumbhar, A.A.; Khan, A.A.; Joshi, P.V.; Puranik, V.G. Monitoring Cellular Uptake and Cytotoxicity of Copper(II) Complex Using a Fluorescent Anthracene Thiosemicarbazone Ligand. Bioconjugate Chem. 2014, 25, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-H.; Khuat, T.-T.-H.; Do, D.-Q.; Hung-Huy Nguyen, H.-H.; Dinh, T.-H. Anthracene-based Ni(II) thiosemicarbazones with novel intramolecular π–π stackings. Inorg. Chem. Commun. 2020, 118, 107994. [Google Scholar] [CrossRef]
- Pelosi, G.; Pinelli, S.; Bisceglie, F. DNA and BSA Interaction Studies and Antileukemic Evaluation of Polyaromatic Thiosemicarbazones and Their Copper Complexes. Compounds 2022, 2, 144–162. [Google Scholar] [CrossRef]
- Reimers, J.R.; Ford, M.J.; Marcuccio, S.M.; Ulstrup, J.; Hush, N.S. Competition of van der Waals and chemical forces on gold–sulfur surfaces and nanoparticles. Nat. Rev. Chem. 2017, 1, 0017. [Google Scholar] [CrossRef]
- Kastrati, A.; Oswald, F.; Scalabre, A.; Fromm, K.M. Photophysical Properties of Anthracene Derivatives. Photochem 2023, 3, 227–273. [Google Scholar] [CrossRef]
- Haseloer, A. Personal communication–Synthesis of Amino Acid Containing Thiosemicarbazones and their Transition Metal Complexes. Master’s Thesis, University of Cologne, Cologne, Germany, 2017. [Google Scholar]
- Taschner, E.; Chimiak, A.; Bator, B.; Sokołowska, T. Neue Veresterungsmethoden in der Peptidchemie, VIII. Darstellung von tert.-Butylestern freier Aminosäuren. Liebigs Ann. Chem. 1961, 646, 134. [Google Scholar] [CrossRef]
- Ton, X.-A.; Bui, B.T.S.; Resmini, M.; Bonomi, P.; Dika, I.; Soppera, O.; Haupt, K. A Versatile Fiber-Optic Fluorescence Sensor Based on Molecularly Imprinted Microstructures Polymerized in Situ. Angew. Chem. Int. Ed. 2013, 52, 8317–8321. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, B.; Korbus, M.; Friedmann, T.; Wolff, C.; Thiele, C.M.; Meyer-Almes, F.-J. Synthesis of azobenzenealkylmaleimide probes to photocontrol the enzyme activity of a bacterial histone deacetylase-like amidohydrolase. Bioorg. Chem. 2014, 57, 155–161. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Hao, G.; Xin, W.; Yang, F.; Zhu, M.; Zhou, H. Design, Synthesis, and Structure–Activity Relationship of 7-Propanamide Benzoxaboroles as Potent Anticancer Agents. J. Med. Chem. 2019, 62, 6765–6784. [Google Scholar] [CrossRef]
- Patra, M.; Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. A Potent Glucose–Platinum Conjugate Exploits Glucose Transporters and Preferentially Accumulates in Cancer Cells. Angew. Chem. Int. Ed. 2016, 55, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Sun, X. The Warburg effect and glucose-derived cancer theranostics. Drug Discov. Today 2017, 22, 1637–1653. [Google Scholar] [CrossRef]
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, J.; Wang, J.; Gong, X.-J.; Liu, W.-X.; Wang, S.; Zhang, Y.; Yang, S.; Zhang, Q.-S.; Wie, J.-X.; et al. Antitumor effects of new glycoconjugated PtII agents dual-targeting GLUT1 and Pgp proteins. Dalton Trans. 2022, 51, 16082–16092. [Google Scholar] [CrossRef] [PubMed]
- Bisceglie, F.; Tavone, M.; Mussi, F.; Azzoni, S.; Montalbano, S.; Franzoni, S.; Tarasconi, P.; Buschini, A.; Pelosi, G. Effects of polar substituents on the biological activity of thiosemicarbazone metal complexes. J. Inorg. Biochem. 2018, 79, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Misra, A.K.; Bhatia, G.; Khan, M.M.; Khanna, A.K. Syntheses and evaluation of glucosyl aryl thiosemicarbazide and glucosyl thiosemicarbazone derivatives as antioxidant and anti-dyslipidemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Tenchiu, A.-C.; Kostas, I.D.; Kovala-Demertzi, D.; Terzis, A. Synthesis and characterization of new aromatic aldehyde/ketone 4-(β-D-glucopyranosyl)thiosemicarbazones. Carbohydr. Res. 2009, 344, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Tajima, H.; Li, G. Synthesis of Hydroxyalkyl Isothiocyanates. Synlett 1997, 7, 773–774. [Google Scholar] [CrossRef]
- Shultz, M.D.; Reveles, J.U.; Khanna, S.N.; Carpenter, E.E. Reactive Nature of Dopamine as a Surface Functionalization Agent in Iron Oxide Nanoparticles. J. Am. Chem. Soc. 2007, 129, 2482–2487. [Google Scholar] [CrossRef]
- Ilyas, S.; Ullah, N.K.; Ilyas, M.; Wennhold, K.; Iqbal, M.; Schlößer, H.A.; Hussain, M.S.; Mathur, S. Mediating the Fate of Cancer Cell Uptake: Dual-Targeted Magnetic Nanovectors with Biotin and Folate Surface Ligands. ACS Biomater. Sci. Eng. 2020, 6, 6138–6147. [Google Scholar] [CrossRef]
- Ghosh, S.; Tochtrop, G.P. A new strategy for the synthesis of β-benzylmercaptoethylamine derivatives. Tetrahedron Lett. 2009, 50, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Akao, A.; Nonoyama, N.; Yasuda, N. A mild and practical deprotection method for benzyl thioethers. Tetrahedron Lett. 2006, 47, 5337–5340. [Google Scholar] [CrossRef]
- Kocieński, P.J. Thiol Protecting Groups. In Protecting Groups; Georg Thieme: Stuttgart, Germany, 2005. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A Strain-Promoted [3 + 2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Viart, H.M.; Larsen, T.S.; Tassone, C.; Andresen, T.L.; Clausen, M.H. Propargylamine–isothiocyanate reaction: Efficient conjugation chemistry in aqueous media. Chem. Commun. 2014, 50, 7800–7802. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, S.; Vessally, E.; Edjlali, L.; Hosseinzadeh-Khanmiri, R.; Ghorbani-Kalhor, E. N-Propargylamines: Versatile building blocks in the construction of thiazole cores. Beilstein J. Org. Chem. 2017, 13, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, R.C.; Khan, U.; Coleman, J.N.; Adronov, A. Polymer Grafting to Single-Walled Carbon Nanotubes: Effect of Chain Length on Solubility, Graft Density and Mechanical Properties of Macroscopic Structures. Small 2013, 9, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnezhad, G.; Amirian, A.M.; Görls, H.; Plass, W.; Sandleben, A.; Schäfer, S.; Klein, A. Redox Instability of Copper(II) Complexes of a Triazine-Based PNP Pincer. Eur. J. Inorg. Chem. 2021, 2021, 1140–1151. [Google Scholar] [CrossRef]
- Yang, X.; Dai, C.; Molina, A.D.D.C.; Wang, B. Boronic acid-modified DNA that changes fluorescent properties upon carbohydrate binding. Chem. Commun. 2010, 46, 1073–1075. [Google Scholar] [CrossRef]
- Vassiliou, S.; Węglarz-Tomczak, E.; Berlicki, L.; Pawełczak, M.; Nocek, B.; Mulligan, R.; Joachimiak, A.; Mucha, A. Structure-Guided, Single-Point Modifications in the Phosphinic Dipeptide Structure Yield Highly Potent and Selective Inhibitors of Neutral Aminopeptidases. J. Med. Chem. 2014, 57, 8140–8151. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kluger, R. Efficient CuAAC click formation of functional hemoglobin bis-tetramers. Chem. Commun. 2010, 46, 7557–7559. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Mishra, D.; Dhak, P.; Gupta, S.; Maiti, T.K.; Basak, A.; Pramanik, P. Biofunctionalized, Phosphonate-Grafted, Ultrasmall Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Multimodal Imaging. Small 2009, 5, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- De Rosales, R.T.M.; Tavaré, R.; Glaria, A.; Varma, G.; Protti, A.; Blower, P.J. 99mTc-Bisphosphonate-Iron Oxide Nanoparticle Conjugates for Dual-Modality Biomedical Imaging. Bioconjugate Chem. 2011, 22, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Sudo, T.; Kamiya, H.; Okada, Y. Ligand Exchange Reactions between Phosphonic Acids at TiO2 Nanoparticle Surfaces. ChemSelect 2021, 6, 2923–2927. [Google Scholar] [CrossRef]
- Orm, N.B.; Trieu, Q.A.; Daniele, S. TiO2-Based Hybrid Nanocomposites Modified by Phosphonate Molecules as Selective PAH Adsorbents. Molecules 2018, 23, 3046. [Google Scholar] [CrossRef]
- Bachinger, A.; Kickelbick, G. Photocatalytic stability of organic phosphonates and phosphates on TiO2 Nanoparticles. Appl. Catal. A General 2011, 409–410, 122–132. [Google Scholar] [CrossRef]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef]
- Urlam, M.K.; Pireddu, R.; Ge, Y.; Zhang, X.; Sun, Y.; Lawrence, H.R.; Guida, W.C.; Sebti, S.M.; Lawrence, N.J. Development of new N-arylbenzamides as STAT3 dimerization inhibitors. Med. Chem. Comm. 2013, 4, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Németh, G.; Greff, Z.; Sipos, A.; Varga, Z.; Székely, R.; Sebestyén, M.; Jászay, Z.; Béni, S.; Nemes, Z.; Pirat, J.-L.; et al. Synthesis and Evaluation of Phosphorus Containing, Specific CDK9/CycT1 Inhibitors. J. Med. Chem. 2014, 57, 3939–3965. [Google Scholar] [CrossRef] [PubMed]


| Compound | TiO2 (Aeroxide P25) | [Dipy–TSC–Ph–Phos–TiO2] | [Anthr–TSC–Ph–Phos–TiO2] |
|---|---|---|---|
| Z-Average (nm) | 537 | 185 | 275 |
| Polydispersity Index | 0.718 | 0.266 | 0.252 |
| Zeta potential (mV) | 10.3 | –19.4 | –35.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohnsen, J.; Rryci, L.; Obretenova, D.; Friedel, J.; Jouchaghani, S.; Klein, A. Functionalizing Thiosemicarbazones for Covalent Conjugation. Molecules 2024, 29, 3680. https://doi.org/10.3390/molecules29153680
Hohnsen J, Rryci L, Obretenova D, Friedel J, Jouchaghani S, Klein A. Functionalizing Thiosemicarbazones for Covalent Conjugation. Molecules. 2024; 29(15):3680. https://doi.org/10.3390/molecules29153680
Chicago/Turabian StyleHohnsen, Johannes, Lukas Rryci, Diana Obretenova, Joshua Friedel, Shahab Jouchaghani, and Axel Klein. 2024. "Functionalizing Thiosemicarbazones for Covalent Conjugation" Molecules 29, no. 15: 3680. https://doi.org/10.3390/molecules29153680
APA StyleHohnsen, J., Rryci, L., Obretenova, D., Friedel, J., Jouchaghani, S., & Klein, A. (2024). Functionalizing Thiosemicarbazones for Covalent Conjugation. Molecules, 29(15), 3680. https://doi.org/10.3390/molecules29153680







