Modification of the Protein Amino Acid Content in Hen Eggs as a Consequence of Different Concentrations of Lupine and Soy in Feed
Abstract
1. Introduction
2. Results
2.1. Performance Results
2.2. Total Protein Analysis
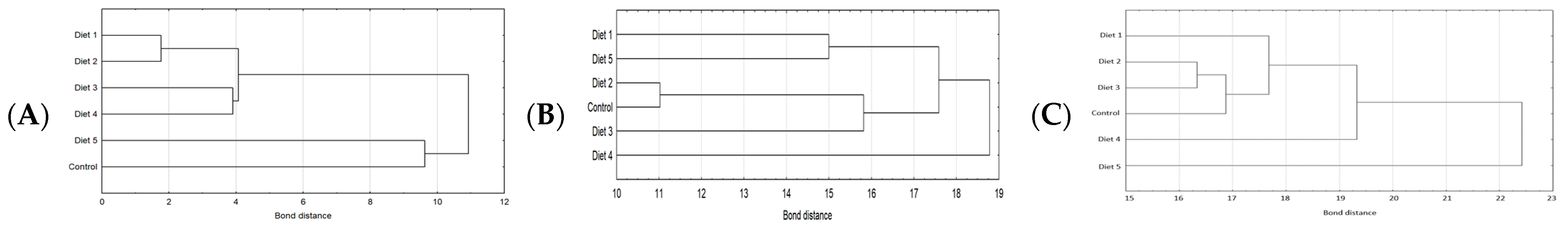
2.3. Amino Acids Content
3. Discussion
4. Materials and Methods
4.1. Laying Hens Experiment
4.2. Legume Seed Analysis
4.3. Sample Preparation
4.4. Protein Quantification
4.5. UHPLC Method
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wasielewska, M. Influence of Feed Additives for Chickens on the Quality of Eggs Laid and Related Risks. J. Educ. Health Sport 2021, 11, 246–258. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, J.; Li, F.; Zheng, J.; Xu, G. Effect of Oils in Feed on the Production Performance and Egg Quality of Laying Hens. Animals 2021, 11, 3482. [Google Scholar] [CrossRef] [PubMed]
- Mottet, A.; Tempio, G. Global Poultry Production: Current State and Future Outlook and Challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Alessandri, C.; Calvani, M.; Rosengart, L.; Madella, C. Anaphylaxis to Quail Egg. Allergy 2005, 60, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Galea, F. Nutrition and Food Management and Their Influence on Egg Quality. In Proceedings of the XLVIII Simposio Scientífico de Avicultura, Santiago de Compostela, Spain, 5–7 October 2011. [Google Scholar]
- Kowalska, E.; Kucharska-Gaca, J.; Kuźniacka, J.; Lewko, L.; Gornowicz, E.; Biesek, J.; Adamski, M. Egg Quality Depending on the Diet with Different Sources of Protein and Age of the Hens. Sci. Rep. 2021, 11, 2638. [Google Scholar] [CrossRef]
- Ahn, D.U.; Kim, S.M.; Shu, H. Effect of Egg Size and Strain and Age of Hens on the Solids Content of Chicken Eggs. Poult. Sci. 1997, 76, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Grobas, S.; Méndez, J.; Lázaro, R.; de Blas, C.; Mateo, G.G. Influence of Source and Percentage of Fat Added to Diet on Performance and Fatty Acid Composition of Egg Yolks of Two Strains of Laying Hens. Poult. Sci. 2001, 80, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.M.O.; Laniyan, T.F.; Kuye, O.A.; Oladele-Ojo, V.O.; Eruvbetine, D. Effect of Copper Salts on Performance, Cholesterol, Residues in Liver, Eggs and Excreta of Laying Hens. Arch. Zootec. 2006, 55, 327–338. [Google Scholar] [CrossRef]
- Leeson, S.; Caston, L.J. Vitamin Enrichment of Eggs. J. Appl. Poult. Res. 2003, 12, 24–26. [Google Scholar] [CrossRef]
- Sari, M.; Aksit, M.; Ozdogan, N.; Basmacioglu, H. Effects of Addition of Flaxseed to Diets of Laying Hens on Some Production Characteristics, Levels of Yolk and Serum Cholesterol, and Fatty Acid Composition of Yolk. Arch. Geflügelkd. 2002, 66, 75–79. [Google Scholar]
- Alagawany, M.; El-Hindawy, M.M.; El-Hack, M.E.A.; Arif, M.; El-Sayed, S.A. Influence of Low-Protein Diet with Different Levels of Amino Acids on Laying Hen Performance, Quality and Egg Composition. An. Acad. Bras. Cienc. 2020, 92, e20180230. [Google Scholar] [CrossRef] [PubMed]
- Drażbo, A.; Mikulski, D.; Zdunczyk, Z.; Szmatowicz, B.; Rutkowski, A.; Jankowski, J. Fatty Acid Composition, Physicochemical and Sensory Properties of Eggs from Laying Hens Fed Diets Containing Blue Lupine Seeds. Arch. Geflügelkd. 2014, 78. [Google Scholar] [CrossRef]
- Mikulski, D.; Jankowski, J.; Zdunczyk, Z.; Juskiewicz, J.; Slominski, B.A. The Effect of Different Dietary Levels of Rapeseed Meal on Growth Performance, Carcass Traits, and Meat Quality in Turkeys. Poult. Sci. 2012, 91, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Al-Sagan, A.A.; Al-Yemni, A.H.; Al-Abdullatif, A.A.; Attia, Y.A.; Hussein, E.O.S. Effects of Different Dietary Levels of Blue Lupine (Lupinus angustifolius) Seed Meal With or Without Probiotics on the Performance, Carcass Criteria, Immune Organs, and Gut Morphology of Broiler Chickens. Front. Vet. Sci. 2020, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future After the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Straková, E.; Všetičková, L.; Kutlvašr, M.; Timová, I.; Suchý, P. Beneficial Effects of Substituting Soybean Meal for White Lupin (Lupinus albus, Cv. Zulika) Meal on the Biochemical Blood Parameters of Laying Hens. Ital. J. Anim. Sci. 2021, 20, 352–358. [Google Scholar] [CrossRef]
- Timová, I.; Straková, E.; Všetičková, L.; Suchý, P. Impact of Feeding Mixture Containing Lupin Meal on Improvement of Polyunsaturated Fatty Acids in Egg Yolk. Czech J. Anim. Sci. 2020, 65, 311–321. [Google Scholar] [CrossRef]
- Mori, H.; Takaya, M.; Nishimura, K.; Goto, T. Breed and Feed Affect Amino Acid Contents of Egg Yolk and Eggshell Color in Chickens. Poult. Sci. 2020, 99, 172–178. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Panaite, T.D.; Saracila, M. Rosehip (Rosa canina L.) Meal as a Natural Antioxidant on Lipid and Protein Quality and Shelf-Life of Polyunsaturated Fatty Acids Enriched Eggs. Antioxidants 2022, 11, 1948. [Google Scholar] [CrossRef]
- Kodani, S.; Msamala, D.; Chigumira, R.; Shumba, T.; Muzofa, P. Implications of Diet and Quality Consistence of Feed on Poultry Layers Egg Quality. Afr. J. Agric. Res. 2022, 18, 617–631. [Google Scholar] [CrossRef]
- Revathy, S. A Study on the Influence of Poultry Feed on the Size, Weight and Protein Content of Egg. J. Food Anim. Sci. 2020, 1, 121–125. [Google Scholar] [CrossRef]
- Kubiś, M.; Kaczmarek, S.; Nowaczewski, S.; Adamski, M.; Hejdysz, M.; Rutkowski, A. Influence of Graded Inclusion of White Lupin (Lupinus albus) Meal on Performance, Nutrient Digestibility, and Ileal Viscosity of Laying Hens. Br. Poult. Sci. 2018, 59, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, S.A.; Hejdysz, M.; Kubiś, M.; Rutkowski, A. Influence of Graded Inclusion of White Lupin (Lupinus albus) Meal on Performance, Nutrient Digestibility and Intestinal Morphology of Broiler Chickens. Br. Poult. Sci. 2016, 57, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, S.A.; Hejdysz, M.; Kubis, M.; Kasprowicz-Potocka, M.; Rutkowski, A. The Nutritional Value of Yellow Lupin (Lupinus luteus L.) for Broilers. Anim. Feed Sci. Technol. 2016, 222, 43–53. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAQ Expert Consultation; FAO Food and Nutrition Paper; FAO: Rome, Italy, 2013; Volume 92, pp. 1–66. [Google Scholar]
- Kopeć, W.; Skiba, T.; Korzeniowska, M.; Bobak, L.; Trziszka, T. Activity of Protease Inhibitors and Lysozyme of Hen’s Egg White Depending on Feed Modification and Egg Storage. Pol. J. Food Nutr. Sci. 2005, 14, S1. [Google Scholar]
- Kawamura, N.; Yokoyama, R.; Takaya, M.; Ono, R.; Goto, T. Combined Effect of Feed and Housing System Affects Free Amino Acid Content of Egg Yolk and Albumen in Brown Layer Chickens. J. Poult. Sci. 2023, 60, 2023007. [Google Scholar] [CrossRef] [PubMed]
- Hejdysz, M.; Kaczmarek, S.A.; Rogiewicz, A.; Rutkowski, A. Influence of Graded Levels of Meals from Three Lupin Species on Growth Performance and Nutrient Digestibility in Broiler Chickens. Br. Poult. Sci. 2019, 60, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, A.; Kaczmarek, S.; Nowaczewski, S.; Jamroz, D. Concentrates Made from: Legume Seeds (Lupinus angustifolius, Lupinus luteus and Pisum sativum) and Rapeseed Meal as Protein Sources in Laying Hen Diets. Ann. Anim. Sci. 2014, 15, 129–142. [Google Scholar] [CrossRef]
- AOAC 976.05-1977(1996), Protein (Crude) in Animal Feed and Pet f. AOAC Official Method. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=2265 (accessed on 11 March 2024).
- AOAC 973.18-1977, Fiber (Acid Detergent) and Lignin (H2SO4). AOAC Official Method. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=1165 (accessed on 11 March 2024).
- AOAC 996.11-2005, Starch (Total) in Cereal Products. Amyloglucos. AOAC Official Method. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=1546 (accessed on 11 March 2024).
- Zalewski, K.; Lahuta, L.B.; Horbowicz, M. The Effect of Soil Drought on the Composition of Carbohydrates in Yellow Lupin Seeds and Triticale Kernels. Acta Physiol. Plant 2001, 23, 73–78. [Google Scholar] [CrossRef]
- Slominski, B.A.; Campbell, L.D. Non-Starch Polysaccharides of Canola Meal: Quantification, Digestibility in Poultry and Potential Benefit of Dietary Enzyme Supplementation. J. Sci. Food Agric. 1990, 53, 175–184. [Google Scholar] [CrossRef]
- Scott, R.W. Colorimetric Determination of Hexuronic Acids in Plant Materials. Anal. Chem. 1979, 51, 936–941. [Google Scholar] [CrossRef]
- Kuźniacka, J.; Hejdysz, M.; Banaszak, M.; Biesek, J.; Kaczmarek, S.; Grabowicz, M.; Rutkowski, A.; Adamski, M. Quality and Physicochemical Traits of Carcasses and Meat from Geese Fed with Lupin-Rich Feed. Animals 2020, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- AOAC 925.31-1925, Nitrogen in Eggs. AOAC Official Method. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&cPath=1&products_id=3065 (accessed on 11 March 2024).
- AOAC 994.12-1997, Amino Acids in Feeds. Performic Acid Oxidation. AOAC Official Method. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=545 (accessed on 11 March 2024).
- Tomczak, A.; Zielińska-Dawidziak, M.; Piasecka-Kwiatkowska, D.; Lampart-Szczapa, E. Blue Lupine Seeds Protein Content and Amino Acids Composition. Plant Soil Environ. 2018, 64, 147–155. [Google Scholar] [CrossRef]


| Experimental Group | Egg Weight (g) | Laying Rate (%) | Feed Intake (g/day) | FCR 1 (kg·kg−1 Eggs) |
|---|---|---|---|---|
| 1 | 60 ± 2 a,* | 95 ± 7 a | 119 ± 8 b | 2.1 ± 0.3 c |
| 2 | 61 ± 2 a | 97 ± 2 a | 116 ± 5 b | 2.1 ± 0.1 c |
| 3 | 61 ± 2 a | 96 ± 3 a | 119 ± 5 b | 2.0 ± 0.1 b,c |
| 4 | 60 ± 2 a | 97 ± 4 a | 115 ± 4 b | 2.0 ± 0.1 a,b |
| 5 | 60 ± 2 a | 96 ± 4 a | 119 ± 6 b | 2.0 ± 0.2 b,c |
| C | 60 ± 2 a | 97 ± 3 a | 110 ± 6 a | 1.9 ± 0.1 a |
| Experimental Diets | Protein Content |
|---|---|
| 1 | 20 ± 1 c,* |
| 2 | 17.7 ± 0.3 a |
| 3 | 18.4 ± 0.9 a,b |
| 4 | 18.1 ± 0.1 a |
| 5 | 18.4 ± 0.6 a,b |
| C | 20 ± 1 c |
| (A) | ||||||||
| Diet | Protein Content in Egg Yolk [%] | |||||||
| 1 C,* | 2 C | 3 A | 4 A,B | 5 B,C | 6 B,C | 7 B,C | Mean Value | |
| 1 | 16.1 ± 0.2 | 17.2 ± 0.3 | 15.36 ± 0.07 | 16.3 ± 0.1 | 15.73 ± 0.03 | 15.67 ± 0.06 | 15.4 ± 0.2 | 16.0 ± 0.6 a,* |
| 2 | 16.9 ± 0.1 | 16.5 ± 0.6 | 15.35 ± 0.05 | 15.39 ± 0.06 | 16.8 ± 0.3 | 16.57 ± 0.03 | 16.9 ± 0.4 | 16.4 ± 0.7 a,b |
| 3 | 16.8 ± 0.3 | 16.67 ± 0.04 | 16.6 ± 0.3 | 15.7 ± 0.1 | 16.5 ± 0.2 | 16.4 ± 0.2 | 16.4 ± 0.6 | 16.4 ± 0.4 a,b |
| 4 | 16.5 ± 0.3 | 16.3 ± 0.2 | 15.75 ± 0.07 | 16.79 ± 0.07 | 16.6 ± 0.2 | 16.00 ± 0.09 | 16.7 ± 0.2 | 16.4 ± 0.4 a,b |
| 5 | 16.76 ± 0.02 | 16.47 ± 0.02 | 16.5 ± 0.2 | 16.2 ± 0.1 | 16.61 ± 0.06 | 17.44 ± 0.01 | 16.98 ± 0.01 | 16.7 ± 0.4 c |
| C | 16.6 ± 0.1 | 16.22 ± 0.06 | 15.69 ± 0.03 | 15.5 ± 0.1 | 16.7 ± 0.1 | 16.5 ± 0.2 | 16.5 ± 0.1 | 16.2 ± 0.5 a |
| (B) | ||||||||
| Diet | Protein Content in Egg White [%] | |||||||
| 1 A,* | 2 A | 3 A | 4 A | 5 A | 6 A | 7 A | Mean Value | |
| 1 | 11.3 ± 0.2 | 11.5 ± 0.3 | 11.7 ± 0.2 | 11.4 ± 0.2 | 11.40 ± 0.04 | 11.90 ± 0.01 | 11.26 ± 0.08 | 11.5 ±0.3 b |
| 2 | 11.85 ± 0.05 | 11.52 ± 0.08 | 11.43 ± 0.06 | 11.26 ± 0.06 | 10.94 ± 0.03 | 11.1 ± 0.1 | 11.8 ± 0.1 | 11.4 ± 0.3 b |
| 3 | 11.56 ± 0.08 | 11.98 ± 0.01 | 11.3 ± 0.1 | 11.6 ± 0.2 | 11.1 ± 0.1 | 11.18 ± 0.04 | 11.5 ± 0.2 | 11.5 ± 0.3 b |
| 4 | 11.4 ± 0.3 | 11.5 ± 0.6 | 11.8 ± 0.1 | 11.81 ± 0.03 | 11.32 ± 0.06 | 11.5 ± 0.1 | 11.9 ± 0.7 | 11.6 ± 0.4 b,c |
| 5 | 10.76 ± 0.04 | 10.47 ± 0.00 | 10.41 ± 0.00 | 10.48 ± 0.07 | 10.61 ± 0.07 | 10.62 ± 0.06 | 10.85 ± 0.06 | 10.6 ± 0.2 a |
| C | 11.8 ± 0.2 | 11.6 ± 0.2 | 11.72 ± 0.04 | 11.67 ± 0.04 | 11.67 ± 0.08 | 11.82 ± 0.06 | 12.2 ± 0.2 | 11.8 ± 0.2 c |
| Sample | Amino Acid | Total NAA *** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SER | ARG ** | ASP + ASN | GLU + GLN | ALA | PRO | TYR | CYS | ||
| Y1 | 7.9 ± 0.3 a,* | 7.4 ± 0.3 a | 9.6 ± 0.2 a | 11.4 ± 0.4 a | 4.7 ± 0.2 a | 3.6 ± 0.1 a | 1.5 ± 0.1 a | 1.19 ± 0.2 a | 37.69 |
| Y2 | 8.0 ± 0.3 a | 7.3 ± 0.2 a | 9.9 ± 0.3 a | 11.4 ± 0.2 a | 4.8 ± 0.1 a | 4.1 ± 0.1 a | 1.7 ± 0.1 a | 1.2 ± 0.1 a,b | 48.4 |
| Y3 | 8.3 ± 0.3 a | 7.6 ± 0.4 a | 9.4 ± 0.3 a | 11.0 ± 0.6 a | 4.7 ± 0.1 a | 3.9 ± 0.0 a | 1.4 ± 0.3 a | 0.8 ± 0.1 a | 38.01 |
| Y4 | 7.6 ± 0.1 a | 7.0 ± 0.5 a | 9.7 ± 0.5 a | 11.2 ± 0.2 a | 4.7 ± 0.1 a | 4.0 ± 0.2 a | 1.5 ± 0.4 a | 0.9 ± 0.2 a | 46.6 |
| Y5 | 8.6 ± 0.3 a | 7.6 ± 0.1 a | 10.6 ± 0.1 a | 12.0 ± 0.5 a | 5.1 ± 0.2 a | 4.2 ± 0.1 a | 1.5 ± 0.2 a | 1.3 ± 0.2 a,b | 50.9 |
| YC | 7.7 ± 0.2 a | 8.8 ± 0.3 a | 12.2 ± 0.2 a | 11.6 ± 0.1 a | 4.9 ± 0.1 a | 4.1 ± 0.1 a | 1.8 ± 0.2 a | 2.3 ± 0.1 b | 53.4 |
| Sample | Amino Acid | Total EAA *** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ILE | LEU | LYS | THR | VAL | HIS ** | PHE | TRP | MET | ||
| Y1 | 4.5 ± 0.1 a,* | 8.00 ± 0.00 a | 5.4 ± 0.2 a | 5.0 ± 0.3 a | 4.5 ± 0.3 a | 3.5 ± 0.1 b,c | 4.1 ± 0.1 a | 1.1 ± 0.1 a | 2.0 ± 0.1 a,b | 38.10 |
| Y2 | 4.3 ± 0.3 a | 8.0 ± 0.2 a | 5.5 ± 0.1 a | 4.9 ± 0.2 a | 4.5 ± 0.5 a | 3.2 ± 0.3 a,b,c | 4.1 ± 0.2 a | 1.1 ± 0.2 a | 2.5 ± 0.2 b | 38.10 |
| Y3 | 4.5 ± 0.4 a | 7.9 ± 0.3 a | 5.8 ± 0.5 a | 4.8 ± 0.4 a | 5.0 ± 0.5 a,b | 3.9 ± 0.2 c | 4.2 ± 0.2 a | 1.2 ± 0.2 a | 2.4 ± 0.4 b | 39.70 |
| Y4 | 4.2 ± 0.1 a | 7.7 ± 0.2 a | 5.2 ± 0.1 a | 4.6 ± 0.1 a | 4.7 ± 0.3 a,b | 2.6 ± 0.5 a | 4.3 ± 0.4 a | 1.1 ± 0.1 a | 1.3 ± 0.2 a | 35.70 |
| Y5 | 4.8 ± 0.4 a | 8.4 ± 0.3 a | 8.1 ± 0.4 b | 5.1 ± 0.2 a | 5.2 ± 0.5 a,b | 2.96 ± 0.00 a,b | 4.20 ± 0.00 a | 1.1 ± 0.1 a | 2.0 ± 0.1 a,b | 41.86 |
| YC | 5.7 ± 0.2 a | 10.1 ± 0.1 a | 7.1 ± 0.2 b | 6.6 ± 0.1 b | 6.2 ± 0.3 b | 3.56 ± 0.00 b,c | 5.36 ± 0.00 a | 1.2 ± 0.2 a | 1.4 ± 0.5 a | 47.22 |
| Sample | Amino Acid | Total NAA *** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SER | ARG ** | ASP + ASN | GLU + GLN | ALA | PRO | TYR | CYS | ||
| W1 | 7.4 ± 0.3 c,* | 6.7 ± 0.5 b | 4.1 ± 0.4 b,c | 15.1 ± 0.6 c | 16.3 ± 0.2 a | 5.1 ± 0.1 a,b | 3.5 ± 0.2 a,b | 2.1 ± 0.1 a | 60.3 |
| W2 | 6.9 ± 0.3 a,c | 6.4 ± 0.3 b | 3.2 ± 0.2 a,b | 9.7 ± 0.1 a | 13.6 ± 0.5 a | 5.2 ± 0.2 a,b | 3.2 ± 0.2 a,b | 2.6 ± 0.4 a | 50.8 |
| W3 | 5.2 ± 0.2 a | 4.4 ± 0.2 a | 2.7 ± 0.1 a | 8.8 ± 0.1 a | 12.7 ± 0.2 a | 4.5 ± 0.1 a | 2.9 ± 0.1 a | 2.2 ± 0.1 a | 43.4 |
| W4 | 7.89 ± 0.00 c | 6.41 ± 0.00 b | 3.5 ± 0.1 a,b,c | 12.8 ± 0.1 a,b,c | 16.0 ± 0.2 a | 6.8 ± 0.1 c | 4.0 ± 0.1 b | 4.1 ± 0.1 b | 61.5 |
| W5 | 7.8 ± 0.2 c | 6.5 ± 0.3 b | 4.5 ± 0.3 c | 14.3 ± 1.0 b,c | 17.4 ± 0.8 a | 6.0 ± 0.2 b,c | 4.1 ± 0.2 b | 2.5 ± 0.3 a | 63.1 |
| WC | 5.8 ± 0.5 a,b | 5.7 ± 0.6 a,b | 3.0 ± 0.3 a,b | 10.1 ± 0.1 a,b | 11.7 ± 0.6 a | 5.20 ± 0.00 a,b | 3.3 ± 0.2 a,b | 2.4 ± 0.2 a | 47.2 |
| Sample | Amino Acid | Total EAA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ILE | LEU | LYS | THR | VAL | HIS ** | PHE | TRP | MET | ||
| W1 | 4.1 ± 0.1 a,* | 6.8 ± 0.3 a,b | 4.2 ± 0.1 a,b | 4.2 ± 0.2 a,b | 5.1 ± 0.1 a,b | 3.3 ± 0.2 b,c | 5.4 ± 0.3 a,b | 1.4 ± 0.1 a | 2.1 ± 0.2 a | 36.6 |
| W2 | 4.24 ± 0.00 a,b | 7.0 ± 0.1 a,b | 4.2 ± 0.2 a,b | 4.2 ± 0.2 a,b | 5.7 ± 0.1 b,c | 3.3 ± 0.1 b,c | 6.6 ± 0.5 b,c | 1.4 ± 0.1 a | 2.5 ± 0.1 a | 39.14 |
| W3 | 3.5 ± 0.1 a | 6.2 ± 0.2 a | 3.8 ± 0.1 a | 3.4 ± 0.1 a | 4.2 ± 0.2 a | 1.9 ± 0.1 a | 5.0 ± 0.3 a | 1.36 ± 0.00 a | 2.8 ± 0.2 a | 32.16 |
| W4 | 5.8 ± 0.1 c | 9.59 ± 0.00 c | 6.0 ± 0.1 c | 5.4 ± 0.1 c | 8.02 ± 0.00 d | 3.70 ± 0.00 c | 7.2 ± 0.1 c | 1.2 ± 0.1 a | 2.3 ± 0.1 a | 49.21 |
| W5 | 5.0 ± 0.4 b,c | 8.0 ± 0.4 b | 5.1 ± 0.5 b,c | 5.2 ± 0.1 b,c | 6.3 ± 0.4 c | 3.4 ± 0.2 b,c | 6.2 ± 0.5 a,b,c | 1.2 ± 0.1 a | 2.2 ± 0.1 a | 42.6 |
| WC | 4.1 ± 0.1 a | 6.9 ± 0.2 a,b | 4.4 ± 0.1 a,b | 3.8 ± 0.3 a | 5.0 ± 0.3 a,b | 2.7 ± 0.1 b | 5.1 ± 0.4 a | 1.4 ± 0.1 a | 2.5 ± 0.1 a | 35.9 |
| Component | 1 | 2 | 3 | 4 | 5 | C |
|---|---|---|---|---|---|---|
| Wheat (CP 118) | 58.15 | 53.67 | 50.28 | 48.37 | 47.98 | 30.00 |
| Corn (CP 94) | - | - | - | - | - | 23.19 |
| Soybean meal | 15.43 | 11.00 | 8.60 | 5.00 | - | - |
| Blue lupine | - | 10.00 | 15.00 | 20.00 | 25.00 | - |
| Peas | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10 |
| Rapeseed oil | 4.15 | 4.70 | 5.50 | 6.00 | 6.30 | 6.02 |
| Limestone (fine to coarse-40:60) | 9.15 | 8.10 | 8.10 | 8.05 | 8.06 | - |
| Rapeseed meal (CP 349) | - | - | - | - | - | 5.00 |
| Sunflower meal (CP 340) | - | - | - | - | - | 5.00 |
| Corn gluten | - | - | - | - | - | 5.00 |
| Potato protein | - | - | - | - | - | 2.00 |
| Monocalcium phosphate | 1.28 | 1.28 | 1.29 | 1.30 | 1.31 | 1.52 |
| Premix 0.5% | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 1.00 |
| NaCl | 0.18 | 0.19 | 0.20 | 0.20 | 0.19 | 0.61 |
| NaHCO3 | 0.35 | 0.30 | 0.29 | 0.29 | 0.29 | - |
| DL-Methionine (98%) | 0.15 | 0.15 | 0.15 | 0.20 | 0.15 | 0.15 |
| HCl-lysine (78%) | 0.15 | 0.02 | - | 0.01 | 0.08 | 0.35 |
| L-Threonine (98%) | 0.05 | 0.02 | 0.02 | 0.01 | 0.04 | 0.02 |
| L-Tryptophan (98%) | - | - | - | - | - | 0.03 |
| L-Valine (98%) | 0.10 | 0.07 | 0.07 | 0.07 | 0.10 | - |
| Metabolizable energy (MJ/kg) | 11.61 | 11.62 | 11.68 | 11.64 | 11.62 | 11.80 |
| Components% | ||||||
| Crude protein | 16.36 | 16.42 | 16.43 | 16.49 | 16.41 | 17.02 |
| Ca | 3.5 | 3.53 | 3.52 | 3.5 | 3.5 | 4.3 |
| P-available | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.46 |
| Na | 0.18 | 0.17 | 0.17 | 0.18 | 0.18 | 0.24 |
| Cl | 0.18 | 0.16 | 0.16 | 0.17 | 0.17 | 0.46 |
| Lys. digest. | 0.76 | 0.75 | 0.75 | 0.75 | 0.75 | 0.69 |
| Met + Cys digest. | 0.65 | 0.64 | 0.65 | 0.67 | 0.65 | 0.62 |
| Thr digest. | 0.53 | 0.53 | 0.54 | 0.54 | 0.54 | 0.49 |
| Tyr digest. | 0.16 | 0.17 | 0.17 | 0.17 | 0.17 | 0.15 |
| Val Tot. | 0.71 | 0.71 | 0.72 | 0.72 | 0.72 | - |
| Arg Tot. | 0.98 | 1.25 | 1.34 | 1.43 | 1.52 | - |
| Linol. Acid | 1.73 | 1.67 | 1.84 | 2.01 | 2.16 | - |
| Component | Narrow-Leaved Lupin | Soybean Meal |
|---|---|---|
| Dry matter | 879 | 916 |
| Crude protein | 263 | 481 |
| Starch | 10.7 | 54.6 |
| Neutral Detergent Fiber | 211 | 237 |
| Total oligosaccharides (RFO 2) | 59.5 | 43.7 |
| Alkaloids | 0.41 | ND 4 |
| Total NSP 3 | 406 | 170 |
| Soluble NSP | 195 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, A.; Zielińska-Dawidziak, M.; Klimowicz, P.; Hejdysz, M.; Kaczmarek, S.; Siger, A.; Cieślak, A. Modification of the Protein Amino Acid Content in Hen Eggs as a Consequence of Different Concentrations of Lupine and Soy in Feed. Molecules 2024, 29, 3727. https://doi.org/10.3390/molecules29163727
Tomczak A, Zielińska-Dawidziak M, Klimowicz P, Hejdysz M, Kaczmarek S, Siger A, Cieślak A. Modification of the Protein Amino Acid Content in Hen Eggs as a Consequence of Different Concentrations of Lupine and Soy in Feed. Molecules. 2024; 29(16):3727. https://doi.org/10.3390/molecules29163727
Chicago/Turabian StyleTomczak, Aneta, Magdalena Zielińska-Dawidziak, Piotr Klimowicz, Marcin Hejdysz, Sebastian Kaczmarek, Aleksander Siger, and Adam Cieślak. 2024. "Modification of the Protein Amino Acid Content in Hen Eggs as a Consequence of Different Concentrations of Lupine and Soy in Feed" Molecules 29, no. 16: 3727. https://doi.org/10.3390/molecules29163727
APA StyleTomczak, A., Zielińska-Dawidziak, M., Klimowicz, P., Hejdysz, M., Kaczmarek, S., Siger, A., & Cieślak, A. (2024). Modification of the Protein Amino Acid Content in Hen Eggs as a Consequence of Different Concentrations of Lupine and Soy in Feed. Molecules, 29(16), 3727. https://doi.org/10.3390/molecules29163727








