Influence of Ca3(PO4)2 on the Surface Morphology and Properties of a CaO-Al2O3-SiO2-Fe2O3-Based High Temperature Phase Reconstructed Complex
Abstract
:1. Introduction
2. Experiment
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
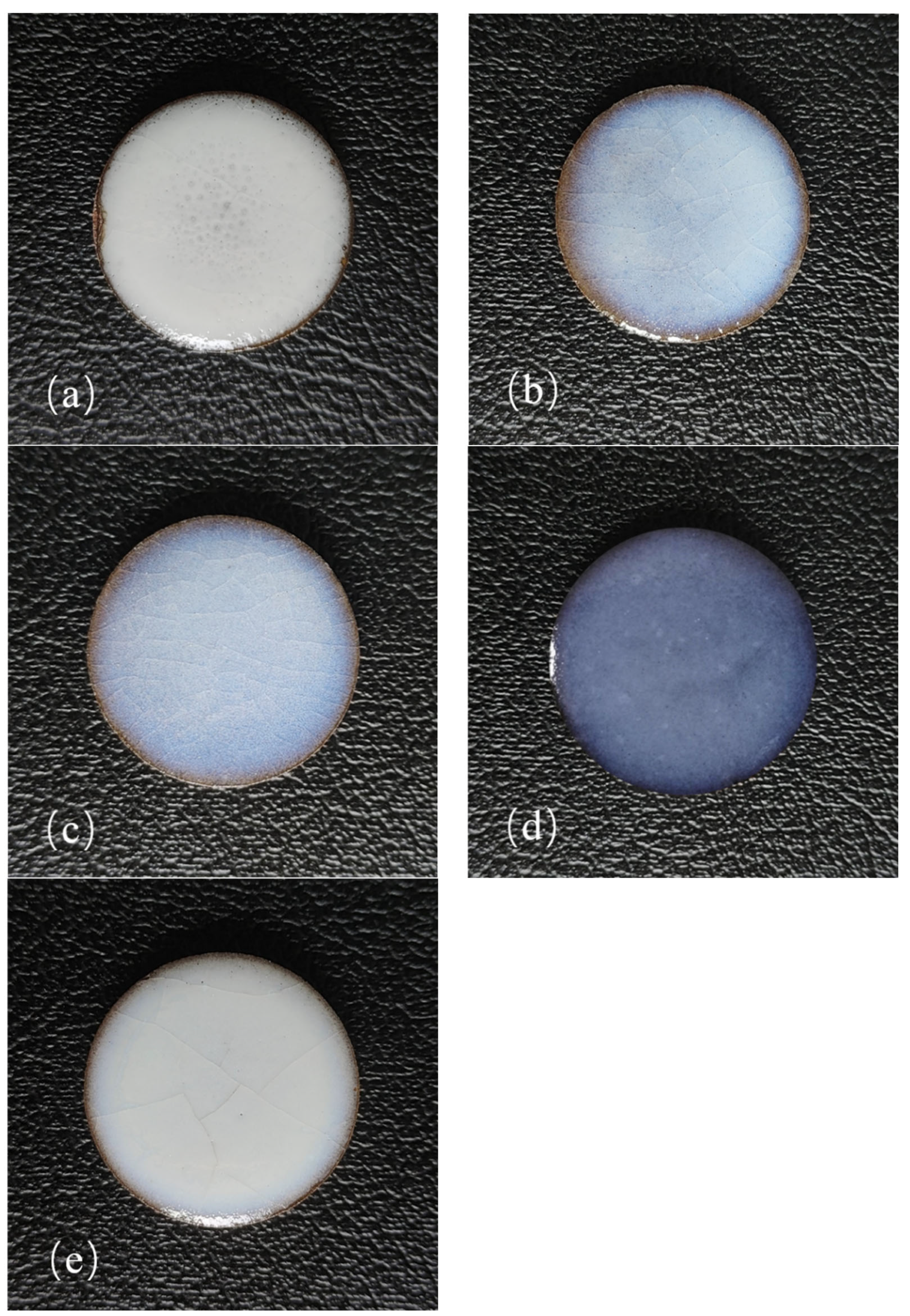
3.1. Surface Morphology
3.2. Phase Analysis
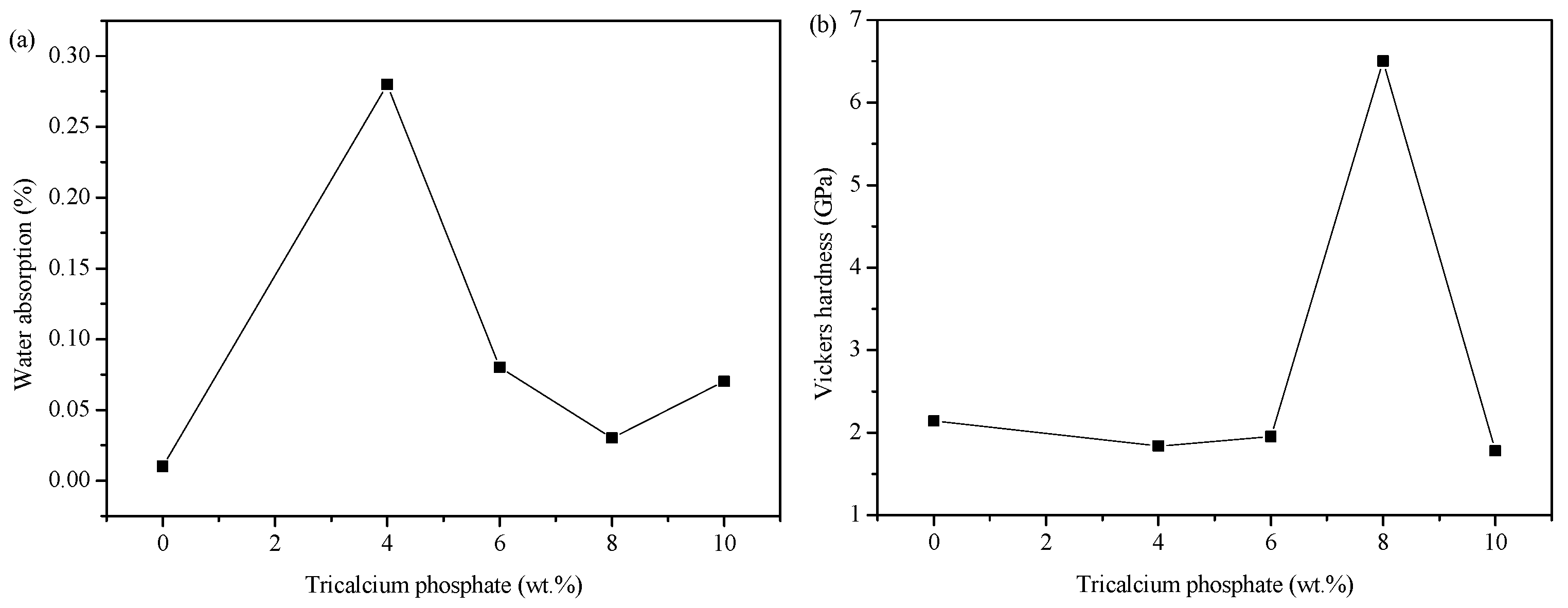
3.3. Physical and Mechanical Properties
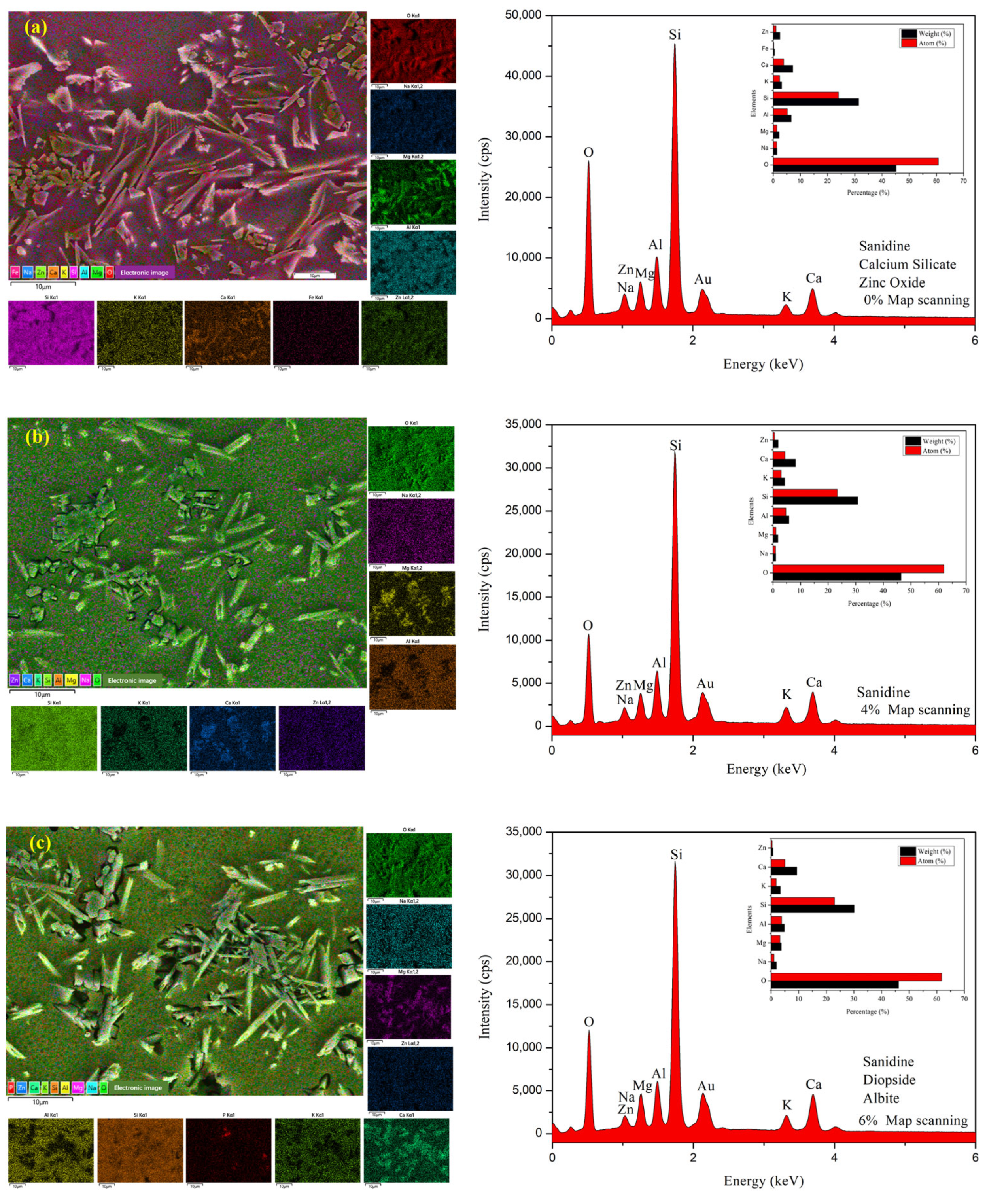
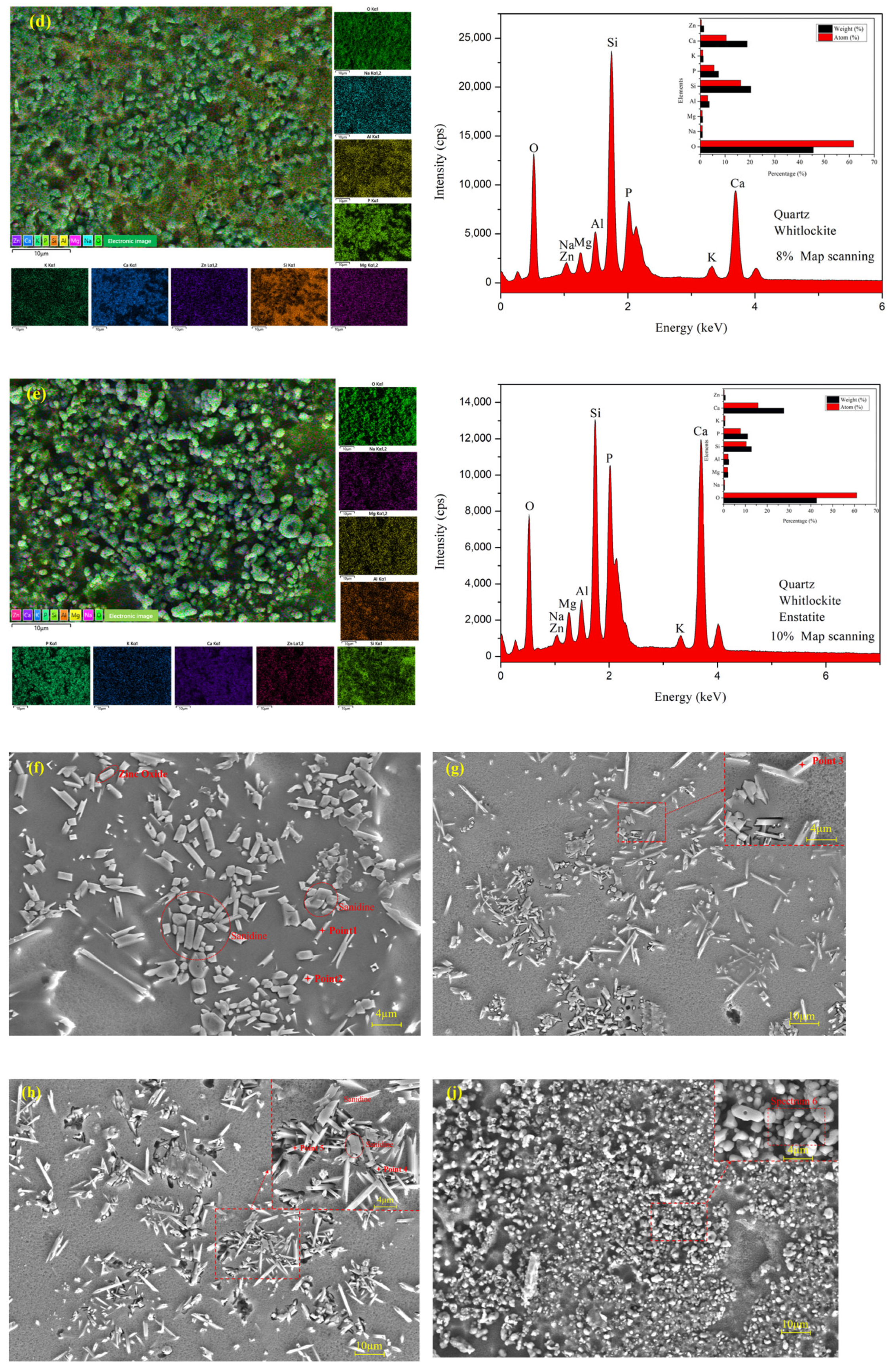
3.4. Microstructure and EDS Analysis
3.5. Opacification Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, H.R.; Tulyaganov, D.U.; Ferreira, J.M.F. Preparation and Characterization of Foams from Sheet Glass and Fly Ash Using Carbonates as Foaming Agents. Ceram. Int. 2009, 35, 229–235. [Google Scholar] [CrossRef]
- Yan, M.; Wen, X.; Sun, Y.; Zhou, Z.; Jiang, J.; Hu, R.; Zhang, Y.; Hantoko, D. Fly Ash Treatment by Desalination Coupled with Solidification of Heavy Metals. Process Saf. Environ. Prot. 2023, 172, 157–164. [Google Scholar] [CrossRef]
- Iyer, R.S.; Scott, J.A. Power Station Fly Ash—A Review of Value-Added Utilization Outside of the Construction Industry. Resour. Conserv. Recycl. 2001, 31, 217–228. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Ye, J.W.; Lu, X.B.; Liu, M.G.; Lin, Y.; Gong, W.T.; Ning, G.L. Preparation of Sintered Foam Materials by Alkali-Activated Coal Fly Ash. J. Hazard. Mater. 2010, 174, 108–112. [Google Scholar] [CrossRef]
- Zhu, M.G.; Ji, R.; Li, Z.M.; Wang, H.; Liu, L.L.; Zhang, Z.T. Preparation of Glass Ceramic Foams for Thermal Insulation Applications from Coal Fly Ash and Waste Glass. Constr. Build. Mater. 2016, 112, 398–405. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhang, Y.H.; Huang, H.W.; Hu, P. Effect of Heat Treatment Process on the Preparation of Foamed Glass Ceramic from Red Mud and Fly Ash. Appl. Mech. Mater. 2014, 670–671, 201–204. [Google Scholar] [CrossRef]
- Barbieri, L.; Lancellotti, I.; Manfredini, T.; Queralt, I.; Rincon, J.M.; Romero, M. Design, Obtainment, and Properties of Glasses and Glass–Ceramics from Coal Fly Ash. Fuel 1999, 78, 271–276. [Google Scholar] [CrossRef]
- Albertini, A.V.P.; Silva, J.L.; Freire, V.N.; Santos, R.P.; Martins, J.L.; Cavada, B.S.; Cadena, P.G.; Rolim Neto, P.J.; Pimentel, M.C.B.; Martinez, C.R.; et al. Immobilized Invertase Studies on Glass–Ceramic Support from Coal Fly Ashes. Chem. Eng. J. 2013, 214, 91–96. [Google Scholar] [CrossRef]
- Wang, S.M.; Zhang, C.X.; Chen, J.D. Utilization of Coal Fly Ash for the Production of Glass-Ceramics with Unique Performances: A Brief Review. J. Mater. Sci. Technol. 2014, 30, 1208–1212. [Google Scholar] [CrossRef]
- Li, B.W.; Deng, L.B.; Zhang, X.F.; Jia, X.L. Structure and Performance of Glass–Ceramics Obtained by Bayan Obo Tailing and Fly Ash. J. Non Cryst. Solids 2013, 380, 103–108. [Google Scholar]
- Yang, S.M.; Zhang, W. Research on Glass Ceramics of Multi-Solid Waste Slag by Blast Furnace Slag and Fly Ash. Bull. Chin. Ceram. Soc. 2015, 34, 487–491. [Google Scholar]
- Lu, Z.Y.; Lu, J.S.; Li, X.B.; Shao, G.Q. Effect of MgO Addition on Sinterability, Crystallization Kinetics, and Flexural Strength of Glass–Ceramics from Waste Materials. Ceram. Int. 2016, 42, 3452–3459. [Google Scholar] [CrossRef]
- Zhao, X.Z. Review and Prospect of Modern Crystalline Glazes in China. Shandong Ceram. 2016, 39, 3–10. [Google Scholar]
- Pekkan, K.; Tasci, E.; Gun, Y. The Effect of ZnO on Development of Crystals in Crystal Glaze Applications. J. Fac. Eng. Archit. Gazi Univ. 2015, 30, 281–287. [Google Scholar]
- Liu, P.D.; Yu, P.L. Crystalline-Glaze Series of Calcium-Magnesium Silicate—Possible Minerals of Calcium-Magnesium Silicate in Crystalline-Glaze. Ceram. Hanyang 2002, 3, 29–32. [Google Scholar]
- Chen, C.M.; Han, X.W.; Zhao, Y.H. The Study of Tea Dust Crystalline Glaze on Non-Ferrous. China Ceram. 2011, 47, 58–60. [Google Scholar]
- Jamaludin, A.R.; Kasim, S.R.; Ahmad, Z.A. The Effect of CaCo3 Addition on the Crystallization Behavior of ZnO Crystal Glaze Fired at Different Gloss Firing and Crystallization Temperatures. Sci. Sinter. 2010, 42, 345–355. [Google Scholar] [CrossRef]
- Li, M.; Li, W.D.; Lu, X.K. Controllable Preparation and Decorative Effect of Iron-Based Crystalline Glazes. J. Chin. Ceram. Soc. 2020, 48, 1134–1144. [Google Scholar]
- Gajek, M.; Partyka, J.; Leśniak, M.; Rapacz-Kmita, A.; Wojcik, L. Gahnite White Colour Glazes in ZnO–R2O–RO–Al2O3–SiO2 System. Ceram. Int. 2018, 44, 15845–15850. [Google Scholar] [CrossRef]
- Qiu, B.X.; Gu, X.Y.; Dong, W.X.; Luo, T.; He, M.K.; Wu, P.Y. Preparation of Black Glaze Using Ferrochromium Slag Waste. J. Chin. Ceram. Soc. 2019, 47, 396–402. [Google Scholar]
- Wang, Y.; Yu, S.H. Coloring Characteristics and Fambe Mechanism of Iron and Copper Coloring Glaze. J. Chin. Ceram. Soc. 2018, 46, 1273–1279. [Google Scholar]
- Zhu, J.F.; Shi, P.; Wang, F.; Zhao, T.; Jiang, H. Preparation of Separative-Phase Fancy Glaze Derived from Iron Ore Slag. Ceram. Int. 2016, 42, 5250–5257. [Google Scholar] [CrossRef]
- Shi, P.; Wang, F.; Zhu, J.F.; Yang, H.B.; Wang, Y.; Fang, Y.; Zhang, B.; Wang, J.H. Amorphous Photonic Crystals and Structural Colors in the Phase Separation Glaze. J. Eur. Ceram. Soc. 2018, 38, 2228–2233. [Google Scholar] [CrossRef]
- Shi, P.; Jin, Z.W.; Wang, F.; Luo, H.J.; Zhu, J.F.; Yang, C.G.; Liu, M.; Chen, K.Y.; Zhang, B. Preparation of a Highly Color-Saturated Amorphous Photonic Crystal Structural Color Glaze by Imitating the Multilayered Structure of Jun Porcelain. Ceram. Int. 2023, 49, 4677–4685. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.L.; Ning, H.J.; Wang, F.; Zhu, J.F.; Luo, H.J.; Fei, G.Q.; Takeoka, Y.; Shi, P. Study on the Coupling Effect of Chemical Color and Structural Color for Lushan Speckle Porcelain Glaze in the Tang Dynasty (618–907AD). J. Eur. Ceram. Soc. 2024, 44, 3410–3417. [Google Scholar] [CrossRef]
- Shi, X.T.; Yu, Y.G.; Sun, Q.; Zhu, W.Y.; Peng, C.; Wu, J.Q. Mechanisms of Pattern and Colour Generation of Chinese Tianmu Glaze. RSC Adv. 2019, 9, 41927–41933. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Guo, H.L.; Sun, H.J.; Peng, T.J. Surface Modification Study of CaO-Al2O3-SiO2-Fe2O3 Base System High Temperature Phase Reconstruction. Front. Mater. 2023, 9, 1109363. [Google Scholar] [CrossRef]
- Capoglu, A. Elimination of Discolouration in Reformulated Bone China Bodies. J. Eur. Ceram. Soc. 2005, 25, 3157–3164. [Google Scholar] [CrossRef]
- Plesingerova, B.; Klapac, M.; Kovalcikova, M. Moisture Expansion of Porous Biscuit Bodies—Reason of Glaze Cracking. Ceram. Silik. 2002, 46, 159–165. [Google Scholar]
- Wu, J.Q. The Effect of CaF2 and Ca3(PO4)2 on Property of Barium Calcium Silicate Glass. Chin. Ceram. 2008, 44, 41–42. [Google Scholar]
- Chen, T.T.; Gong, B.; Tang, C.A. Origin and Evolution of Cracks in the Glaze Surface of a Ceramic during the Cooling Process. Materials 2023, 16, 5508. [Google Scholar] [CrossRef]
- Zhang, C.S.; Wang, X.; Zhu, H.J.; Wu, Q.S.; Hu, Z.C.; Feng, Z.Z.; Jia, Z. Preparation and Properties of Foam Ceramic from Nickel Slag and Waste Glass Powder. Ceram. Int. 2020, 46, 23623–23628. [Google Scholar] [CrossRef]
- Hui, T.; Sun, H.J.; Peng, T.J.; Liu, L.; Ding, W.J.; Wang, C.; Liu, B. Recycling of Extracted Titanium Slag and Gold Tailings for Preparation of Self-Glazed Ceramic Foams. Ceram. Int. 2022, 48, 23415–23427. [Google Scholar] [CrossRef]
- Wang, S.L.; Bao, Z.H.; Miao, L.F. Effect of MgO on Crystallization and Properties of Anorthite Glass-Ceramic Glaze. J. Ceram. 2023, 44, 1024–1030. [Google Scholar]
- Chi, Z.X. Ceramic Glaze Colorants and Decoration; China Building Materials Press: Beijing, China, 2015; ISBN 978-7-5160-1296-3. [Google Scholar]
- de Souza-Dal Bó, G.C.; Dal Bó, M.; Bernardin, A.M. Reuse of Laminated Glass Waste in The Manufacture of Ceramic Frits and Glazes. Mater. Chem. Phys. 2021, 257, 123847. [Google Scholar] [CrossRef]
- Gol, F.; Yilmaz, A.; Kacar, E.; Simsek, S.; Sarıtas, Z.G.; Ture, C.; Arslan, M.; Bekmezci, M.; Burhan, H.; Sen, F. Reuse of Glass Waste in The Manufacture of Ceramic Tableware Glazes. Ceram. Int. 2021, 47, 21061–21068. [Google Scholar] [CrossRef]
- Yang, C.A. Research on Separative-Phase Glaze and Its Structural Color. Ph.D. Dissertation, Shanxi University of Science and Technology, Xi’an, China, 2016. (In Chinese). [Google Scholar]



| SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | ZnO | Fe2O3 | TiO2 | BaO | ZrO2 | P2O5 | MnO | SrO | Others | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 39.13 | 16.74 | 7.70 | 0.33 | 2.91 | 0.28 | 0.07 | 22.97 | 5.25 | 0.16 | 0.33 | 0.44 | 0.17 | 0.26 | — | 3.01 |
| SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | ZnO | Fe2O3 | TiO2 | BaO | ZrO2 | P2O5 | MnO | SrO | Others | SO3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaoline | 54.82 | 38.19 | 1.38 | — | 0.30 | 0.19 | 0.01 | 1.31 | 3.16 | — | 0.21 | — | — | 0.04 | 0.39 | — |
| Feldspar | 62.08 | 10.54 | 1.13 | — | 22.10 | 0.31 | 0.02 | 1.05 | — | 1.07 | 0.23 | 0.12 | 0.10 | 0.24 | 1.01 | — |
| Fired Talc | 81.96 | 0.29 | 5.75 | 10.21 | — | 0.11 | — | 0.69 | — | — | — | 0.48 | — | — | 0.51 | — |
| wollastonite | 51.75 | — | 48.25 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Quartz | 99.21 | 0.24 | 0.12 | 0.06 | 0.07 | 0.10 | — | 0.05 | — | 0.02 | 0.00 | — | — | — | 0.19 | 0.02 |
| Trialcium Phosphate | Other Raw Materials | |
|---|---|---|
| G1 | 0 | same |
| G2 | 4 | same |
| G3 | 6 | same |
| G4 | 8 | same |
| G5 | 10 | same |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Guo, H.; Sun, H.; Peng, T. Influence of Ca3(PO4)2 on the Surface Morphology and Properties of a CaO-Al2O3-SiO2-Fe2O3-Based High Temperature Phase Reconstructed Complex. Molecules 2024, 29, 3740. https://doi.org/10.3390/molecules29163740
Yang H, Guo H, Sun H, Peng T. Influence of Ca3(PO4)2 on the Surface Morphology and Properties of a CaO-Al2O3-SiO2-Fe2O3-Based High Temperature Phase Reconstructed Complex. Molecules. 2024; 29(16):3740. https://doi.org/10.3390/molecules29163740
Chicago/Turabian StyleYang, Huanyin, Hongli Guo, Hongjuan Sun, and Tongjiang Peng. 2024. "Influence of Ca3(PO4)2 on the Surface Morphology and Properties of a CaO-Al2O3-SiO2-Fe2O3-Based High Temperature Phase Reconstructed Complex" Molecules 29, no. 16: 3740. https://doi.org/10.3390/molecules29163740








