Abstract
Nucleosides, nucleotides, and their analogues are an important class of molecules that are used as substrates in research of enzymes and nucleic acid, or as antiviral and antineoplastic agents. Nucleoside phosphorylation is usually achieved with chemical methods; however, enzymatic phosphorylation is a viable alternative. Here, we present a chemoenzymatic synthesis of modified cytidine monophosphates, where a chemical synthesis of novel N4-modified cytidines is followed by an enzymatic phosphorylation of the nucleosides by nucleoside kinases. To enlarge the substrate scope, multiple mutant variants of Drosophila melanogaster deoxynucleoside kinase (DmdNK) (EC:2.7.1.145) and Bacillus subtilis deoxycytidine kinase (BsdCK) (EC:2.7.1.74) have been created and tested. It has been determined that certain point mutations in the active sites of the kinases alter their substrate specificities noticeably and allow phosphorylation of compounds that had been otherwise not phosphorylated by the wild-type DmdNK or BsdCK.
1. Introduction
Nucleoside and nucleotide analogues are an invaluable asset in the fields of molecular biology, biotechnology, and pharmacology. Modified nucleosides and their phosphates are employed in enzyme or nucleic acid research, and some are key building blocks of modern RNA vaccines [1,2,3,4,5]. In addition, certain nucleoside analogues are known to possess antiviral or anticancer properties [6,7]. Due to the immense interest in such compounds, the need for efficient nucleotide preparation is ever-growing. Generally, nucleoside phosphorylation is achieved utilizing chemical methods; however, they are not without drawbacks and must be chosen with caution depending on the target nucleoside. Synthetic approaches usually rely on extremely reactive phosphochloride compounds [8]. Such reactions are infamous for their harsh conditions, the usage of harmful reagents, and the need for protective group chemistry. A welcome alternative to chemical phosphorylation is the use of phosphorylating enzymes. Nucleoside phosphorylation can be achieved by the help of nucleoside kinases [9,10,11,12,13]. Some examples are nucleoside kinases such as Homo sapiens uridine–cytidine kinase (HsUCK), Homo sapiens adenosine kinase (HsAK), Escherichia coli guanosine–inosine kinase, and deoxynucleoside kinases such as Homo sapiens deoxycytidine kinase (HsdCK), herpes simplex virus 1 thymidine kinase (HSV-1 TK), Drosophila melanogaster deoxynucleoside kinase (DmdNK), and Bacillus subtilis deoxycytidine kinase (BsdCK) [14,15,16,17,18,19,20].
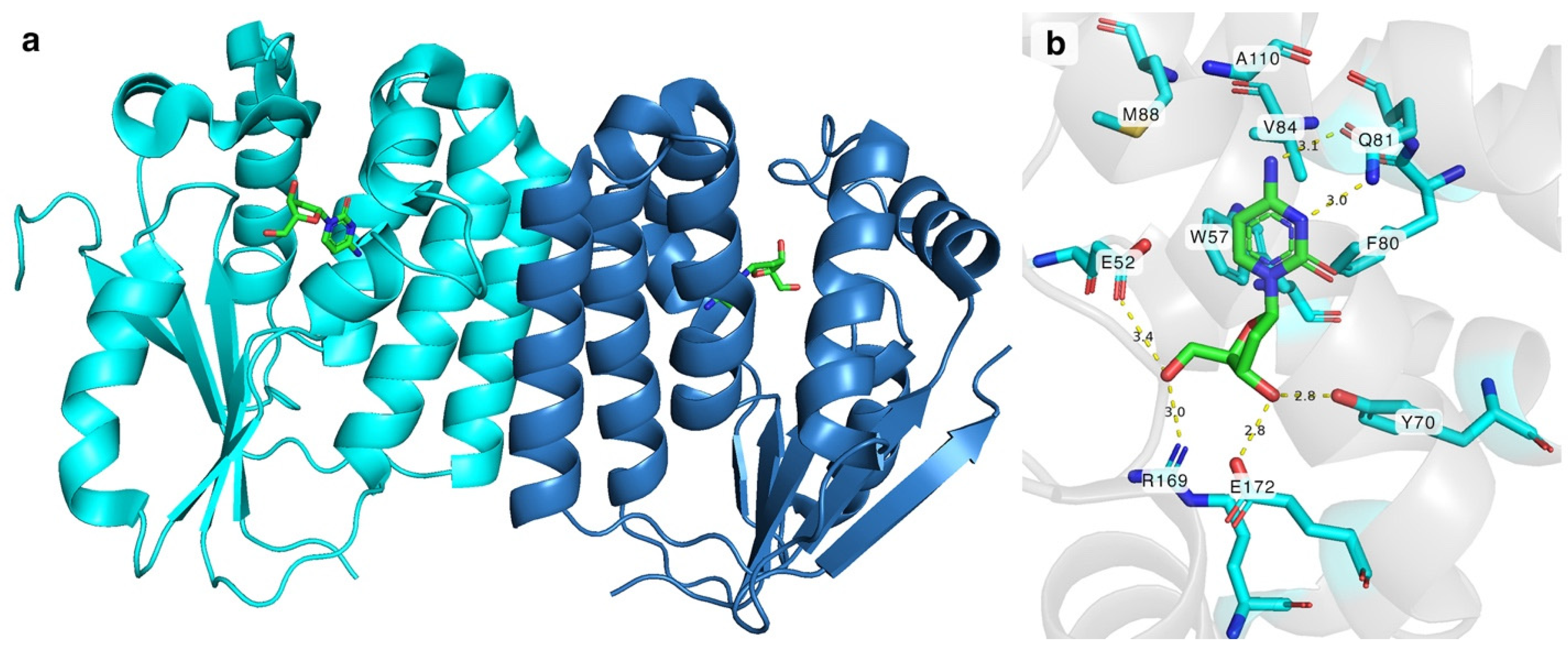
DmdNK (EC:2.7.1.145) is a multisubstrate kinase that phosphorylates all canonical deoxy- and ribonucleosides with a biased affinity towards pyrimidine nucleobase-bearing deoxyribonucleosides [21]. In addition to canonical nucleosides, nucleoside analogues with various modifications at nucleobase or sugar are also acceptable substrates to the kinase [22,23,24]. Crystallographic structures have been solved with different substrates, including but not limited to 2′-deoxycytidine, 2′-deoxythymidine, 5-fluoro-2′-deoxycytidine, (E)-5-(2-bromovinyl)-2′-deoxyuridine, and 2′-deoxythymidine 5′-triphosphate [25,26,27]. The active form of the enzyme is a homodimer (Figure 1a). The monomer of DmdNK (29 kDa) consists of eight α-helices and a central five-strand parallel β-sheet. Crystallographic data of DmdNK with a bound 2′-deoxycytidine has revealed that the active site is lined with hydrophobic residues (Figure 1b). When a substrate is bound, the nucleobase is stabilized by aromatic residues W57, F80, and F114. In addition, Q81 interacts with N3 and N4 atoms of the nucleobase. Polar residues Y70 and E172 form hydrogen bonds with the 3′-hydroxy group of the ribose moiety, and E52, R169, and a water molecule anchors the 5′-OH in place. The E52 residue together with R105 acts as a reaction initiator by deprotonating the 5′-hydroxygroup of the ribose. The deep cavity, lined with hydrophobic residues V84, M88, and A110, and located near the C5 position of cytosine moiety, might offer an explanation as to why the kinase accepts such a variety of substrates with modifications at positions N4 or C5 [25].
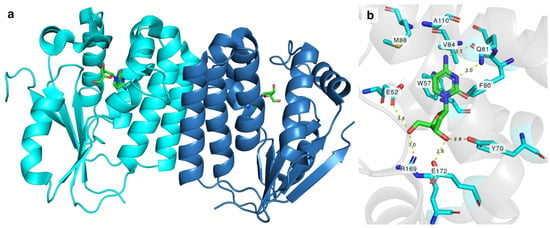
Figure 1.
(a) A dimer of DmdNK with 2′-deoxycytidine molecules (green) bound in the active centres. Each monomer is coloured in different shades of blue. (b) Interactions between 2′-deoxycytidine (green) and amino acid residues (cyan) in the active site of deoxynucleoside kinase. F114 is omitted for a clearer view. Yellow dashed lines represent hydrogen bonds between the nucleoside and the residues. PDB ID: 1j90.
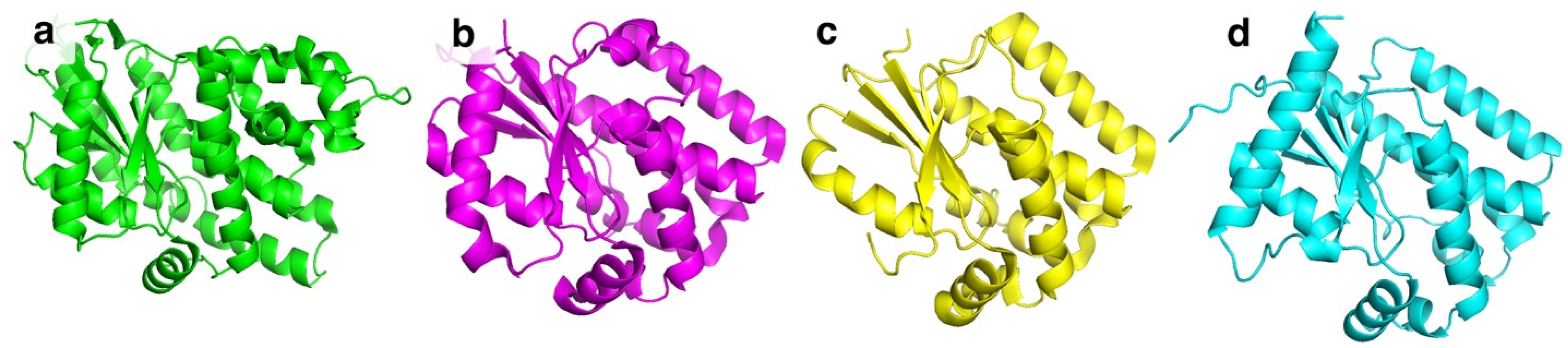
BsdCK (EC:2.7.1.74) is a nucleoside kinase that belongs to the non-TK1-like kinase family together with HSV-1 TK, HsdCK, and DmdNK [10]. All kinases belonging to this family share a similar fold (Figure 2a–d) and are found as homodimers. In contrast to the aforementioned kinases, BsdCK has not been studied thoroughly and its crystal structure has yet to be determined. The mass of its monomer is 25 kDa. Sequence analysis has revealed that it has a 25.6% identity to DmdNK and a 25.3% identity to HsdCK. The main substrates of BsdCK are nucleosides of cytosine and adenine [28]. The specificity toward sugar moiety is quite lenient. Substrates bearing ribose, 2′-deoxyribose, and arabinose moieties are accepted, although deoxyribonucleosides are favoured. As discovered in our previous study, the substrate specificity of BsdCK is not as limited as initially believed [23]. Furthermore, BsdCK phosphorylates all canonical ribo- and 2′-deoxyribonucleosides; however, phosphorylation of thymidine is poor. The kinase also accepts a variety of pyrimidine nucleoside analogues bearing modifications at the nucleobase such as N4-acetyl-2′-deoxycytidine, 5-fluorocytidine, N1-methylpseudouridine, or 2-thiouridine. Moreover, modifications of the sugar moiety are also tolerated. Some examples include 2′-O-methyluridine, 2′,3′-O-isopropylidineuridine, 2′-N-acetyl-2′-amino-2′-deoxyuridine, and 2′,3′-dideoxyuridine [23].
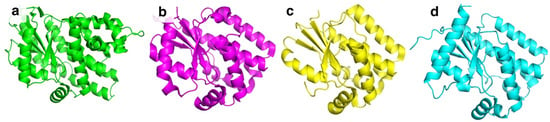
Figure 2.
Tertiary structures of non-TK1-like family nucleoside kinases. (a) HSV-1 TK monomer. PDB ID: 4IVP. (b) HsdCK monomer. PDB ID: 1p61. (c) DmdNK monomer. PDB ID: 1j90. (d) BsdCK monomer. Model generated by ColabFold v1.5.5.
Even though the mild reaction conditions and high regioselectivity make enzymatic nucleoside phosphorylation a compelling alternative to chemical nucleotide synthesis, it is far from perfect. Enzymatic phosphorylation is limited by the substrate specificity of the utilized enzymes and is often plagued with low to moderate product yields.
This work is a continuation of our previous study where we applied DmdNK and BsdCK for the phosphorylation of base- and sugar-modified nucleosides and discovered that even though the kinases possess a broad substrate specificity, a number of modified nucleosides remained non-phosphorylated [23]. To expand the substrate scope, we have created 16 DmdNK and 3 BsdCK mutant variants and tested them towards various N4-modified cytidine nucleosides.
2. Results and Discussion
The aim of our study was to increase the activities of DmdNK and BsdCK towards nucleobase-modified cytidine nucleosides. As we have determined in our previous investigation of wild-type DmdNK and BsdCK, the two kinases can phosphorylate a variety of pyrimidine analogues with small to medium-sized nucleobase or sugar modifications [23]. In the current study, we have focused on cytidine and 2′-deoxycytidine nucleosides bearing modifications at the N4-position.
2.1. Substrates Exploited in the Study
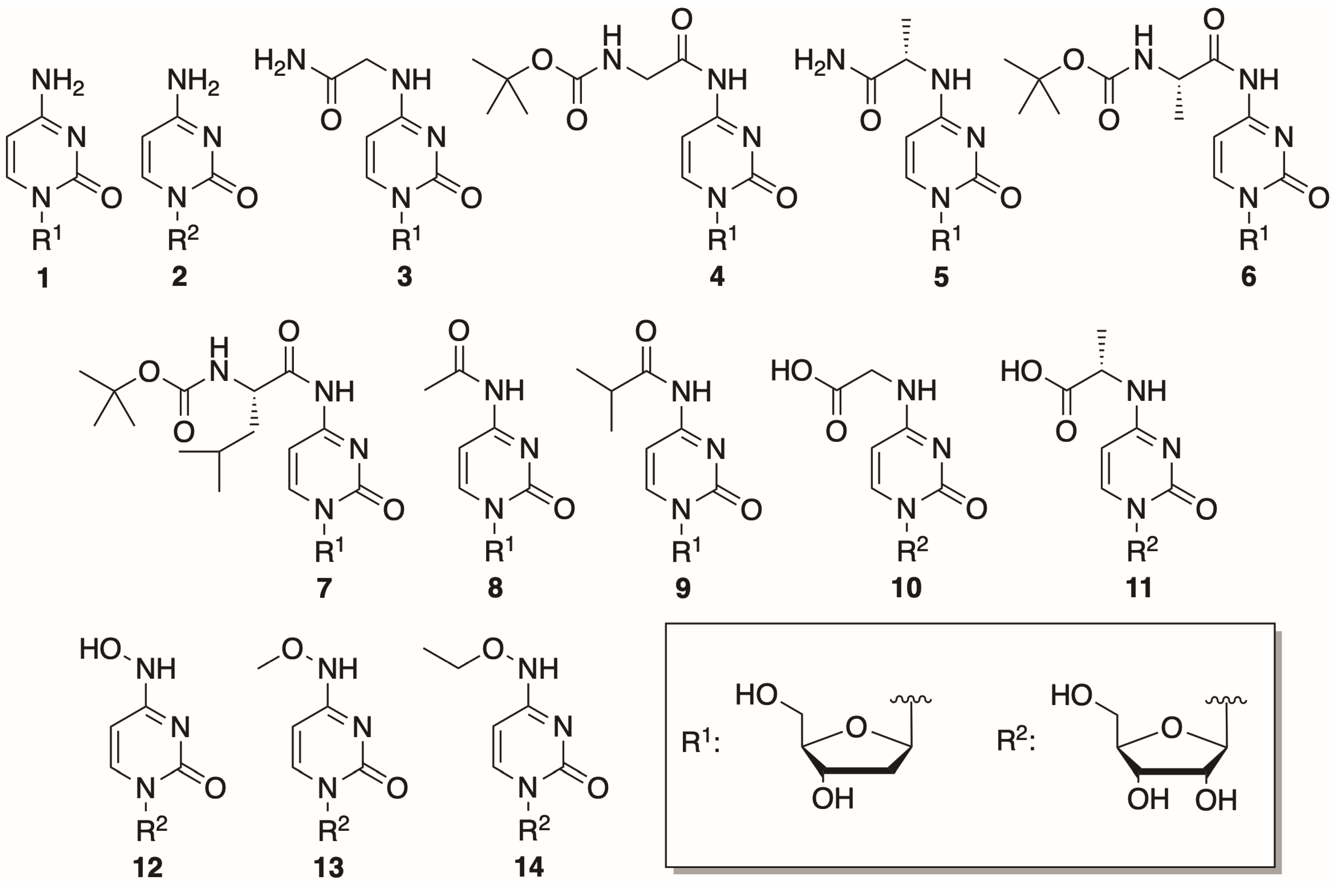
In this study, 14 cytosine nucleosides were chosen to be tested as phosphorylation substrates. The structures of the nucleosides are provided in Figure 3. Two canonical nucleosides, 2′-deoxycytidine and cytidine, were picked to assess the activity of the mutant variants since they are known to be efficiently phosphorylated by both DmdNK-WT and BsdCK-WT [20,21]. The remaining 12 nucleosides were chosen to be as diverse as possible and had modifications at the N4-position of the cytosine nucleobase, that ranged from small to bulky, from polar to hydrophobic. Nucleosides 1, 2, 8, and 9 were obtained commercially, while the rest were synthesized in our laboratory. Preparation of the N4-amino acid-modified nucleosides (3–7) was described previously [29]. Syntheses of the acidic nucleosides (10 and 11), and N4-hydroxy- (12) and N4-alkoxy-modified cytidines (13 and 14) are provided below.
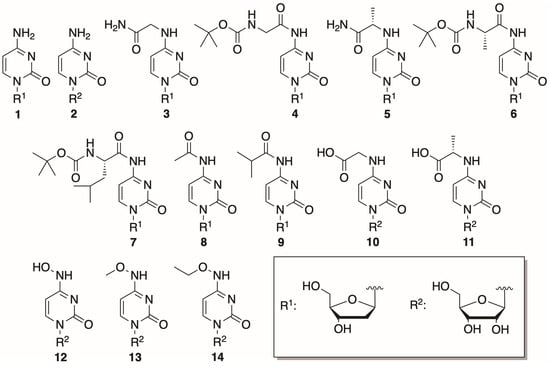
Figure 3.
Structures of the canonical and N4-modified nucleosides used in the study.
2.1.1. Synthesis of N-[1-(β-D-Ribofuranosyl)-2-oxo-4-pyrimidinyl]-amino Acids
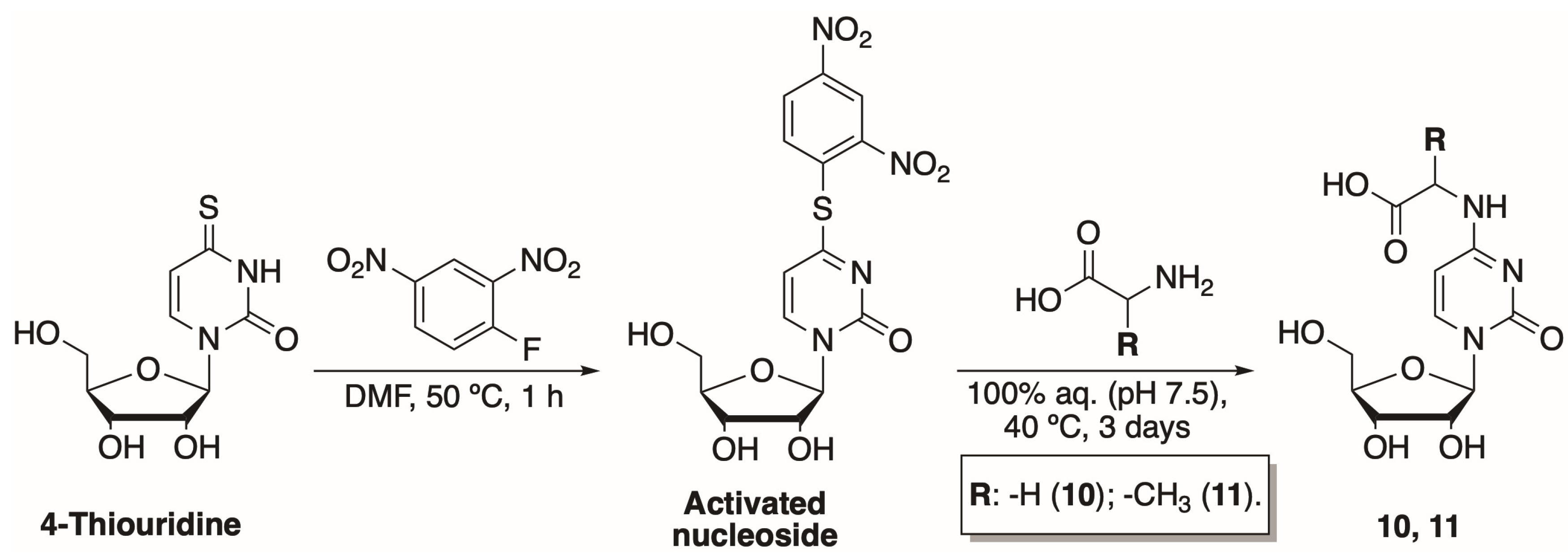
To further investigate the influence of the modifications at N4-position on nucleoside phosphorylation, we have synthesized two N-[1-(β-D-ribofuranosyl)-2-oxo-4-pyrimidinyl]-amino acids. These compounds are analogues to our previously synthesized N4-amino acid-modified 2′-deoxycytidines [29]. The newly synthesized compounds had exposed carboxyl instead of amide functional groups. The two-step synthesis of N-[1-(β-D-ribofuranosyl)-2-oxo-4-pyrimidinyl]-amino acids has been adapted from Ohashi et al. (Scheme 1) [30]. Treatment of commercially available 4-thiouridine with 2,4-dinitrofluorbenzene in N,N-dimethylformamide (DMF) for 1 h at 50 °C resulted in an activated nucleoside. The compound was purified using normal phase column chromatography and obtained in an overall yield of 24%.
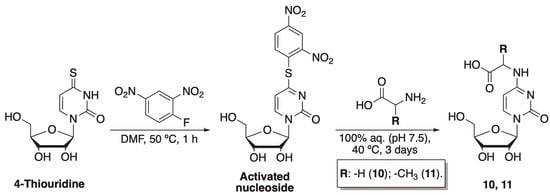
Scheme 1.
Preparation of N-[1-(β-D-ribofuranosyl)-2-oxo-4-pyrimidinyl]-amino acids.
The activated nucleoside was then treated with the corresponding amino acid (glycine or alanine) at 40 °C using 50 mM potassium phosphate buffer (pH 7.5) as a solvent. Products 10 and 11 were purified using two different chromatographic techniques. Hence, N-[1-(β-D-ribofuranosyl)-2-oxo-4-pyrimidinyl]-glycine (10) was purified by ion-exchange chromatography, while N-[1-(β-D-ribofuranosyl)-2-oxo-4-pyrimidinyl]-L-alanine (11) was separated using reversed-phase and hydrophilic interaction liquid chromatography methods. Both products were obtained in 13% yields.
2.1.2. Synthesis of N4-Hydroxy- and N4-Alkoxycytidines
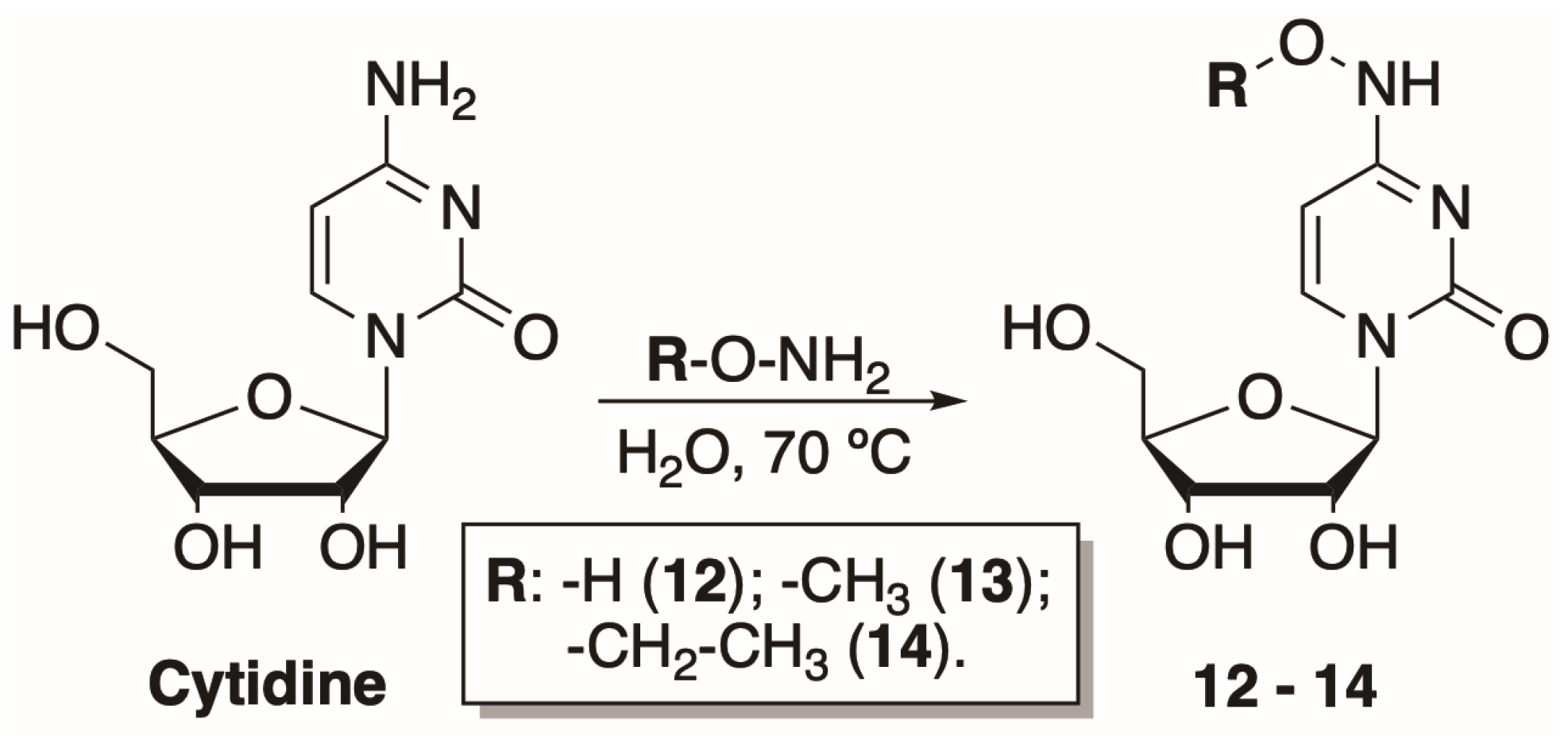
To explore how the bulkiness of the N4 modifications of the cytosine nucleobase affects the phosphorylation efficiency, three additional cytidine nucleoside analogues were synthesized. The preparation of N4-alkoxycytidines was based on N4-hydroxycytidine synthesis described by Paymode et al. [31]. The reaction between cytidine and amine hydrochloride salt in water at 70 °C resulted in corresponding hydroxy-, methoxy-, and ethoxy- modifications at the N4-position of cytidine (12–14) (Scheme 2). The products were purified by normal phase chromatography and obtained in a moderate 56–76% yield.
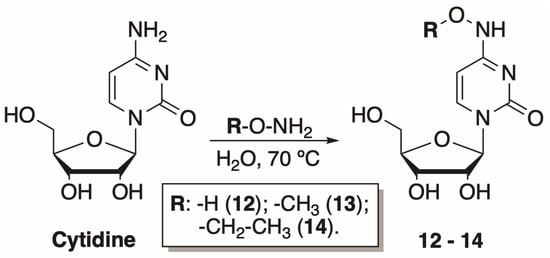
Scheme 2.
Preparation of N4-hydroxy- and N4-alkoxycytidines.
2.2. Synthesis and Application of the Mutant Kinases for the Phosphorylation of Canonical and N4-Modified Cytidine Nucleosides
Site-directed mutagenesis was carried out for the creation of mutant DmdNK and BsdCK variants, which were generated with histidine tags at the N-termini and applied for phosphorylation of nucleosides without further removal of the tags after purification. The preparation of the recombinant kinases is described in Materials and Methods. Sixteen mutant DmdNK variants, carrying a single or a double mutation, were generated: DmdNK-W57F, DmdNK-W57V, DmdNK-Q81A, DmdNK-Q81A+V84G, DmdNK-Q81A+M88G, DmdNK-A110G, DmdNK-V84A, DmdNK-V84A+M88A, DmdNK-V84A+A110D, DmdNK-V84G, DmdNK-M88A, DmdNK-M88G, DmdNK-M88R, DmdNK-M88R+A110G, DmdNK-A110D, and DmdNK-A110G. Three BsdCK mutants, carrying a single or a double mutation, were created: BsdCK-R70M, BsdCK-R70M+D93A, and BsdCK-D93A. Mutant variants DmdNK-Q81A+V84G, DmdNK-Q81A+M88G, DmdNK-Q81A+A110G, DmdNK-V84G, DmdNK-M88G, and DmdNK-A110G were found to be unstable and precipitated after an overnight dialysis. The solubility of those proteins was partially overcome by replacing E. coli BL-21(DE3) with E. coli KRX strain for gene expression, and cultivation of the transformants at 20 °C instead of 37 °C for 20 h.
The mutants were employed for the phosphorylation of cytidine nucleosides, whose structures are provided in Figure 3 above. The conditions for the phosphorylation reactions are given in Section 3. The results are provided in Table 1 and Table 2.

Table 1.
Phosphorylation efficiencies of pyrimidine nucleosides by DmdNK wild-type and mutant variants. Number is the phosphorylation efficiency (%) calculated using HPLC-MS chromatogram areas. Cells are coloured based on mutant performance in comparison to the wild-type kinase with the particular substrate (a margin of error of ±3 was applied). Rose—phosphorylation efficiency decreased ≥2-fold compared to DmdNK-WT; orange—phosphorylation efficiency decreased ≥1.5-fold but <2-fold compared to DmdNK-WT; lime—phosphorylation efficiency increased ≥1.5-fold but <2-fold compared to DmdNK-WT; green—phosphorylation efficiency increased ≥2-fold compared to DmdNK-WT; white—phosphorylation efficiency was similar to DmdNK-WT.

Table 2.
Phosphorylation efficiencies of pyrimidine nucleosides by BsdCK wild-type and mutant variants. Number is the phosphorylation efficiency (%) calculated using HPLC-MS chromatogram areas. Cells are coloured based on mutant performance in comparison to the wild-type kinase with the particular substrate (a margin of error of ±3 was applied). Rose—phosphorylation efficiency decreased ≥2-fold compared to BsdCK-WT; orange—phosphorylation efficiency decreased ≥1.5-fold but <2-fold compared to BsdCK-WT; lime—phosphorylation efficiency increased ≥1.5-fold but <2-fold compared to BsdCK-WT; green—phosphorylation efficiency increased ≥2-fold compared to BsdCK-WT; white—phosphorylation efficiency was similar to BsdCK-WT.
2.3. Mutations in the Hydrophobic Cavity of DmdNK Active Site
As has been previously mentioned, in addition to canonical nucleosides, wild-type DmdNK (DmdNK-WT) is efficient at phosphorylating a broad range of modified pyrimidine nucleosides [21,23,32]. One of the reasons why such a wide variety of nucleoside analogues is accepted by the kinase is the voluminous cavity found in the active site of the enzyme, that allows for accommodation of cytosine nucleosides bearing modifications at C5 or N4 positions [27]. The residues that line the cavity are V84, M88, and A110. Knecht and colleagues have examined the significance of the residues by creating point mutations at the aforementioned positions [33]. It has been discovered that the replacement of V84 with alanine decreases the affinity towards deoxythymidine and makes deoxycytidine the preferred substrate of DmdNK mutant variant V84A. The shift in affinity occurs due to the loss of favourable interactions between valine and the methyl group of deoxythymidine. In addition, a triple mutant DmdNK-V84S+M88R+A110D, which mimics active sites of deoxyguanosine kinases (dGK), has displayed substrate preferences similar to those of dGK, and favoured deoxyguanosine over deoxycytidine as a substrate [25]. In another study, a triple mutant DmdNK-V84A+M88R+A110D enabled phosphorylation of purine nucleoside analogues, 9-β-D-arabinofuranosyladenine, 9-β-D-arabinofuranosylguanine, and 2′,2′-difluorodeoxyguanosine, which were not accepted as substrates by the DmdNK-WT [34].
To investigate whether modifications of the hydrophobic cavity can lead to an increased activity towards N4-modified cytosine nucleosides, we have created point mutations at the positions V84, M88, and A110, respectively. Our hypothesis was that the extra space gained by replacing these residues with smaller alanine or glycine residues would allow the entering of substrates with bulkier modifications. In total, seven single mutant variants with modifications at the hydrophobic cavity were generated. V84 was replaced with alanine (DmdNK-V84A) and glycine (DmdNK-V84G); M88 was replaced with alanine (DmdNK-M88A), glycine (DmdNK-M88G), and arginine (DmdNK-M88R); A110 was replaced with aspartate (DmdNK-A110D) and glycine (DmdNK-A110G). In addition, three double mutants were created (DmdNK-V84A+M88A, DmdNK-V84A+A110D, and DmdNK-M88R+A110G) to investigate whether any synergistic effects were present. The data on the activity of the developed mutants is presented in Table 1.
When tested with canonical deoxycytidine (1), all variants displayed similar performance to DmdNK-WT and converted the compound to its monophosphate with 79–98% efficiency, with only a couple of poor performers (DmdNK-V84A+A110D and DmdNK-M88R-A110G), whose efficiencies reached 22–34%. Mutants DmdNK-V84A, DmdNK-V84A+M88A, and DmdNK-A110G retained the same activities towards cytidine (2) as DmdNK-WT and phosphorylated the compound with 65–75% efficiencies, while the activities of the remaining mutants decreased to 1–41%.
As evident from our results, replacing V84 with either alanine (DmdNK-V84A) or glycine (DmdNK-V84G) more than triples the activity of the kinase towards deoxycytidines modified at N4-position. Mutants DmdNK-V84A and DmdNK-V84G presented phosphorylation efficiencies of up to 98% for deoxycytidines modified with glycine (3), alanine (5), or isobutyryl- group (9), whereas DmdNK-WT phosphorylated the compounds with much lower efficiencies that were between 0% and 31%. The mutants retained the same activity towards N4-acetyl-2′-deoxycytidine (8) as DmdNK-WT and converted the compound into its monophosphate fully.
Replacement of the methionine at position 88 with either alanine (DmdNK-M88A) or glycine (DmdNK-M88G) resulted in a 2- to 6-fold decrease in activity towards all our tested N4-modified deoxycytidines (3–9). Phosphorylation efficiencies reached up to 54%, whereas the wild-type enzyme converted the same compounds to their monophosphate forms with efficiencies of up to 100%. In comparison, the double mutant DmdNK-V84A+M88A performed slightly better than the single M88A mutation-carrying variant as conversions of the N4-modified nucleosides 3, 5, and 8 reached 56–100% compared to 1–54%. However, N4-isobutyryl-2′-deoxycytidine (9) was not efficiently phosphorylated by either of the variants carrying an M88 mutation. Variant DmdNK-M88R, which had arginine instead of methionine in position 88, as is natively found in many deoxyguanosine kinases, presented with a complete loss of activity towards all tested N4-modified nucleosides, as did the double mutant DmdNK-M88R+A110G.
Although alanine is already a small residue, the replacement of A110 to glycine (DmdNK-A110G) led to a significantly higher activity towards the N4-modified deoxycytidines: phosphorylation efficiencies of 3, 5, and 9 increased up to 10-fold compared to DmdNK-WT and reached 30–98%. While replacing A110 with glycine increased the activity of the kinase towards N4-modified pyrimidine nucleosides, mutation A110D did the opposite. Phosphorylation of substrates 3, 8, and 12 by DmdNK-A110D decreased to 0–27%, which were otherwise phosphorylated with efficiencies of 31–100% by DmdNK-WT. In addition, the double mutant DmdNK-V84A+A110D, which presented with a 5-fold decrease in activity towards deoxycytidine compared to DmdNK-WT, was unable to phosphorylate any of the N4-modified cytidines.
N4-amino acid-modified deoxycytidines that had a Boc-protecting group (4, 6, and 7) were not acceptable substrates to the tested DmdNK mutants. Since the modifications are rather large, we believe that they would simply not fit in the active centres of the kinases. In addition, neither of the examined variants with mutations at V84, M88, and A110 positions showed high activity towards the acidic nucleosides 10 and 11, and N4-hydroxy- (12) and N4-alkoxy-modified cytidines (13, 14). Phosphorylation efficiencies of the compounds reached up to 24% compared to up to 46% of DmdNK-WT, although in most cases the compounds were not accepted as substrates at all. The modifications are relatively small sized which leads to believe that it is unfavourable interactions and not a lack of space that prevents the compounds from being efficiently phosphorylated.
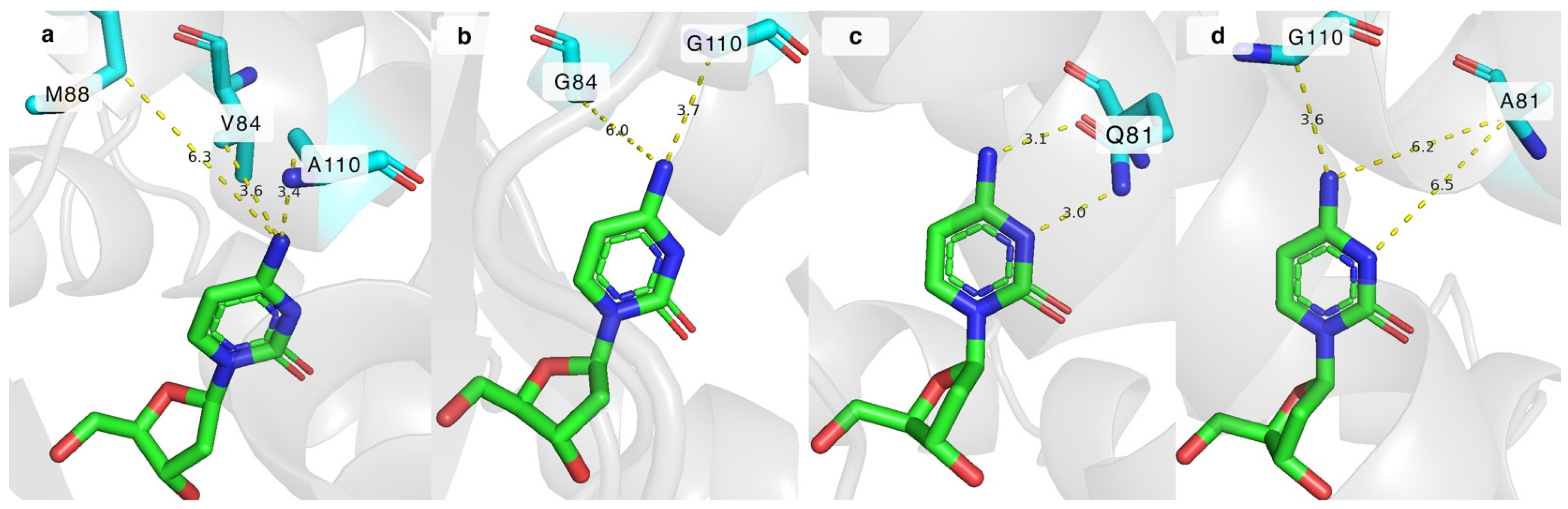
The impact of the mutations can be explained by analysing the crystal structure of DmdNK-WT with deoxycytidine in its active site (PDB ID: 1j90). As depicted in Figure 4a, residues V84 and A110 are in close proximity to the N4 atom of deoxycytidine, 3.6 Å and 3.4 Å, respectively. The extra space gained by replacing these residues with smaller amino acids allows the accommodation of bulkier nucleoside analogues (Figure 4b). In addition to the increased physical capacity of the substrate pocket, non-covalent interactions also play a role. The loss of the hydrophobic side chain of valine in the case of DmdNK-V84A or DmdNK-V84G, or methyl in the case of DmdNK-A110G, increases the affinity towards substrates with polar modifications such as N4-glycinoyl-2′-deoxycytidine (3). The methionine at position 88 is 6.3 Å away from the N4 atom, thus replacing the residue with a more compact group is expected to have less impact on the specificity of DmdNK. As evident from our results, swapping M88 with either a smaller residue such as alanine (DmdNK-M88A) or glycine (DmdNK-M88G) or a positively charged arginine (DmdNK-M88R) had a negative effect on the phosphorylation efficiency—the activity of all variants towards the tested compounds decreased two- to threefold compared to wild-type DmdNK.
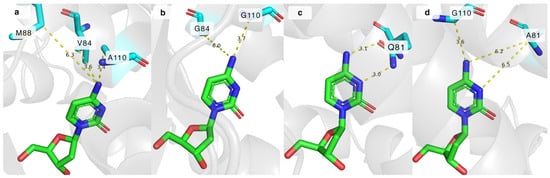
Figure 4.
The active site of DmdNK with bound deoxycytidine. (a) Distances between N4 atom of deoxycytidine and V84, M88, and A110 residues of DmdNK-WT. PDB ID: 1j90. (b) Predicted distances between N4 atom of deoxycytidine and residues at positions 84 and 110, where V84 and A110 are replaced by glycine. Model generated by ColabFold v1.5.5d. (c) Hydrogen bonds between N3 and N4 atoms of deoxycytidine and Q81 residue in DmdNK-WT. PDB ID: 1j90. (d) Predicted distances between deoxycytidine and the residues at positions 81 and 110, where Q81 is replaced by alanine, and A110 is replaced by glycine. Model generated by ColabFold v1.5.5.
To sum up, residues V84 and A110 have significant effects on the phosphorylation efficiency: replacing them with less bulky amino acids creates extra space in the active centre and increases the activity of the kinase towards substrates bearing N4-modifications. Since residue M88 is rather far away from the N4-position of cytosine, replacing it with a smaller residue had a negative effect on its activity: a decrease in phosphorylation efficiency was observed.
2.4. Significance of the Q81 Residue in the Active Site of DmdNK
Another position of interest was Q81, which forms two hydrogen bonds with the N3 and N4 atoms of cytosine and assists in stabilizing the substrate in the active site [25]. Some DmdNK-Q81 mutants have already been described. Replacing the positive charge of glutamine with the negative charge from glutamate was shown to enable phosphorylation of an unnatural pyrimidine nucleoside, 6-amino-5-nitro-3-(1′-β-D-2′-deoxyribofuranosyl)-2(1H)-pyridone, that was otherwise repulsed by the wild-type kinase due to the N3 atom bearing a hydrogen atom and acting as a proton donor instead of an acceptor [35]. In addition, shortening the residue by one carbon atom by replacing glutamine with asparagine caused the enzyme to favour purine nucleosides over pyrimidines [36]. To gain a deeper understanding of the importance of the glutamine residue at position 81, we have created a mutant in which the bulkier residue was replaced with the residue of alanine (DmdNK-Q81A). Our hypothesis was that the removal of the hydrogen bonds between the nucleobase and the residue should allow more flexibility of the nucleobase moiety in the active centre, thus nucleosides with larger modifications would fit more easily. As illustrated in Figure 4c, in the wild-type enzyme the distances between the N3 and N4 atoms of the nucleobase and Q81 are 3.0 Å and 3.1 Å. However, according to the in silico ColabFold v1.5.5 model, when Q81 is replaced by alanine (DmdNK-Q81A), the distances increase to over 6.2 Å (Figure 4d) and all interactions are eliminated. As can be seen from Table 1, the mutation alone did not cause the kinase to behave in a drastically different way to the wild-type variant. It retained the same activity towards 2′-deoxycytidine (1) and N4-acetyl-2′-deoxycytidine (8) as DmdNK-WT; however, phosphorylation of cytidine (2) decreased more than 5-fold. Conversions of the N4-modified deoxycytidines 3, 5, and 9 into the corresponding monophosphates did not differ significantly from DmdNK-WT and reached 0–20%. Just like the wild-type kinase, DmdNK-Q81A was unable to phosphorylate the acidic nucleosides 10 and 11, and nucleosides bearing the Boc-protecting group (4, 6, and 7). Even though phosphorylation of N4-hydroxycytidine (12) decreased more than 20-fold, a slight improvement was noticed with N4-methoxy- (13) and N4-ethoxycytidine (14). The compounds were phosphorylated with 4–11% efficiencies, whereas DmdNK-WT reached efficiencies of 0–5%.
The Q81A mutation was paired with the V84G, M88G, or A110G mutations to test whether any cumulative effects were present, but the double mutants DmdNK-Q81A+V84G, DmdNK-Q81A+M88G, and DmdNK-Q81A+A110G did not exhibit improved performance compared to the single-mutation carrying variants DmdNK-V84G, DmdNK-M88G, and DmdNK-A110G. Nevertheless, a double mutant DmdNK-Q81A+A110G showed an improvement in phosphorylating N4-alkoxy-modified cytidines (13, 14) compared to DmdNK-WT and DmdNK-A110G as conversions of the compounds increased from 0–5% to 30%. Since the compounds were poorly accepted by both DmdNK-WT and all mutant variants, the extra space provided by the A110G mutation, and the additional flexibility of the substrate provided by the removal of the bonds to Q81 may be the reasons (Figure 4d).
2.5. Significance of the W57 Residue in the Active Site of DmdNK
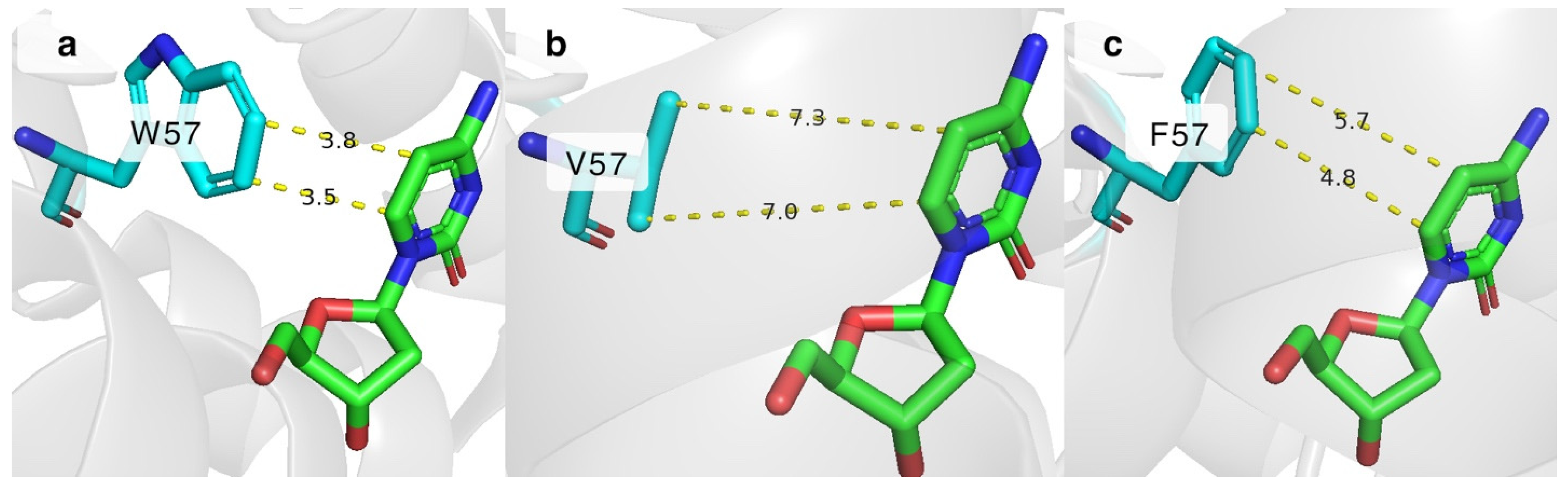
Stacking interactions are important for stabilizing the cytosine nucleobase in place. One of the residues responsible for π-stacking in the active site of DmdNK is W57, which interacts with the C5 and C6 atoms of cytosine as depicted in Figure 5a [25]. It is currently unknown how important this interaction is for the substrate specificity of the enzyme. To gain insight into this issue, we have created two DmdNK mutants in which W57 was replaced by the hydrophobic residues, phenylalanine (DmdNK-W57F) or valine (DmdNK-W57V). The phosphorylation efficiencies of the mutants are provided in Table 1. Compared to DmdNK-WT, the activities of the mutants towards deoxycytidine remained largely unchanged, but the phosphorylation efficiencies of cytidine and all nucleoside analogues tested decreased drastically (from 0–100% to 0–51%). According to the active site models generated by ColabFold v.1.5.5, the distances between cytosine and valine in the case of DmdNK-W57V become too large (over 7.0 Å) for any kind of interaction to occur (Figure 5b). Nonetheless, the distance between the aromatic ring of phenylalanine and cytosine might still allow weak non-covalent interactions (Figure 5c). This explains why the mutant DmdNK-W57F, which had a phenylalanine residue, retained slightly higher activity compared to that of the mutant DmdNK-W57V, which had a valine residue. The results suggest that the stabilizing interaction between the nucleobase and the aromatic system of W57 is crucial for the overall performance of the kinase.
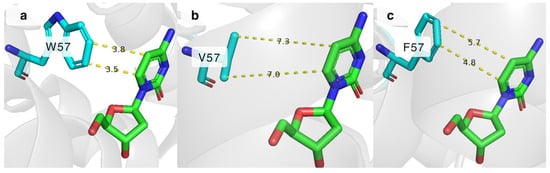
Figure 5.
The active site of DmdNK with bound deoxycytidine. (a) Stacking interaction between W57 and cytosine atoms. PDB ID: 1j90. (b) Predicted distances between C5 and C6 atoms of deoxycytidine and residue at position 57, where W57 is replaced by valine. Model generated by ColabFold v1.5.5. (c) Possible interactions between C5 and C6 atoms of deoxycytidine and residue at position 57, where W57 is replaced by phenylalanine. Model generated by ColabFold v1.5.5.
2.6. Enhancing BsdCK Substrate Diversity via Single-Site Mutagenesis
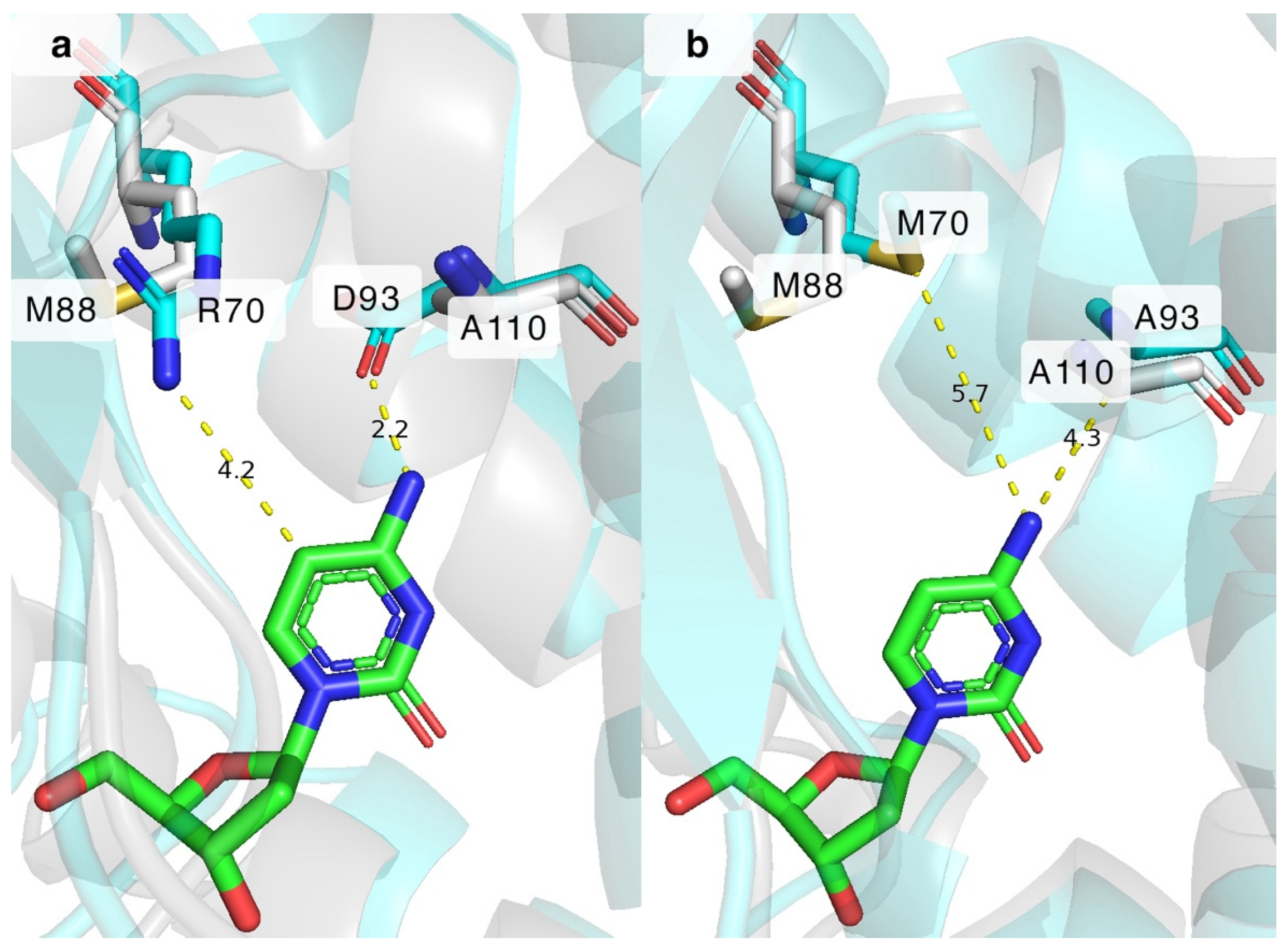
BsdCK is known to favour nucleosides of adenine and cytosine, and, as we have discovered in our previous study, it can also phosphorylate various pyrimidine nucleosides bearing small modifications at the nucleobase or sugar [20,23]. While BsdCK demonstrates a relatively broad substrate acceptance, it is still outperformed by the extensively researched DmdNK, which has one of the broadest substrate specificities known for nucleoside kinases [12,19,21,27,35,36,37,38]. Thus, we decided to modify the wild-type BsdCK (BsdCK-WT) to make it more similar to the DmdNK-WT in terms of substrate scope. Although the crystal structure of BsdCK has not been solved, we employed the protein structure prediction software ColabFold v.1.5.5 to determine potentially important residues. The generated model of BsdCK-WT monomer was superimposed on DmdNK-WT, and the residues lining the substrate pockets were compared. Since our goal was to increase the activity of BsdCK towards pyrimidine nucleosides bearing modifications at the N4 position, we have chosen residues that resided close to this group. As discussed previously, in the active site of DmdNK, residues M88 and A110 are located near the N4 position of the cytidine nucleobase. In the case of BsdCK, these residues correspond to R70 and D93 (Figure 6a). Single-site mutagenesis was used to generate three mutant variants of BsdCK: two single-mutation carrying variants BsdCK-R70M and BsdCK-D93A, and a double mutant BsdCK-R70M+D93A. The results are provided in Table 2.
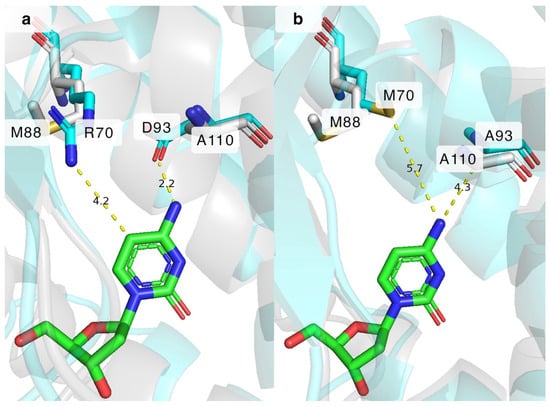
Figure 6.
Superimposition of BsdCK (cyan) on DmdNK (grey) with 2′-deoxycytidine (green) in the active centres. (a) M88 and A110 residues of DmdNK-WT, and corresponding R70 and D93 residues of BsdCK-WT near the N4 and C5 positions of 2′-deoxycytidine. (b) M88 and A110 residues of DmdNK-WT, and M70 and A93 residues of BsdCK-R70M+D93A mutant variant near the N4 and C5 positions of 2′-deoxycytidine. Grey residues belong to DmdNK. Cyan residues belong to BsdCK. Yellow dashed lines show predicted distances between the residues of BsdCK and 2′-deoxycytidine. DmdNK PDB ID: 1j90. BsdCK models generated using ColabFold v.1.5.5 software.
The mutant BsdCK-R70M, which had methionine instead of arginine, displayed extremely low activity. Compared to BsdCK-WT, phosphorylation of canonical deoxycytidine (1) and cytidine (2) decreased from 93–100% to 2–7%. Traces of N4-glycinoyl-2′-deoxycytidine (3) monophosphate were detected in the reaction mixture, although a 5-fold decrease in phosphorylation efficiency was observed when compared to that of the wild-type kinase. Variant BsdCK-R70M exhibited no activity towards the remainder of the N4-modified nucleosides and will not be further discussed.
The other single mutant, BsdCK-D93A, retained similar activity towards deoxycytidine (1) and N4-acetyl-2′-deoxycytidine (8) compared to that of BsdCK-WT, but phosphorylation of cytidine (2) still decreased significantly (from 93% to 4%). However, an improvement in the phosphorylation of N4-modified deoxycytidines was observed. Conversion of N4-amino acid modified nucleosides 3 and 5 increased from 0–11% to 16–20% compared to that of BsdCK-WT, and deoxycytidine bearing the N4-isobutyryl modification (9) was phosphorylated with 79% efficiency, whereas it was barely accepted by the wild-type kinase (2%).
However, the greatest increase in activity was noticed with the double-mutant BsdCK-R70M+D93A. Similarly to the single-mutation carrying BsdCK-D93A, it retained the same activities as those of BsdCK-WT towards deoxycytidine (1) and N4-acetyl-2′-deoxycytidine (8), but a 2-fold decrease in cytidine (2) phosphorylation efficiency was observed. The N4-modified deoxycytidines 3, 5, and 9 were converted into their monophosphate forms with high efficiencies, ranging from 37% to 98%. BsdCK-R70M+D93A even outperformed the DmdNK-WT, after which the double mutant was modelled. The mutant exhibited efficiencies comparable to those of the best-performing DmdNK-V84A and DmdNK-V84G variants, even though the corresponding position (F66) in the active site of BsdCK-R70M+D93A remained unchanged.
The introduced mutations of BsdCK were not enough to overcome the spatial limitations of the substrate pocket and/or unfavourable interactions between the residues of the active centre and the substrates: neither of the BsdCK mutants gained the ability to phosphorylate the nucleoside analogues bearing Boc-protecting group (4, 6 and 7), or the acidic nucleosides 10 and 11. A decrease in activity towards N4-hydroxy- (12) and N4-alkoxy-modified nucleosides (13 and 14) was observed, as well. The best-performing double mutant BsdCK-R70M+D93A displayed a 2- to 7-fold decline in phosphorylation efficiency when compared to that of BsdCK-WT (conversion went from 1–18% to 0–9%).
The increased activity towards N4-modified deoxycytidines 3, 5, and 9 can be partially explained by the extra space gained by replacing the bulkier aspartate with the smaller alanine at position 93. As depicted in Figure 6a, the aspartate residue in the BsdCK-WT is expected to be located 2.2 Å from the cytosine nucleobase and form hydrogen bonds with its N4 atom. This explains why cytosine moiety-bearing nucleosides are such excellent substrates to the wild-type enzyme. The aspartate, however, is not so great for the N4-modified deoxycytidines. Both the proximity and the negative charge of the aspartate residue prevent many nucleoside analogues from fitting into the active site of the enzyme. The issues are remedied by replacing the residue with alanine as it is found in DmdNK-WT. As can be seen from Figure 6b, replacing the D93 residue with alanine lengthens the distance from 2.2 Å to 4.3 Å, which in turn allows for the accommodation of nucleoside analogues that are modified at the N4-position. In the case of arginine at position 70, the residue is expected to be located 4.2 Å from the C5 carbon (Figure 6a) which is far enough to accommodate canonical pyrimidine nucleosides in the active site. However, the positively charged tail of arginine prevents nucleosides with certain modifications at positions N4 or C5 from entering the active site, both by spatial limitation and by the positive charge. This explains why, as was discovered in our previous study, thymidine is a poor substrate for the BsdCK-WT. Replacing R70 with a hydrophobic methionine, as is natively found in DmdNK-WT, provides additional space in the active centre (Figure 6b), as well as removes the positive charge provided by arginine. In conclusion, mimicking the active site of DmdNK by replacing both R70 with methionine and D93 with alanine has proven to be an excellent way to increase the activity of BsdCK towards N4-modified cytosine nucleosides.
2.7. Differences in Phosphorylating N4-Amino Acid Modified Nucleosides Bearing Amide and Carboxyl Functional Groups
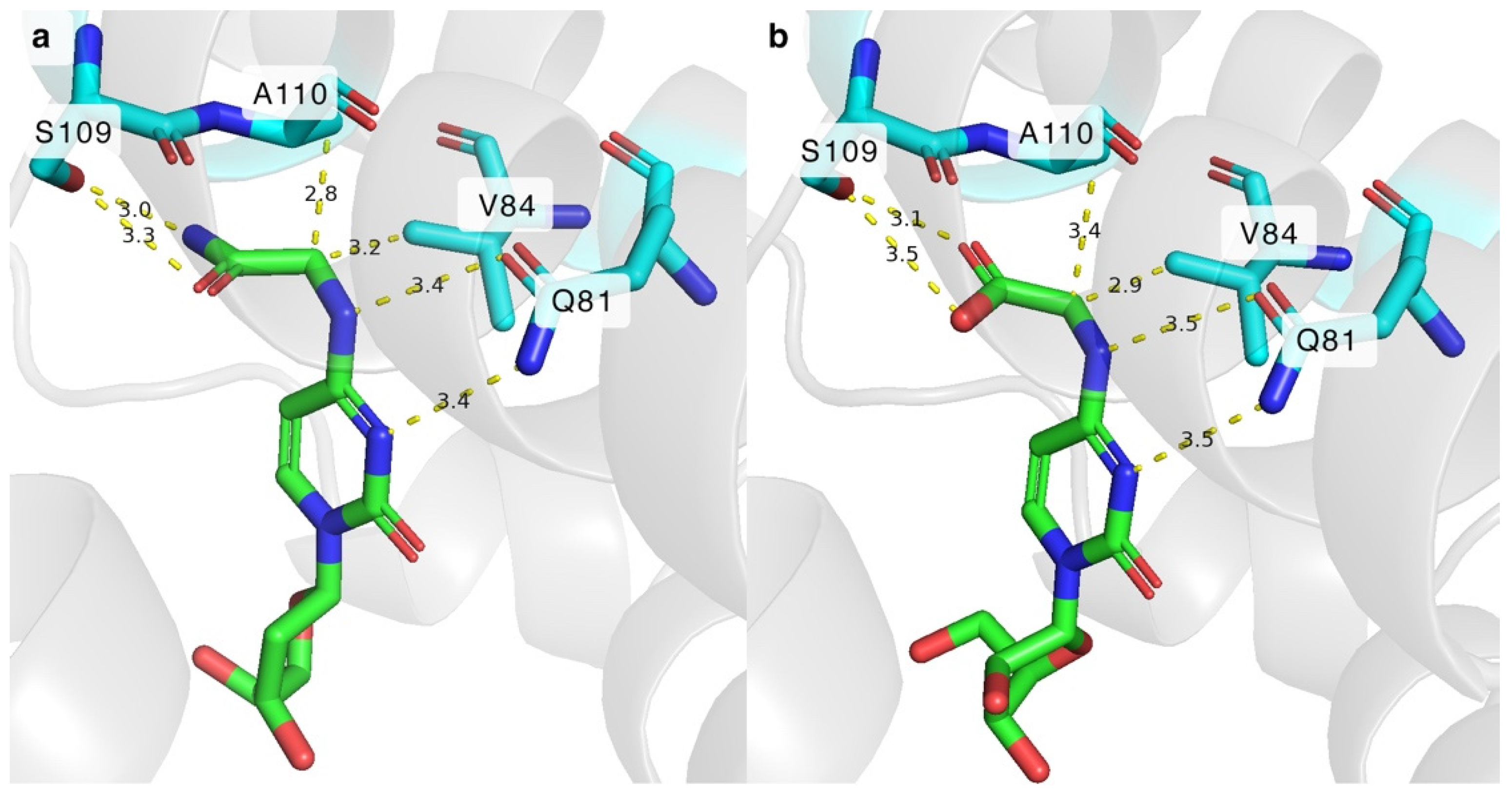
As it became apparent that the primary amide group bearing nucleosides N4-glycinoyl-2′-deoxycytidine (3) and N4-alaninoyl-2′-deoxycytidine (5) were successfully phosphorylated by certain DmdNK and BsdCK mutants, we decided to test whether analogues with carboxyl groups instead of amide functional groups were suitable substrates for the mutants, as well. Hence, two acidic cytidines bearing glycine and alanine residues (10, 11) were synthesized. To our surprise, none of the DmdNK or BsdCK variants were capable of phosphorylating the compounds. Since the amide and carboxyl groups are similar in size, we hypothesized that the issue was not a lack of space, but rather unfavourable interactions between the substrate and the residues of the enzymes’ active sites. To gain insight into how the N4-modified nucleoside might bind to the enzyme’s active centre, DmdNK-WT was docked with its substrate N4-glycinoyl-2′-deoxycytidine (3) and a non-accepted N4-glycine-modified acidic nucleoside (10). As depicted in Figure 7a,b, both compounds form very similar interactions with neighbouring residues Q81, V84, and A110. In addition, the amide and carboxyl groups of the nucleosides form interactions with the residue S109, which we expect to be the determining factor in whether the N4-amino acid-modified nucleoside is phosphorylated or not. The reason why 3 was efficiently phosphorylated by certain DmdNK mutants was favourable interactions. The amine of the amide group forms hydrogen bonds with the hydroxyl group of the serine 109 residue. On the other hand, 10 was not a suitable substrate for any of the kinase variants tested, and we believe that the same serine residue is the culprit. Under the conditions we tested (pH 7.5), the carboxyl group of glycine in the acidic nucleoside 10 is expected to be deprotonated and carry a negative charge, creating an unfavourable repulsive interaction between the nucleoside and the S109 residue [39]. In the given in silico models, the carboxyl group bearing nucleoside can be seen to be shifted slightly further away from S109, and closer to V84, compared to the nucleoside bearing the N4-amide functional group. We believe that the repulsive force prevents the acidic nucleoside from entering the active sites of the kinases and it is the reason why one compound is accepted as a substrate and the other one is not. To prove whether this theory is correct, additional research should be performed.
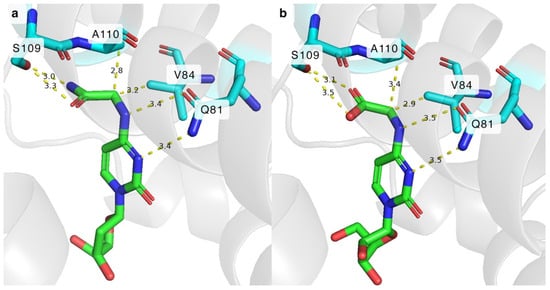
Figure 7.
(a) Molecular docking of N4-glycinoyl-2′-deoxycytidine in the active centre of DmdNK-WT. (b) Molecular docking of N-[1-(β-D-ribofuranosyl)-2-oxo-4-pyrimidinyl]-glycine in the active centre of DmdNK-WT. PDB ID: 1j90. Molecular docking performed by DiffDock software (https://huggingface.co/spaces/reginabarzilaygroup/DiffDock-Web; accessed on 6 August 2024).
3. Materials and Methods
3.1. General Information
Chemicals and solvents were purchased from Sigma–Aldrich (Darmstadt, Germany), Alfa Aesar (Kandel, Germany), Honeywell (Machelen, Belgium), Fluka (Buchs, Switzerland), Merck (Vilnius, Lithuania) and GE Healthcare (Freiburg, Germany), and were of analytical grade or higher. A full list of nucleosides, utilized for the study, is provided in Supplementary Data. Thin-layer chromatography (TLC) was carried out on 25 TLC aluminium sheets coated with silica gel 60 F254 (Merck, Vilnius, Lithuania). Ion exchange chromatography was carried out on Dowex 1 × 8 formate form (Sigma–Aldrich, Darmstadt, Germany). Flash chromatography was performed using Buchi Pure C-815 Flash system, equipped with a diode array detector (Buchi AG, Flawil, Switzerland). NMR spectra were recorded in DMSO-d6 on a Bruker Ascend 400 1H NMR—400 MHz, 13C NMR—101 MHz, 31P NMR—202 MHz (Bruker BioSpin, Rheinstetten, Germany). Chemical shifts are reported in ppm relative to the solvent resonance signal as an internal standard. UV spectra were recorded on a Lambda 25 Perkin Elmer UV/VIS spectrometer (PerkinElmer, Beaconsfield, UK). HPLC-MS analyses were performed using a high-performance liquid chromatography system, equipped with a photodiode array detector (SPD-M20A) and a mass spectrometer (LCMS-2020, Shimadzu, Kyoto, Japan) equipped with an ESI source. The chromatographic separation was conducted using a YMC Pack Pro column, 3 × 150 mm (YMC, Kyoto, Japan) at 40 °C and a mobile phase that consisted of 0.1% formic acid water solution (solvent A), and acetonitrile (solvent B). Mass spectrometry data was acquired in both positive and negative ionization mode and analysed using the LabSolutions LCMS software (version 5.82 SP1). Protein structures were visualized using the PyMOL Molecular Graphics System, Version 3.0 Schrödinger, LLC. Protein structure models were generated by ColabFold v1.5.5 software [40]. Molecular docking was performed utilizing DiffDock software (https://huggingface.co/spaces/reginabarzilaygroup/DiffDock-Web; accessed on 6 August 2024) [41].
3.2. Synthesis of N-[1-(β-D-Ribofuranosyl)-2-oxo-4-pyrimidinyl]-amino Acids
4-Thiouridine (3.85 mmol) was dissolved in 8 mL N,N-dimethylformamide, 5.8 mmol 2,4-dinitrofluorobenzene was added and the reaction mixture was stirred for 1 h at 50 °C temperature. The reaction was monitored by TLC analysis. After the reaction was completed, the reaction mixture was concentrated using a rotary evaporator and the residue was purified by column chromatography (silica gel, chloroform/methanol mixture, 100:0 → 93:7).
Appropriate amino acid (10 mmol) was dissolved in 10 mL 50 mM potassium phosphate buffer (pH 7.5) and activated thioether (220 mg, 5 mmol) was added to the reaction mixture which was then stirred for 3 days at 40 °C. The reaction was monitored with TLC analysis. After the reaction was completed, the reaction mixture was washed with chloroform to eliminate residual activated thioether.
N-[1-(β-D-ribofuranosyl)-2-oxo-4-pyrimidinyl]-glycine was purified by ion-exchange chromatography using Dowex I column (formate, 2.5 × 12 cm), washed with water, and eluted with 0.1 M formic acid. The fractions containing the product were combined, washed with ether, and concentrated to obtain a white solid compound.
N-[1-(β-D-ribofuranosyl)-2-oxo-4-pyrimidinyl]-L-alanine was purified by flash chromatography. Firstly, the product was separated using FlashPure Select C18 30µm spherical 20 g column (water/methanol mixture, 95:5 → 0:100). The fractions containing the product were combined and concentrated to 1 mL using a rotary evaporator. To remove residue amino acid, a second purification was performed using FlashPure EcoFlex Amino 50 µm spherical column (acetonitrile/10 mM ammonium carbonate (pH 8.0) buffer, 95:5 → 60:40).
Synthesized nucleosides were characterized by NMR spectroscopy and HPLC-MS analysis.
3.3. Synthesis of N4-Hydroxy- and N4-Alkoxycytidines
Cytidine (8.23 mmol) was dissolved in 0.4 mL water, 9.05 mmol of corresponding amine hydrochloride salt (hydroxylamine, methoxyamine, or ethoxyamine) was added and the reaction mixture was heated at 70 °C temperature. The reaction was monitored with TLC analysis. After the reaction was completed, the reaction mixture was concentrated using a rotary evaporator and the residue was purified by column chromatography (silica gel, chloroform/methanol mixture, 100:0 → 80:20). The products were obtained in 56–76% yields. Synthesized nucleosides were characterized by NMR spectroscopy and HPLC-MS analysis.
3.4. Bacterial Strains, Plasmids, and Reagents
Construction of plasmids, that contained DmdNK-WT and BsdCK-WT genes, has been previously described [23]. Escherichia coli DH5α was used for plasmid amplification. Escherichia coli BL21 (DE3) and KRX strains were employed for the expression of target genes. All three strains were purchased from Novagen (Darmstadt, Germany). Thermo Scientific aLICator LIC cloning and expression kit was purchased from ThermoFisher Scientific (Vilnius, Lithuania). Phusion Plus PCR Master Mix, utilized for gene amplification, was purchased from ThermoFisher Scientific (Vilnius, Lithuania). Restriction enzyme DpnI (10 U/μL) was purchased from ThermoFisher Scientific (Vilnius, Lithuania).
3.5. Site-Directed Mutagenesis
The mutant variants of D. melanogaster dNK and B. subtilis dCK were created using site-directed mutagenesis. Plasmids pLATE31-dNK and pLATE31-dCK were used as starting templates [23]. All templates and primers utilized in the construction of the mutants are listed in Table S1. The gene syntheses were performed by PCR using Phusion Plus polymerase. DmdNK variants V84A, V84A+A110D, V84A+M88A, M88A, M88R, M88R+A110D, and A110D, and BsdCK variants R70M, R70M+D93M, and D93M were synthesized conventionally, using a forward and a reverse primer, both carrying the same mutation. DmdNK variants W57F, W57V, Q81A, Q81A+V84G, Q81A+M88G, Q81A+A110G, V84G, M88G, and A110G were created using a single-primer site-directed mutagenesis protocol as described by Huang and Zhang [42]. PCR mixtures were treated with 1 µL of DpnI to remove the template DNA. The amplified genes were purified from 1% agarose gel and cloned into pLATE31 plasmid according to the manufacturer’s protocol. The resulting plasmids were chemically transformed into competent E. coli DH5α cells. Transformants were selected on LB agar plates (0.1 mg/mL ampicillin). The plasmids were isolated and sequenced to confirm that the desired mutations were present. The plasmids with correct sequences were named pLATE31-dNK-W57F, pLATE31-dNK-W57V, pLATE31-dNK-Q81A, pLATE31-dNK-Q81A+V84G, pLATE31-dNK-Q81A+M88G, pLATE31-dNK-Q81A+A110G, pLATE31-dNK-V84A, pLATE31-dNK-V84G, pLATE31-dNK-V84A+M88A, pLATE31-dNK-V84A+A110D, pLATE31-dNK-M88A, pLATE31-dNK-M88G, pLATE31-dNK-M88R, pLATE31-dNK-M88R+A110D, pLATE31-dNK-A110D, pLATE31-dNK-A110G, pLATE31-dCK-R70M, pLATE31-dCK-R70M+D93M, and pLATE31-dCK-D93M.
3.6. Expression of Target Genes and Purification of Proteins
The recombinant plasmids, except for DmdNK-Q81A+V84G, DmdNK-Q81A+M88G, DmdNK-Q81A+A110G, DmdNK-V84G, DmdNK-M88G, and DmdNK-A110G, for which E. coli KRX strain was used, were transformed into E. coli BL21 (DE3) cells by electroporation. Transformants, selected on 0.1 mg/mL ampicillin LB agar plates, were inoculated into 200 mL of LB medium containing 0.1 mg/mL ampicillin and incubated at 37 °C and a shaking speed of 180 rpm for 2–4 h. At an OD600 of 0.6, gene expression was induced with 1 mM of IPTG for 3 h. In the case of E. coli KRX transformants, at an OD600 of 0.6 the temperature was decreased to 20 °C and gene expression was induced with 0.5 mM of IPTG for 20 h. Cells were harvested by centrifugation at 4000× g at 4 °C for 10 min. The supernatant was discarded, and the pellet was suspended in 6 mL of 50 mM potassium phosphate buffer (pH 7.5). Cells were lysed by sonication (5 min total time; 2 s disruption, 8 s cooling; 22 kHz at 40% amplitude) on ice. Cell-free fractions were recovered by centrifugation at 16,000× g at 4 °C for 10 min. The obtained lysate was loaded onto 1 mL Ni2+ HiTrap chelating HP column equilibrated with 50 mM potassium phosphate buffer (pH 7.5). The enzymes were eluted using 50 mM potassium phosphate buffer containing 0.5 M imidazole (pH 7.5) gradient from 0% to 100%, at a flow rate of 0.7 mL/min. Protein purification was monitored by 14% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Fractions containing target protein were pooled, placed into dialysis bags, and dialyzed overnight at 4 °C in 1 L of 50 mM potassium phosphate buffer (pH 7.5). The precipitated proteins were removed by centrifugation at 16,000× g at 4 °C for 10 min. Concentrations of the purified enzymes were determined to be between 0.04 and 2.4 mg/mL. Solutions of the purified enzymes were stored at −20 °C until further use.
3.7. Substrate Specificity Assay
The substrate specificity assays were performed in 50 µL reaction mixtures that contained 10 mM nucleoside, 15 mM GTP, 15 mM MgCl2, and 0.69 nmol/mL DmdNK or 2.69 nmol/mL BsdCK in 50 mM potassium phosphate buffer (pH 7.5). The phosphorylation reactions were carried out at 37 °C and a mixing speed of 300 rpm for 24 h. After the reactions were complete, 50 µL of acetonitrile was added. The mixtures were centrifuged at 16,000× g at 4 °C for 10 min, supernatants were analysed using HPLC-MS. The substrate specificities of the kinases were determined based on the formation of corresponding nucleoside 5′-monophosphates. The phosphorylation efficiencies were calculated from HPLC-MS chromatogram areas (data provided in Supplementary Materials). Substrates utilized in the study are provided in Figure 3.
4. Conclusions
In this study, single-site mutagenesis was applied to two nucleoside kinases DmdNK and BsdCK in order to expand their substrate scope. It was discovered that modifying the substrate pocket of DmdNK by replacing residues V84 with alanine or glycine, and A110 with glycine had the most prominent effects on substrate selectivity. The kinase gained the ability to phosphorylate N4-modified cytidine nucleosides, such as N4-glycinoyl-2′-deoxycytidine and N4-isobutyryl-2′-deoxycytidine, that were otherwise phosphorylated poorly or not at all by the wild-type variant.
Mimicking the active site of DmdNK by replacing residues R70 and D93 with methionine and alanine, respectively, in BsdCK was found to widen the range of its suitable substrates. The double mutant acquired the ability to phosphorylate cytidine analogues, such as N4-alaninoyl-2′-deoxycytidine and N4-isobutyryl-2′-deoxycytidine, that were too bulky for BsdCK-WT.
This study proves that manipulating the active sites of nucleoside kinases is a viable option to expand the variety of accepted substrates. With the help of in silico modelling, important residues in the active centres can be recognized and, consequentially, modified to fit a target nucleoside. The strategy can be employed for enzymatic nucleoside 5′-triphosphate synthesis, where the first phosphorylation step is usually performed by nucleoside kinases [17,23,24,43,44]. The following steps are performed using nucleoside monophosphate and nucleoside diphosphate kinases. Similar to nucleoside kinases, nucleoside monophosphate kinases are known to be substrate-specific. For example, viral thymidylate kinases HSV-1 TK and VZV TK phosphorylate 2′-deoxythymidine, dTMP and dUMP, while mammalian UMP-CMP kinase accepts CMP, dCMP, UMP, dUMP, AMP, and dAMP as substrates [11,45]. Nucleoside diphosphate kinases, on the other hand, possess a wide-substrate cleft and can phosphorylate an abundance of substrates [11,46,47]. In addition to nucleotide synthesis, nucleoside kinases, such as DmdNK or HSV1-TK, are often utilized in suicide gene therapy where they activate nucleoside prodrugs that are targeted towards tumour cells [9,48,49,50]. Modifying the kinases can be an effective strategy for improving their activity towards target nucleosides and, in turn, increasing the efficacy of cancer treatment [34,51]. Furthermore, the kinases could be utilized in the field of nucleic acid labelling. For example, HsdCK and HsUCK2 have been successfully applied in cell-specific metabolic labelling of RNA, where the kinases phosphorylated 2′-azidocytidine and 2′-azidouridine, respectively, into their monophosphate forms, which were then subsequently phosphorylated into NTPs by cellular NMP and NDP kinases. Incorporation of these NTPs by RNA polymerases allowed for effective RNA probing with low cytotoxicity [4,5].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29163767/s1, a list of used reagents, 1H-NMR (400 MHz), 13C-NMR (101 MHz) and MS spectra of compounds. Table S1: PCR primers used for site-directed mutagenesis of DmdNK and BsdCK. Figure S1: HPLC chromatogram of DmdNK-V84A catalysed N4-isobutyryl-deoxycytidine phosphorylation reaction. Table S2: HPLC data of DmdNK catalysed reactions. Table S3: HPLC data of BsdCK catalysed reactions.
Author Contributions
Conceptualization, M.K., K.B., J.S. and R.M.; methodology, M.K. and K.B.; validation, M.K. and K.B.; formal analysis, M.K., K.B., J.S. and R.M.; investigation, M.K., K.B., J.S. and R.M.; resources, R.M.; data curation, M.K., K.B., J.S. and R.M.; writing—original draft preparation, M.K.; writing—review and editing, M.K., K.B., J.S. and R.M.; visualization, M.K.; supervision, R.M.; project administration, R.M.; funding acquisition, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the Research Council of Lithuania (LMTLT), agreement No. S-MIP-22-6.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saito, Y.; Hudson, R.H.E. Base-Modified Fluorescent Purine Nucleosides and Nucleotides for Use in Oligonucleotide Probes. J. Photochem. Photobiol. C Photochem. Rev. 2018, 36, 48–73. [Google Scholar] [CrossRef]
- Urbelienė, N.; Tiškus, M.; Tamulaitienė, G.; Gasparavičiūtė, R.; Lapinskaitė, R.; Jauniškis, V.; Sūdžius, J.; Meškienė, R.; Tauraitė, D.; Skrodenytė, E.; et al. Cytidine Deaminases Catalyze the Conversion of N(S,O)4-Substituted Pyrimidine Nucleosides. Sci. Adv. 2023, 9, eade4361. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A Multivalent Nucleoside-Modified MRNA Vaccine against All Known Influenza Virus Subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Kleiner, R.E. Cell- and Polymerase-Selective Metabolic Labeling of Cellular RNA with 2′-Azidocytidine. J. Am. Chem. Soc. 2020, 142, 14417–14421. [Google Scholar] [CrossRef] [PubMed]
- Nainar, S.; Cuthbert, B.J.; Lim, N.M.; England, W.E.; Ke, K.; Sophal, K.; Quechol, R.; Mobley, D.L.; Goulding, C.W.; Spitale, R.C. An Optimized Chemical-Genetic Method for Cell-Specific Metabolic Labeling of RNA. Nat. Methods 2020, 17, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Zenchenko, A.A.; Drenichev, M.S.; Il’icheva, I.A.; Mikhailov, S.N. Antiviral and Antimicrobial Nucleoside Derivatives: Structural Features and Mechanisms of Action. Mol. Biol. 2021, 55, 786–812. [Google Scholar] [CrossRef] [PubMed]
- Guinan, M.; Benckendorff, C.; Smith, M.; Miller, G.J. Recent Advances in the Chemical Synthesis and Evaluation of Anticancer Nucleoside Analogues. Molecules 2020, 25, 2050. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Depaix, A.; Périgaud, C.; Peyrottes, S. Recent Trends in Nucleotide Synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef]
- Slot Christiansen, L.; Munch-Petersen, B.; Knecht, W. Non-Viral Deoxyribonucleoside Kinases—Diversity and Practical Use. J. Genet. Genom. 2015, 42, 235–248. [Google Scholar] [CrossRef]
- Sandrini, M.P.B.; Piškur, J. Deoxyribonucleoside Kinases: Two Enzyme Families Catalyze the Same Reaction. Trends Biochem. Sci. 2005, 30, 225–228. [Google Scholar] [CrossRef]
- Deville-Bonne, D.; El Amri, C.; Meyer, P.; Chen, Y.; Agrofoglio, L.A.; Janin, J. Human and Viral Nucleoside/Nucleotide Kinases Involved in Antiviral Drug Activation: Structural and Catalytic Properties. Antivir. Res. 2010, 86, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.F.; Winiger, C.B.; Shaw, R.W.; Kim, M.-J.; Kim, M.-S.; Daugherty, A.B.; Chen, F.; Moussatche, P.; Moses, J.D.; Lutz, S.; et al. A Single Deoxynucleoside Kinase Variant from Drosophila Melanogaster Synthesizes Monophosphates of Nucleosides That Are Components of an Expanded Genetic System. ACS Synth. Biol. 2017, 6, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Abraham, C.; Suslov, O.; Yaren, O.; Shaw, R.W.; Kim, M.-J.; Wan, S.; Marliere, P.; Benner, S.A. Synthetic Biology Pathway to Nucleoside Triphosphates for Expanded Genetic Alphabets. ACS Synth. Biol. 2023, 12, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Van Rompay, A.R.; Norda, A.; Lindén, K.; Johansson, M.; Karlsson, A. Phosphorylation of Uridine and Cytidine Nucleoside Analogs by Two Human Uridine-Cytidine Kinases. Mol. Pharmacol. 2001, 59, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Boison, D. Adenosine Kinase: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2013, 65, 906–943. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Iida, A.; Teshiba, S.; Fujio, T. Cloning of a Guanosine-Inosine Kinase Gene of Escherichia Coli and Characterization of the Purified Gene Product. J. Bacteriol. 1995, 177, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Hellendahl, K.F.; Kamel, S.; Wetterwald, A.; Neubauer, P.; Wagner, A. Human Deoxycytidine Kinase Is a Valuable Biocatalyst for the Synthesis of Nucleotide Analogues. Catalysts 2019, 9, 997. [Google Scholar] [CrossRef]
- Wild, K.; Bohner, T.; Folkers, G.; Schulz, G.E. The Structures of Thymidine Kinase from Herpes Simplex Virus Type 1 in Complex with Substrates and a Substrate Analogue. Protein Sci. 1997, 6, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Munch-Petersen, B.; Knecht, W.; Lenz, C.; Søndergaard, L.; Piškur, J. Functional Expression of a Multisubstrate Deoxyribonucleoside Kinase from Drosophila Melanogaster and Its C-Terminal Deletion Mutants. J. Biol. Chem. 2000, 275, 6673–6679. [Google Scholar] [CrossRef]
- Andersen, R.B.; Neuhard, J. Deoxynucleoside Kinases Encoded by the YaaG AndyaaF Genes of Bacillus Subtilis. J. Biol. Chem. 2001, 276, 5518–5524. [Google Scholar] [CrossRef]
- Johansson, M.; Van Rompay, A.R.; Degrève, B.; Balzarini, J.; Karlsson, A. Cloning and Characterization of the Multisubstrate Deoxyribonucleoside Kinase of Drosophila Melanogaster. J. Biol. Chem. 1999, 274, 23814–23819. [Google Scholar] [CrossRef]
- Wu, Y.; Fa, M.; Tae, E.L.; Schultz, P.G.; Romesberg, F.E. Enzymatic Phosphorylation of Unnatural Nucleosides. J. Am. Chem. Soc. 2002, 124, 14626–14630. [Google Scholar] [CrossRef]
- Koplūnaitė, M.; Butkutė, K.; Špelveris, D.; Urbelienė, N.; Meškys, R. Enzymatic Synthesis of Modified Nucleoside 5′-Monophosphates. Catalysts 2022, 12, 1401. [Google Scholar] [CrossRef]
- Serra, I.; Conti, S.; Piškur, J.; Clausen, A.R.; Munch-Petersen, B.; Terreni, M.; Ubiali, D. Immobilized Drosophila Melanogaster Deoxyribonucleoside Kinase (Dm DNK) as a High Performing Biocatalyst for the Synthesis of Purine Arabinonucleotides. Adv. Synth. Catal. 2014, 356, 563–570. [Google Scholar] [CrossRef]
- Johansson, K.; Ramaswamy, S.; Ljungcrantz, C.; Knecht, W.; Piskur, J.; Munch-Petersen, B.; Eriksson, S.; Eklund, H. Structural Basis for Substrate Specificities of Cellular Deoxyribonucleoside Kinases. Nat. Struct. Biol. 2001, 8, 616–620. [Google Scholar] [CrossRef]
- Mikkelsen, N.E.; Johansson, K.; Karlsson, A.; Knecht, W.; Andersen, G.; Piškur, J.; Munch-Petersen, B.; Eklund, H. Structural Basis for Feedback Inhibition of the Deoxyribonucleoside Salvage Pathway: Studies of the Drosophila Deoxyribonucleoside Kinase. Biochemistry 2003, 42, 5706–5712. [Google Scholar] [CrossRef]
- Mikkelsen, N.E.; Munch-Petersen, B.; Eklund, H. Structural Studies of Nucleoside Analog and Feedback Inhibitor Binding to Drosophila Melanogaster Multisubstrate Deoxyribonucleoside Kinase. FEBS J. 2008, 275, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Møllgaard, H. Deoxyadenosine/Deoxycytidine Kinase from Bacillus Subtilis. Purification, Characterization, and Physiological Function. J. Biol. Chem. 1980, 255, 8216–8220. [Google Scholar] [CrossRef] [PubMed]
- Koplūnaitė, M.; Butkutė, K.; Meškys, R.; Tauraitė, D. Synthesis of Pyrimidine Nucleoside and Amino Acid Conjugates. Tetrahedron Lett. 2020, 61, 152598. [Google Scholar] [CrossRef]
- Ohashi, S.; Nakamura, M.; Acharyya, S.; Inagaki, M.; Abe, N.; Kimura, Y.; Hashiya, F.; Abe, H. Development and Comparison of 4-Thiouridine to Cytidine Base Conversion Reaction. ACS Omega 2024, 9, 9300–9308. [Google Scholar] [CrossRef]
- Paymode, D.J.; Vasudevan, N.; Ahmad, S.; Kadam, A.L.; Cardoso, F.S.P.; Burns, J.M.; Cook, D.W.; Stringham, R.W.; Snead, D.R. Toward a Practical, Two-Step Process for Molnupiravir: Direct Hydroxamination of Cytidine Followed by Selective Esterification. Org. Process Res. Dev. 2021, 25, 1822–1830. [Google Scholar] [CrossRef]
- Knecht, W.; Munch-Petersen, B.; Piškur, J. Identification of Residues Involved in the Specificity and Regulation of the Highly Efficient Multisubstrate Deoxyribonucleoside Kinase from Drosophila Melanogaster. J. Mol. Biol. 2000, 301, 827–837. [Google Scholar] [CrossRef]
- Knecht, W. A Few Amino Acid Substitutions Can Convert Deoxyribonucleoside Kinase Specificity from Pyrimidines to Purines. EMBO J. 2002, 21, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Solaroli, N.; Johansson, M.; Balzarini, J.; Karlsson, A. Enhanced Toxicity of Purine Nucleoside Analogs in Cells Expressing Drosophila Melanogaster Nucleoside Kinase Mutants. Gene Ther. 2007, 14, 86–92. [Google Scholar] [CrossRef][Green Version]
- Chen, F.; Zhang, Y.; Daugherty, A.B.; Yang, Z.; Shaw, R.; Dong, M.; Lutz, S.; Benner, S.A. Biological Phosphorylation of an Unnatural Base Pair (UBP) Using a Drosophila Melanogaster Deoxynucleoside Kinase (DmdNK) Mutant. PLoS ONE 2017, 12, e0174163. [Google Scholar] [CrossRef] [PubMed]
- Solaroli, N.; Bjerke, M.; Amiri, M.H.; Johansson, M.; Karlsson, A. Active Site Mutants of Drosophila Melanogaster Multisubstrate Deoxyribonucleoside Kinase. Eur. J. Biochem. 2003, 270, 2879–2884. [Google Scholar] [CrossRef]
- Knecht, W.; Mikkelsen, N.E.; Clausen, A.R.; Willer, M.; Eklund, H.; Gojković, Z.; Piškur, J. Drosophila Melanogaster Deoxyribonucleoside Kinase Activates Gemcitabine. Biochem. Biophys. Res. Commun. 2009, 382, 430–433. [Google Scholar] [CrossRef]
- Egeblad-Welin, L.; Sonntag, Y.; Eklund, H.; Munch-Petersen, B. Functional Studies of Active-site Mutants from Drosophila Melanogaster Deoxyribonucleoside Kinase. FEBS J. 2007, 274, 1542–1551. [Google Scholar] [CrossRef]
- Larsen, P.O. Physical and Chemical Properties of Amino Acids. In Amino Acids and Derivatives; Elsevier: Amsterdam, The Netherlands, 1980; pp. 225–269. [Google Scholar]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Stärk, H.; Jing, B.; Barzilay, R.; Jaakkola, T. DiffDock: Diffusion Steps, Twists, and Turns for Molecular Docking. arXiv 2022, arXiv:2210.01776. [Google Scholar]
- Huang, Y.; Zhang, L. An In Vitro Single-Primer Site-Directed Mutagenesis Method for Use in Biotechnology. Methods Mol. Biol. 2017, 1498, 375–383. [Google Scholar] [PubMed]
- Fehlau, M.; Kaspar, F.; Hellendahl, K.F.; Schollmeyer, J.; Neubauer, P.; Wagner, A. Modular Enzymatic Cascade Synthesis of Nucleotides Using a (d)ATP Regeneration System. Front. Bioeng. Biotechnol. 2020, 8, 854. [Google Scholar] [CrossRef]
- Zou, Z.; Ding, Q.; Ou, L.; Yan, B. Efficient Production of Deoxynucleoside-5′-Monophosphates Using Deoxynucleoside Kinase Coupled with a GTP-Regeneration System. Appl. Microbiol. Biotechnol. 2013, 97, 9389–9395. [Google Scholar] [CrossRef] [PubMed]
- Van Rompay, A.R.; Johansson, M.; Karlsson, A. Phosphorylation of Nucleosides and Nucleoside Analogs by Mammalian Nucleoside Monophosphate Kinases. Pharmacol. Ther. 2000, 87, 189–198. [Google Scholar] [CrossRef]
- Lascu, I.; Gonin, P. The Catalytic Mechanism of Nucleoside Diphosphate Kinases. J. Bioenerg. Biomembr. 2000, 32, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Chen, Y.; Feng, J.Y.; Borroto-Esoda, K.; Deville-Bonne, D.; Janin, J.; Moréra, S. Nucleoside Diphosphate Kinase and the Activation of Antiviral Phosphonate Analogs of Nucleotides: Binding Mode and Phosphorylation of Tenofovir Derivatives. Nucleosides Nucleotides Nucleic Acids 2009, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, M.; Zhang, Y.; Zhong, L.; Zheng, X. Novel Combination Oncolytic Adenoviral Gene Therapy Armed with Dm-DNK and CD40L for Breast Cancer. Curr. Gene Ther. 2019, 19, 54–65. [Google Scholar] [CrossRef]
- Fillat, C.; Carrio, M.; Cascante, A.; Sangro, B. Suicide Gene Therapy Mediated by the Herpes Simplex Virus Thymidine Kinase Gene/Ganciclovir System: Fifteen Years of Application. Curr. Gene Ther. 2003, 3, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhao, L.; Zhu, Z.; Liu, Q.; Xu, H.; Johansson, M.; Karlsson, A.; Zheng, X. The Multisubstrate Deoxyribonucleoside Kinase of Drosophila Melanogaster as a Therapeutic Suicide Gene of Breast Cancer Cells. J. Gene Med. 2011, 13, 305–311. [Google Scholar] [CrossRef]
- Knecht, W.; Rozpedowska, E.; Le Breton, C.; Willer, M.; Gojkovic, Z.; Sandrini, M.P.B.; Joergensen, T.; Hasholt, L.; Munch-Petersen, B.; Piskur, J. Drosophila Deoxyribonucleoside Kinase Mutants with Enhanced Ability to Phosphorylate Purine Analogs. Gene Ther. 2007, 14, 1278–1286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).