A Theoretical Exploration of the Photoinduced Breaking Mechanism of the Glycosidic Bond in Thymine Nucleotide
Abstract
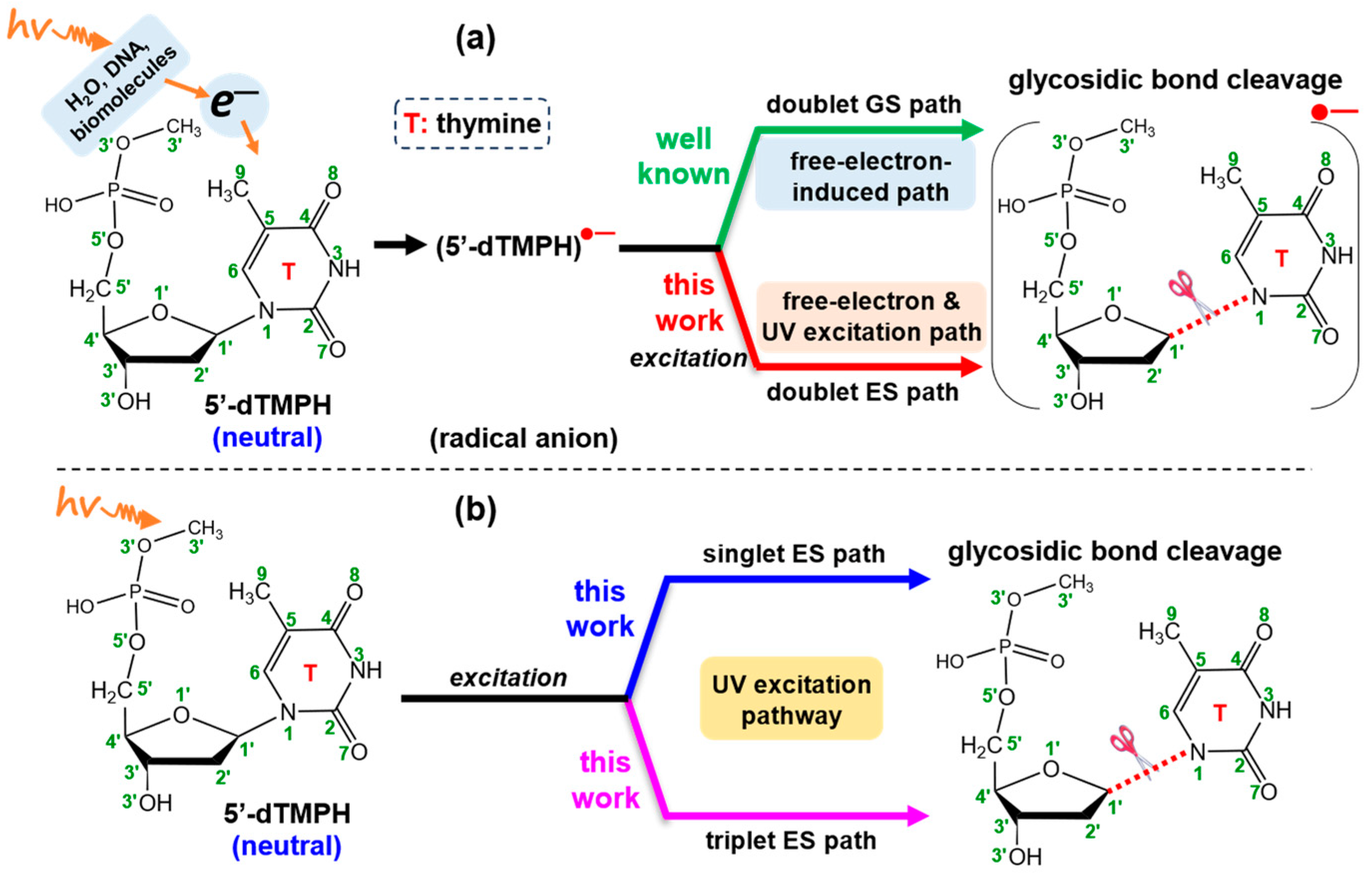
1. Introduction
2. Results and Discussion
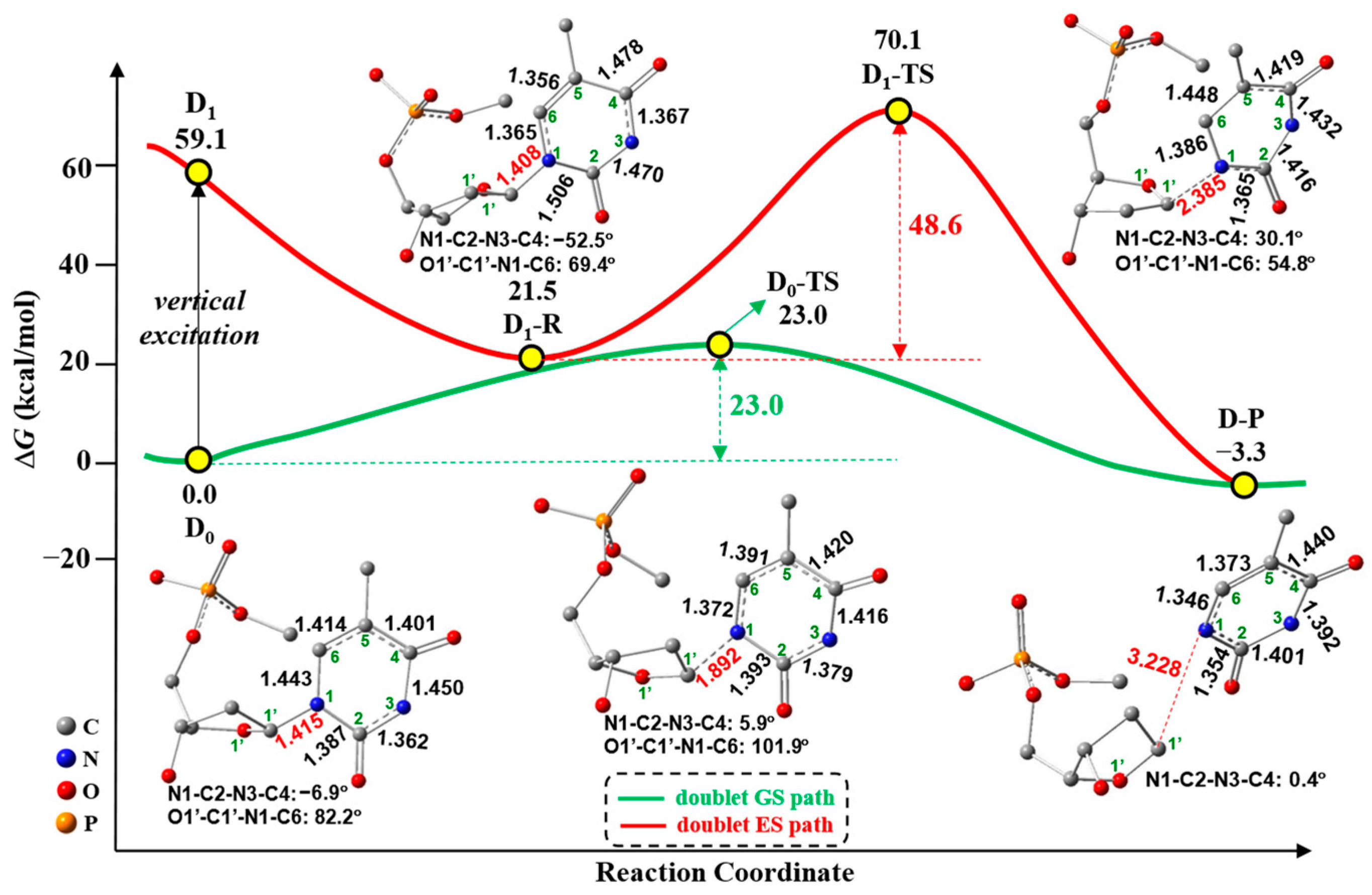
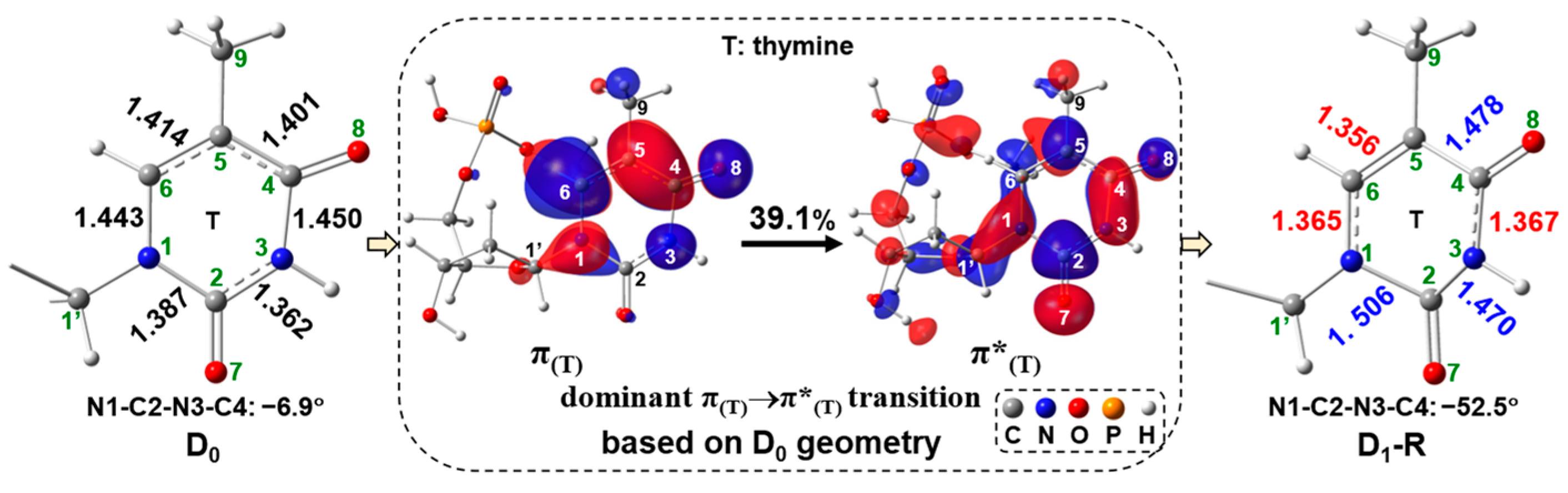
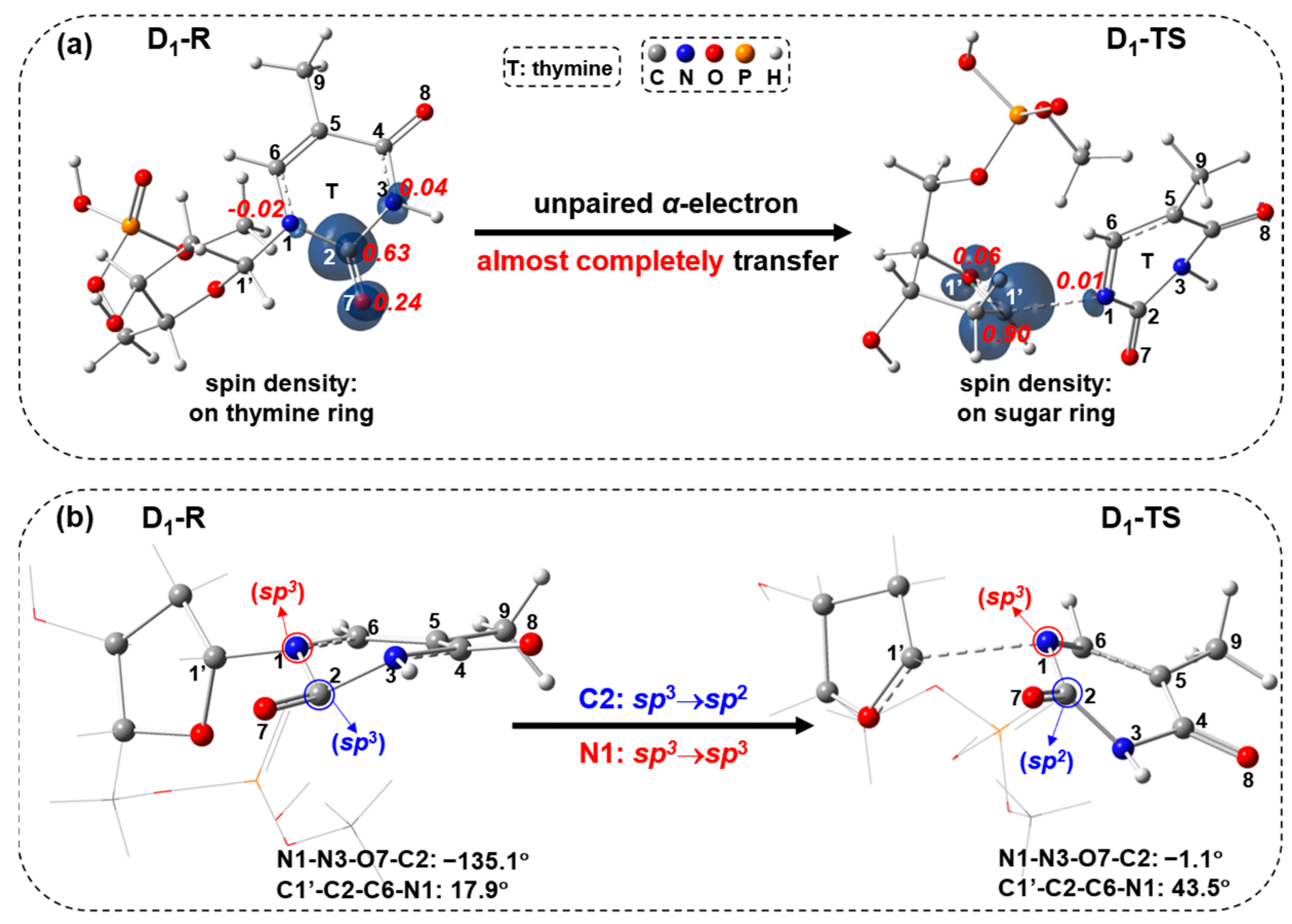
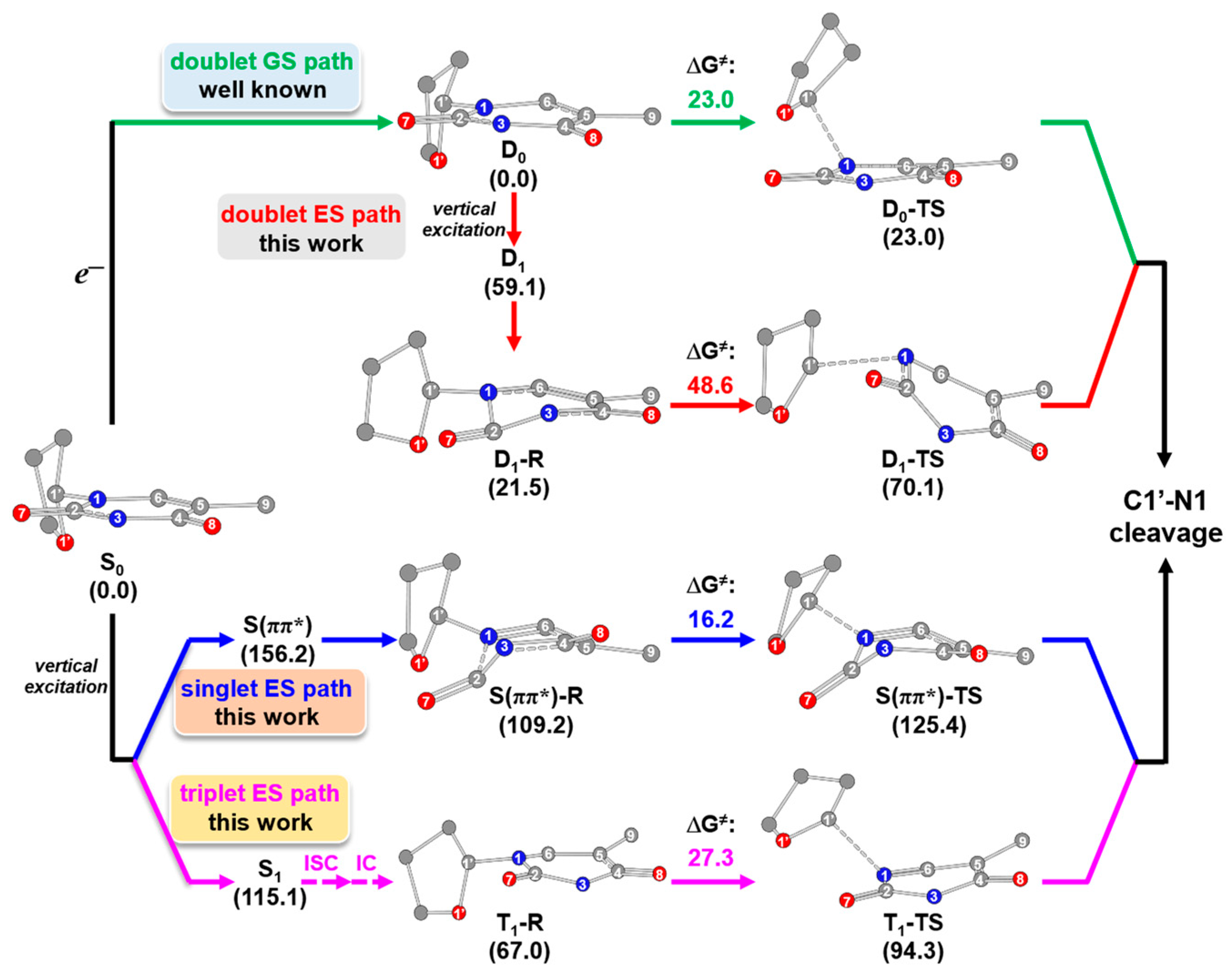
2.1. Free-Electron-Induced Path and Free-Electron and UV Excitation Path of C1′-N1 Bond Cleavage
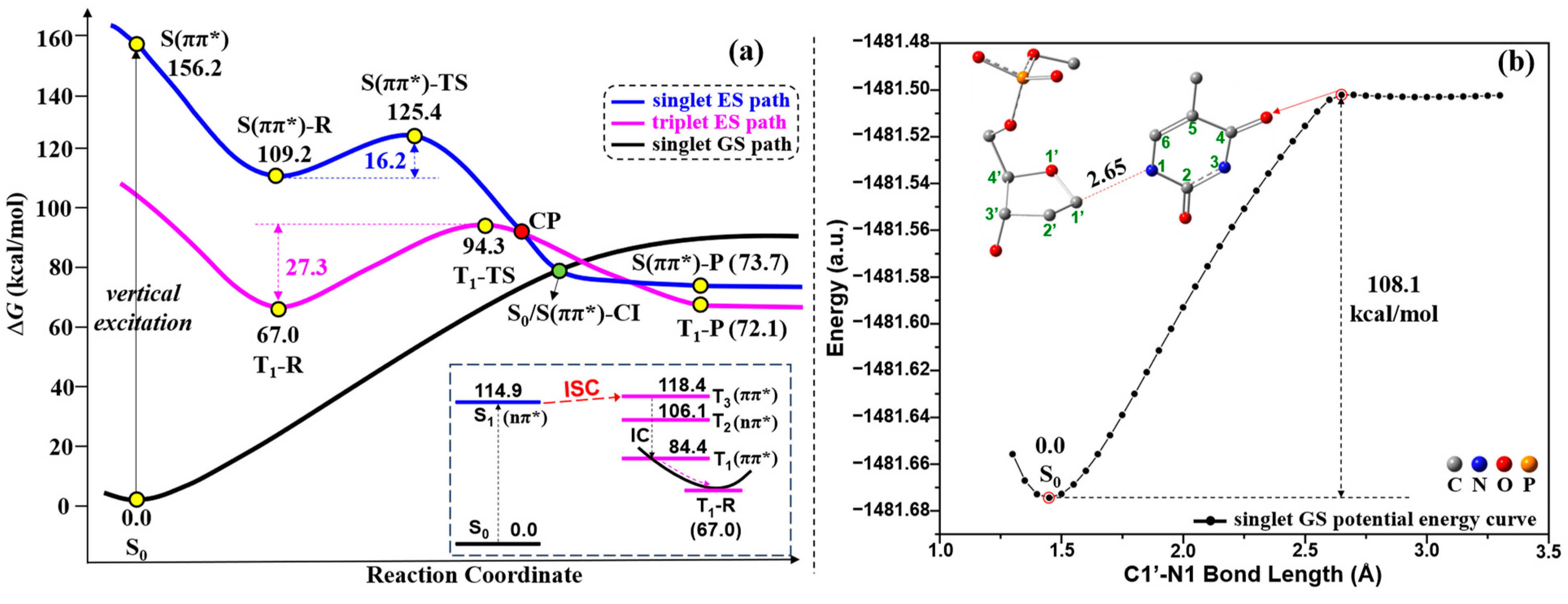
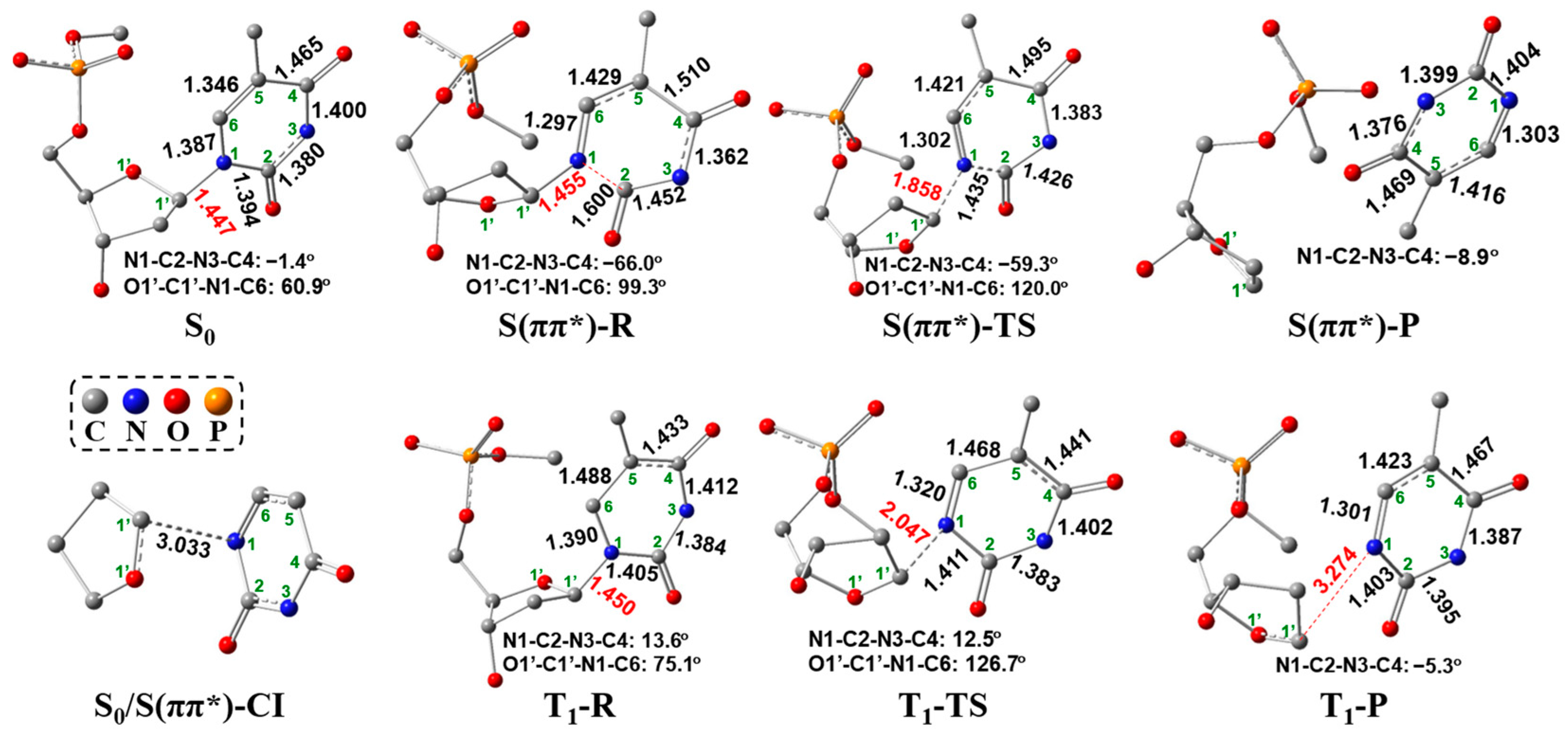
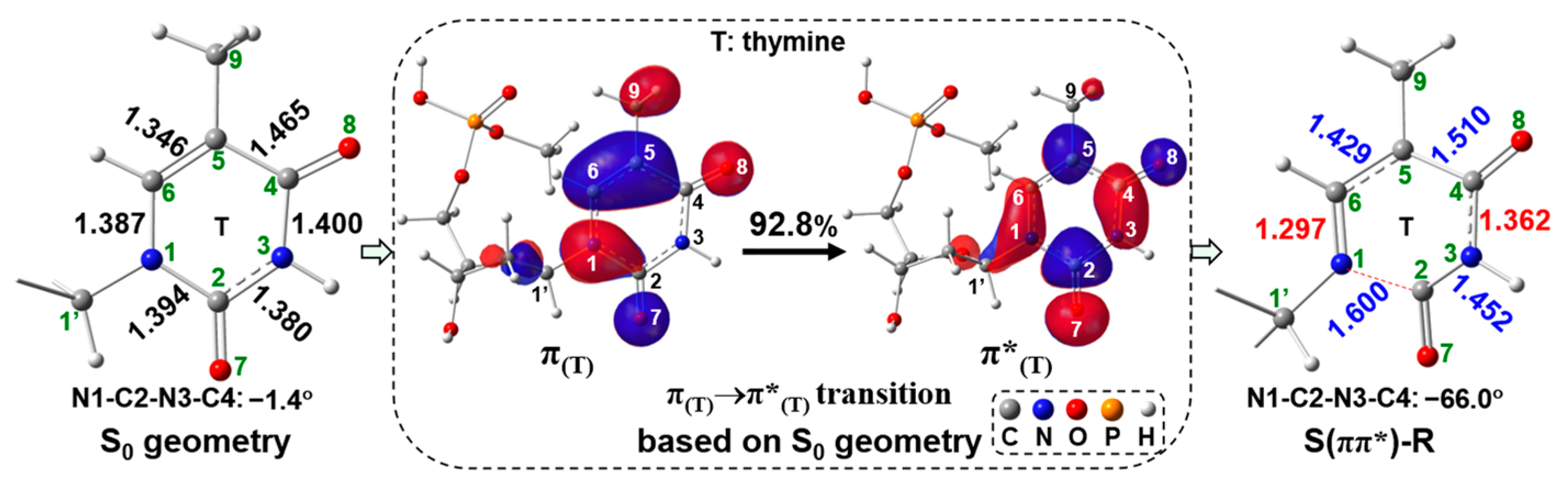
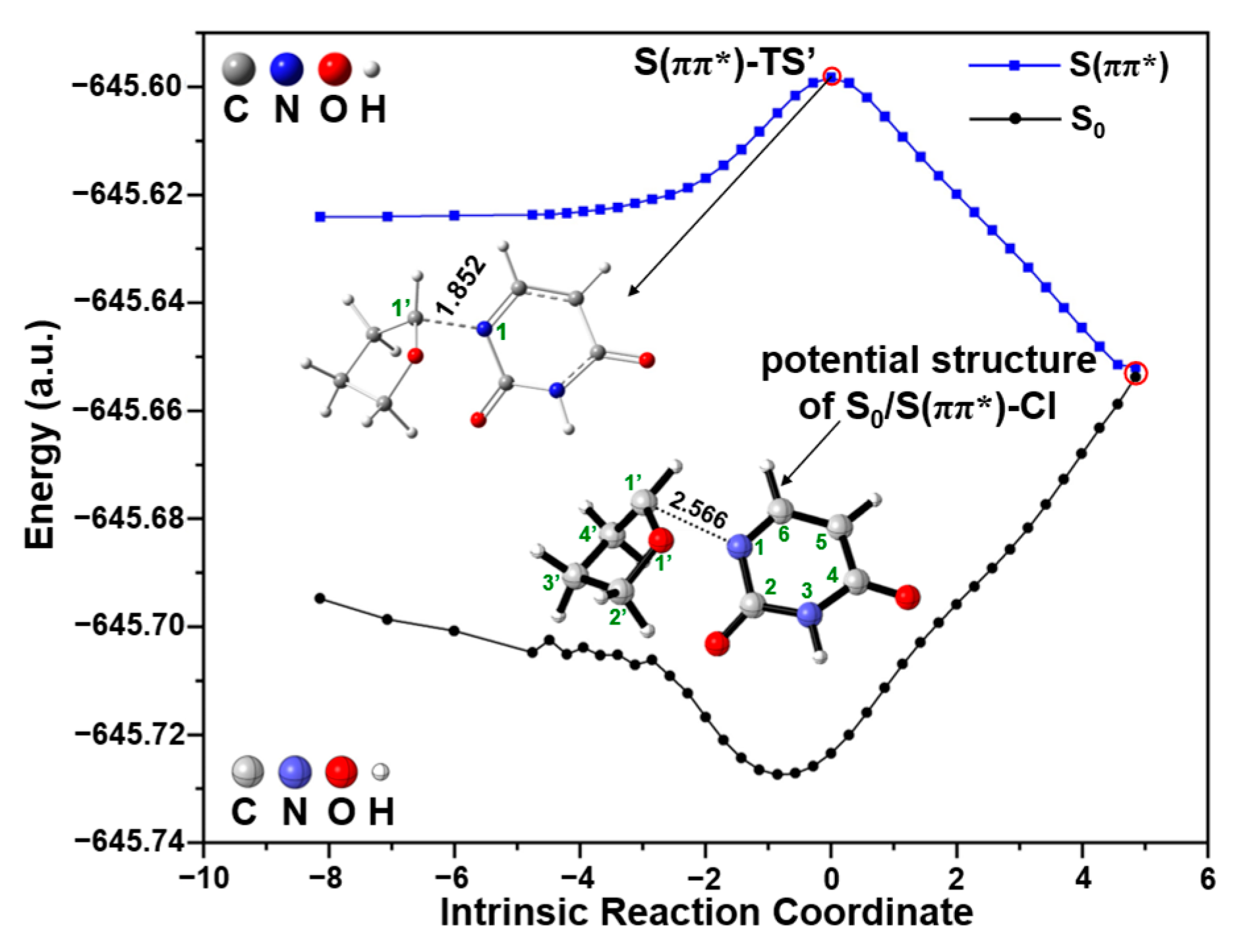
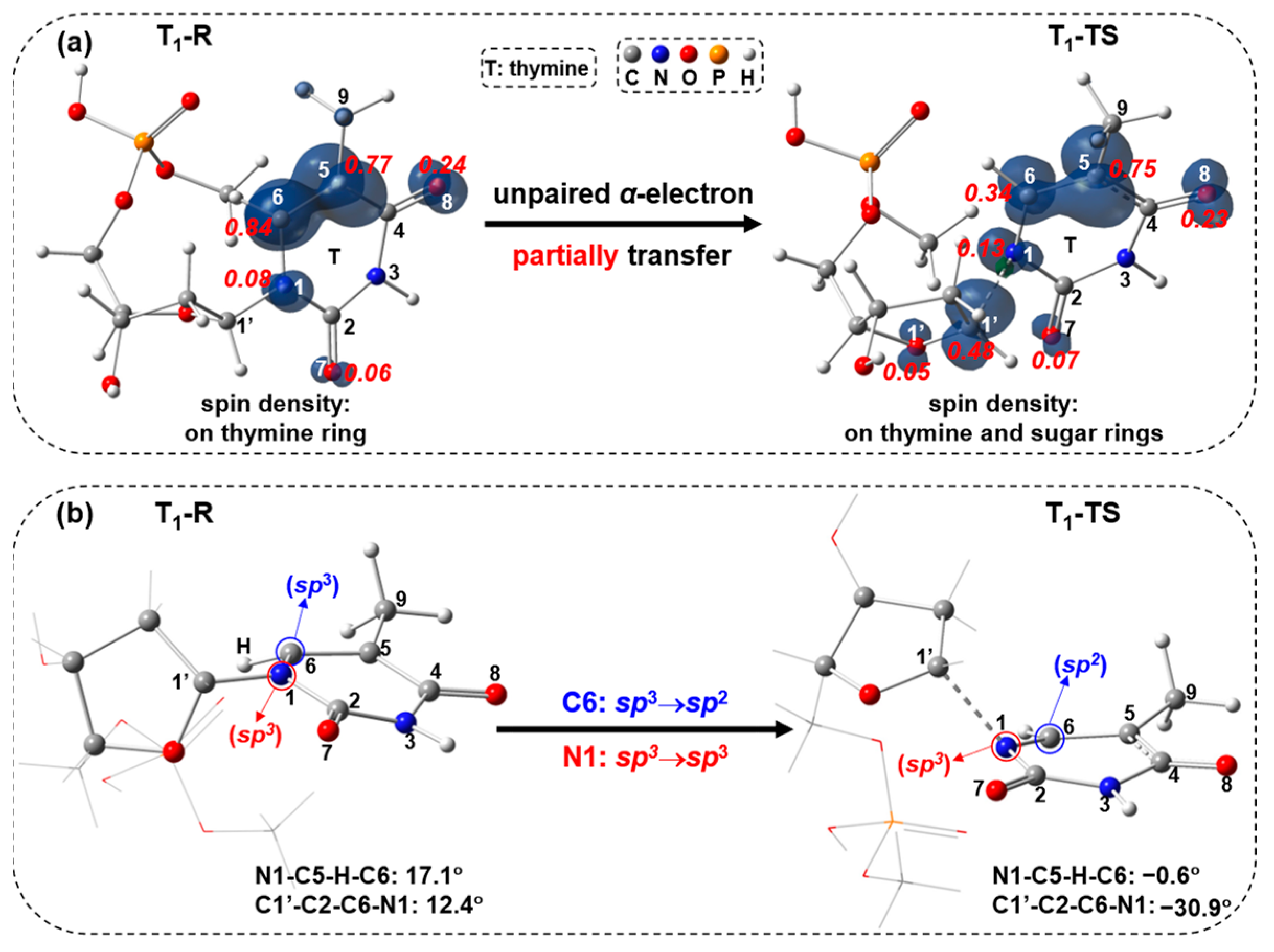
2.2. UV Excitaiton Pathway of C1′-N1 Bond Cleavage
2.3. Overall Mechanism of C1′-N1 Bond Cleavage
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanche, L. Low Energy Electron-Driven Damage in Biomolecules. Eur. Phys. J. D 2005, 35, 367–390. [Google Scholar] [CrossRef]
- Simons, J. How Do Low-Energy (0.1–0.2 eV) Electrons Cause DNA-Strand Breaks? Acc. Chem. Res. 2006, 39, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Becker, D.; Adhikary, A.; Sevilla, M.D. Reaction of Electrons with DNA: Radiation Damage to Radiosensitization. Int. J. Mol. Sci. 2019, 20, 3998. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Denisov, S.A.; Adhikary, A.; Mostafavi, M. Ultrafast Processes Occurring in Radiolysis of Highly Concentrated Solutions of Nucleosides/Tides. Int. J. Mol. Sci. 2019, 20, 4963. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kumar, A.; Muroya, Y.; Yamashita, S.; Sakurai, T.; Denisov, S.A.; Sevilla, M.D.; Adhikary, A.; Seki, S.; Mostafavi, M. Observation of Dissociative Quasi-Free Electron Attachment to Nucleoside via Excited Anion Radical in Solution. Nat. Commun. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, Y.; Sanche, L. Damage Induced to DNA and Its Constituents by 0–3 eV UV Photoelectrons. Photochem. Photobiol. 2022, 98, 546–563. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Huwaidi, A.; Robert, G.; Cloutier, P.; Bass, A.D.; Sanche, L.; Wagner, J.R. Shape Resonances in DNA: Nucleobase Release, Reduction, and Dideoxynucleoside Products Induced by 1.3 to 2.3 eV Electrons. J. Phys. Chem. B 2022, 126, 5175–5184. [Google Scholar] [CrossRef] [PubMed]
- Narayanan SJ, J.; Tripathi, D.; Verma, P.; Adhikary, A.; Dutta, A.K. Secondary Electron Attachment-Induced Radiation Damage to Genetic Materials. ACS Omega 2023, 8, 10669–10689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.B.; Eriksson, L.A. A Triplet Mechanism for the Formation of Cyclobutane Pyrimidine Dimers in UV–Irradiated DNA. J. Phys. Chem. B 2006, 110, 7556–7562. [Google Scholar] [CrossRef]
- Improta, R. Photophysics and Photochemistry of Thymine Deoxy–Dinucleotide in Water: A PCM/TD–DFT Quantum Mechanical Study. J. Phys. Chem. B 2012, 116, 14261–14274. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, K.; Song, D.; Su, H. Physical Quenching in Competition with the Formation of Cyclobutane Pyrimidine Dimers in DNA Photolesion. J. Phys. Chem. A 2014, 118, 9105–9112. [Google Scholar] [CrossRef] [PubMed]
- Pimblott, S.M.; LaVerne, J.A. Production of Low–Energy Electrons by Ionizing Radiation. Radiat. Phys. Chem. 2007, 76, 1244–1247. [Google Scholar] [CrossRef]
- Zhang, Y.; Cloutier, P.; Hunting, D.J.; Wagner, J.R.; Sanche, L. Glycosidic Bond Cleavage of Thymidine by Low-Energy Electrons. J. Am. Chem. Soc. 2004, 126, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Henle, E.S.; Roots, R.; Holley, W.R.; Chatterjee, A. DNA Strand Breakage Is Correlated with Unaltered Base Release after Gamma Irradiation. Radiat. Res. 1995, 143, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Razskazovskiy, Y.; Debije, M.G.; Bernhard, W.A. Direct Radiation Damage to Crystalline DNA: What is the Source of Unaltered Base Release? Radiat. Res. 2000, 153, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Carime, H.; Gohlke, S.; Fischbach, E.; Scheike, J.; Illenberger, E. Thymine Excision from DNA by Subexcitation Electrons. Chem. Phys. Lett. 2004, 387, 267–270. [Google Scholar] [CrossRef]
- Gomes, P.J.; Ferraria, A.M.; Botelho do Rego, A.M.; Hoffmann, S.V.; Ribeiro, P.A.; Raposo, M. Energy Thresholds of DNA Damage Induced by UV Radiation: An XPS Study. J. Phys. Chem. B 2015, 119, 5404–5411. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xie, Y.; Schaefer, H.F., III. Near 0 eV Electrons Attach to Nucleotides. J. Am. Chem. Soc. 2006, 128, 1250–1252. [Google Scholar] [CrossRef]
- Gu, J.; Xie, Y.; Schaefer, H.F., III. Glycosidic Bond Cleavage of Pyrimidine Nucleosides by Low–Energy Electrons: A Theoretical Rationale. J. Am. Chem. Soc. 2005, 127, 1053–1057. [Google Scholar] [CrossRef]
- Meißner, R.; Makurat, S.; Kozak, W.; Limão-Vieira, P.; Rak, J.; Denifl, S. Electron-Induced Dissociation of the Potential Radiosensitizer 5-Selenocyanato-2′-deoxyuridine. J. Phys. Chem. B 2019, 123, 1274–1282. [Google Scholar] [CrossRef]
- Khorsandgolchin, G.; Sanche, L.; Cloutier, P.; Wagner, J.R. Strand Breaks Induced by Very Low Energy Electrons: Product Analysis and Mechanistic Insight into the Reaction with TpT. J. Am. Chem. Soc. 2019, 141, 10315–10323. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, M.; Dang, T.P.; Sobczak, A.J.; Lumpuy, D.A.; Dutta, P.; Ward, S.; Ward, K.; Alahmadi, M.; Kumar, A.; Sevilla, M.D.; et al. Site of Azido Substitution in the Sugar Moiety of Azidopyrimidine Nucleosides Influences the Reactivity of Aminyl Radicals Formed by Dissociative Electron Attachment. J. Phys. Chem. B 2020, 124, 11357–11370. [Google Scholar] [CrossRef] [PubMed]
- Adjei, D.; Reyes, Y.; Kumar, A.; Ward, S.; Denisov, S.A.; Alahmadi, M.; Sevilla, M.D.; Wnuk, S.F.; Mostafavi, M.; Adhikary, A. Pathways of the Dissociative Electron Attachment Observed in 5- and 6-Azidomethyluracil Nucleosides: Nitrogen (N2) Elimination vs Azide Anion (N3–) Elimination. J. Phys. Chem. B 2023, 127, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, Y.; Liu, W.; Gao, T.; Zheng, Y.; Sanche, L. Clustered DNA Damage Induced by 2–20 eV Electrons and Transient Anions: General Mechanism and Correlation to Cell Death. J. Phys. Chem. Lett. 2019, 10, 2985–2990. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Leszczynski, J. Electron Attachment–Induced DNA Single–Strand Breaks at the Pyrimidine Sites. Nucleic Acids Res. 2010, 38, 5280–5290. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Leszczynski, J.; Schaefer, H.F., III. Interactions of Electrons with Bare and Hydrated Biomolecules: From Nucleic Acid Bases to DNA Segments. Chem. Rev. 2012, 112, 5603–5640. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sanche, L.; Sevilla, M.D. Base Release in Nucleosides Induced by Low–Energy Electrons: A DFT Study. Radiat. Res. 2006, 165, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.J.; Ribeiro, P.A.; Shaw, D.; Mason, N.J.; Raposo, M. UV Degradation of Deoxyribonucleic Acid. Polym. Degrad. Stab. 2009, 94, 2134–2141. [Google Scholar] [CrossRef][Green Version]
- Marcum, J.C.; Kaufman, S.H.; Weber, J.M. UV-Photodissociation of Non-Cyclic and Cyclic Mononucleotides. Int. J. Mass Spectrom. 2011, 303, 129–136. [Google Scholar] [CrossRef]
- Gross, E.K.U.; Kohn, W. Time-Dependent Density-Functional Theory. Adv. Quantum Chem. 1990, 21, 255–291. [Google Scholar]
- Chen, H.-Y.; Yang, P.-Y.; Chen, H.-F.; Kao, C.-L.; Liao, L.-W. DFT Reinvestigation of DNA Strand Breaks Induced by Electron Attachment. J. Phys. Chem. B 2014, 118, 11137–11144. [Google Scholar] [CrossRef] [PubMed]
- Improta, R.; Santoro, F.; Blancafort, L. Quantum Mechanical Studies on the Photophysics and the Photochemistry of Nucleic Acids and Nucleobases. Chem. Rev. 2016, 116, 3540–3593. [Google Scholar] [CrossRef]
- Barbatti, M.; Aquino, A.J.A.; Szymczak, J.J.; Nachtigallová, D.; Hobza, P.; Lischka, H. Relaxation Mechanisms of UV-Photoexcited DNA and RNA Nucleobases. Proc. Natl. Acad. Sci. USA 2010, 107, 21453–21458. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Zuquin, J.; Pepino, A.J.; Galván, I.F.; Rivalta, I.; Aquilante, F.; Garavelli, M.; Lindh, R.; Segarra-Martí, J. Characterizing Conical Intersections in DNA/RNA Nucleobases with Multiconfigurational Wave Functions of Varying Active Space Size. J. Chem. Theory Comput. 2023, 19, 8258–8272. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Lee, S.; Huix-Rotllant, M.; Filatov, M.; Choi, C.H. Impact of the Dynamic Electron Correlation on the Unusually Long Excited-State Lifetime of Thymine. J. Phys. Chem. Lett. 2021, 12, 4339–4346. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, J.J.; Barbatti, M.; Hoo, J.T.S.; Adkins, J.A.; Windus, T.L.; Nachtigallová, D.; Lischka, H. Photodynamics Simulations of Thymine: Relaxation into the First Excited Singlet State. J. Phys. Chem. A 2009, 113, 12686–12693. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Tully, J.C.; Granucci, G. Photochemistry of DNA Fragments via Semiclassical Nonadiabatic Dynamics. J. Phys. Chem. B 2010, 114, 12116–12128. [Google Scholar] [CrossRef]
- Turro, N.J. Modern Molecular Photochemistry; University Science Book: Mill Valley, CA, USA, 1991. [Google Scholar]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA Dodecamer: Conformation and Dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Kumar, A.; Sevilla, M.D. The Role of πσ* Excited States in Electron-Induced DNA Strand Break Formation: A Time-Dependent Density Functional Theory Study. J. Am. Chem. Soc. 2008, 130, 2130–2131. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results Obtained with the Correlation Energy Density Functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. The Path of Chemical Reactions—The IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Legault, C.Y. CYLview; Version 1.0b; Université de Sherbrooke: Sherbrooke, QC, Canada, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, 2016. [Google Scholar]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Hegarty, D.; Robb, M.A. Application of Unitary Group Methods to Configuration Interaction Calculations. Mol. Phys. 1979, 38, 1795–1812. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Martin, H.; Lázaro, L.R.; Gunnlaugsson, T.; Scanlan, E.M. Glycosidase Activated Prodrugs for Targeted Cancer Therapy. Chem. Soc. Rev. 2022, 51, 9694–9716. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Orimoto, Y.; Aoki, Y. A Theoretical Exploration of the Photoinduced Breaking Mechanism of the Glycosidic Bond in Thymine Nucleotide. Molecules 2024, 29, 3789. https://doi.org/10.3390/molecules29163789
Huang X, Orimoto Y, Aoki Y. A Theoretical Exploration of the Photoinduced Breaking Mechanism of the Glycosidic Bond in Thymine Nucleotide. Molecules. 2024; 29(16):3789. https://doi.org/10.3390/molecules29163789
Chicago/Turabian StyleHuang, Xiao, Yuuichi Orimoto, and Yuriko Aoki. 2024. "A Theoretical Exploration of the Photoinduced Breaking Mechanism of the Glycosidic Bond in Thymine Nucleotide" Molecules 29, no. 16: 3789. https://doi.org/10.3390/molecules29163789
APA StyleHuang, X., Orimoto, Y., & Aoki, Y. (2024). A Theoretical Exploration of the Photoinduced Breaking Mechanism of the Glycosidic Bond in Thymine Nucleotide. Molecules, 29(16), 3789. https://doi.org/10.3390/molecules29163789





