Improved Anti-Oxidant and Anti-Bacterial Capacities of Skim Milk Fermented by Lactobacillus plantarum
Abstract
1. Introduction
2. Results
2.1. Identification and Quantification of Potential Active Ingredients
2.1.1. Composition Analysis of Protease Hydrolysate (PM) and Fermentation Supernatant (FM)
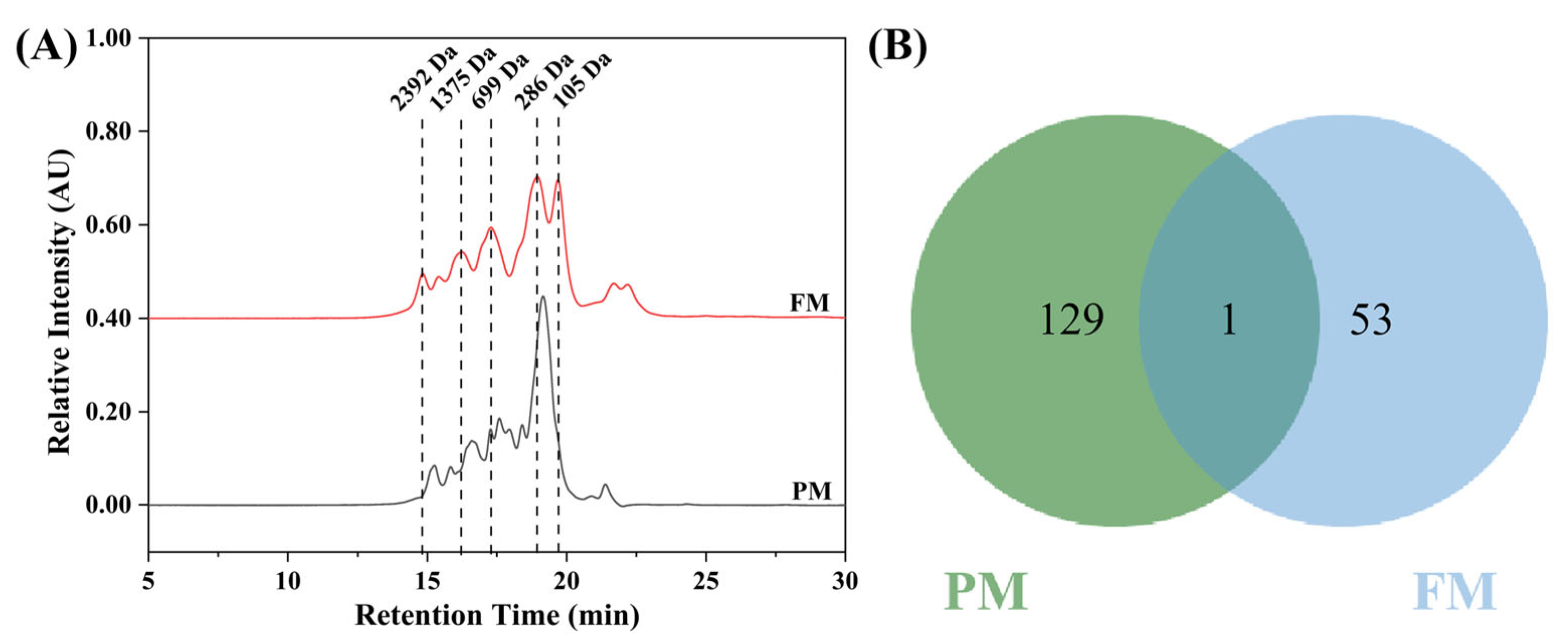
2.1.2. Molecular Weight Distribution and Peptidomics of Peptides
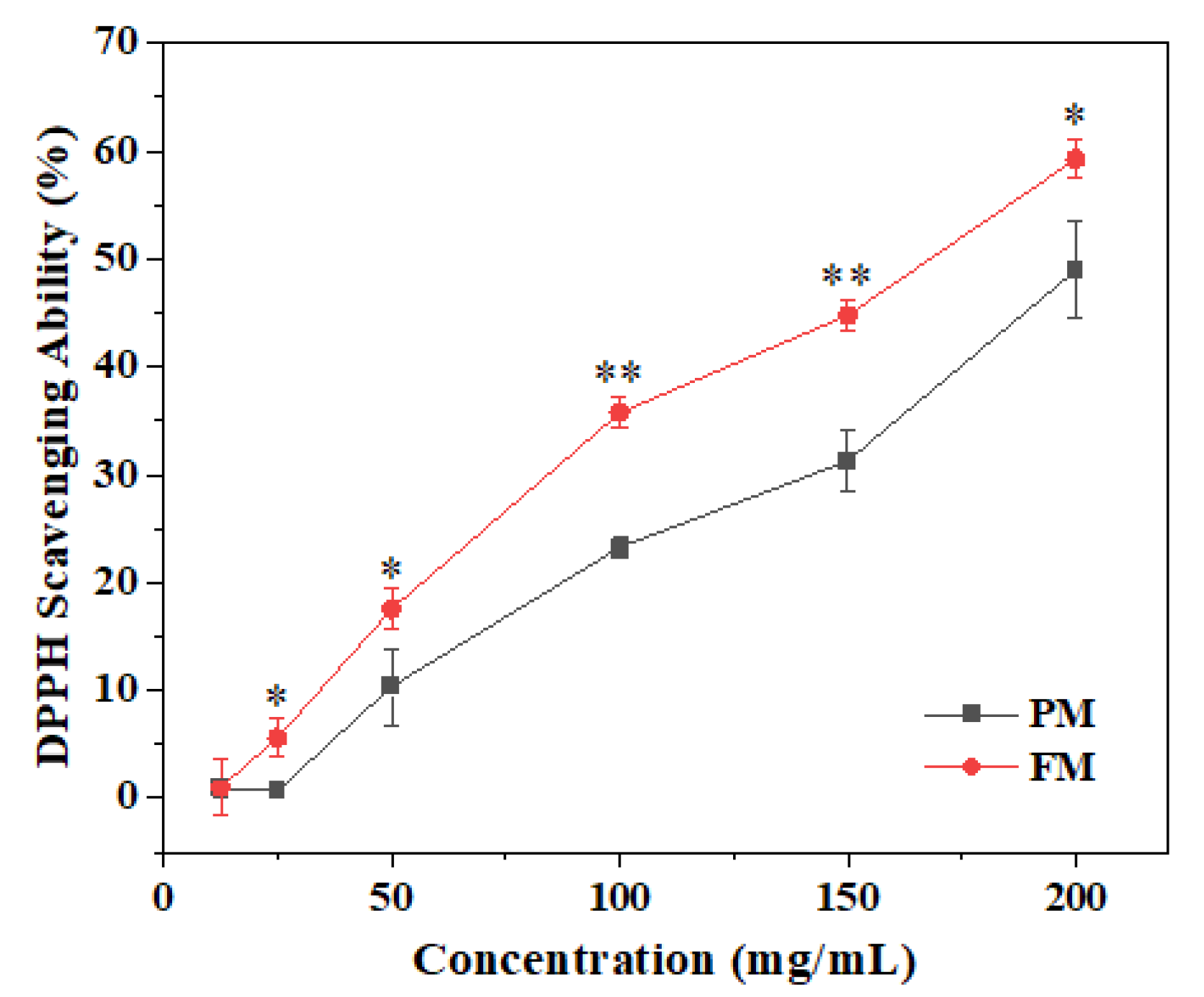
2.2. Anti-Oxidant Activity
2.3. Anti-Bacterial Activity
2.3.1. Minimum Inhibitory Concentration (MIC)
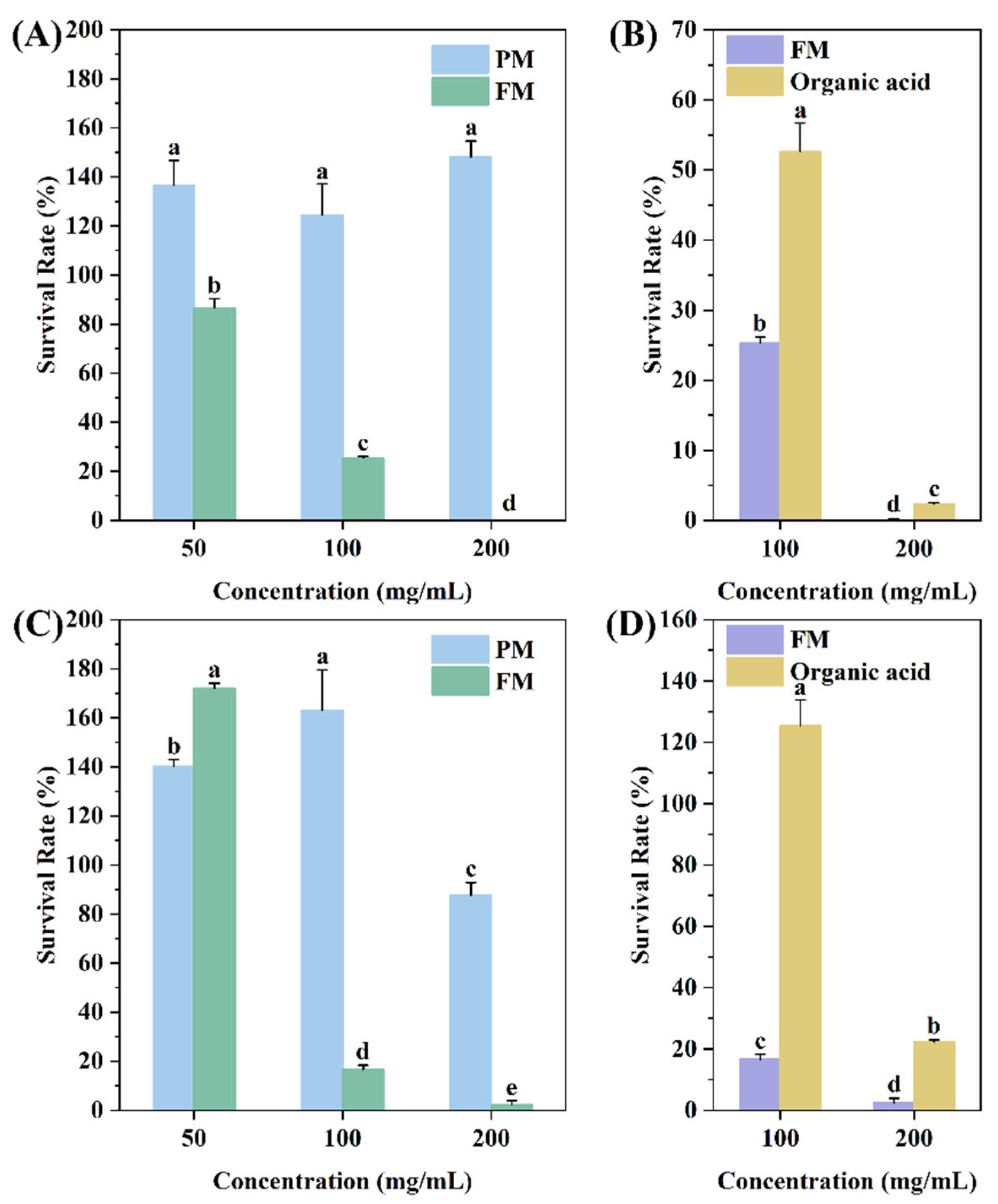
2.3.2. Bacterial Survival Analysis
2.3.3. Cytoplasmic Membrane Depolarization
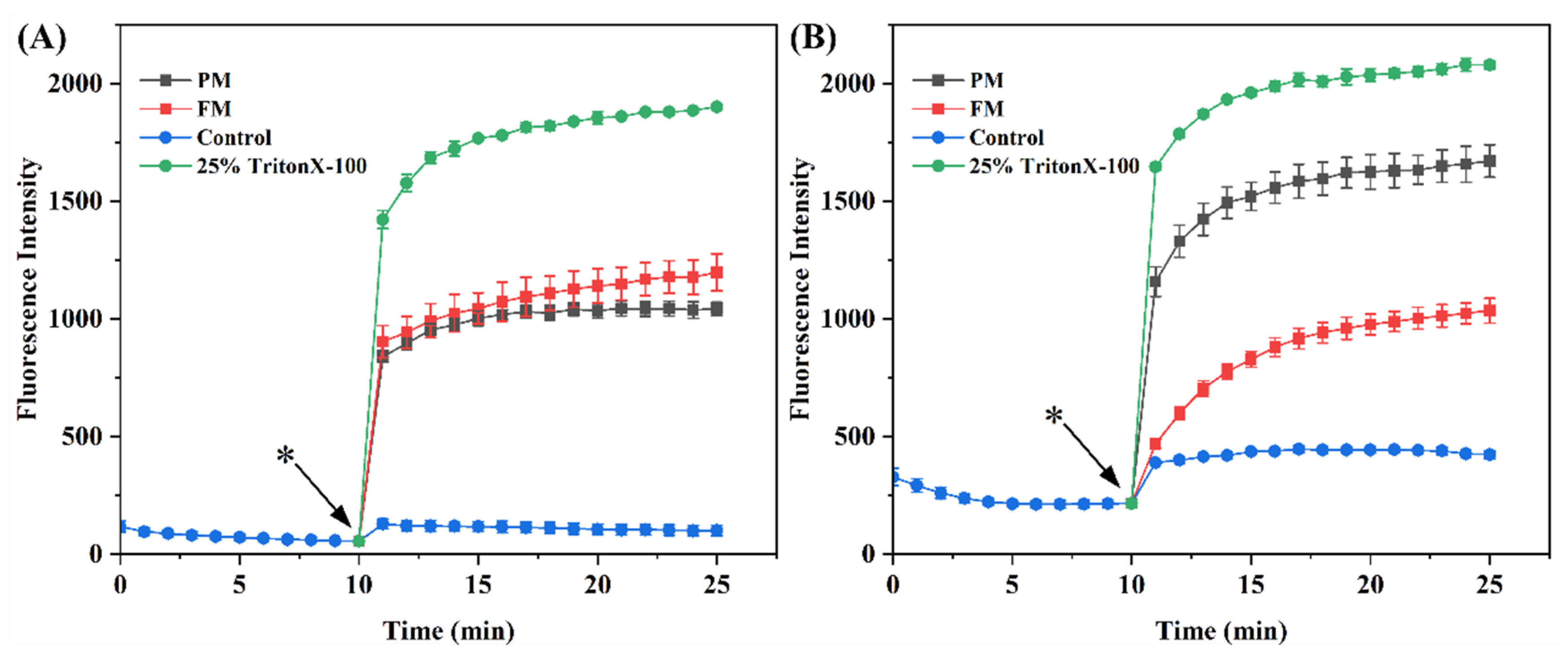
2.3.4. Bacterial Cell Membrane Permeability
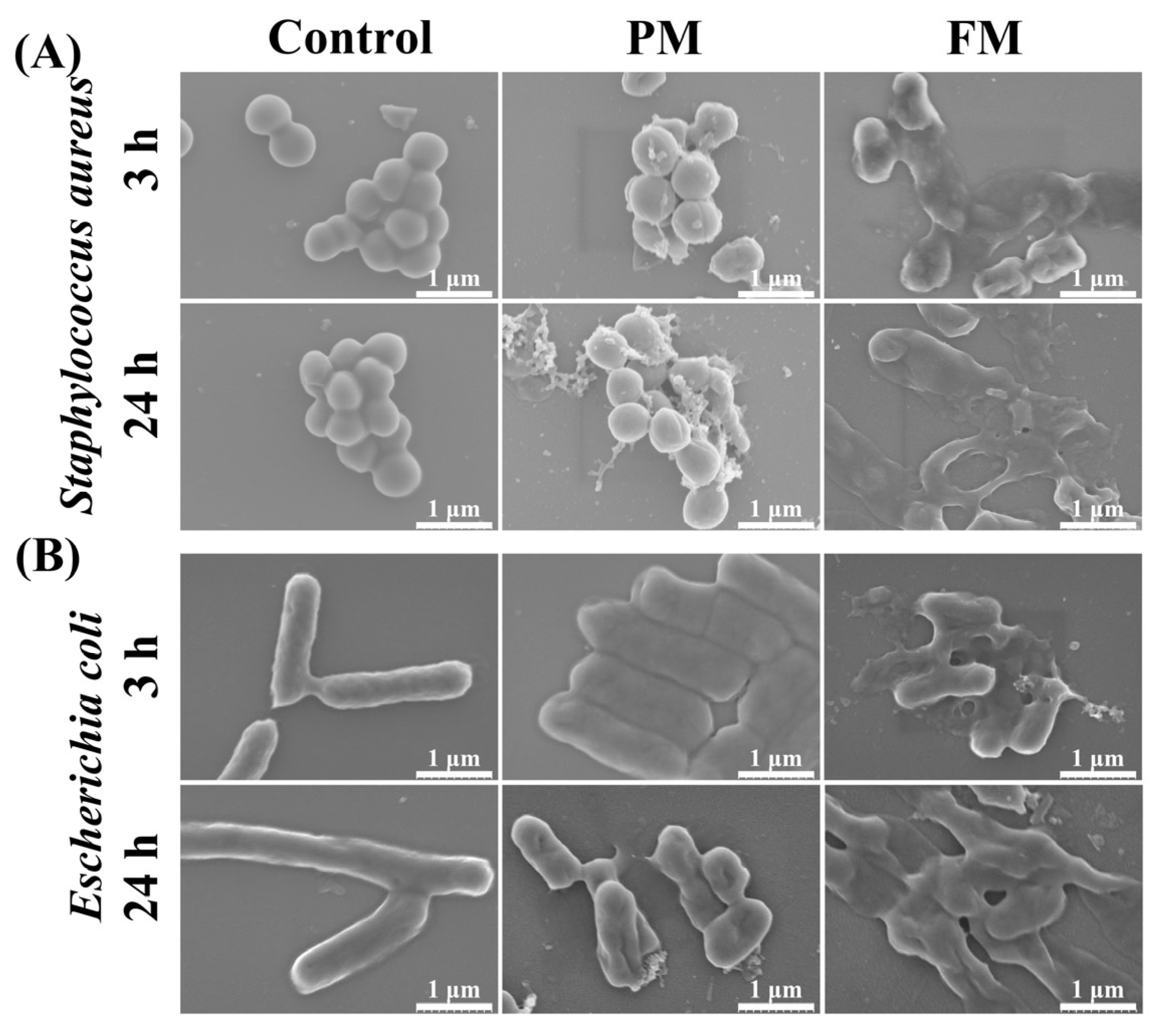
2.3.5. Morphological Observation of Bacteria
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of PM and FM
4.2.1. Preparation of PM
4.2.2. Preparation of FM
4.3. Measurement of Protein, Peptide, and Amino Acid Contents
4.4. Measurement of Organic Acid Content
4.5. Measurement of Polysaccharide Content
4.6. Molecular Weight Distribution of Peptides
4.7. Peptidomics Analysis of PM and FM
4.8. Anti-Oxidant Activity
4.9. Anti-Bacterial Activity
4.9.1. The MIC Values of FM
4.9.2. Bacterial Survival Analysis
4.9.3. Cytoplasmic Membrane Depolarization
4.9.4. Bacterial Cell Membrane Permeability
4.9.5. Observation of Bacteria Morphology by SEM
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, T.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Bioactives in Bovine Milk: Chemistry, Technology, and Applications. Nutr. Rev. 2021, 79, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, W.; Zhang, J.; Bao, X.; Feng, H.; Sun, J.; Liu, X.; Sun, L. Milk Casein Hydrolysate Peptides Regulate Starch Digestion through Inhibition of α-glucosidase: An Insight into the Active Oligopeptide Screening, Enzyme Inhibition Behaviors, and Oligopeptide-enzyme Binding Interactions. Food Hydrocoll. 2024, 152, 109926–109938. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin Research, Technology and Applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Kazimierska, K.; Kalinowska-Lis, U. Milk Proteins—Their Biological Activities and Use in Cosmetics and Dermatology. Molecules 2021, 26, 3253. [Google Scholar] [CrossRef]
- Esmaeilpour, M.; Ehsani, M.R.; Aminlari, M.; Shekarforoush, S.; Hoseini, E. Antimicrobial Activity of Peptides Derived from Enzymatic Hydrolysis of Goat Milk Caseins. Comp. Clin. Pathol. 2016, 25, 599–605. [Google Scholar] [CrossRef]
- Kang, L.; Han, T.; Cong, H.; Yu, B.; Shen, Y. Recent Research Progress of Biologically Active Peptides. Biofactors 2022, 48, 575–596. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, L.; Li, M.; Zhu, Y.; Liang, D.; Ma, Y.; Sun, L.; Zhao, G.; Tu, Q. Probiotic Bacillus as Fermentation Agents: Status, Potential Insights, and Future Perspectives. Food Chem. X 2024, 22, 101465–101479. [Google Scholar] [CrossRef]
- Yang, H.Y.; Han, L.; Lin, Y.Q.; Li, T.; Wei, Y.; Zhao, L.H.; Tong, X.L. Probiotic Fermentation of Herbal Medicine: Progress, Challenges, and Opportunities. Am. J. Chin. Med. 2023, 51, 1105–1126. [Google Scholar] [CrossRef]
- Begunova, A.V.; Savinova, O.S.; Glazunova, O.A.; Moiseenko, K.V.; Rozhkova, I.V.; Fedorova, T.V. Development of Antioxidant and Antihypertensive Properties During Growth of Lactobacillus helveticus, Lactobacillus rhamnosus and Lactobacillus reuteri on Cow’s Milk: Fermentation and Peptidomics Study. Foods 2020, 10, 17. [Google Scholar] [CrossRef]
- Sun, L.; Gong, M.; Lv, X.; Huang, Z.; Gu, Y.; Li, J.; Du, G.; Liu, L. Current Advance in Biological Production of Short-chain Organic Acid. Appl. Microbiol. Biotechnol. 2020, 104, 9109–9124. [Google Scholar] [CrossRef]
- Garnier, L.; Penland, M.; Thierry, A.; Maillard, M.B.; Jardin, J.; Coton, M.; Leyva Salas, M.; Coton, E.; Valence, F.; Mounier, J. Antifungal Activity of Fermented Dairy Ingredients: Identification of Antifungal Compounds. Int. J. Food Microbiol. 2020, 322, 108574–108582. [Google Scholar] [CrossRef] [PubMed]
- Izawa, N.; Sone, T. Cosmetic Ingredients Fermented by Lactic Acid Bacteria. In Microbial Production; Anazawa, H., Shimizu, S., Eds.; Springer Japan: Tokyo, Japan, 2014; pp. 233–242. [Google Scholar]
- Picon, A.; García-Casado, M.A.; Nuñez, M. Proteolytic Activities, Peptide Utilization and Oligopeptide Transport Systems of Wild Lactococcus lactis Strains. Int. Dairy J. 2010, 20, 156–162. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Santiago-López, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Assessment of Multifunctional Activity of Bioactive Peptides Derived from Fermented Milk by Specific Lactobacillus plantarum Strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, H.; Liu, G.; Gong, H.; Li, Y.; Chen, Y.; Yang, Y. Compound Probiotics Producing Cellulase Could Replace Cellulase Preparations During Solid-state Fermentation of Millet Bran. Bioresour. Technol. 2023, 385, 129457–129469. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S.; Schuh, S.; Dutter, T.; Heine, R.G.; Kuslys, M. Design, Quality, Safety and Efficacy of Extensively Hydrolyzed Formula for Management of Cow’s milk Protein Allergy: What are the Challenges? Adv. Food Nutr. Res. 2020, 93, 147–204. [Google Scholar] [PubMed]
- Gooran, N.; Tan, S.W.; Frey, S.L.; Jackman, J.A. Unraveling the Biophysical Mechanisms of How Antiviral Detergents Disrupt Supported Lipid Membranes: Toward Replacing Triton X-100. Langmuir 2024, 40, 6524–6536. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.M.B.; Franquelim, H.G.; Castanho, M.A.R.B.; Veiga, A.S. Molecular Interaction Studies of Peptides Using Steady-state Fluorescence Intensity. Static (De)quenching Revisited. J. Pept. Sci. 2007, 14, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Duan, Y.; Zhang, M.; Liang, S.; Sun, Y.; Cheng, J.; Guo, M. Peptide Profiles and Antioxidant Capacity of Extensive Hydrolysates of Milk Protein Concentrate. J. Dairy Sci. 2022, 105, 7972–7985. [Google Scholar] [CrossRef]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Activity of Milk and Dairy Products. Animals 2022, 12, 245. [Google Scholar] [CrossRef]
- Cirrincione, S.; Luganini, A.; Lamberti, C.; Manfredi, M.; Cavallarin, L.; Giuffrida, M.G.; Pessione, E. Donkey Milk Fermentation by Lactococcus lactis subsp. cremoris and Lactobacillus rhamnosus Affects the Antiviral and Antibacterial Milk Properties. Molecules 2021, 26, 5100. [Google Scholar] [CrossRef]
- Pihurov, M.; Păcularu-Burada, B.; Cotârleț, M.; Bahrim, G.E. Tailoring the Optimized Fermentation Conditions of SCOBY-Based Membranes and Milk Kefir Grains to Promote Various Functional Properties. Foods 2022, 11, 3107. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Bhushan, B.; Vij, S. Antioxidative, ACE Inhibitory and Antibacterial Activities of Soy Milk Fermented by Indigenous Strains of Lactobacilli. Legume Sci. 2020, 2, 1–7. [Google Scholar] [CrossRef]
- Wei, C.; Cui, P.; Liu, X. Antibacterial Activity and Mechanism of Madecassic Acid Against Staphylococcus aureus. Molecules 2023, 28, 1895. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Yan, C.; Shi, C.; Xu, Y.; Ma, Y.; Zhang, C.; Peng, X.; Xia, X. Inhibitory Effect of Coenzyme Q0 on the Growth of Staphylococcus aureus. Foodborne Pathog. Dis. 2019, 16, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, Y.; Ren, W.C.; Su, Y.; Li, J.; Du, Y.Q.; Wang, Q.H.; Kuang, H.X. Rapid Determination and Origin Identification of Total Polysaccharides Contents in Schisandra Chinensis by Near-infrared Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120327–120334. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Kurgan, L.; Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, 45012–45034. [Google Scholar] [CrossRef]
- Adolfsson, K.H.; Huang, P.; Golda-Cepa, M.; Xu, H.; Kotarba, A.; Hakkarainen, M. Scavenging of DPPH by Persistent Free Radicals in Carbonized Particles. Adv. Sustain. Syst. 2023, 7, 2200425–2200432. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. Antibacterial Activity of Acidified Sodium Benzoate against Escherichia coli O157: H7, Salmonella enterica, and Listeria Monocytogenes in Tryptic Soy Broth and on Cherry Tomatoes. Int. J. Food Microbiol. 2018, 274, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, L.; Li, M.; Dong, W.; Luo, X.; Shang, D. Membrane-active and DNA Binding Related Double-action Antimycobacterial Mechanism of Antimicrobial Peptide W3R6 and Its Synthetic Analogs. Biochim. Biophys. Acta (BBA) Gen. Subj. 2023, 1867, 130415–130427. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, W.; Wang, L.; Zhu, C.; Xue, X.; Xia, X.; Wu, X.; Bai, Y.; Hu, J. Antimicrobial Peptide Mastoparan X Has Good Activity Against Escherichia coli in Vitro and Alleviates Its Pathogenicity in Mice. Transl. Med. Commun. 2023, 8, 22–35. [Google Scholar] [CrossRef]
- Boix-Lemonche, G.; Lekka, M.; Skerlavaj, B. A Rapid Fluorescence-Based Microplate Assay to Investigate the Interaction of Membrane Active Antimicrobial Peptides with Whole Gram-Positive Bacteria. Antibiotics 2020, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Senthil, B.; Devasena, T.; Prakash, B.; Rajasekar, A. Non-cytotoxic Effect of Green Synthesized Silver Nanoparticles and Its Antibacterial Activity. J. Photochem. Photobiol. B Biol. 2017, 177, 1–7. [Google Scholar] [CrossRef] [PubMed]

| Sample | Protein (%) | Peptide (%) | Amino Acid (%) | Organic Acid (%) | Polysaccharide (%) |
|---|---|---|---|---|---|
| PM | 3.84 ± 1.04 | 39.94 ± 1.74 | 1.93 ± 0.06 | N.D. | 12.24 ± 0.09 |
| FM | 1.36 ± 0.44 | 30.76 ± 1.95 | 2.12 ± 0.15 | 33.73 ± 2.83 | 11.98 ± 0.09 |
| NO. | Protein Name | Peptide Sequences | Molecular Weight (Da) | Predicted Activity Score | Bioactive | Proportion (%) |
|---|---|---|---|---|---|---|
| 1 | β-casein | SLVYPFPGPIH | 1225.65 | 0.76 | Anti-oxidative | 0.34 |
| 2 | β-casein | LLYQEPVLGPVRGPFPIIV | 2106.22 | 0.72 | Anti-oxidative | 0.04 |
| 3 | α-S2-casein | INPSKENLCSTFCKEVVRN | 2294.11 | 0.69 | Anti-oxidative | 2.97 |
| 4 | α-S2-casein | INPSKENLCSTFCKEVVR | 2180.07 | 0.65 | Anti-oxidative | 0.81 |
| 5 | β-casein | KEMPFPKYPVEPF | 1607.81 | 0.64 | Anti-oxidative; Anti-bacterial | 2.75 |
| 6 | α-S2-casein | INPSKENLCSTFCKE | 1825.83 | 0.64 | Anti-oxidative | 1.40 |
| 7 | α-lactalbumin | HKALCSEKLDQWLCEKL | 2157.07 | 0.64 | Anti-bacterial | 0.21 |
| 8 | α-S2-casein | PSKENLCSTFCKEVVRNA | 2138.03 | 0.61 | Anti-oxidative | 0.07 |
| 9 | β-casein | VYPFPGPIHNSLPQ | 1564.80 | 0.61 | Anti-oxidative | 0.03 |
| 10 | α-S2-casein | SKENLCSTFCKEVVRN | 1969.93 | 0.59 | Anti-oxidative | 1.87 |
| 11 | α-S2-casein | SKENLCSTFCKEVVR | 1855.89 | 0.55 | Anti-oxidative | 0.19 |
| 12 | β-casein | PKHKEMPFPKYPVEPF | 1970.01 | 0.54 | Anti-oxidative; Anti-bacterial | 1.60 |
| 13 | β-casein | PKHKEMPFPKYP | 1497.78 | 0.53 | Anti-bacterial | 5.94 |
| 14 | β-casein | LPVPQKAVPYPQRDMP | 1834.98 | 0.53 | Anti-oxidative | 0.45 |
| 15 | β-casein | KHKEMPFPKYPVEPF | 1872.96 | 0.51 | Anti-oxidative; Anti-bacterial | 41.99 |
| 16 | α-S2-casein | SKENLCSTFCKEVVRNA | 2040.97 | 0.51 | Anti-oxidative | 0.29 |
| Type | Strains | MIC (mg/mL) |
|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus | 200 |
| Gram-negative bacteria | Escherichia coli | 150 |
| Samples | PM | FM | Control | 25% TritonX-100 |
|---|---|---|---|---|
| Fluorescence intensity | 1039.28 ± 99.08 | 395.38 ± 24.09 | 166.61 ± 7.80 | 585.37 ± 34.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, B.; Ding, Y.; Liu, N.; Yang, C.; Sun, Y. Improved Anti-Oxidant and Anti-Bacterial Capacities of Skim Milk Fermented by Lactobacillus plantarum. Molecules 2024, 29, 3800. https://doi.org/10.3390/molecules29163800
Wang Y, Zhao B, Ding Y, Liu N, Yang C, Sun Y. Improved Anti-Oxidant and Anti-Bacterial Capacities of Skim Milk Fermented by Lactobacillus plantarum. Molecules. 2024; 29(16):3800. https://doi.org/10.3390/molecules29163800
Chicago/Turabian StyleWang, Ying, Bingtian Zhao, Yun Ding, Nan Liu, Cheng Yang, and Yajuan Sun. 2024. "Improved Anti-Oxidant and Anti-Bacterial Capacities of Skim Milk Fermented by Lactobacillus plantarum" Molecules 29, no. 16: 3800. https://doi.org/10.3390/molecules29163800
APA StyleWang, Y., Zhao, B., Ding, Y., Liu, N., Yang, C., & Sun, Y. (2024). Improved Anti-Oxidant and Anti-Bacterial Capacities of Skim Milk Fermented by Lactobacillus plantarum. Molecules, 29(16), 3800. https://doi.org/10.3390/molecules29163800






