Study on Calcination Characteristics of Diaspore-Kaolin Bauxite Based on Machine Vision
Abstract
:1. Introduction
2. Results and Discussion
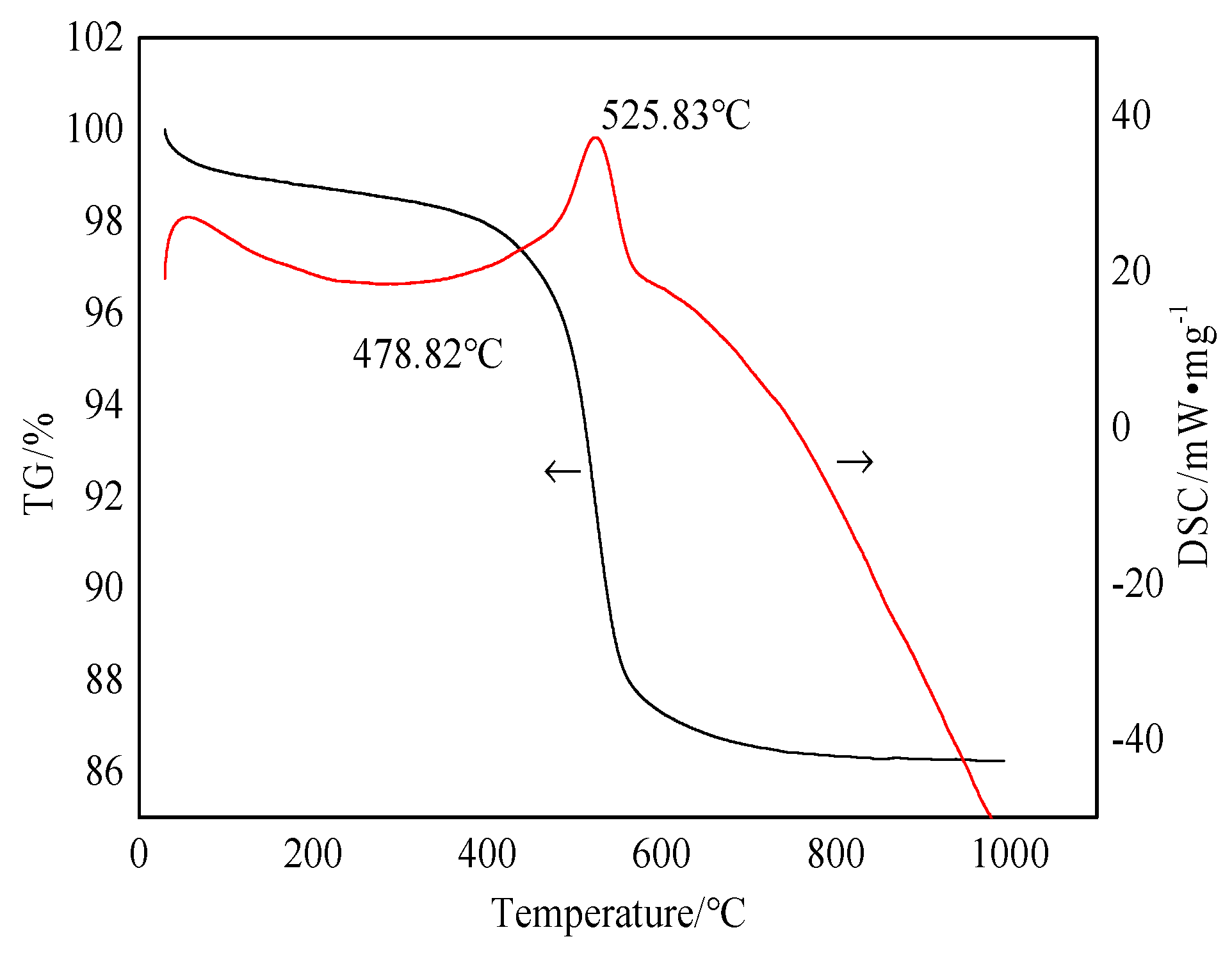
2.1. Analysis of the Properties of Experimental Materials
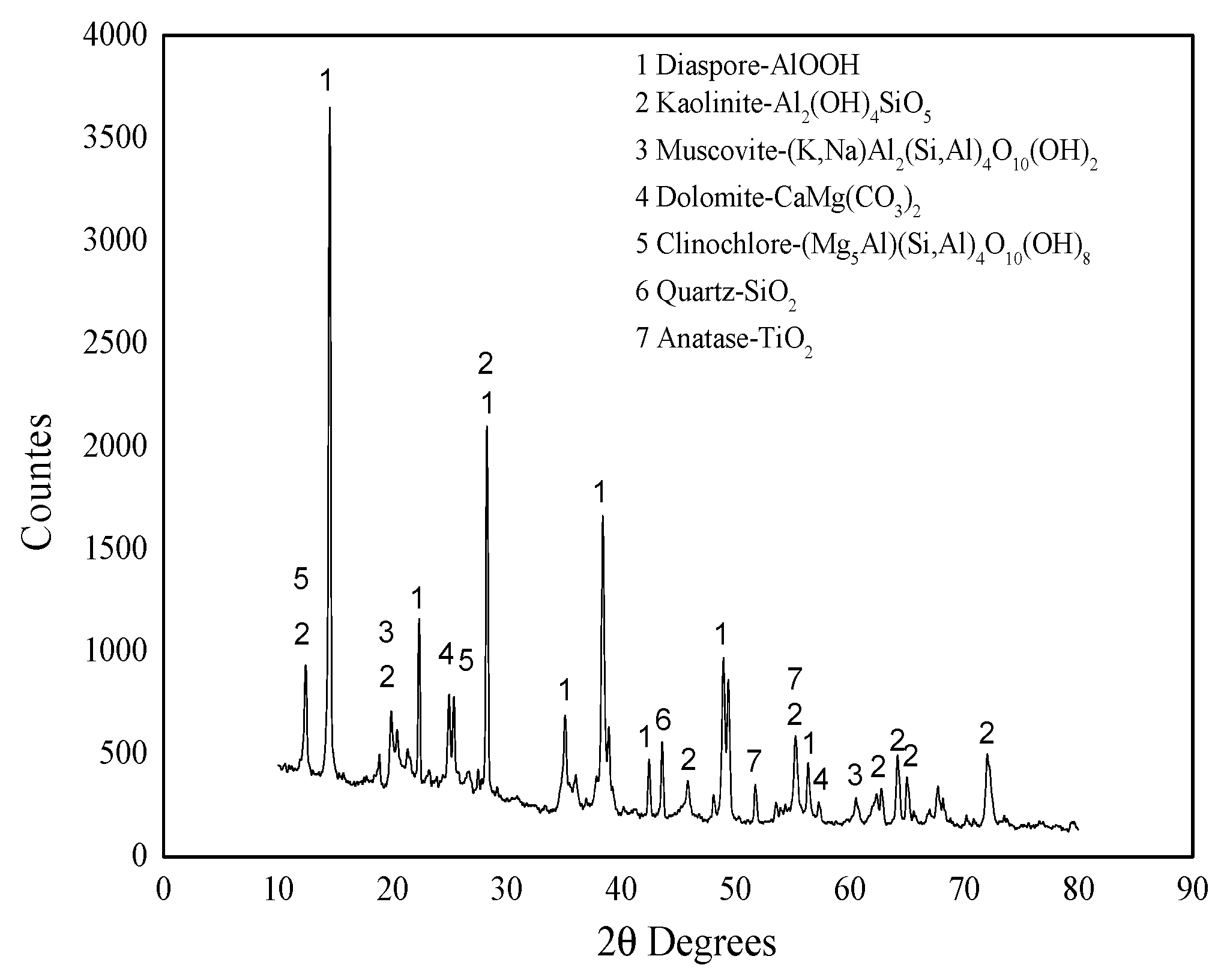
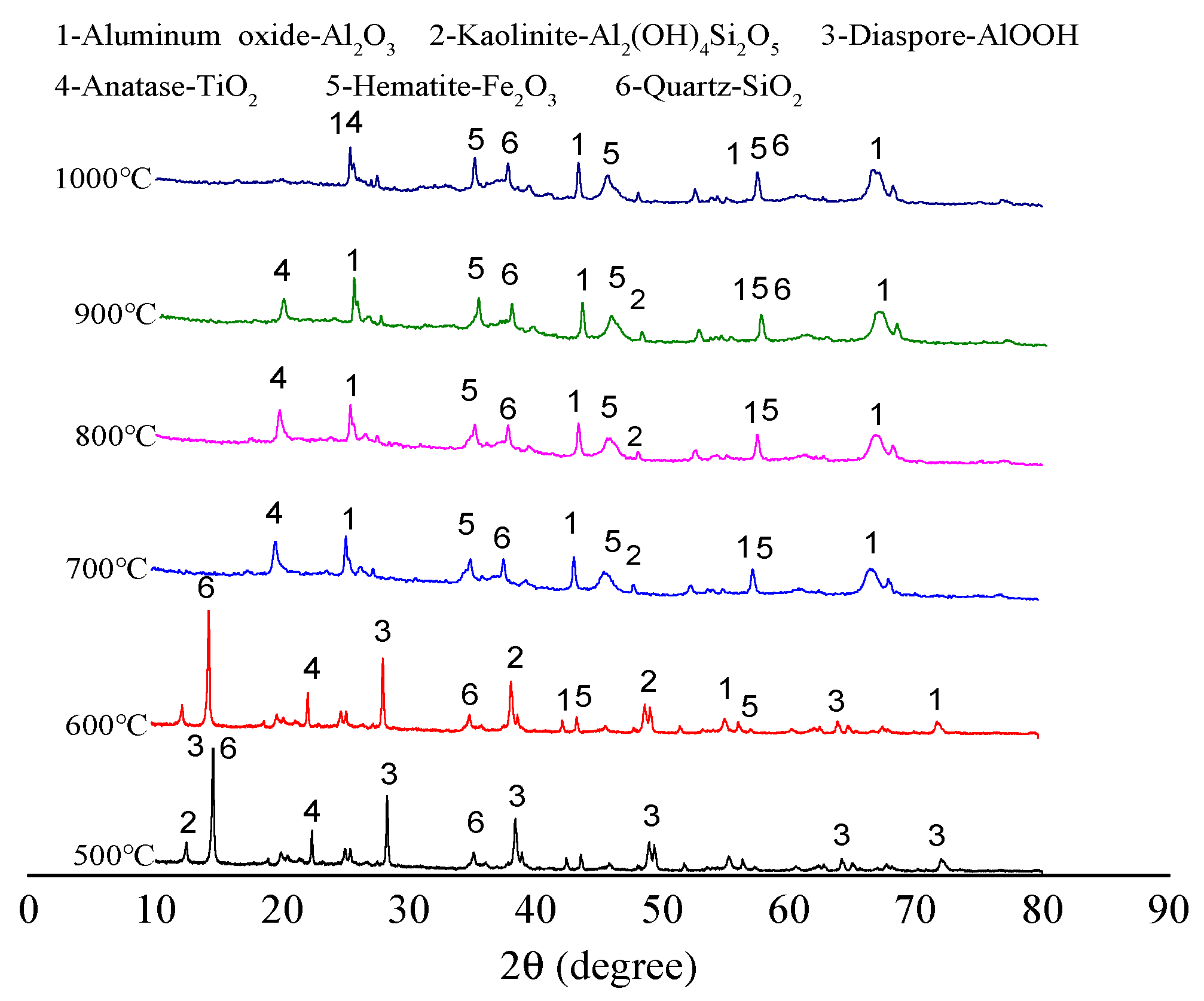
2.2. XRD Pattern Analysis
2.3. Contrastive Analysis of X-ray Fluorescence Spectra
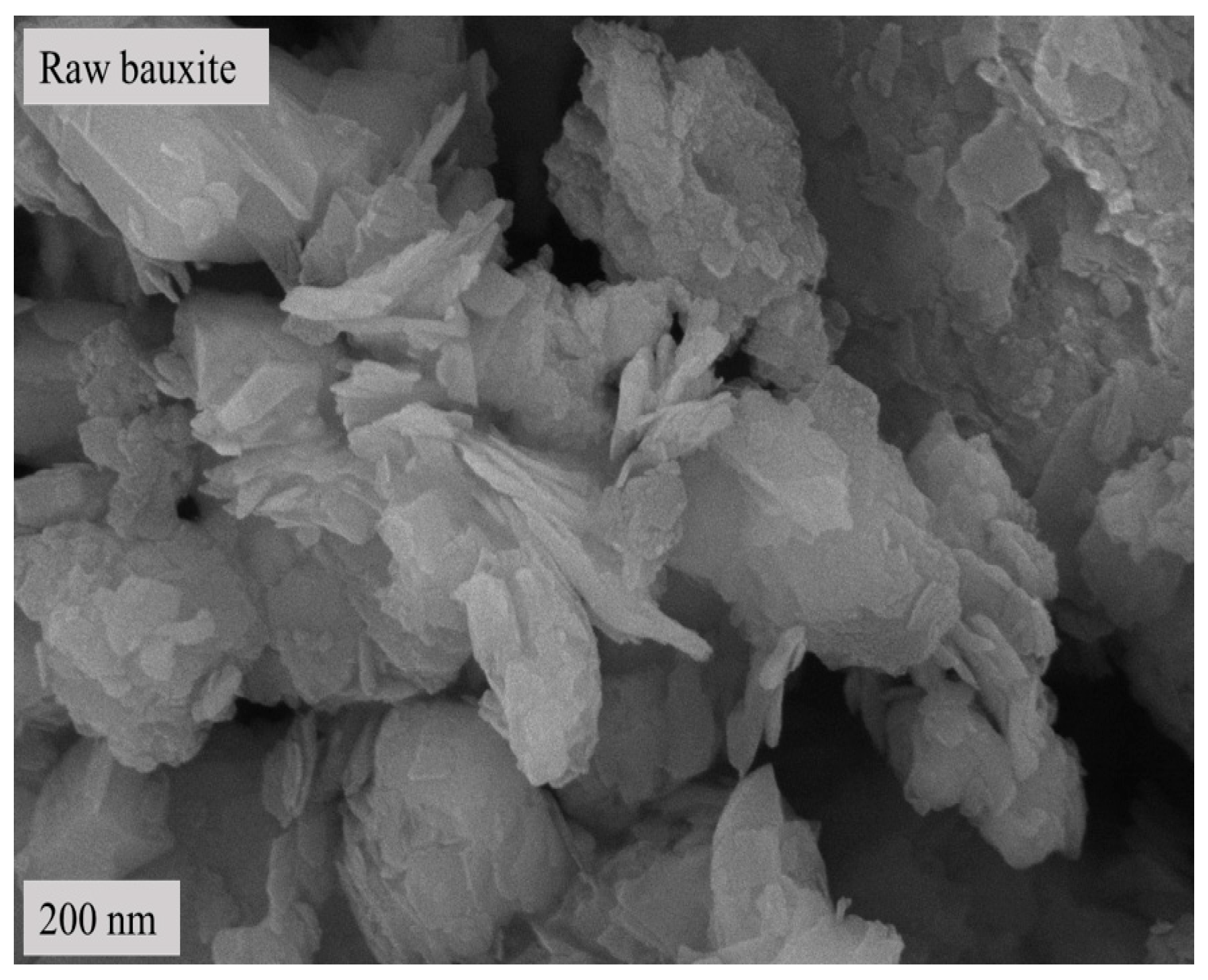
2.4. Contrastive Analysis by Scanning Electron Microscopy
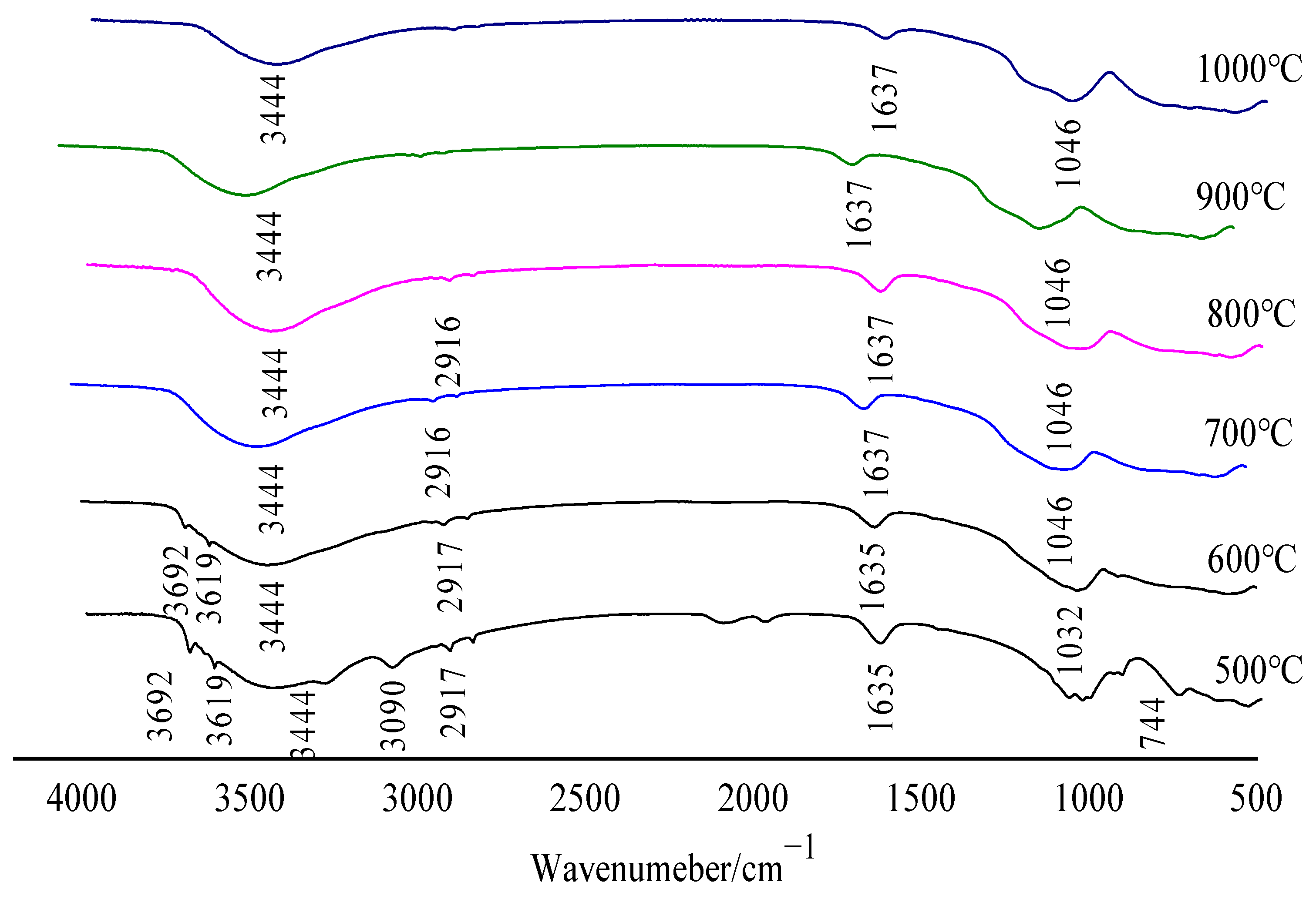
2.5. Contrastive Analysis of Fourier Infrared Spectroscopy
2.6. Comparative Analysis of Organic Carbon Content
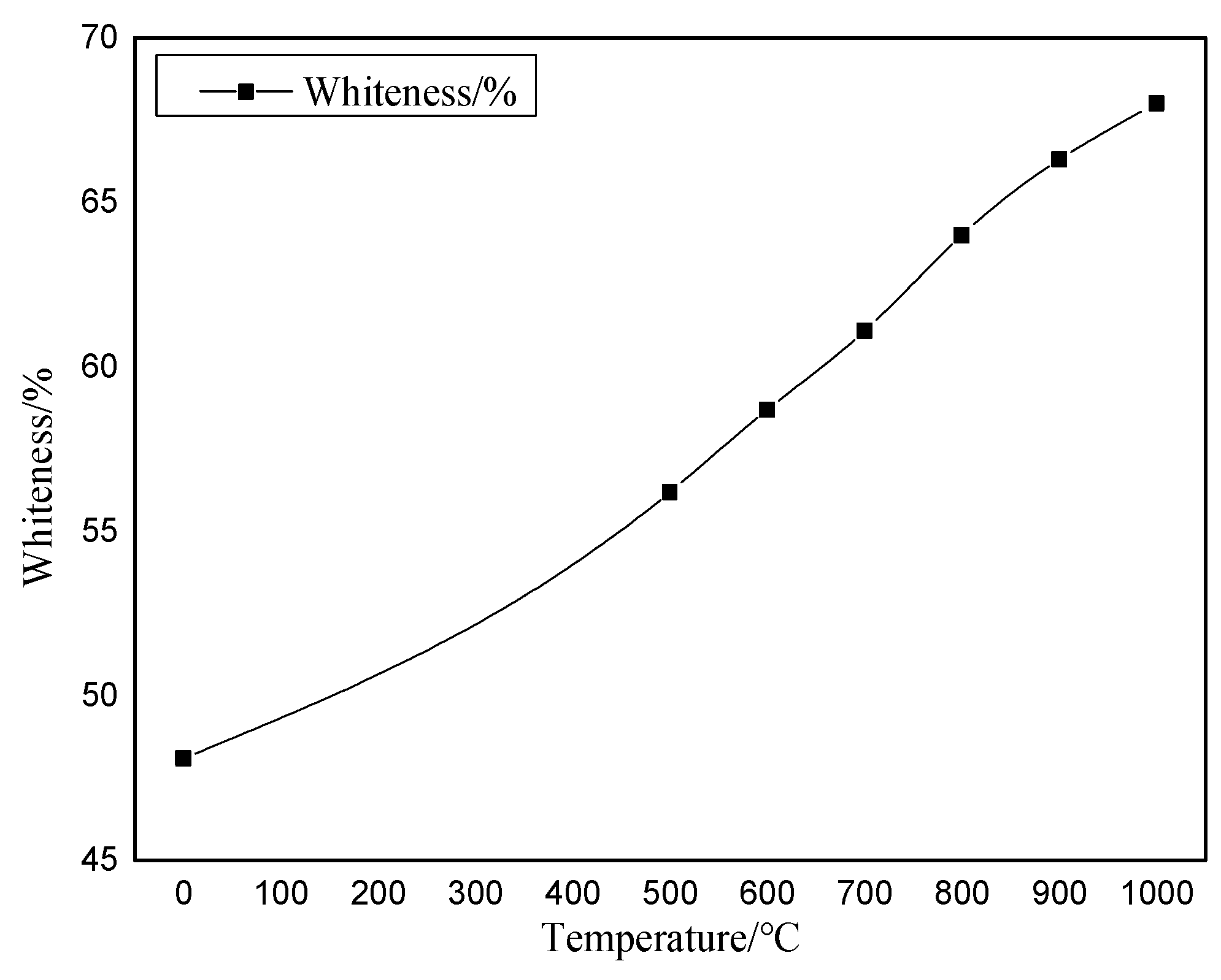
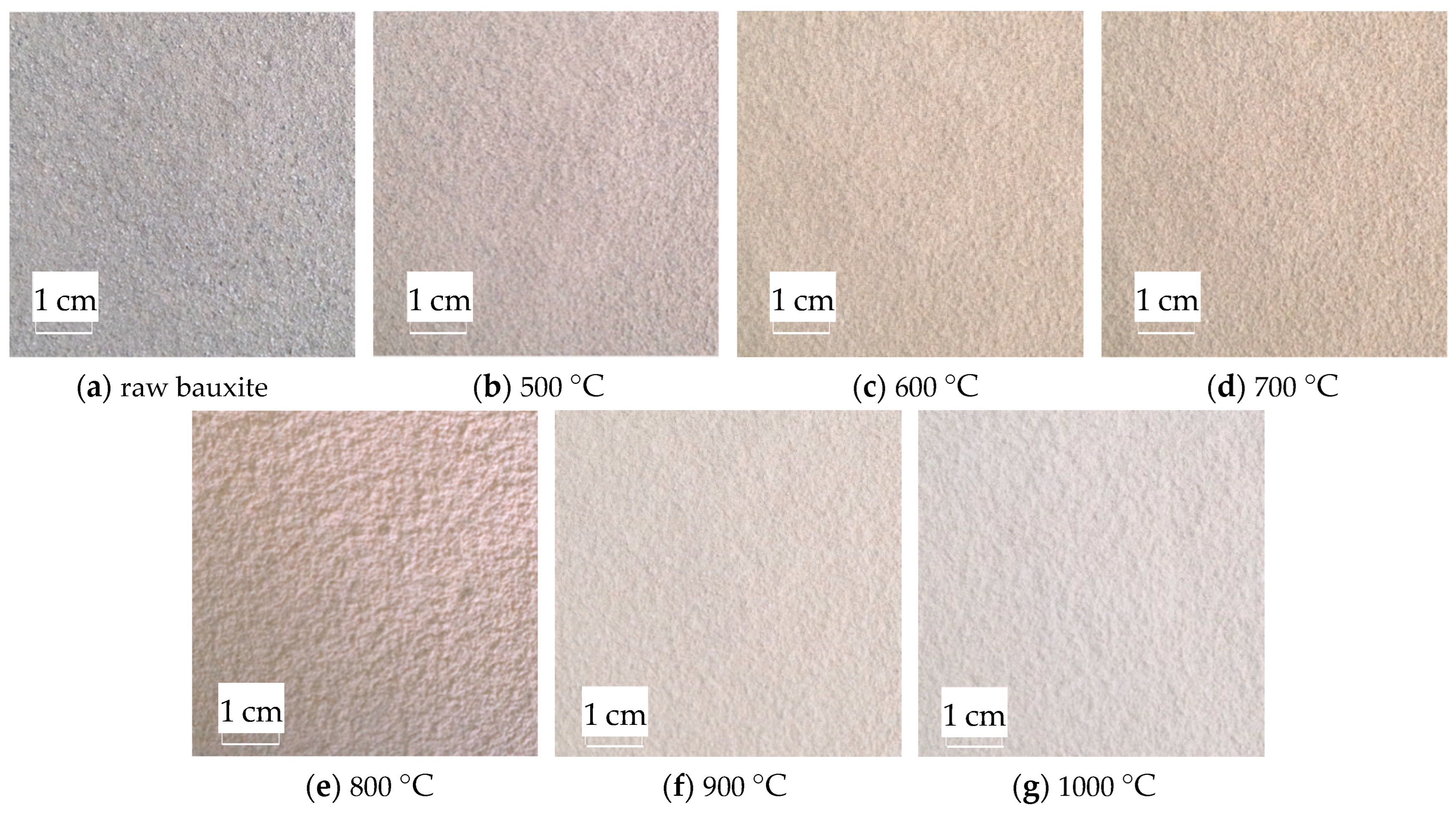
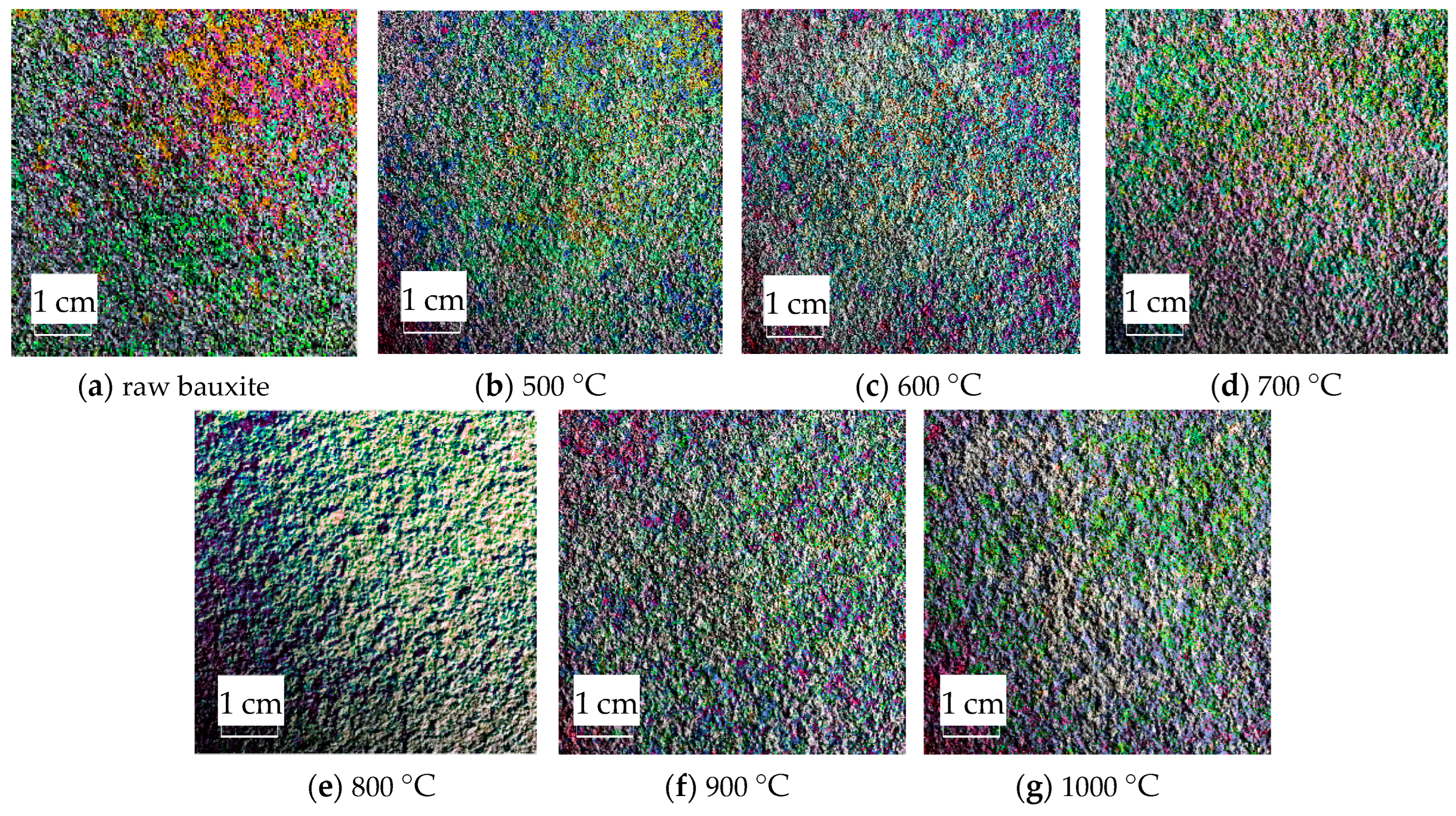
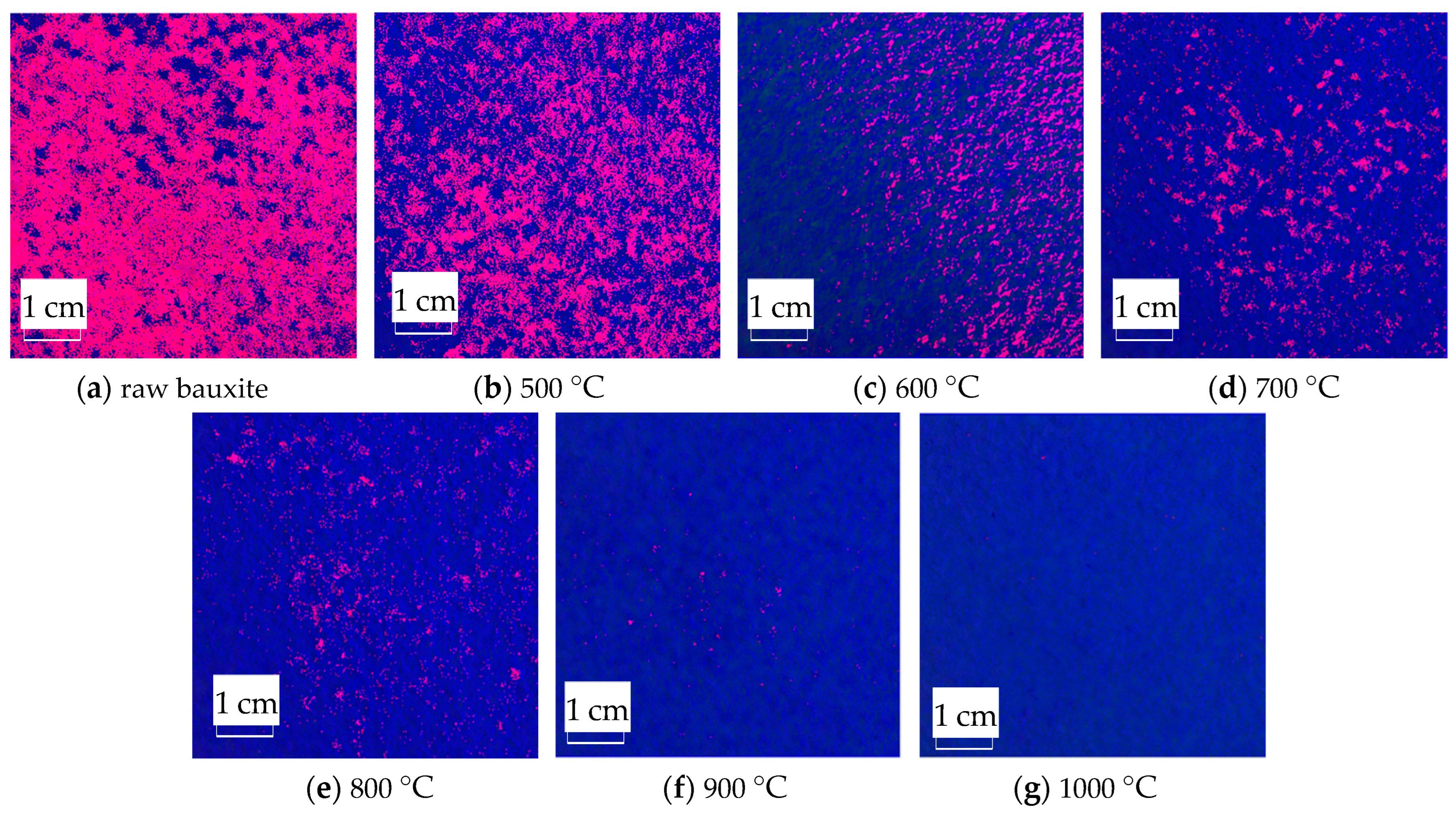
2.7. Image Color Feature Extraction
3. Sampling and Methods
3.1. Sample Preparation
3.2. Test Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, L.; Zhang, S.; Zhang, S.H.; Liu, J.N.; Xiao, K.Y. Geologic characteristics and potential of bauxite in China. Ore Geol. Rev. 2019, 23, 29–33. [Google Scholar] [CrossRef]
- Han, Y.X.; Liu, X.; He, F.Y.; Gao, P.; Ma, S.J. Current situation of bauxite resource and its beneficiation technology in China. Prot. Util. Miner. Resour. 2019, 39, 151–158. [Google Scholar]
- Wang, X.M.; Jiao, Y.Q.; Du, Y.H.; Ling, W.L.; Wu, L.Q.; Cui, T.; Zhou, Q.; Jin, Z.G.; Lei, Z.Y.; Weng, S.F. REE mobility and Ce anomaly in bauxite deposit of WZD area. Northern Guizhou, China. J. Geochem. Explor. 2013, 133, 56–64. [Google Scholar] [CrossRef]
- Ren, Y.H.; Ren, Q.; Wu, X.L.; Zheng, J.L.; Ou, H. Mechanism of low temperature sintered high-strength ferric-rich ceramics using bauxite tailings. Mater. Chem. Phys. 2019, 238, 23–29. [Google Scholar] [CrossRef]
- Palchikova, I.G.; Smirnov, E.S.; Palchikov, E.I. Quantization noise as a determinant for color thresholds in machine vision. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2018, 35, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, X.; Zhang, L.; Jia, L.; Jiang, J.; Wang, L. A mineral feature extraction method based on virtual band simulation. Remote Sens. Lett. 2017, 8, 547–556. [Google Scholar] [CrossRef]
- Bai, X.Z. Enhancing microscopy images of minerals through morphological center operator-based feature extraction. Microsc. Res. Tech. 2013, 76, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, J.P.; Gui, W.H. Flotation froth state recognition based on the statistical distribution of image spatial structure. CIESC J. 2014, 64, 4296–4303. [Google Scholar]
- Lv, S.; Feng, Y.B.; Zhang, S.L.; Wu, X.J.; Wang, Y.H. Extraction and analysis of mineral phase features based on image recognition. China Plant Eng. 2018, 31, 132–134. [Google Scholar]
- Yuan, H.; Ding, Y. Grey correlation method to determine the meso-level evaluation index of the mineral aggregate gradation of asphalt mixture. Shanxi Archit. 2017, 43, 94–96. [Google Scholar]
- Su, B.X. Quantitative analysis of sinter mineral composition based on comprehensive image processing technology. Min. Metall. 2015, 24, 64–70+74. [Google Scholar]
- Zhou, H.W. Research on real-time detection system of FeO content in sinter based on image processing and fuzzy C-means clustering. Anhui Univ. 2012, 19, 31–39. [Google Scholar]
- Chen, Z.M.; Wang, F.Z.; Zhang, P.; Ke, C.D.; Zhu, Y.; Cao, W.X.; Jiang, H.D. Skewed distribution of leaf color RGB model and application of skewed parameters in leaf color description model. Plant Methods 2020, 16, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.M. Availability of Bauxite Reserves. Nat. Resour. Res. 2004, 13, 64–73. [Google Scholar] [CrossRef]
- Palmer, S.J.; Frost, R.L. Characterisation of bauxite and seawater neutralised bauxite residue using XRD and vibrational spectroscopic techniques. J. Mater. Sci. 2009, 44, 64–72. [Google Scholar] [CrossRef]
- Guatame-Garcia, A.; Buxton, M.; Deon, F.; Lievens, C.; Hecker, C. Toward an on-line characterization of kaolin calcination process using short-wave infrared spectroscopy. Miner. Process. Extr. Metall. Rev. 2018, 39, 26–33. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, G.; Gómez-Gil, J.; Navas-Gracia, L.M. Testing different color spaces based on hue for the environmentally adaptive segmentation algorithm (EASA). Comput. Electron. Agric. 2009, 68, 131–137. [Google Scholar] [CrossRef]
- Hong, F.W.; Jun, Q.L.; Chao, Y.C.; Fei, L.X.; Zhen, S.X. Suspension Calcination and Alkali Leaching of Low-grade High-sulfur Bauxite: Desulfurization, Mineralogical Evolution and Desilication. Min. Miner. 2020, 65, 141–149. [Google Scholar]
- Mendelovici, E. Acid and Thermal Treatments of Lateritic Bauxites. J. Therm. Anal. Calorim. 2004, 75, 121–125. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, W.; Zhang, Y.Y. Crystallization Properties Research of High Alumina Bauxite at Low Calcination Temperature. Met. Mine 2019, 59, 100–104. [Google Scholar]
- Gómez-Polo, C.; Casado, A.M.; Gómez-Polo, M.; Montero, J. Colour thresholds of the gingival chromatic space. J. Dent. 2020, 103, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Sun, H.; Li, M.Z.; Michihisa, L. Reports Outline Remote Sensing Study Findings from China Agricultural University (Application of Color Featuring and Deep Learning in Maize Plant Detection). J. Technol. 2020, 68, 175–181. [Google Scholar]
- Hong, Z.; Wei, G.W.; Tao, W.; Zhao, B.C.; Xiang, Y.Z. Key-Frame Extraction Based on HSV Histogram and Adaptive Clustering. J. Math. 2019, 85, 115–123. [Google Scholar]
- Guan, S. Fabric defect delaminating detection based on visual saliency in HSV color space. J. Text. Inst. 2018, 109, 221–227. [Google Scholar] [CrossRef]




| Component | Al2O3 | SiO2 | TiO2 | K2O | Fe2O3 | MgO | SO3 | CaO | P2O5 | ZrO2 | Else | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 60.39 | 19.17 | 2.84 | 1.03 | 1.08 | 0.77 | 0.14 | 0.16 | 0.10 | 0.05 | 0.51 | 13.76 |
| Content/% | Al2O3 | SiO2 | TiO2 | K2O | Fe2O3 | MgO | CaO | P2O5 | ZrO2 | Else | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | |||||||||||
| 500 °C | 62.18 | 20.36 | 3.16 | 1.41 | 1.18 | 0.87 | 0.19 | 0.11 | 0.07 | 6.47 | |
| 600 °C | 63.08 | 20.67 | 3.25 | 1.43 | 1.28 | 0.88 | 0.20 | 0.12 | 0.08 | 5.01 | |
| 700 °C | 66.59 | 21.04 | 3.47 | 1.46 | 1.29 | 0.92 | 0.22 | 0.13 | 0.09 | 2.79 | |
| 800 °C | 67.99 | 22.62 | 3.55 | 1.49 | 1.31 | 0.93 | 0.23 | 0.15 | 0.09 | 1.64 | |
| 900 °C | 68.51 | 22.75 | 3.58 | 1.51 | 1.34 | 0.94 | 0.24 | 0.17 | 0.09 | 0.87 | |
| 1000 °C | 68.53 | 22.83 | 3.68 | 1.52 | 1.35 | 0.95 | 0.25 | 0.18 | 0.1 | 0.61 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, J. Study on Calcination Characteristics of Diaspore-Kaolin Bauxite Based on Machine Vision. Molecules 2024, 29, 3813. https://doi.org/10.3390/molecules29163813
Li L, Liu J. Study on Calcination Characteristics of Diaspore-Kaolin Bauxite Based on Machine Vision. Molecules. 2024; 29(16):3813. https://doi.org/10.3390/molecules29163813
Chicago/Turabian StyleLi, Longjiang, and Jun Liu. 2024. "Study on Calcination Characteristics of Diaspore-Kaolin Bauxite Based on Machine Vision" Molecules 29, no. 16: 3813. https://doi.org/10.3390/molecules29163813
APA StyleLi, L., & Liu, J. (2024). Study on Calcination Characteristics of Diaspore-Kaolin Bauxite Based on Machine Vision. Molecules, 29(16), 3813. https://doi.org/10.3390/molecules29163813






