Synthesis of High-Purity Hydroxyapatite and Phosphoric Acid Derived from Moroccan Natural Phosphate Rocks by Minimizing Cation Content Using Dissolution–Precipitation Technique
Abstract
1. Introduction
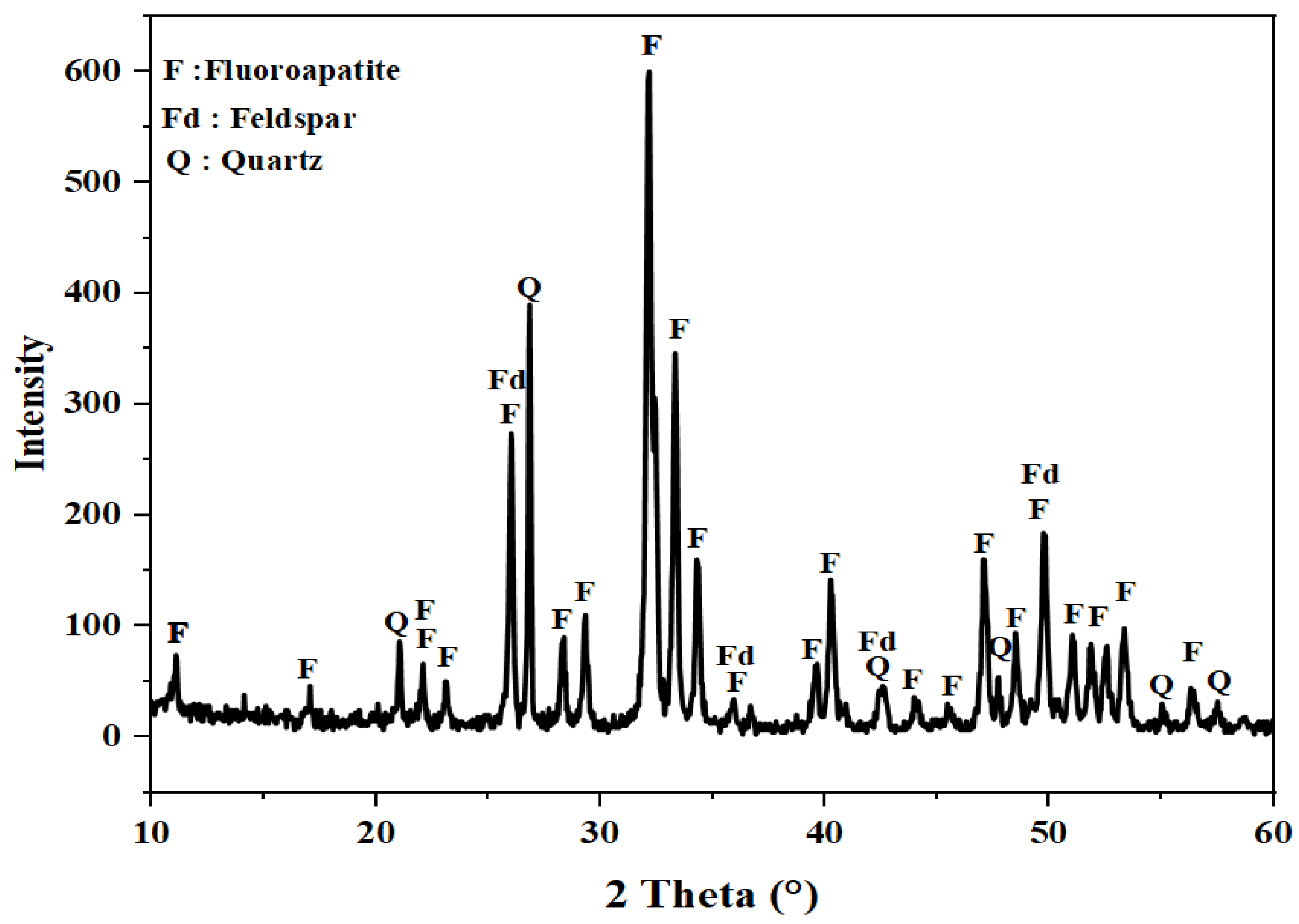
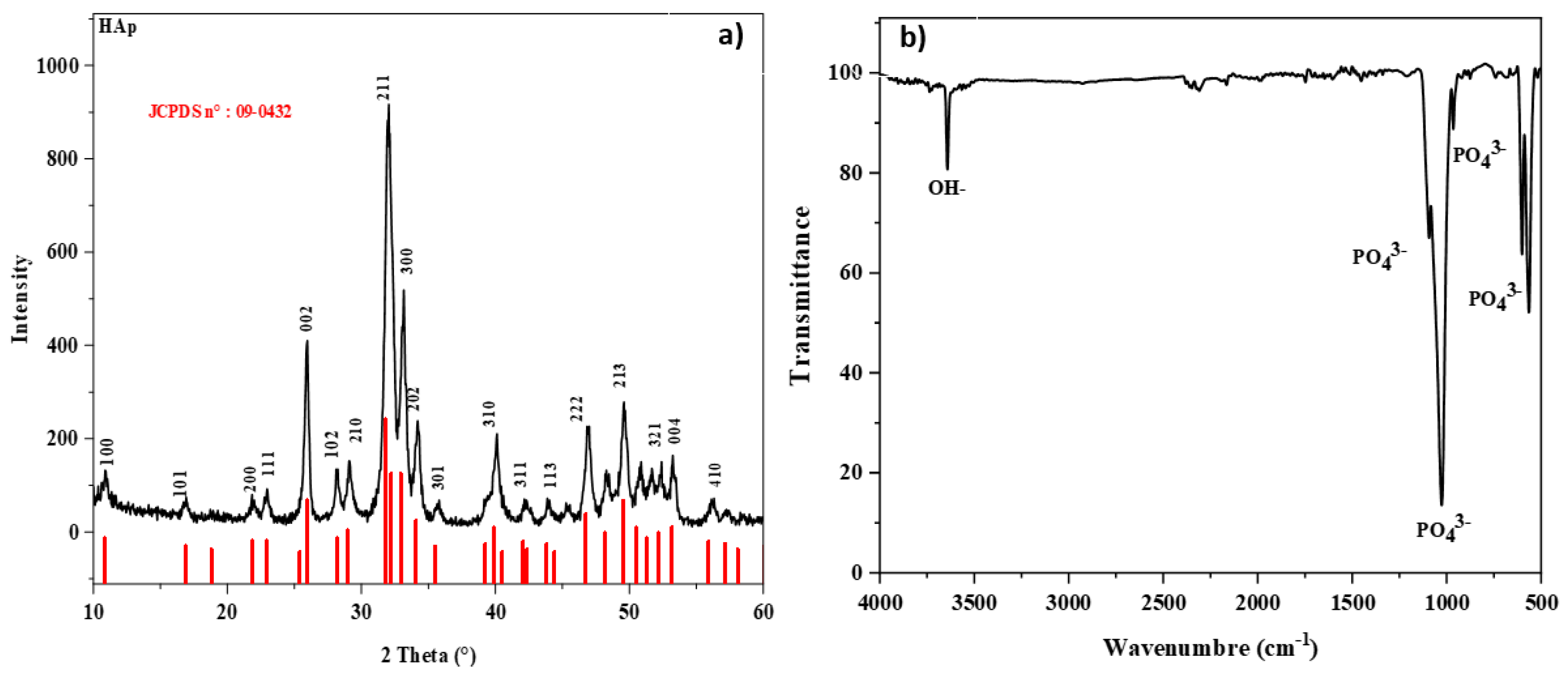
2. Results and Discussion
3. Materials and Methods
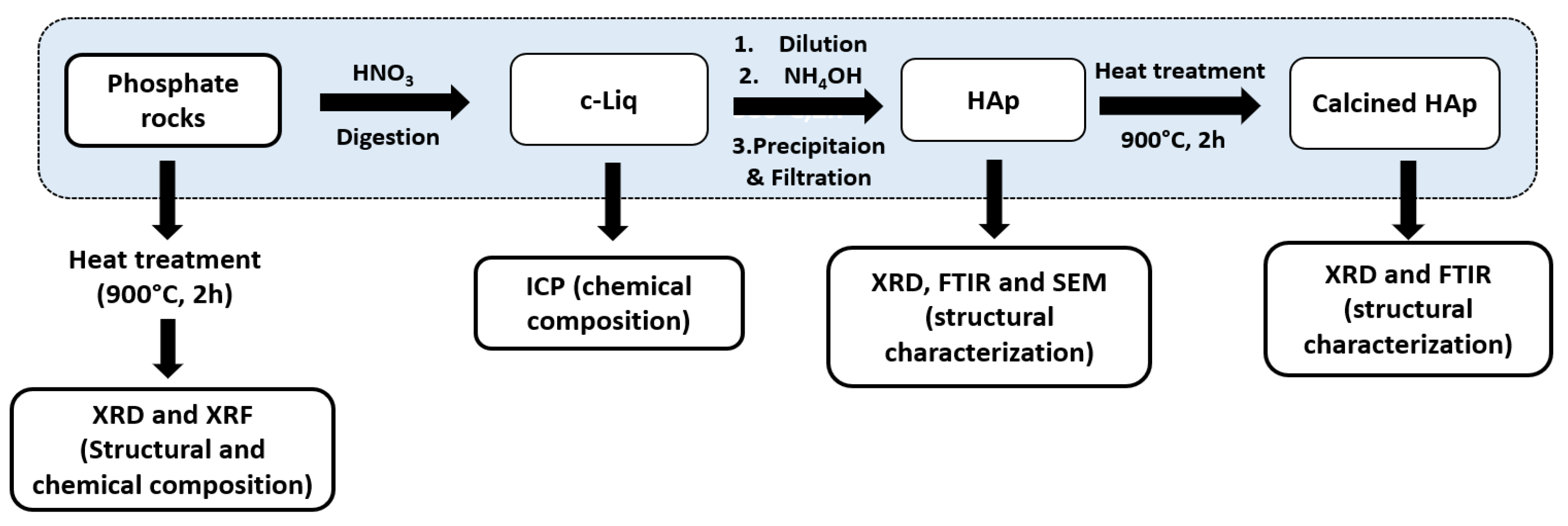
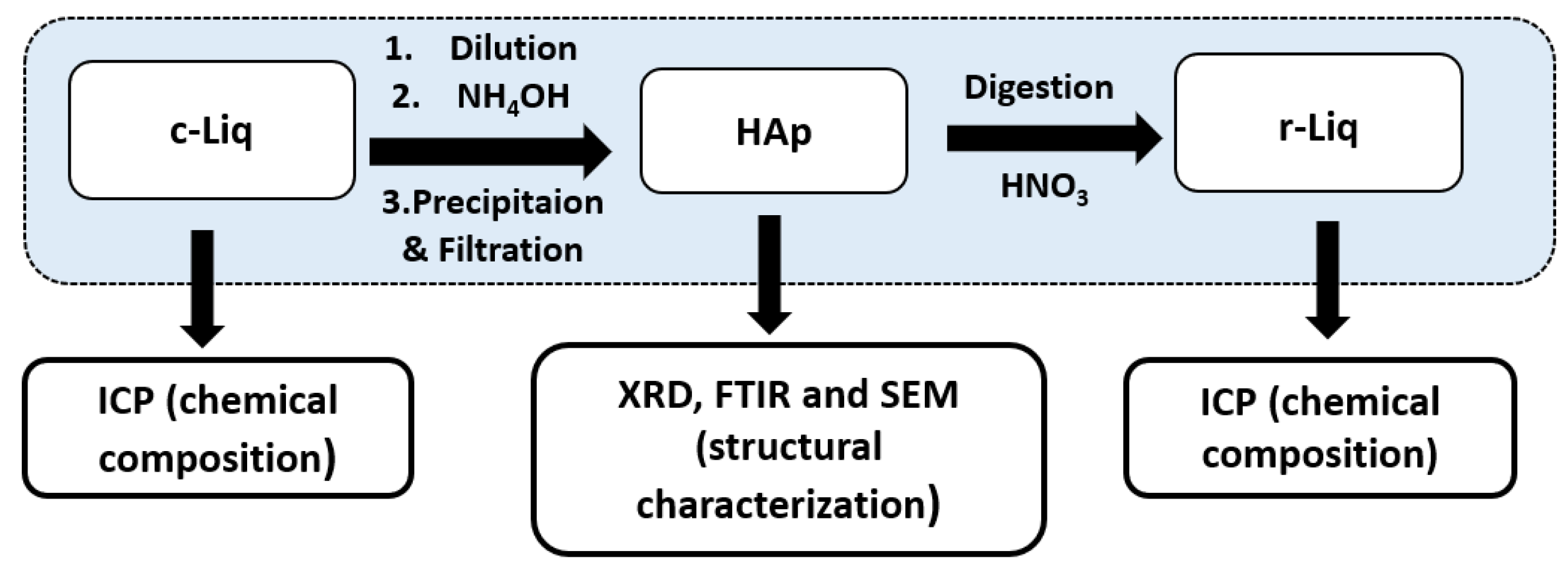
3.1. Hydroxyapatite Precipitation
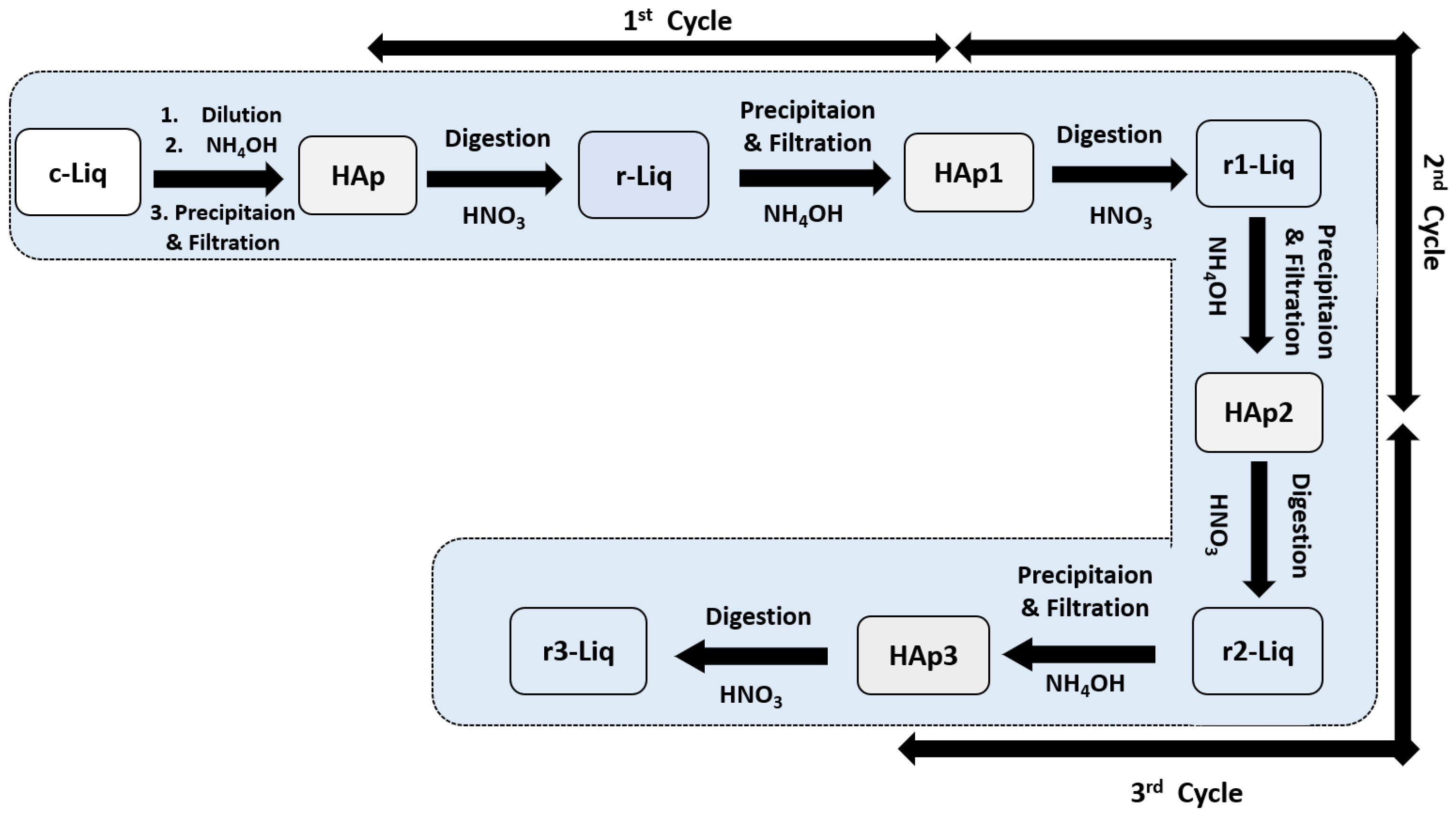
3.2. Purification Process of Hydroxyaptite
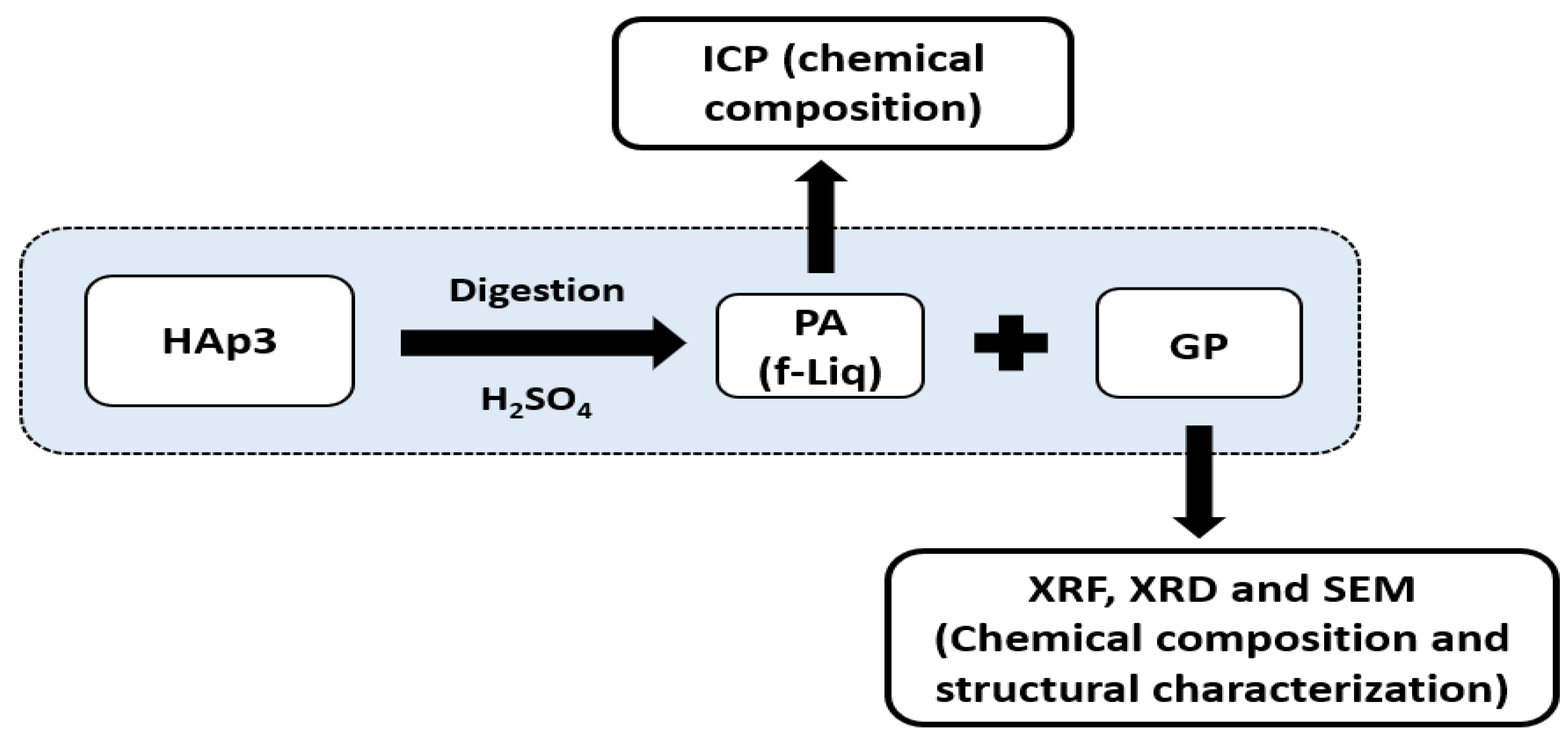
3.3. Preparation of High-Quality PA and GP
3.4. Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent Advances in Biomedical Engineering of Nano-Hydroxyapatite Including Dentistry, Cancer Treatment and Bone Repair. Compos. B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Dental Applications of Calcium Orthophosphates (CaPO4). J. Dent. Res. 2019, 1, 1007. [Google Scholar]
- Zhang, G.; Lu, Y.; Song, J.; Huang, D.; An, M.; Chen, W.; Han, P.; Yao, X.; Zhang, X. A Multifunctional Nano-Hydroxyapatite/MXene Scaffold for the Photothermal/Dynamic Treatment of Bone Tumours and Simultaneous Tissue Regeneration. J. Colloid. Interface Sci. 2023, 652, 1673–1684. [Google Scholar] [CrossRef]
- Machado, T.R.; Leite, I.S.; Inada, N.M.; Li, M.S.; da Silva, J.S.; Andrés, J.; Beltrán-Mir, H.; Cordoncillo, E.; Longo, E. Designing Biocompatible and Multicolor Fluorescent Hydroxyapatite Nanoparticles for Cell-Imaging Applications. Mater. Today Chem. 2019, 14, 100211. [Google Scholar] [CrossRef]
- Safitri, N.; Rauf, N.; Tahir, D. Enhancing Drug Loading and Release with Hydroxyapatite Nanoparticles for Efficient Drug Delivery: A Review Synthesis Methods, Surface Ion Effects, and Clinical Prospects. J. Drug Deliv. Sci. Technol. 2023, 90, 105092. [Google Scholar] [CrossRef]
- Widayat, W.; Hadiyanto, H.; Wardani, P.W.A.; Zuhra, U.A.; Prameswari, J. Preparation of KI/Hydroxyapatite Catalyst from Phosphate Rocks and Its Application for Improvement of Biodiesel Production. Molecules 2020, 25, 2565. [Google Scholar] [CrossRef] [PubMed]
- Ulas, B.; Yilmaz, Y.; Koc, S.; Kivrak, H. Hydroxyapatite Supported PdxIn100-x as a Novel Electrocatalyst for High-Efficiency Glucose Electrooxidation. Int. J. Hydrogen Energy 2023, 48, 6798–6810. [Google Scholar] [CrossRef]
- U. Tosun, G.; Sakhno, Y.; Jaisi, D.P. Synthesis of Hydroxyapatite Nanoparticles from Phosphorus Recovered from Animal Wastes. ACS Sustain. Chem. Eng. 2021, 9, 15117–15126. [Google Scholar] [CrossRef]
- Balasooriya, I.L.; Chen, J.; Gedara, S.M.K.; Han, Y.; Wickramaratne, M.N. Applications of Nano Hydroxyapatite as Adsorbents: A Review. Nanomaterials 2022, 12, 2324. [Google Scholar] [CrossRef]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-Hydroxyapatite in Oral Care Cosmetics: Characterization and Cytotoxicity Assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef]
- Rhaiti, H.; Laghzizil, A.; Saoiabi, A.; El Asri, S.; Lahlil, K.; Gacoin, T. Surface Properties of Porous Hydroxyapatite Derived from Natural Phosphate. Mater. Chem. Phys. 2012, 136, 1022–1026. [Google Scholar] [CrossRef]
- Zhang, H.B.; Zhou, K.C.; Li, Z.Y.; Huang, S.P. Plate-like Hydroxyapatite Nanoparticles Synthesized by the Hydrothermal Method. J. Phys. Chem. Solids 2009, 70, 243–248. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Wang, H.; Zhu, M.; Yan, H. Rapid Formation of Hydroxyapatite Nanostructures by Microwave Irradiation. Chem. Phys. Lett. 2004, 396, 429–432. [Google Scholar] [CrossRef]
- Cho, J.S.; Rhee, S.H. The Size Control of Hydroxyapatite Particles during Spray Pyrolysis. Key Eng. Mater. 2013, 529–530, 66–69. [Google Scholar] [CrossRef]
- Delgadillo-Velasco, L.; Hernández-Montoya, V.; Montes-Morán, M.A.; Gómez, R.T.; Cervantes, F.J. Recovery of Different Types of Hydroxyapatite by Precipitation of Phosphates of Wastewater from Anodizing Industry. J. Clean. Prod. 2020, 242, 118564. [Google Scholar] [CrossRef]
- Marrane, S.E.; Dänoun, K.; Allouss, D.; Sair, S.; Channab, B.E.; Rhihil, A.; Zahouily, M. A Novel Approach to Prepare Cellulose-g-Hydroxyapatite Originated from Natural Sources as an Efficient Adsorbent for Heavy Metals: Batch Adsorption Optimization via Response Surface Methodology. ACS Omega 2022, 7, 28076–28092. [Google Scholar] [CrossRef] [PubMed]
- Billah, R.E.K.; Abdellaoui, Y.; Anfar, Z.; Giácoman-Vallejos, G.; Agunaou, M.; Soufiane, A. Synthesis and Characterization of Chitosan/Fluorapatite Composites for the Removal of Cr (VI) from Aqueous Solutions and Optimized Parameters. Water Air Soil. Pollut. 2020, 231, 163. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.M.; Lamonier, J.F. Hydroxyapatite, a Multifunctional Material for Air, Water and Soil Pollution Control: A Review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Mandalunis, P.M. A Review of Metal Exposure and Its Effects on Bone Health. J. Toxicol. 2018, 2018, 4854152. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Liu, H.; Cai, Y.; Qi, X.; Dang, Z. Multicomponent Adsorption of Heavy Metals onto Biogenic Hydroxyapatite: Surface Functional Groups and Inorganic Mineral Facilitating Stable Adsorption of Pb(II). J. Hazard. Mater. 2023, 443, 130167. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Ammanoeil, R.N.; Saad, E.A.; Daoud, J.A. Purification of Crude Phosphoric Acid and Leached Apatite by Solvent Extraction with CYANEX 923 in Kerosene. Period. Polytech. Chem. Eng. 2019, 63, 122–129. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of Rare Earth Elements from Phosphate Rock by Hydrometallurgical Processes—A Critical Review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Reyes, L.H.; Medina, I.S.; Mendoza, R.N.; Vázquez, J.R.; Rodríguez, M.A.; Guibal, E. Extraction of Cadmium from Phosphoric Acid Using Resins Impregnated with Organophosphorus Extractants. Ind. Eng. Chem. Res. 2001, 40, 1422–1433. [Google Scholar] [CrossRef]
- Khaless, K.; Chanouri, H.; Amal, S.; Ouaattou, A.; Mounir, E.M.; Haddar, H.; Benhida, R. Wet Process Phosphoric Acid Purification Using Functionalized Organic Nanofiltration Membrane. Separations 2022, 9, 100. [Google Scholar] [CrossRef]
- Abderrahim, N.; Djellabi, R.; Amor, H.B.; Fellah, I.; Giordana, A.; Cerrato, G.; Di Michele, A.; Bianchi, C.L. Sustainable Purification of Phosphoric Acid Contaminated with Cr(VI) by Ag/Ag3PO4 Coated Activated Carbon/Montmorillonite under UV and Solar Light: Materials Design and Photocatalytic Mechanism. J. Environ. Chem. Eng. 2022, 10, 107870. [Google Scholar] [CrossRef]
- Zieliński, J.; Huculak-Mączka, M.; Kaniewski, M.; Nieweś, D.; Hoffmann, K.; Hoffmann, J. Kinetic Modelling of Cadmium Removal from Wet Phosphoric Acid by Precipitation Method. Hydrometallurgy 2019, 190, 105157. [Google Scholar] [CrossRef]
- Norwood, V.M.; Tate, L.R. Removing Heavy Metals from Phosphoric Acid and Phosphate Fluid Fertilizers. ACS Symp. Ser. 1992, 509, 147–160. [Google Scholar] [CrossRef]
- Freitas, A.M.B.; Giulietti, M. Production of Defluorinated Dicalcium Phosphate from Phosphate Rock Concentrate. Nutr. Cycl. Agroecosyst 1997, 48, 235–240. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of Metal Sulphide Precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Abdel-Ghafar, H.M.; Abdel-Aal, E.A.; Ibrahim, M.A.M.; El-Shall, H.; Ismail, A.K. Purification of High Iron Wet-Process Phosphoric Acid via Oxalate Precipitation Method. Hydrometallurgy 2019, 184, 1–8. [Google Scholar] [CrossRef]
- Forouzesh, M.; Fatehifar, E.; Khoshbouy, R.; Daryani, M. Experimental Investigation of Iron Removal from Wet Phosphoric Acid through Chemical Precipitation Process. Chem. Eng. Res. Des. 2023, 189, 308–318. [Google Scholar] [CrossRef]
- Mahrou, A.; Jouraiphy, R.; Mazouz, H.; Boukhair, A.; Fahad, M. Magnesium Removal from Phosphoric Acid by Precipitation: Optimization by Experimental Design. Chem. Ind. Chem. Eng. Q. 2021, 27, 113–119. [Google Scholar] [CrossRef]
- Kaba, O.B.; Filippov, L.O.; Filippova, I.V.; Badawi, M. Interaction between Fine Particles of Fluorapatite and Phosphoric Acid Unraveled by Surface Spectroscopies. Powder Technol. 2021, 382, 368–377. [Google Scholar] [CrossRef]
- Lakrat, M.; Azzaoui, K.; Jodeh, S.; Akartasse, N.; Mejdoubi, E.; Lamhamdi, A. The Removal of Methyl Orange by Nanohydroxyapatite from Aqueous Solution: Isotherm, Kinetics and Thermodynamics Studies. Desalin. Water Treatm. 2017, 85, 237–249. [Google Scholar] [CrossRef]
- Lakrat, M.; Fadlaoui, S.; Aaddouz, M.; Asri, O.E.; Melhaoui, M.; Mejdoubi, E.M. Synthesis and Characterization of Composites Based on Hydroxyapatite Nanoparticles and Chitosan Extracted from Shells of the Freshwater Crab Potamon Algeriense. Prog. Chem. Appl. Chitin Deriv. 2020, 25, 132–142. [Google Scholar] [CrossRef]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Lakrat, M.; Hamed, O.; Jodeh, S.; Berrabah, M.; Elidrissi, A.; el Meskini, I.; Daoudi, M. Preparation of Hydroxyapatite Biobased Microcomposite Film for Selective Removal of Toxic Dyes from Wastewater. Desalin. Water Treatm. 2019, 149, 28. [Google Scholar] [CrossRef]
- Akartasse, N.; Azzaoui, K.; Mejdoubi, E.; Hanbali, G.; Elansari, L.L.; Jodeh, S.; Hammouti, B.; Jodeh, W.; Lamhamdi, A. Study and Optimization of the Synthesis of Apatitic Nanoparticles by the Dissolution/Precipitation Method. Arab. J. Sci. Eng. 2022, 47, 7035–7051. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Self-Setting Calcium Orthophosphate (CaPO) Formulations. Adv. Nano-Bio. Mater. Dev. 2018, 3, 41–146. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic Substituted Hydroxyapatite for Bone Regeneration Applications: A Review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; pp. 145–211. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, B.; Wang, C.; Shi, B.; Chen, Y. Thermodynamic Analysis and Application for Preparing FePO4 from Nitric Acid Pressure Leach Laterite Residue by Selective Leaching in Phosphoric Acid and Induced Precipitation. Hydrometallurgy 2022, 212, 105896. [Google Scholar] [CrossRef]
- Lakrat, M.; Jodati, H.; Mejdoubi, E.M.; Evis, Z. Synthesis and Characterization of Pure and Mg, Cu, Ag, and Sr Doped Calcium-Deficient Hydroxyapatite from Brushite as Precursor Using the Dissolution-Precipitation Method. Powder Technol. 2023, 413, 118026. [Google Scholar] [CrossRef]
- Elsayed, A.A.A.; EL-Gohary, A.; Taha, Z.K.; Farag, H.M.; Hussein, M.S.; AbouAitah, K. Hydroxyapatite Nanoparticles as Novel Nano-Fertilizer for Production of Rosemary Plants. Sci. Hortic. 2022, 295, 110851. [Google Scholar] [CrossRef]
- Noruzi, M.; Hadian, P.; Soleimanpour, L.; Ma’mani, L.; Shahbazi, K. Hydroxyapatite Nanoparticles: An Alternative to Conventional Phosphorus Fertilizers in Acidic Culture Media. Chem. Biol. Technol. Agric. 2023, 10, 71. [Google Scholar] [CrossRef]
- Ahmed, I. Overview on the Removal of Iron from Phosphoric Acid: A Comparative Study. Arab. J. Nucl. Sci. Appl. 2021, 54, 37–49. [Google Scholar] [CrossRef]
- Hagag, M.S.; Morsy, A.M.A.; Ali, A.H.; El-Shiekh, A.S. Adsorption of Rare Earth Elements onto the Phosphogypsum a Waste Byproduct. Water Air Soil. Pollut. 2019, 230, 308. [Google Scholar] [CrossRef]
- El-Asmy, A.A.; Serag, H.M.; Mahdy, M.A.; Amin, M.I. Purification of Phosphoric Acid by Minimizing Iron, Copper, Cadmium and Fluoride. Sep. Purif. Technol. 2008, 61, 287–292. [Google Scholar] [CrossRef]
- Cao, W.; Yi, W.; Peng, J.; Yin, S. Relationship between the Evolution of Organic Impurities and Properties of β-Hemihydrate Phosphogypsum. Constr. Build. Mater. 2023, 409, 134125. [Google Scholar] [CrossRef]
- Ma, B.; Jin, Z.; Su, Y.; Lu, W.; Qi, H.; Hu, P. Utilization of Hemihydrate Phosphogypsum for the Preparation of Porous Sound Absorbing Material. Constr. Build. Mater. 2020, 234, 117346. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; He, X.; Su, Y.; Strnadel, B.; Miao, W. Hydration and Compressive Strength of Supersulfated Cement with Low-Activity High Alumina Ferronickel Slag. Cem. Concr. Compos. 2023, 136, 104892. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Kang, X.; Yu, J.; Fan, Y.; Dang, Y.; Zhang, W.; Wang, S. Belite-Calcium Sulfoaluminate Cement Prepared with Phosphogypsum: Influence of P2O5 and F on the Clinker Formation and Cement Performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, X. Preparation of Gypsum with High Purity and Whiteness from Phosphogypsum for CO2 Mineral Sequestration. Sci. Rep. 2023, 13, 4156. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Teng, L.; Rohani, S.; Qin, Z.; Zhao, B.; Xu, C.C.; Ren, S.; Liu, Q.; Liang, B. CO2 Mineral Carbonation Using Industrial Solid Wastes: A Review of Recent Developments. Chem. Eng. J. 2021, 416, 129093. [Google Scholar] [CrossRef]
- Awwad, N.S.; El-Nadi, Y.A.; Hamed, M.M. Successive Processes for Purification and Extraction of Phosphoric Acid Produced by Wet Process. Chem. Eng. Process. Process Intensif. 2013, 74, 69–74. [Google Scholar] [CrossRef]




| Compound | CaO | P2O5 | SiO2 | F | Al2O3 | MnO | MgO | Fe2O3 | Na2O | SO3 | TiO2 | Other * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (wt%) | 44.01 | 39.58 | 5.26 | 5.12 | 1.50 | 0.56 | 0.40 | 0.30 | 0.22 | 0.64 | 0.06 | 0.17 |
| Elements | Ca | P | Al | Fe | Mg | Mn | Ti | Cd | Sn | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| c-Liq (mg/L) | 142.4 | 101 | 22.983 | 17.134 | 14.438 | 0.369 | 0.5133 | 0.1954 | 0.1 | 0.05 |
| Solution | Elements (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sn | Pb | Cd | Mn | Ti | Mg | Al | Fe | |
| c-Liq | 0.1 | 0.05 | 0.1954 | 0.369 | 0.3234 | 14.438 | 22.983 | 17.134 |
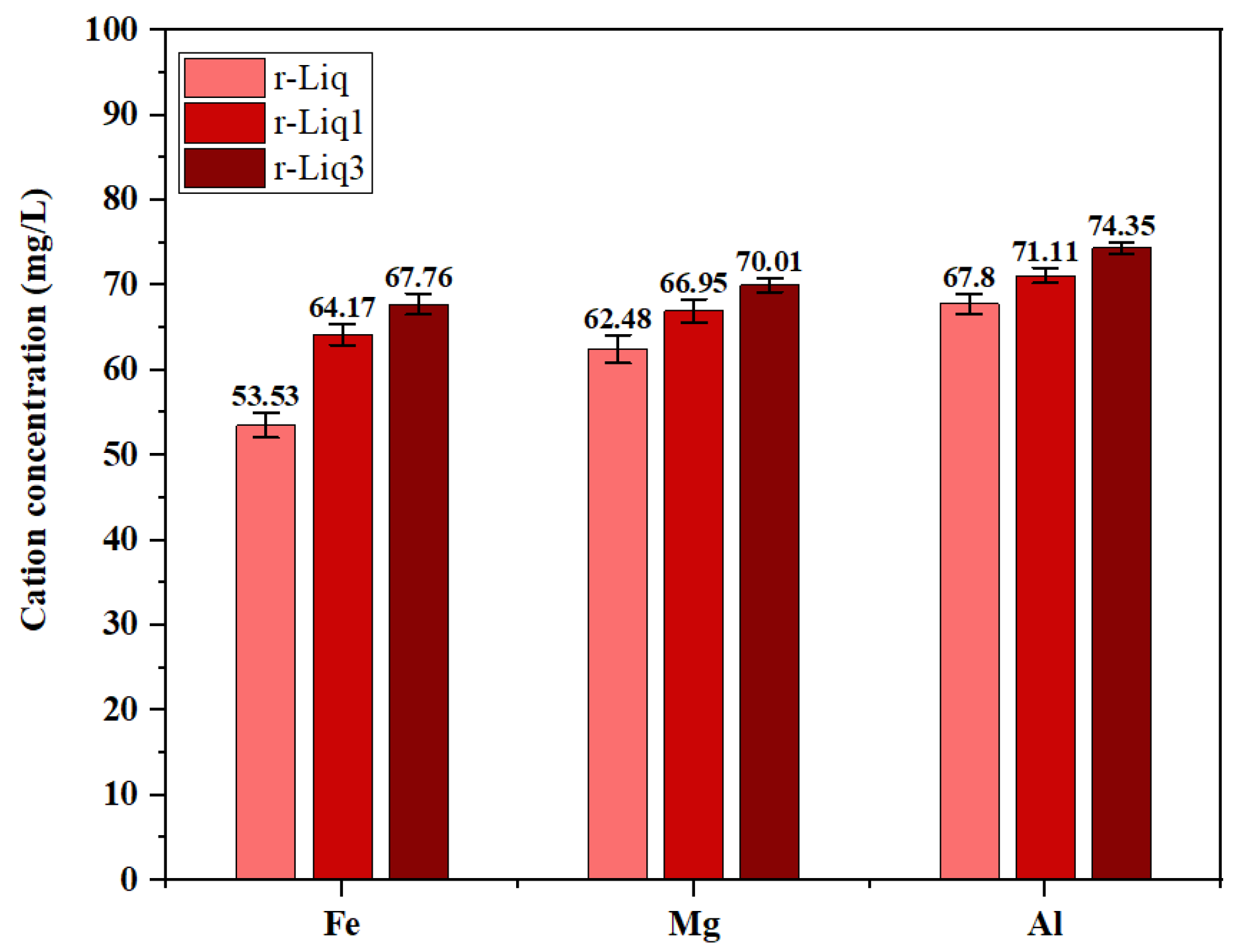
| r-Liq | 0.053 | 0.0001 | 0.0209 | 0.1315 | 0.1311 | 5.416 | 7.399 | 7.962 |
| r1-Liq | - | 0.0023 | 0.1151 | 0.09 | 4.771 | 6.138 | 6.513 | |
| r3-Liq | - | - | - | 0.1012 | 0.007 | 4.642 | 5.294 | 5.013 |
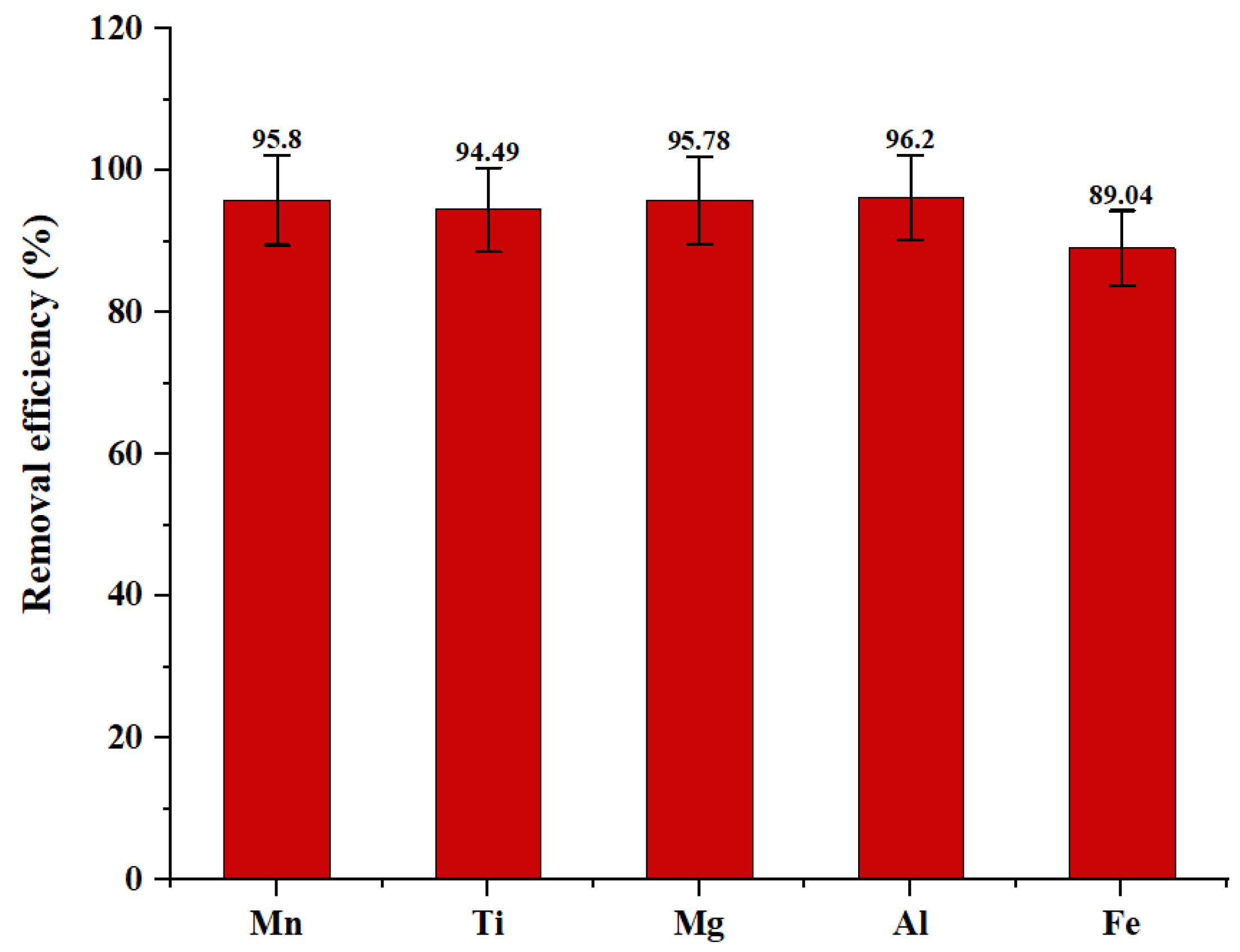
| Solution | Elements (mg/L) | ||||
|---|---|---|---|---|---|
| Mg | Al | Fe | Mn | Ti | |
| r3-Liq | 4.242 | 5.894 | 5.013 | 0.1012 | 0.007 |
| PA | 0.4647 | 0.3242 | 0.1897 | 0.0009 | - |
| Compound | SO3 | CaO | Al2O3 | P2O5 | Fe2O3 | Other | LOI a |
|---|---|---|---|---|---|---|---|
| Concentration (wt.%) | 48.70 | 29.74 | 0.096 | 0.01602 | 0.09 | 0.129 | 21.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benataya, K.; Lakrat, M.; Hammani, O.; Aaddouz, M.; Ait Yassine, Y.; Abuelizz, H.A.; Zarrouk, A.; Karrouchi, K.; Mejdoubi, E. Synthesis of High-Purity Hydroxyapatite and Phosphoric Acid Derived from Moroccan Natural Phosphate Rocks by Minimizing Cation Content Using Dissolution–Precipitation Technique. Molecules 2024, 29, 3854. https://doi.org/10.3390/molecules29163854
Benataya K, Lakrat M, Hammani O, Aaddouz M, Ait Yassine Y, Abuelizz HA, Zarrouk A, Karrouchi K, Mejdoubi E. Synthesis of High-Purity Hydroxyapatite and Phosphoric Acid Derived from Moroccan Natural Phosphate Rocks by Minimizing Cation Content Using Dissolution–Precipitation Technique. Molecules. 2024; 29(16):3854. https://doi.org/10.3390/molecules29163854
Chicago/Turabian StyleBenataya, Karim, Mohammed Lakrat, Othmane Hammani, Mohamed Aaddouz, Youssef Ait Yassine, Hatem A. Abuelizz, Abdelkader Zarrouk, Khalid Karrouchi, and Elmiloud Mejdoubi. 2024. "Synthesis of High-Purity Hydroxyapatite and Phosphoric Acid Derived from Moroccan Natural Phosphate Rocks by Minimizing Cation Content Using Dissolution–Precipitation Technique" Molecules 29, no. 16: 3854. https://doi.org/10.3390/molecules29163854
APA StyleBenataya, K., Lakrat, M., Hammani, O., Aaddouz, M., Ait Yassine, Y., Abuelizz, H. A., Zarrouk, A., Karrouchi, K., & Mejdoubi, E. (2024). Synthesis of High-Purity Hydroxyapatite and Phosphoric Acid Derived from Moroccan Natural Phosphate Rocks by Minimizing Cation Content Using Dissolution–Precipitation Technique. Molecules, 29(16), 3854. https://doi.org/10.3390/molecules29163854








