Effect of the Addition of Inorganic Fillers on the Properties of Degradable Polymeric Blends for Bone Tissue Engineering
Abstract
:1. Introduction
2. Results
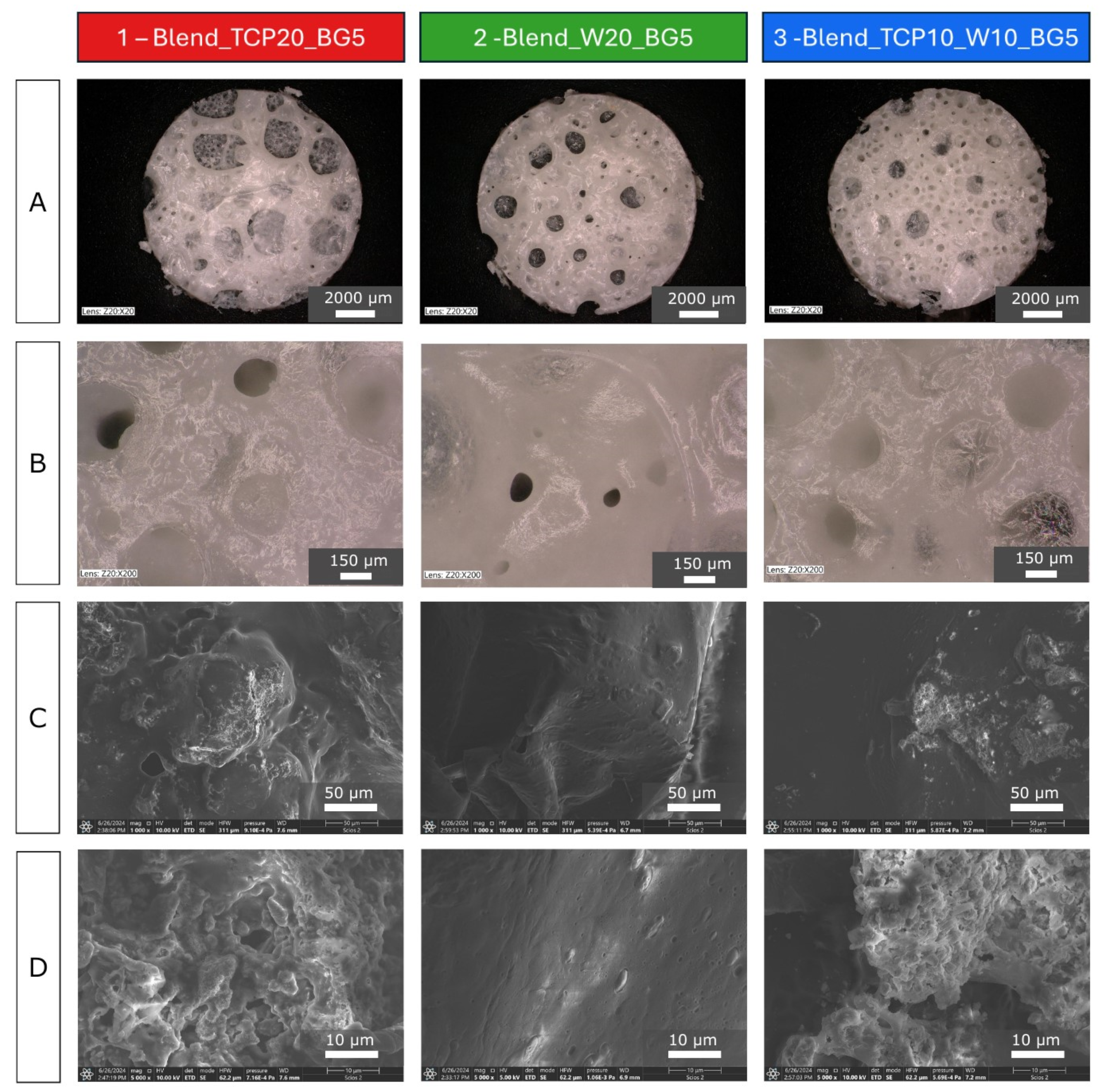
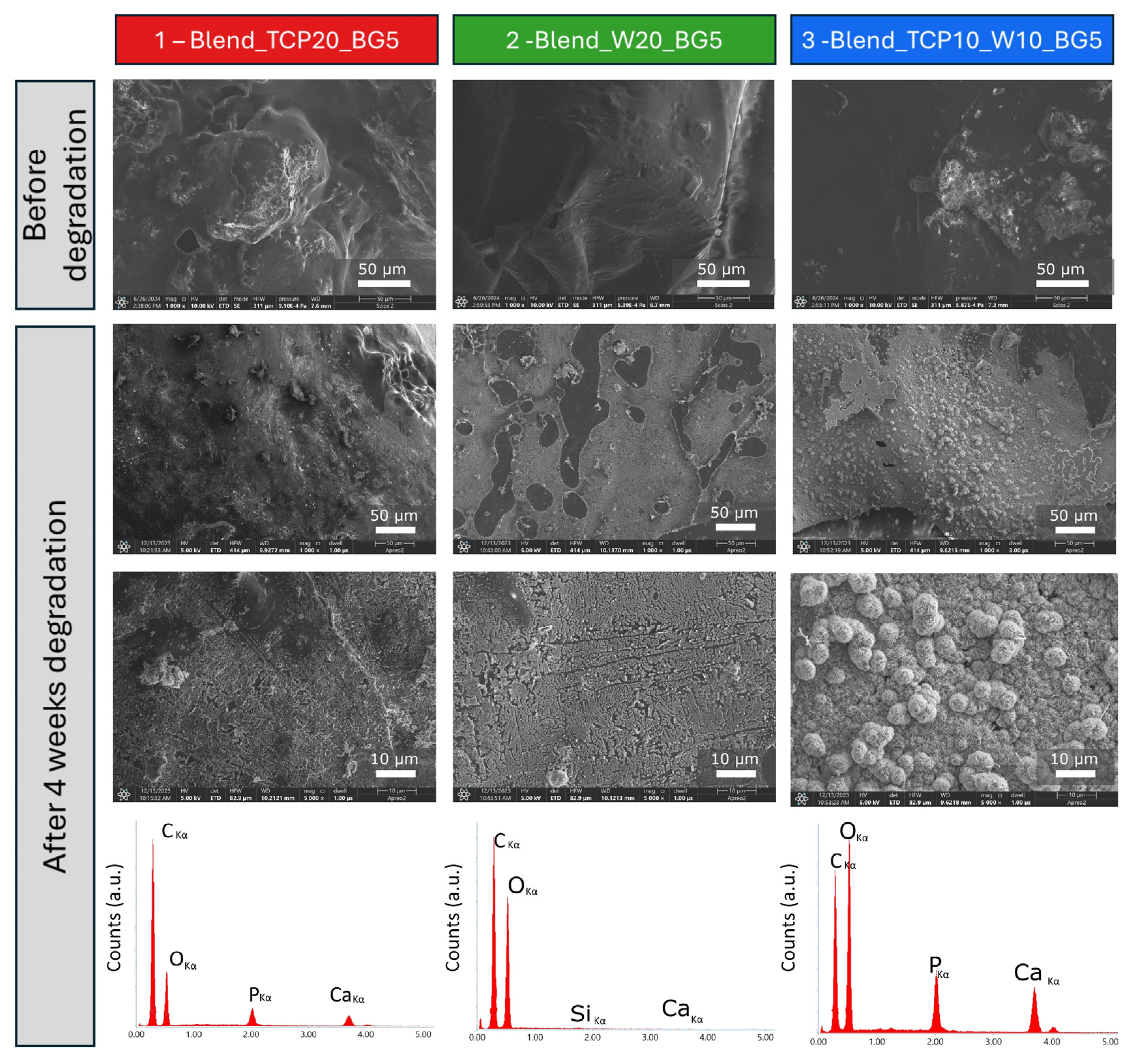
2.1. Morphology and Microstructure of the Fabricated Scaffolds
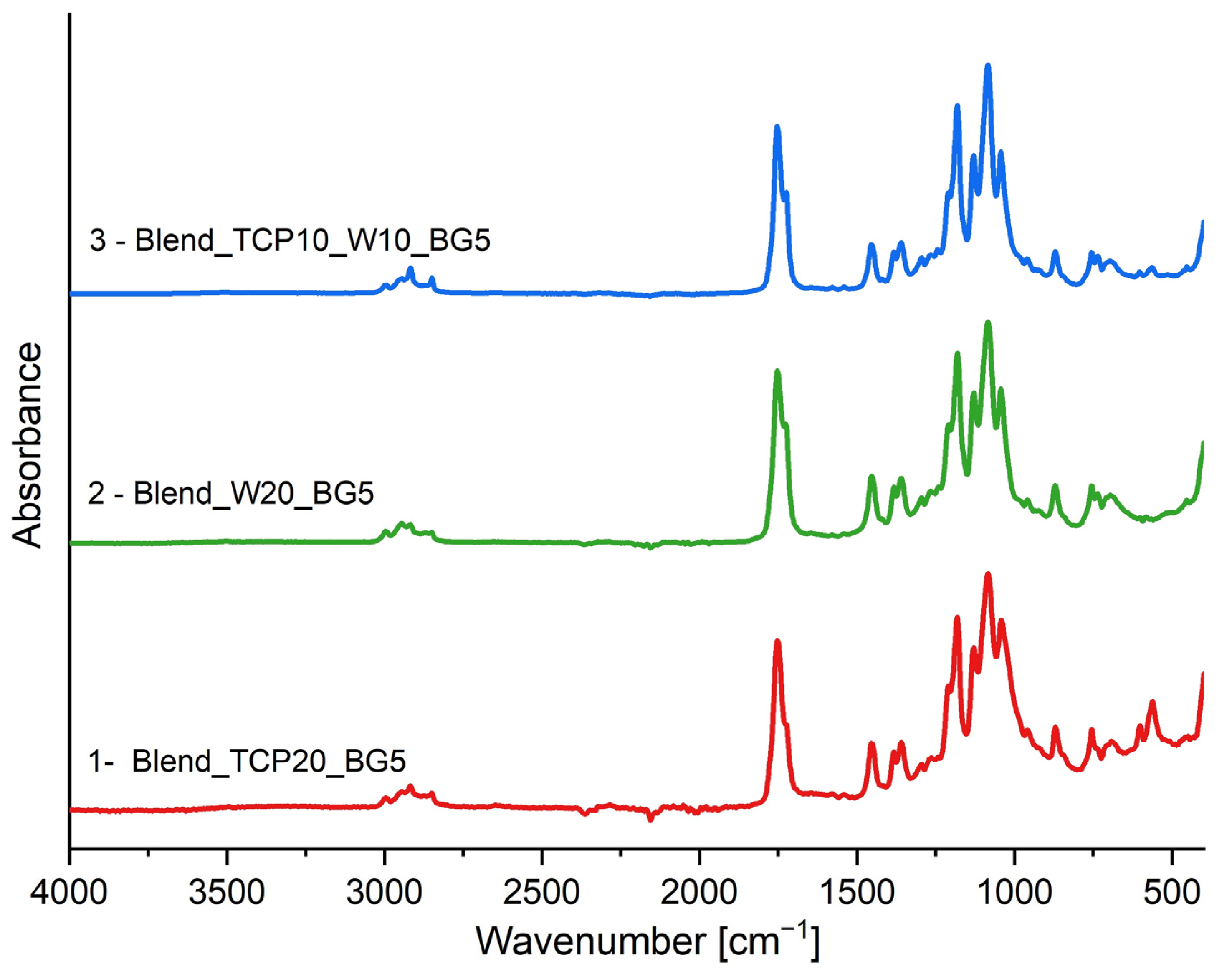
2.2. FTIR-ATR Spectroscopy
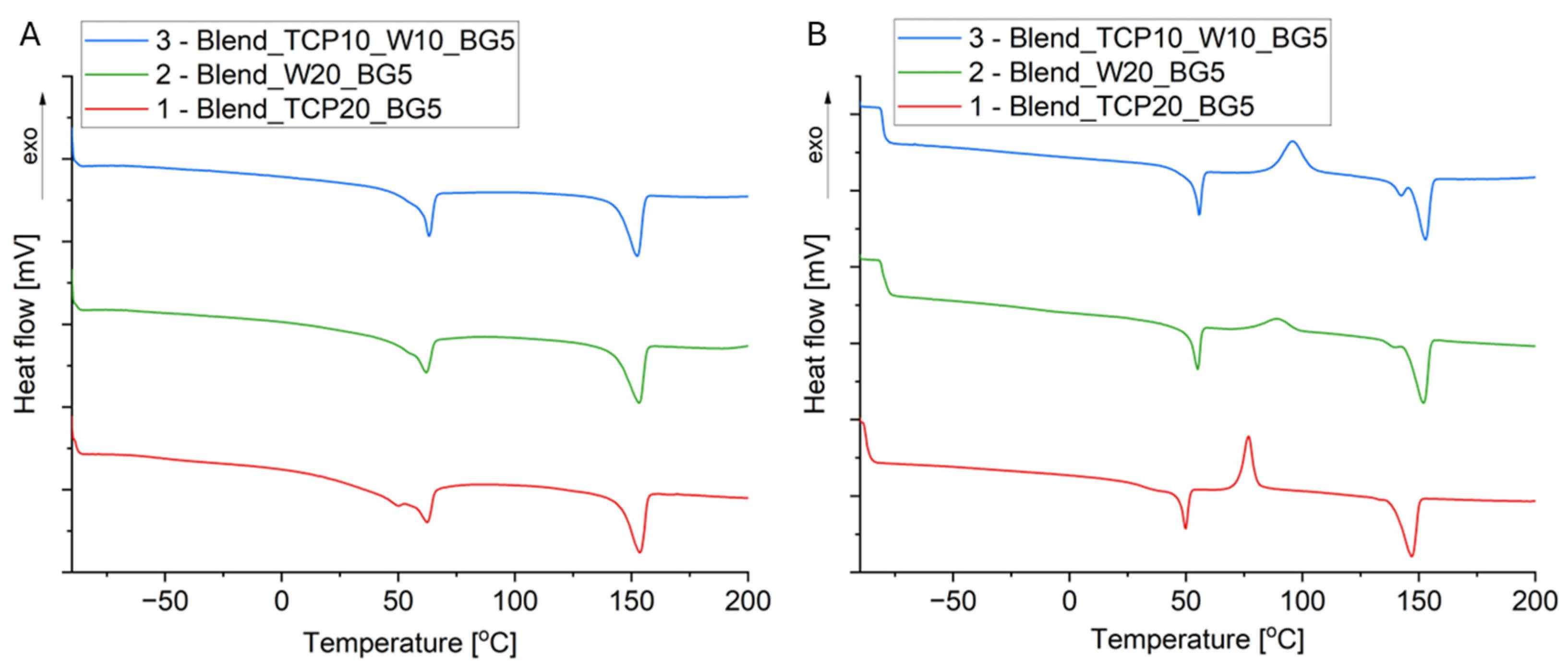
2.3. Differential Scanning Calorimetry
- —melting enthalpy of PLA;
- —total mass fraction of the additives, PPF, PCL, and PEG;
- —melting enthalpy of 100% crystalline PLA.
2.4. Thermogravimetry
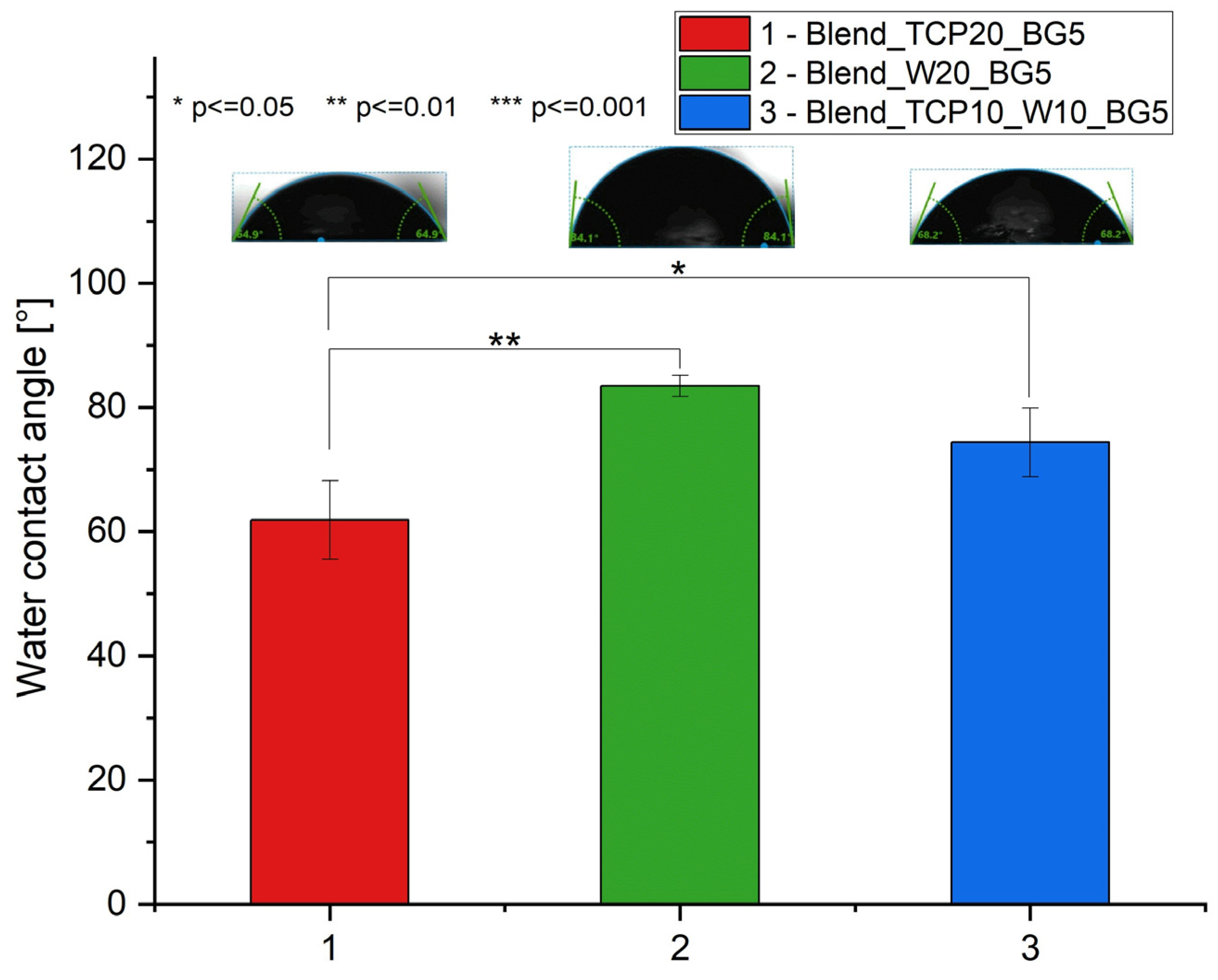
2.5. Water Contact Angle Measurements
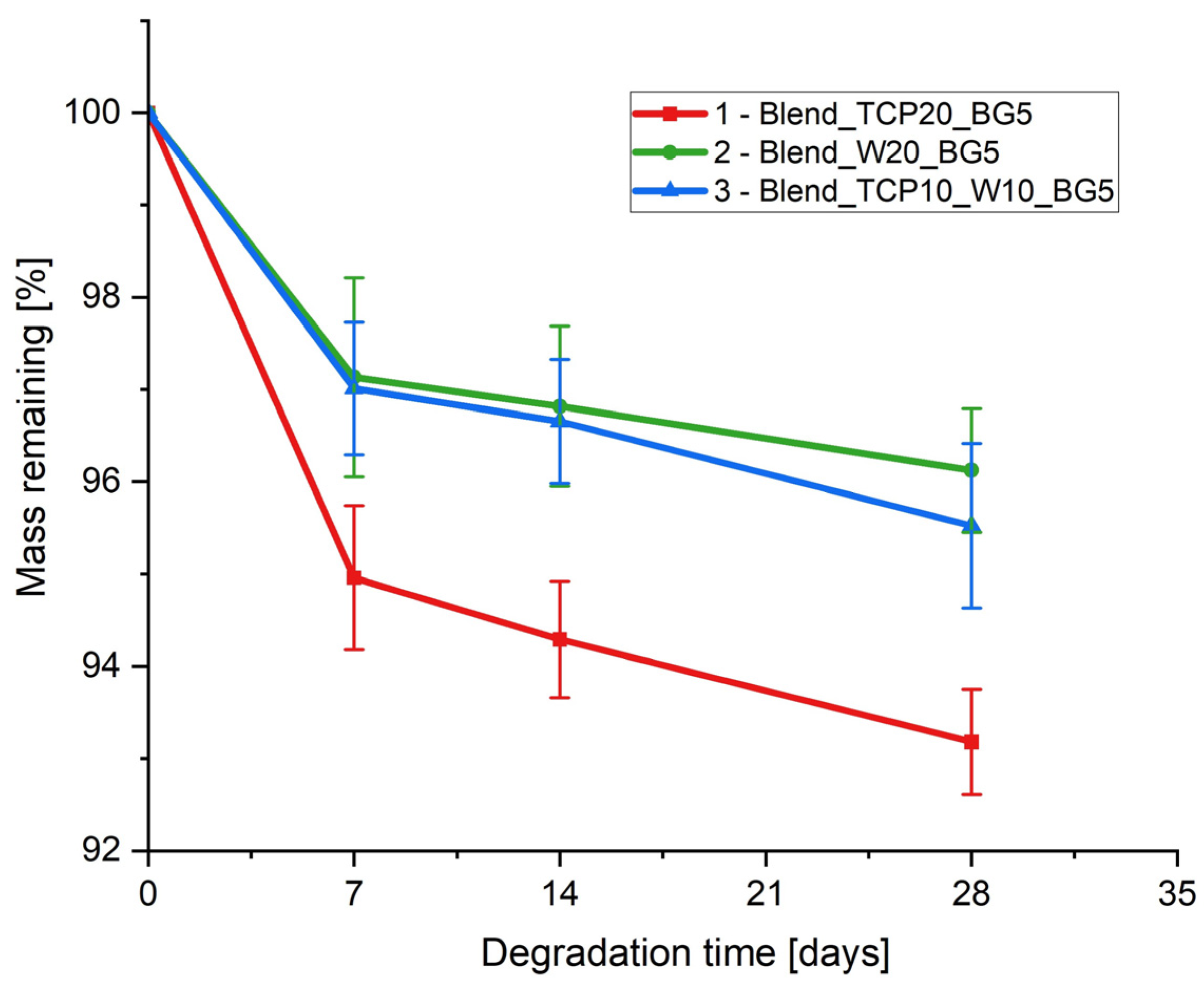
2.6. Degradation Study
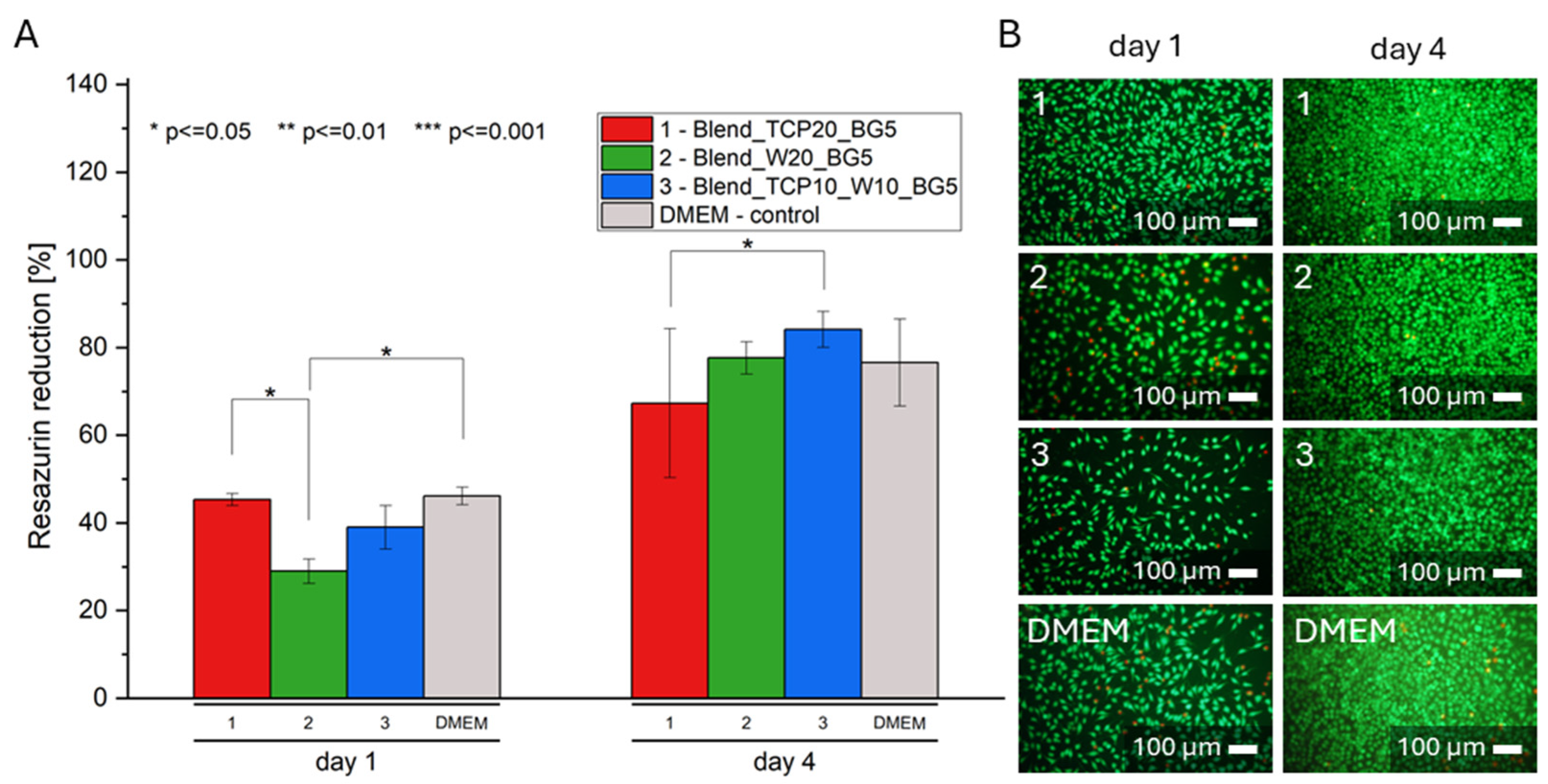
2.7. Biological Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Scaffolds Manufacturing
4.3. Optical and Scanning Electron Microscopy
4.4. Fourier Transform Infrared Spectroscopy
4.5. Differential Scanning Calorimetry
4.6. Thermogravimetry
4.7. Contact Angle
4.8. Degradation Rate
- M0—initial mass of the sample;
- Mi—mass of the sample after incubation.
4.9. Biological Evaluation on Extracts
- Fi—fluorescence of the sample;
- F0—fluorescence of a non-reduced solution;
- F100—fluorescence of 100% reduced solution.
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, 7–11. [Google Scholar] [CrossRef]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef]
- Qu, H. Additive Manufacturing for Bone Tissue Engineering Scaffolds. Mater. Today Commun.S 2020, 24, 101024. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Castañeda-Rodríguez, S.; González-Torres, M.; Ribas-Aparicio, R.M.; Del Prado-Audelo, M.L.; Leyva-Gómez, G.; Gürer, E.S.; Sharifi-Rad, J. Recent Advances in Modified Poly (Lactic Acid) as Tissue Engineering Materials. J. Biol. Eng. 2023, 17, 21. [Google Scholar] [CrossRef]
- Prasad, A. State of Art Review on Bioabsorbable Polymeric Scaffolds for Bone Tissue Engineering. Mater. Today Proc. 2021, 44, 1391–1400. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, Y.; Hu, J.; Wu, Z.; Chen, H. Fabrication and Characterization of Polylactic Acid/Polycaprolactone Composite Macroporous Micro-Nanofiber Scaffolds by Phase Separation. New J. Chem. 2020, 44, 17382–17390. [Google Scholar] [CrossRef]
- Kozaniti, F.K.; Deligianni, D.D.; Georgiou, M.D.; Portan, D.V. The Role of Substrate Topography and Stiffness on MSC Cells Functions: Key Material Properties for Biomimetic Bone Tissue Engineering. Biomimetics 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak Simunec, D.; Kyratzis, I.L.; Sola, A.; Wen, C. Biodegradable PLA-ZnO Nanocomposite Biomaterials with Antibacterial Properties, Tissue Engineering Viability, and Enhanced Biocompatibility. Smart Mater. Manuf. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Ye, G.; Gu, T.; Chen, B.; Bi, H.; Hu, Y. Mechanical, Thermal Properties and Shape Memory Behaviors of PLA/PCL/PLA-g-GMA Blends. Polym. Eng. Sci 2023, 63, 2084–2092. [Google Scholar] [CrossRef]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt Electrowriting of PLA, PCL, and Composite PLA/PCL Scaffolds for Tissue Engineering Application. Sci. Rep. 2022, 12, 19935. [Google Scholar]
- Ghodrati, M.; Rafiaei, S.M.; Tayebi, L. Fabrication and Evaluation of PLA/MgAl2O4 Scaffolds Manufactured through 3D Printing Method. J. Mech. Behav. Biomed. Mater. 2023, 145, 106001. [Google Scholar] [CrossRef]
- Mathieu, P.; Bascou, R.; Navarro Oliva, F.S.; Nesterenko, A.; Ngo, A.; Lisiecki, I.; Guénin, E.; Bedoui, F. Electrospinning of Ultrafine Non-hydrolyzed Silk Sericin/PEO Fibers on PLA: A Bilayer Scaffold Fabrication. Polym. Eng. Sci 2023, 63, 830–840. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic Degradation of Polylactic Acid (PLA) and Its Composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Rohner, D.; Hutmacher, D.W.; Cheng, T.K.; Oberholzer, M.; Hammer, B. In vivo Efficacy of Bone-marrow-coated Polycaprolactone Scaffolds for the Reconstruction of Orbital Defects in the Pig. J. Biomed. Mater. Res. 2003, 66B, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M. In Vitro Biocompatibility Assessment of Poly(ε-Caprolactone) Films Using L929 Mouse Fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Wang, J.-Z.; You, M.-L.; Ding, Z.-Q.; Ye, W.-B. A Review of Emerging Bone Tissue Engineering via PEG Conjugated Biodegradable Amphiphilic Copolymers. Mater. Sci. Eng. C 2019, 97, 1021–1035. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Song, J. Biodegradable PEG-Based Amphiphilic Block Copolymers for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2015, 1, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Bennett, J.; Treasure, T.; Voytik-Harbin, S.; Goebel, W.S.; Chu, T.G. Poly(Propylene Fumarate) Reinforced Dicalcium Phosphate Dihydrate Cement Composites for Bone Tissue Engineering. J. Biomed. Mater. Res 2012, 100A, 1792–1802. [Google Scholar] [CrossRef]
- Lee, K.-W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly(Propylene Fumarate) Bone Tissue Engineering Scaffold Fabrication Using Stereolithography: Effects of Resin Formulations and Laser Parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, L.; Yaszemski, M.J. Bone-Tissue-Engineering Material Poly(Propylene Fumarate): Correlation between Molecular Weight, Chain Dimensions, and Physical Properties. Biomacromolecules 2006, 7, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wan, Y.; Becker, M.L.; Long, Y.-Z.; Dean, D. Poly(Propylene Fumarate)-Based Materials: Synthesis, Functionalization, Properties, Device Fabrication and Biomedical Applications. Biomaterials 2019, 208, 45–71. [Google Scholar] [CrossRef]
- Engebretson, B.; Sikavitsas, V.I. Long-Term In Vivo Effect of Peg Bone Tissue Engineering Scaffolds. J. Long Term Eff. Med. Implant. 2012, 22, 211–218. [Google Scholar] [CrossRef]
- Distler, T.; Fournier, N.; Grünewald, A.; Polley, C.; Seitz, H.; Detsch, R.; Boccaccini, A.R. Polymer-Bioactive Glass Composite Filaments for 3D Scaffold Manufacturing by Fused Deposition Modeling: Fabrication and Characterization. Front. Bioeng. Biotechnol. 2020, 8, 552. [Google Scholar] [CrossRef]
- Dong, J.; Uemura, T.; Shirasaki, Y.; Tateishi, T. Promotion of Bone Formation Using Highly Pure Porous β-TCP Combined with Bone Marrow-Derived Osteoprogenitor Cells. Biomaterials 2002, 23, 4493–4502. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S. Osteoclastic Resorption of Calcium Phosphate Ceramics with Different Hydroxyapatite/β-Tricalcium Phosphate Ratios. Biomaterials 1997, 18, 1037–1041. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Himani, J.; Purnima, J. Development of Glass Fiber, Wollastonite Reinforced Polypropylene Hybrid Composite: Mechanical Properties and Morphology. Mater. Sci. Eng. A 2010, 527, 1946–1951. [Google Scholar] [CrossRef]
- Maxim, L.D.; McConnell, E.E. A Review of the Toxicology and Epidemiology of Wollastonite. Inhal. Toxicol. 2005, 17, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.Y.; Sulong, A.B.; Muhamad, N.; Raza, M.R.; Ramli, M.I. Incorporation of Wollastonite Bioactive Ceramic with Titanium for Medical Applications: An Overview. Mater. Sci. Eng. C 2019, 97, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Naciri, M.; Al-Rubeai, M. Osteoconductivity and Growth Factor Production by MG63 Osteoblastic Cells on Bioglass-coated Orthopedic Implants. Biotechnol. Bioeng. 2011, 108, 454–464. [Google Scholar] [CrossRef]
- Savaris, M.; Braga, G.L.; Dos Santos, V.; Carvalho, G.A.; Falavigna, A.; Machado, D.C.; Viezzer, C.; Brandalise, R.N. Biocompatibility Assessment of Poly(Lactic Acid) Films after Sterilization with Ethylene Oxide in Histological Study In Vivo with Wistar Rats and Cellular Adhesion of Fibroblasts In Vitro. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Benkaddour, A.; Jradi, K.; Robert, S.; Daneault, C. Grafting of Polycaprolactone on Oxidized Nanocelluloses by Click Chemistry. Nanomaterials 2013, 3, 141–157. [Google Scholar] [CrossRef]
- Chopra, S.; Pande, K.; Puranam, P.; Deshmukh, A.D.; Bhone, A.; Kale, R.; Galande, A.; Mehtre, B.; Tagad, J.; Tidake, S. Explication of Mechanism Governing Atmospheric Degradation of 3D-Printed Poly(Lactic Acid) (PLA) with Different in-Fill Pattern and Varying in-Fill Density. RSC Adv. 2023, 13, 7135–7152. [Google Scholar] [CrossRef]
- Elhattab, K.; Bhaduri, S.B.; Sikder, P. Influence of Fused Deposition Modelling Nozzle Temperature on the Rheology and Mechanical Properties of 3D Printed β-Tricalcium Phosphate (TCP)/Polylactic Acid (PLA) Composite. Polymers 2022, 14, 1222. [Google Scholar] [CrossRef]
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable Magnesium-Doped Brushite Cement for Controlled Drug Release Application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Afriani, F.; Dahlan, K.; Nikmatin, S.; Zuas, O. Alginate affecting the characteristics of porous β-tcp/alginate composite scaffolds. J. Optoelectron. Biomed. Mater. 2015, 7, 67–76. [Google Scholar]
- Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.; Silvestros, S.; Sagnou, M. Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and In Vitro Studies. Materials 2018, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Herth, E.; Zeggari, R.; Rauch, J.-Y.; Remy-Martin, F.; Boireau, W. Investigation of Amorphous SiOx Layer on Gold Surface for Surface Plasmon Resonance Measurements. Microelectron. Eng. 2016, 163, 43–48. [Google Scholar] [CrossRef]
- Chen, W.; Liang, Y.; Hou, X.; Zhang, J.; Ding, H.; Sun, S.; Cao, H. Mechanical Grinding Preparation and Characterization of TiO2-Coated Wollastonite Composite Pigments. Materials 2018, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Y.; Moztarzadeh, F.; Shahabi, S.; Tahriri, M. Synthesis, Characterization, and In Vitro Bioactivity of Sol-Gel-Derived SiO2 –CaO–P2 O5–MgO-SrO Bioactive Glass. Synth. React. Inorg. M 2014, 44, 692–701. [Google Scholar] [CrossRef]
- Askadskii, A.; Popova, M.; Matseevich, T.; Kurskaya, E. The Influence of the Degree of Crystallinity on the Glass Transition Temperature of Polymers. Adv. Mater. Res. 2013, 864–867, 751–754. [Google Scholar] [CrossRef]
- Sabater I Serra, R.; Kyritsis, A.; Escobar Ivirico, J.L.; Gómez Ribelles, J.L.; Pissis, P.; Salmerón-Sánchez, M. Molecular Mobility in Biodegradable Poly(-Caprolactone)/Poly(Hydroxyethyl Acrylate) Networks. Eur. Phys. J. E 2011, 34, 37. [Google Scholar] [CrossRef] [PubMed]
- Utomo, E.; Domínguez-Robles, J.; Moreno-Castellanos, N.; Stewart, S.A.; Picco, C.J.; Anjani, Q.K.; Simón, J.A.; Peñuelas, I.; Donnelly, R.F.; Larrañeta, E. Development of Intranasal Implantable Devices for Schizophrenia Treatment. Int. J. Pharm. 2022, 624, 122061. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Sánchez, L.; Bagudanch, I.; Sustaita, A.; Iturbe-Ek, J.; Elizalde, L.; Garcia-Romeu, M.; Elías-Zúñiga, A. Single-Point Incremental Forming of Two Biocompatible Polymers: An Insight into Their Thermal and Structural Properties. Polymers 2018, 10, 391. [Google Scholar] [CrossRef]
- Eren Boncu, T.; Ozdemir, N. Electrospinning of Ampicillin Trihydrate Loaded Electrospun PLA Nanofibers I: Effect of Polymer Concentration and PCL Addition on Its Morphology, Drug Delivery and Mechanical Properties. Int. J. Polym. Mater. 2022, 71, 669–676. [Google Scholar] [CrossRef]
- Shekhar, N.; Mondal, A. Synthesis, Properties, Environmental Degradation, Processing, and Applications of Polylactic Acid (PLA): An Overview. Polym. Bull. 2024, 81, 1–37. [Google Scholar] [CrossRef]
- Maity, N.; Bruchiel-Spanier, N.; Sharabani-Yosef, O.; Mandler, D.; Eliaz, N. Zinc Oxide Nanoparticles Embedded Photo-Crosslinkable PLA-Block-PEG toward Effective Antibacterial Coatings. Mater. Adv. 2023, 4, 3026–3036. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Pérez-Camargo, R.A.; Coulembier, O.; Sangroniz, L.; Aranburu, N.; Guerrica-Echevarria, G.; Liu, G.; Wang, D.; Cavallo, D.; Müller, A.J. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly(ε-Caprolactone) (PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Ji, T.; Feng, B.; Shen, J.; Zhang, M.; Hu, Y.; Jiang, A.; Zhu, D.; Chen, Y.; Ji, W.; Zhang, Z.; et al. An Avascular Niche Created by Axitinib-Loaded PCL/Collagen Nanofibrous Membrane Stabilized Subcutaneous Chondrogenesis of Mesenchymal Stromal Cells. Adv. Sci. 2021, 8, 2100351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, D.; Zhao, L.; Lin, J. Composite Fibrous Membrane Comprising PLA and PCL Fibers for Biomedical Application. Compos. Commun. 2022, 34, 101268. [Google Scholar] [CrossRef]
- More, N.; Avhad, M.; Utekar, S.; More, A. Polylactic Acid (PLA) Membrane—Significance, Synthesis, and Applications: A Review. Polym. Bull. 2023, 80, 1117–1153. [Google Scholar] [CrossRef]
- Iqbal, M.; Valour, J.-P.; Fessi, H.; Elaissari, A. Preparation of Biodegradable PCL Particles via Double Emulsion Evaporation Method Using Ultrasound Technique. Colloid Polym. Sci. 2015, 293, 861–873. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Zhou, L.; Bi, Z.; Shi, M.; Wang, D.; Li, Q. Fabrication of a Novel Three-Dimensional Porous PCL/PLA Tissue Engineering Scaffold with High Connectivity for Endothelial Cell Migration. Eur. Polym. J. 2021, 161, 110834. [Google Scholar] [CrossRef]
- Meyhami, T.; Hassanajili, S.; Tanideh, N.; Taheri, E. Three Dimensional Scaffolds of Hybrid PLA/PCL/HA/Silica Nanocomposites for Bone Tissue Engineering. Polym. Bull. 2024, 81, 6025–6053. [Google Scholar] [CrossRef]
- Samourides, A.; Browning, L.; Hearnden, V.; Chen, B. The Effect of Porous Structure on the Cell Proliferation, Tissue Ingrowth and Angiogenic Properties of Poly(Glycerol Sebacate Urethane) Scaffolds. Mater. Sci. Eng. C 2020, 108, 110384. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; et al. Influence of the Pore Size and Porosity of Selective Laser Melted Ti6Al4V ELI Porous Scaffold on Cell Proliferation, Osteogenesis and Bone Ingrowth. Mater. Sci. Eng. C 2020, 106, 110289. [Google Scholar] [CrossRef]
- Mohammadi, H.; Sepantafar, M.; Muhamad, N.; Bakar Sulong, A. How Does Scaffold Porosity Conduct Bone Tissue Regeneration? Adv. Eng. Mater. 2021, 23, 2100463. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Jodati, H.; Yılmaz, B.; Evis, Z. A Review of Bioceramic Porous Scaffolds for Hard Tissue Applications: Effects of Structural Features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Lin, L.; Li, S.; Hou, W.; He, Y.; Sheng, L.; Yi, C.; Zhang, X.; Li, H.; et al. Large-Pore-Size Ti6Al4V Scaffolds with Different Pore Structures for Vascularized Bone Regeneration. Mater. Sci. Eng. C 2021, 131, 112499. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sanchez, C.; Al Mushref, F.R.A.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The Effect of Pore Size and Porosity on Mechanical Properties and Biological Response of Porous Titanium Scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Pudełko-Prażuch, I.; Balasubramanian, M.; Ganesan, S.M.; Marecik, S.; Walczak, K.; Pielichowska, K.; Chatterjee, S.; Kandaswamy, R.; Pamuła, E. Characterization and In Vitro Evaluation of Porous Polymer-Blended Scaffolds Functionalized with Tricalcium Phosphate. J. Funct. Biomater. 2024, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Winnett, J.; Jumbu, N.; Cox, S.; Gibbons, G.; Grover, L.M.; Warnett, J.; Williams, M.A.; Dancer, C.E.J.; Mallick, K.K. In-Vitro Viability of Bone Scaffolds Fabricated Using the Adaptive Foam Reticulation Technique. Biomater Adv. 2022, 136, 212766. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Huang, P.; Prasopthum, A.; Parsons, A.J.; Ai, F.; Yang, J. Characterisation of Bone Regeneration in 3D Printed Ductile PCL/PEG/Hydroxyapatite Scaffolds with High Ceramic Microparticle Concentrations. Biomater. Sci. 2022, 10, 138–152. [Google Scholar] [CrossRef]
- Di, W.; Ren, H.; Li, W.; Liu, D.; Sun, X. Fabrication and Characterization of β-TCP/Zn-1Mg Composite Scaffolds for Orthopedic Applications. Mater. Today Commun. 2024, 39, 109059. [Google Scholar] [CrossRef]
- Wang, B.; Ye, X.; Chen, G.; Zhang, Y.; Zeng, Z.; Liu, C.; Tan, Z.; Jie, X. Fabrication and Properties of PLA/β-TCP Scaffolds Using Liquid Crystal Display (LCD) Photocuring 3D Printing for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2024, 12, 1273541. [Google Scholar] [CrossRef] [PubMed]
- Zenebe, C.G. A Review on the Role of Wollastonite Biomaterial in Bone Tissue Engineering. BioMed Res. Int. 2022, 2022, 4996530. [Google Scholar] [CrossRef] [PubMed]
- Arpornmaeklong, P.; Pressler, M.J. Effects of SS-TCP Scaffolds on Neurogenic and Osteogenic Differentiation of Human Embryonic Stem Cells. Anat. Anz. 2018, 215, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Graça, M.P.F.; Gavinho, S.R.; Jakka, S.K.; Borges, J.P.; Silva, J.C.; Costa, L.C. Exploring the Impact of Copper Oxide Substitution on Structure, Morphology, Bioactivity, and Electrical Properties of 45S5 Bioglass®. Biomimetics 2024, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.H.; Magalhães, J.A.; Gouveia, R.F.; Bertran, C.A.; Motisuke, M.; Camargo, S.E.A.; Trichês, E.D.S. Hierarchical Structures of β-TCP/45S5 Bioglass Hybrid Scaffolds Prepared by Gelcasting. J. Mech. Behav. Biomed. Mater. 2016, 62, 10–23. [Google Scholar] [CrossRef]
- Wong, J.F.; Chan, J.X.; Hassan, A.B.; Mohamad, Z.B.; Othman, N.B. Thermal and Flammability Properties of Wollastonite-Filled Thermoplastic Composites: A Review. J. Mater. Sci. 2021, 56, 8911–8950. [Google Scholar] [CrossRef]
- Canales, D.; Saavedra, M.; Flores, M.T.; Bejarano, J.; Ortiz, J.A.; Orihuela, P.; Alfaro, A.; Pabón, E.; Palza, H.; Zapata, P.A. Effect of Bioglass Nanoparticles on the Properties and Bioactivity of Poly(Lactic Acid) Films. J. Biomed. Mater. Res. 2020, 108, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, L.; Gruetzmacher, J.A.; Currier, B.L.; Yaszemski, M.J. A Biodegradable and Cross-Linkable Multiblock Copolymer Consisting of Poly(Propylene Fumarate) and Poly(ε-Caprolactone): Synthesis, Characterization, and Physical Properties. Macromolecules 2005, 38, 7358–7370. [Google Scholar] [CrossRef]
- Matumba, K.I.; Motloung, M.P.; Ojijo, V.; Ray, S.S.; Sadiku, E.R. Investigation of the Effects of Chain Extender on Material Properties of PLA/PCL and PLA/PEG Blends: Comparative Study between Polycaprolactone and Polyethylene Glycol. Polymers 2023, 15, 2230. [Google Scholar] [CrossRef]
- Ju, Z.; Brosse, N.; Hoppe, S.; Wang, Z.; Ziegler-Devin, I.; Zhang, H.; Shu, B. Thermal and Mechanical Properties of Polyethylene Glycol (PEG)-Modified Lignin/Polylactic Acid (PLA) Biocomposites. Int. J. Biol. Macromol. 2024, 262, 129997. [Google Scholar] [CrossRef]
- Santos, G.G.D.; Vasconcelos, L.Q.; Barreto, I.C.; Miguel, F.B.; Araújo, R.P.C.D. Wollastonite and Tricalcium Phosphate Composites for Bone Regeneration. Res. Soc. Dev. 2022, 11, e12011931662. [Google Scholar] [CrossRef]
- Gonçalves Dos Santos, G.; Borges Miguel, I.R.J.; De Almeida Barbosa Junior, A.; Teles Barbosa, W.; Vieira De Almeida, K.; García-Carrodeguas, R.; Lia Fook, M.; Rodríguez, M.A.; Borges Miguel, F.; Correia De Araújo, R.P.; et al. Bone Regeneration Using Wollastonite/β-TCP Scaffolds Implants in Critical Bone Defect in Rat Calvaria. Biomed. Phys. Eng. Express 2021, 7, 055015. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M. Mechanism of Bone Mineralization. Cold Spring Harb. Perspect. Med. 2018, 8, a031229. [Google Scholar] [CrossRef] [PubMed]
- Łukowicz, K.; Zagrajczuk, B.; Nowak, A.; Niedźwiedzki, Ł.; Laczka, M.; Cholewa-Kowalska, K.; Osyczka, A.M. The Role of CaO/SiO2 Ratio and P2O5 Content in Gel-Derived Bioactive Glass-Polymer Composites in the Modulation of Their Bioactivity and Osteoinductivity in Human BMSCs. Mater. Sci. Eng. C 2020, 109, 110535. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, W.M.; Kyriakides, T.R. Cell Interactions with Polymers. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–293. ISBN 978-0-12-818422-6. [Google Scholar]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Kasper, F.K.; Tanahashi, K.; Fisher, J.P.; Mikos, A.G. Synthesis of Poly(Propylene Fumarate). Nat. Protoc. 2009, 4, 518–525. [Google Scholar] [CrossRef] [PubMed]

| Sample | Tg [°C] | Tcc [°C] | Tm [°C] | Xc [%] | ||||
|---|---|---|---|---|---|---|---|---|
| 1st Heating | 2nd Heating | 1st Heating | 2nd Heating | 1st Heating | 2nd Heating | 1st Heating | 2nd Heating | |
| 1 (Blend_TCP20_BG5) | −51 (PCL) 48 (PLA) | 39 (PLA) | nd | 83 (PLA) | 62 (PCL) 153 (PLA) | 53 (PCL) 145 (PLA) | 43 | 35 |
| 2 (Blend_W20_BG5) | nd | nd | nd | 89 (PLA) | 142 (PLA) | 144 (PLA) | 41 | 39 |
| 3 (Blend_TCP10_W10_BG5) | nd | nd | nd | 96 (PLA) | 143 (PLA) | 146 (PLA) | 37 | 34 |
| Sample | Mass Loss [%] | Char Residue at 600 °C [%] | T1% [°C] | T3% [°C] | T5% [°C] | T10% [°C] | T50% [°C] | TDTGmax [°C] |
|---|---|---|---|---|---|---|---|---|
| 1 (Blend_TCP20_BG5) | 78 | 22 | 230 | 287 | 308 | 329 | 366 | 351 (PCL) 383 (PLA) 392 (PEG) |
| 2 (Blend_W20_BG5) | 79 | 21 | 226 | 287 | 297 | 307 | 341 | 315 (PCL) 333 (PLA) 394 (PEG) |
| 3 (Blend_TCP10_W10_BG5) | 79 | 21 | 254 | 308 | 318 | 335 | 369 | 324 (PEG) 366 (PLA) 391 (PEG) |
| Sample | Component [wt. %] | ||||||
|---|---|---|---|---|---|---|---|
| PLA | PCL | PEG | PPF | TCP | W | BG | |
| 1—Blend_TCP20_BG5 | 55 | 12 | 5 | 3 | 20 | - | 5 |
| 2—Blend_W20_BG5 | 55 | 12 | 5 | 3 | - | 20 | 5 |
| 3—Blend_TCP10_W10_BG5 | 55 | 12 | 5 | 3 | 10 | 10 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marecik, S.; Pudełko-Prażuch, I.; Balasubramanian, M.; Ganesan, S.M.; Chatterjee, S.; Pielichowska, K.; Kandaswamy, R.; Pamuła, E. Effect of the Addition of Inorganic Fillers on the Properties of Degradable Polymeric Blends for Bone Tissue Engineering. Molecules 2024, 29, 3826. https://doi.org/10.3390/molecules29163826
Marecik S, Pudełko-Prażuch I, Balasubramanian M, Ganesan SM, Chatterjee S, Pielichowska K, Kandaswamy R, Pamuła E. Effect of the Addition of Inorganic Fillers on the Properties of Degradable Polymeric Blends for Bone Tissue Engineering. Molecules. 2024; 29(16):3826. https://doi.org/10.3390/molecules29163826
Chicago/Turabian StyleMarecik, Stanisław, Iwona Pudełko-Prażuch, Mareeswari Balasubramanian, Sundara Moorthi Ganesan, Suvro Chatterjee, Kinga Pielichowska, Ravichandran Kandaswamy, and Elżbieta Pamuła. 2024. "Effect of the Addition of Inorganic Fillers on the Properties of Degradable Polymeric Blends for Bone Tissue Engineering" Molecules 29, no. 16: 3826. https://doi.org/10.3390/molecules29163826







