Study on the Gas-Chromic Character of Pd/TiO2 for Fast Room-Temperature CO Detection
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Pd/TiO2
2.2. DFT Computational Analysis
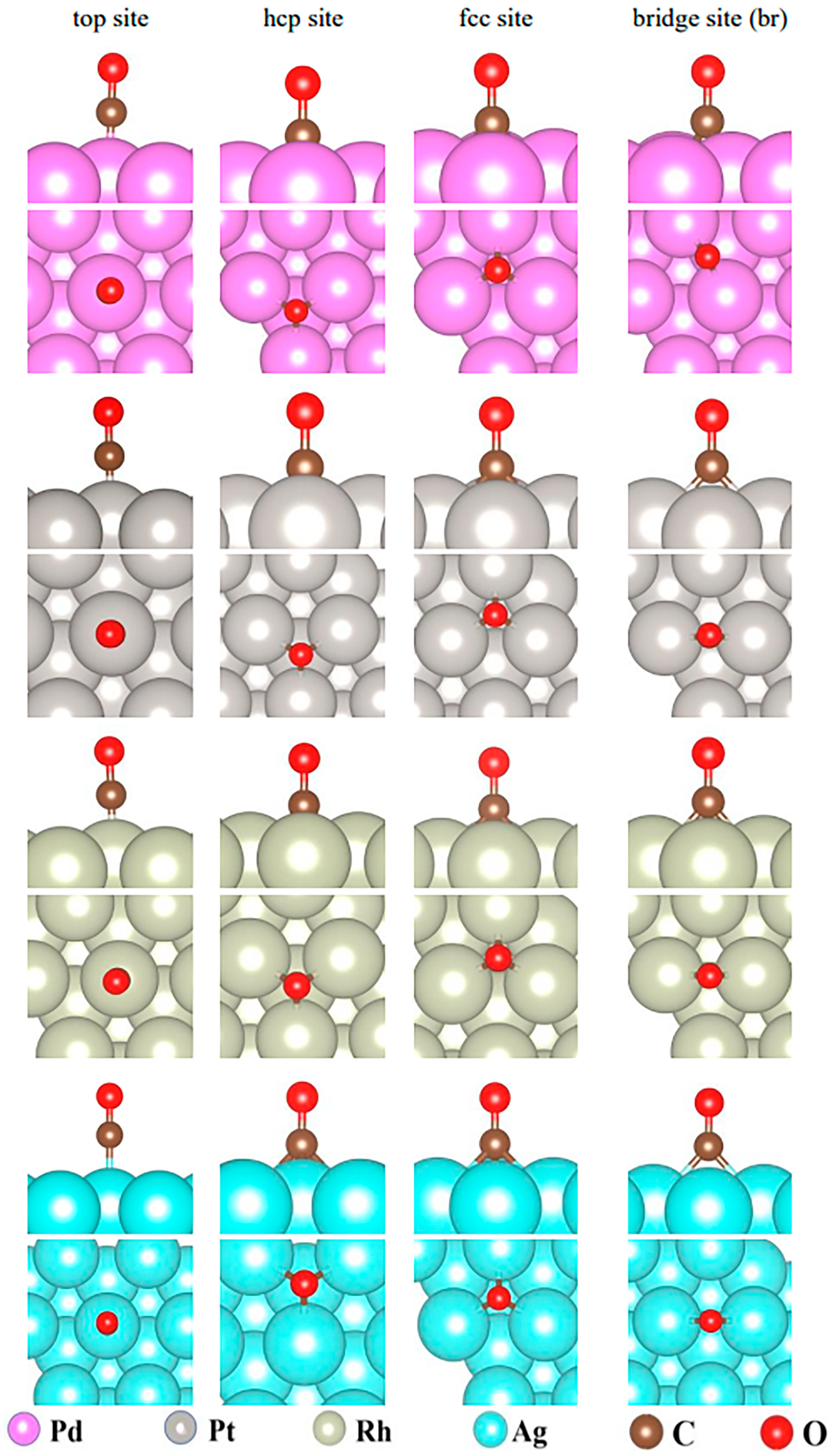
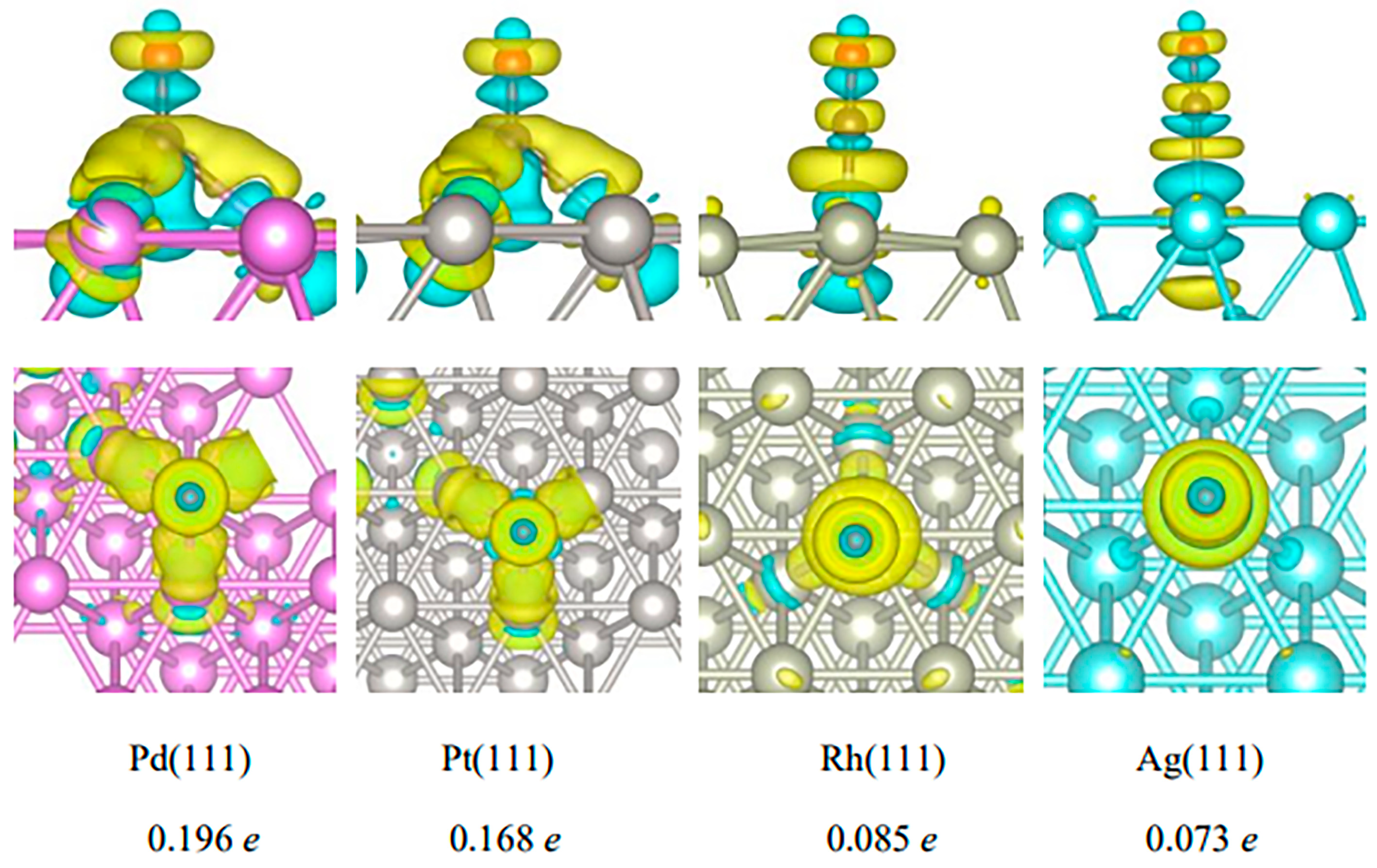
2.2.1. Adsorption of CO on the Surfaces of Pd(111), Pt(111), Rh(111), and Ag(111)
2.2.2. Adsorption of CO on the Surface of PdO
2.3. Catalytic Chromic Experiments
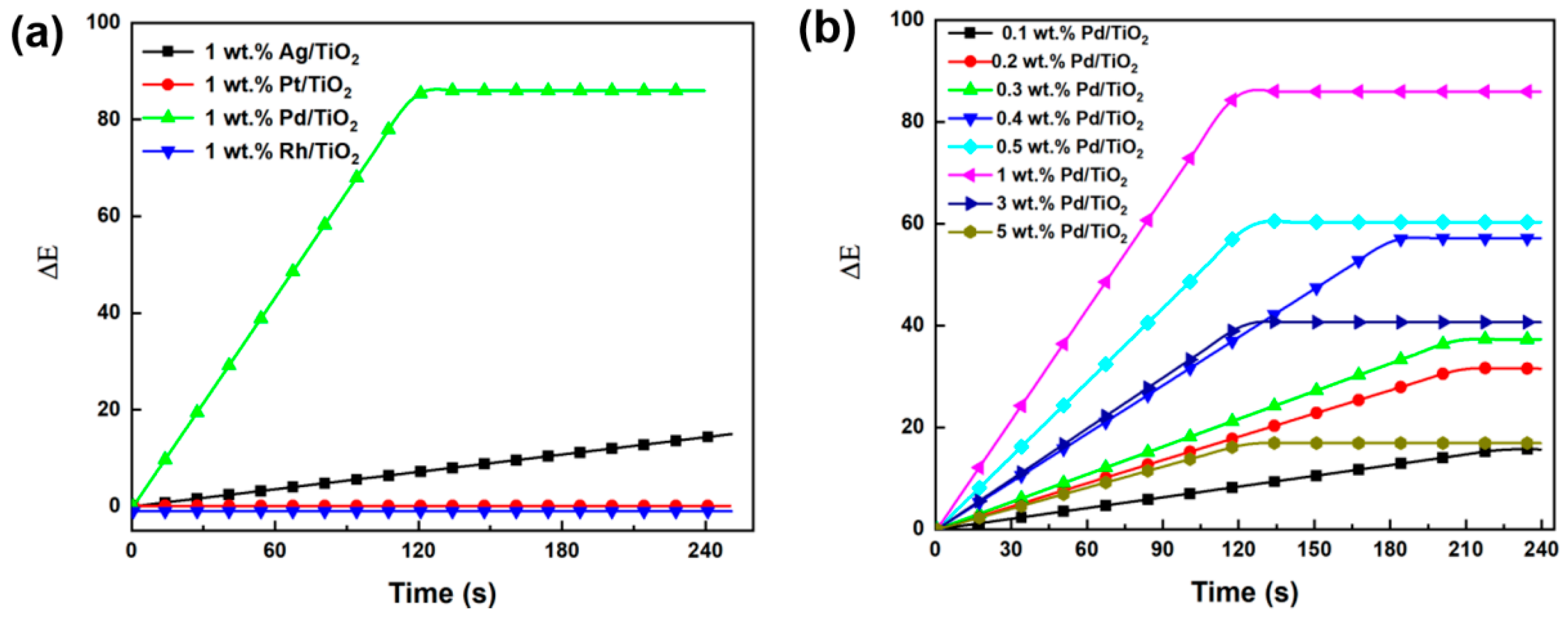
2.3.1. Effect of Noble Metals
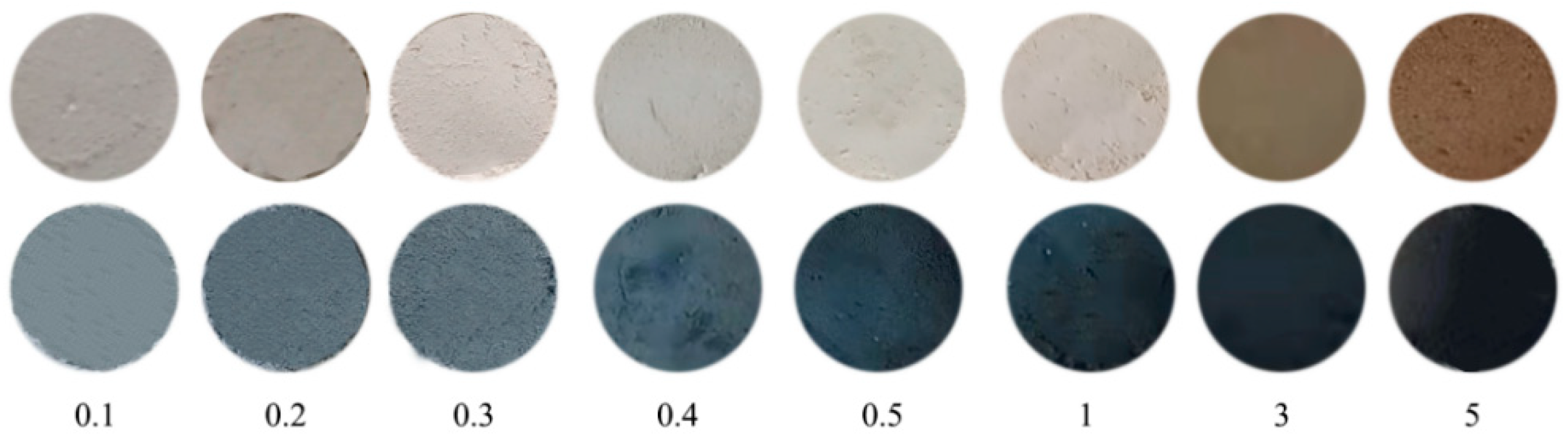
2.3.2. Effect of Pd Loading Content
3. Experimental
3.1. Synthesis of Pd/TiO2 Catalyst
3.2. CO Gasochromic Evaluation
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soto-Garcia, L.; Huang, X.; Thimmaiah, D.; Rossner, A.; Hopke, P.K. Exposures to Carbon Monoxide from Off-Gassing of Bulk Stored Wood Pellets. Energy Fuels 2015, 29, 218–226. [Google Scholar] [CrossRef]
- Hampson, N.B.; Weaver, L.K. Carbon monoxide poisoning and risk for ischemic stroke. Eur. J. Intern. Med. 2016, 31, e7. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.H.; Hoshi, T.; Westerhausen, M.; Schiller, A. Carbon Monoxide—Physiology, Detection and Controlled Release. ChemInform 2014, 50, 3644–3660. [Google Scholar] [CrossRef] [PubMed]
- Marks, G.S.; Vreman, H.J.; McLaughlin, B.E.; Brien, J.F.; Nakatsu, K. Measurement of Endogenous Carbon Monoxide Formation in Biological Systems. Antioxid. Redox Signal. 2002, 4, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Prockop, L.D.; Chichkova, R.I. Carbon monoxide intoxication: An updated review. J. Neurol. Sci. 2007, 262, 122–130. [Google Scholar] [CrossRef]
- Raub, J. Health effects of exposure to ambient carbon monoxide. Chemosphere 1999, 1, 331–351. [Google Scholar] [CrossRef]
- Yan, T.; Chen, J.; Wu, S.; Mao, Z.; Liu, Z. A Rationally Designed Fluorescence Chemosensor for On-Site Monitoring of Carbon Monoxide in Air. Org. Lett. 2014, 16, 3296–3299. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Hu, J.; Wang, J.; Ong, C.; Zhu, F. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B Chem. 2005, 115, 247–251. [Google Scholar] [CrossRef]
- Mohammad, D.S.; Masoudi, A.A.; Mortezaali, A.; Hosseini, Z.S. Enhanced Visible Light Pho-tocatalytic Activity of Nanostructured SiO2-TiO2 Thin Films through Optimizing Thickness. Phys. Scr. 2023, 98, 105904. [Google Scholar]
- Dinh, N.; Oanh, N.; Long, P.; Bernard, M.; Goff, A.H.-L. Electrochromic properties of TiO2 anatase thin films prepared by a dipping sol–gel method. Thin Solid Film. 2003, 423, 70–76. [Google Scholar] [CrossRef]
- Hwang, K.; T-Raissi, A.; Qin, N. Effect of pigment concentration and particle size of TiO2 support on performance of chemochromic hydrogen tape sensor. Int. J. Hydrogen Energy 2018, 43, 9877–9883. [Google Scholar] [CrossRef]
- Jenkins, A.H.; Medlin, J.W. Controlling Heterogeneous Catalysis with Organic Monolayers on Metal Oxides. Acc. Chem. Res. 2021, 54, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Sambare, A.A.; Pawar, R.; Shirsat, M. A DFT investigation on transition metal (Co, Cr, Cu, Mn, Mo and Nb)-doped bismuth ferrite oxide (BiFeO3) for CO gas adsorption. Theor. Chem. Acc. 2023, 142, 61. [Google Scholar] [CrossRef]
- Promthong, N.; Tabtimsai, C.; Rakrai, W.; Wanno, B. Transition metal-doped graphene nanoflakes for CO and CO2 storage and sensing applications: A DFT study. Struct. Chem. 2020, 31, 2237–2247. [Google Scholar] [CrossRef]
- Liu, H.M.; Wu, S.P.; Sun, C.; Huang, Z.; Xu, H.L.; Shen, W. Fabricating Uniform TiO2-CeO2 Solid Solution Supported Pd Catalysts by an In Situ Capture Strategy for Low-Temperature Co Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 10795–10802. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, W.; Ye, H.; Li, Y. Enhanced carbon monoxide sensing properties of TiO2 with exposed (0 0 1) facet: A combined first-principle and experimental study. Appl. Surf. Sci. 2018, 442, 507–516. [Google Scholar] [CrossRef]
- Chumakova, V.; Marikutsa, A.; Platonov, V.; Khmelevsky, N.; Rumyantseva, M. Distinct Roles of Additives in the Improved Sensitivity to CO of Ag- and Pd-Modified Nanosized LaFeO3. Chemosensors 2023, 11, 60. [Google Scholar] [CrossRef]
- Gupta, S.; Knoepfel, A.; Zou, H.; Ding, Y. Investigations of Methane Gas Sensor Based on Biasing Operation of N-Zno Nanorods/P-Si Assembled Diode and Pd Functionalized Schottky Junctions. Sens. Actuators B Chem. 2023, 392, 134030. [Google Scholar] [CrossRef]
- Kumar, A.; Mohammadi, M.M.; Swihart, M.T. Synthesis, growth mechanisms, and applications of palladium-based nanowires and other one-dimensional nanostructures. Nanoscale 2019, 11, 19058–19085. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, Y.; Xu, L.; Chen, X. Adsorption and sensing performances of transition metal (Ag, Pd, Pt, Rh, and Ru) modified WSe2 monolayer upon SF6 decomposition gases (SOF2 and SO2F2). Appl. Surf. Sci. 2022, 581, 152365. [Google Scholar] [CrossRef]
- Ndaya, C.C.; Javahiraly, N.; Brioude, A. Recent Advances in Palladium Nanoparticles-Based Hydrogen Sensors for Leak Detection. Sensors 2019, 19, 4478. [Google Scholar] [CrossRef] [PubMed]
- Nihal; Sharma, R.; Kaur, N.; Choudhary, B.C.; Goswamy, J.K. DFT based study of transition metals (Au,Ag,Pd& Pt) doped graphitic carbon nitride (gCN) monolayer as a CO gas sensor. Phys. Scr. 2022, 97, 065706. [Google Scholar] [CrossRef]
- Sharma, B.; Myung, J. Pd-based ternary alloys used for gas sensing applications: A review. Int. J. Hydrogen Energy 2019, 44, 30499–30510. [Google Scholar] [CrossRef]
- Kumar, A.; Zhao, Y.; Mohammadi, M.M.; Liu, J.; Thundat, T.; Swihart, M.T. Palladium Nanosheet-Based Dual Gas Sensors for Sensitive Room-Temperature Hydrogen and Carbon Monoxide Detection. ACS Sens. 2022, 7, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.J.; DeLaRiva, A.T.; Lin, S.; Johnson, R.S.; Guo, H.; Miller, J.T.; Kwak, J.H.; Peden, C.H.F.; Kiefer, B.; Allard, L.F.; et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 2014, 5, 4885. [Google Scholar] [CrossRef] [PubMed]
- Kopelent, R.; Tereshchenko, A.; Guda, A.; Smolentsev, G.; Artiglia, L.; Sushkevich, V.L.; Bugaev, A.; Sadykov, I.I.; Baidya, T.; Bodnarchuk, M.; et al. Enhanced Reducibility of the Ceria–Tin Oxide Solid Solution Modifies the CO Oxidation Mechanism at the Platinum–Oxide Interface. ACS Catal. 2021, 11, 9435–9449. [Google Scholar] [CrossRef]
- Lin, L.; Hu, C.; Xu, S.; Jia, H.; Tao, H.; Zhang, Z. First-principle investigation of CO adsorption on Pd-loaded VO2 monolayer. Mater. Today Commun. 2022, 32, 103681. [Google Scholar] [CrossRef]
- Siemer, M.; Tomaschun, G.; Kluener, T.; Christopher, P.; Al-Shamery, K. Insights into Spec-tator-Directed Catalysis: Co Adsorption on Amine-Capped Platinum Nanoparticles on Oxide Supports. ACS Appl. Mater. Interfaces 2020, 27765–27776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, X.; Liu, F.; Liu, C.; Su, T.; Lin, J.; Sun, P.; Sun, Y.; Liu, F.; Lu, G. The enhanced CO gas sensing performance of Pd/SnO2 hollow sphere sensors under hydrothermal conditions. RSC Adv. 2016, 6, 80455–80461. [Google Scholar] [CrossRef]
- Beketov, G.; Heinrichs, B.; Pirard, J.-P.; Chenakin, S.; Kruse, N. XPS structural characterization of Pd/SiO2 catalysts prepared by cogelation. Appl. Surf. Sci. 2013, 287, 293–298. [Google Scholar] [CrossRef]
- Grissa, R.; Abramova, A.; Tambio, S.-J.; Lecuyer, M.; Deschamps, M.; Fernandez, V.; Greneche, J.-M.; Guyomard, D.; Lestriez, B.; Moreau, P. Thermomechanical Polymer Binder Reactivity with Positive Active Materials for Li Metal Polymer and Li-Ion Batteries: An XPS and XPS Imaging Study. ACS Appl. Mater. Interfaces 2019, 11, 18368–18376. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.F.; Gaber, S.; AlJaber, A.S.; Polychronopoulou, K. CO Oxidation at Near-Ambient Temperatures over TiO2-Supported Pd-Cu Catalysts: Promoting Effect of Pd-Cu Nanointerface and TiO2 Morphology. Nanomaterials 2021, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-A, E.; Rangel, R.; Solís-Garcia, A.; Venezia, A.M.; Zepeda, T.A. Ftir Investigation under Reaction Conditions during Co Oxidation over Rux-CeO2 Catalysts. Mol. Catal. 2020, 493, 111086. [Google Scholar] [CrossRef]
- Tokranov, A.A.; Tokranova, E.O.; Shafigulin, R.V.; Bulanova, A.V. Study of the Adsorption Properties of Lantha-num-Doped Mesoporous Silica Gels, Modified with Nickel and Silver, by the Method of Gas Chromatography. Prot. Met. Phys. Chem. 2022, 58, 1164–1170. [Google Scholar]
- Chauhan, N.; Singh, V.; Kumar, S.; Kumari, M. Preparation, Characterization and Evaluations of Carbon-Doped Ag/Fe/TiO2 Mesoporous Nanocomposite Photocatalyst for Degradation of Methylene Blue and Congo Red. J. Nanosci. Nanotechnol. 2021, 21, 5344–5351. [Google Scholar] [CrossRef] [PubMed]
- Ahasan, R.; Wang, Y.; Wang, R. In situ DRIFTS and CO-TPD studies of CeO2 and SiO2 supported CuOx catalysts for CO oxidation. Mol. Catal. 2022, 518, 112085. [Google Scholar] [CrossRef]
- Liu, X.; Xu, M.; Wan, L.; Zhu, H.; Yao, K.; Linguerri, R.; Chambaud, G.; Han, Y.; Meng, C. Superior catalytic performance of atomically dispersed palladium on graphene in CO oxidation. ACS Catal. 2020, 10, 3084–3093. [Google Scholar] [CrossRef]
- Hu, Z.; Xiang, Y.; Li, Y.; Long, Z.; Liu, A.; Dai, X.; Lei, X.; Tang, Z. Research on Identification Technology of Field Pests with Protective Color Characteristics. Appl. Sci. 2022, 12, 3810. [Google Scholar] [CrossRef]
- Wang, N.; Qian, T.; Yang, J.; Li, L.; Zhang, Y.; Zheng, X.; Xu, Y.; Zhao, H.; Zhao, J. An Enhanced YOLOv5 Model for Greenhouse Cucumber Fruit Recognition Based on Color Space Features. Agriculture 2022, 12, 1556. [Google Scholar] [CrossRef]
- Yao, J.; Fan, X.; Li, B.; Qin, W. Adverse Weather Target Detection Algorithm Based on Adaptive Color Levels and Improved YOLOv5. Sensors 2022, 22, 8577. [Google Scholar] [CrossRef]


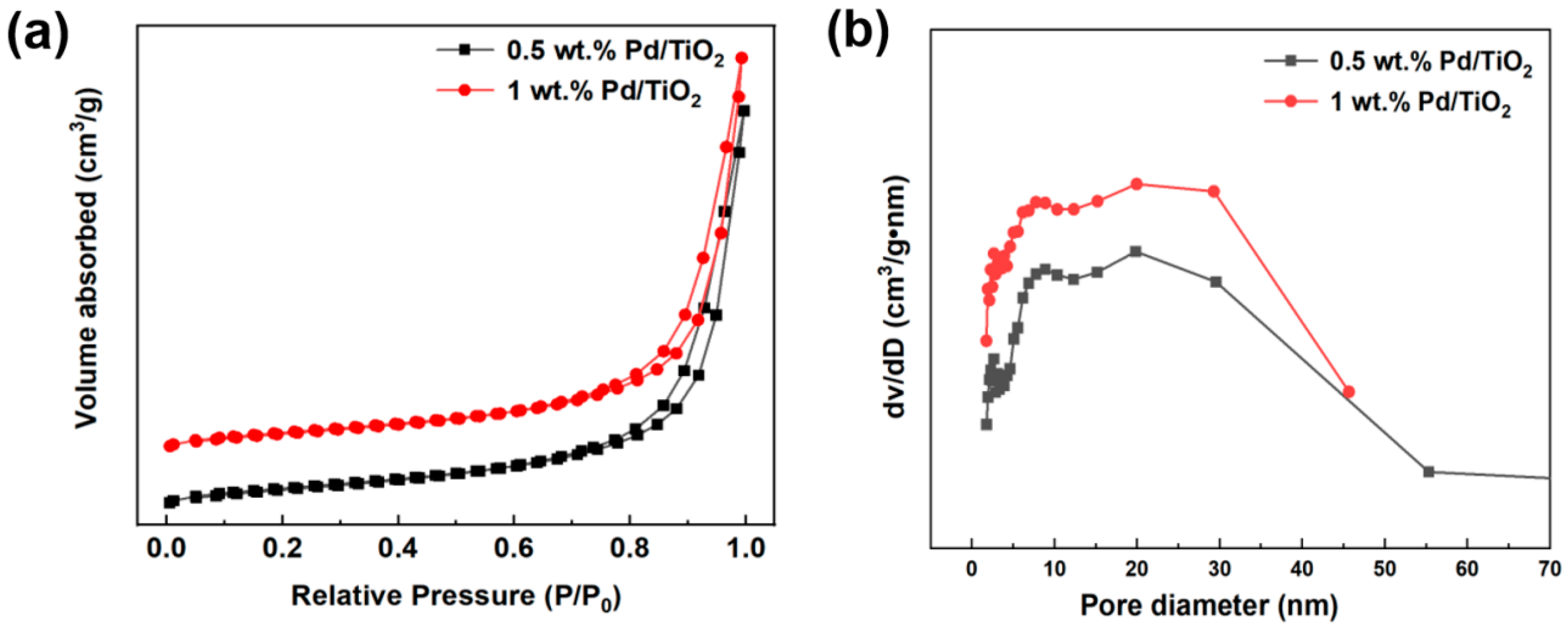


| Catalyst | SBET (m2·g−1) | Vtotal (cm3·g−1) | DP (nm) |
|---|---|---|---|
| 0.5 wt.% Pd/TiO2 | 75.2 | 0.44 | 23.6 |
| 1 wt.% Pd/TiO2 | 76.8 | 0.45 | 23.3 |
| Metal Surface | Adsorption Site | Ead (eV) | DM-C (Å) |
|---|---|---|---|
| Pd(111) | top | −1.43 | 1.85 |
| hcp | −1.99 | 2.07, 2.07, 2.08 | |
| fcc | −1.94 | 2.08, 2.08, 2.08 | |
| br | −1.83 | 1.99, 2.00 | |
| Pt(111) | top | −1.72 | 1.84 |
| hcp | −1.85 | 2.10, 2.12, 2.12 | |
| fcc | −1.78 | 2.12, 2.13, 2.13 | |
| br | −1.83 | 2.02, 2.02 | |
| Rh(111) | top | −1.81 | 1.84 |
| hcp | −1.79 | 2.09, 2.09, 2.10 | |
| fcc | −1.68 | 2.10, 2.10, 2.14 | |
| br | −1.70 | 2.03, 2.03 | |
| Ag(111) | top | −0.07 | 2.17 |
| hcp | −0.06 | 2.34, 2.32, 2.32 | |
| fcc | −0.07 | 2.32, 2.31, 2.32 | |
| br | −0.06 | 2.25, 2.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Sun, K.; Chen, Y.; Yuan, Y. Study on the Gas-Chromic Character of Pd/TiO2 for Fast Room-Temperature CO Detection. Molecules 2024, 29, 3843. https://doi.org/10.3390/molecules29163843
Li X, Sun K, Chen Y, Yuan Y. Study on the Gas-Chromic Character of Pd/TiO2 for Fast Room-Temperature CO Detection. Molecules. 2024; 29(16):3843. https://doi.org/10.3390/molecules29163843
Chicago/Turabian StyleLi, Xinbao, Kai Sun, Ying Chen, and Ye Yuan. 2024. "Study on the Gas-Chromic Character of Pd/TiO2 for Fast Room-Temperature CO Detection" Molecules 29, no. 16: 3843. https://doi.org/10.3390/molecules29163843
APA StyleLi, X., Sun, K., Chen, Y., & Yuan, Y. (2024). Study on the Gas-Chromic Character of Pd/TiO2 for Fast Room-Temperature CO Detection. Molecules, 29(16), 3843. https://doi.org/10.3390/molecules29163843






