Synthesis, Photoluminescence, and Electroluminescence of Phosphorescent Dipyrido[3,2-a;2′3′-c]phenazine–Platinum(II) Complexes Bearing Hole-Transporting Acetylide Ligands
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. UV-vis Absorption and Photoluminescence Properties
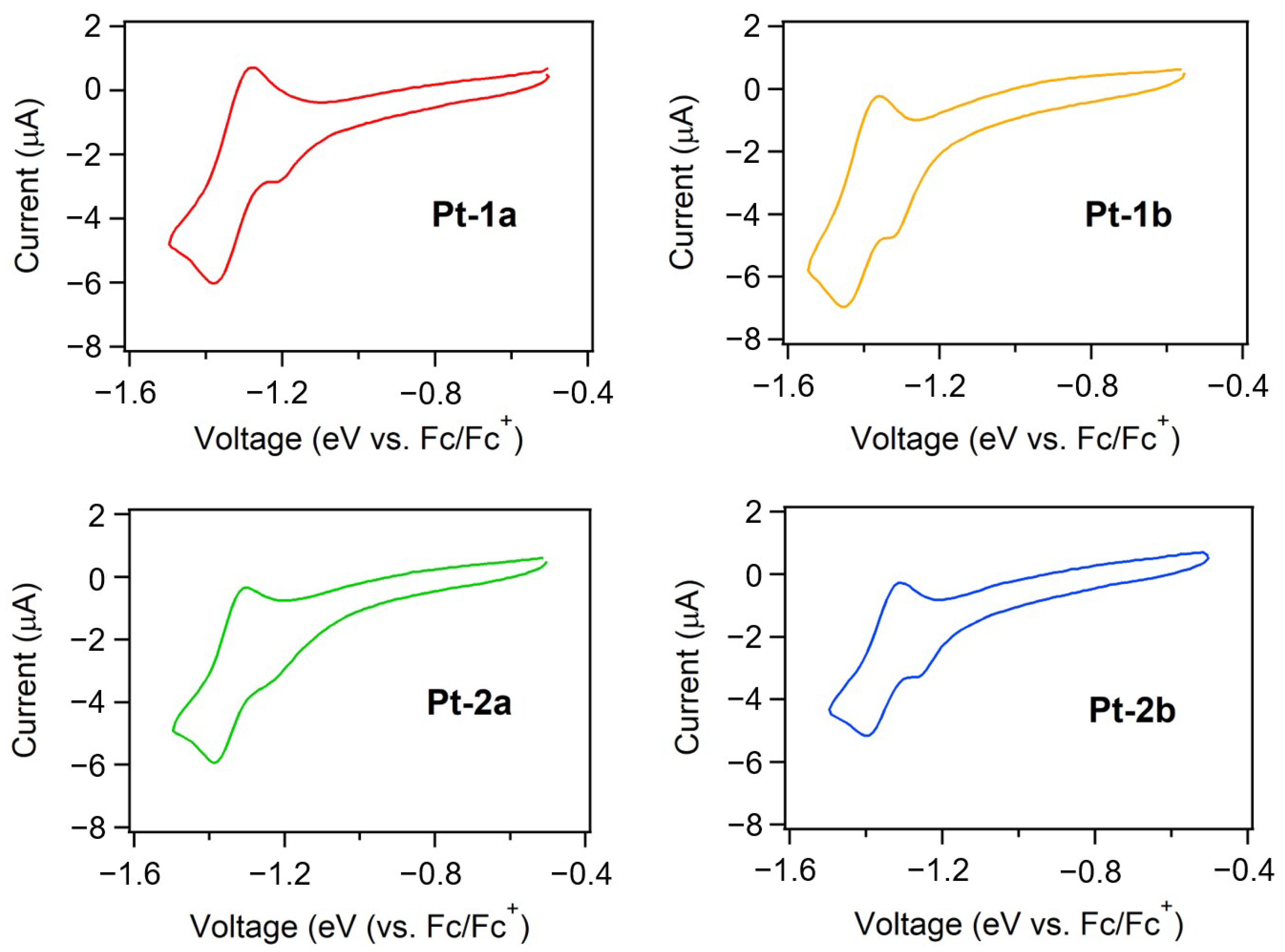
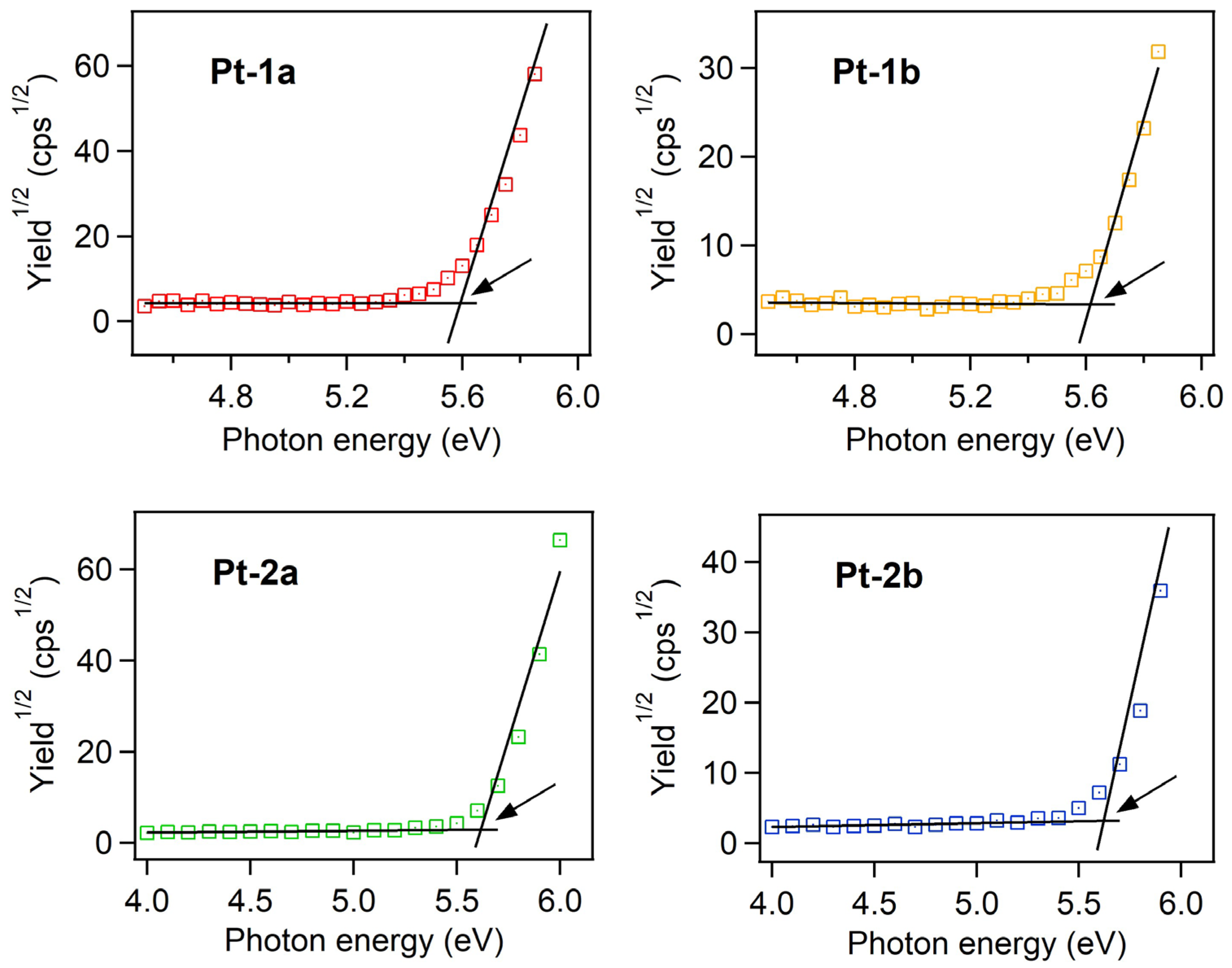
2.3. Evaluation of HOMO and LUMO Energy Levels
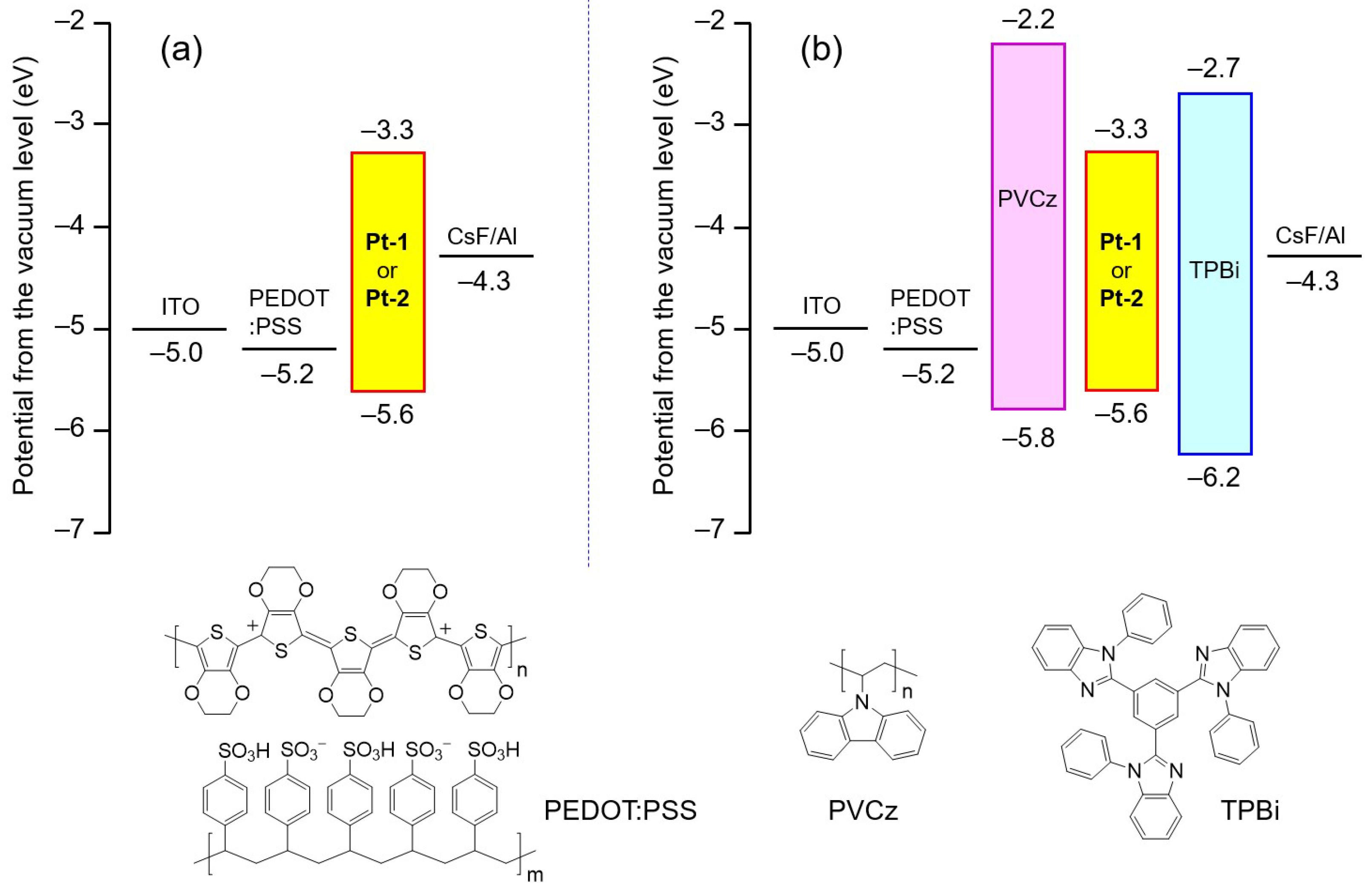
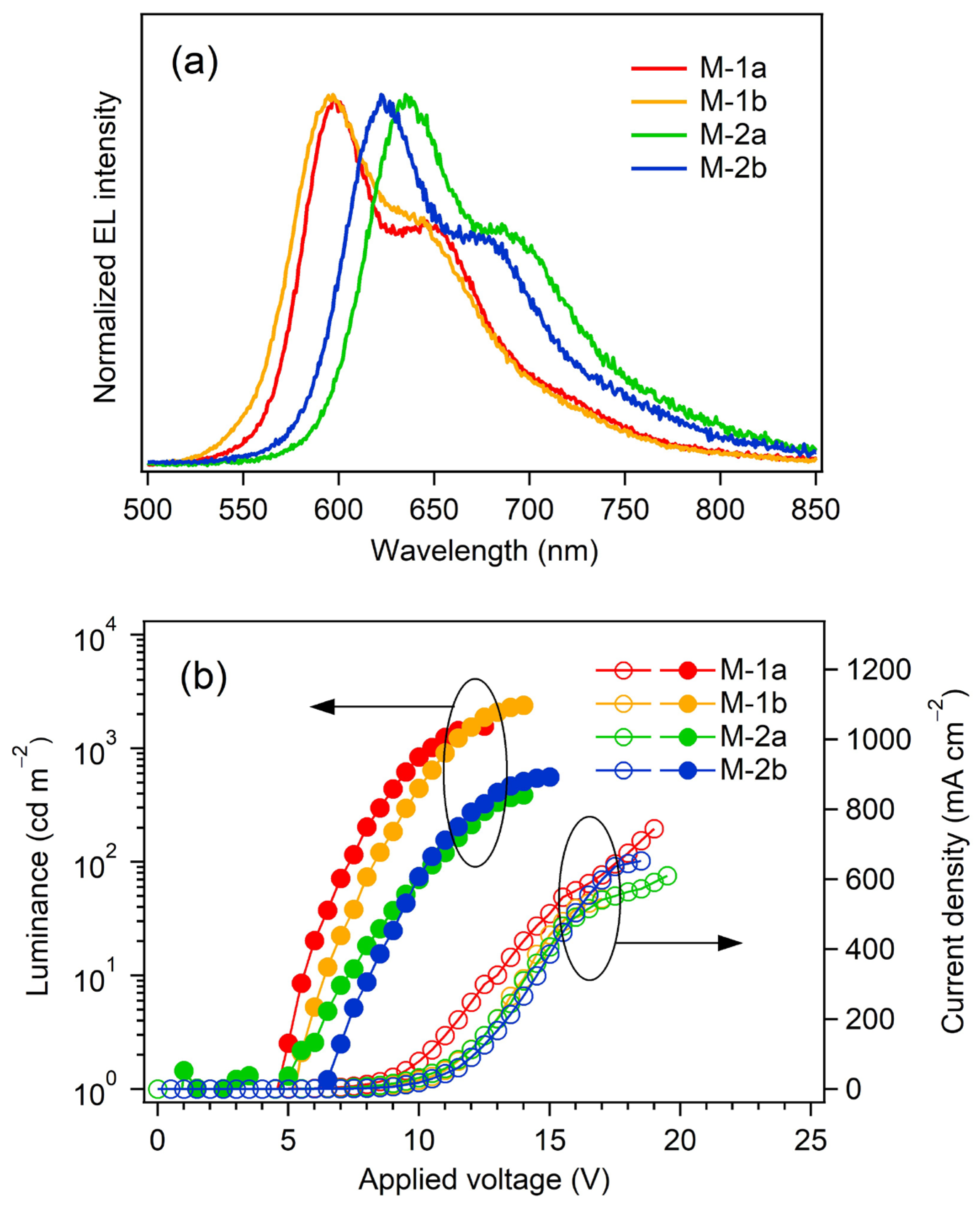
2.4. Fabrication of OLEDs
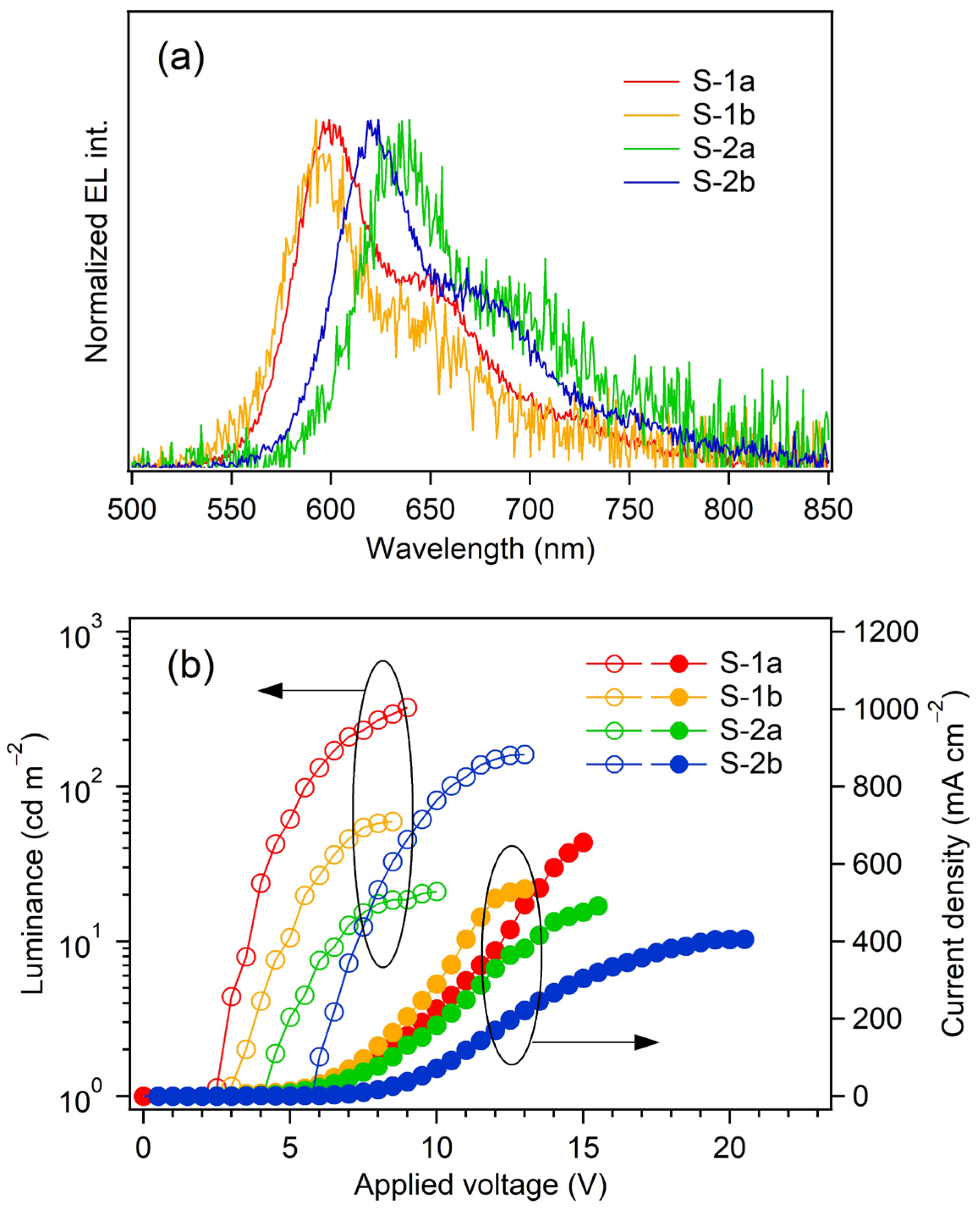
2.4.1. Fabrication of Non-Doped OLEDs with a Simple Device Structure
2.4.2. Fabrication of Non-Doped OLED with a Multilayer Structure
3. Methods
3.1. Evaluation of Optical and Photophysical Properties
3.2. Evaluation of Electrochemical Properties
3.3. IP Measurements
3.4. Fabrication of OLEDs
3.4.1. Fabrication of a Single-Layer Device
3.4.2. Fabrication of a Multilayer Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A.; Chen, C.H. Electroluminescence of Doped Organic Thin Films. J. Appl. Phys. 1989, 65, 3610–3616. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent Progress in Metal–Organic Complexes for Optoelectronic Applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef]
- Farinola, G.M.; Ragni, R. Electroluminescent Materials for White Organic Light Emitting Diodes. Chem. Soc. Rev. 2011, 40, 3467–3482. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, X.; Kon, F.; Tang, J.; Yan, D.; Wang, J.; Liu, Y.; Sun, Y.; Sheng, R.; Chen, P. Progress in Research on White Organic Light-Emitting Diodes Based on Ultrathin Emitting Layers. Micromachines 2024, 15, 626. [Google Scholar] [CrossRef] [PubMed]
- Liguori, R.; Nunziata, F.; Aprano, S.; Maglione, M.G. Overcoming Challenges in OLED Technology for Lighting Solutions. Electronics 2024, 13, 1299. [Google Scholar] [CrossRef]
- Miao, W.-C.; Hsiao, F.-H.; Sheng, Y.; Lee, T.-Y.; Hong, Y.-H.; Tsai, C.-W.; Chen, H.-L.; Liu, Z.; Lin, C.-L.; Chung, R.J.; et al. Microdisplays: Mini-LED, Micro-OLED, and Micro-LED. Adv. Optical Mater. 2024, 12, 2300112. [Google Scholar] [CrossRef]
- Woo, J.-Y.; Park, M.-H.; Jeong, S.-H.; Kim, Y.-H.; Kim, B.; Lee, T.-W.; Han, T.-H. Advances in Solution-Processed OLEDs and their Prospects for Use in Displays. Adv. Mater. 2023, 35, 2207454. [Google Scholar] [CrossRef]
- Murawski, C.; Gather, M.C. Emerging Biomedical Applications of Organic Light-Emitting Diodes. Adv. Optical Mater. 2021, 9, 2100269. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% Internal Phosphorescence Efficiency in an Organic Light Emitting Device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef]
- Brooks, J.; Babayan, Y.; Lamansky, S.; Djurovich, P.I.; Tsyba, I.; Bau, R.; Thompson, M.E. Synthesis and Characterization of Phosphorescent Cyclometalated Platinum Complexes. Inorg. Chem. 2002, 41, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, V.; Brooks, J.; Tamayo, A.; Alexander, A.M.; Djurovich, P.I.; D’Andrade, B.W.; Adachi, C.; Forrest, S.R.; Thompson, M.E. High Efficiency Single Dopant White Electrophosphorescent Light Emitting Diodes. New J. Chem. 2002, 26, 1171–1178. [Google Scholar] [CrossRef]
- Lu, W.; Mi, B.-X.; Chan, M.C.W.; Hui, Z.; Che, C.-M.; Zhu, N.; Lee, S.T. Light-Emitting Tridentate Cyclometalated Platinum(II) Complexes Containing σ-Alkynyl Auxiliaries: Tuning of Photo- and Electrophosphorescence. J. Am. Chem. Soc. 2004, 126, 4958–4971. [Google Scholar] [CrossRef]
- Unger, Y.; Meyer, D.; Molt, O.; Schildknecht, C.; Münster, I.; Wagenblast, G.; Strassner, T. Green–Blue Emitters: NHC-Based Cyclometalated [Pt(C^C*)(acac)] Complexes. Angew. Chem. Int. Ed. 2010, 49, 10214–10216. [Google Scholar] [CrossRef]
- Rossi, E.; Murphy, L.; Brothwood, P.L.; Colombo, A.; Dragonetti, C.; Roberto, D.; Ugo, R.; Cocchi, M.; Williams, J.A.G. Cyclometallated Platinum(II) Complexes of 1,3-Di(2-pyridyl)benzenes: Tuning Excimer Emission from Red to Near-Infrared for NIR-OLEDs. J. Mater. Chem. 2011, 21, 15501–15510. [Google Scholar] [CrossRef]
- Fukagawa, H.; Shimizu, T.; Hanashima, H.; Osada, Y.; Suzuki, M.; Fujikake, H. Highly Efficient and Stable Red Phosphorescent Organic Light-Emitting Diodes Using Platinum Complexes. Adv. Mater. 2012, 24, 5099–5103. [Google Scholar] [CrossRef]
- Soellner, J.; Strassner, T. The “Enders Triazole” Revisited: Highly Efficient, Blue Platinum(II) Emitters. Organometallics 2018, 37, 1821–1824. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef]
- Tamayo, A.B.; Alleyne, B.D.; Djurovich, P.I.; Lamansky, S.; Tsyba, I.; Ho, N.N.; Bau, R.; Thompson, M.E. Synthesis and Characterization of Facial and Meridional Tris-Cyclometalated Iridium(III) Complexes. J. Am. Chem. Soc. 2003, 125, 7377–7387. [Google Scholar] [CrossRef]
- Chang, C.-F.; Cheng, Y.-M.; Chi, Y.; Chiu, Y.-C.; Lin, C.-C.; Lee, G.-H.; Chou, P.-T.; Chen, C.-C.; Chang, C.-H.; Wu, C.-C. Highly Efficient Blue-Emitting Iridium(III) Carbene Complexes and Phosphorescent OLEDs. Angew. Chem. Int. Ed. 2008, 47, 4542–4545. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, K.-M.; Yang, K.; Kang, Y. Blue Phosphorescent Ir(III) Complex with High Color Purity: Fac-Tris(2′,6′-difluoro-2,3′-bipyridinato-N,C4′)iridium(III). Inorg. Chem. 2009, 48, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Yagi, S.; Asuka, H.; Inui, Y.; Ikawa, S.; Maeda, T.; Nakazumi, H.; Sakurai, Y. Pure Red Electrophosphorescence from Polymer Light-Emitting Diodes Doped with Highly Emissive Bis-Cyclometalated Iridium(III) Complexes. J. Organomet. Chem. 2010, 695, 1972–1978. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, N.S.; Oh, H.-Y.; Yang, J.H.; Jeon, W.S.; Park, J.S.; Suh, M.C.; Kwon, J.H. Highly Efficient Red Phosphorescent Dopants in Organic Light-Emitting Devices. Adv. Mater. 2011, 23, 2721–2726. [Google Scholar] [CrossRef]
- Kang, Y.; Chang, Y.-L.; Lu, J.-S.; Ko, S.-B.; Rao, Y.; Varlan, M.; Luc, Z.-H.; Wang, S. Highly Efficient Blue Phosphorescent and Electroluminescent Ir(III) Compounds. J. Mater. Chem. C 2013, 1, 441–450. [Google Scholar] [CrossRef]
- Duan, L.; Hou, L.; Lee, T.-W.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Solution Processable Small Molecules for Organic Light-Emitting Diodes. J. Mater. Chem. 2010, 20, 6392–6407. [Google Scholar] [CrossRef]
- Yook, K.S.; Lee, J.Y. Small Molecule Host Materials for Solution Processed Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2014, 26, 4218–4233. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-Y.; Tang, Y.-Q.; Cai, X.-Y.; Tang, J.-X.; Li, Y.-Q. Solution-Processed OLEDs for Printing Displays. Mater. Chem. Front. 2023, 7, 1166–1196. [Google Scholar] [CrossRef]
- Aizawa, N.; Pu, Y.-J.; Watanabe, M.; Chiba, T.; Ideta, K.; Toyota, N.; Igarashi, M.; Suzuri, Y.; Sasabe, H.; Kido, J. Solution-Processed Multilayer Small-Molecule Light-Emitting Devices with High-Efficiency White-Light Emission. Nat. Commun. 2014, 5, 5756. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Moses, D.; Heeger, A.J. Polymer-Based Light-Emitting Diodes (PLEDs) and Displays Fabricated from Arrays of PLEDs. In Organic Light Emitting Devices: Synthesis, Properties, and Applications; Müllen, K., Scherf, U., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 151–180. [Google Scholar]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Cardone, A.; Martinelli, C.; Farinola, G.M.; Babudri, F.; Naso, F.; Büchel, M.; Bruno, G. Blue-Gap Poly(p-phenylene vinylene)s with Fluorinated Double Bonds: Interplay Between Supramolecular Organization and Optical Properties in Thin Films. Adv. Mater. 2009, 21, 1115–1120. [Google Scholar] [CrossRef]
- Pu, Y.-J.; Morishita, N.; Chiba, T.; Ohisa, S.; Igarashi, M.; Masuhara, A.; Kido, J. Efficient Electron Injection by Size- and Shape-Controlled Zinc Oxide Nanoparticles in Organic Light-Emitting Devices. ACS Appl. Mater. Interfaces 2015, 7, 25373–25377. [Google Scholar] [CrossRef]
- Xu, F.; Kim, H.U.; Kim, J.-H.; Jung, B.J.; Grimsdale, A.C.; Hwang, D.-H. Progress and Perspective of Iridium-Containing Phosphorescent Polymers for Light-Emitting Diodes. Prog. Polym. Sci. 2015, 47, 92–121. [Google Scholar] [CrossRef]
- Liu, B.; Dang, F.; Tian, Z.; Feng, Z.; Jin, D.; Dang, W.; Yang, X.; Zhou, G.; Wu, Z. High Triplet Energy Level Achieved by Tuning the Arrangement of Building Blocks in Phosphorescent Polymer Backbones for Furnishing High Electroluminescent Performances in Both Blue and White Organic Light-Emitting Devices. ACS Appl. Mater. Interfaces 2017, 9, 16360–16374. [Google Scholar] [CrossRef]
- Shao, S.; Wang, S.; Xu, X.; Yang, Y.; Lv, J.; Ding, J.; Wang, L.; Jing, X.; Wang, F. Realization of High-Power-Efficiency White Electroluminescence from a Single Polymer by Energy-Level Engineering. Chem. Sci. 2018, 9, 8656–8664. [Google Scholar] [CrossRef]
- Ding, J.; Wang, B.; Yue, Z.; Yao, B.; Xie, Z.; Cheng, Y.; Wang, L.; Jing, X.; Wang, F. Bifunctional Green Iridium Dendrimers with a “Self-Host” Feature for Highly Efficient Nondoped Electrophosphorescent Devices. Angew. Chem. Int. Ed. 2009, 48, 6664–6666. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, N.; Pu, Y.-J.; Nakayama, K.-i.; Yokoyama, M.; Kido, J. Synthesis, Photoluminescence and Electroluminescence Properties of Iridium Complexes with Bulky Carbazole Dendrons. Org. Electron. 2009, 10, 465–472. [Google Scholar] [CrossRef]
- Okamura, N.; Maeda, T.; Yagi, S. Sky-Blue Phosphorescence from Bis- and Tris-Cyclometalated Iridium(III) Complexes Bearing Carbazole-Based Dendrons: Fabrication of Non-doped Multilayer Organic Light-Emitting Diodes by Solution Processing. New J. Chem. 2017, 41, 10357–10366. [Google Scholar] [CrossRef]
- Okamura, N.; Maeda, T.; Yagi, S. Janus-Type Dendritic Organoiridium(III) Complex Bearing Hole- and Electron-Transporting Moieties: Synthesis, Luminescence Properties, and OLED Applications. Bull. Chem. Soc. Jpn. 2018, 91, 1419–1428. [Google Scholar] [CrossRef]
- Okamura, N.; Egawa, K.; Maeda, T.; Yagi, S. Control of Excimer Phosphorescence by Steric Effects in Cyclometalated Platinum(II) Diketonate Complexes Bearing Peripheral Carbazole Moieties towards Application in Non-Doped White OLEDs. New J. Chem. 2018, 42, 11583–11592. [Google Scholar] [CrossRef]
- Shigehiro, T.; Kawai, Y.; Yagi, S.; Maeda, T.; Nakazumi, H.; Sakurai, Y. Novel Phosphorescent Platinum(II) Dipyrido [3,2-a:2′,3′-c]phenazine Bis(acetylide) Complexes Bearing Electron-donating Side-Arms at the 2,7-Positions. Chem. Lett. 2015, 44, 288–290. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Hofkens, J.; Scheblykin, I.G.; Kruk, M.; Dehaen, W. Click Reaction Synthesis and Photophysical Studies of Dendritic Metalloporphyrins. Eur. J. Org. Chem. 2014, 2014, 1766–1777. [Google Scholar] [CrossRef]
- Lim, Y.-K.; Jiang, X.; Bollinger, J.C.; Lee, D. Molecular Engineering of Two-Dimensional π-Conjugation: Expected and Unexpected Photophysical Consequences of a Simple Particle-in-a-Box Approach. J. Mater. Chem. 2007, 17, 1969–1980. [Google Scholar] [CrossRef]
- Okamura, N.; Maeda, T.; Fujiwara, H.; Soman, A.; Unni, K.N.N.; Ajayaghosh, A.; Yagi, S. Photokinetic Study on Remarkable Excimer Phosphorescence from Cyclometalated Platinum(II) Complexes Bearing a Benzoylated 2-Phenylpyridinate Ligand. Phys. Chem. Chem. Phys. 2018, 20, 542–552. [Google Scholar] [CrossRef]
- Sprick, R.S.; Cheetham, K.J.; Bai, Y.; Fernandes, J.A.; Barnes, M.; Bradley, J.W.; Cooper, A.I. Polymer Photocatalysts with Plasma-Enhanced Activity. J. Mater. Chem. A 2020, 8, 7125–7129. [Google Scholar] [CrossRef]
- Asoh, T.; Kawabata, K.; Takimiya, K. Carbonyl-Terminated Quinoidal Oligothiophenes as p-Type Organic Semiconductors. Materials 2020, 13, 3020. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Heo, J.H.; Cheon, C.H.; Kim, H.; Im, S.H.; Son, H.J. A [2,2]Paracyclophane Triarylamine-Based Hole-Transporting Material for High Performance Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 24215–24220. [Google Scholar] [CrossRef]
- Kim, H.; Byun, Y.; Das, R.R.; Choi, B.-K.; Ahn, P.-S. Small Molecule Based and Solution Processed Highly Efficient Red Electrophosphorescent Organic Light Emitting Devices. Appl. Phys. Lett. 2007, 91, 093512. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, J.Y.; Park, T.J.; Jeon, W.S.; Kennedy, G.P.; Kwon, J.H. Small Molecule Host System for Solution-Processed Red Phosphorescent OLEDs. Synth. Met. 2010, 160, 631–635. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Zhou, W.; Zeng, Q.; Gong, Y.; Guo, S.; Xie, G.; Liu, Y. Anthraquinonyl Isoquinoline-Based Deep-Red Emissive Neutral Iridium(III) Complexes for Solution-Processed Organic Light-Emitting Diodes. Inorg. Chem. Commun. 2024, 166, 112616. [Google Scholar] [CrossRef]
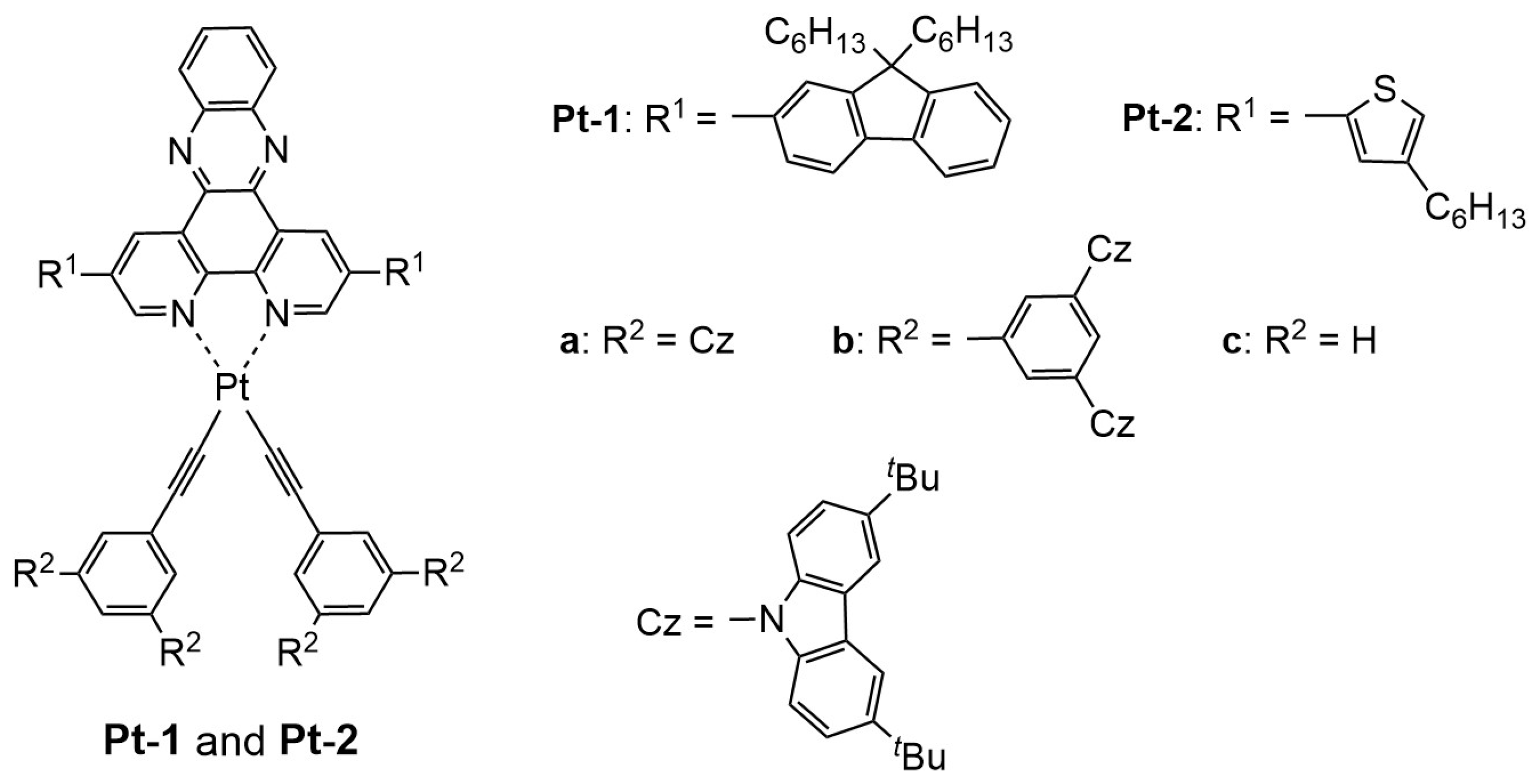

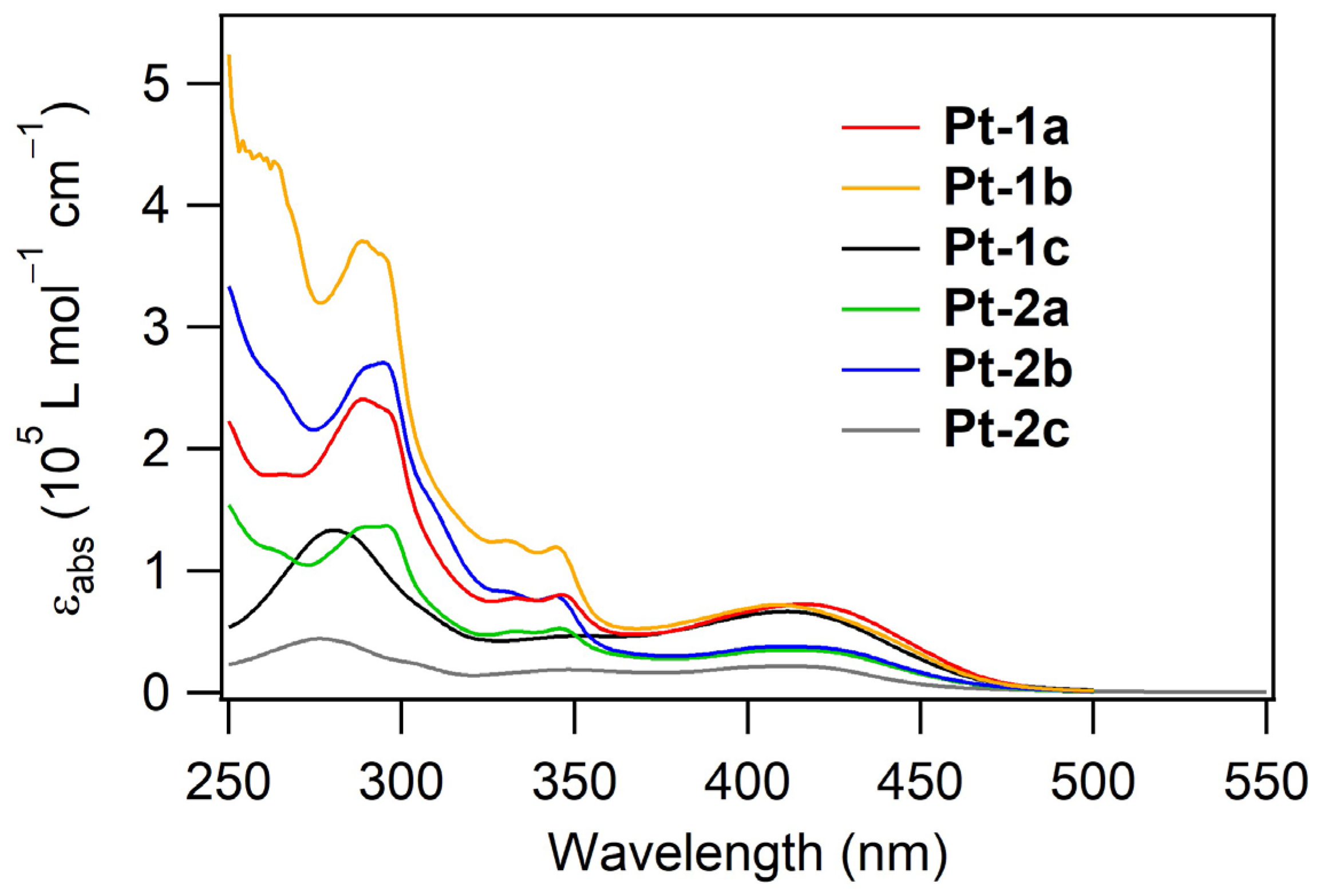
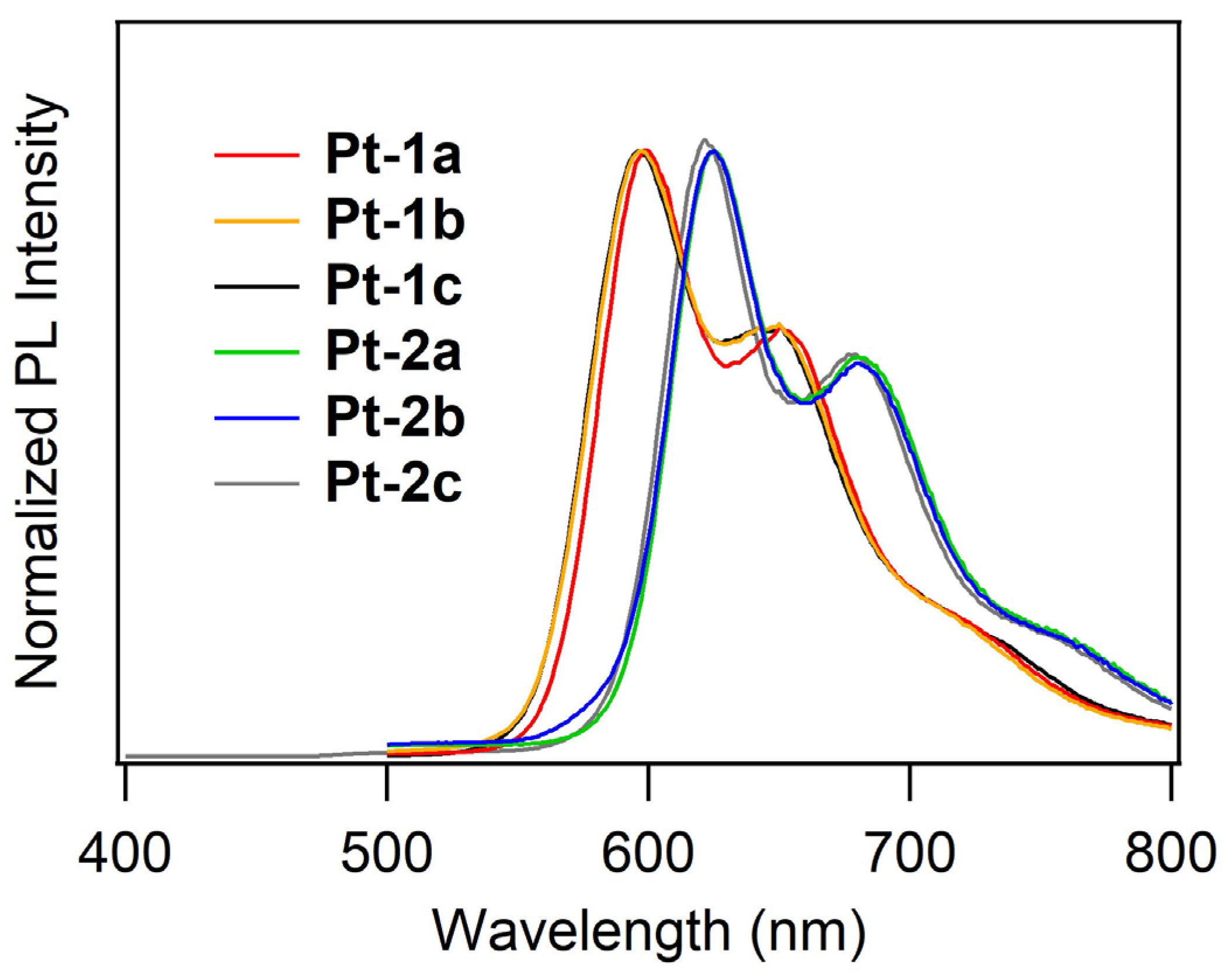
| Compd | λabs/nm (log [εabs/(L mol−1 cm−1)]) 1 | λPL/nm 1,2 | ΦPL 1,2 | τPL/μs 3 | kr/μs−1 4 | knr/μs−1 5 |
|---|---|---|---|---|---|---|
| Pt-1a | 289 (5.38), 346 (4.91), 416 (4.86) | 599, 653 | 0.033 | 2.36 | 0.014 | 0.41 |
| Pt-1b | 289 (5.56), 344 (5.08), 408 (4.86) | 597, 650 | 0.18 | 1.18 | 0.15 | 0.69 |
| Pt-1c | 280 (5.12), 411 (4.82) | 597, 650 | 0.14 | 2.31 | 0.061 | 0.37 |
| Pt-2a | 295 (5.14), 346 (4.72), 413 (4.54) | 625, 680 | 0.068 | 1.04 | 0.065 | 0.90 |
| Pt-2b | 295 (5.43), 344 (4.90), 410 (4.58) | 625, 680 | 0.11 | 2.42 | 0.045 | 0.37 |
| Pt-2c | 276 (4.43), 412 (2.15) | 622, 679 | 0.14 | 1.63 | 0.083 | 0.53 |
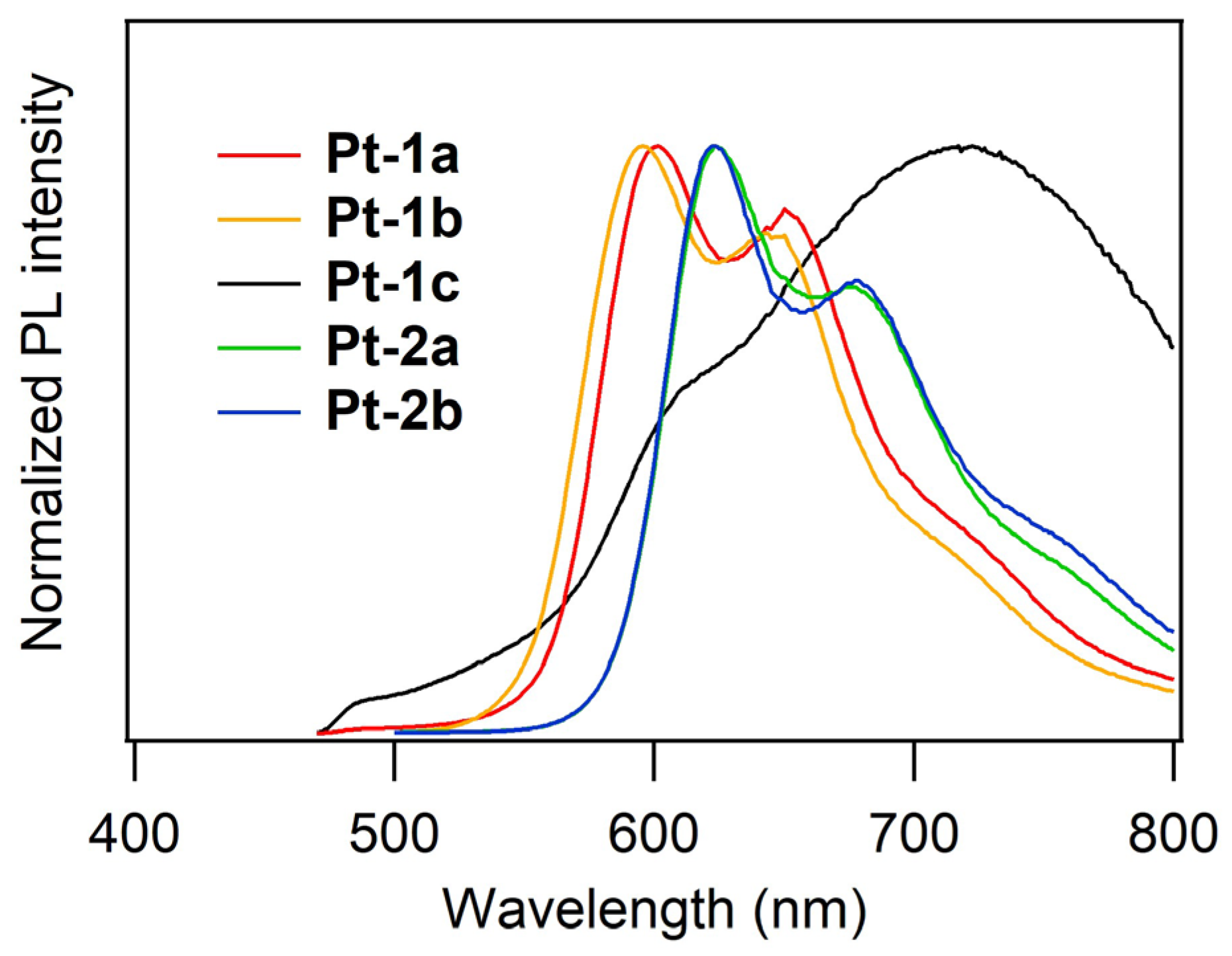
| Compd | λPL/nm | ΦPL |
|---|---|---|
| Pt-1a | 601, 650 | 0.094 |
| Pt-1b | 596, 643 | 0.083 |
| Pt-1c | 722 | 0.024 |
| Pt-2a | 624, 676 | 0.042 |
| Pt-2b | 622, 677 | 0.069 |
| Pt-2c | N.D. 1 | N.D. 1 |
| Compd | EHOMO 1 | ELUMO 2 | Egap 3 |
|---|---|---|---|
| Pt-1a | −5.59 | −3.31 | 2.28 |
| Pt-1b | −5.61 | −3.30 | 2.31 |
| Pt-2a | −5.61 | −3.30 | 2.31 |
| Pt-2b | −5.62 | −3.29 | 2.33 |
| OLED | EML | Von 1 /V | λEL /nm | Lmax 2 /cd m−2 | ηj max 3 /cd A−1 | ηp max 4 /lm W−1 | ηext max 5 /% | CIE (x, y) 6 | γ 7 /% |
|---|---|---|---|---|---|---|---|---|---|
| S-1a | Pt-1a | 2.5 | 599 | 324 | 0.59 | 0.46 | 0.37 | (0.61, 0.39) | 20 |
| S-1b | Pt-1b | 3.0 | 592 | 59.4 | 0.096 | 0.059 | 0.052 | (0.59, 0.42) | 3.1 |
| S-2a | Pt-2a | 4.0 | 639 | 21.0 | 0.039 | 0.027 | 0.062 | (0.67, 0.32) | 7.4 |
| S-2b | Pt-2b | 5.5 | 624 | 162 | 0.13 | 0.053 | 0.14 | (0.66, 0.34) | 10 |
| OLED | EML | Von 1 /V | λEL /nm | Lmax 2 /cd m−2 | ηj max 3 /cd A−1 | ηp max 4 /lm W−1 | ηext max 5 /% | CIE (x, y) 6 | γ 7 /% |
|---|---|---|---|---|---|---|---|---|---|
| M-1a | Pt-1a | 4.5 | 597 | 1570 | 1.54 | 0.67 | 1.02 | (0.60, 0.39) | 43 |
| M-1b | Pt-1b | 5.0 | 597 | 2390 | 1.77 | 0.60 | 1.04 | (0.58, 0.40) | 50 |
| M-2a | Pt-2a | 5.0 | 635 | 391 | 0.28 | 0.13 | 0.44 | (0.65, 0.31) | 42 |
| M-2b | Pt-2b | 6.0 | 622 | 561 | 0.39 | 0.12 | 0.48 | (0.62, 0.32) | 28 |
| Device Structure | EML | Processing for EML | ηext 1 /% | Reference |
|---|---|---|---|---|
| ITO/PEDOT:PSS/EML/B3PYMPM 2/LiF/Al | Dendritic iridium(III) emitter, Green EL | Solution, Non-doped | 8.3 13 | 36 |
| ITO/PEDOT:PSS/PVCz/EML/BPOPB/LiF/Al | Dendritic iridium(III) emitter, Sky-blue EL | Solution, Non-doped | 5.72 14 | 37 |
| ITO/PEDOT:PSS/PVCz/EML/TPBi/LiF/Al | Platinum(II) emitter bearing hole-transporting units, Reddish Orange Excimer EL | Solution, Non-doped | 6.5 14 | 39 |
| ITO/PEDOT:PSS/EML/TPBi/LiF/Al | Ir(piq)3 7 doped into NPB 8:TPBi, Red EL | Solution, Doped | 15.1 15 | 47 |
| ITO/PEDOT:PSS/TCTA 3/EML/TPBi/LiF/Al | Ir(piq)2acac 9 doped into 2-TNATA 10:TPBi, Red EL | Solution, Doped | 7.7 | 48 |
| ITO/PEDOT:PSS/EML/DPEPO 4/TmPyPB 5/Liq 6/Al | mCP 11:Ir(III) phosphor 12, Red EL | Solution, Doped | 5.94 14 | 49 |
| ITO/PEDOT:PSS/EML/TPBi/CsF/Al (Device M-1b) | Pt-1a | Solution, Non-doped | 1.04 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, H.; Okamura, N.; Nagaoka, M.; Suzuki, N.; Kodama, S.; Maeda, T.; Yagi, S. Synthesis, Photoluminescence, and Electroluminescence of Phosphorescent Dipyrido[3,2-a;2′3′-c]phenazine–Platinum(II) Complexes Bearing Hole-Transporting Acetylide Ligands. Molecules 2024, 29, 3849. https://doi.org/10.3390/molecules29163849
Matsuura H, Okamura N, Nagaoka M, Suzuki N, Kodama S, Maeda T, Yagi S. Synthesis, Photoluminescence, and Electroluminescence of Phosphorescent Dipyrido[3,2-a;2′3′-c]phenazine–Platinum(II) Complexes Bearing Hole-Transporting Acetylide Ligands. Molecules. 2024; 29(16):3849. https://doi.org/10.3390/molecules29163849
Chicago/Turabian StyleMatsuura, Hiroki, Naoki Okamura, Masaki Nagaoka, Naoya Suzuki, Shintaro Kodama, Takeshi Maeda, and Shigeyuki Yagi. 2024. "Synthesis, Photoluminescence, and Electroluminescence of Phosphorescent Dipyrido[3,2-a;2′3′-c]phenazine–Platinum(II) Complexes Bearing Hole-Transporting Acetylide Ligands" Molecules 29, no. 16: 3849. https://doi.org/10.3390/molecules29163849






