Effects of Clay Nanosheets on the Photostability of Cationic Porphyrin
Abstract
:1. Introduction
2. Results and Discussion
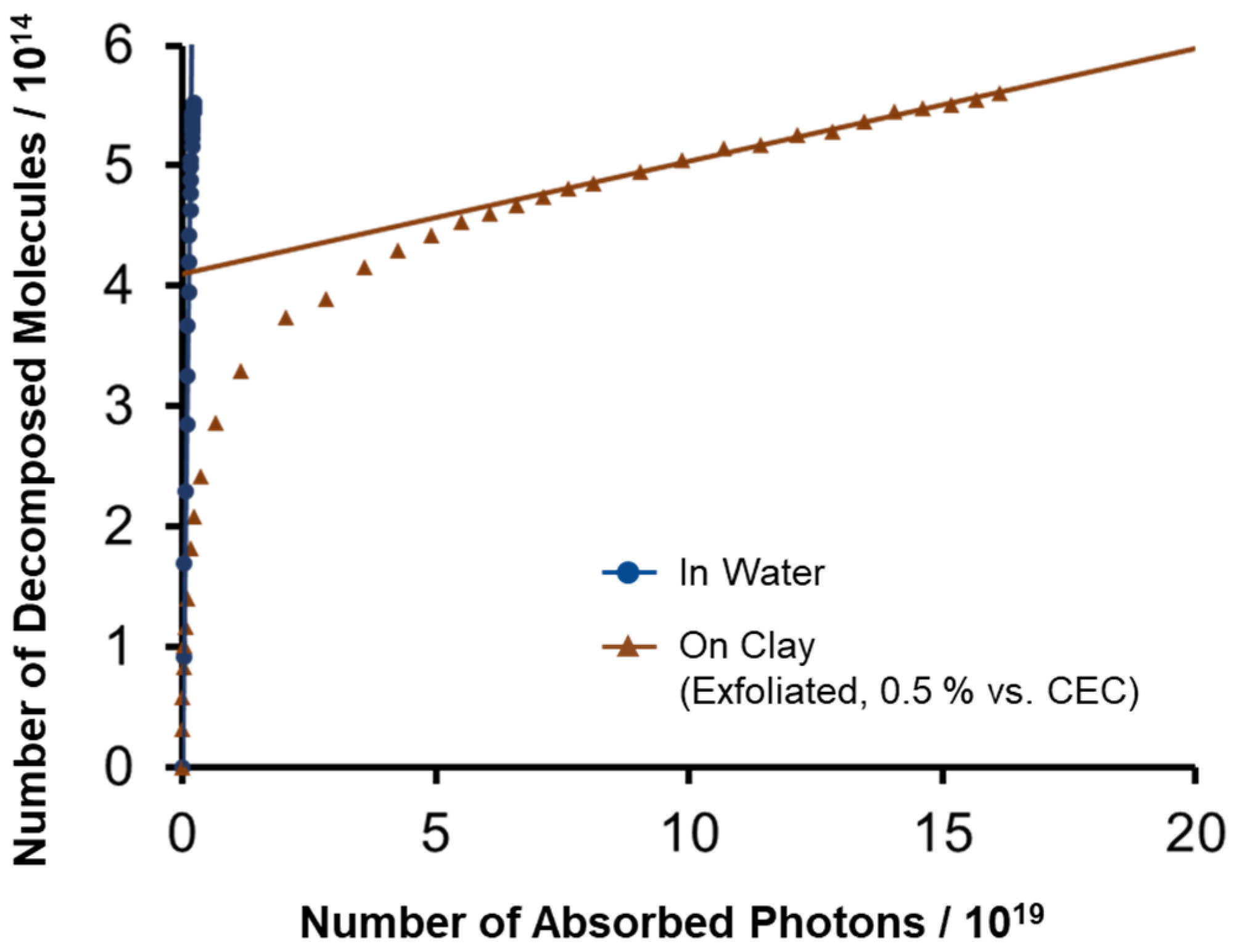
2.1. Decomposition Behavior of ZnTMAP4+ in Water and on Exfoliated Clay under Light Irradiation
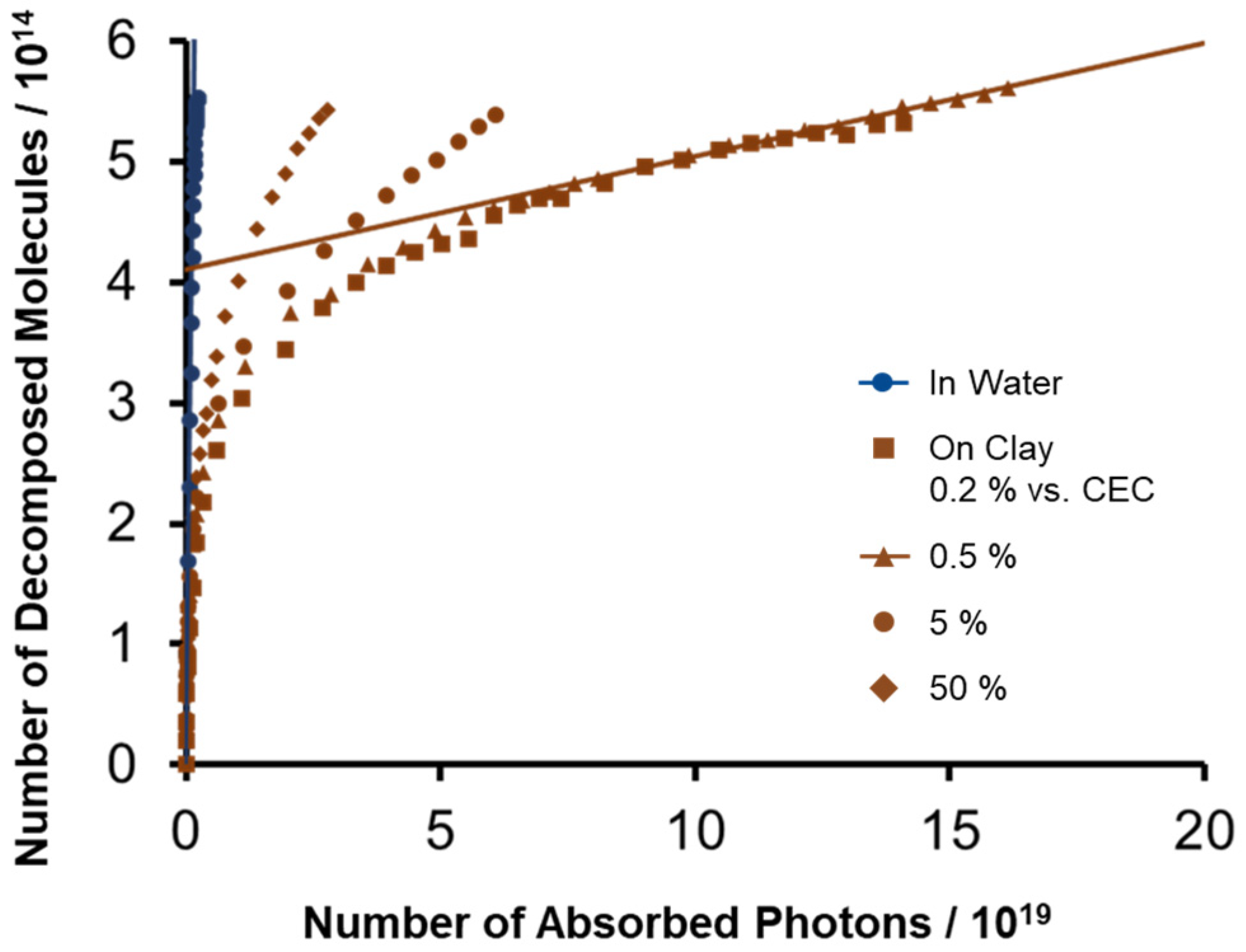
2.2. Effect of Adsorption Density of ZnTMAP4+ on Decomposition Behavior under Light Irradiation
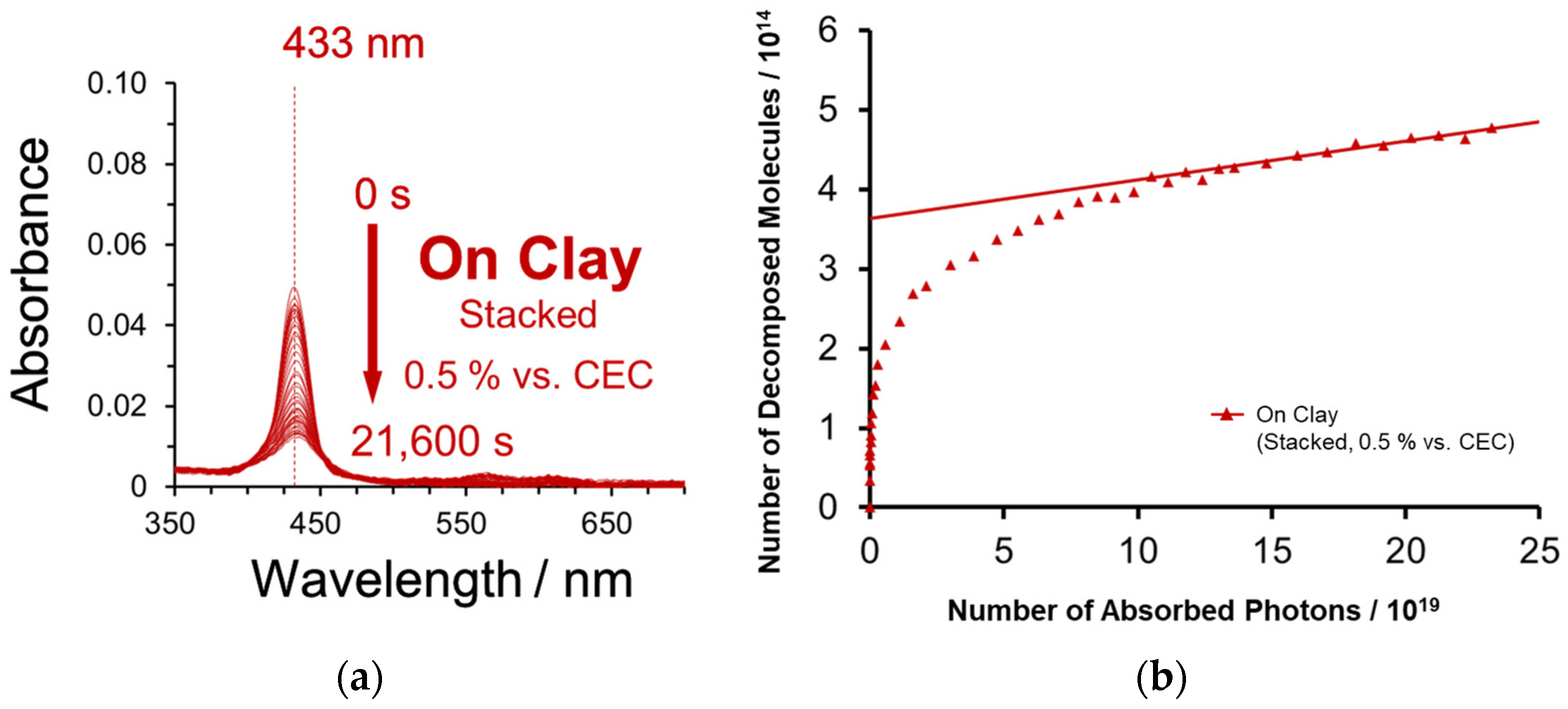
2.3. Decomposition Behavior of ZnTMAP4+ between the Stacked Clay Layers
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Clay–Dye Complexes
3.3. Light Irradiation
3.4. Estimation of the Number of Absorbed Photons
3.5. Photodecomposition Quantum Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Wu, Q.; Petit, S.; Gates, W.P.; Yang, H.; Yu, W.; Zhou, C. Insights into the Rheological Behavior of Aqueous Dispersions of Synthetic Saponite: Effects of Saponite Composition and Sodium Polyacrylate. Langmuir 2019, 35, 13040–13052. [Google Scholar] [CrossRef] [PubMed]
- Dazas, B.; Lanson, B.; Delville, A.; Robert, J.L.; Komarneni, S.; Michot, L.J.; Ferrage, E. Influence of Tetrahedral Layer Charge on the Organization of Interlayer Water and Ions in Synthetic Na-Saturated Smectites. J. Phys. Chem. C 2015, 119, 4158–4172. [Google Scholar] [CrossRef]
- Vogels, R.J.M.J.; Kloprogge, J.T.; Geus, J.W. Synthesis and characterization of saponite clays. Am. Miner. 2005, 90, 931–944. [Google Scholar] [CrossRef]
- Ogawa, M.; Kuroda, K. Photofunctions of Intercalation Compounds. Chem. Rev. 1995, 95, 399–438. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tenma, Y.; Nishioka, Y.; Kawamata, J. Efficient Nonlinear Optical Properties of Dyes Confined in Interlayer Nanospaces of Clay Minerals. Chem.-Asian J. 2012, 7, 1170–1179. [Google Scholar] [CrossRef]
- Yamagishi, A.; Tamura, K.; Yamamoto, S.; Sato, F.; Yoshida, J. Up-conversion of photon energy in colloidal clay systems. Appl. Clay Sci. 2024, 255, 107397. [Google Scholar] [CrossRef]
- Ishida, Y.; Kulasekharan, R.; Shimada, T.; Ramamurthy, V.; Takagi, S. Supramolecular-Surface Photochemistry: Supramolecular Assembly Organized on a Clay Surface Facilitates Energy Transfer between an Encapsulated Donor and a Free Acceptor. J. Phys. Chem. C 2014, 118, 10198–10203. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hartman, H. Clays and the Origin of Life: The Experiments. Life 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takimoto, K.; Kato, M.; Nagaoka, S.; Tamura, K.; Yamagishi, A. Real-Time Monitoring of Low Pressure Oxygen Molecules over Wide Temperature Range: Feasibility of Ultrathin Hybrid Films of Iridium(III) Complexes and Clay Nanosheets. Bull. Chem. Soc. Jpn. 2020, 93, 194–199. [Google Scholar] [CrossRef]
- Okada, T.; Watanabe, Y.; Ogawa, M. Photoregulation of the intercalation behavior of phenol for azobenzene–clay intercalation compounds. J. Mater. Chem. 2005, 15, 987–992. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Sivaguru, J. Supramolecular Photochemistry as a Potential Synthetic Tool: Photocycloaddition. Chem. Rev. 2016, 116, 9914–9993. [Google Scholar] [CrossRef] [PubMed]
- Liu Robert, S.H.; Hammond, G.S. Reflection on Medium Effects on Photochemical Reactivity. Acc. Chem. Res. 2005, 38, 396–403. [Google Scholar] [PubMed]
- Li, S.; Mu, B.; Wang, X.; Wang, A. Recent researches on natural pigments stabilized by clay minerals: A review. Dye. Pigment. 2021, 190, 109322. [Google Scholar] [CrossRef]
- Kohno, Y.; Kinoshita, K.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of natural anthocyanin by intercalation into montmorillonite. Appl. Clay Sci. 2009, 42, 519–523. [Google Scholar] [CrossRef]
- Kohno, Y.; Totsuka, K.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Photostability enhancement of anionic natural dye by intercalation into hydrotalcite. J. Colloid Interface Sci. 2009, 337, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Teepakakorn, A.P.; Bureekaew, S.; Ogawa, M. Adsorption-Induced Dye Stability of Cationic Dyes on Clay Nanosheets. Langmuir 2018, 34, 14069–14075. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Itoh, T.; Kageyama, H.; Mizoguchi, T.; Kodera, Y.; Matsushima, A.; Torii, K.; Inada, Y. Photostabilization of Chlorophyll a Adsorbed onto Smectite. Dye. Pigment. 1995, 28, 77–82. [Google Scholar] [CrossRef]
- Saito, T.; Fukui, K.; Kodera, Y.; Matsushima, A.; Nishimura, H.; Inada, Y. Photostability of biliverdin bound to smectite, clay mineral. Dye. Pigment. 2005, 65, 21–24. [Google Scholar] [CrossRef]
- Cheng, M.; Song, W.; Ma, W.; Chen, C.; Zhao, J.; Lin, J.; Zhu, H. Catalytic activity of iron species in layered clays for photodegradation of organic dyes under visible irradiation. Appl. Catal. B Environ. 2008, 77, 355–363. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Yue, P.; Zhu, H.; Lu, G. Degradation of Azo-dye Orange II by a Photoassisted Fenton Reaction Using a Novel Composite of Iron Oxide and Silicate Nanoparticles as a Catalyst. Ind. Eng. Chem. Res. 2003, 42, 2058–2066. [Google Scholar] [CrossRef]
- Cheng, M.; Ma, W.; Chen, C.; Yao, J.; Zhao, J. Photocatalytic degradation of organic pollutants catalyzed by layered iron(II) bipyridine complex–clay hybrid under visible irradiation. Appl. Catal. B Environ. 2006, 65, 217–226. [Google Scholar] [CrossRef]
- Tani, S.; Yamaki, H.; Sumiyoshi, A.; Suzuki, Y.; Hasegawa, S.; Yamazaki, S.; Kawamata, J. Enhanced Photodegradation of Organic Dyes Adsorbed on a Clay. J. Nanosci. Nanotechnol. 2009, 9, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Masui, D.; Shimada, T.; Tachibana, H.; Inoue, H.; Takagi, S. The Mechanism of the Porphyrin Spectral Shift on Inorganic Nanosheets: The Molecular Flattening Induced by the Strong Host–Guest Interaction due to the “Size-Matching Rule”. J. Phys. Chem. C 2012, 116, 7879–7885. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Shimada, T.; Takagi, S. Unique Photochemical Properties of p-Substituted Cationic Triphenylbenzene Derivatives on a Clay Layer Surface. J. Phys. Chem. C 2013, 117, 2774–2779. [Google Scholar] [CrossRef]
- Ishida, Y.; Shimada, T.; Takagi, S. “Surface-Fixation Induced Emission” of Porphyrazine Dye by a Complexation with Inorganic Nanosheets. J. Phys. Chem. C 2014, 118, 20466–20471. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Shimada, T.; Takagi, S. Structure resembling effect of clay surface on photochemical properties of meso-phenyl or pyridyl-substituted monocationic antimony(V) porphyrin derivatives. RSC Adv. 2015, 5, 8479–8485. [Google Scholar] [CrossRef]
- Umemoto, T.; Ohtani, Y.; Tsukamoto, T.; Shimada, T.; Takagi, S. Pinning effect for photoisomerization of a dicationic azobenzene derivative by anionic sites of the clay surface. Chem. Commun. 2014, 50, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Shimada, T.; Ishida, T.; Takagi, S. “In-water” Dehydration Reaction of an Aromatic Diol on an Inorganic Surface. Langmuir 2021, 19, 11978–11985. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Shimada, T.; Ishida, Y.; Fujimura, T.; Masui, D.; Tachibana, H.; Eguchi, M.; Inoue, H. Size-Matching Effect on Inorganic Nanosheets: Control of Distance, Alignment, and Orientation of Molecular Adsorption as a Bottom-Up Methodology for Nanomaterials. Langmuir 2013, 29, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Shimada, T.; Eguchi, M.; Yui, T.; Yoshida, H.; Tryk, D.A.; Inoue, H. High-Density Adsorption of Cationic Porphyrins on Clay Layer Surfaces without Aggregation: The Size-Matching Effect. Langmuir 2002, 18, 2265–2272. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Shimada, T.; Shiragami, T.; Takagi, S. Photochemical Chlorination and Oxygenation Reaction of Cyclohexene Sensitized by Ga(III) Porphyrin–Clay Minerals System with High Durability and Usability. Bull. Chem. Soc. Jpn. 2015, 88, 578–583. [Google Scholar] [CrossRef]
- Golec, B.; Buczyńska, J.; Nawara, K.; Gorski, A.; Waluk, J. Photodegradation of free base and zinc porphyrins in the presence and absence of oxygen. Photochem. Photobiol. Sci. 2023, 22, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Mizuno, J.; Ohtani, Y.; Shimada, T.; Takagi, S. Elucidation of the Adsorption Distribution of Cationic Porphyrin on the Inorganic Surface by Energy Transfer as a Molecular Ruler. J. Phys. Chem. C 2018, 122, 4365–4371. [Google Scholar] [CrossRef]
- Ishida, Y.; Shimada, T.; Tachibana, H.; Inoue, H.; Takagi, S. Regulation of the Collisional Self-Quenching of Fluorescence in Clay/Porphyrin Complex by Strong Host–Guest Interaction. J. Phys. Chem. A 2012, 116, 12065–12072. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.S.; Neves, M.G.P.M.S.; Martins, R.R.L.; Cavaleiro, J.A.S.; Boschi, T.; Tagliatesta, P. Photo-oxygenation of meso-tetraphenylporphyrin derivatives: The influence of the substitution pattern and characterization of the reaction products. J. Porphyr. Phthalocyanines 1998, 2, 45–51. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Mondal, B. Supramolecular photochemistry concepts highlighted with select examples. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 68–102. [Google Scholar] [CrossRef]




| ZnTMPyP4+ | ϕdec/10−7 |
|---|---|
| Water | 3400 |
| Clay (Exfoliated) | 9.4 |
| Clay (Stacked) | 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahara, Y.; Hirade, Y.; Arakawa, K.; Shimada, T.; Ishida, T.; Tachibana, H.; Takagi, S. Effects of Clay Nanosheets on the Photostability of Cationic Porphyrin. Molecules 2024, 29, 3738. https://doi.org/10.3390/molecules29163738
Tahara Y, Hirade Y, Arakawa K, Shimada T, Ishida T, Tachibana H, Takagi S. Effects of Clay Nanosheets on the Photostability of Cationic Porphyrin. Molecules. 2024; 29(16):3738. https://doi.org/10.3390/molecules29163738
Chicago/Turabian StyleTahara, Yoshinori, Yugo Hirade, Kyosuke Arakawa, Tetsuya Shimada, Tamao Ishida, Hiroshi Tachibana, and Shinsuke Takagi. 2024. "Effects of Clay Nanosheets on the Photostability of Cationic Porphyrin" Molecules 29, no. 16: 3738. https://doi.org/10.3390/molecules29163738
APA StyleTahara, Y., Hirade, Y., Arakawa, K., Shimada, T., Ishida, T., Tachibana, H., & Takagi, S. (2024). Effects of Clay Nanosheets on the Photostability of Cationic Porphyrin. Molecules, 29(16), 3738. https://doi.org/10.3390/molecules29163738







