Progress on the Anti-Inflammatory Activity and Structure–Efficacy Relationship of Polysaccharides from Medical and Edible Homologous Traditional Chinese Medicines
Abstract
1. Introduction
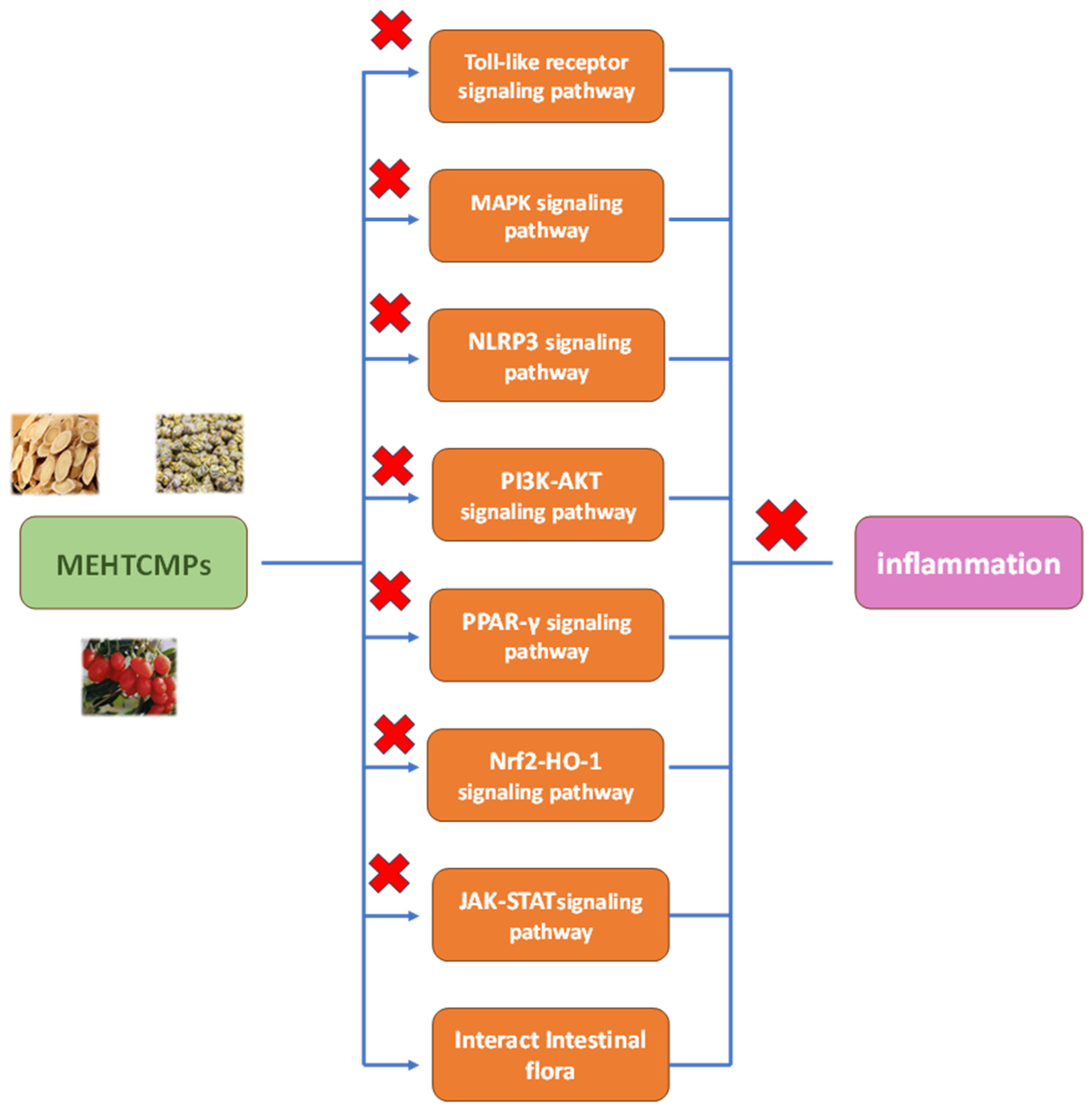
2. Anti-Inflammatory Mechanism of MEHTCMPs
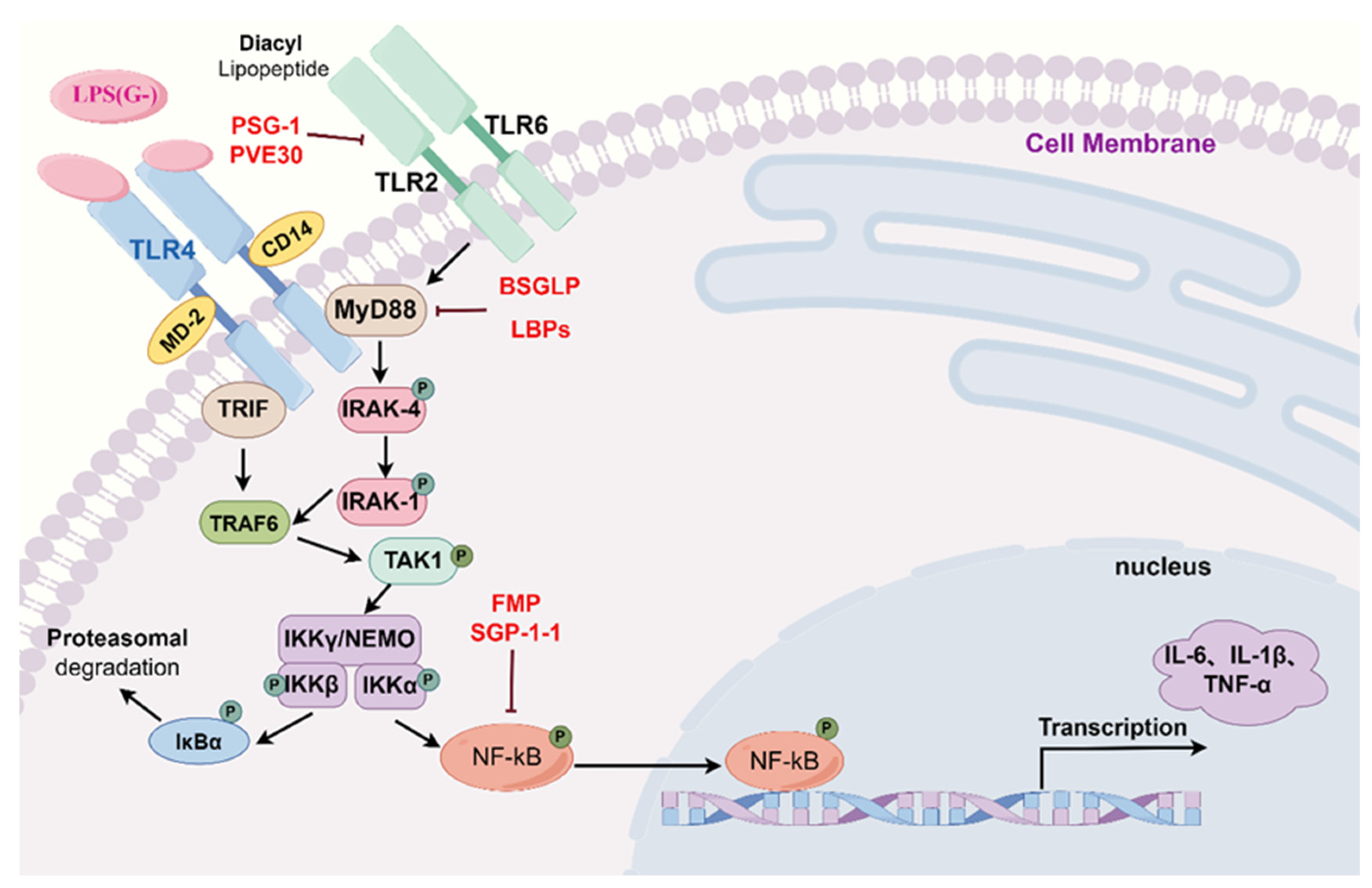
2.1. Toll-like Receptor Signaling Pathways
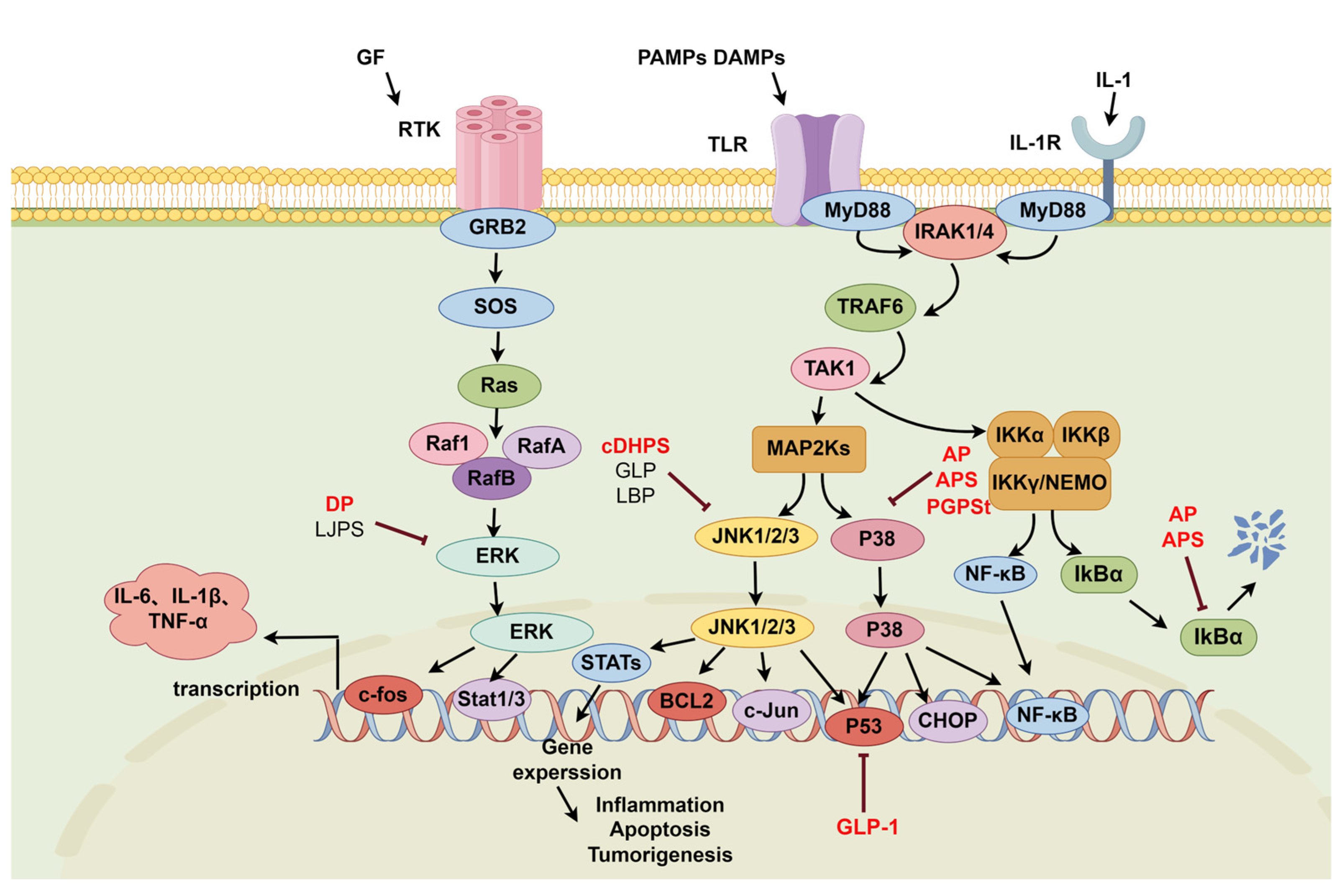
2.2. MAPK Signaling Pathway
2.2.1. P38-NF-κB Signaling Pathway
2.2.2. JNK-NF-κB Signaling Pathway
2.2.3. ERK-NF-κB Signaling Pathway
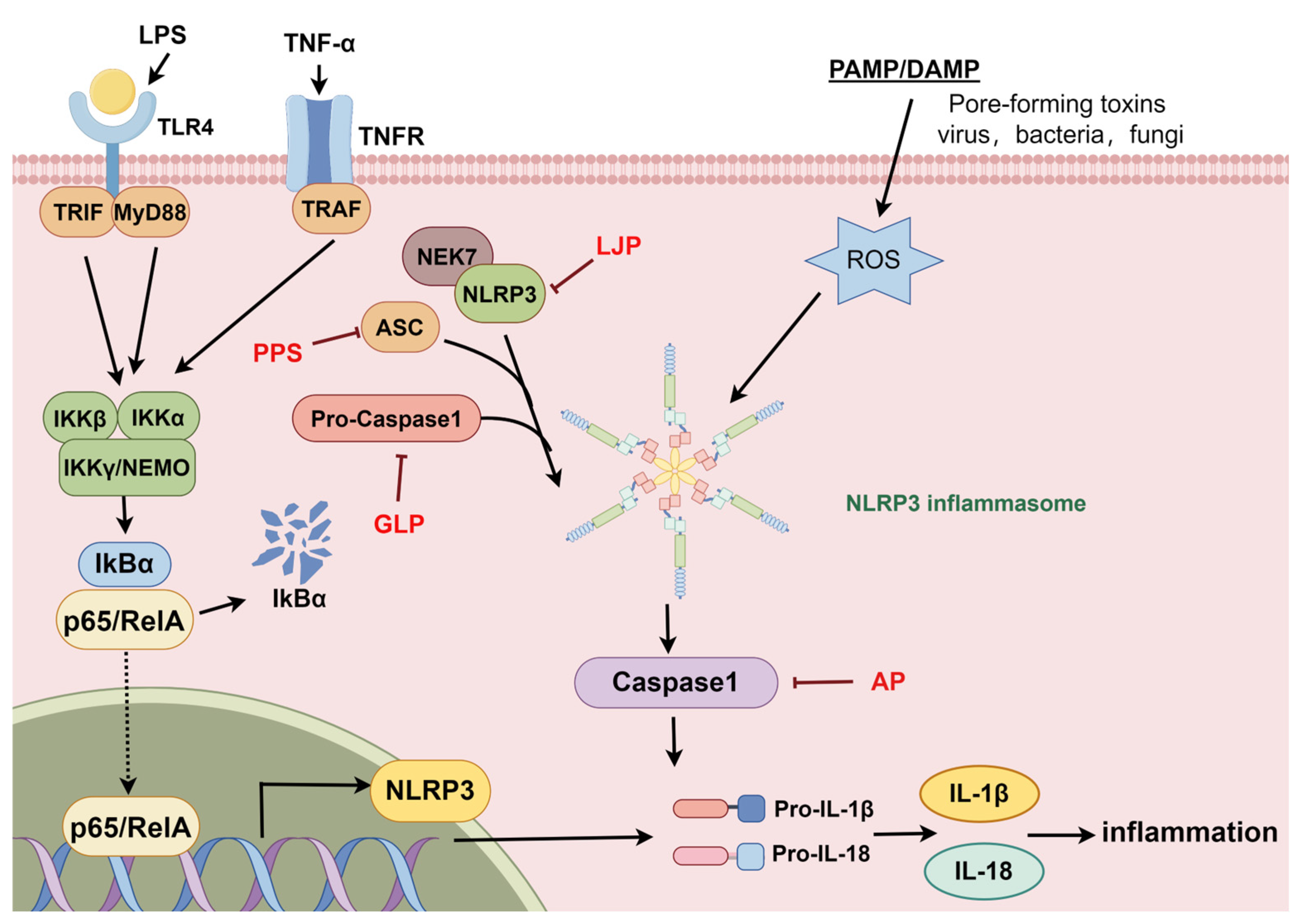
2.3. The NLRP3 Signaling Pathway
2.4. The PI3K-AKT Signaling Pathway
2.4.1. PI3K-AKT-GSK3β
2.4.2. PI3K-AKT-mTOR
2.5. PPAR Signaling Pathway
2.6. Nrf2-HO-1 Signaling Pathway
2.7. JAK-STAT Signaling Pathway
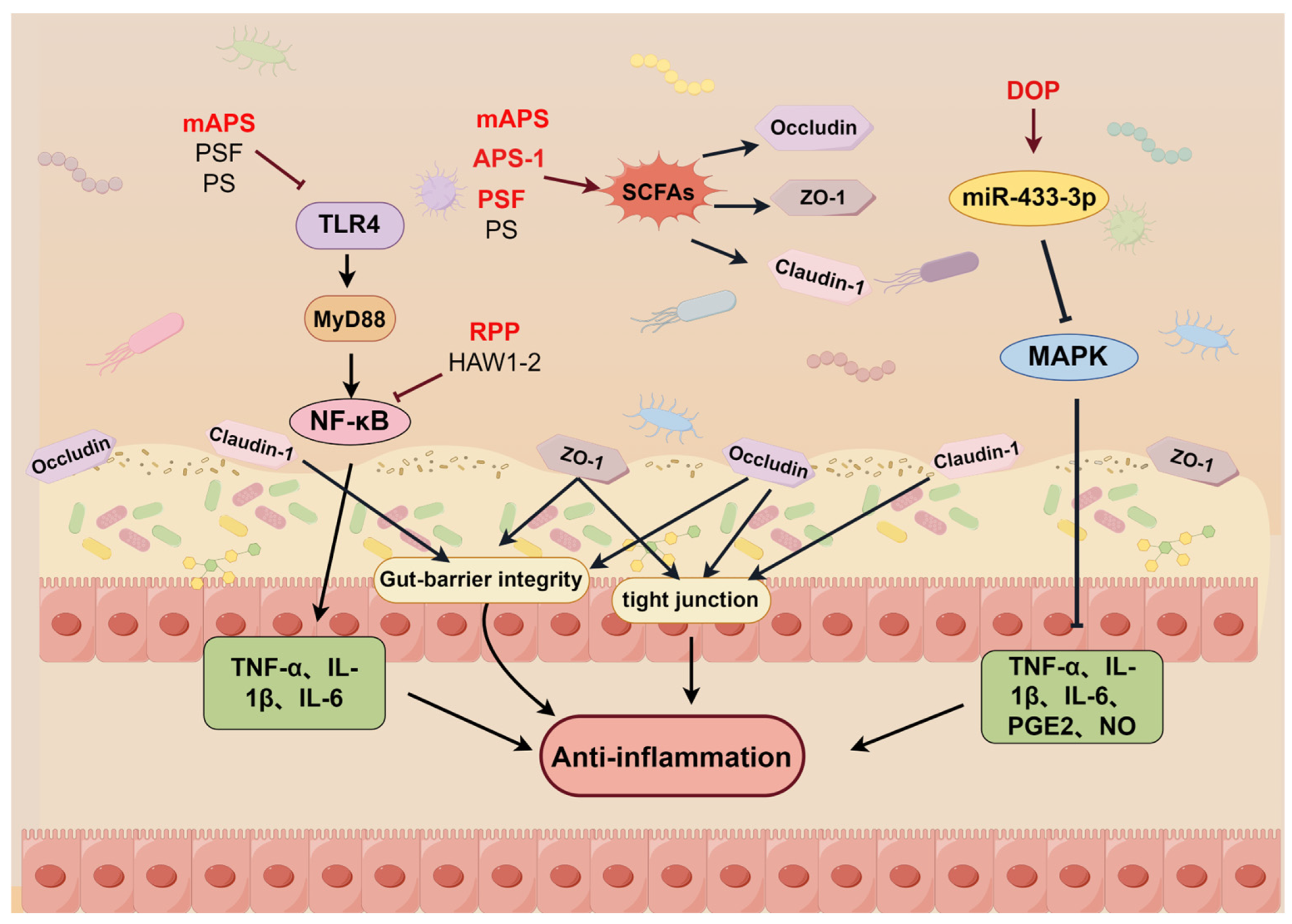
2.8. Regulation of the Intestinal Flora
3. Relationship between the Structures and Anti-Inflammatory Activities of MEHTCMPs
3.1. Primary Structure
3.1.1. Molecular Weight
3.1.2. Composition and Proportion of Monosaccharides
3.1.3. Glycosidic Bonds
3.2. Advanced Structure
3.3. Structural Modification
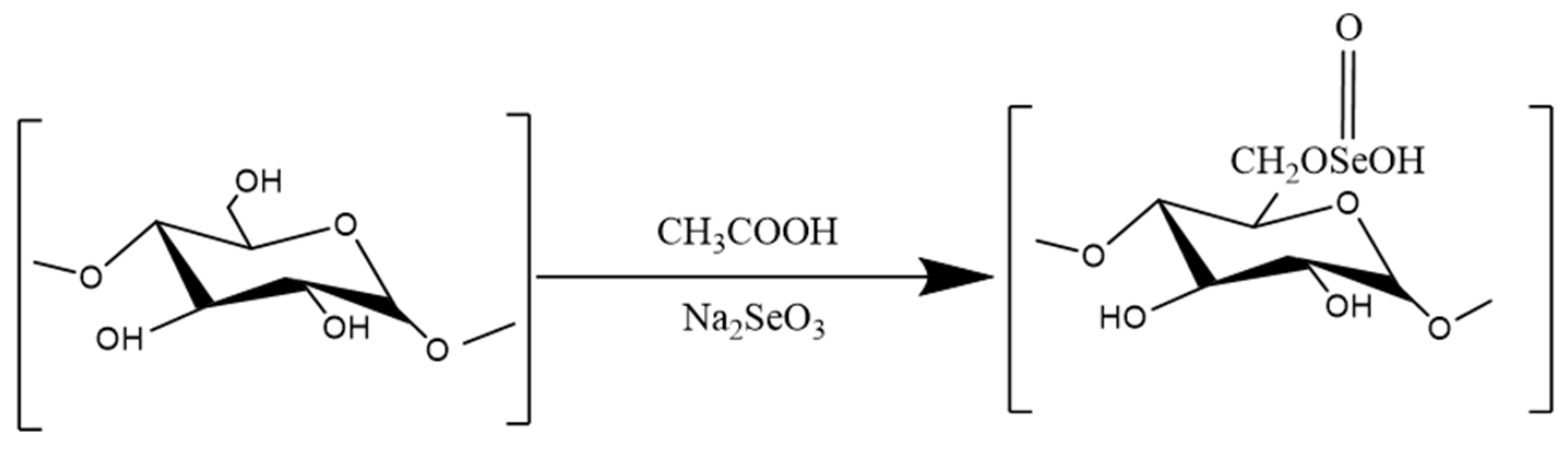
3.3.1. Selenization
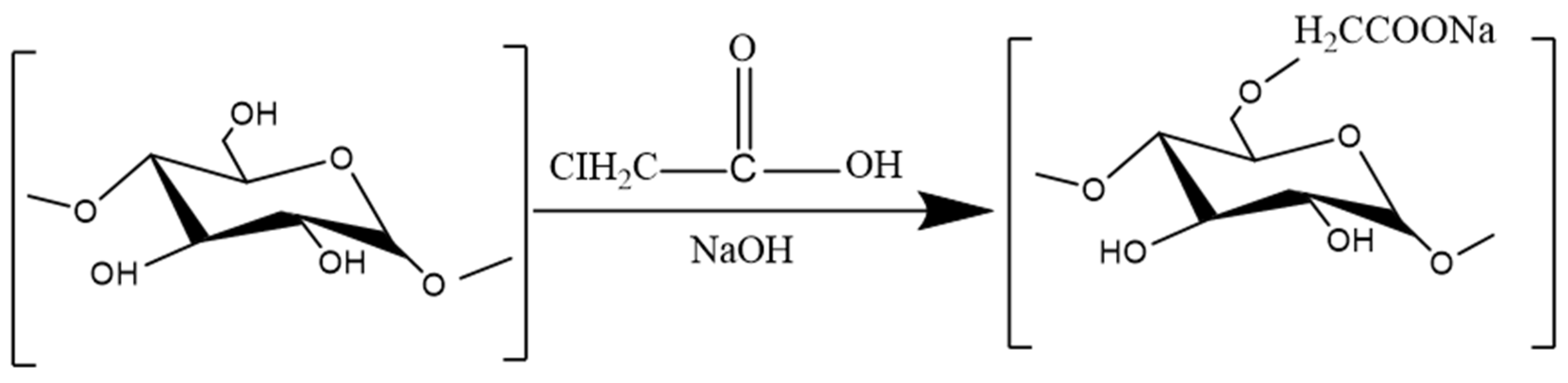
3.3.2. Carboxymethylation
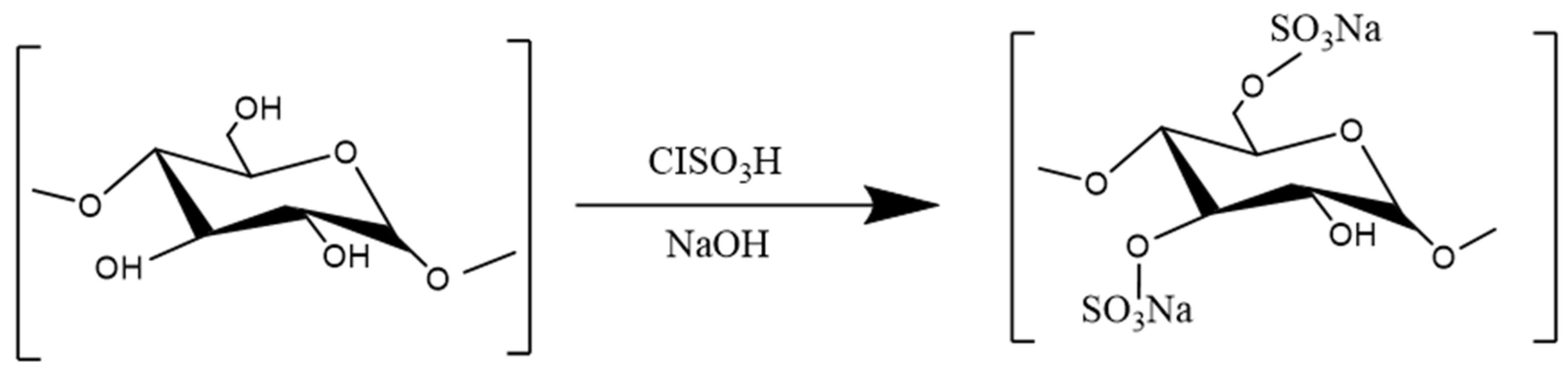
3.3.3. Sulfation
3.3.4. Complexation with Metal Ions
| Source | Compound Name | Structural Modification | Effects | References |
|---|---|---|---|---|
| Glycyrrhiza uralensis | Se-GPS | Selenization | TNF-α ↓, IL-1β ↓ | [191] |
| Astragalus membranaceus | sAPS3 | Selenization | TNF-α ↓, IL-1β ↓ | [130] |
| Eucommia ulmoides | EUP-SeNP | Selenization | IL-1β ↓, IL-6 ↓, IL-12 ↓, IL-17 ↓, TNF-α ↓, P-IκB/IκB ↓, p-p65/p65 ↓, TLR-4 ↓, IL-10 ↑ | [192] |
| Angelica sinensis | sCAP | Selenization | TP ↑, SOD ↑, T-AOC ↓, ALT ↓, AST ↓, ALP ↓, MDA ↓, ROS ↓, p-ERK ↓, p-JNK ↓, p-p38 ↓ | [140] |
| Poria cocos | CMP33 | Carboxymethylation | NO ↓, IL-1β ↓, IL-6 ↓, TNF-α ↓ | [148] |
| Pseudocydonia sinensis | CSP | Carboxymethylation | TNF-α ↓, IL-1β ↓, IL-6 ↓ | [178] |
| Ganoderma lucidum | CM-GLP | Carboxymethylation | NF-κB ↓, TNF-α ↓, IL-1 ↓, IL-6 ↓ | [134] |
| Astragalus membranaceus | SAPS | Sulfation | TNF-α ↓, IL-1β ↓, IL-8 ↓, TLR4 | [131] |
| Laminaria japonica | SLJP1 | Sulfation | TNF-α ↓, IL-1β ↓, IL-6 ↓, PPAR-γ ↓ | [201,202] |
| Laminaria japonica | SLJP2 | Sulfation | TNF-α ↓, IL-1β ↓, IL-6 ↓, PPAR-γ ↓ | [201,202] |
| Laminaria japonica | SLJP3 | Sulfation | TNF-α ↓, IL-1β ↓, IL-6 ↓, PPAR-γ ↓ | [201,202] |
| Ganoderma lucidum | SGRP | Sulfation | TNF-α ↓, IL-1β ↓, IL-6 ↓, TLR4 ↓, NF-κB ↓ | [132] |
| Ganoderma lucidum | GLP | Sulfation | NO ↓ | [135] |
| ginger | GP-Zn(II) | Introduce Zn | IL-1β ↓, IL-6 ↓, IL-8 ↓, IL-12 ↓, TNF-α ↓, IL-10 ↑ | [179] |
| Laminarin | LP-SR | Introduce SR | IL-6 ↓ | [206] |
| Eucommia ulmoides | EUP-Sr | Introduce SR | IL-1β ↓ | [207] |
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Glc | Glucose |
| Gal | Galactose |
| Rha | Rhamnose |
| Ara | Arabinose |
| Man | Mannose |
| GalA | Galacturonic acid |
| Xyl | Xylose |
| Rib | Ribose |
| GlcA | Glucuronic acid |
| Fuc | Fucuronic |
| Fru | Fructose |
| Idoa | Iduronic acid |
| NF-κB | Nuclear factor-κB |
| NLRP3 | Nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing 3 |
| TNF-α | Tumor necrosis factor-α |
| Caspase-1 | Cysteinyl aspartate specific proteinase-1 |
| IL-1β | Interleukin |
| ERK | Extracellular signal-regulated protein kinase |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| Bcl2 | B-cell lymphoma-2 |
| Bax | bcl2-Associated X |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NQO-1 | NAD(P)H quinone dehydrogenase-1 |
| HO-1 | Heme oxygenase-1 |
| SOD | Superoxide dismutase |
| Interleukin | (IL)-1β/4/6/10/12/B |
| AP-1 | Activator protein-1 |
| PYY | Peptide YY |
| SCFAs | Short chain fatty acids |
| ZO-1 | Zona occludens 1 |
| GPR41/43 | G-protein-coupled receptor 41/43 |
| TLR4 | Toll-like receptor 4 |
| LPS | Lipopolysaccharide |
| PPARs/PPARα/PPARγ | Peroxisome proliferators-activated receptor-s/α/γ |
| PI3K | Phosphoinositide-3 kinase |
| Akt | Protein kinase B |
| GSK3β | Glycogen synthase kinase |
| mTOR | Mammalian target of rapamycin |
| JAK | Janus activated kinase |
| STAT | Signal transducer and activator of transcription |
References
- Shan, F.; Huang, L.; Guo, J.; Chen, M. History and development of the homology of medicine and food. Life Sci. 2015, 27, 1061–1069. [Google Scholar]
- Wang, Y.; Wu, Y.; Xiu, F.; Xiao, G.; Zhang, Y.; Xu, D. Study on the origin and Development of Chinese medicinal materials with the same origin as medicine and food. Life Sci. 2023, 42, 65–71. [Google Scholar]
- Chittasupho, C.; Junmahasathien, T.; Chalermmongkol, J.; Wongjirasakul, R.; Leesawat, P.; Okonogi, S. Suppression of Intracellular Reactive Oxygen Species in Human Corneal Epithelial Cells via the Combination of Quercetin Nanoparticles and Epigallocatechin Gallate and In Situ Thermosensitive Gel Formulation for Ocular Drug Delivery. Pharmaceuticals 2021, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Knorr, J.; Wree, A.; Tacke, F.; Feldstein, A.E. The NLRP3 Inflammasome in Alcoholic and Nonalcoholic Steatohepatitis. Semin. Liver Dis. 2020, 40, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Stappenbeck, T.S. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Pathol. 2016, 11, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.A.; McInnes, I.B.; Brewer, J.M.; Garside, P. Cellular imaging in rheumatic diseases. Nat. Rev. Rheumatol. 2015, 11, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Tai, F.W.D.; McAlindon, M.E. NSAIDs and the small bowel. Curr. Opin. Gastroenterol. 2018, 34, 175–182. [Google Scholar] [CrossRef]
- Watanabe, T.; Fujiwara, Y.; Chan, F.K.L. Current knowledge on non-steroidal anti-inflammatory drug-induced small-bowel damage: A comprehensive review. J. Gastroenterol. 2020, 55, 481–495. [Google Scholar]
- Park, J.; Jeon, S.R.; Kim, J.O.; Kim, H.G.; Lee, T.H.; Cho, J.H.; Ko, B.M.; Lee, J.S.; Lee, M.S. Rebleeding rate and risk factors in nonsteroidal anti-inflammatory drug-induced enteropathy. J. Dig. Dis. 2018, 19, 279–287. [Google Scholar]
- Maseda, D.; Ricciotti, E. NSAID-Gut Microbiota Interactions. Front. Pharmacol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Su, G.Y.; Li, Z.Y.; Wang, R.; Lu, Y.Z.; Nan, J.X.; Wu, Y.L.; Zhao, Y.Q. Signaling pathways involved in p38-ERK and inflammatory factors mediated the anti-fibrosis effect of AD-2 on thioacetamide-induced liver injury in mice. Food Funct. 2019, 10, 3992–4000. [Google Scholar] [CrossRef]
- Lv, X.C.; Wu, Q.; Cao, Y.J.; Lin, Y.C.; Guo, W.L.; Rao, P.F.; Zhang, Y.Y.; Chen, Y.T.; Ai, L.Z.; Ni, L. Ganoderic acid A from Ganoderma lucidum protects against alcoholic liver injury through ameliorating the lipid metabolism and modulating the intestinal microbial composition. Food Funct. 2022, 13, 5820–5837. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Liu, B.; Shang, Z.Z.; Li, Q.M.; Zha, X.Q.; Wu, D.L.; Yu, N.J.; Han, L.; Peng, D.Y.; Luo, J.P. Structural features and anti-gastric cancer activity of polysaccharides from stem, root, leaf and flower of cultivated Dendrobium huoshanense. Int. J. Biol. Macromol. 2020, 143, 651–664. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Liang, C.Y.; Liu, T.T.; Liang, Y.M.; Li, S.J.; Lu, Y.Y.; Liang, J.; Yuan, X.; Li, C.J.; Hou, S.Z.; et al. Protective roles and mechanisms of polysaccharides from Dendrobium officinal on natural aging-induced premature ovarian failure. Biomed. Pharmacother. 2018, 101, 953–960. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, X.; Liu, Y. Hypoglycemic and hypolipidemic effects of a polysaccharide from flower buds of Lonicera japonica in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2017, 102, 396–404. [Google Scholar] [CrossRef]
- González, M.E.; Alarcón, B.; Carrasco, L. Polysaccharides as antiviral agents: Antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987, 31, 1388–1393. [Google Scholar] [CrossRef]
- Feng, H.; Yang, J.; Zhi, H.; Hu, X.; Yang, Y.; Zhang, L.; Liu, Q.; Feng, Y.; Wu, D.; Li, H. Eucommia ulmoides Leaf Polysaccharide in Conjugation with Ovalbumin Act as Delivery System Can Improve Immune Response. Pharmaceutics 2021, 13, 1384. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Niu, H.; Liu, J.; Ruilian, M.; Wang, Y.; Xiao, Y.; Xiao, Z.; Sun, J.; Dong, Y.; et al. Astragalus polysaccharide from Astragalus Melittin ameliorates inflammation via suppressing the activation of TLR-4/NF-κB p65 signal pathway and protects mice from CVB3-induced virus myocarditis. Int. J. Biol. Macromol. 2019, 126, 179–186. [Google Scholar] [CrossRef]
- Qi, C.; Li, L.; Cheng, G.; Xiao, B.; Xing, Y.; Zhao, X.; Liu, J. Platycodon grandiflorus Polysaccharide with Anti-Apoptosis, Anti-Oxidant and Anti-Inflammatory Activity Against LPS/D-GalN Induced Acute Liver Injury in Mice. J. Polym. Environ. 2021, 29, 4088–4097. [Google Scholar] [CrossRef]
- Shang, Z.Z.; Qin, D.Y.; Li, Q.M.; Zha, X.Q.; Pan, L.H.; Peng, D.Y.; Luo, J.P. Dendrobium huoshanense stem polysaccharide ameliorates rheumatoid arthritis in mice via inhibition of inflammatory signaling pathways. Carbohydr. Polym. 2021, 258, 117657. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.L.; Ma, J.M.; Fan, Y.N.; Zhang, Y.N.; Ge, R.; Tao, X.J.; Zhang, M.W.; Gao, Q.H.; Yang, J.J. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int. J. Biol. Macromol. 2021, 183, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Rodrigues, F.; Saavedra, M.J.; Nunes, F.M.; Marques, G. Bioactive polysaccharides from medicinal mushrooms: A review on their isolation, structural characteristics and antitumor activity. Food Biosci. 2022, 49, 101955. [Google Scholar] [CrossRef]
- Huang, S.; Chen, F.; Cheng, H.; Huang, G. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yan, L.; Guo, S.; Wen, L.; Yu, M.; Feng, L.; Jia, X. Structural Elucidation, Modification, and Structure-Activity Relationship of Polysaccharides in Chinese Herbs: A Review. Front. Nutr. 2022, 9, 908175. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M.; Kagan, J.C. A cell biological view of Toll-like receptor function: Regulation through compartmentalization. Nat. Rev. Immunol. 2009, 9, 535–542. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflammation 2019, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Pflug, K.M.; Sitcheran, R. Targeting NF-κB-Inducing Kinase (NIK) in Immunity, Inflammation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8470. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Sang, T.; Guo, C.; Guo, D.; Wu, J.; Wang, Y.; Wang, Y.; Chen, J.; Chen, C.; Wu, K.; Na, K.; et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, J.; Fu, X.; Wang, P.; Chen, C. Fructus mori polysaccharide alleviates diabetic symptoms by regulating intestinal microbiota and intestinal barrier against TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2023, 249, 126038. [Google Scholar] [CrossRef]
- Gong, P.; Cui, D.; Guo, Y.; Wang, M.; Wang, Z.; Huang, Z.; Yang, W.; Chen, F.; Chen, X. A novel polysaccharide obtained from Siraitia grosvenorii alleviates inflammatory responses in a diabetic nephropathy mouse model via the TLR4-NF-κB pathway. Food Funct. 2021, 12, 9054–9065. [Google Scholar] [CrossRef]
- Ying, M.; Zheng, B.; Yu, Q.; Hou, K.; Wang, H.; Zhao, M.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Ganoderma atrum polysaccharide ameliorates intestinal mucosal dysfunction associated with autophagy in immunosuppressed mice. Food Chem. Toxicol. 2020, 138, 111244. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, Y.; Chang, C.; Qiu, Z.; Hu, J.; Wu, Y.; Zhang, B.; Zheng, G. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Wang, X.; Wang, Z.; Wang, J.; Zhen, W.; Huang, S.; Li, T.; Fan, H.; Ma, Y.; et al. Effects of Glycyrrhiza polysaccharide on growth performance, appetite, and hypothalamic inflammation in broilers. J. Anim. Sci. 2023, 101, skad027. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, Y.; Yuan, M.; Xu, L.; Luo, X.; Wu, R.; Xi, Z.; Li, Y.; Xu, H. Prunella vulgaris polysaccharide inhibits herpes simplex virus infection by blocking TLR-mediated NF-κB activation. Chin. Med. 2024, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Liao, H.Y.; Bai, D.Y.; Wang, Z.Q.; Xie, X.W. MAPK/ERK signaling pathway: A potential target for the treatment of intervertebral disc degeneration. Biomed. Pharmacother. 2021, 143, 112170. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, H.; Guo, W.; Yu, L. Potential role of ghrelin in the regulation of inflammation. Faseb J. 2022, 36, e22508. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in LPS-Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef]
- Mishra, V.; Banga, J.; Silveyra, P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol. Ther. 2018, 181, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Song, L.; Xue, J.; Wang, X.; Song, S.; Wang, S. Polysaccharide from Ganoderma lucidum ameliorates cognitive impairment by regulating the inflammation of the brain-liver axis in rats. Food Funct. 2021, 12, 6900–6914. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Li, K.; Liu, R.; Du, J.; Zou, D.; Ma, Y. Angelica polysaccharide attenuates LPS-induced inflammation response of primary dairy cow claw dermal cells via NF-κB and MAPK signaling pathways. BMC Vet. Res. 2021, 17, 248. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Li, X.; Xue, C.; Zhang, L.; Wang, C.; Xu, X.; Shan, A. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J. Cell Physiol. 2020, 235, 5525–5540. [Google Scholar] [CrossRef] [PubMed]
- Chen, F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 2012, 72, 379–386. [Google Scholar] [CrossRef]
- Kennedy, N.J.; Davis, R.J. Role of JNK in tumor development. Cell Cycle 2003, 2, 199–201. [Google Scholar]
- Karin, M.; Lawrence, T.; Nizet, V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liong, E.C.; Ching, Y.P.; Chang, R.C.; Fung, M.L.; Xu, A.M.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr. Diabetes 2013, 3, e81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ouyang, H.; Mei, X.; Lu, B.; Yu, Z.; Chen, K.; Wang, Z.; Ji, L. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway. Faseb J. 2019, 33, 11776–11790. [Google Scholar] [CrossRef]
- Moon, S.K.; Cha, B.Y.; Kim, C.H. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J. Cell Physiol. 2004, 198, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Jia, H.; Xu, L.; Cao, Y.; Zhai, M.; Li, K.; Xia, L.; Jiang, L.; Li, X.; et al. Tetrahydrocurcumin improves lipopolysaccharide-induced myocardial dysfunction by inhibiting oxidative stress and inflammation via JNK/ERK signaling pathway regulation. Phytomedicine 2022, 104, 154283. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, Z.; Nie, C.; Chang, L.; Jiang, T. Targeting Src homology phosphatase 2 ameliorates mouse diabetic nephropathy by attenuating ERK/NF-κB pathway-mediated renal inflammation. Cell Commun. Signal 2023, 21, 362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, D.; Huang, Y.; Dai, Y.; Wang, Y.; Liu, M.; Wang, N.; Yin, T.; Du, W.; He, K.; et al. Oral administration of punicalagin attenuates imiquimod-induced psoriasis by reducing ROS generation and inflammation via MAPK/ERK and NF-κB signaling pathways. Phytother. Res. 2024, 38, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Huang, Y. Effects of dandelion polysaccharide on gastric mucosal inflammatory response and MAPK/ERK pathway in rats with Helicobacter pylori associated gastritis. Mod. J. Integr. Tradit. Chin. West. Med. 2019, 28, 3877–3880. [Google Scholar]
- Wang, S.; Ni, L.; Fu, X.; Duan, D.; Xu, J.; Gao, X. A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-κB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo. Mar. Drugs 2020, 18, 593. [Google Scholar] [CrossRef]
- Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Therapeutic regulation of the NLRP3 inflammasome in chronic inflammatory diseases. Arch. Pharm. Res. 2021, 44, 16–35. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.G.; Brough, D.; Freeman, S. Inhibiting the Inflammasome: A Chemical Perspective. J. Med. Chem. 2016, 59, 1691–1710. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Bai, X.; Zhang, T.; Zhou, L.; Li, J.; Zhang, L. The protective effect of Lonicera japonica polysaccharide on mice with depression by inhibiting NLRP3 inflammasome. Ann. Transl. Med. 2019, 7, 811. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, Y. Pachymaran regulates anxiety and depression-like behaviors induced by lipopolysaccharide through NF-κB and NLRP3 signaling pathways. Sci. Technol. Food Ind. 2023, 44, 371–377. [Google Scholar]
- Han, L. Ganoderma Lucidum Polysaccharide Regulates Dectin-1 Receptor to Inhibit NF-κB/NLRP3 Inflammasome Signaling Mediated Myelin Regeneration. Master’s Thesis, Yunnan University, Yunnan, China, 2022. [Google Scholar]
- Xiao, L.; Qi, L.; Zhang, G.; Liu, H.; Gu, Y.; Zhang, L.; Zhang, M.; Wu, H. Polygonatum sibiricum Polysaccharides Attenuate Lipopoly-Saccharide-Induced Septic Liver Injury by Suppression of Pyroptosis via NLRP3/GSDMD Signals. Molecules 2022, 27, 5999. [Google Scholar] [CrossRef]
- Wan, H.; Zheng, Y.; Liang, Y.; Lu, L. Effect of Angelica polysaccharide on NLRP3 inflammatory signaling pathway in renal tissue of rats with chronic renal failure. Henan Med. Res. 2023, 32, 1000–1005. [Google Scholar]
- Jeong, S.J.; Pise-Masison, C.A.; Radonovich, M.F.; Park, H.U.; Brady, J.N. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene 2005, 24, 6719–6728. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthritis Cartilage 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Acosta-Martinez, M.; Cabail, M.Z. The PI3K/Akt Pathway in Meta-Inflammation. Int. J. Mol. Sci. 2022, 23, 15330. [Google Scholar] [CrossRef]
- Lappas, M. GSK3β is increased in adipose tissue and skeletal muscle from women with gestational diabetes where it regulates the inflammatory response. PLoS ONE 2014, 9, e115854. [Google Scholar] [CrossRef]
- Lin, J.; Song, T.; Li, C.; Mao, W. GSK-3β in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118659. [Google Scholar] [CrossRef]
- Huang, H.; An, J.; Fan, X. To investigate the effects of Codonopsis polysaccharides on the neural function and brain protection of herpes simplex virus encephalitis type I mice based on PI3K/AKT/GSK3β pathway. CVD J. ICM 2023, 21, 4347–4352. [Google Scholar]
- Vangan, N.; Cao, Y.; Jia, X.; Bao, W.; Wang, Y.; He, Q.; Binderiya, U.; Feng, X.; Li, T.; Hao, H.; et al. mTORC1 mediates peptidoglycan induced inflammatory cytokines expression and NF-κB activation in macrophages. Microb. Pathog. 2016, 99, 111–118. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Qi, X.; Lu, X.; Han, Y.; Xing, Y.; Zheng, Y.; Cui, C. Ginseng polysaccharide reduces autoimmune hepatitis inflammatory response by inhibiting PI3K/AKT and TLRs/NF-κB signaling pathways. Phytomedicine 2023, 116, 154859. [Google Scholar] [CrossRef]
- Zhang, X. Polygonatum Polysaccharide Alleviates CCL4-Induced Acute Liver Injury In Rats Through PI3K/AKT/mTOR Pathway. Master’s Thesis, Anhui Agricultural University, Anhui, China, 2023. [Google Scholar]
- Stark, J.M.; Coquet, J.M.; Tibbitt, C.A. The Role of PPAR-γ in Allergic Disease. Curr. Allergy Asthma Rep. 2021, 21, 45. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Sivarajah, A.; Thiemermann, C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc. Res. 2005, 65, 772–781. [Google Scholar] [CrossRef]
- Xu, T.; Liu, R.; Lu, X.; Wu, X.; Heneberg, P.; Mao, Y.; Jiang, Q.; Loor, J.; Yang, Z. Lycium barbarum polysaccharides alleviate LPS-induced inflammatory responses through PPARγ/MAPK/NF-κB pathway in bovine mammary epithelial cells. J. Anim. Sci. 2022, 100, skab345. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Xue, Q.L.; Han, S.; Li, W.; Zhao, T. Study on the effect and mechanism of sea-buckthorn polysaccharide on reducing sepsis induced liver injury in mice with liver-specific PPARγ knockout. CJI Chin. J. Immunol. 2022, 38, 789–794. [Google Scholar]
- Zhang, H. Effects of Astragalus Polysaccharides on Myocardial Protection and PPARγ/NF-κB Signaling Pathway in db/db Mice with Diabetic Cardiomyopathy. Ph.D. Thesis, Gansu University of Chinese Medicine, Lanzhou, China, 2018. [Google Scholar]
- Hu, T. Evaluation of Anti-Inflammatory and Lipid-Lowering Functions of Mulberry Yellow Polysaccharide and Its Molecular Mechanism. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2020. [Google Scholar]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 108073. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yuan, W.; Xiong, X.; Zhang, Z.; Liu, J.; Zheng, Y.; Wang, J.; Liu, J. HO-1 in Bone Biology: Potential Therapeutic Strategies for Osteoporosis. Front. Cell Dev. Biol. 2021, 9, 791585. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme Oxygenases in Cardiovascular Health and Disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Wang, P.; Ma, Z.; Peng, L.; Wang, Z.; Chen, Z. Ultrasonic treatment of Dendrobium officinale polysaccharide enhances antioxidant and anti-inflammatory activity in a mouse D-galactose-induced aging model. Food Sci. Nutr. 2022, 10, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, S.; Hu, Y.; Yang, Y.; Yuan, J.; Wu, Y.; Li, S.; Lin, J.; He, L.; Hou, S.; et al. Protective roles and mechanisms of Dendrobium officinal polysaccharides on secondary liver injury in acute colitis. Int. J. Biol. Macromol. 2018, 107 Pt B, 2201–2210. [Google Scholar] [CrossRef]
- Liang, J.; Wu, Y.; Yuan, H.; Yang, Y.; Xiong, Q.; Liang, C.; Li, Z.; Li, C.; Zhang, G.; Lai, X.; et al. Dendrobium officinale polysaccharides attenuate learning and memory disabilities via anti-oxidant and anti-inflammatory actions. Int. J. Biol. Macromol. 2019, 126, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zou, D.; Liu, M.; Ma, Y. Protective effect of Poria polysaccharide on renal tubular epithelial cell injury induced by magnesium ammonium phosphate in sheep. Chin. J. Vete Sci. 2022, 42, 294–298. [Google Scholar]
- Xie, P.; Chen, L.; Wang, J.; Wang, X.; Yang, S.; Zhu, G. Polysaccharides from Polygonatum cyrtonema Hua prevent post-traumatic stress disorder behaviors in mice: Mechanisms from the perspective of synaptic injury, oxidative stress, and neuroinflammation. J. Ethnopharmacol. 2024, 319 Pt 1, 117165. [Google Scholar] [CrossRef]
- Li, H.N.; Zhao, L.L.; Zhou, D.Y.; Chen, D.Q. Ganoderma Lucidum Polysaccharides Ameliorates Hepatic Steatosis and Oxidative Stress in db/db Mice via Targeting Nuclear Factor E2 (Erythroid-Derived 2)-Related Factor-2/Heme Oxygenase-1 (HO-1) Pathway. Med. Sci. Monit. 2020, 26, e921905. [Google Scholar] [CrossRef] [PubMed]
- Imada, K.; Leonard, W.J. The Jak-STAT pathway. Mol. Immunol. 2000, 37, 1–11. [Google Scholar] [CrossRef]
- Li, W.X. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008, 18, 545–551. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, M.; Muroi, M.; Tanamoto, K.-i.; Suzuki, T.; Azuma, H.; Ikeda, H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: Roles of the receptor complex. Pharmacol. Ther. 2003, 100, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhong, Z.; Darnell, J.E., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 1995, 82, 241–250. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, X.; Chen, W.; Liu, J. Angelica polysaccharide mitigates lipopolysaccharide-evoked inflammatory injury by regulating microRNA-10a in neuronal cell line HT22. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3194–3201. [Google Scholar] [CrossRef]
- Wang, K.; Song, Z.; Wang, H.; Li, Q.; Cui, Z.; Zhang, Y. Angelica sinensis polysaccharide attenuates concanavalin A-induced liver injury in mice. Int. Immunopharmacol. 2016, 31, 140–148. [Google Scholar] [CrossRef]
- Wu, T.; Lin, H.; Lin, W.; Zhu, F.; Long, D. Effects of Dendrobium polysaccharide on JAK/STAT3 signaling pathway in cerebral tissue of ischemic stroke rats. Chin. J. Arterioscler. 2023, 31, 225–230. [Google Scholar]
- Sun, Y.; Shi, Z.; Liu, M.; Zhao, Y. Effects of Yam polysaccharide on myocardial injury and JAK2/STAT3 signaling pathway in sepsis rats. Chin. J. Arterioscler. 2022, 30, 669–675. [Google Scholar]
- Wang, Q.; Bie, Y.; Wang, D.; Fan, W. Effects of dandelion polysaccharides on IL-6/STAT3 signaling pathway in rats with ulcerative colitis. Chin. J. Appl. Physiol. 2017, 33, 422–425. [Google Scholar]
- Fan, W.; Wang, P.; Wang, Q. Effects of Purslane polysaccharide on IL-6/STAT3 and NF-κB in intestinal tissues of rats with ulcerative colitis. Chin. J. Appl. Physiol. 2018, 34, 263–267. [Google Scholar]
- Shen, H.; Zhao, Z.; Zhao, Z.; Chen, Y.; Zhang, L. Native and Engineered Probiotics: Promising Agents against Related Systemic and Intestinal Diseases. Int. J. Mol. Sci. 2022, 23, 594. [Google Scholar] [CrossRef]
- Rui, Z.; Zhang, L.; Li, X.; Han, J.; Yuan, Y.; Ding, H.; Liu, Y.; Ding, X. Pterostilbene exert an anti-arthritic effect by attenuating inflammation, oxidative stress, and alteration of gut microbiota. J. Food Biochem. 2022, 46, e14011. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, M.; Wang, L.; Wang, G. Polysaccharides and glycosides from Aralia echinocaulis protect rats from arthritis by modulating the gut microbiota composition. J. Ethnopharmacol. 2021, 269, 113749. [Google Scholar] [CrossRef]
- Pang, J.; Ma, S.; Xu, X.; Zhang, B.; Cai, Q. Effects of rhizome of Atractylodes koreana (Nakai) Kitam on intestinal flora and metabolites in rats with rheumatoid arthritis. J. Ethnopharmacol. 2021, 281, 114026. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Yan, Y.; Yuan, H.; Rong, A.; Xu, G.; Cai, F.; Yang, Y.; Wang, Y.; Zhang, W. Astragalus mongholicus polysaccharides ameliorate hepatic lipid accumulation and inflammation as well as modulate gut microbiota in NAFLD rats. Food Funct. 2022, 13, 7287–7301. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, W.; Feng, Y.; Hou, H.; Zhang, Z.; Yu, Q.; Zhou, Y.; Luo, Q.; Luo, Y.; Ouyang, H.; et al. Radix Puerariae thomsonii polysaccharide (RPP) improves inflammation and lipid peroxidation in alcohol and high-fat diet mice by regulating gut microbiota. Int. J. Biol. Macromol. 2022, 209 Pt A, 858–870. [Google Scholar] [CrossRef]
- Yang, B.; Xiong, Z.; Lin, M.; Yang, Y.; Chen, Y.; Zeng, J.; Jia, X.; Feng, L. Astragalus polysaccharides alleviate type 1 diabetes via modulating gut microbiota in mice. Int. J. Biol. Macromol. 2023, 234, 123767. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Zhong, Y.; Xiao, G.; Efferth, T.; Georgiev, M.I.; Vargas-De-La-Cruz, C.; Bajpai, V.K.; Caprioli, G.; Liu, J.; et al. Dendrobium officinale Polysaccharide Alleviates Intestinal Inflammation by Promoting Small Extracellular Vesicle Packaging of miR-433-3p. J. Agric. Food Chem. 2021, 69, 13510–13523. [Google Scholar] [CrossRef]
- Gu, W.; Wang, Y.; Zeng, L.; Dong, J.; Bi, Q.; Yang, X.; Che, Y.; He, S.; Yu, J. Polysaccharides from Polygonatum kingianum improve glucose and lipid metabolism in rats fed a high fat diet. Biomed. Pharmacother. 2020, 125, 109910. [Google Scholar] [CrossRef]
- Fan, X.H.; Li, K.; Yang, Y.; Qin, X.; Li, Z.; Li, X. Screening of anti-inflammatory active components of Astragalus polysaccharides based on molecular weight distribution and metabolomic regulation mechanism. APSB Acta Pharm. Sin. B 2022, 57, 783–792. [Google Scholar]
- Chen, G.; Jiang, N.; Zheng, J.; Hu, H.; Yang, H.; Lin, A.; Hu, B.; Liu, H. Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2023, 241, 124386. [Google Scholar] [CrossRef]
- Wang, J.H.; Dong, J.C.; Zhao, J.X.; Jin, S.Z.; Zhang, H.Y.; Zhang, S.Y. ffects of Astragalus membranaceus polysaccharide with different relative molecular weight on expression of inflammatory factors in RAW264.7 cells. J. Jilin Univ. (Med. Ed.) 2011, 37, 1051–1056. [Google Scholar]
- Hamid, M.; Liu, D.; Abdulrahim, Y.; Khan, A.; Qian, G.; Huang, K. Inactivation of Kupffer Cells by Selenizing Astragalus Polysaccharides Prevents CCl4-Induced Hepatocellular Necrosis in the Male Wistar Rat. Biol. Trace Elem. Res. 2017, 179, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Li, Y.; Wang, F.; Yang, X.; Yao, J. Sulfated Astragalus polysaccharide can regulate the inflammatory reaction induced by LPS in Caco2 cells. Int. J. Biol. Macromol. 2013, 60, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cui, W.; Gao, Z.; Zhang, J.; Jia, L. Structural characterization and amelioration of sulfated polysaccharides from Ganoderma applanatum residue against CCl4-induced hepatotoxicity. Int. Immunopharmacol. 2021, 96, 107554. [Google Scholar] [CrossRef]
- Gao, Z.; Yuan, F.; Li, H.; Feng, Y.; Zhang, Y.; Zhang, C.; Zhang, J.; Song, Z.; Jia, L. The ameliorations of Ganoderma applanatum residue polysaccharides against CCl4 induced liver injury. Int. J. Biol. Macromol. 2019, 137, 1130–1140. [Google Scholar] [CrossRef]
- Li, Y.W.; Chen, L.H.; Hang, L.Q.; Zhou, D.P.; Liu, P.; Jin, Y.; Zhu, W.H. Effect of carboxymethylated Ganoderma lucidum polysaccharide pretreatment on cerebral ischemia-reperfusion injury in rats and its mechanism. Shandong Med. 2018, 58, 12–16. [Google Scholar]
- Liu, Y.F. Structural and Conformational Characterization of Polysaccharides from Ganoderma lucidum and Study on Structure-Activity Relationship of Immune Regulation. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2018. [Google Scholar]
- Chen, H.; Shi, X.; Zhang, L.; Yao, L.; Cen, L.; Li, L.; Lv, Y.; Wei, C. Ultrasonic Extraction Process of Polysaccharides from Dendrobium nobile Lindl.: Optimization, Physicochemical Properties and Anti-Inflammatory Activity. Foods 2022, 11, 2957. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Luo, J. Optimization of semi-bionic extraction process and evaluation of anti-inflammatory activity of Den-drobium huoshanense polysaccharide. Anhui Agric. Sci. 2018, 46, 151–154+183. [Google Scholar]
- Zhang, X.; Liu, Y.; Cheng, Y.R.; Wang, J.X.; Gong, Y.T.; Li, Y.T.; Yang, D.P.; Li, T.; Dong, L. Correlation between molecular weight distribution and anti-inflammatory activity of Lycium barbarum polysaccharide. J. Beijing Univ. TCM 2020, 43, 959–964. [Google Scholar]
- Liu, W.J. Structure of Angelica Polysaccharide and Its Mechanism of Inhibiting LPS-Induced Macrophage Activation. Ph.D. Thesis, Northwest Agriculture & Forestry University, Xianyang, China, 2023. [Google Scholar]
- Gao, Z.; Zhang, C.; Tian, W.; Liu, K.; Hou, R.; Yue, C.; Wu, Y.; Wang, D.; Liu, J.; Hu, Y.; et al. The antioxidative and hepatoprotective effects comparison of Chinese angelica polysaccharide(CAP)and selenizing CAP (sCAP) in CCl4 induced hepatic injury mice. Int. J. Biol. Macromol. 2017, 97, 46–54. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, X.; Cao, M.; Zheng, S.; Ma, Y.; Huang, Q. NF-κB and AMPK-Nrf2 pathways support the protective effect of polysaccharides from Polygonatum cyrtonema Hua in lipopolysaccharide-induced acute lung injury. J. Ethnopharmacol. 2022, 291, 115153. [Google Scholar] [CrossRef]
- Shen, F.; Song, Z.; Xie, P.; Li, L.; Wang, B.; Peng, D.; Zhu, G. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef]
- Zhao, T. Study on the Preparation Process of Polysaccharide Granules of Mordant Yellow and Its Anti-Inflammatory Mechanism on Ulcerative Colitis in C57BL/6 Mice. Master’s Thesis, Changchun University of Chinese Medicine, Changchun, China, 2023. [Google Scholar]
- Sun, Y.; Huo, J.; Zhong, S.; Zhu, J.; Li, Y.; Li, X. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohydr. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef]
- Liu, L.N.; Wang, R.Z.; Wu, S.Y.; Jin, C.S. Structural analysis and anti-inflammatory activity of two polysaccharides from mulberry yellow. J. Anhui Univ. TCM 2021, 1, 107–112. [Google Scholar]
- Cheng, Y.; Xie, Y.; Ge, J.C.; Wang, L.; Peng, D.Y.; Yu, N.J.; Zhang, Y.; Jiang, Y.H.; Luo, J.P.; Chen, W.D. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos. Carbohydr. Polym. 2021, 263, 117979. [Google Scholar] [CrossRef]
- Liu, X.; Hu, S.; Zhang, X. Structure and biological activity of a carboxymethyl poria polysaccharide. Mod. Food Sci. Technol. 2018, 34, 42–49+47. [Google Scholar]
- Liu, X.; Wang, X.; Xu, X.; Zhang, X. Purification, antitumor and anti-inflammation activities of an alkali-soluble and carboxymethyl polysaccharide CMP33 from Poria cocos. Int. J. Biol. Macromol. 2019, 127, 39–47. [Google Scholar] [CrossRef]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008, 58, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, X.; Xie, H.; Li, X.; Shi, L. Structural characterization and antitumor activity of a polysaccharide from ramulus mori. Carbohydr. Polym. 2018, 190, 232–239. [Google Scholar] [CrossRef]
- Morales, D.; Rutckeviski, R.; Villalva, M.; Abreu, H.; Soler-Rivas, C.; Santoyo, S.; Iacomini, M.; Smiderle, F.R. Isolation and comparison of α- and β-D-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities. Carbohydr. Polym. 2020, 229, 115521. [Google Scholar] [CrossRef]
- Zhao, T.; Guo, Y.; Yan, S.; Li, N.; Ji, H.; Hu, Q.; Zhang, M.; Li, Q.; Gao, H.; Yang, L.; et al. Preparation, structure characterization of carboxymethylated schisandra polysaccharides and their intervention in immunotoxicity to polychlorinated biphenyls. Process Biochem. 2022, 115, 30–41. [Google Scholar] [CrossRef]
- Zhan, Q.; Chen, Y.; Guo, Y.; Wang, Q.; Wu, H.; Zhao, L. Effects of selenylation modification on the antioxidative and immunoregulatory activities of polysaccharides from the pulp of Rose laevigata Michx fruit. Int. J. Biol. Macromol. 2022, 206, 242–254. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. Preparation, structure and activity of polysaccharide phosphate esters. Biomed. Pharmacother. 2021, 144, 112332. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Shu, Z.; Zheng, Y.; Hu, X.; Zhang, P.; Huang, H.; Sheng, L.; Zhang, P.; Wang, Q.; et al. Regulation strategy, bioactivity, and physical property of plant and microbial polysaccharides based on molecular weight. Int. J. Biol. Macromol. 2023, 244, 125360. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ren, W.; Li, G.; Yang, P.; Chen, R.; He, H. The effect of structure and preparation method on the bioactivity of polysaccharides from plants and fungi. Food Funct. 2022, 13, 12541–12560. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, X.; Wang, F.; Zhang, Q.; Zhang, Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 2013, 61, 270–275. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Luo, S.; Zheng, Y.; Luo, X.; Zhou, L. Antitumor activity of Lycium barbarum polysaccharides with different molecular weights: An in vitro and in vivo study. Food Nutr. Res. 2017, 61, 1399770. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Miao, J.; Jing, S.; Li, X.; Huang, L.; Gao, W. The effect of different extraction techniques on property and bioactivity of polysaccharides from Dioscorea hemsleyi. Int. J. Biol. Macromol. 2017, 102, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Li, C.Y.; Fu, Y.P.; Jiang, Q.X.; Peng, X.; Li, L.X.; Song, X.; Zhao, X.H.; Li, Y.P.; Chen, X.F.; et al. The comparison of preliminary structure and intestinal anti-inflammatory and anti-oxidative activities of polysaccharides from different root parts of Angelica sinensis (Oliv.) Diels. J. Ethnopharmacol. 2022, 295, 115446. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Hua, Y.; Xue, W.; Zhang, M.; Wei, Y.; Ji, P. Metabonomics study on the hepatoprotective effect of polysaccharides from different preparations of Angelica sinensis. J. Ethnopharmacol. 2014, 151, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xiao, N.; Zeng, L.; Xiao, J.; Huang, J.; Xu, Y.; Chen, Y.; Ren, Y.; Du, B. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr. Polym. 2020, 250, 116958. [Google Scholar] [CrossRef]
- Zhao, G.; Kan, J.; Li, Z.; Chen, Z. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydr. Polym. 2005, 61, 125–131. [Google Scholar] [CrossRef]
- Zhang, T.T.; Lu, C.L.; Jiang, J.G.; Wang, M.; Wang, D.M.; Zhu, W. Bioactivities and extraction optimization of crude polysaccharides from the fruits and leaves of Rubus chingii Hu. Carbohydr. Polym. 2015, 130, 307–315. [Google Scholar] [CrossRef]
- Kang, S.-M.; Kim, K.-N.; Lee, S.-H.; Ahn, G.; Cha, S.-H.; Kim, A.-D.; Yang, X.-D.; Kang, M.-C.; Jeon, Y.-J. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS-stimulated RAW 264.7 macrophages. Carbohydr. Polym. 2011, 85, 80–85. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Gao, Q.; Zou, Y. Hypoglycemic Effect of Chinese Yam (Dioscorea opposita rhizoma) Polysaccharide in Different Structure and Molecular Weight. J. Food Sci. 2017, 82, 2487–2494. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, T.; Wang, H.; Cui, Z.; Cheng, F.; Wang, K.P. Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2016, 147, 401–408. [Google Scholar] [CrossRef]
- Chen, Z.H.; Chen, Y.L.; Wu, T.; Hua, Y.; Zhang, M. Structural characteristics and immune activity of DMP4a-1 polysaccharide from Dendrobium leptocaulis. Chin. J. Pharma Sci. 2005, 23, 1781–1784. [Google Scholar]
- Li, Q.; Liu, W.; Zhang, H.; Chen, C.; Liu, R.; Hou, H.; Luo, Q.; Yu, Q.; Ouyang, H.; Feng, Y.; et al. α-D-1,3-glucan from Radix Puerariae thomsonii improves NAFLD by regulating the intestinal flora and metabolites. Carbohydr. Polym. 2023, 299, 120197. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Yang, S.; Zhao, D.; Wang, M. A polysaccharide from cultured mycelium of Hericium erinaceus relieves ulcerative colitis by counteracting oxidative stress and improving mitochondrial function. Int. J. Biol. Macromol. 2019, 125, 572–579. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Xu, D.; Gao, Q. A polysaccharide from cultured mycelium of Hericium erinaceus and its anti-chronic atrophic gastritis activity. Int. J. Biol. Macromol. 2015, 81, 656–661. [Google Scholar] [CrossRef]
- Song, J.; Chen, Y.; Lv, Z.; Taoerdahong, H.; Li, G.; Li, J.; Zhao, X.; Jin, X.; Chang, J. Structural characterization of a polysaccharide from Alhagi honey and its protective effect against inflammatory bowel disease by modulating gut microbiota dysbiosis. Int. J. Biol. Macromol. 2024, 259 Pt 1, 128937. [Google Scholar] [CrossRef]
- Wu, Y.S.; Ho, S.Y.; Nan, F.H.; Chen, S.N. Ganoderma lucidum beta 1,3/1,6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement. Altern. Med. 2016, 16, 500. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between Modification, Conformation and Functional Activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef]
- Meng, Y.; Lyu, F.; Xu, X.; Zhang, L. Recent Advances in Chain Conformation and Bioactivities of Triple-Helix Polysaccharides. Biomacromolecules 2020, 21, 1653–1677. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, T.; Li, Y.; Xue, H.; Zou, J.; Cai, J.; Shi, R.; Wu, J.; Ma, Y. Gardenia jasminoides Ellis polysaccharide ameliorates cholestatic liver injury by alleviating gut microbiota dysbiosis and inhibiting the TLR4/NF-κB signaling pathway. Int. J. Biol. Macromol. 2022, 205, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiao, Y.; Shen, S.; Zhao, W.; Zhang, Q.; Zhang, S. Chaenomeles sinensis polysaccharide and its carboxymethylated derivative alleviate dextran sulfate sodium-induced ulcerative colitis via suppression of inflammation and oxidative stress. Biomed. Pharmacother. 2023, 169, 115941. [Google Scholar] [CrossRef]
- Li, W.; Qiu, Z.; Ma, Y.; Zhang, B.; Li, L.; Li, Q.; He, Q.; Zheng, Z. Preparation and Characterization of Ginger Peel Polysaccharide-Zn (II) Complexes and Evaluation of Anti-Inflammatory Activity. Antioxidants 2022, 11, 2331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mo, Q.; Li, Z.; Lai, H.; Lou, J.; Liu, S.; Mao, J. Effects of degree of carboxymethylation on physicochemical and biological properties of pachyman. Int. J. Biol. Macromol. 2012, 51, 1052–1056. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Feng, X.; Ibrahim, S.A.; Huang, W. Structure characterization and in vitro immunomodulatory activities of carboxymethyl pachymaran. Int. J. Biol. Macromol. 2021, 178, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhang, M.-W.; Liao, S.-T.; Zhang, R.-F.; Deng, Y.-Y.; Wei, Z.-C.; Yang, B. Effects of alkali dissociation on the molecular conformation and immunomodulatory activity of longan pulp polysaccharide (LPI). Carbohydr. Polym. 2012, 87, 1311–1317. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.; Huang, J.; Wei, Y.; Yang, Y.; Li, X.; Luo, H.; Qin, A.; Gan, R. Optimization of acetylation process and antioxidant activity of polysaccharide from longan meat by response surface test. Food Sci. 2016, 37, 63–68. [Google Scholar]
- Ming, K.; Chen, Y.; Yao, F.; Shi, J.; Yang, J.; Du, H.; Wang, X.; Wang, Y.; Liu, J. Phosphorylated Codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis A virus compared with Codonopsis pilosula polysaccharide. Int. J. Biol. Macromol. 2017, 94 Pt A, 28–35. [Google Scholar] [CrossRef]
- Hou, R.; Chen, J.; Yue, C.; Li, X.; Liu, J.; Gao, Z.; Liu, C.; Lu, Y.; Wang, D.; Li, H.; et al. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohydr. Polym. 2016, 142, 73–81. [Google Scholar] [CrossRef]
- Wang, K.-W.; Yang, C.; Yan, S.-N.; Wang, H.; Cao, X.-J.; Cheng, Y. Dendrobium hancockii polysaccharides, structure characterization, modification, antioxidant and antibacterial activity. Ind. Crops Prod. 2022, 188, 115565. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef]
- Li, Z.W.; Du, Z.M.; Wang, Y.W.; Feng, Y.X.; Zhang, R.; Yan, X.B. Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review. Polymers 2022, 14, 4161. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, W.; Huang, G. Preparation and activities of selenium polysaccharide from plant such as Grifola frondosa. Carbohydr. Polym. 2020, 242, 116409. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Lian, K.X.; Zhang, H.J.; Liu, C.B. Study on anti-inflammatory activity of selenated Glycyrrhiza uralensis polysaccharide. Heilongjiang Anim. Sci. Veter Med. 2018, 4, 178–181. [Google Scholar]
- Ye, R.; Guo, Q.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Eucommia ulmoides polysaccharide modified nano-selenium effectively alleviated DSS-induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity. J. Nanobiotechnol. 2023, 21, 222. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Wang, Z.; Xie, J. Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. Trends Food Sci. Technol. 2021, 118, 539–557. [Google Scholar] [CrossRef]
- Liu, Y.; You, Y.; Li, Y.; Zhang, L.; Tang, T.; Duan, X.; Li, C.; Liu, A.; Hu, B.; Chen, D. Characterization of carboxymethylated polysaccharides from Catathelasma ventricosum and their antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 355–362. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165 Pt B, 2425–2431. [Google Scholar] [CrossRef]
- Wang, H.X. Chemical Composition Analysis of Different Medicinal Parts of Poria Cocos and Study on Quality Standard of Red Poria Cocos. Master’s Thesis, Hebei Medical University, Shijiazhuang, China, 2016. [Google Scholar]
- Kang, Y.; Wang, W. Determination of water-soluble and alkali-soluble polysaccharides in Poria Cocos by near infrared diffuse reflection method. Chin. J. ETMF 2016, 22, 80–83. [Google Scholar]
- Yuan, J.-J. Comparative study on the relationship between content of Poria polysaccharide and its origin in Poria poria. J. China Prescr. Drug 2016, 14, 18–19. [Google Scholar]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Li, Q.M.; Teng, H.; Zha, X.Q.; Pan, L.H.; Luo, J.P. Sulfated Laminaria japonica polysaccharides inhibit macrophage foam cell formation. Int. J. Biol. Macromol. 2018, 111, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, H.R.; Zha, X.Q.; Chen, S.; Pan, L.H.; Li, Q.M.; Luo, J.P. Prevention and possible mechanism of a purified Laminaria japonica polysaccharide on adriamycin-induced acute kidney injury in mice. Int. J. Biol. Macromol. 2020, 148, 591–600. [Google Scholar] [CrossRef]
- Jing, Y.-S.; Zhang, R.-J.; Wu, L.-F.; Zheng, Y.; Gao, X.; Hao, T.; Zhang, D. Advances in structural characteristics and physiological activities of polysaccharide iron complexes. Food R&D 2019, 40, 203–208. [Google Scholar]
- Qian, Y.; Wang, D.; Fan, M.; Sun, X.; Li, X. Effects of metal ions in polysaccharides on their antioxidant and antitumor activities. J. Chin. Inst. Food Sci. 2020, 20, 52–60. [Google Scholar]
- Li, X.; Jiang, F.; Liu, M.; Qu, Y.; Lan, Z.; Dai, X.; Huang, C.; Yue, X.; Zhao, S.; Pan, X.; et al. Synthesis, Characterization, and Bioactivities of Polysaccharide Metal Complexes: A Review. J. Agric. Food Chem. 2022, 70, 6922–6942. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Y.; Hu, L.; Peng, Y.; Deng, Y.; He, W.; Ge, Y.; Tang, B. Strontium Laminarin polysaccharide modulates osteogenesis-angiogenesis for bone regeneration. Int. J. Biol. Macromol. 2021, 181, 452–461. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, F.; Ruiz-Ortega, L.I.; Peng, Y.; Tian, Y.; He, W.; Tang, B. Fabrication of strontium Eucommia ulmoides polysaccharides and in vitro evaluation of their osteoimmunomodulatory property. Int. J. Biol. Macromol. 2019, 140, 727–735. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Li, C.; Wu, G.; Wu, Y.; Huang, P.; Zhao, Y.; Zhang, X.; Tang, B. Surface modification of polyetheretherketone (PEEK) to enhance osteointegration by grafting strontium Eucommia ulmoides polysaccharides. Int. J. Biol. Macromol. 2022, 211, 230–237. [Google Scholar]

| Number | Chinese Name | English Name | Latin Name | Name of Family | Part Used |
|---|---|---|---|---|---|
| 1 | Baibiandou | Semen Dolichoris Album | Dolichos lablab L. | Fabaceae | Mature seed |
| 2 | Baibiandouhua | Flower of Hyacinth Dolichos | Dolichos lablab L. | Fabaceae | Flower |
| 3 | Baiguo | ginkgo seed | Ginkgo biloba L | Ginkgoaceae | Mature seed |
| 4 | Baihe | lily | Lilium lancifolium Thunb. Lilium brownie F.E.Brown var.viridulum Baker Lilium pumilum DC. | Liliaceae | Fleshly scale leaf |
| 5 | Baimaogen | rhizoma imperatae | Imperata cylindrica Beauv.var. major (Nees) C.E.Hubb. | Poaceae Barnhart | Rhizome |
| 6 | Baizhi | angelica | Angelica dahurica (Fisch.ex Hoffm.) Benth.et Hook.f Angelica dahurica (Fisch.ex Hoffm.) Benth. et Hook.f.var.formosana (Boiss.) Shan et Yuan | Apiaceae | Root |
| 7 | Bajiaohuixiang | Anisi Stellati Fructus | Illicium verum Hook.f. | Magnoliaceae | Ripe fruit |
| 8 | Biba | long pepper | Piper longum L. | Piperaceae Giseke | Fruit/ripe ear |
| 9 | Bohe | mint | Mentha canadensis L. | Lamiaceae | Overground part |
| 10 | Buzhaye | leaf of paniculate microcos | Microcos paniculata L. | Tiliaceae | Leaf |
| 11 | Caoguo | Amomum tsao-ko | Amomum tsao-ko Crevost et Lemaire | zingiberaceae | Fruit |
| 12 | Chenpi | dried tangerine peel | Citrus reticulata Blanco | Rutaceae | Ripe peel |
| 13 | Chixiaodou | ricebean | Vigna umbellate (Thunb.) Ohwi & Ohashi | Fabaceae | Mature seed |
| 14 | Daidaihua | seville orange flower | Citrus aurantium L.var.amara Engl. | Rutaceae | Flower bud |
| 15 | Dandouchi | fermented soybean | Glycine max (L.) Merr. | Fabaceae | Mature seeds |
| 16 | Danggui | Angelica sinensis | Angelica sinensis (Oliv.) Diels | Apiaceae | Root |
| 17 | Dangshen | Salvia miltiorrhiza | Codonopsis pilosula (Franch.) Nannf. | Campanulaceae | Root |
| 18 | Danzhuye | Lophatherum gracile | Lophatherum gracile Brongn. | Poaceae Barnhart | Stem leaf |
| 19 | Daodou | blade bean | Canavalia gladiate (Jacq.) DC | Fabaceae | Mature seed |
| 20 | Dingxiang | clove | Eugenia caryophyllata Thunb | Myrtaceae | Bud |
| 21 | Duzhongye | folium cortex eucommiae | Eucommia ulmoides Oliv. | Eucommiaceae | Leaf |
| 22 | Ejiao | donkey-hide gelatin | Equus asinus L. | Equidae | skin |
| 23 | Feizi | Chinese torreya | Torreya grandis Fort. | Taxaceae Gray | Mature seed |
| 24 | Fenge | Pueraria kudzu | Pueraria montana var. thomsonii (Benth.) Wiersema ex D. B. Ward | Fabaceae | Root |
| 25 | Fengmi | honey | Apis cerana Fabricius | Apoidea | Nectar, secreta |
| 26 | Foshou | fingered citron | Citrus medica L.var.sarcodactylis Swingle | Rutaceae | Fruit |
| 27 | Fuling | Poria cocos | Poria cocos(Schw.)Wolf | Polyporaceae | Sclerotium |
| 28 | Fupenzi | raspberry | Rubus chingii Hu | Rosaceae | Fruit |
| 29 | Gaoliangjiang | Alpinia officinarum | Alpinia officinarum Hance | zingiberaceae | Rhizom |
| 30 | Gegen | lobed Kudzuvine root | Puerariae Lobatae Radix | Fabaceae | Root |
| 31 | Gouqizi | Chinese wolfberry | Lycium chinense Miller | Solanaceae | Ripe fruit |
| 32 | Gancao | liquorice root | Glycyrrhiza uralensis Fisch. Glycyrrhiza inflata Bat. Glycyrrhiza glabra L | Fabaceae | Root/rhizome |
| 33 | Heihujiao | black pepper | Piper nigrum L. | Piperaceae Giseke | Near ripe/ripe fruit |
| 34 | Heizhima | Semen sesami nigrum | Sesamum indicum L | Pedaliaceae | Mature seed |
| 35 | Heye | lotus leaf | Nelumbo nucifera Gaertn. | Nymphaeaceae | Leaf |
| 36 | Huaihua | Sophora flower | Sophora japonica Linn | Fabaceae | Flower |
| 37 | Huaimi | Sophora flower-bud | Sophora japonica Linn | Fabaceae | Flower bud |
| 38 | Huajiao | Sichuan pepper | Zanthoxylum bungeanum Maxim. | Rutaceae | Ripe peel |
| 39 | Huangjiezi | yellow mustard | Brassica juncea (L.) Czern.et Coss | Brassicaceae | Mature seed |
| 40 | Huangjing | rhizoma polygonati | Polygonatum kingianum Coll.et Hemsl. Polygonatum sibiricum Red. Polygonatum cyrtonema Hua | Liliaceae | Rhizome |
| 41 | Huangqi | milk vetch root | Astragalus membranaceus (Fisch.) Bunge | Fabaceae | Root |
| 42 | Huomaren | Semen Cannabis | Cannabis sativa L. | Moraceae | Ripe fruit |
| 43 | Huoxiang | Agastache rugosus | Agastache rugosa (Fisch. & C. A. Mey.) Kuntze | Lamiaceae | Overground part |
| 44 | Jiang | ginger | Zingiber officinale Roscoe | zingiberaceae | Rhizom |
| 45 | Jianghuang | turmeric | Curcuma longa L. | zingiberaceae | Rhizome |
| 46 | Jiegeng | Platycodon grandiflorus | Platycodon grandifloras (Jacq.) A.DC. | Campanulaceae | Root |
| 47 | Jineijin | endothelium corneum gigeriae galli | Gallusgallusdomesticus Brisson | Phasianidae | Inner wall of gizzard |
| 48 | Jinyinhua | honeysuckle | Lonicera japonica Thunb. | Caprifoliaceae | Buds/budding Flowers |
| 49 | Juemingnzi | Cassia seed | Cassia obtusifolia L. Cassia tora L. | Fabaceae | Mature seed |
| 50 | Juhong | exocarpium | Citrus reticulata Blanco | Rutaceae | Outer peel |
| 51 | Juhua | chrysanthemum | Chrysanthemum morifolium Ramat | Asteraceae | Capitulum |
| 52 | Juju | witloof | Cichorium intybus L. | Asteraceae | Anaerial part/root |
| 53 | Kunbu | kombucha | Ecklonia kurome Okam. Laminaria japonica Aresch. | Laminariaceae | Thallus |
| 54 | Laifuzi | radish seed | Raphanus sativus L. | Brassicaceae | Mature seed |
| 55 | Lianzi | lotus seed | Nelumbo nucifera Gaertn. | Nymphaeaceae | Mature seed |
| 56 | Lingzhi | Ganoderma lucidum | Ganoderma lucidum (Curtis) P. Karst. | Polyporaceae | Fruiting body |
| 57 | Longyanrou | longan flesh | Dimocarpus lon.gan Lour. | Sapindaceae | Aril |
| 58 | Lugen | rhizoma phragmitis | Phragmites communis Trin. | Poaceae Barnhart | Rhizome |
| 59 | Luohanguo | Momordica grosvenori | Siraitia grosvenorii (Swingle) C. Jeffrey ex Lu et Z. Y. Zhang | Cucurbitaceae | Fruit |
| 60 | Machixian | purslane | Portulaca oleracea L | Portulacaceae | Overground part |
| 61 | Maiya | malt | Hordeum vulgare L. | Poaceae Barnhart | Ripe fruit |
| 62 | Meiguihua | rose | Rosa rugosa Thunb or Rose rugosa cv. Plena | Rosaceae | Flower bud |
| 63 | Mugua | pawpaw | Chaenomeles speciosa (Sweet) Nakai | Rosaceae | Near ripe fruit |
| 64 | Muli | oyster | Ostreidae | Ostreidae | Shell |
| 65 | Pangdahai | sterculia scaphigera | Sterculia lychnophora Hance | Sterculiaceae | Mature seed |
| 66 | Pugongying | dandelion | Taraxacum mongolicum Hand.-Mazz. | Asteraceae | Whole herb |
| 67 | Qianshi | Semen Euryales | Euryale ferox Salisb. ex Konig et Sims | Nymphaeaceae | Mature seed kernel |
| 68 | Qingguo | Chinese white olive | Canarium album Raeusch | Burseraceae | Ripe fruit |
| 69 | Qishe | long-noded pit viper | Agkistrodon acutus (Guenther) | Viperidae | Dried body |
| 70 | Renshen | ginseng | Panax ginseng C. A. Mey. | Araliaceae | Root/rhizome |
| 71 | Roucongrong | cistanche | Cistanche deserticola Ma | Orobanchaceae | Succulent stem |
| 72 | Roudoukou | myristica fragrans | Myristica fragrans Houtt. | Myristicaceae | Kernel/seed coat |
| 73 | Rougui | cinnamon | Cinnamomum cassia Presl | Lauraceae | Bark |
| 74 | Sangshen | mulberry | Morus alba L. | Moraceae | Ruit ear |
| 75 | Sangye | folium mori | Morus alba L. | Moraceae | Leaf |
| 76 | Shaji | sea-buckthorn | Hippophae rhamnoidese L. | Elaeagnaceae | Ripe fruit |
| 77 | Shannai | rhizoma kaempferiae | Kaempferia galanga L. | zingiberaceae | Rhizome |
| 78 | Shanyao | Chinese yam | Dioscorea opposita Thunb. | Dioscoreaceae | Rhizome |
| 79 | Shanyinhua | lonicerae flos | Lonicera macranthoides Hand.-Mazz | Caprifoliaceae | Buds/budding Flowers |
| 80 | Shanzha | hawthorn | Crataegus pinnatifida Bge.var.major N.E.Br. Crataegus pinnatifida Bge. | Rosaceae | Ripe fruit |
| 81 | Shanzhuyu | dogwood | Cornus officinalis Sieb. et Zucc. | Cornaceae | Fruit |
| 82 | Sharen | fructus amomi | Amomum villosum Lour.var.xanthioides T.L.Wu et Senjen | zingiberaceae | Ripe fruit |
| 83 | Songhuafen | pollen pini | Pinus massoniana Lamb. | Pinaceae | Dried pollen |
| 84 | Suanzaoren | spina date seed | Ziziphus jujuba Mill.var.spinosa (Bunge) Hu ex H.F.Chou | Rhamnaceae | Pulp/mature seeds |
| 85 | Taoren | peach kernel | Prunus persica (L.) Batsch Prunus davidiana (Carr.) Franch. | Rosaceae | Mature seed |
| 86 | Tianma | gastrodia elata | Gastrodia elata Bl. | Orchidaceae | Tuber |
| 87 | Tiepishihu | Dendrobium officinale | Dendrobium officinale Kimura & Migo | Orchidaceae | Stem |
| 88 | Wumei | black plum | Prunus mume (Sieb.) Sieb.et Zucc | Rosaceae | Near ripe fruit |
| 89 | Wushaoshe | zaocys dhumnade | Zaocys dhumnades | Colubridae | Dried body |
| 90 | Xiakucao | selfheal | Prunella vulgaris L. | Lamiaceae | Fruit ear |
| 91 | Xiangru | elsholtzia | Elsholtzia ciliata (Thunb.) Hyl. | Lamiaceae | Overground part |
| 92 | Xiangyuan | citron | Citrus medica L. | Rutaceae | Ripe fruit |
| 93 | Xiaohuixiang | fennel | Foeniculum vulgare Mill. | Apiaceae | Ripe fruit |
| 94 | Xiaoji | artichoke | Cirsium setosum (Willd.) MB. | Asteraceae | Overground part |
| 95 | Xiebai | allium macrostemon | Allium macrostemon Bunge | Liliaceae | Bulb |
| 96 | Xihonghua | stigma croci | Crocus sativus L | Iridaceae | Stigma |
| 97 | Xingren | almond | Prunus armeniaca L.var.ansu Maxim Prunus sibirica L. Prunus mandshurica (Maxim) Koehne Prunus armeniaca L. | Rosaceae | Mature seed |
| 98 | Xiyangshen | American ginseng | Panax quinquefoliu L. | Araliaceae | Root/rhizome |
| 99 | Yansui | coriander | Coriandrum sativum L. | Apiaceae | Fruit/seed |
| 100 | Yiyiren | semen coicis | Coix lacryma-jobi L.var.mayuen (Roman.) Stapf | Poaceae Barnhart | Mature seed kernel |
| 101 | Yizhiren | fructus Alpiniae oxyphyllae | Alpinia oxyphylla Miq. | zingiberaceae | Nuts/fruit |
| 102 | Yuganzi | emblic leafflower fruit | Phyllanthus emblica L. | Euphorbiaceae | Ripe fruit |
| 103 | Yuliren | bunge cherry seed | Prunus humilis Bge. Prunus japonica Thunb. Prunus pedunculata Maxim. | Rosaceae | Mature seed |
| 104 | Yuxingcao | fish mint | Houttuynia cordata Thunb. | Saururaceae | Whole grass/ground parts |
| 105 | Yuzhu | radix polygonati officinalis | Polygonatum odoratum (Mill.) Druce | Liliaceae | Rhizome |
| 106 | Zao | jujube | Ziziphus jujuba Mill. | Rhamnaceae | Ripe fruit |
| 107 | Zhijuzi | Turnjujube | Hovenia dulcis Thunb. | Rhamnaceae | Rachis, leaves, and stem branches |
| 108 | Zhizi | Cape jasmine | Gardenia jasminoides J.Ellis | Rubiaceae | Ripe fruit |
| 109 | Zisu | purple perilla | Perilla frutescens (L.) Britt. | Lamiaceae | Leaf/twigs |
| 110 | Zisuzi | perilla seed | Perilla frutescens (L.) Brit | Lamiaceae | Ripe fruit |
| Source | Compound Name | Model | Dose | Molecular Weight | Monosaccharide Composition and Ratio | Glycosidic Bond | Effects | Mechanisms | References |
|---|---|---|---|---|---|---|---|---|---|
| Astragalus membranaceus | APS | IPEC-J2 cell BALB/c mice (LPS-induced inflammation model) | 0.2 mL 200 mg/kg 7 days | p-p38 ↓, ERK1/2 ↓, IκB-α ↑, IL-6 ↓, IL-1α ↓, TNF-α ↓, IL-1β ↓, CXCL8 ↓, TNFAIP3 ↓, CXCL2 ↓, BCL3 ↓, BNIP3 ↓ | Alleviating LPS-induced inflammation by inhibiting the MAPK and NF-κB signaling pathways | [57] | |||
| Astragalus membranaceu | APS-I | RAW264.7 cell (LPS-induced inflammation model) | 10, 25, 50, 100 μg/mL | >2000 kDa | Man, Rha, GalA, Glu, Gal, Ara 0.54∶0.26∶12.24∶17.24∶8.46∶1 | NO ↓, TNF-α ↓, IL-10 ↑ | Closely related to amino acid metabolism and energy metabolism | [127] | |
| Astragalus membranaceu | APS-II | RAW264.7 cell (LPS-induced inflammation model) | 10, 25, 50, 100 μg/mL | 10 kDa | Rha, GalA, Glu, Gal, Ara 0.26∶0.14∶24.04∶0.62∶1 | NO ↓, TNF-α ↓, IL-10 ↑ | Closely related to amino acid metabolism and energy metabolism | [127] | |
| Astragalus membranaceu | APS-1 | C57BL/6 mice (T1D model) | 200 mg/kg 8 consecutive weeks | IL-10 ↑, IL-6 ↓, TNF-α ↓, SCFAs ↑, BCFAs ↓, GPR41 ↑, HDAC2 ↑, ZO-1 ↑, occludin ↑, claudin-1 ↑ | Alleviates T1D system inflammation by reducing inflammatory factors and regulating gut microbes | [123] | |||
| Astragalus membranaceu | APS-A1 | RAW264.7 cell (LPS-induced inflammation model) | 50, 100, 200 μg/mL dependent manner | 2620 KDa | Glu, Gal, Ara 52.3:1.0:1.3. | 1,4-α-D-Glcp | TNF-α ↓, IL-1β ↓, IL-6 ↓, MCP-1 ↓, NLRP3 ↓, iNOS ↓, COX-2 ↓, p-JNK ↓, p-ERK ↓, p-p38 ↓, P65 ↓ | Alleviates LPS-induced inflammation by inhibiting the MAPK and NF-κB signaling pathways | [128] |
| Astragalus membranaceu | APS-B1 | RAW264.7 cell (LPS-induced inflammation model) | 50, 100, 200 μg/mL Dependent manner | 4950 KDa. | Glu, Gal, Ara, Man, Rha, GalA 75.2:17.3:19.4:1.0:1.1:1.3 | 1,4-α-D-Glcp,1,4,6-α-D-Glcp,1,5-α-L-Araf | TNF-α ↓, IL-1β ↓, IL-6 ↓, MCP-1 ↓, NLRP3 ↓, iNOS, ↓COX-2 ↓, p-JNK ↓, p-ERK ↓, p-p38 ↓, P65 ↓ | Alleviates LPS-induced inflammation by inhibiting the MAPK and NF-κB signaling pathways | [128] |
| Astragalus membranaceu | AP | C57BL/6 mice (CVB3-induced viral myocarditis model) | 200 mg/kg | IL-1β ↓, IL-6 ↓, TNF-α ↓, INF-γ ↓, MCP-1 ↓, TLR-4 ↓, p-NF-κB p65 ↓ | Alleviation of CVB3-induced viral myocarditis by inhibiting the TLR-4/NF-κB p65 signaling pathway | [20] | |||
| Astragalu membranaceu | APSI-C | RAW264.7 cell (LPS-induced inflammation model) | 12.5, 25, 50 mg/L | 4.5 KDa | TNF-α ↓, NO ↓, IL-10 ↑ | Alleviating LPS-induced inflammation by inhibiting inflammatory factors and increasing levels of pro-inflammatory factors | [129] | ||
| Astragalus membranaceu | sAPS3 | Wistar rats (CCl4-induced hepatocellular necrosis model) | 40 mg/kg 3 weeks | TNF-α ↓, IL-β1 ↓, ATG7 ↓, CD68 ↓, LC3II ↓ | Alleviating CCl4-induced liver injury by inhibiting inflammatory factors and decreasing the expression levels of ATG7 or LC3II, key regulators of Kupffer (KCs) autophagy | [130] | |||
| Astragalus membranaceu | SAPS | Caco2 cell (LPS-induced inflammation model) | 25, 50, 100 μg/mL | TLR4 ↓, TNF-α ↓, IL-1β ↓, IL-8 ↓, ZO-1 ↑, Occludin ↑ | Alleviating LPS-induced inflammation by inhibiting inflammatory factors and modulating intestinal flora | [131] | |||
| Ganoderma lucidum | BSGLP | C57BL/6 J mice (HFD-induced obesity model) | 100, 300 mg/kg | 26.0 kDa | Glu, Man, Gal 87.4:4.81:8.14 | (1→3)-β-D-Glcp, (1→6)-β-D-Glcp, (1→3,6)-β-D-Glcp | IL-1β ↓, IL-6 ↓, MCP-1 ↓, Occludin ↑, ZO-1 ↑, Claudin-1 ↑, SCFAs ↑, LBP ↓, CD14 ↓, Myd88 ↓, TLR4 ↓, p-NF-κB ↓, GPR43 ↑, Firmicutes/Bacteroidetes ↓, Reg3γ ↓ | Alleviation of inflammation through the modulation of gut microbes and inhibition of the TLR4/Myd88/NF-κB signaling pathway | [36] |
| Ganoderma lucidum | GLP-1 | Wistar rats (D-gal induced cognitive impairment model) | 20 mg/kg 10 mL/kg 60 days | 107 KDa | (1→, and →3)-β-D-Glcp | p-p38MAPK ↓, p-p53 ↓, p-JNK1+JNK2+JNK3 ↓, TNF-α ↓, IL-6 ↓, IL-10 ↑, TGF-β1 ↑ | Alleviating D-gal-induced systemic inflammation by inhibiting the MAPK signaling pathway and reducing inflammatory factors | [55] | |
| Ganoderma lucidum | SGRP | Kunming mice (CCl4-induced chronic liver injury model) | 400, 200, 100 mg/kg 6 weeks | 15.542 KDa | Fuc, Xyl, Man, Gal, Glu 4.8:0.9:4.9:9.9:11.6 | (1 → 6)-linked glycoside | TNF-α ↓, IL-1β ↓, IL-6 ↓, TLR4 ↓, p-NF-κB p65 ↓, IκBα ↑ | Alleviation of liver fibrosis by inhibiting the TLR4/NF-κB signaling pathway | [132] |
| Ganoderma lucidum | GRP | Kunming mice (CCl4-induced chronic liver injury model) | 400, 200, 100 mg/kg 4 weeks | 12.2 kDa | Rha, Fuc, Man, Glu 1.99:1.21:6.33:6.78 | TNF-α ↓, IL-6 ↓, IL-10 ↓, p-p65 ↓, TGF-β ↓, IκBα ↑ | Alleviating chronic liver injury by reducing pro- and anti-inflammatory factors | [133] | |
| Ganoderma lucidum | PSG-1 | BALB/c mice (cyclophosphamide-induced intestinal mucosal dysfunction model) | 25, 50, 100 mg/kg 7 days | TLR-2 ↓, TLR-4 ↓, TLR-6 ↓, IFN-γ ↑, IL-2 ↑, IL-12p70 ↑, IL-4 ↑, IL-1β ↑, IL-17 ↑, IL-21 ↑, IL-23 ↑, TGF-β3 ↑, T-bet ↑, GATA-3 ↑, RORγt ↑, Foxp3 ↑, ZO-1 ↑, occludin ↑, claudin-1 ↑, LC3 ↑, Beclin-1 ↑, Atg5 ↑, Atg7 ↑ | Alleviating cyclophosphamide (Cy)-induced intestinal mucosal dysfunction by regulating intestinal flora and improving intestinal immunity | [39] | |||
| Ganoderma lucidum | GLP | C57BL/6 mice (AOM/DSS-induced inflammation, tumorigenesis model) RAW264.7, HT-29, NCM460 cell (LPS-induced inflammation model) | 200, 300 mg/kg 0.8 mg/mL | 25.0 kDa | Ara, Man, Glu, Gal (4.19%), (15.69%), (78.15%), (1.97%) | TLR4 ↓, p-NF-κB p65 ↓, Myd88 ↓, IL-1β ↓, iNOS ↓, COX-2 ↓, p-JNK ↓, p-ERK ↓, IL-6 ↓, IL-1β5, TNF-α ↓, SCFAs ↑, occludin ↑, ZO-1 ↑ | Regulation of intestinal flora through inhibition of MAPK and NF-κB and increased production of SCFAs to alleviate colitis and tumors | [61] | |
| Ganoderma lucidum | GLP | C57BL/6 mice (CPZ-induced CNS demyelinating disease model) (MOG35-55 induces the development of an experimental autoimmune encephalomyelitis disease model) BV2cell (LPS-induced neuroinflammation model) | 5 mg/kg 50 μg/mL | NF-κB ↓, NLRP3 ↓, ASC ↓, pro-caspase-1 ↓, caspase-1 ↓, IL-1β ↓, TNFα ↓, IL-17 ↓, Dectin-1 ↑, IL-10 ↑ | Regulation of the Dectin-1 receptor inhibits NF-κB/NLRP3 inflammatory vesicle signaling and thus suppresses neuroinflammation | [75] | |||
| Ganoderma lucidum | CM-GLP | SD rats (cerebral ischemia-reperfusion model) | 40 mg/kg | MDA ↓, NF-κB ↓, TNF-α ↓, IL-1 ↓, IL-6 ↓, SOD ↑, HSP-70 ↑, p-Akt ↑ | Alleviating cerebral ischemia-reperfusion injury by modulating the HSP70/PI3K/Akt signaling pathway | [134] | |||
| Ganoderma lucidum | GLPN | C57 mice (DSS-induced colitis model) | 200 mg/kg 17 days | 35 KDa | Glc | (1→3)-β-D- glucan, (1→6)-β-D- l side-branching unit on every third residue | TNF-α ↓, IL-1β ↓, IL-6 ↓ | Relief of colitis by inhibiting L-selectin binding to ligands | [135] |
| Dendrobium nobile | DNP1 | RAW264.7 cells (LPS-induced inflammation model) | 200 μg/mL | 67.72 kDa | Man, Glc (75.86%), (24.14%) | β-1,4-ᴅ-Manp, β-1,4-ᴅ-Glcp residues | NO ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, IL-10 ↑ | Alleviating LPS-induced inflammation by modulating pro- and anti-inflammatory factors | [136] |
| Dendrobium nobile | DNP2 | RAW264.7 cells (LPS-induced inflammation model) | 200 μg/mL | 37.45 kDa | Man, Glc (72.32%), (27.68%) | β-1,4-ᴅ-Manp, β-1,4-ᴅ-Glcp residues | NO ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, IL-10 ↑ | Alleviating LPS-induced inflammation by modulating pro- and anti-inflammatory factors | [136] |
| Dendrobium huoshanense | cDHPS | DBA/1J male mice (type II collagen-induced arthritis model) | 0.1095, 0.4380 g/kg 30 days dose-dependent manner | p-IκB ↓, p- p65 ↓, p-JNK ↓, p-p38 ↓, p-ERK1/2 ↓, p-PI3K ↓, p- AKT ↓, p-JAK1 ↓, p- STAT3 ↓, IL-1β ↓, IL-6 ↓, IL-17 ↓, TNF-α ↓, GM-CSF ↓, M-CSF ↓, CXCL12 ↓, CCL ↓5, MMP3 ↓, MMP8 ↓, MMP9 ↓, VEGF ↓, IL-10 ↑, TGF-β ↑, HIF-1α ↓ | Alleviation of rheumatoid arthritis through inhibition of the NF-κB, MAPK, PI3K/AKT, and JAK1/STAT3 signaling pathways | [22] | |||
| Dendrobium officinale | DOPS | BalB/c mice (4% DSS-induced secondary liver injury in an acute colitis model) RAW264.7 cells (LPS-induced inflammation model) | 50, 100, 200 mg/kg 14 days 50, 100, 200 μg/mL | 393.8 kDa | Man, Glu 5.83:1.05 | IL-1β ↓, TNF-α ↓, MDA ↓, SOD ↑, GSH-Px ↑, Nrf-2 ↑, HO-1 ↑, NQO-1 ↑ | Alleviation of liver injury secondary to colitis by activation of the Nrf-2 signaling pathway | [101] | |
| Dendrobium officinale | DOPS | Kunming mice (ovariectomy, D-gal-induced learning and memory impairment model) | 140 mg/kg | MDA ↓, TNF-α ↓, IL-1β ↓, Nrf2 ↑, HO-1 ↑ | Improving Learning Memory Disorders by Activating the Nrf2/HO-1 Signaling Pathway | [102] | |||
| Dendrobium officinale | M-DOP | Kunming mice (D-Gal-induced aging model) | 250, 500, 1000 mg/kg | 75.41 kDa | Ara, Gal, Glc, Man, Rha 0.38:0.40:1.00:0.12:0.02 | SOD ↑, CAT ↑, GSH-Px ↑, Nrf2 ↑, HO-1 ↑, NQO1 ↑, IL-6 ↓, IL-1β ↓, NO ↓ | Amelioration of liver injury by activation of Nrf2/HO-1/NQO1 signaling pathway | [100] | |
| Dendrobium officinale | DOP | SD rats (middle cerebral artery occlusion model) | 25, 50, 100 μg/g | IFN-γ ↓, COX-2 ↓, IL-6 ↓, p-JAK/JAK ↓, p-STAT3/STAT3 ↓ | Reduces brain inflammation and repairs neurological function by inhibiting JAK/STAT3 signaling pathway activation | [113] | |||
| Dendrobium officinale | DOP | BALB/c mice (DSS-induced colitis model) Caco-2, RAW264.7 cells (LPS-induced inflammation model) | 200 mg/kg 20 days 0.5 mg/mL | 618 kDa | 1,4-β-D-mannopyranosyl residues, β-D glucopyranosyl residue | miR-433-3p ↑, NO ↓, TNF-α ↓, IL-6 ↓, PGE2 ↓, MAPK8 ↓ | Alleviating intestinal inflammation by inhibiting the MAPK signaling pathway | [125] | |
| Dendrobium huoshanense | DHP-1 | RAW264.7 cells (LPS-induced inflammation model) | 25, 50, 100, 200, 400 μg/mL | 262.50 kDa | Gal, Man, Glc, 1.00:1.89:22.66 | NO ↓, IL-1β ↓ | Alleviating LPS-induced inflammation by inhibiting pro-inflammatory factors | [137] | |
| Dendrobium huoshanense | DHP-2 | RAW264.7 cells (LPS-induced inflammation model) | 25, 50, 100, 200, 400 μg/mL | 521.37 kDa | Gal, Man, Glc, 2.80:1.00:10.93 | NO ↓, IL-1β ↓ | Alleviating LPS-induced inflammation by inhibiting pro-inflammatory factors | [137] | |
| Lycium chinense | LBPs | SD rats (nonalcoholic fatty liver disease model) | 50 mg/kg 8 weeks | Man, Rha, Glu, Gal, Ara 1.00:0.93:12.55:0.31:0.53 | IL-6 ↓, TNF-α ↓, IL-1β ↓, MCP-1 ↓, IL-10 ↑, TLR4 ↓, MyD88 ↓, IKK ↓, IκB ↓, p38MAPK ↓, NF-κBp65 ↓, occludin ↑, ZO-1 ↑ | Alleviation of NAFLD by inhibition of TLR4/MyD88/NF-κB and MAPK and modulation of intestinal flora | [23] | ||
| Lycium chinense | LBPs | SD rats (nonalcoholic fatty liver disease model) | 1 mg/kg 8 weeks | iNOS ↓, COX-2 ↓, IL-1β ↓, SOCS-3 ↓, TGF-β1 ↓, a-SMA ↓, p-JNK ↓, p-c-Jun ↓, p-ERK ↓, p-MEK ↓ | Alleviating NAFLD by inhibiting the MAPK signaling pathway | [62] | |||
| Lycium chinense | LBP | Bovine mammary epithelial cells (LPS-induced inflammation model) | 100, 300 μg/mL 24 h | COX-2 ↓, NLRP3 ↓, TNF-α ↓, IL-1β, IL-6 ↓, IκBα ↓, p65 ↓, p38 ↓, JNK ↓, ERK ↓, PPARγ ↑ | Mitigation of mastitis by inhibiting the MAPK/NF-κB signaling pathway in a PPARγ-dependent manner | [90] | |||
| Lycium chinense | GDLP | C57BL/KsJ mice (T2DM model) | 400 mg/kg 8 weeks | TNF-α ↓, Nrf2 ↓, HO-1 ↓ | Alleviating type 2 diabetes-induced liver inflammation by inhibiting the Nrf2/HO-1 signaling pathway | [105] | |||
| Lycium chinense | LBPs | RAW264.7 cell (LPS-induced inflammation model) | 1 g/L 24 h | 34.6 KDa | NO ↓ | Alleviating LPS-induced inflammation by inhibiting NO secretion levels | [138] | ||
| Angelica sinensis | AP | HT22 cell (LPS-induced inflammation model) | 80μg/mL | IL-1β ↓, TNF-α ↓, IL-6 ↓, miR-10a ↑, p-IκBa ↓, p-p65 ↓, pJAK2 ↓, p-STAT3 ↓, p53 ↓, p21 ↓, cleaved PARP ↓, cleaved caspase-3/9 ↓ | Alleviating LPS-induced inflammatory injury by inhibiting the NF-κB and JAK2/STAT3 signaling pathways and modulating miR-10a | [111] | |||
| Angelica sinensis | APS-2I | BalB/c mice (septicemia model) RAW264.7 cell (LPS-induced inflammation model) | 5, 10 mg/L 20, 40 mg/L | 720 KDa | Man, Rha, Glc, Gal, Ara, GalA (4.9%), (6.5%), (1.2%), (12.2%), (28.0%), (47.2%) | α-1,5-Araf, α-1,3-Araf, α-1,3,5-Araf, β-1,4-Galp, β-1,6-Galp | TNF-α ↓, IFN-β ↓, NO ↓, TIRAP ↓, MyD88 ↓, TRAM ↓, TRIF ↓, TLR4 ↓, MD-2 ↓ | Relief of sepsis by inhibition of the TLR4/Myd88/NF-κB signaling pathway and TRAM/TRIF signaling pathway | [139] |
| Angelica sinensis | APS-3I | BalB/c mice (septicemia model) RAW264.7 cell (LPS-induced inflammation model) | 5, 10 mg/L 20, 40 mg/L | 590 KDa | Mainly Glc | α-1,6-Glcp, α-1,2-Glcp, α-1,3-Glcp | TNF-α ↓, IFN-β ↓, NO ↓, TIRAP ↓, MyD88 ↓, TRAM ↓, TRIF ↓, TLR4 ↓, MD-2 ↓ | Relief of sepsis by inhibition of the TLR4/Myd88/NF-κB signaling pathway and TRAM/TRIF signaling pathway | [139] |
| Angelica sinensis | AP | Primary claw dermal cells (LPS-induced inflammation model) | 10, 50, 100 µg/mL | p-IκBα ↓, p-p65 ↓, p-ERK ↓, p-JNK ↓, p-p38 ↓, CCL2 ↓, CCL20 ↓, CXCL2 ↓, CXCL8 ↓, CXCL10 ↓, TLR4 ↓, MyD88 ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, NO ↓ | Alleviating LPS-induced inflammation by inhibiting the NF-κB and MAPK signaling pathways | [56] | |||
| Angelica sinensis | AP | SD rats (chronic renal failure model) | 10, 20, 40 mg/mL dose-dependent manner | IL-18 ↓, IL-1β ↓, IL-6 ↓, NLRP3 ↓, caspase-1 ↓ | Alleviating chronic functional renal failure by inhibiting NLRP3 inflammasome signaling activation | [77] | |||
| Angelica sinensis | sCAP | ICR mice (CCl4-induced hepatic injury model) | 0.05, 0.1, 0.15 mg/mL | p-ERK ↓, p-JNK ↓, p-p38 ↓, MDA ↓, ROS ↓, SOD ↑, T-AOC ↑ | Mitigation of CCl4-induced liver injury by MAPK inhibition | [140] | |||
| Angelica sinensis | CAP | ICR mice (CCl4-induced hepatic injury model) | 0.05, 0.1, 0.15 mg/mL | p-ERK ↓, p-JNK ↓, p-p38 ↓, MDA ↓, ROS ↓, SOD ↑, T-AOC ↑ | Mitigation of CCl4-induced liver injury by MAPK inhibition | [140] | |||
| Polygonatum sibiricum | PSP | BALB/c mice (septic acute liver injury model) | 150, 300, 600 mg/kg | TNF-α ↓, IL-6 ↓, MPO ↓, IL-18 ↓, IL-1β ↓, NLRP3 ↓, ASC ↓, caspase-1 ↓, AST ↓, ALT ↓, ALP ↓, TBIL ↓ | Treatment of septic acute liver injury by inhibiting the NLRP3/GSDMD signaling pathway | [76] | |||
| Polygonatum sibiricum | PCP | KM mice (LPS-induced acute lung injury model) | 400, 800 mg/kg/dw 7 consecutive days | 8.842 KDa | Fru, Glu, Gal 92.73:6.37:0.90 | β-D, α-D | IL-1β, IL-6, TNF-α, MPO ↓, SOD ↑, p-IKKβ ↓, p-IκBα ↓, p-p65 ↓, HO-1 ↓, NQO-1 ↓, Nrf2 ↓, p-AMPK ↓ | Lung protection through inhibition of the NF-κB and AMPK-Nrf2 signaling pathways | [141] |
| Polygonatum sibiricum | HPCP | KM mice (LPS-induced acute lung injury model) | 400, 800 mg/kg/dw 7 consecutive days | 5.521 KDa | Fru, Glu, Gal, Ara, Xyl 60.16:22.35:13.03:1.35:3.12 | β-D, α-D | IL-1β, IL-6, TNF-α, MPO ↓, SOD ↑, p-IKKβ ↓, p-IκBα ↓, p-p65 ↓, HO-1 ↓, NQO-1 ↓, Nrf2 ↓, p-AMPK ↓ | Lung protection through inhibition of the NF-κB and AMPK-Nrf2 signaling pathways | [141] |
| Polygonatum sibiricum | PCP | SD rats (CCl4-induced acute liver injury model) | 400 mg/kg 7 consecutive days | GSH ↑, SOD ↑, ROS ↓, MDA ↓, p-PI3K/PI3K ↓, p-AKT/AKT ↓, p-m TOR/mTOR ↓, LC3II/LC3I ↑ | Attenuating CCl4-induced acute liver injury by activating autophagy through inhibition of the PI3K/AKT/mTOR pathway | [87] | |||
| Polygonatum sibiricum | PSP | C57BL/6 mice (Single prolonged stress model) | 200, 400, 800 mg/kg | 6–14 kD | IL-1β ↓, TNF-α ↓, NLRP3 ↓, ASC ↓, SOD ↑, MDA ↓, HO-1 ↓, Nrf2 ↓, BDNF ↑, p-TrkB ↑, PSD95 ↑, Arc ↑, GluA1 ↑, GluN2B ↓ | Attenuating PTSD-like behaviors by inhibiting activation of Nrf2/HO-1, inhibiting the NLRP3 signaling pathway | [104] | ||
| Polygonatum sibiricum | PSP | C57BL/6 mice (LPS and chronic unpredictable mild stress-induced depression model) | 100, 200, 400 mg/kg | 6–14 kD | Ara, Glu, GluA, Gal, GalA, Man, Rha, Rib 13.7:82.9:3.7:36.2:4.3:52.5:3.3:1.0 | GluA1 ↑, GluA2 ↑, GluN2A ↓, GluN2B ↓, p-AKT/AKT ↑, p-mTOR/mTOR ↑, caspase-3 ↓, IL-1β ↓, TNF-α ↓, p-ERK ↓, NF-κB ↓, SOD ↑, MDA ↓, CORT ↓, 5-HT ↑ | Prevent depression by reducing inflammation by inhibiting the NF-κB and MAPK signaling pathways | [142] | |
| Polygonatum sibiricum | PS | SD rat (HFD-induced obesity model) | 120, 240, 480 mg/kg 14 weeks | 134.7 kDa | Man, Rha, GalA, Gal, Glc, GlcA, Xyl, Ara, Fuc, idoA | ZO-1 ↑, occludin ↑, TLR4 ↓, IL-1β ↓, IL-10 ↑, IκB-α ↑, SCFA ↑ | Alleviating inflammation by inhibiting TLR4/NFκB and modulating intestinal flora | [126] | |
| Polygonatum sibiricum | PSF | SD rat (HFD-induced obesity model) | 120, 240, 480 mg/kg 14 weeks | 178.6 kD | Man, Rha, GalA, Gal, Glc, GlcA, Xyl, Ara, Fuc, idoA | ZO-1 ↑, occludin ↑, TLR4 ↓, IL-1β ↓, IL-10 ↑, IκB-α ↑, SCFA ↑ | Alleviating inflammation by inhibiting TLR4/NFκB and modulating intestinal flora | [126] | |
| Phellinus igniarius | S-A3 | C57BL/6 mice (ulcerative colitis model) RAW264.7 (LPS-induced inflammation model) | 50, 100 mg/kg 31.25, 15.625, 7.8125 μg/mL | 3.3 KDa | Gal, Glc, Man, GlcA contain small amounts of Fuc, Xyl, GalA, Rha | TNF-α ↓, IL-6 ↓, IL-1β ↓, p65 ↓, AKT ↓, JNK ↓, P38 ↓ | Inhibit ulcerative colitis by inhibiting the NF-κB, MAPK, and AKT signaling pathways | [143] | |
| Phellinus igniarius | SHPS-1 | C57BL/6 mice (ulcerative colitis model) RAW264.7 cells (LPS-induced inflammation model) | 100 mg/kg 28 day 250 μg/mL 24 h | 46 kDa | Ara, Man, Glu, Gal 2.2:15.7:49.3:32.8 | 1,3-linked β-D-Glcp 1,6-linked α-D-Galp residues | IL-1β ↓, TNF-α ↓, IL-10 ↑, iNOS ↓, INF-β ↓, INF-γ ↓, MCP-1 ↓, CXCL-1 ↓, CD 86 ↓, IL-4 ↑, Occludin ↑, Claudin-4 ↑, ZO-1 ↑, CD 206 ↑, p-STAT-1 ↓ | Ulcerative colitis is inhibited by reducing the phosphorylation level of STAT-1 and the expression level of STAT-1 target genes such as iNOS and TNF-α, as well as increasing the anti-inflammatory factor and CD206 | [144] |
| Phellinus igniarius | PLP | ICR mice (enteritis model) RAW264.7 cells (LPS-induced inflammation model) | 500 mg/kg 25, 50, 100 μg/mL | NO ↓, MPO ↓, MDA ↓, IL-1β ↓, TNF-α ↓, iNOS ↓, IL-6 ↓, p38 ↓, JNK ↓, ERK ↓, PPARα ↑, PPARγ ↑ | By activating PPARα and PPARγ, MAPK signaling pathway is blocked to alleviate inflammation | [93] | |||
| Phellinus igniarius | SHP-1-1 | RAW264.7 cells (LPS-induced inflammation model) | 25, 50, 100 μg/mL | 333.599 kDa | Fuc, Ara, Rha, Gal, Glu, Xyl, Man, Glu 11.48∶0.18∶0.28∶17.86∶27.71∶0.64∶11.75∶0.94 | α-Glycosidic bond | NO ↓, TNF-α ↓, IL-6 ↓, IL-1β ↓ | Reduces inflammation by inhibiting pro-inflammatory factors | [145] |
| Phellinus igniarius | SHP-2-1 | RAW264.7 cells (LPS-induced inflammation model) | 25, 50, 100 μg/mL | 563.032 kDa | Fuc, Ara, Rha, Gal, Glu, Xyl, Man, Glu 0.73∶0.14∶0.32∶1.13:14.96∶1.04∶3.79∶1.50 | β-Glycosidic bond | NO ↓, TNF-α ↓, IL-6 ↓, IL-1β ↓ | Reduces inflammation by inhibiting pro-inflammatory factors | [145] |
| Poria cocos | PPS | KM mice BV-2 cell (LPS-induced anxiety and depression-like behavior model) | 20, 80 mg/kg 4, 8, 16 μmol/L | ROS ↓, NO ↓, TNF-α ↓, IL-1β ↓, CD16/32 ↓, NF-κB p65 ↓, CD206 ↑, NLRP3 ↓, ASC ↓, cleaved caspase-1 ↓ | Attenuating LPS-induced anxiety and depression-like behaviors by inhibiting NF-κB and NLRP3 signaling pathways. | [74] | |||
| Poria cocos | PCP | Sheep renal tubular epithelial cells (MAP-induced inflammation and oxidative stress model) | 5, 10, 50 mg/L | MDA ↓, SOD ↑, IL-6 ↓, TNF-α ↓, Nrf2 ↑, HO-1 ↑, NQO1 ↑ | Reduces inflammation by activating the Nrf2/HO-1 signaling pathway | [103] | |||
| Poria cocos | PCP-1C | KM mice (CCl4-induced liver injury model) | 50, 100, 200 mg/kg Two consecutive weeks | 17 kDa | Man, Gal, Glc, Fuc 17.4, 43.5, 24.4, 14.6 | 1,3-β-D-Glcp, 1,4-β-D-Glcp, 1,6-β-D-Glcp, | IL-1β ↓, IL-6 ↓, TNF-α ↓, SOD ↑, GSH-Px ↑, MDA ↓, CAR ↓, CYP2E1 ↓ | Alleviation of CCl4-induced liver injury by inhibiting CAR/CYP2E1 signaling pathway | [146] |
| Poria cocos | CMP44 | RAW264.7 cell (LPS-induced inflammation model) | 31.25–1000 μg/mL | 209.6 KDa | D-glucose | β- (1, 3) | NO ↓, TNF-α ↓, IL-6 ↓, IL-1β ↓ | Reduces inflammation by inhibiting pro-inflammatory factors | [147] |
| Poria cocos | CMP33 | RAW264.7 cell (LPS-induced inflammation model) | 31.25–1000 μg/mL | 152.3 KDa | (1 → 3), (1→6), (1→2)-linked glucose residues | NO ↓, TNF-α ↓, IL-6 ↓, IL-1β ↓ | Reduces inflammation by inhibiting pro-inflammatory factors | [148] |
| Source | Compound Name | Molecular Weight | Effects | References |
|---|---|---|---|---|
| Lycium barbarum | LBP | 34.6 KDa | NO ↓ | [138] |
| Angelica sinensis | ASP-Hb | 67.9 KDa | IL-6 ↓, IL-1β ↓, TNF-α ↓, TLR4 ↓ | [160] |
| honey of Polygonatum sibiricum Delar. ex Redoute | HPCP | 5521 KDa | p-IKKβ ↓, p-IκBα ↓, p-p65 ↓, IL-1β ↓, TNF-α ↓, IL-6 ↓, p-AMPK ↑, Nrf2 ↑, HO-1 ↑, NQO-1 ↑ | [141] |
| Astragalusmembranaceus | APSI-C | 4.5 KDa | NO ↓, TNF-α ↓ | [129] |
| Dendrobium huoshanense | DHP-1 | 521.37 KDa | NO ↓, IL-1β ↓ | [137] |
| Dendrobium huoshanense | DHP-2 | 262.50 KDa | NO ↓, IL-1β ↓ | [137] |
| Source | Compound Name | Composition and Proportion of Monosaccharides | Effects | References |
|---|---|---|---|---|
| Dioscorea polystachya | CYP-1 | Rib, Rha, Ara, Xyl | TNF-α ↓, IL-1β ↓ | [163] |
| Rubusidaeus | L-Ps-1 | Rha, Ara, Xyl, glucose, galactose 2.47:4.75:4.12:1:2.48 | TNF-α ↓, iNOS ↓, IL-6 ↓ | [165] |
| Rubusidaeus | F-Ps-3 | Rha, Ara, Xyl, Glu, Gal 4.21:14.72:1.63:1:3.22 | TNF-α ↓, iNOS ↓, IL-6 ↓ | [165] |
| Astragalus membranaceus | APS-I | Man, Rha, Gal A, Glu, Gal, Ara 0.54:0.26:12.24:17.24:8.46:1 | NO ↓, TNF-α ↓, IL-10 ↑ | [127] |
| Astragalus membranaceus | APS-II | Rha, Gal A, Glu, Gal, Ara 0.26:0.14:24.04:0.62:1 | NO ↓, TNF-α ↓, IL-10 ↑ | [127] |
| Phellinus igniarius | SHP-2-1 | Fuc, Ara, Rha, Gal, Glucose, Xyl, Man, Glu A 0.73:0.14:0.32:1.13:14.96:1.04:3.79:1.50 | NO ↓, IL-1β ↓ | [145] |
| Phellinus igniarius | SHP-1-1 | Fuc, Ara, Rha, Gal, Glu, Xyl, Man, Glu A 11.48:0.18:0.28:17.86:27.71:0.64:11.75:0.94 | NO ↓, IL-1β ↓ | [145] |
| Sargassum pallidum | PPS | Fucose | NO ↓ | [166] |
| Dendrobium nobile | DNP1 | Man (75.86%), Glc (24.14%) | NO ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, IL-10 ↑ | [136] |
| Dendrobium nobile | DNP2 | Man (72.32%), Glc (27.68%) | NO ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, IL-10 ↑ | [136] |
| Source | Compound Name | Glucosidic Bond | Effects | References |
|---|---|---|---|---|
| Pueraria montana var. thomsonii | RPP-2 | α-D-1,3-glucan | TNF-α ↓ | [170] |
| Hericium erinaceus | EP-1 | β-d-Glc(1→3) | SOD ↑, ROS ↓ | [171,172] |
| Phellinus igniarius | SHPS-1 | 1, 3-β-D-GLCP residue | STAT-1 ↓, iNOS ↓, TNF-α ↓ | [144] |
| Phellinus igniarius | A3 | α-1, 6-D-GALp | IL-6 ↓, IL-1β ↓, TNF-α ↓, P65 ↓, p-P38 ↓, p-ERK ↓, p-JNK ↓, p-AKT ↓ | [143] |
| honey | AHPN50-1a | (1→6) -α-GlcP | IL-1β ↓, IL-6 ↓, TNF-α ↓ | [173] |
| Poria cocos | PCP-1C | 1,3-β-D-Glcp | IL-1β ↓, IL-6 ↓, TNF-α ↓, SOD ↑, GSH-Px ↑ | [146] |
| Ganoderma lucidum | MBG | β-1→3 and β-1→6 glucan | IgA ↑, IgG ↑, poly-Ig ↑, IL-2 ↑ | [174] |
| Angelica sinensis | APS-2I | α-D-β-Galp-(1→6) | MyD88 ↓, TLR4 ↓, TNF-α ↓, IFN-β ↓, IL-6 ↓, NO ↓ | [139] |
| Poria cocos | CMP44 | (1→3) -β-d-glucan, (1→6)-β,(1→2)-β glucoside bonds | NO ↓, TNF-α ↓, IL-6 ↓, IL-1β ↓ | [147] |
| Ganoderma lucidum | BSGLP | (1→3)-β-D-Glcp, (1→6)-β-D-Glcp | TLR4 ↓, Myd88 ↓, NF-κB ↓ | [36] |
| Source | Compound Name | Conformation | Appearance Characteristics | Effects | References |
|---|---|---|---|---|---|
| Poria cocos | CMP33 | triple helix structure | IL-6 ↓, TNF-α ↓, IL-1β | [148] | |
| Ganoderma lucidum | GLP | triple helix structure | TNF-α ↓, IL-1β ↓, IL-6 ↓, L-selectin ↓ | [135] | |
| Pueraria montana var. thomsonii | RPP-2 | smooth, clean, and irregular sheet structure | TNF-α ↓ | [170] | |
| Gardenia jasminoides | GPS | irregular, thin, randomly distributed, and amorphous structures | TLR4 ↓, NF-κB ↓, MyD88 ↓, MCP-1 ↓, IL-6 ↓ | [177] | |
| Pseudocydonia sinensis | CSP-M | a sheet surface and porous structures | MPO ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, NO ↓, MDA ↓, SOD ↑, GSH ↑ | [178] | |
| ginger | GP-Zn(II) | flat surface, sheet structure, and partial dendritic fragments | IL-1β ↓, IL-6 ↓, IL-8 ↓, IL-12 ↓, TNF-α ↓, IL-10 ↑ | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lin, X.; Xia, L.; Xiong, S.; Xia, B.; Xie, J.; Lin, Y.; Lin, L.; Wu, P. Progress on the Anti-Inflammatory Activity and Structure–Efficacy Relationship of Polysaccharides from Medical and Edible Homologous Traditional Chinese Medicines. Molecules 2024, 29, 3852. https://doi.org/10.3390/molecules29163852
Zhang Y, Lin X, Xia L, Xiong S, Xia B, Xie J, Lin Y, Lin L, Wu P. Progress on the Anti-Inflammatory Activity and Structure–Efficacy Relationship of Polysaccharides from Medical and Edible Homologous Traditional Chinese Medicines. Molecules. 2024; 29(16):3852. https://doi.org/10.3390/molecules29163852
Chicago/Turabian StyleZhang, Yuanyuan, Xiulian Lin, Li Xia, Suhui Xiong, Bohou Xia, Jingchen Xie, Yan Lin, Limei Lin, and Ping Wu. 2024. "Progress on the Anti-Inflammatory Activity and Structure–Efficacy Relationship of Polysaccharides from Medical and Edible Homologous Traditional Chinese Medicines" Molecules 29, no. 16: 3852. https://doi.org/10.3390/molecules29163852
APA StyleZhang, Y., Lin, X., Xia, L., Xiong, S., Xia, B., Xie, J., Lin, Y., Lin, L., & Wu, P. (2024). Progress on the Anti-Inflammatory Activity and Structure–Efficacy Relationship of Polysaccharides from Medical and Edible Homologous Traditional Chinese Medicines. Molecules, 29(16), 3852. https://doi.org/10.3390/molecules29163852






