Synergistic Ag/g–C3N4 H2O2 System for Photocatalytic Degradation of Azo Dyes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization
2.1.1. XRD Analysis
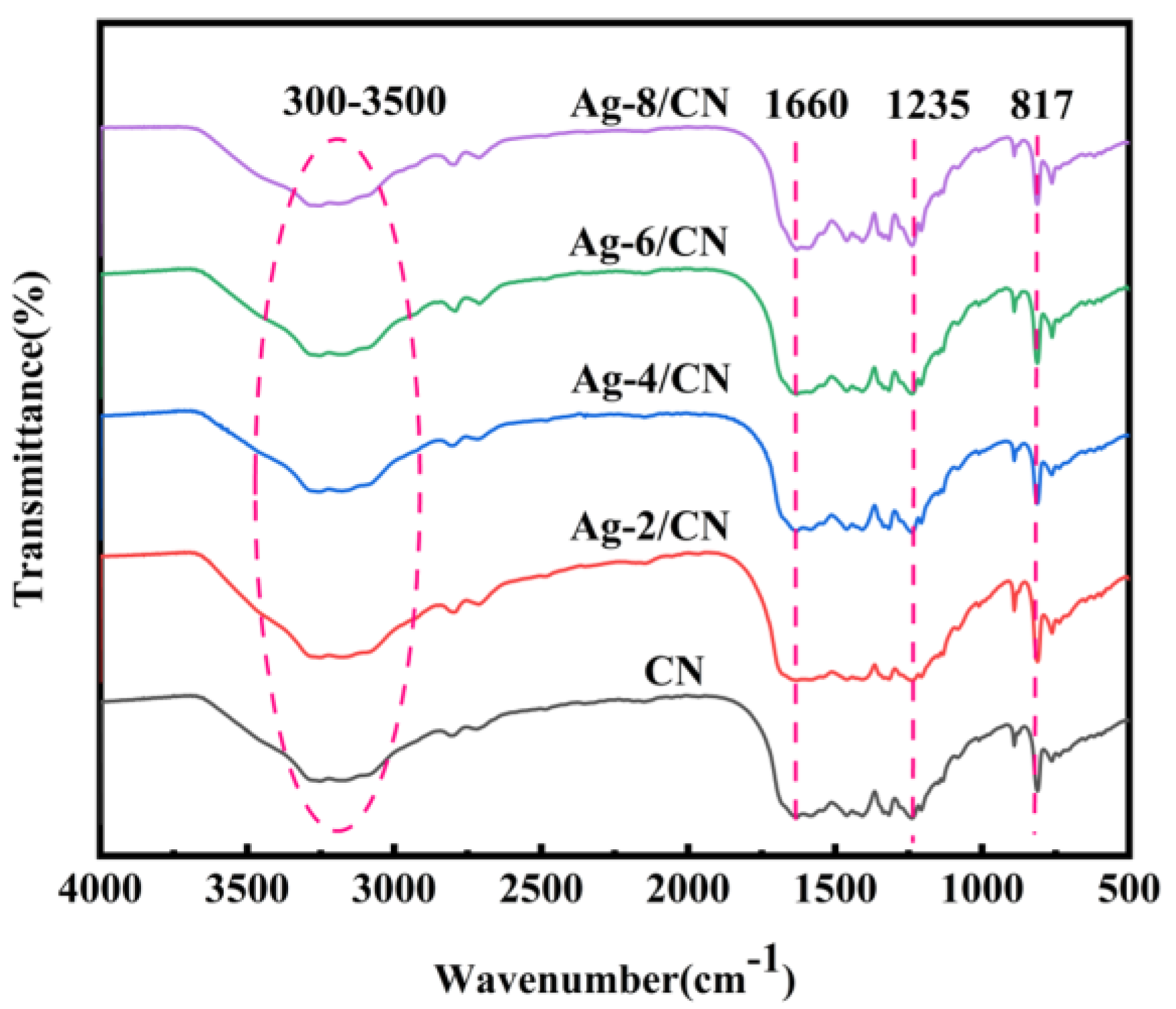
2.1.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
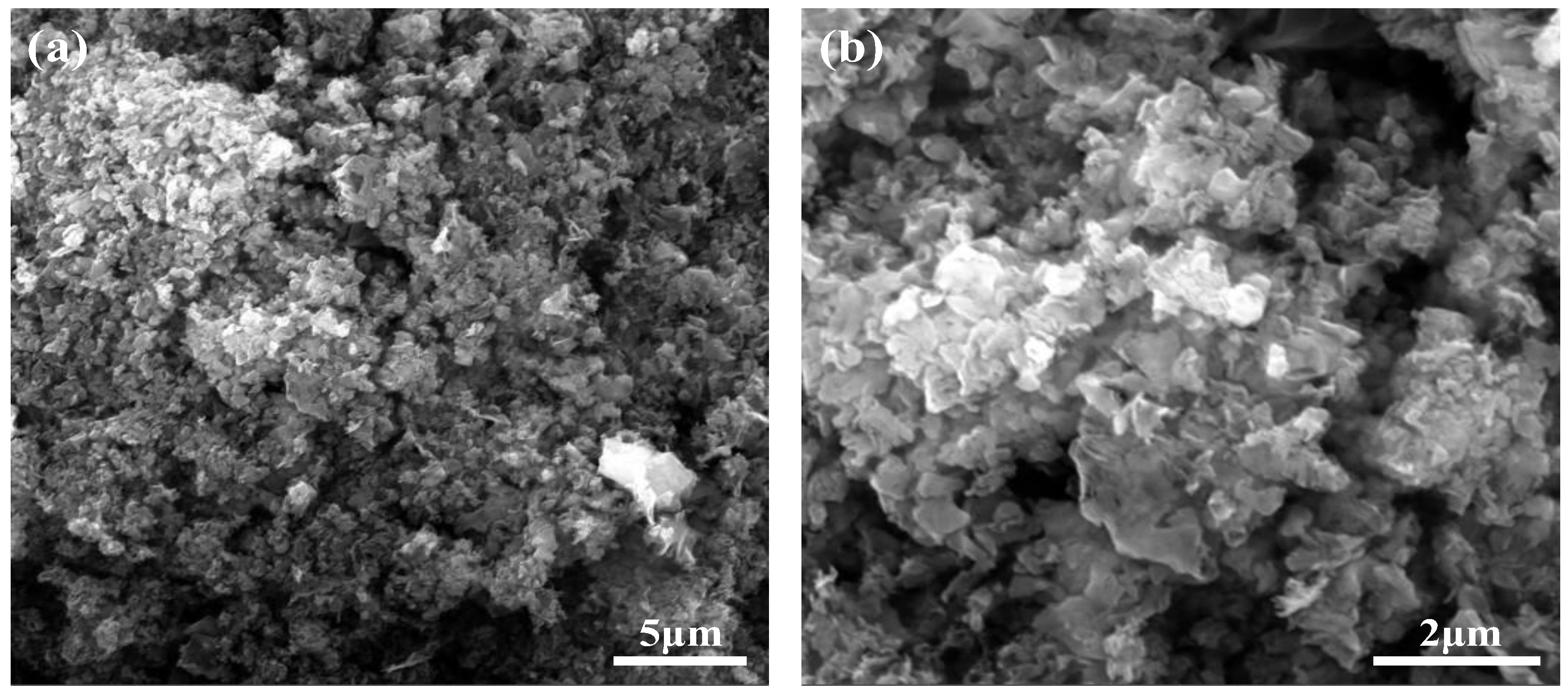
2.1.3. Scanning Electron Microscopy (SEM)
2.1.4. Ultraviolet–Visible Diffuse Reflectance Analysis (UV–Vis/DRS)
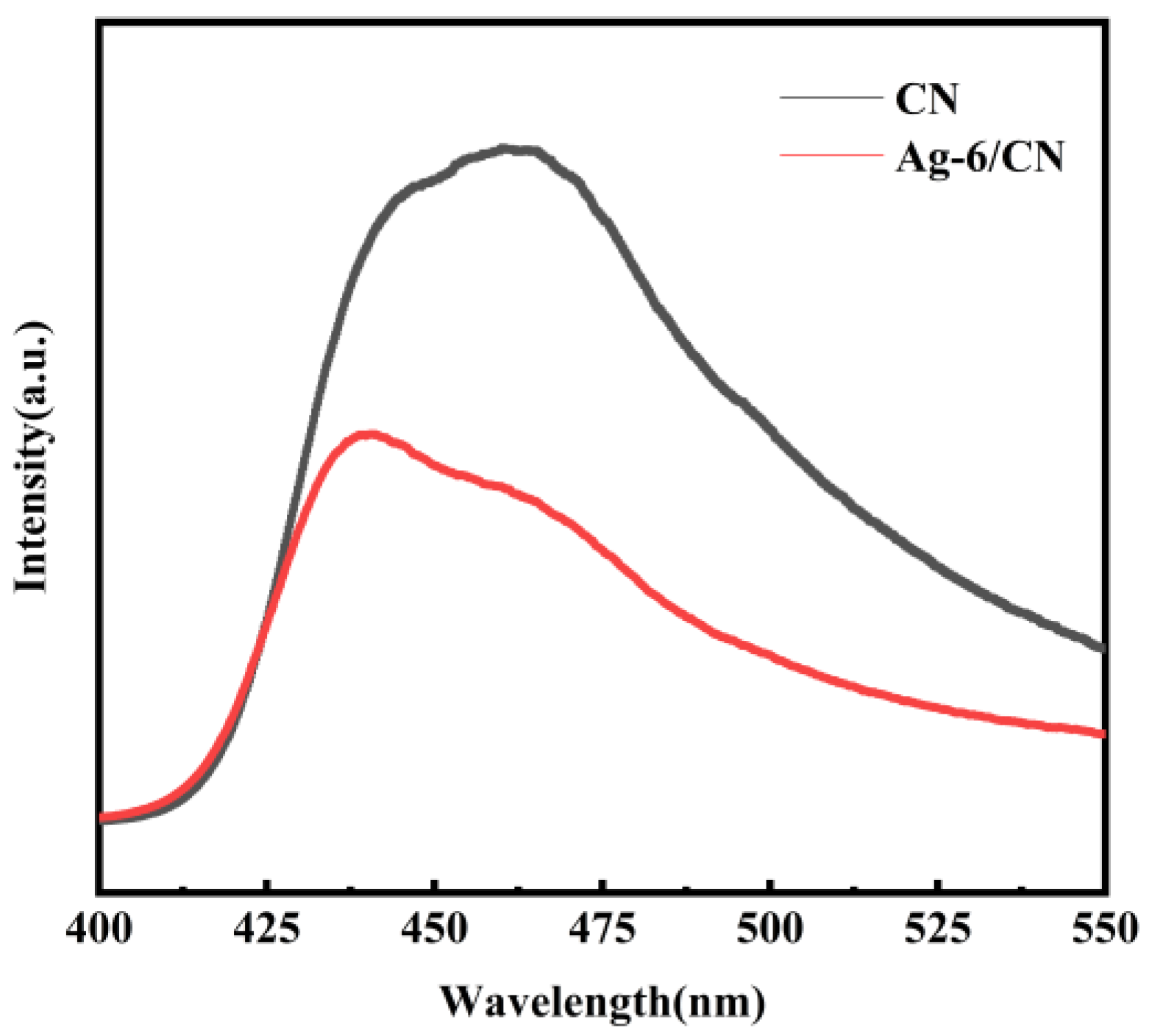
2.1.5. Photoluminescence (PL) Spectroscopy Analysis
2.2. Study on Photocatalytic Effects of Composite Materials
2.2.1. Effect of Ag-X/CN Composite Ratio on Photocatalysis
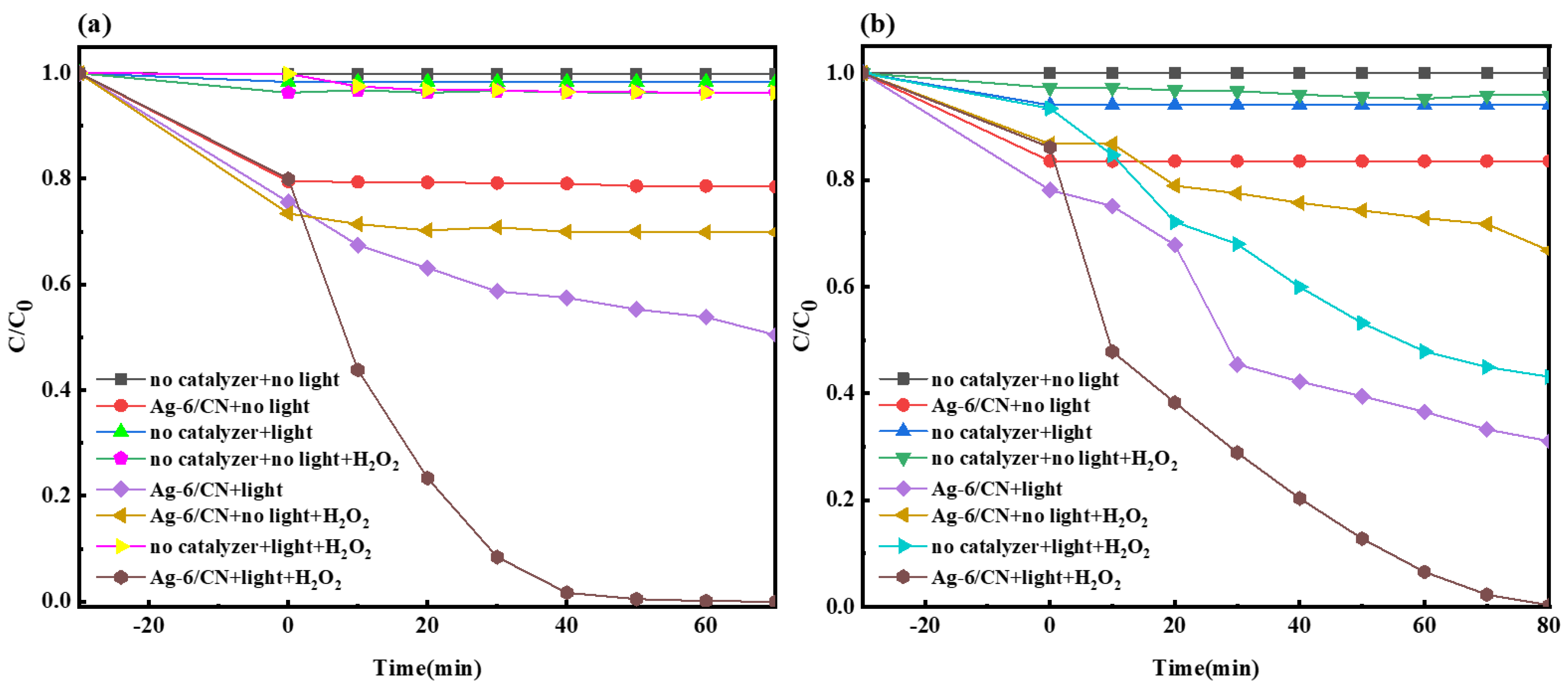
2.2.2. The Impact of Catalyst and Illumination on Photocatalysis
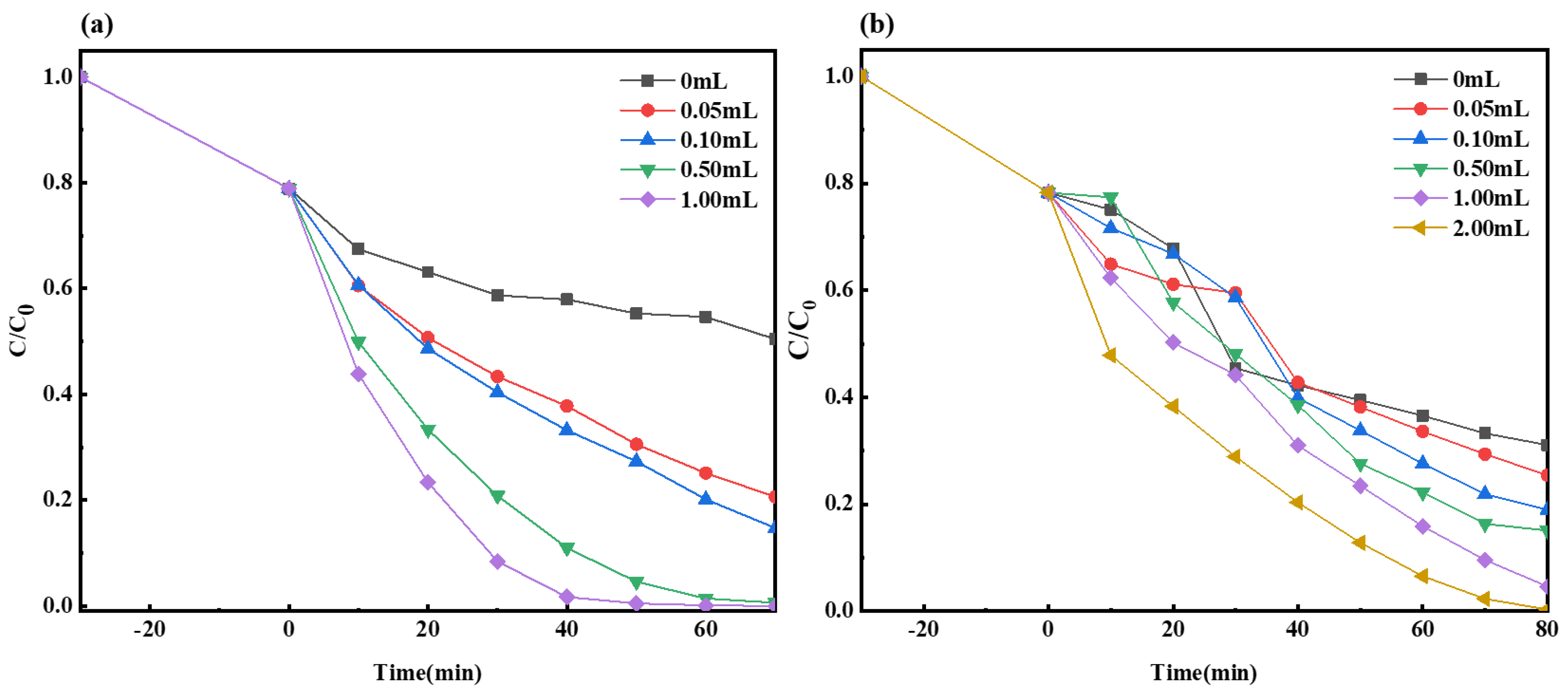
2.2.3. The Impact of H2O2 Dosage on Photocatalysis
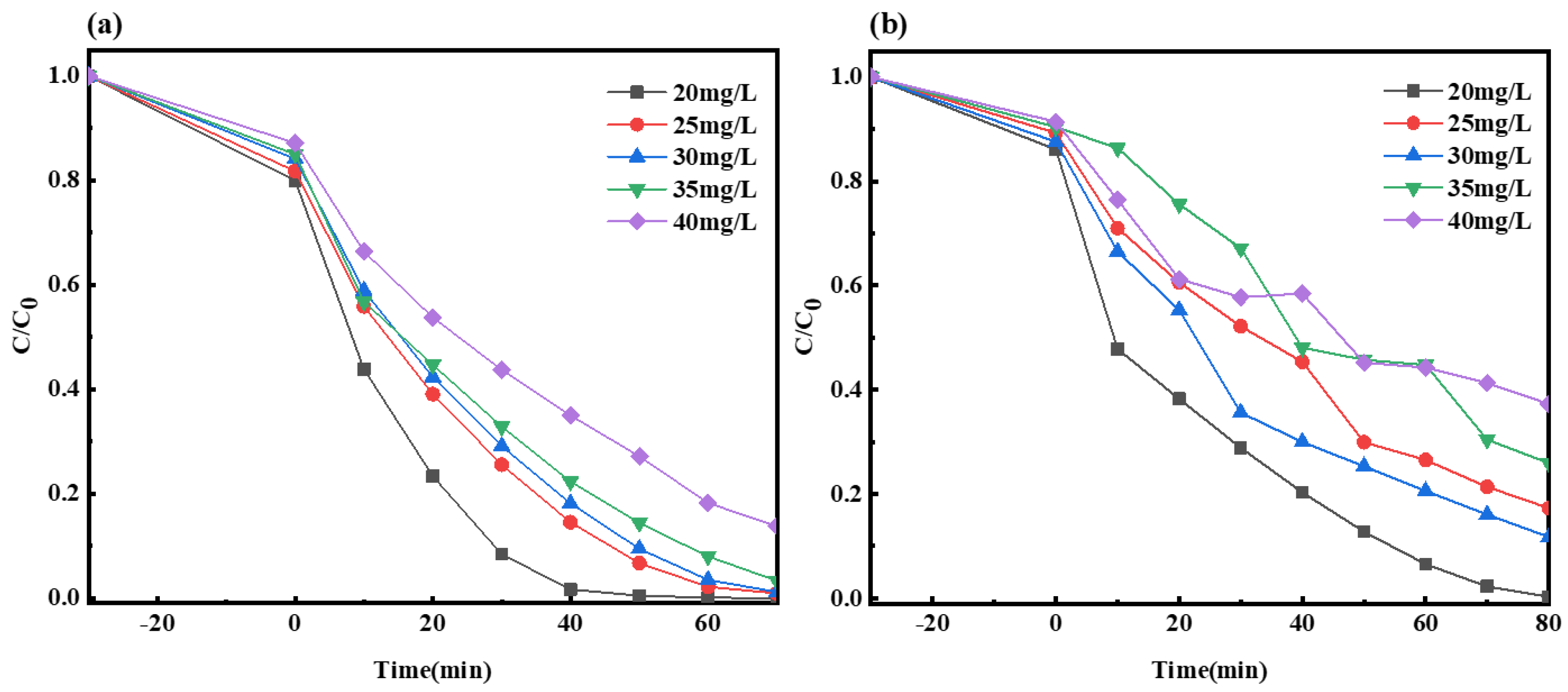
2.2.4. Impact of Initial Solution Concentration on the Photocatalysis by Composite Material
2.2.5. Impact of Composite Material Dosage on Photocatalysis
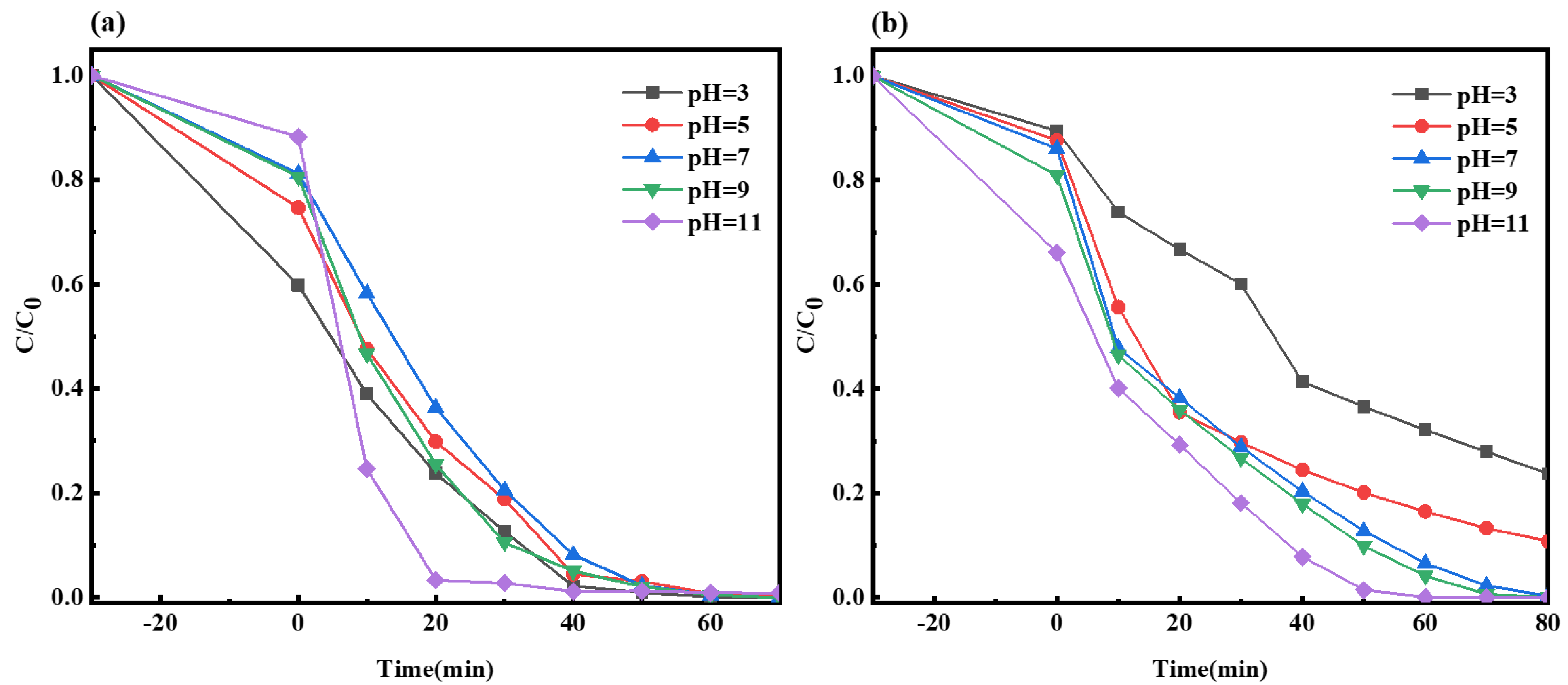
2.2.6. Impact of Solution pH on the Photocatalysis of Composite Material
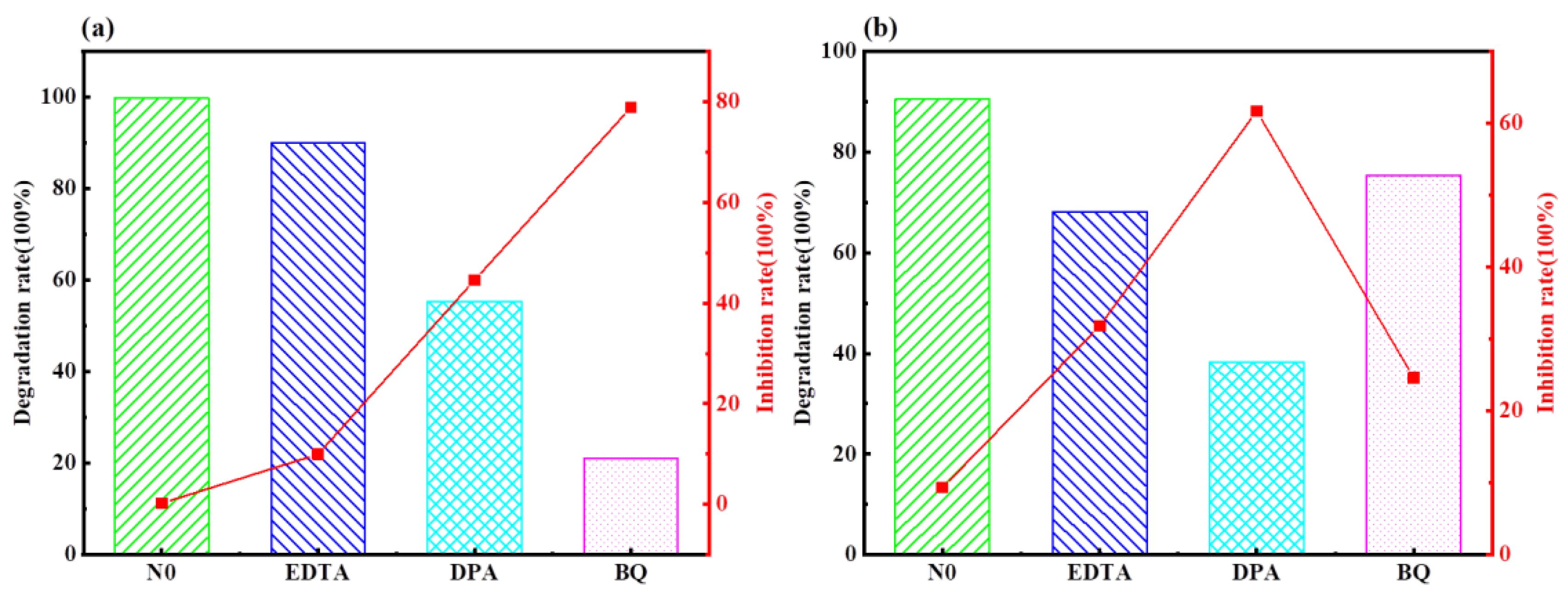
2.3. Mechanism of Photocatalytic Degradation of MO and MB by Ag-6/CN with H2O2 System
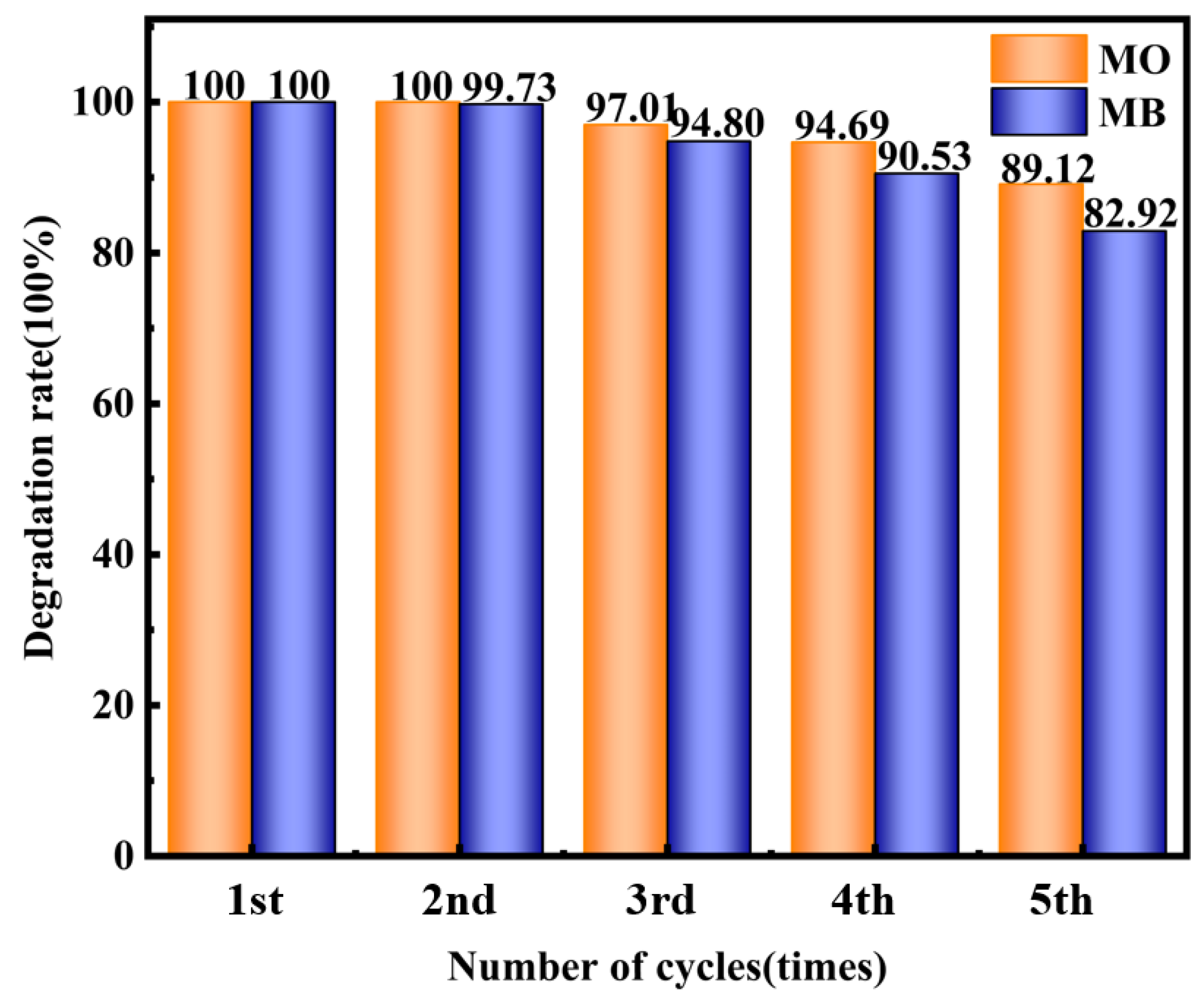
2.4. Stability Test of Composite Material
3. Experimental Section
3.1. Experimental Reagents
3.2. Experimental Apparatus
3.3. Catalyst Preparation
3.4. Structural Characterization Methods
3.5. Degradation Experiment Method
3.5.1. Preparation of Methyl Orange and Methylene Blue Wastewater
3.5.2. Activity Test of Photocatalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.-B.; Zheng, Y.-Q.; Lee, C.-C. Statistical analysis of the regional air quality index of Yangtze River Delta based on complex network theory. Appl. Energy 2024, 357, 122529. [Google Scholar] [CrossRef]
- Liu, J.B.; Liu, B.R.; Lee, C. Efficiency evaluation of China’s transportation system considering carbon emissions: Evidence from big data analytics methods. Sci. Total Environ. 2024, 922, 171031. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Yuan, X.Y.; Lee, C. Prediction of carbon emissions in China’s construction industry using an improved grey prediction model. Sci. Total Environ. 2024, 938, 173351. [Google Scholar] [CrossRef] [PubMed]
- Chung, K. Azo dyes and human health: A review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, W.; Gao, S.; Chen, D. Immobilized CeO2 for adsorption of azo dye. J. Exp. Nanosci. 2019, 14, 107–115. [Google Scholar] [CrossRef]
- Ajaz, M.; Shakeel, S.; Rehman, A. Microbial use for azo dye degradation-a strategy for dye bioremediation. Int. Microbiol. 2020, 23, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Abbas, R.; Sadik, W.A.; Omer, A.M.; Abd-Ellatif, M.M.; Mohy-Eldin, M.S. Development of novel amino-ethyl chitosan hydrogel for the removal of methyl orange azo dye model. Sci. Rep. 2024, 14, 1284. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; William, W.Y.; Wang, Q. Enhanced catalytic degradation performance of azo dyes based on Janus emulsification. Appl. Surf. Sci. 2023, 637, 157925. [Google Scholar] [CrossRef]
- Tarbajova, V.; Kolackova, M.; Chaloupsky, P.; Dobesova, M.; Capal, P.; Pilat, Z.; Samek, O.; Zemanek, P.; Svec, P.; Sterbova, D.S.; et al. Physiological and transcriptome profiling of Chlorella sorokiniana: A study on azo dye wastewater decolorization. J. Hazard. Mater. 2023, 460, 132450. [Google Scholar] [CrossRef]
- Kun, L.; Zhang, Q. Nano-TiO2 photocatalytic technology and atmospheric pollution control. China Environ. Sci. 2018, 38, 852–861. [Google Scholar]
- Zeng, P.; Wang, Q.Y.; Zhang, H.J.; Wang, J.Q.; Chen, X.Y. Application of photocatalysis techniques on treatment of wastewater with medium and low concentration of ammonium nitrogen. Sci. China Chem. 2019, 4, 78–82. [Google Scholar]
- Li, Y.; Xia, Z.; Yang, Q.; Wang, L.; Xing, Y. Review on g-C3N4-based S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144. [Google Scholar] [CrossRef]
- Du, J.; Wang, Z.; Li, Y.; Li, R.; Li, X.; Wang, K. Establishing WO3/g-C3N4 Composite for “Memory” Photocatalytic Activity and Enhancement in Photocatalytic Degradation. Catal. Lett. 2019, 149, 1167–1173. [Google Scholar] [CrossRef]
- Liu, J.B.; Bao, Y.; Zheng, W.T. Analyses of some structural properties on a class of hierarchical scale-free networks. Fractals 2022, 30, 2250136. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Elchalakani, M.; Liu, H.; Briseghella, B.; Sun, C. Photocatalytic concrete for degrading organic dyes in water. Environ. Sci. Pollut. Res. 2022, 29, 39027–39040. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Mishra, P.K.; Tiwary, D. Enhanced photocatalytic performance of NiS/ZnO nanocomposite for the remediation of PNP and RhB dye. J. Environ. Chem. Eng. 2022, 10, 107459. [Google Scholar] [CrossRef]
- Li, Z.Q.; Chen, X.W.; Wang, Y.; Cheng, T.; Huang, Y.; Dong, P.Y.; Wang, W.Y.; Zhang, B.B.; Xi, X.G. Preparation of CeTiO4/g-C3N4 Composite with Efficient Photocatalytic Activity for Dye⁃Degradation. Chin. J. Inorg. Chem. 2022, 38, 53–62. [Google Scholar]
- Mohammadzadeh, A.; Khoshghadam-Pireyousefan, M.; Shokrianfard-Ravasjan, B.; Azadbeh, M.; Rashedi, H.; Dibazar, M.; Mostafaei, A. Synergetic photocatalytic effect of high purity ZnO pod shaped nanostructures with H2O2 on methylene blue dye degradation. J. Alloys Compd. 2020, 845, 156333. [Google Scholar] [CrossRef]
- Carmen, M.; Nicoleta, C.; Georgiana, B.; Dobromir, M.; Girtan, M.; Doroshkevich, A.; Gyorgy, E.; Mardare, D. The enhancement of the photocatalytic properties of SmFe0.7Co0.3O3 thin films by synergistic effect of Sr doping and H2O2 as co-catalyst. Ceram. Int. 2023, 49, 14225–14237. [Google Scholar]
- Gong, C.; Zhai, J.; Wang, X.; Zhu, W.; Yang, D.; Luo, Y.; Gao, X. Synergistic improving photo-Fenton and photo-catalytic degradation of carbamazepine over FeS2/Fe2O3/organic acid with H2O2 in-situ generation. Chemosphere 2022, 307, 136199. [Google Scholar] [CrossRef] [PubMed]
- Merdoud, R.; Aoudjit, F.; Mouni, L.; Ranade, V.V. Degradation of methyl orange using hydrodynamic Cavitation, H2O2, and photo-catalysis with TiO2-Coated glass Fibers: Key operating parameters and synergistic effects. Ultrason. Sonochemistry 2024, 103, 106772. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hu, X.; Bao, M.; Li, F.; Li, Y.; Lu, J. Fabrication of MIL-Fe (53)/modified g-C3N4 photocatalyst synergy H2O2 for degradation of tetracycline. Sep. Purif. Technol. 2021, 279, 119661. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, P.; Yang, J.; Xu, G.; Yang, H.; Shi, Z.; Hu, Q.; Dong, B.; Guo, Z. Photocatalytic degradation of organic dye and phytohormone by a Cu (II) complex powder catalyst with added H2O2. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125147. [Google Scholar] [CrossRef]
- Yang, W.; Ding, K.; Chen, G.; Wang, H.; Deng, X. Synergistic multisystem photocatalytic degradation of anionic and cationic dyes using graphitic phase carbon nitride. Molecules 2023, 28, 2796. [Google Scholar] [CrossRef] [PubMed]
- Shvalagin, V.V.; Kompanets, M.O.; Kutsenko, O.S.; Kuchmy, S.Y.; Skoryk, M.A. Photocatalytic Activity of g-C3N4 in the Partial Oxidation of Benzyl Alcohol Under Visible Light. Theor. Exp. Chem. 2020, 56, 111–116. [Google Scholar] [CrossRef]
- Yao, G.Y.; Liu, Y.Q.; Liu, J.C.; Xu, Y. Synthesis of Porous g-C3N4 with Enhanced Visible-Light Photoactivity. Molecules 2022, 27, 1754. [Google Scholar] [CrossRef] [PubMed]
- Shankarling, G.S.; Deshmukh, P.P.; Joglekar, A.R. Process intensification in azo dyes. J. Environ. Chem. Eng. 2017, 5, 3302–3308. [Google Scholar] [CrossRef]
- Zhu, X.D.; Qin, F.Q.; Luo, Y.H.; Zhang, L.; Yang, D.; Feng, W.; Chen, S. Enhanced photocatalytic activity of p (BaSnO3)-n (anatase/rutile/brookite Tio2) heterojunction composites by efficient inter facial charge transfer. J. Mol. Struct. 2023, 1294, 136440. [Google Scholar] [CrossRef]
- Zhu, X.; Qin, F.; Xia, Y.; Zhong, Y.; Zhang, X.; Feng, W.; Jiao, Y. Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property. Nanotechnol. Rev. 2022, 11, 2916–2927. [Google Scholar] [CrossRef]
- Wong, K.T.; Kim, S.C.; Yun, K.; Choong, C.E.; Nah, I.W.; Jeon, B.-H.; Yoon, Y.; Jang, M. Understanding the potential band position and e−/h+ separation lifetime for Z-scheme and type-II heterojunction mechanisms for effective micropollutant mineralization: Comparative experimental and DFT studies. Appl. Catal. B Environ. 2020, 273, 119034. [Google Scholar] [CrossRef]
- Wu, L.; Hu, M.L.; Wang, L.P.; Huang, S.; Zhou, X. Preparation of TiHAP@g-C3N4 heterojunction and photocatalytic degradation of methyl Orange. J. Inorg. Mater. 2022, 38, 503. [Google Scholar] [CrossRef]
- Song, Y.H.; Dai, W.L.; Xu, H.; Zhao, J. Preparation and photocatalytic properties of g-C3N4/Bi12O17Cl2 composites. Chin. J. Mater. Res. 2021, 35, 911–917. [Google Scholar]
- Ying, L.W.; Liu, Y.; Dong, W.B.; Yuan, H.; Ma, L. Effect of bromide ions on the UV/H2O2 oxidation of alanine in aqueous solutions. Chem. Eng. J. 2016, 285, 172–179. [Google Scholar] [CrossRef]



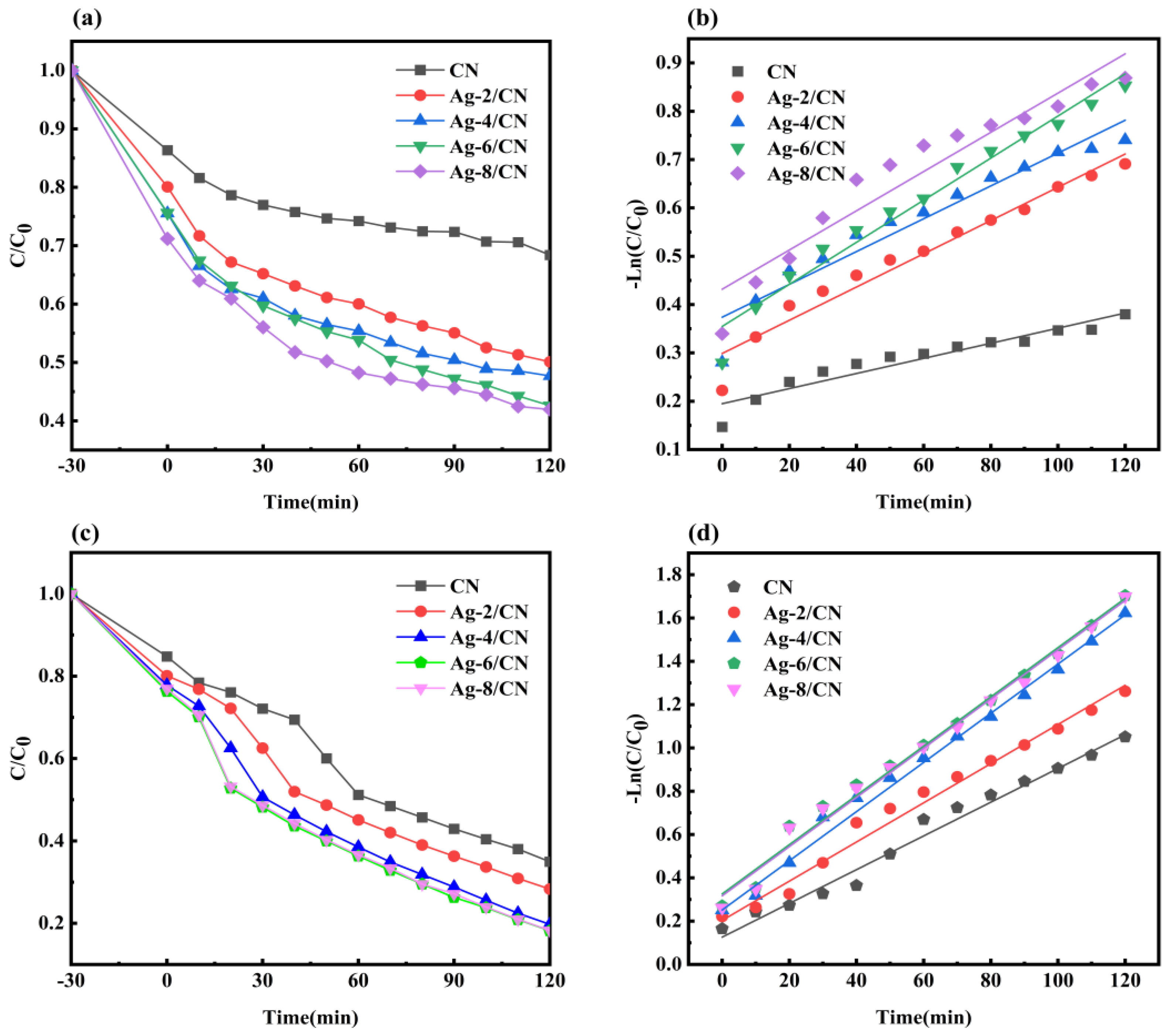


| Methyl Orange (MO) | Methylene Blue (MB) | |||
|---|---|---|---|---|
| K (×10−2/min−1) | R2 | K (×10−2/min−1) | R2 | |
| CN | 0.156 | 0.902 | 0.779 | 0.968 |
| Ag-2/CN | 0.344 | 0.955 | 0.905 | 0.978 |
| Ag-4/CN | 0.340 | 0.928 | 1.136 | 0.987 |
| Ag-6/CN | 0.435 | 0.969 | 1.138 | 0.987 |
| Ag-8/CN | 0.406 | 0.915 | 1.137 | 0.987 |
| Name | Chemical Formula | Manufacturer |
|---|---|---|
| Urea | CH4N2O | Tianjin Damao Chemical Reagent Factory, Tianjin, China |
| Silver Nitrate | AgNO3 | Sinopharm Chemical Reagent Co., Ltd, Shanghai, China |
| Anhydrous Ethanol | C2H6O | Wuxi Zhanwang Chemical Reagent Co., Ltd, Wuxi, China |
| Methyl Orange | C14H14N3NaO3S | Tianjin Guangfu Technology Development Co., Ltd, Tianjin, China |
| Methylene Blue | C16H18ClN3S·3H2O | Shanghai Zhanyun Chemical Co., Ltd, Shanghai, China |
| Hydrochloric Acid | HCl | Sinopharm Chemical Reagent Co., Ltd, Shanghai, China |
| Sodium Hydroxide | NaOH | Xilong Scientific Co., Ltd, Shantou, China |
| p-Benzoquinone | C6H4O2 | Shanghai McLean Biochemical Technology Co., Ltd, Shanghai, China |
| Diphenylamine | C12H11N | Tianjin Siyou Fine Chemicals Co., Ltd, Tianjin, China |
| Methanol | CH3OH | Tianjin Damao Chemical Reagent Factory, Tianjin, China |
| EDTA | C10H16N2O8 | Shanghai McLean Biochemical Technology Co., Ltd, Shanghai, China |
| 30% Hydrogen Peroxide | H2O2 | Sinopharm Chemical Reagent Co., Ltd, Shanghai, China |
| Name | Model | Manufacturer |
|---|---|---|
| Magnetic Stirrer | 8S-1 | Changzhou Guoyu Instrument Manufacturing, Changzhou, China |
| Electronic Balance | FA2004 | Shanghai Shunyu Hengping Scientific Instrument Co., Ltd, Shanghai, China |
| Electric Constant Temperature Blast Drying Oven | DHG-9101-1SA | Shanghai Sanfa Scientific Instrument Co., Ltd, Shanghai, China |
| Muffle Furnace | KSL-1100X | Hefei Kejing Material Technology Co., Ltd, Hefei, China |
| Ultrasonic Cleaner | KQ-100B | Kunshan Jielimei Ultrasonic Instrument Co., Ltd, Kunshan, China |
| Xenon Lamp Light Source | CEL-HXF300 | Beijing Zhongjiaojin Yuan Technology Co., Ltd, Beijing, China |
| Optical Dark Box | GXAS345 | Beijing Newbit Technology Co., Ltd, Beijing, China |
| Photocatalytic Glass Reactor | KW100 | Beijing Newbit Technology Co., Ltd, Beijing, China |
| UV–Vis Spectrophotometer | UV-26001 | Suzhou Shimadzu Instrument Co., Ltd, Suzhou, China |
| Vacuum Freeze-Dryer | FD-1A-50 | Beijing Boyikang Experimental Instrument Co., Ltd, Beijing, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, W.; Ding, K. Synergistic Ag/g–C3N4 H2O2 System for Photocatalytic Degradation of Azo Dyes. Molecules 2024, 29, 3871. https://doi.org/10.3390/molecules29163871
Wang Y, Yang W, Ding K. Synergistic Ag/g–C3N4 H2O2 System for Photocatalytic Degradation of Azo Dyes. Molecules. 2024; 29(16):3871. https://doi.org/10.3390/molecules29163871
Chicago/Turabian StyleWang, Yajing, Wen Yang, and Kun Ding. 2024. "Synergistic Ag/g–C3N4 H2O2 System for Photocatalytic Degradation of Azo Dyes" Molecules 29, no. 16: 3871. https://doi.org/10.3390/molecules29163871





