Hydrogels and Aerogels for Versatile Photo-/Electro-Chemical and Energy-Related Applications
Abstract
:1. Introduction
2. Hydrogels
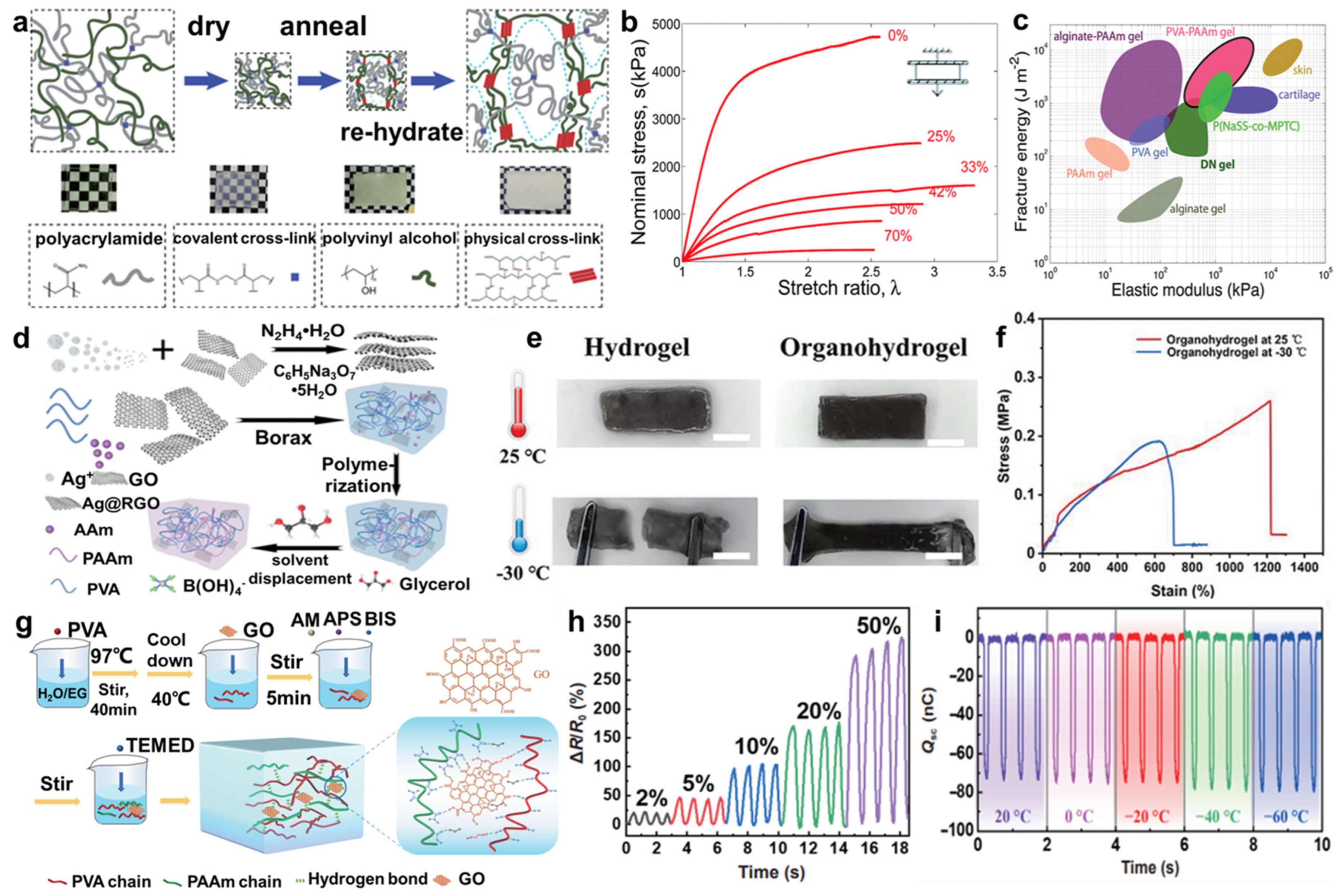
2.1. PAAm-Based Hydrogels

2.2. PVA-Based Hydrogels
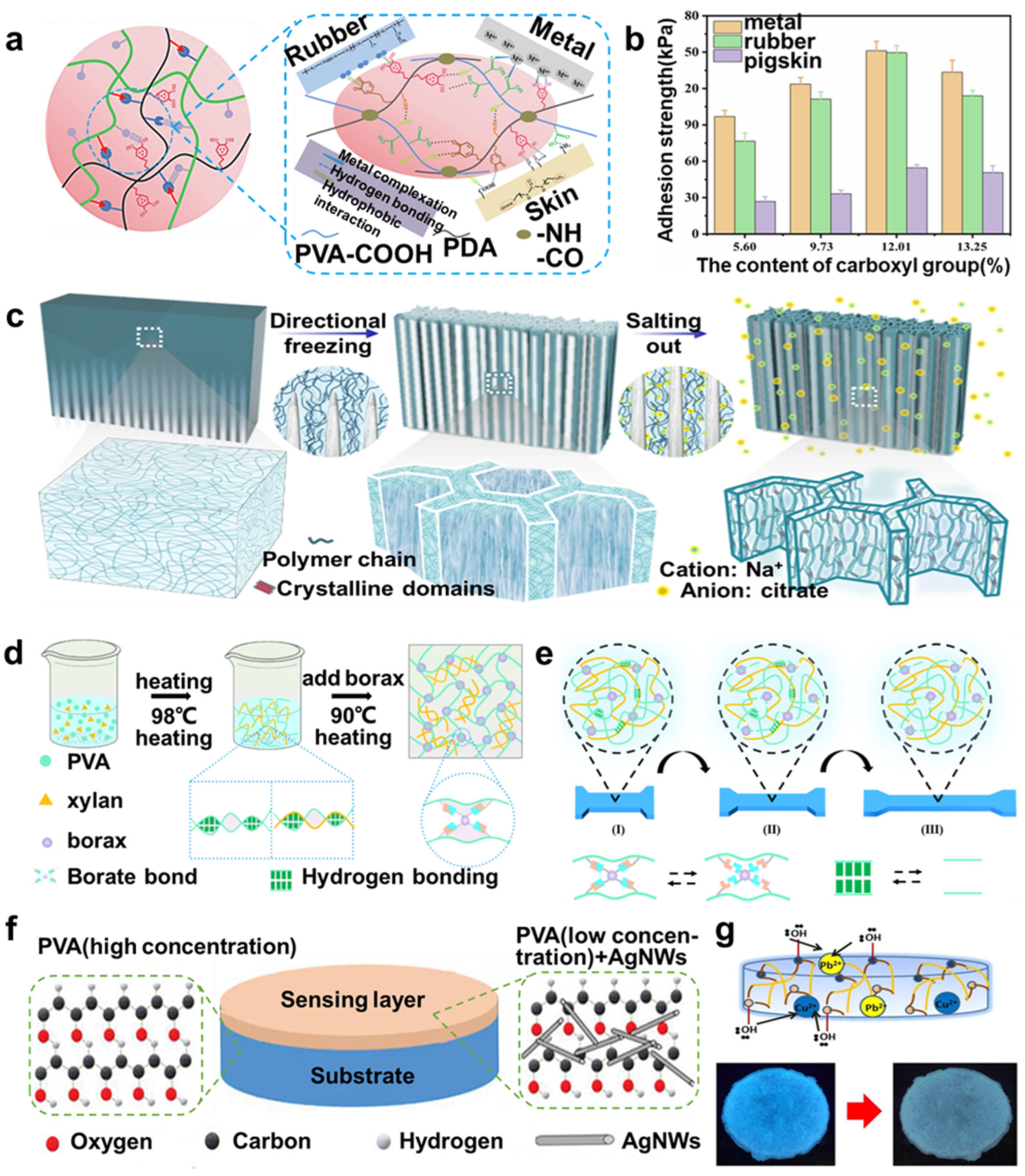
2.3. Copolymer-Based Hydrogels
2.4. Graphene Hydrogels
3. Aerogels
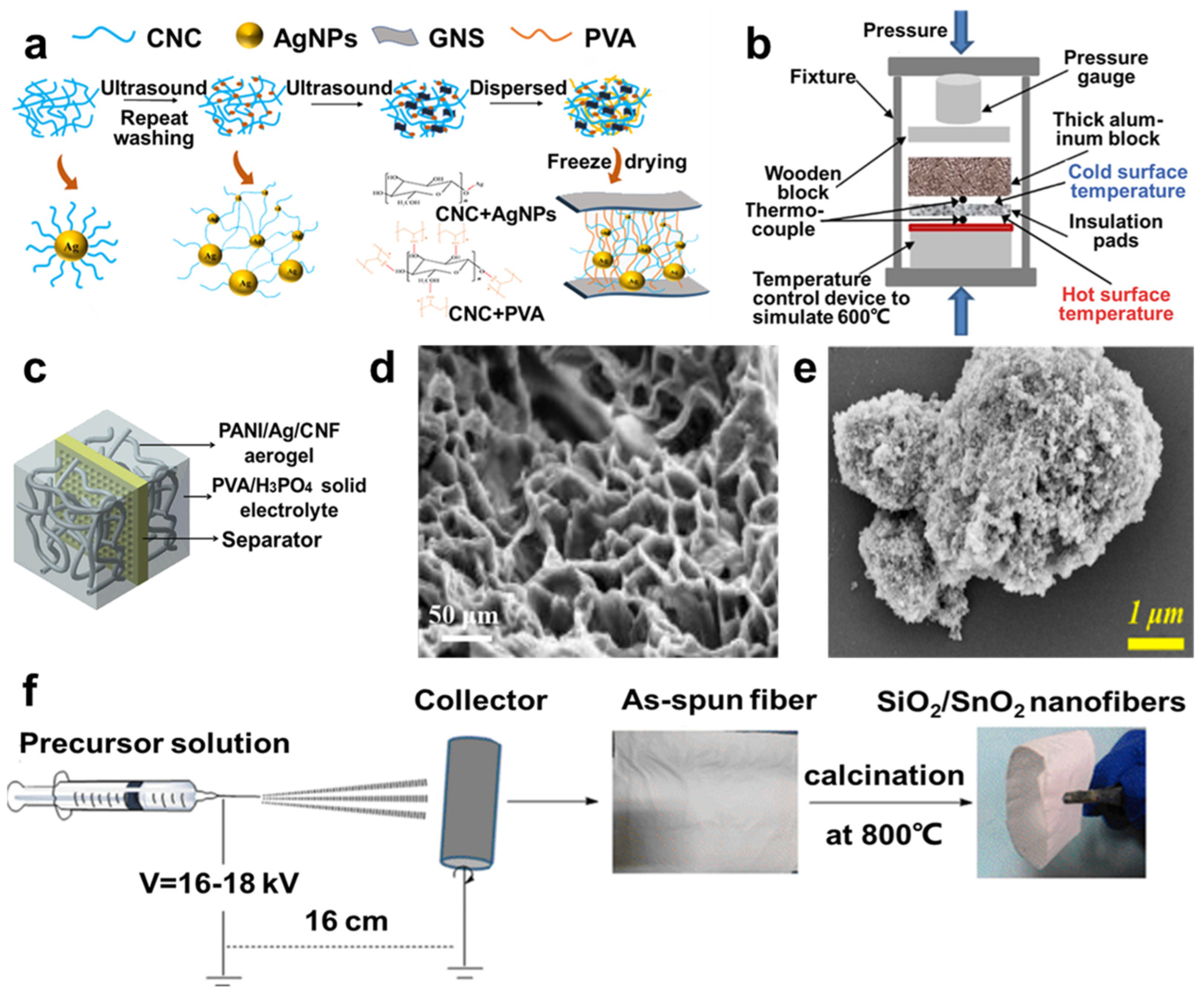
3.1. Carbon Aerogels
3.1.1. Resorcinol Formaldehyde (RF) Aerogels
3.1.2. Carbon Nanotube (CNT) Aerogel
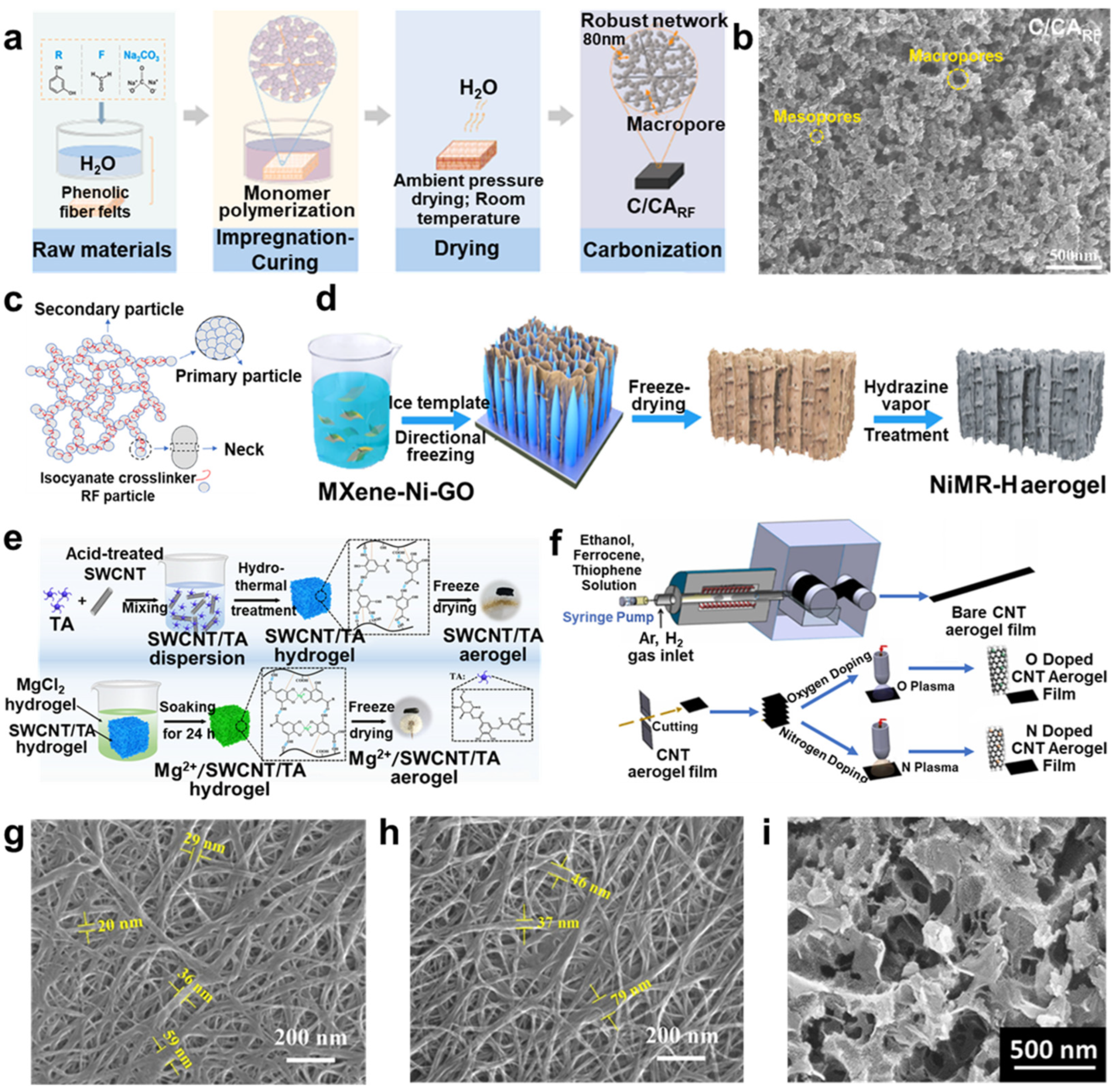
3.1.3. Graphene Aerogel
3.2. Organic Aerogels
3.3. Oxide Aerogels
3.3.1. SiO2 Aerogels
3.3.2. Al2O3 Aerogels
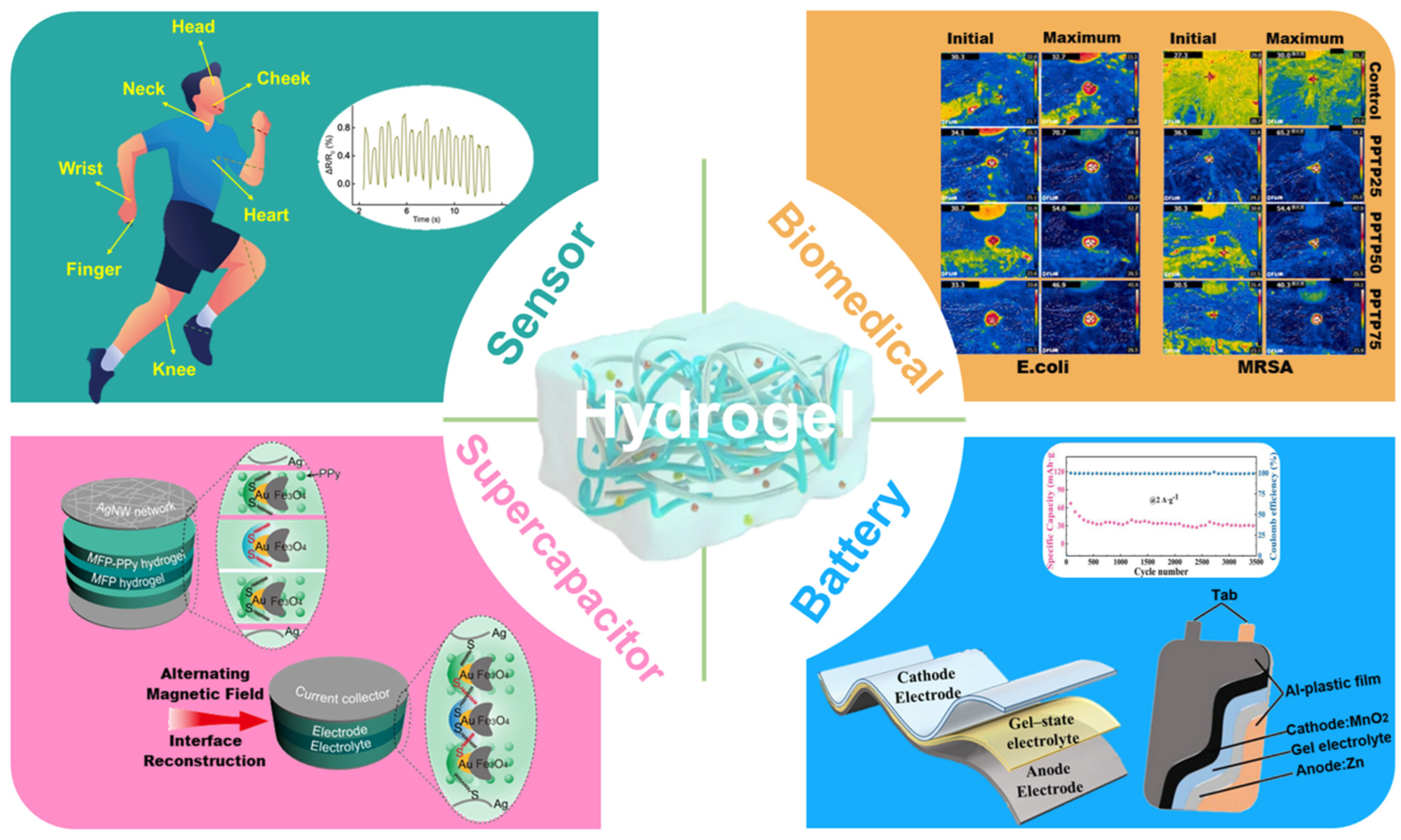
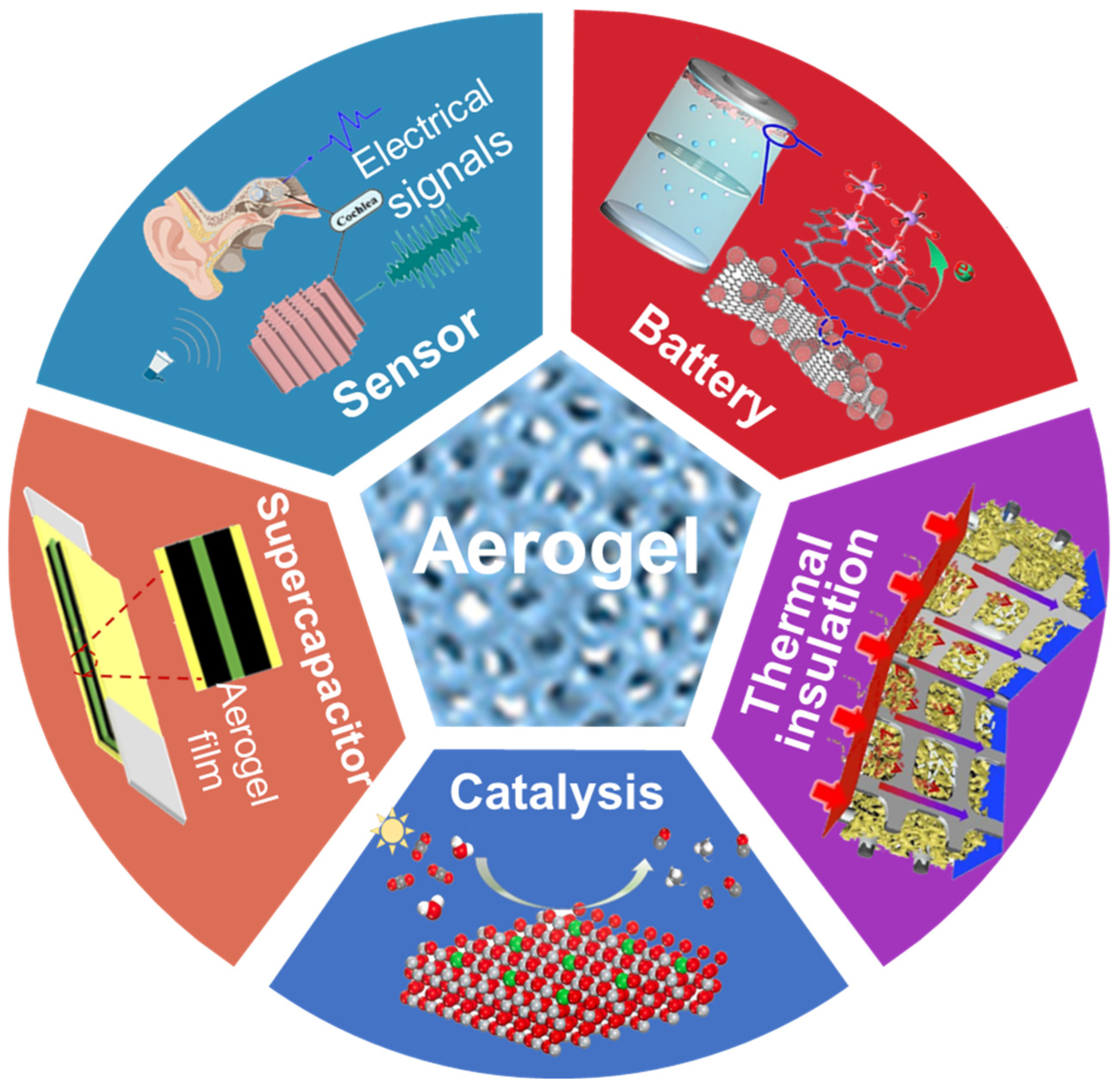
4. Applications
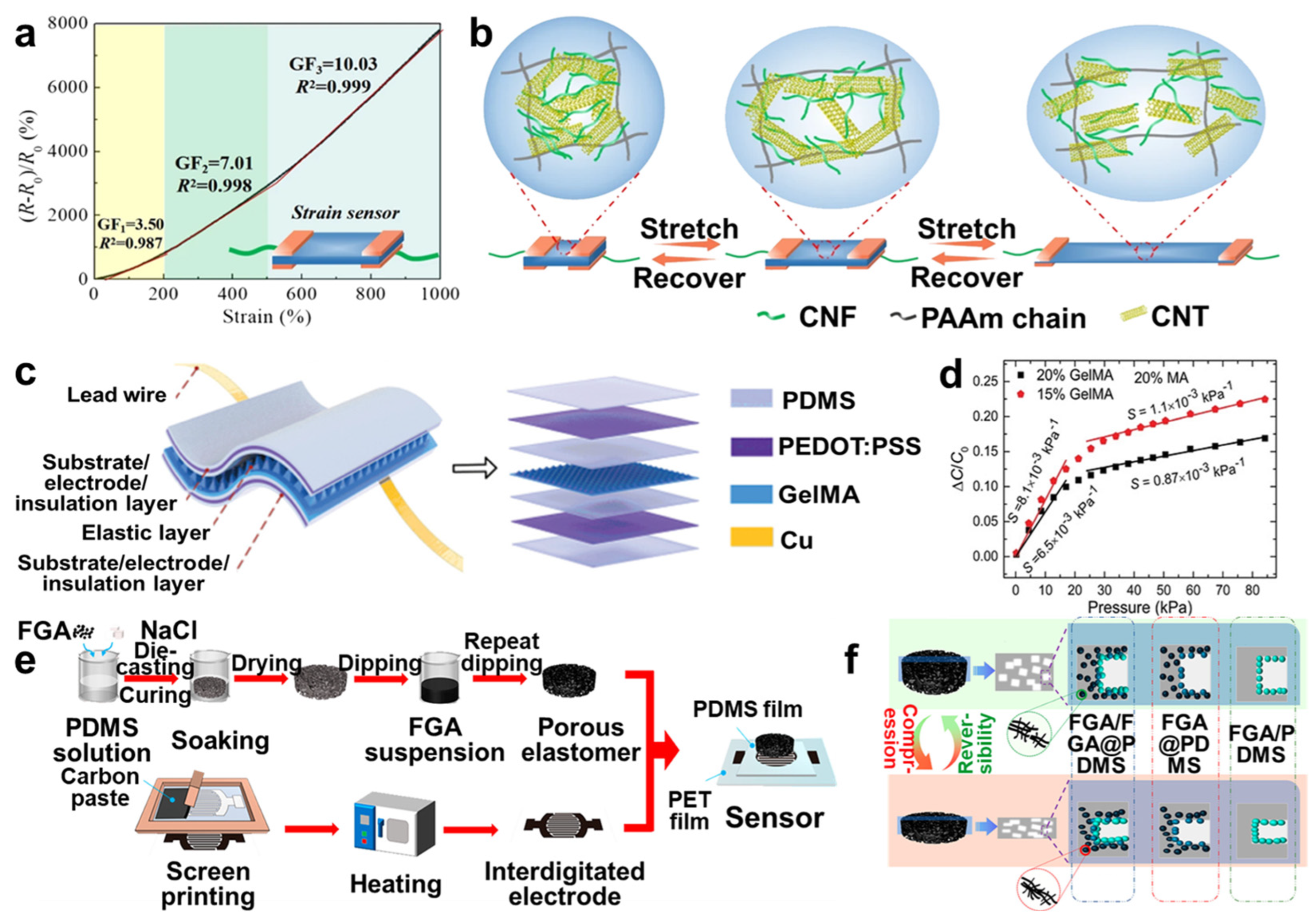
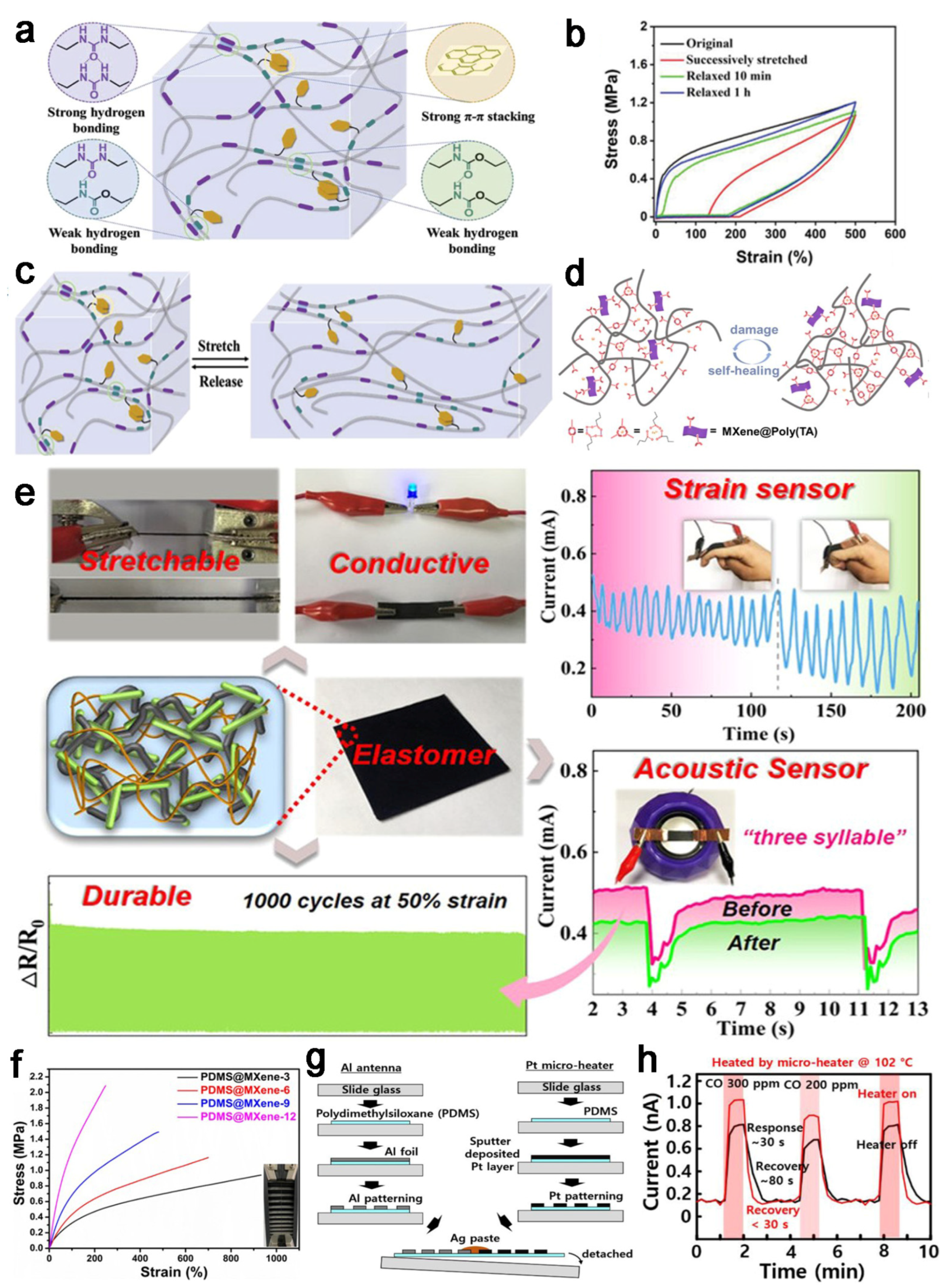
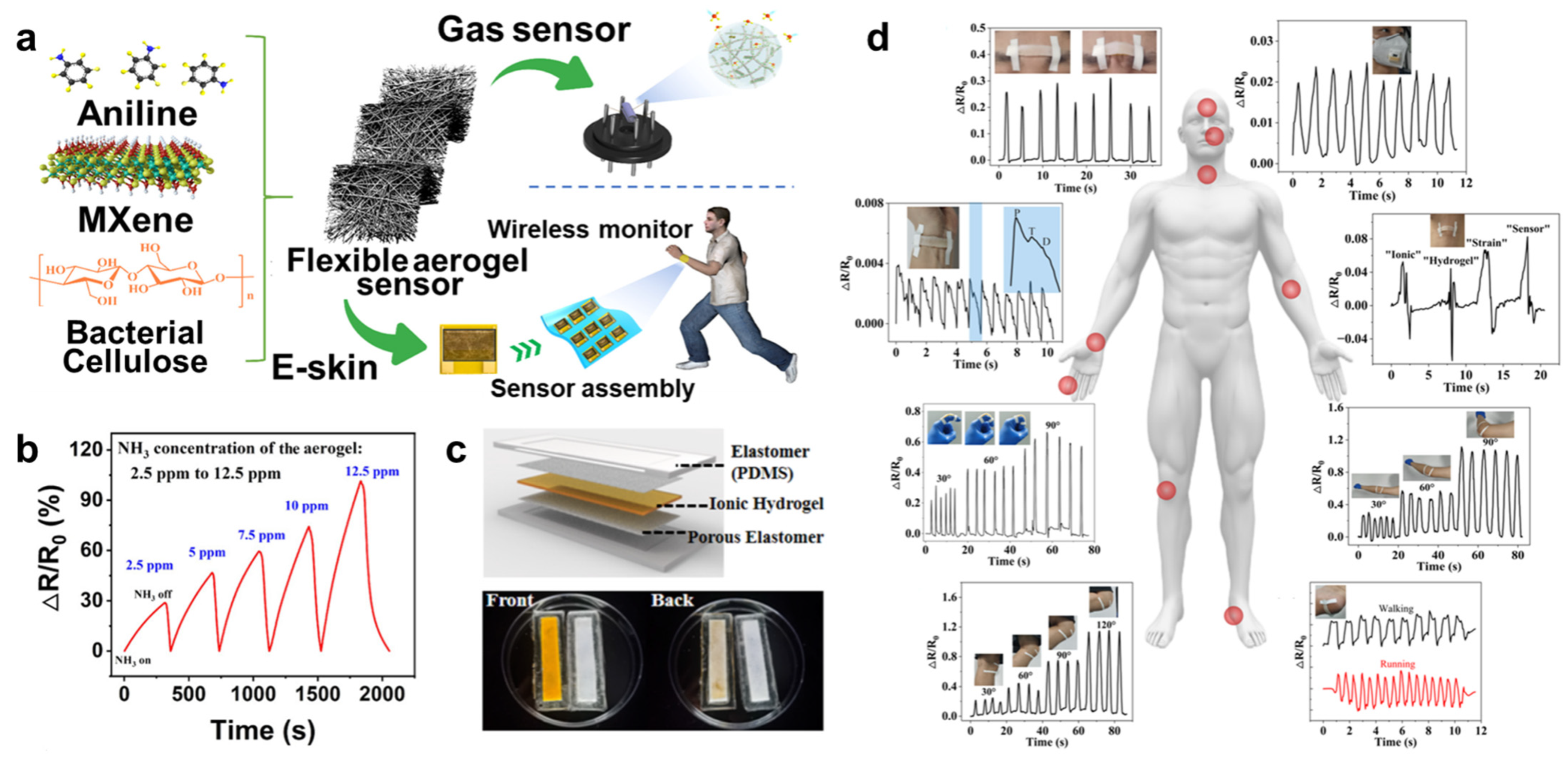
4.1. Sensors
4.2. Batteries
4.2.1. Electrolytes
4.2.2. Electrodes
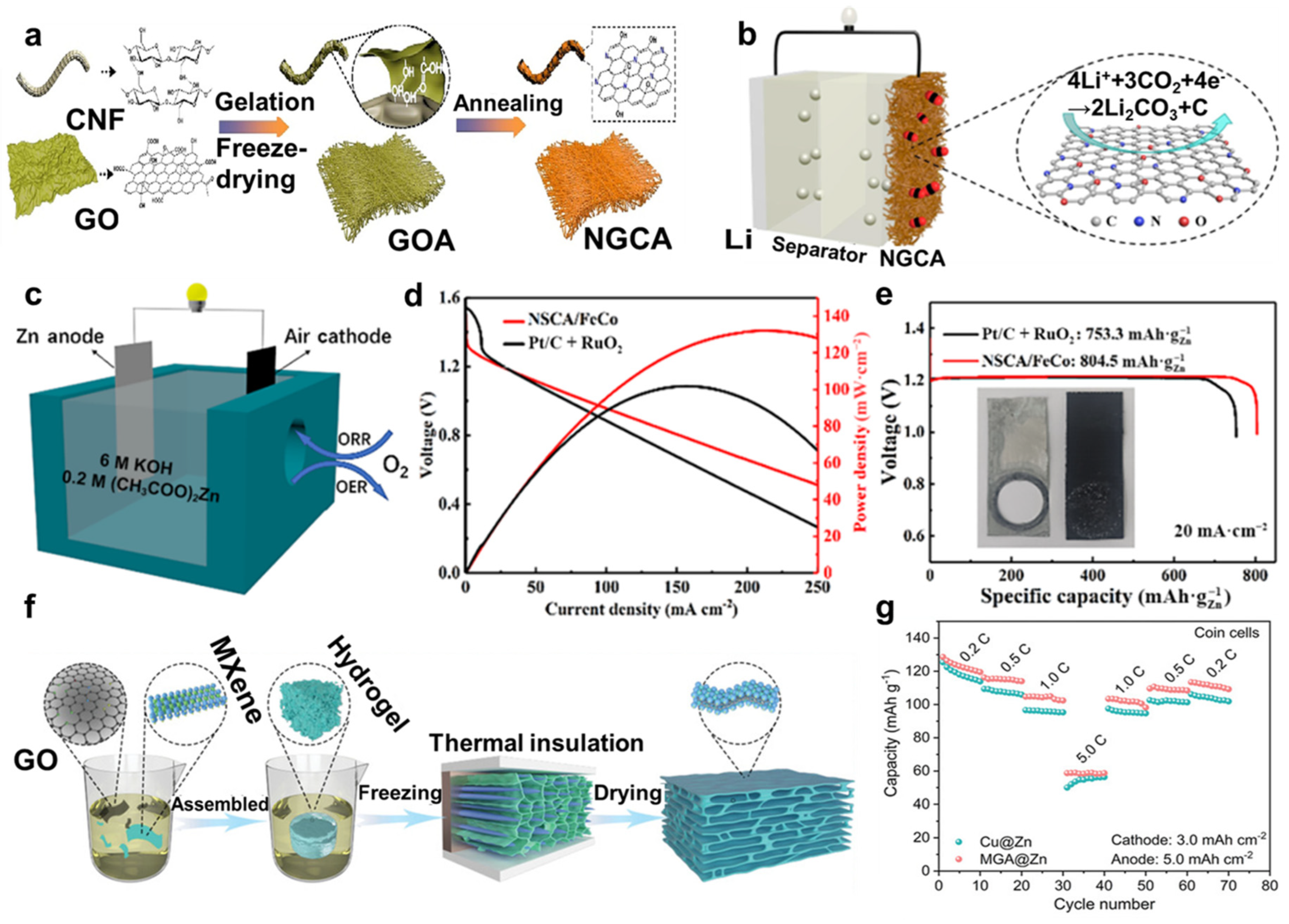
4.3. Supercapacitors
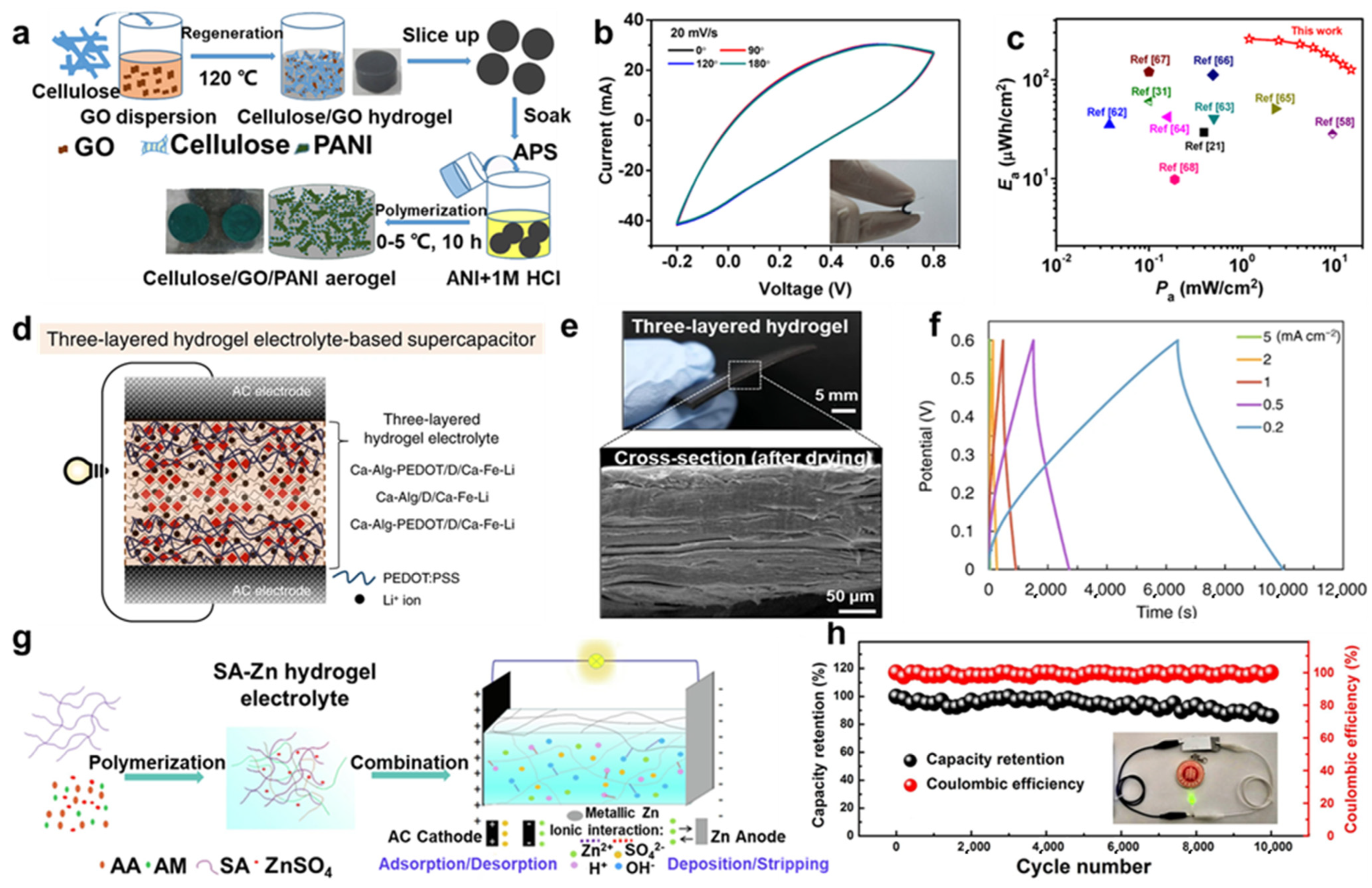
4.4. Photo-/Electro-Chemical Catalysis
4.5. Biomedical Applications
4.6. Thermal Insulation
5. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, B.; Yan, J.; Long, F.; Qiu, W.; Meng, G.; Zeng, Z.; Huang, H.; Wang, H.; Lin, N.; Liu, X.-Y. Bioinspired Conductive Enhanced Polyurethane Ionic Skin as Reliable Multifunctional Sensors. Adv. Sci. 2023, 10, 2300857. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Zhang, L.; Zhou, J.; Lu, A. Transparent, Ultra-Stretching, Tough, Adhesive Carboxyethyl Chitin/Polyacrylamide Hydrogel Toward High-Performance Soft Electronics. Nano-Micro Lett. 2022, 15, 8. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Z.; Zheng, J.; Cheng, Q.; Tan, Y.; Huang, S.; Zhang, L.; Wang, Y.; Zhou, H. A Directional Chitosan Sound Sensor Based on Piezoelectric–Triboelectric Sensing. ACS Macro Lett. 2023, 12, 577–582. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, Z.; Shen, M.; Chowdhury, S.P.; Ignaszak, A.; Sun, S.; Ni, Y. Cellulose Nanofibers/Reduced Graphene Oxide/Polypyrrole Aerogel Electrodes for High-Capacitance Flexible All-Solid-State Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11175–11185. [Google Scholar] [CrossRef]
- Du, Z.; Liu, W.; Liu, J.; Chu, Z.; Qu, F.; Li, L.; Shen, G. A Thermally Chargeable Supercapacitor based on the g-C3N4-Doped PAMPS/PAA Hydrogel Solid Electrolyte and 2D MOF@Ti3C2Tx MXene Heterostructure Composite Electrode. Adv. Mater. Interfaces 2023, 10, 2300266. [Google Scholar] [CrossRef]
- Qin, H.; Liu, P.; Chen, C.; Cong, H.-P.; Yu, S.-H. A multi-responsive healable supercapacitor. Nat. Commun. 2021, 12, 4297. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, X.; Ma, T.; Zhang, C.; Chen, X.; Zhang, X.; Huang, T.; Li, W.; Yu, A. Ultradispersed titanium dioxide nanoparticles embedded in a three- dimensional graphene aerogel for high performance sulfur cathodes. RSC Adv. 2019, 9, 6568–6575. [Google Scholar] [CrossRef]
- Ho, J.; Hatakeyama Sato, K.; Chiba, A.; Hayashi, M.; Igarashi, Y.; Oyaizu, K.; Chen, W.-C. Sandwich Configuration of Zinc Anode, Gel Electrolyte, and Radical Polymer Cathode for Fully Stretch-Rechargeable Battery. Adv. Sustain. Syst. 2023, 7, 2300080. [Google Scholar] [CrossRef]
- Cao, G.; Zhao, L.; Ji, X.; Peng, Y.; Yu, M.; Wang, X.; Li, X.; Ran, F. “Salting out” in Hofmeister Effect Enhancing Mechanical and Electrochemical Performance of Amide-based Hydrogel Electrolytes for Flexible Zinc-Ion Battery. Small 2023, 19, 2207610. [Google Scholar] [CrossRef]
- Guo, X.; Li, C.; Wang, X.; Li, Z.; Zeng, H.; Hou, P.; Xie, M.; Li, Y.; Shi, Z.; Feng, S. Rational design of interfacial bonds within dual carbon-protected manganese oxide towards durable aqueous zinc ion battery. Sci. China Chem. 2023, 66, 1406–1416. [Google Scholar] [CrossRef]
- Xia, Y.; Man, J.; Wu, X.; Huang, S.; Lu, A.; Shen, X.; Cui, S.; Chen, X.; Fu, G. Oxygen-vacancy-assisted construction of Ce–TiO2 aerogel for efficiently boosting photocatalytic CO2 reduction without any sacrifice agent. Ceram. Int. 2023, 49, 6100–6112. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Hu, Y.; Zheng, T.; An, J. Constructions of synergistic photothermal therapy antibacterial hydrogel based on polydopamine, tea polyphenols and polyvinyl alcohol and effects on wound healing in mouse. Colloids Surf. B Biointerfaces 2022, 219, 112831. [Google Scholar] [CrossRef]
- Jia, X.; Hua, C.; Yang, F.; Li, X.; Zhao, P.; Zhou, F.; Lu, Y.; Liang, H.; Xing, M.; Lyu, G. Hydrophobic aerogel-modified hemostatic gauze with thermal management performance. Bioact. Mater. 2023, 26, 142–158. [Google Scholar] [CrossRef]
- Pan, Y.; Jin, X.; Wang, H.; Huang, H.; Wu, C.; Yan, X.; Hong, C.; Zhang, X. Nano-TiO2 coated needle carbon fiber reinforced phenolic aerogel composite with low density, excellent heat-insulating and infrared radiation shielding performance. J. Mater. Sci. Technol. 2023, 152, 181–189. [Google Scholar] [CrossRef]
- Song, X.; Qiu, X.; Huang, X.; Tu, Y.; Zhao, Q.; Sun, R.; Zhang, L. Waxy rice amylopectin towards stretchable elastic conductive hydrogel for human motion detection. New J. Chem. 2021, 45, 4210–4218. [Google Scholar] [CrossRef]
- Prakash, J.; Rao, P.T.; Ghorui, S.; Bahadur, J.; Jain, V.; Dasgupta, K. Tailoring surface properties with O/N doping in CNT aerogel film to obtain sensitive and selective sensor for volatile organic compounds detection. Sens. Actuators B-Chem. 2022, 359, 131606. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, C.; Wang, Y.; Li, T.; Zhai, K.; Zhang, F.; Bai, Y.; Tan, Y.; Ma, Y.; Xu, K.; et al. A facile method to synthesize strong salt-enhanced hydrogels based on reversible physical interaction. Soft Matter 2020, 16, 738–746. [Google Scholar] [CrossRef]
- Hynd, M.R.; Turner, J.N.; Shain, W. Applications of hydrogels for neural cell engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 1223–1244. [Google Scholar] [CrossRef]
- Dragan, E.S.; Lazar, M.M.; Dinu, M.V.; Doroftei, F. Macroporous composite IPN hydrogels based on poly(acrylamide) and chitosan with tuned swelling and sorption of cationic dyes. Chem. Eng. J. 2012, 204, 198–209. [Google Scholar] [CrossRef]
- Asher, S.A.; Kimble, K.W.; Walker, J.P. Enabling Thermoreversible Physically Cross-Linked Polymerized Colloidal Array Photonic Crystals. Chem. Mater. 2008, 20, 7501–7509. [Google Scholar] [CrossRef]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super flexible, fatigue resistant, self-healing PVA/xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, W.; Li, X.; Gao, X. Highly stretchable and sensitive strain sensors based on modified PDMS and hybrid particles of AgNWs/graphene. Nanotechnology 2023, 34, 06LT01. [Google Scholar] [CrossRef]
- Li, F.; Xie, L.; Sun, G.; Kong, Q.; Su, F.; Cao, Y.; Wei, J.; Ahmad, A.; Guo, X.; Chen, C.-M. Resorcinol-formaldehyde based carbon aerogel: Preparation, structure and applications in energy storage devices. Microporous Mesoporous Mater. 2019, 279, 293–315. [Google Scholar] [CrossRef]
- Ciszewski, M.; Szatkowska, E.; Koszorek, A.; Majka, M. Carbon aerogels modified with graphene oxide, graphene and CNT as symetric supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2017, 28, 4897–4903. [Google Scholar] [CrossRef]
- Nargatti, K.I.; Subhedar, A.R.; Ahankari, S.S.; Grace, A.N.; Dufresne, A. Nanocellulose-based aerogel electrodes for supercapacitors: A review. Carbohydr. Polym. 2022, 297, 120039. [Google Scholar] [CrossRef]
- Sen, S.; Singh, A.; Bera, C.; Roy, S.; Kailasam, K. Recent developments in biomass derived cellulose aerogel materials for thermal insulation application: A review. Cellulose 2022, 29, 4805–4833. [Google Scholar] [CrossRef]
- Rahmanian, V.; Pirzada, T.; Wang, S.; Khan, S.A. Cellulose-Based Hybrid Aerogels: Strategies toward Design and Functionality. Adv. Mater. 2021, 33, 2102892. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ji, C.; Fu, R.; Yang, X.; Wan, Z.; Wen, L.; Song, Q.; Liu, Y.; Wang, Y.; Sai, H. Robust SiO2-Al2O3/Agarose Composite Aerogel Beads with Outstanding Thermal Insulation Based on Coal Gangue. Gels 2022, 8, 165. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Shi, F.; Yu, L.; Liu, S.; Hu, S.; Liu, D. Synthesis of mesoporous SiO2 aerogel/WxTiO2 nanocomposites with high adsorptivity and photocatalytic activity. Adv. Powder Technol. 2016, 27, 1781–1789. [Google Scholar] [CrossRef]
- Lin, D.C.; Yurke, B.; Langrana, N.A. Mechanical Properties of a Reversible, DNA-Crosslinked Polyacrylamide Hydrogel. J. Biomech. Eng. 2004, 126, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, S. Removal of humic acid from aqueous solution using polyacrylamide/chitosan semi-IPN hydrogel. Water Sci. Technol. 2018, 2017, 16–26. [Google Scholar] [CrossRef]
- Peng, X.; Peng, Q.; Wu, M.; Wang, W.; Gao, Y.; Liu, X.; Sun, Y.; Yang, D.; Peng, Q.; Wang, T.; et al. A pH and Temperature Dual-Responsive Microgel-Embedded, Adhesive, and Tough Hydrogel for Drug Delivery and Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 19560–19573. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Jiang, S.; He, S.; Wu, Q.; Xiao, H.; Han, J. Environment-tolerant ionic hydrogel–elastomer hybrids with robust interfaces, high transparence, and biocompatibility for a mechanical–thermal multimode sensor. InfoMat 2023, 5, e12409. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, H.D.; Kim, S.H.L.; Kim, I.; Kim, I.G.; Choi, J.S.; Jeong, J.; Kim, J.H.; Kwon, S.K.; Hwang, N.S. Self-Healing and Adhesive Artificial Tissue Implant for Voice Recovery. ACS Appl. Bio Mater. 2018, 1, 1134–1146. [Google Scholar] [CrossRef]
- Xu, Y.; Ghag, O.; Reimann, M.; Sitterle, P.; Chatterjee, P.; Nofen, E.; Yu, H.; Jiang, H.; Dai, L.L. Development of visible-light responsive and mechanically enhanced “smart” UCST interpenetrating network hydrogels. Soft Matter 2018, 14, 151–160. [Google Scholar] [CrossRef]
- Lin, F.; Lu, X.; Wang, Z.; Lu, Q.; Lin, G.; Huang, B.; Lu, B. In situ polymerization approach to cellulose–polyacrylamide interpenetrating network hydrogel with high strength and pH-responsive properties. Cellulose 2019, 26, 1825–1839. [Google Scholar] [CrossRef]
- Ning, X.; Huang, J.; A, Y.; Yuan, N.; Chen, C.; Lin, D. Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels. Int. J. Mol. Sci. 2022, 23, 15757. [Google Scholar] [CrossRef]
- Yan, X.; Yang, J.; Chen, F.; Zhu, L.; Tang, Z.; Qin, G.; Chen, Q.; Chen, G. Mechanical properties of gelatin/polyacrylamide/graphene oxide nanocomposite double-network hydrogels. Compos. Sci. Technol. 2018, 163, 81–88. [Google Scholar] [CrossRef]
- Feng, K.; Hung, G.; Yang, X.; Liu, M. High-strength and physical cross-linked nanocomposite hydrogel with clay nanotubes for strain sensor and dye adsorption application. Compos. Sci. Technol. 2019, 181, 107701. [Google Scholar] [CrossRef]
- Lin, T.; Li, S.; Hu, Y.; Sheng, L.; Chen, X.; Que, X.; Peng, J.; Ma, H.; Li, J.; Zhai, M. Ultrastretchable and adhesive agarose/Ti3C2Tx-crosslinked-polyacrylamide double-network hydrogel for strain sensor. Carbohydr. Polym. 2022, 290, 119506. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiang, L.; Zhu, P.; Qian, Y.; Zhao, B.; Chen, G. Multifunctional Organohydrogel-Based Ionic Skin for Capacitance and Temperature Sensing toward Intelligent Skin-like Devices. Chem. Mater. 2021, 33, 8623–8634. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, X.; Ye, L. A new mussel-inspired highly self-adhesive & conductive poly (vinyl alcohol)-based hydrogel for wearable sensors. Appl. Surf. Sci. 2021, 562, 150162. [Google Scholar]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Azadi, S.; Peng, S.; Moshizi, S.A.; Asadnia, M.; Xu, J.; Park, I.; Wang, C.H.; Wu, S. Biocompatible and Highly Stretchable PVA/AgNWs Hydrogel Strain Sensors for Human Motion Detection. Adv. Mater. Technol. 2020, 5, 2000426. [Google Scholar] [CrossRef]
- Singh, S.; Nwabor, O.; Sukri, D.; Wunnoo, S.; Dumjun, K.; Lethongkam, S.; Kusolphat, P.; Hemtanon, N.; Klinprathum, K.; Sunghan, J.; et al. Poly (vinyl alcohol) copolymerized with xanthan gum/hypromellose/sodium carboxymethyl cellulose dermal dressings functionalized with biogenic nanostructured materials for antibacterial and wound healing application. Int. J. Biol. Macromol. 2022, 216, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Priya; Kaith, B.S.; Singh, A.; Isha; Vipula; Chandel, K. Enzymatic construction of quinine derivative of dextrin/PVA based hybrid gel film for the simultaneous detection and removal of copper and lead ions in real water samples. Chem. Eng. J. 2020, 382, 122891. [Google Scholar] [CrossRef]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Wang, Z.; Liu, Z.; Yao, S.; Li, L. Antibacterial, Antifreezing, Stretchable, and Self-Healing Organohydrogel Electrode Based Triboelectric Nanogenerator for Self-Powered Biomechanical Sensing. Adv. Mater. Interfaces 2022, 9, 2200290. [Google Scholar] [CrossRef]
- Dai, X.; Long, Y.; Jiang, B.; Guo, W.; Sha, W.; Wang, J.; Cong, Z.; Chen, J.; Wang, B.; Hu, W. Ultra-antifreeze, ultra-stretchable, transparent, and conductive hydrogel for multi-functional flexible electronics as strain sensor and triboelectric nanogenerator. Nano Res. 2022, 15, 5461–5468. [Google Scholar] [CrossRef]
- Dong, X.; Tong, S.; Dai, K.; Ren, D.; Wang, T.; Wang, Y.; Leng, Y.; Li, Q.; Wang, S. Preparation of PVA/PAM/Ag strain sensor via compound gelation. J. Appl. Polym. Sci. 2022, 139, e51883. [Google Scholar] [CrossRef]
- Chen, G.; Huang, J.; Gu, J.; Peng, S.; Xiang, X.; Chen, K.; Yang, X.; Guan, L.; Jiang, X.; Hou, L. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 2020, 8, 6776–6784. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Fan, M.; Tang, P.; Yang, S.; Pan, L.; Wang, H.; Bin, Y. Strong and tough PVA/PAA hydrogel fiber with highly strain sensitivity enabled by coating MWCNTs. Compos. Part A-Appl. Sci. Manuf. 2020, 138, 106050. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, C.; Xing, M.; Wang, L.; Guan, G. Surface modification of polyvinyl alcohol (PVA)/polyacrylamide (PAAm) hydrogels with polydopamine and REDV for improved applicability. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2020, 108, 117–127. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, G.; Wang, S.; Zhang, Y.; Jian, Y.; He, L.; Yu, T.; Luo, H.; Kong, D.; Xianyu, Y.; et al. Stretchable graphene–hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. 2024, 7, 51–65. [Google Scholar] [CrossRef]
- Hauck, M.; Saure, L.M.; Zeller-Plumhoff, B.; Kaps, S.; Hammel, J.; Mohr, C.; Rieck, L.; Nia, A.S.; Feng, X.; Pugno, N.M.; et al. Overcoming Water Diffusion Limitations in Hydrogels via Microtubular Graphene Networks for Soft Actuators. Adv. Mater. 2023, 35, 2302816. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Liang, C.; Sha, G.; Guo, S. Resorcinol–formaldehyde aerogels prepared by supercritical acetone drying. J. Non-Cryst. Solids 2000, 271, 167–170. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, K.N.; Lee, H.J.; Kim, J.H. Resorcinol/formaldehyde aerogel fine powders with nanopore structure prepared by supercritical CO2 drying techniques. J. Ind. Eng. Chem. 2002, 8, 546–551. [Google Scholar]
- Zhang, W.; Hu, C.; Li, J.; Zhao, R.; Pang, S.; Liang, B.; Tang, S. Insight into the origin of oxidation behaviors of carbon fiber reinforced carbon aerogel composites with different porous skeletons. Carbon 2023, 209, 118008. [Google Scholar] [CrossRef]
- Aghabararpour, M.; Mohsenpour, M.; Motahari, S.; Ghahreman, A. Mechanical and thermal insulation properties of isocyanate crosslinked resorcinol formaldehyde aerogel: Effect of isocyanate structure. J. Appl. Polym. Sci. 2019, 136, 48196. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zheng, L.; Li, Z.; Wu, L.; Wang, X. Superelastic, Anticorrosive, and Flame-Resistant Nitrogen-Containing Resorcinol Formaldehyde/Graphene Oxide Composite Aerogels. ACS Sustain. Chem. Eng. 2019, 7, 10873–10879. [Google Scholar] [CrossRef]
- Alshrah, M.; Naguib, H.E.; Park, C.B. Reinforced resorcinol formaldehyde aerogel with Co-assembled polyacrylonitrile nanofibers and graphene oxide nanosheets. Mater. Des. 2018, 151, 154–163. [Google Scholar] [CrossRef]
- Lin, Z.; Zeng, Z.; Gui, X.; Tang, Z.; Zou, M.; Cao, A. Carbon Nanotube Sponges, Aerogels, and Hierarchical Composites: Synthesis, Properties, and Energy Applications. Adv. Energy Mater. 2016, 6, 1600554. [Google Scholar] [CrossRef]
- Prakash, J.; Uppal, S.; Kaushal, A.; Dasgupta, K. Effect of O/N doping in CNT aerogel film on their nucleic acid hybridization detection ability as electrochemical impedance biosensor. Mater. Today Commun. 2022, 32, 103965. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, J.G.; Ovalle-Robles, R.; Kang, T.J. Aerogel sheet of carbon nanotubes decorated with palladium nanoparticles for hydrogen gas sensing. Int. J. Hydrog. Energy 2018, 43, 6456–6461. [Google Scholar] [CrossRef]
- Liao, W.; Yu, C.; Peng, Z.; Xu, F.; Zhang, Y.; Zhong, W. Ultrasensitive Mg2+-Modulated Carbon Nanotube/Tannic Acid Aerogels for High-Performance Wearable Pressure Sensors. ACS Sustain. Chem. Eng. 2023, 11, 2186–2197. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Liang, L.; Li, Q.; Yan, X.; Feng, Y.; Wang, Y.; Zhang, H.-B.; Zhou, X.; Liu, C.; Shen, C.; Xie, X. Multifunctional Magnetic Ti3C2Tx MXene/Graphene Aerogel with Superior Electromagnetic Wave Absorption Performance. ACS Nano 2021, 15, 6622–6632. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, W.; Bi, S.; Liu, S.; Huang, W.; Zhao, Q. Preparation and application of cellulose gel in flexible supercapacitors. J. Energy Storage 2021, 42, 103058. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Li, J.; Bai, Y.; Tan, X. Preparation of novel composite aerogel with conductive and antibacterial via constructing three-dimensional crosslinked structure. React. Funct. Polym. 2022, 178, 105361. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Z.; Chen, B.; Zhang, W.; Sharma, S.; Gu, W.; Deng, Y. Solid-state flexible polyaniline/silver cellulose nanofibrils aerogel supercapacitors. J. Power Sources 2014, 246, 283–289. [Google Scholar] [CrossRef]
- Niu, Q.; Guo, Y.; Gao, K.; Shao, Z. Polypyrrole/cellulose nanofiber aerogel as a supercapacitor electrode material. RSC Adv. 2016, 6, 109143–109149. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Li, Q.; Wang, W.; Du, Q.; Yang, H.; Qiu, L.; Zhang, Z.; Shen, J. Industrial application of SiO2 aerogel prepared by supercritical ethanol (SCE) drying technique as cold and heat insulation materials. J. Sol-Gel Sci. Technol. 2022, 106, 341–348. [Google Scholar] [CrossRef]
- Zhang, R.; An, Z.; Zhao, Y.; Zhang, L.; Zhou, P. Nanofibers reinforced silica aerogel composites having flexibility and ultra-low thermal conductivity. Int. J. Appl. Ceram. Technol. 2020, 17, 1531–1539. [Google Scholar] [CrossRef]
- Gao, B.; Sun, X.; Yao, C.; Mao, L. A new strategy to obtain thin ZrO2–Al2O3 composite aerogel coating with prominent high–temperature resistance and rapid heat dissipation. J. Solid State Chem. 2022, 314, 123384. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Y.; Wang, F.; Deng, Z.-P. Thermal insulation material based on SiO2 aerogel. Constr. Build. Mater. 2016, 122, 548–555. [Google Scholar] [CrossRef]
- Shen, X.; Mao, T.; Li, C.; Mao, F.; Xue, Z.; Xu, G.; Amirfazli, A. Durable superhydrophobic coatings based on CNTs-SiO2gel hybrids for anti-corrosion and thermal insulation. Prog. Org. Coat. 2023, 181, 107602. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, H.; Wang, X.; Li, C.; Wang, W.; Hong, Z.; Zhi, M. One-step synthesis of monolithic micro-nano yttria stabilized ZrO2-Al2O3 composite aerogel. Microporous Mesoporous Mater. 2018, 259, 26–32. [Google Scholar] [CrossRef]
- Du, Y.; Sun, Y.; Lu, S.; Zhang, K.; Song, C.; Li, B.; He, X.; Li, Q. Ultra-stretchable, anti-freezing conductive hydrogels crosslinked by strong hydrogen bonding for flexible sensors. J. Polym. Sci. 2022, 60, 2733–2740. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, S.; Liu, X.; Chen, Y.; Qi, P.; Liu, X. Molybdenum disulfide enhanced polyacrylamide-acrylic acid-Fe3+ ionic conductive hydrogel with high mechanical properties and anti-fatigue abilities as strain sensors. Colloids Surf. a-Physicochem. Eng. Asp. 2021, 610, 125692. [Google Scholar] [CrossRef]
- Jing, X.; Li, H.; Mi, H.; Liu, Y.-J.; Feng, P.-Y.; Tan, Y.-M.; Turng, L.-S. Highly transparent, stretchable, and rapid self-healing polyvinyl alcohol/cellulose nanofibril hydrogel sensors for sensitive pressure sensing and human motion detection. Sens. Actuators B-Chem. 2019, 295, 159–167. [Google Scholar] [CrossRef]
- Dragoman, M.; Ghimpu, L.; Obreja, C.; Dinescu, A.; Plesco, I.; Dragoman, D.; Braniste, T.; Tiginyanu, I. Ultra-lightweight pressure sensor based on graphene aerogel decorated with piezoelectric nanocrystalline films. Nanotechnology 2016, 27, 475203. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, R.; Pan, F.; Fu, Z. Polyvinyl Alcohol/Graphene Oxide Conductive Hydrogels via the Synergy of Freezing and Salting Out for Strain Sensors. Sensors 2022, 22, 3015. [Google Scholar] [CrossRef]
- Wei, Y.; Qian, Y.; Zhu, P.; Xiang, L.; Lei, C.; Qiu, G.; Wang, C.; Liu, Y.; Liu, Y.; Chen, G. Nanocellulose-templated carbon nanotube enhanced conductive organohydrogel for highly-sensitive strain and temperature sensors. Cellulose 2022, 29, 3829–3844. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Xu, G.; Jiang, Z.; Ge, Z.; Wang, M.; Ge, X. Flexible, high sensitive and radiation-resistant pressure-sensing hydrogel. Chin. Chem. Lett. 2022, 33, 1011–1016. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Chen, Y.; Ling, H.; Zhao, L.; Luo, G.; Wang, X.; Hartel, M.C.; Liu, H.; Xue, Y.; et al. Gelatin Methacryloyl-Based Tactile Sensors for Medical Wearables. Adv. Funct. Mater. 2020, 30, 2003601. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, D.; Jia, H.; Yang, C.; Zheng, Z.; Wang, X. Bio-based visual optical pressure-responsive sensor. Carbohydr. Polym. 2021, 260, 117823. [Google Scholar] [CrossRef]
- Luo, R.; Cui, Y.; Li, H.; Wu, Y.; Du, B.; Zhou, S.; Hu, J. Fragmented Graphene Aerogel/Polydimethylsiloxane Sponges for Wearable Piezoresistive Pressure Sensors. ACS Appl. Nano Mater. 2023, 6, 7065–7076. [Google Scholar] [CrossRef]
- Zhi, H.; Gao, J.; Feng, L. Hydrogel-Based Gas Sensors for NO2 and NH3. ACS Sens. 2020, 5, 772–780. [Google Scholar] [CrossRef]
- Zhi, H.; Zhang, X.; Wang, F.; Wan, P.; Feng, L. Flexible Ti3C2Tx MXene/PANI/Bacterial Cellulose Aerogel for e-Skins and Gas Sensing. ACS Appl. Mater. Interfaces 2021, 13, 45987–45994. [Google Scholar] [CrossRef]
- Zhao, W.; Lin, Z.; Wang, X.; Wang, Z.; Sun, Z. Mechanically Interlocked Hydrogel-Elastomer Strain Sensor with Robust Interface and Enhanced Water-Retention Capacity. Gels 2022, 8, 625. [Google Scholar] [CrossRef] [PubMed]
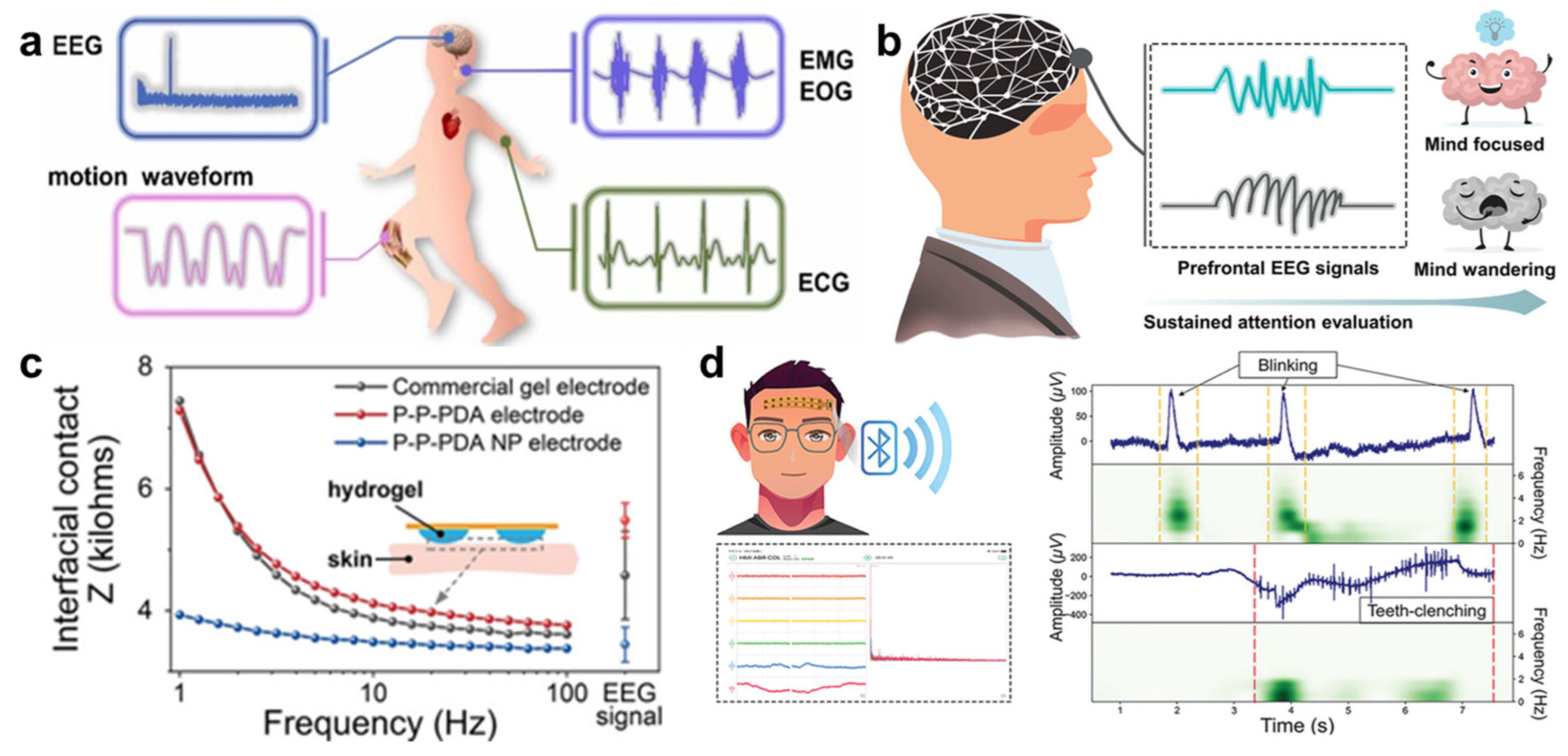
- Hsieh, J.; Alawieh, H.; Li, Y.; Iwane, F.; Zhao, L.; Anderson, R.; Abdullah, S.I.; Kevin Tang, K.W.; Wang, W.; Pyatnitskiy, I.; et al. A highly stable electrode with low electrode-skin impedance for wearable brain-computer interface. Biosens. Bioelectron. 2022, 218, 114756. [Google Scholar] [CrossRef]
- Sheng, X.; Qin, Z.; Xu, H.; Shu, X.; Gu, G.; Zhu, X. Soft ionic-hydrogel electrodes for electroencephalography signal recording. Sci. China-Technol. Sci. 2021, 64, 273–282. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Tang, R.; Ai, J.; Ma, Y.; Chen, Y.; Feng, X. Stable and low-resistance polydopamine methacrylamide-polyacrylamide hydrogel for brain-computer interface. Sci. China Mater. 2022, 65, 2298–2308. [Google Scholar] [CrossRef]
- Kim, Y.; Song, J.; An, S.; Shin, M.; Son, D. Soft Liquid Metal-Based Conducting Composite with Robust Electrical Durability for a Wearable Electrocardiogram Sensor. Polymers 2022, 14, 3409. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Liang, Q.; Sun, X.; Yu, D.; Huang, X.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q. Intrinsically Electron Conductive, Antibacterial, and Anti-swelling Hydrogels as Implantable Sensors for Bioelectronics. Adv. Funct. Mater. 2022, 32, 2208024. [Google Scholar] [CrossRef]
- Liu, J.; Liu, K.; Pan, X.; Bi, K.; Zhou, F.; Lu, P.; Lei, M. A flexible semidry electrode for long-term, high-quality electrocardiogram monitoring. Adv. Compos. Hybrid Mater. 2022, 6, 13. [Google Scholar] [CrossRef]
- Murciego, L.; Komolafe, A.; Peřinka, N.; Nunes-Matos, H.; Junker, K.; Díez, A.G.; Lanceros-Méndez, S.; Torah, R.; Spaich, E.G.; Dosen, S. A Novel Screen-Printed Textile Interface for High-Density Electromyography Recording. Sensors 2023, 23, 1113. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Xue, J.; Huang, G.; Zheng, S.; Zhao, K.; Huang, J.; Wang, Y.; Zhang, Y.; Yin, T.; et al. Body Temperature Enhanced Adhesive, Antibacterial, and Recyclable Ionic Hydrogel for Epidermal Electrophysiological Monitoring. Adv. Healthc. Mater. 2022, 11, 2200653. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, Y.; Chen, B.; Chen, X.; Han, Y.; Guo, M.; Xia, H.-Q.; Song, R.; Zhang, X.; Zhou, J. In Situ Forming Epidermal Bioelectronics for Daily Monitoring and Comprehensive Exercise. ACS Nano 2022, 16, 17931–17947. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Chi, X.; Feng, Z.; Yang, C.; Gao, R.; Li, S.; Zhang, C.; Chen, X.; Huang, P.; et al. Biomimetic integration of tough polymer elastomer with conductive hydrogel for highly stretchable, flexible electronic. Nano Energy 2022, 92, 106735. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Deng, F.; Chen, Y.; Zhang, H. Eye-Movement-Controlled Wheelchair Based on Flexible Hydrogel Biosensor and WT-SVM. Biosensors 2021, 11, 198. [Google Scholar] [CrossRef]
- Hsieh, J.; Li, Y.; Wang, H.; Perz, M.; Tang, Q.; Tang, K.W.K.; Pyatnitskiy, I.; Reyes, R.; Ding, H.; Wang, H. Design of hydrogel-based wearable EEG electrodes for medical applications. J. Mater. Chem. B 2022, 10, 7260–7280. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, C.; Guo, T.; Tian, Y.; Song, W.; Lei, J.; Li, Q.; Wang, A.; Zhang, M.; Bai, S.; et al. Hydrogel Nanoarchitectonics of a Flexible and Self-Adhesive Electrode for Long-Term Wireless Electroencephalogram Recording and High-Accuracy Sustained Attention Evaluation. Adv. Mater. 2023, 35, 202209606. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, H.; Jing, X.; Liu, Y.; Mi, H.-Y. Recent advancements in self-healing composite elastomers for flexible strain sensors: Materials, healing systems, and features. Sens. Actuators A Phys. 2021, 329, 112800. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Liu, Y.; Wang, Z.; Yan, C.; Song, C.; Gao, C.; Wu, Y. A NIR laser induced self-healing PDMS/Gold nanoparticles conductive elastomer for wearable sensor. J. Colloid Interface Sci. 2021, 599, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, H.; Yi, S.; Zou, K.-K.; Zhang, D.; Zhong, G.-J.; Yan, D.-X.; Li, Z.-M. Flexible Polydimethylsiloxane Composite with Multi-Scale Conductive Network for Ultra-Strong Electromagnetic Interference Protection. Nano-Micro Lett. 2022, 15, 15. [Google Scholar] [CrossRef]
- Huang, J.; Cai, Y.; Xue, C.; Ge, J.; Zhao, H.; Yu, S.-H. Highly stretchable, soft and sticky PDMS elastomer by solvothermal polymerization process. Nano Res. 2021, 14, 3636–3642. [Google Scholar] [CrossRef]
- Liu, W.; Liu, D.; Xiao, Y.; Zou, M.; Shi, L.-Y.; Yang, K.-K.; Wang, Y.-Z. Healable, Recyclable, and High-Stretchable Polydimethylsiloxane Elastomer Based on Synergistic Effects of Multiple Supramolecular Interactions. Macromol. Mater. Eng. 2022, 307, 2200310. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, H.; Lu, Y.; Wang, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Fu, Q.; Han, J. Inherently Conductive Poly(dimethylsiloxane) Elastomers Synergistically Mediated by Nanocellulose/Carbon Nanotube Nanohybrids toward Highly Sensitive, Stretchable, and Durable Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 59142–59153. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, K.; Liu, Y.; Zhao, H.; Gao, C.; Wu, Y. Modified MXene-doped conductive organosilicon elastomer with high-stretchable, toughness, and self-healable for strain sensors. Compos. Struct. 2022, 282, 115071. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Michel, S.; Vanderborght, B.; Clemens, F. Piezoresistive sensor fiber composites based on silicone elastomers for the monitoring of the position of a robot arm. Sens. Actuators A Phys. 2021, 318, 112433. [Google Scholar] [CrossRef]
- Cho, J.; Shin, G. Fabrication of a Flexible, Wireless Micro-Heater on Elastomer for Wearable Gas Sensor Applications. Polymers 2022, 14, 1557. [Google Scholar] [CrossRef] [PubMed]
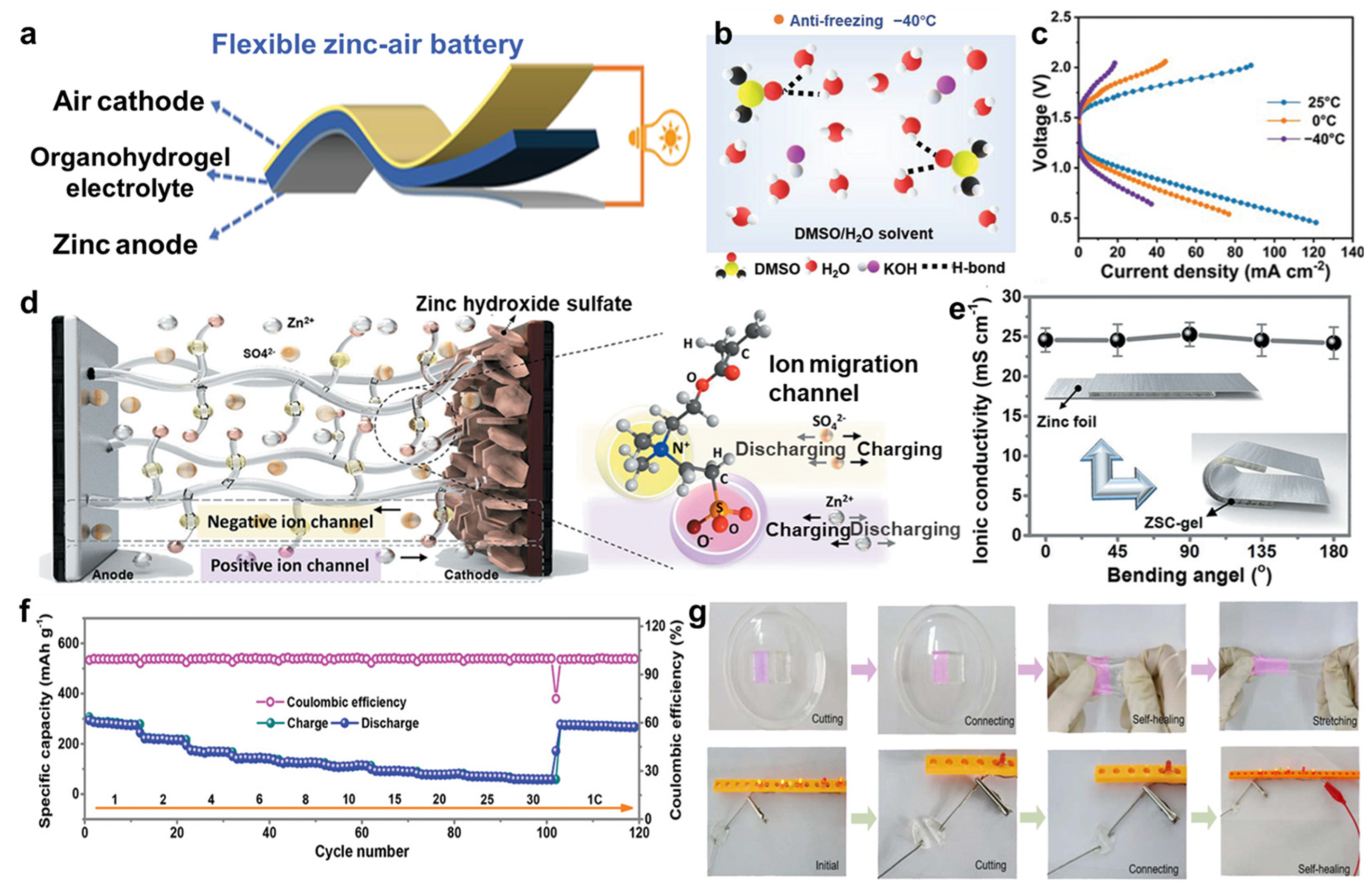
- Jiang, D.; Wang, H.; Wu, S.; Sun, X.; Li, J. Flexible Zinc-Air Battery with High Energy Efficiency and Freezing Tolerance Enabled by DMSO-Based Organohydrogel Electrolyte. Small Methods 2022, 6, 2101043. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Gao, A.; Ling, J.; Yi, F.; Hao, J.; Li, Q.; Shu, D. Fundamental study on Zn corrosion and dendrite growth in gel electrolyte towards advanced wearable Zn-ion battery. Chem. Eng. J. 2022, 446, 137021. [Google Scholar] [CrossRef]
- Mo, F.; Chen, Z.; Liang, G.; Wang, D.; Zhao, Y.; Li, H.; Dong, B.; Zhi, C. Zwitterionic Sulfobetaine Hydrogel Electrolyte Building Separated Positive/Negative Ion Migration Channels for Aqueous Zn-MnO2 Batteries with Superior Rate Capabilities. Adv. Energy Mater. 2020, 10, 2000035. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, A.; Hao, J.; Li, X.; Ling, J.; Yi, F.; Li, Q.; Shu, D. Soaking-free and self-healing hydrogel for wearable zinc-ion batteries. Chem. Eng. J. 2023, 452, 139605. [Google Scholar] [CrossRef]
- Alex, A.S.; Ananda, L.M.S.; Sekkar, V.; John, B.; Gouri, C.; Ilangovan, S.A. Microporous carbon aerogel prepared through ambient pressure drying route as anode material for lithium ion cells. Polym. Adv. Technol. 2017, 28, 1945–1950. [Google Scholar] [CrossRef]
- Yu, W.; Liu, L.; Yang, Y.; Li, N.; Chen, Y.; Yin, X.; Niu, J.; Wang, J.; Ding, S. N, O-diatomic dopants activate catalytic activity of 3D self-standing graphene carbon aerogel for long-cycle and high-efficiency Li-CO2 batteries. Chem. Eng. J. 2023, 465, 142787. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Li, Y.; Wang, J.; Kawi, S.; Zhong, Q. FeCo alloy/N, S co-doped carbon aerogel derived from directional-casting cellulose nanofibers for rechargeable liquid flow and flexible Zn-air batteries. Nano Res. 2023, 16, 6870–6880. [Google Scholar] [CrossRef]
- Yu, J.; Xia, J.; Guan, X.; Xiong, G.; Zhou, H.; Yin, S.; Chen, L.; Yang, Y.; Zhang, S.; Xing, Y.; et al. Self-healing liquid metal confined in carbon nanofibers/carbon nanotubes paper as a free-standing anode for flexible lithium-ion batteries. Electrochim. Acta 2022, 425, 140721. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Li, L.; Chen, R. Encapsulation of Metallic Zn in a Hybrid MXene/Graphene Aerogel as a Stable Zn Anode for Foldable Zn-Ion Batteries. Adv. Mater. 2022, 34, 2106897. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Zhang, Y.; Ni, Y. Recent progress on development of electrolyte and aerogel electrodes applied in supercapacitors. J. Power Sources 2023, 560, 232698. [Google Scholar] [CrossRef]
- Shaikh, J.S.; Shaikh, N.S.; Mishra, Y.K.; Pawar, S.S.; Parveen, N.; Shewale, P.M.; Sabale, S.; Kanjanaboos, P.; Praserthdam, S.; Lokhande, C.D. The implementation of graphene-based aerogel in the field of supercapacitor. Nanotechnology 2021, 32, 362001. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Z.; Gong, Q.; Liu, X.; Yang, Y.; Chen, C.; Qian, C. Green Synthesis of Free Standing Cellulose/Graphene Oxide/Polyaniline Aerogel Electrode for High-Performance Flexible All-Solid-State Supercapacitors. Nanomaterials 2020, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.J.; Lim, M.B.; Zhou, X.; Pauzauskie, P.J. Rapid synthesis of transition metal dichalcogenide–carbon aerogel composites for supercapacitor electrodes. Microsyst. Nanoeng. 2017, 3, 17032. [Google Scholar] [CrossRef]
- Wu, S.; Lou, D.; Wang, H.; Jiang, D.; Fang, X.; Meng, J.; Sun, X.; Li, J. One-pot synthesis of anti-freezing carrageenan/polyacrylamide double-network hydrogel electrolyte for low-temperature flexible supercapacitors. Chem. Eng. J. 2022, 435, 135057. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, T.; Li, Y.; Yang, L.; Ahmad, A.; Jiang, T.; Shu, Y.; Jing, Z.; Luo, H.; Lu, X.; et al. Fabrication of PANI@Ti3C2Tx/PVA hydrogel composite as flexible supercapacitor electrode with good electrochemical performance. Ceram. Int. 2022, 48, 15721–15728. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, J.; Yang, C.; Zang, L.; Zhang, G.; Sakai, E. High-Performance PVA/PEDOT:PSS Hydrogel Electrode for All-Gel-State Flexible Supercapacitors. Adv. Mater. Technol. 2021, 6, 2000919. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Yang, Y.; Qian, C.; Chen, C.; Han, L.; Han, Q. A stretchable and self-healable conductive hydrogels based on gelation/polyacrylamide/polypyrrole for all-in-one flexible supercapacitors with high capacitance. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 636, 128145. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Zhu, X.; Ji, C.; Meng, Q.; Mi, H.; Yang, Q.; Li, Z.; Yang, N.; Qiu, J. Freeze-Tolerant Hydrogel Electrolyte with High Strength for Stable Operation of Flexible Zinc-Ion Hybrid Supercapacitors. Small 2022, 18, 2200055. [Google Scholar] [CrossRef]
- Yang, K.; Hu, L.; Wang, Y.; Xia, J.; Sun, M.; Zhang, Y.; Gou, C.; Jia, C. Redox-active sodium 3,4-dihydroxy anthraquinone-2-sulfonate anchored on reduced graphene oxide for high-performance Zn-ion hybrid capacitors. J. Mater. Chem. A 2022, 10, 12532–12543. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Lin, X.; Lu, J.; Huang, Z.; Sun, P.; Zhang, Y.; Xu, X.; Li, Q.; Liu, H. Dual-network polyvinyl alcohol/polyacrylamide/xanthan gum ionic conductive hydrogels for flexible electronic devices. Int. J. Biol. Macromol. 2023, 233, 123573. [Google Scholar] [CrossRef]
- Wu, D.; Ji, C.; Mi, H.; Guo, F.; Cui, H.; Qiu, P.; Yang, N. A safe and robust dual-network hydrogel electrolyte coupled with multi-heteroatom doped carbon nanosheets for flexible quasi-solid-state zinc ion hybrid supercapacitors. Nanoscale 2021, 13, 15869–15881. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Jiang, D.; Wu, S.; Yi, W.; Sun, X.; Li, J. Organohydrogel electrolyte-based flexible zinc-ion hybrid supercapacitors with dendrite-free anode, broad temperature adaptability and ultralong cycling life. J. Power Sources 2022, 528, 231210. [Google Scholar] [CrossRef]
- Han, L.; Huang, H.; Fu, X.; Li, J.; Yang, Z.; Liu, X.; Pan, L.; Xu, M. A flexible, high-voltage and safe zwitterionic natural polymer hydrogel electrolyte for high-energy-density zinc-ion hybrid supercapacitor. Chem. Eng. J. 2020, 392, 123733. [Google Scholar] [CrossRef]
- El Idrissi, N.; Belachemi, L.; Merle, N.; Zinck, P.; Kaddami, H. Comprehensive preparation and catalytic activities of Co/TEMPO-cellulose nanocomposites: A promising green catalyst. Carbohydr. Polym. 2022, 295, 119765. [Google Scholar] [CrossRef]
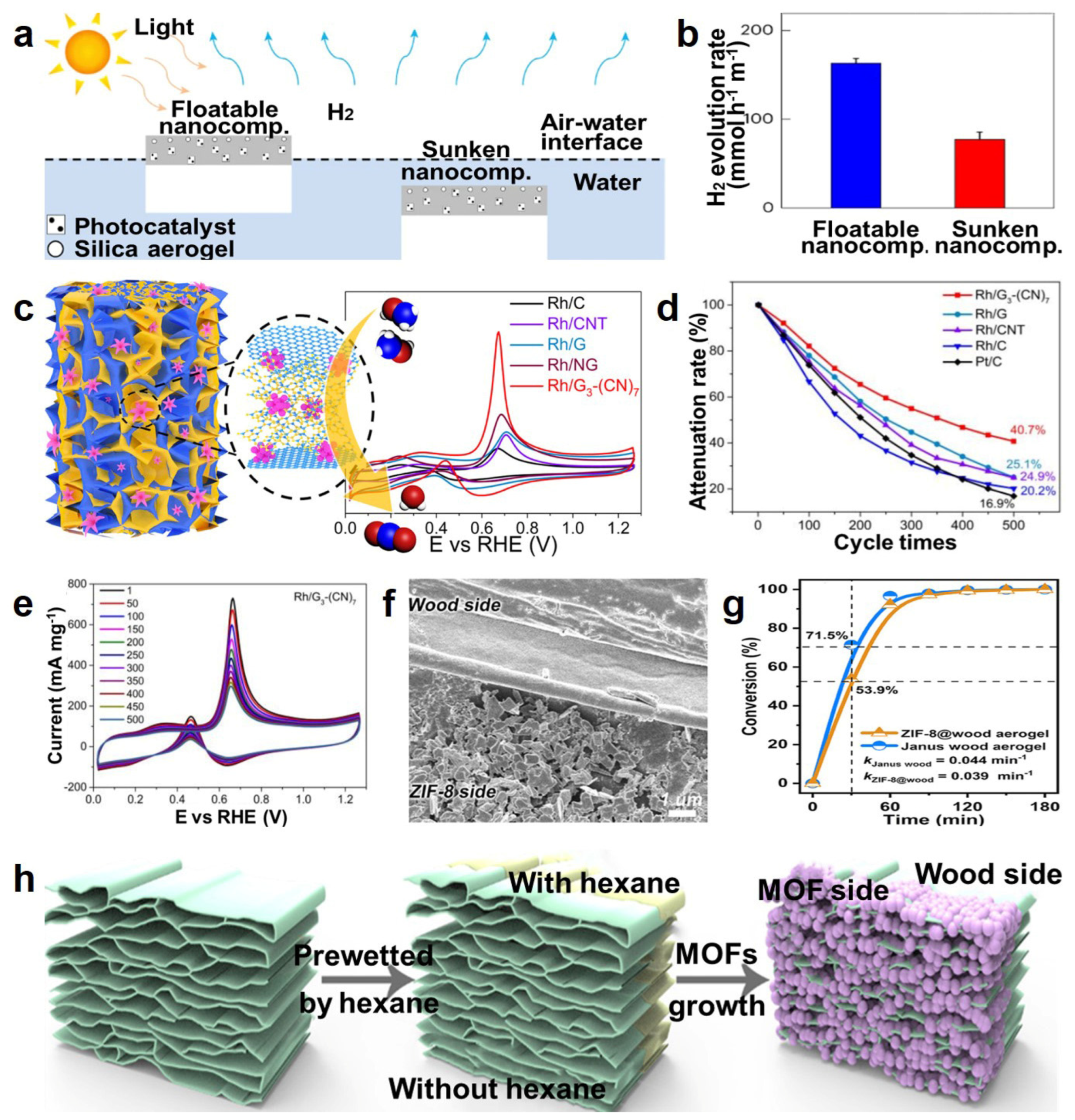
- Lee, W.H.; Lee, C.W.; Cha, G.D.; Lee, B.-H.; Jeong, J.H.; Park, H.; Heo, J.; Bootharaju, M.S.; Sunwoo, S.-H.; Kim, J.H.; et al. Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production. Nat. Nanotechnol. 2023, 18, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, J.; Shi, F.; Song, X.; Zhang, H.; Zhang, H.; Ma, C.; Zhu, K.; Liu, J. Construction of a novel highly porous BiOBr/CsxWO3@SiO2 composite aerogel: Adsorption/self-heating photocatalytic synergistic degradation of antibiotics and mechanism study. J. Environ. Chem. Eng. 2022, 10, 107785. [Google Scholar] [CrossRef]
- Du, X.; Cai, W.; Zhang, Q.; Yang, L.; He, H. Rhodium Nanoflowers on Three-Dimensional Graphitic Carbon Nitride Nanosheets/Graphene Hybrid Aerogels as Electrocatalysts for Methanol Oxidation. ACS Appl. Nano Mater. 2021, 4, 9729–9737. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, C.; Xie, Y.; Huang, S.; Liu, C.; Wu, J.; Xu, Z.-K. Janus Metal–Organic Frameworks/Wood Aerogel Composites for Boosting Catalytic Performance by Le Châtelier’s Principle. ACS Appl. Mater. Interfaces 2021, 13, 51039–51047. [Google Scholar] [CrossRef]
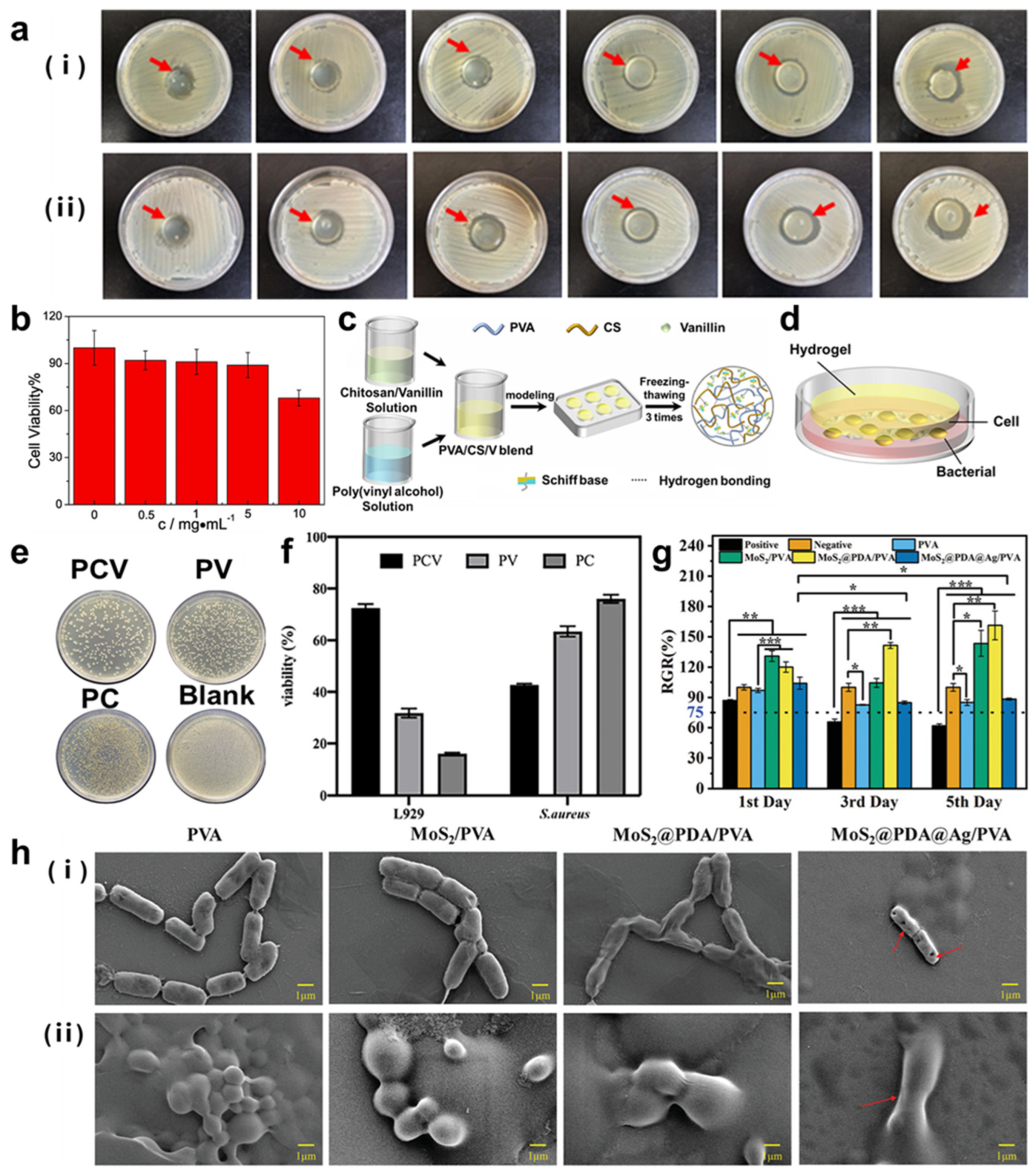
- Li, Z.; Xu, W.; Wang, X.; Jiang, W.; Ma, X.; Wang, F.; Zhang, C.; Ren, C. Fabrication of PVA/PAAm IPN hydrogel with high adhesion and enhanced mechanical properties for body sensors and antibacterial activity. Eur. Polym. J. 2021, 146, 110253. [Google Scholar] [CrossRef]
- Xiong, S.; Li, R.; Ye, S.; Ni, P.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.; Zhang, X. Vanillin enhances the antibacterial and antioxidant properties of polyvinyl alcohol-chitosan hydrogel dressings. Int. J. Biol. Macromol. 2022, 220, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Li, M.; Liu, J.; Hu, Y.; Tang, K. MoS2@PDA@Ag/PVA Hybrid Hydrogel with Excellent Light-Responsive Antibacterial Activity and Enhanced Mechanical Properties for Wound Dressing. Macromol. Mater. Eng. 2022, 307, 2100654. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A rose bengal/graphene oxide/PVA hybrid hydrogel with enhanced mechanical properties and light-triggered antibacterial activity for wound treatment. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111447. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Zhang, Y.; Cao, Q.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. Novel lignin-chitosan-PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104, 110002. [Google Scholar] [CrossRef]
- Olaret, E.; Voicu, S.I.; Oprea, R.; Miculescu, F.; Butac, L.; Stancu, I.-C.; Serafim, A. Nanostructured Polyacrylamide Hydrogels with Improved Mechanical Properties and Antimicrobial Behavior. Polymers 2022, 14, 2320. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. A synergistic antibacterial effect between terbium ions and reduced graphene oxide in a poly( vinyl alcohol)-alginate hydrogel for treating infected chronic wounds. J. Mater. Chem. B 2019, 7, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; An, X.; Fan, Z. Aramid nanofibers reinforced polyvinyl alcohol/tannic acid hydrogel with improved mechanical and antibacterial properties for potential application as wound dressing. J. Mech. Behav. Biomed. Mater. 2021, 118, 104452. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhang, Y.; Xin, H.; Yan, D.; Li, G.; Chen, R. Metal-phenolic network-based polydopamine@Cu within a polyvinyl alcohol hydrogel film for improved infected wound healing through antibacterial and pro-angiogenesis activity. Mater. Des. 2022, 221, 110904. [Google Scholar] [CrossRef]
- Morgado, A.; Soares, A.; Flores-Colen, I.; Veiga, M.D.; Gomes, M.G. Durability of Thermal Renders with Lightweight and Thermal Insulating Aggregates: Regranulated Expanded Cork, Silica Aerogel and Expanded Polystyrene. Gels 2021, 7, 35. [Google Scholar] [CrossRef]
- Peng, T.; Zhu, J.; Huang, T.; Jiang, C.; Zhao, F.; Ge, S.; Xie, L. Facile preparation for gelatin/hydroxyethyl cellulose-SiO2 composite aerogel with good mechanical strength, heat insulation, and water resistance. J. Appl. Polym. Sci. 2021, 138, 50539. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Luo, T.; Zhao, M.; Zhang, L.; Zhao, Z.; Yu, T.; Yan, Y. Hydrogels and Aerogels for Versatile Photo-/Electro-Chemical and Energy-Related Applications. Molecules 2024, 29, 3883. https://doi.org/10.3390/molecules29163883
Sun J, Luo T, Zhao M, Zhang L, Zhao Z, Yu T, Yan Y. Hydrogels and Aerogels for Versatile Photo-/Electro-Chemical and Energy-Related Applications. Molecules. 2024; 29(16):3883. https://doi.org/10.3390/molecules29163883
Chicago/Turabian StyleSun, Jiana, Taigang Luo, Mengmeng Zhao, Lin Zhang, Zhengping Zhao, Tao Yu, and Yibo Yan. 2024. "Hydrogels and Aerogels for Versatile Photo-/Electro-Chemical and Energy-Related Applications" Molecules 29, no. 16: 3883. https://doi.org/10.3390/molecules29163883
APA StyleSun, J., Luo, T., Zhao, M., Zhang, L., Zhao, Z., Yu, T., & Yan, Y. (2024). Hydrogels and Aerogels for Versatile Photo-/Electro-Chemical and Energy-Related Applications. Molecules, 29(16), 3883. https://doi.org/10.3390/molecules29163883






