Potential Pharmacological Properties of Triterpene Derivatives of Ursolic Acid
Abstract
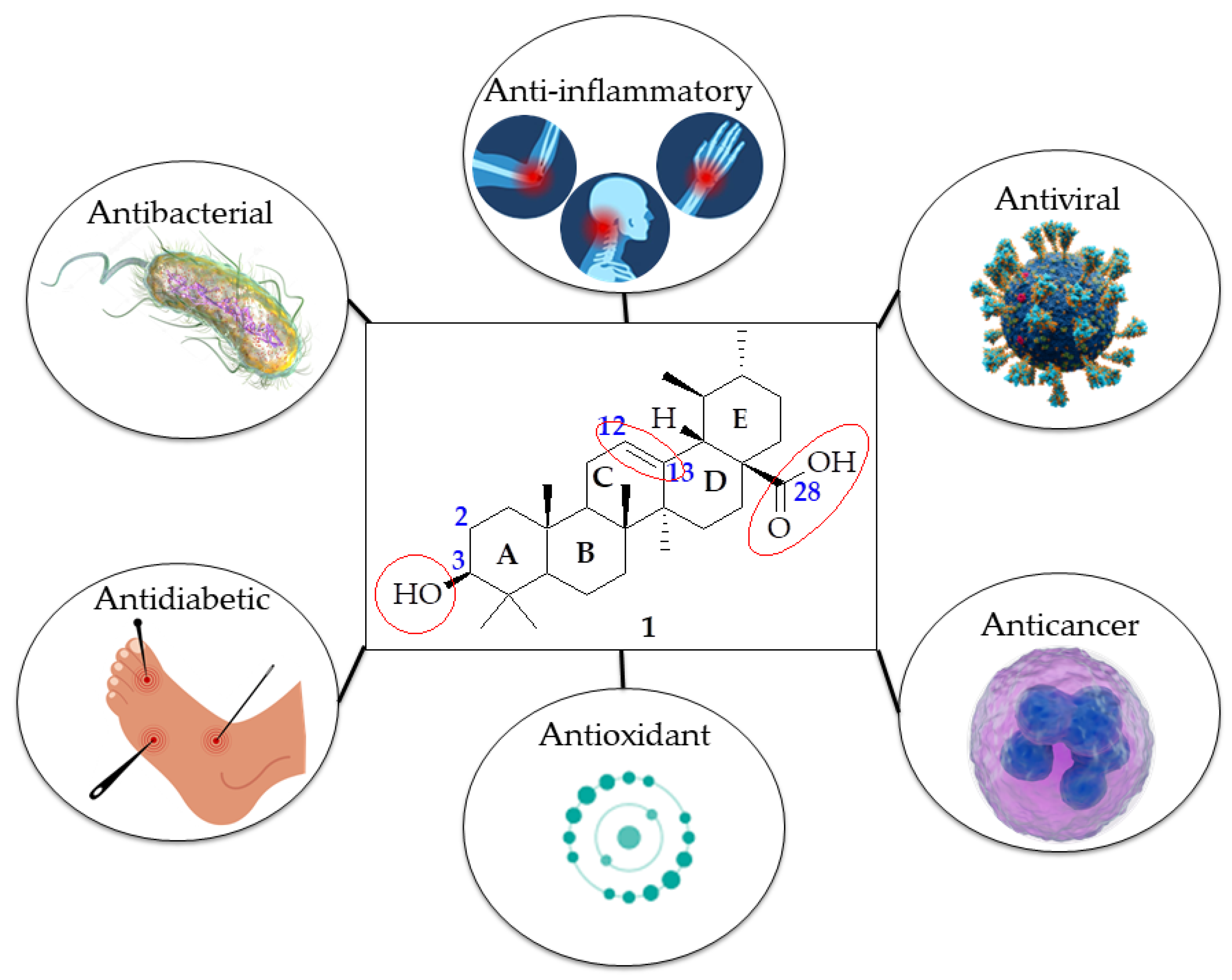
:1. Introduction
2. UA Derivatization
3. Pharmacokinetic Studies
4. Biological Activities
4.1. Anti-Inflammatory Activity
4.2. Anticancer Activity
4.3. Antimicrobial
4.3.1. Antibacterial Activity
4.3.2. Antiviral Activity
Human Immunodeficiency Virus (HIV)
Influenza Virus
4.3.3. Antioxidant Properties
4.3.4. Antidiabetic Activity
4.3.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.; Shen, Q.K.; Guo, H.Y.; Quan, Z.S.; Li, X. Research, Development and Pharmacological Activity of Fusidic Acid and Its Derivatives. J. Mol. Struct. 2023, 1291, 135942. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on Natural Products for Drug Design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Eder, J.; Sedrani, R.; Wiesmann, C. The Discovery of First-in-Class Drugs: Origins and Evolution. Nat. Rev. Drug Discov. 2014, 13, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Di Fabio, G.; Romanucci, V.; De Marco, A.; Zarrelli, A. Triterpenoids from Gymnema sylvestre and Their Pharmacological Activities. Molecules 2014, 19, 10956–10981. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic Triterpenes: New Tools to Fight Metabolic Syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and Its Derivatives as Novel Compounds with Different Pharmacological Effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Luo, J.; Hu, Y.L.; Wang, H. Ursolic Acid Inhibits Breast Cancer Growth by Inhibiting Proliferation, Inducing Autophagy and Apoptosis, and Suppressing Inflammatory Responses via the PI3K/AKT and NF-κB Signaling Pathways In Vitro. Exp. Ther. Med. 2017, 14, 3623–3631. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Alam, P.; Al-Yousef, H.M.; Siddiqui, N.A.; Alhowiriny, T.A.; Alqasoumi, S.I.; Amina, M.; Hassan, W.H.B.; Abdelaziz, S.; Abdalla, R.H. Anticancer Activity and Concurrent Analysis of Ursolic Acid, β-Sitosterol, and Lupeol in Three Different Hibiscus Species (Aerial Parts) by Validated HPTLC Method. Saudi Pharm. J. 2018, 26, 1060–1067. [Google Scholar] [CrossRef]
- Chan, W.E.C.; Soon, Y.C.; Tan, J.B.L.; Yong, S.K.; Hui, Y.W. Ursolic Acid: An Overview on Its Cytotoxic Activities against Breast and Colorectal Cancer Cells. J. Integr. Med. 2019, 17, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Tohmé, M.J.; Giménez, M.C.; Peralta, A.; Colombo, M.I.; Delgui, L.R. Ursolic Acid: A Novel Antiviral Compound Inhibiting Rotavirus Infection In Vitro. Int. J. Antimicrob. Agents 2019, 54, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; et al. Oleanolic Acid and Ursolic Acid: Novel Hepatitis C Virus Antivirals that Inhibit NS5B Activity. Antiviral Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.B.; Giniyatullina, G.V.; Yamansarov, E.Y.; Tolstikov, G.A. Betulin and Ursolic Acid Synthetic Derivatives as Inhibitors of Papilloma Virus. Bioorg. Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, H.; Sui, Z.; Jing, F.; Quan, X.; Zhao, W.; Liu, G. Ursolic Acid Exhibits Anti-Inflammatory Effects through Blocking TLR4-MyD88 Pathway Mediated by Autophagy. Cytokine 2019, 123, 154726. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.K.; Jeong, N.H.; Yoo, J.; Ha, Y.S.; Lee, B.; Choi, H.; Park, P.H.; Shin, T.Y.; Kwon, T.K.; et al. Anti-Inflammatory Effects of Ursolic Acid-3-Acetate on Human Synovial Fibroblasts and a Murine Model of Rheumatoid Arthritis. Int. Immunopharmacol. 2017, 49, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Zahra, W.; Singh, S.S.; Birla, H.; Keswani, C.; Dilnashin, H.; Rathore, A.S.; Singh, R.; Singh, R.K.; Singh, S.P. Anti-Inflammatory Activity of Ursolic Acid in MPTP-Induced Parkinsonian Mouse Model. Neurotox. Res. 2019, 36, 452–462. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant and Anti-Inflammatory Mechanisms of Neuroprotection by Ursolic Acid: Addressing Brain Injury, Cerebral Ischemia, Cognition Deficit, Anxiety, and Depression. Oxid. Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef]
- Yenigün, S.; Başar, Y.; İpek, Y.; Behçet, L.; Özen, T.; Demirtaş, İ. Determination of Antioxidant, DNA Protection, Enzyme Inhibition Potential and Molecular Docking Studies of a Biomarker Ursolic Acid in Nepeta Species. J. Biomol. Struct. Dyn. 2024, 42, 5799–5816. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, X.; Cao, W.; Ren, X.; Ren, G.; Liu, P.; Chen, J. Effects of Different Drying Methods on the Characterization, Dissolution Rate and Antioxidant Activity of Ursolic Acid-Loaded Chitosan Nanoparticles. Foods 2021, 10, 2470. [Google Scholar] [CrossRef]
- Mahlo, S.M.; McGaw, L.J.; Eloff, J.N. Antifungal Activity and Cytotoxicity of Isolated Compounds from Leaves of Breonadia salicina. J. Ethnopharmacol. 2013, 148, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Zhao, H.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Antifungal Efficacy of Ursolic Acid in Control of Alternaria alternata Causing Black Spot Rot on Apple Fruit and Possible Mechanisms Involved. Sci. Hortic. 2019, 256, 108636. [Google Scholar] [CrossRef]
- Zahari, R.; Halimoon, N.; Ahmad, M.F.; Ling, S.K. Antifungal Compound Isolated from Catharanthus roseus L. (Pink) for Biological Control of Root Rot Rubber Diseases. Int. J. Anal. Chem. 2018, 2018, 8150610. [Google Scholar] [CrossRef]
- Shaik, A.B.; Ahil, S.B.; Govardhanam, R.; Senthi, M.; Khan, R.; Sojitra, R.; Kumar, S.; Srinivas, A. Antifungal Effect and Protective Role of Ursolic Acid and Three Phenolic Derivatives in the Management of Sorghum Grain Mold Under Field Conditions. Chem. Biodivers. 2016, 13, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Innocente, A.; Casanova, B.B.; Klein, F.; Lana, A.D.; Pereira, D.; Muniz, M.N.; Sonnet, P.; Gosmann, G.; Fuentefria, A.M.; Gnoatto, S.C. Synthesis of Isosteric Triterpenoid Derivatives and Antifungal Activity. Chem. Biol. Drug Des. 2014, 83, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.Y.; Khalitova, R.R.; Nedopekina, D.A.; Gubaidullin, R.R. Antimicrobial Properties of Amine- and Guanidine-Functionalized Derivatives of Betulinic, Ursolic and Oleanolic Acids: Synthesis and Structure/Activity Evaluation. Steroids 2020, 154, 108530. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of Oxidative Stress on the Antibacterial Activity of Betulin, Betulinic Acid and Ursolic Acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.P.; Zhang, K.; Lu, Y.J.; He, P.; Zhao, S.Q. In Vitro and In Vivo Evaluation of the Antidiabetic Activity of Ursolic Acid Derivatives. Eur. J. Med. Chem. 2014, 80, 502–508. [Google Scholar] [CrossRef]
- Guzmán-Ávila, R.; Flores-Morales, V.; Paoli, P.; Camici, G.; Ramírez-Espinosa, J.J.; Cerón-Romero, L.; Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Yolanda Rios, M.; Villalobos-Molina, R.; et al. Ursolic Acid Derivatives as Potential Antidiabetic Agents: In Vitro, In Vivo, and In Silico Studies. Drug Dev. Res. 2018, 79, 70–80. [Google Scholar] [CrossRef]
- Alkreathy, H.M.; Ahmad, A. Catharanthus roseus Combined with Ursolic Acid Attenuates Streptozotocin-Induced Diabetes through Insulin Secretion and Glycogen Storage. Oxid. Med. Cell. Longev. 2020, 2020, 8565760. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Yan, Y.; Liu, D.; Wang, C.; Wang, H. Inhibition of Glycosidase by Ursolic Acid: In Vitro, In Vivo and In Silico Study. J. Sci. Food Agric. 2020, 100, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Uzaşçı, S.; Dirmenci, T.; Erim, F.B. α-Glucosidase Enzyme Inhibitory Effects and Ursolic and Oleanolic Acid Contents of Fourteen Anatolian Salvia Species. J. Pharm. Biomed. Anal. 2018, 155, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of Oleanolic and Ursolic Acid Derivatives Toward Hepatocellular Carcinoma and Evaluation of NF-κB Involvement. Bioorg. Chem. 2019, 90, 103054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Jin, L.L.; Liu, F.; Jin, C.; Jin, C.M.; Wei, Z.Y. Evaluation of Ursolic Acid Derivatives with Potential Anti-Toxoplasma gondii Activity. Exp. Parasitol. 2020, 216, 107935. [Google Scholar] [CrossRef] [PubMed]
- Frolova, T.S.; Lipeeva, A.V.; Baev, D.S.; Baiborodin, S.I.; Orishchenko, K.E.; Kochetov, A.V.; Sinitsyna, O.I. Fluorescent Labeling of Ursolic Acid with FITC for Investigation of Its Cytotoxic Activity Using Confocal Microscopy. Bioorg. Chem. 2019, 87, 876–887. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, R.Z.; Zhang, J.; Guo, T.; Zhang, M.T.; Huang, X.C.; Zhang, B.; Liao, Z.X.; Sun, J.; Wang, H.S. Discovery of Antitumor Ursolic Acid Long-Chain Diamine Derivatives as Potent Inhibitors of NF-κB. Bioorg. Chem. 2018, 79, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in Medicinal Chemistry of Ursolic Acid Derivatives as Anticancer Agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.H.; Zhang, L.H.; Jin, X.J.; Ma, J.; Piao, H.R. Design, Synthesis, and Screening of Novel Ursolic Acid Derivatives as Potential Anti-Cancer Agents That Target the HIF-1α Pathway. Bioorg. Med. Chem. Lett. 2019, 29, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Zhang, Z.H.; Li, M.Y.; Wei, Z.Y.; Jin, X.J.; Piao, H.R. Synthesis and Evaluation of the HIF-1α Inhibitory Activities of Novel Ursolic Acid Tetrazole Derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 1440–1445. [Google Scholar] [CrossRef]
- Wang, W.Y.; Wu, W.Y.; Li, A.L.; Liu, Q.S.; Sun, Y.; Gu, W. Synthesis, Anticancer Evaluation and Mechanism Studies of Novel Indolequinone Derivatives of Ursolic Acid. Bioorg. Chem. 2021, 109, 104705. [Google Scholar] [CrossRef]
- Bai, K.K.; Yu, Z.; Chen, F.L.; Li, F.; Li, W.Y.; Guo, Y.H. Synthesis and Evaluation of Ursolic Acid Derivatives as Potent Cytotoxic Agents. Bioorg. Med. Chem. Lett. 2012, 22, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, G.R.; Maurya, A. Drug Resistance Reversal Potential of Ursolic Acid Derivatives against Nalidixic Acid- and Multidrug-Resistant Escherichia coli. Chem. Biol. Drug Des. 2014, 83, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Y.; Chi, K.Q.; Wang, K.S.; Wu, J.; Liu, L.P.; Piao, H.R. Design, Synthesis, Evaluation, and Molecular Docking of Ursolic Acid Derivatives Containing a Nitrogen Heterocycle as Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2018, 28, 1797–1803. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, H.; Ren, Y.; Wang, Z.; Zeng, P.; Li, X.; Wang, J.; Zheng, X. Anti-Hepatocellular Carcinoma Activity and Mechanism of Chemopreventive Compounds: Ursolic Acid Derivatives. Pharm. Biol. 2016, 54, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.; Petrova, A.; Zileeva, Z.; Kuzmina, U.; Zainullina, L.; Vakhitova, Y.; Babkov, D.; Kazakova, O. Novel A-Ring Chalcone Derivatives of Oleanolic and Ursolic Amides with Anti-Proliferative Effect Mediated Through ROS-Triggered Apoptosis. Int. J. Mol. Sci. 2021, 22, 9796. [Google Scholar] [CrossRef] [PubMed]
- Sylla, B.; Lavoie, S.; Legault, J.; Gauthier, C.; Pichette, A. Synthesis, Cytotoxicity and Anti-Inflammatory Activity of Rhamnose-Containing Ursolic and Betulinic Acid Saponins. RSC Adv. 2019, 9, 39743–39757. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Song, K.; Zhang, Y.; Yang, M.; Fan, B.; Huang, H.; Chen, G. Biotransformation of Ursolic Acid by Circinella muscae and Their Anti-Neuroinflammatory Activities of Metabolites. Nat. Prod. Res. 2022, 36, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Antonio, E.; Junior, O.D.R.A.; Marcano, R.G.D.J.V.; Diedrich, C.; da Silva Santos, J.; Machado, C.S.; Khalil, N.M.; Mainardes, R.M. Chitosan Modified Poly (Lactic Acid) Nanoparticles Increased the Ursolic Acid Oral Bioavailability. Int. J. Biol. Macromol. 2021, 172, 133–142. [Google Scholar] [CrossRef]
- Shao, J.; Fang, Y.; Zhao, R.; Chen, F.; Yang, M.; Jiang, J.; Chen, Z.; Yuan, X.; Jia, L. Evolution from Small Molecule to Nano-Drug Delivery Systems: An Emerging Approach for Cancer Therapy of Ursolic Acid. Asian J. Pharm. Sci. 2020, 15, 685–700. [Google Scholar] [CrossRef]
- Khan, K.; Aqil, M.; Imam, S.S.; Ahad, A.; Moolakkadath, T.; Sultana, Y.; Mujeeb, M. Ursolic Acid Loaded Intra Nasal Nano Lipid Vesicles for Brain Tumour: Formulation, Optimization, In-Vivo Brain/Plasma Distribution Study and Histopathological Assessment. Biomed. Pharmacother. 2018, 106, 1578–1585. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic Acid from Apple Pomace and Traditional Plants: A Valuable Triterpenoid with Functional Properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef]
- Kashyap, D.; Singh, H.; Sharma, A.K. Ursolic Acid (UA): A Metabolite with Promising Therapeutic Potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, R.; Zhang, J. Repositioning of Antibiotic Levofloxacin as a Mitochondrial Biogenesis Inhibitor to Target Breast Cancer. Biochem. Biophys. Res. Commun. 2016, 471, 639–645. [Google Scholar] [CrossRef]
- Farwick, M.; Köhler, T.; Schild, J.; Mentel, M.; Maczkiewitz, U.; Pagani, V.; Bonfigli, A.; Rigano, L.; Bureik, D.; Gauglitz, G.G. Pentacyclic Triterpenes from Terminalia arjuna Show Multiple Benefits on Aged and Dry Skin. Skin Pharmacol. Physiol. 2014, 27, 71–81. [Google Scholar] [CrossRef] [PubMed]
- López-Hortas, L.; Pérez-Larrán, P.; González-Muñoz, M.J.; Falqué, E.; Domínguez, H. Recent Developments on the Extraction and Application of Ursolic Acid: A Review. Food Res. Int. 2018, 103, 130–149. [Google Scholar] [CrossRef]
- Ramos, A.A.; Pereira-Wilson, C.; Collins, A.R. Protective Effects of Ursolic Acid and Luteolin against Oxidative DNA Damage Include Enhancement of DNA Repair in Caco-2 Cells. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2010, 692, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated Through Suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Takada, K.; Nakane, T.; Masuda, K.; Ishii, H. Ursolic Acid and Oleanolic Acid, Members of Pentacyclic Triterpenoid Acids, Suppress TNF-α-Induced E-Selectin Expression by Cultured Umbilical Vein Endothelial Cells. Phytomedicine 2010, 17, 1114–1119. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Li, C.S.; Cao, L.T.; Bai, X.Q.; Zhao, D.H.; Sun, S.M. New Ursolic Acid Derivatives Bearing 1,2,3-Triazole Moieties: Design, Synthesis and Anti-Inflammatory Activity In Vitro and In Vivo. Mol. Divers. 2022, 26, 1129–1139. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Li, C.S.; Li, P.; Bai, X.Q.; Guo, S.Y.; Jin, Y.; Piao, S.J. Synthesis and Evaluation of Ursolic Acid-Based 1,2,4-Triazolo[1,5-a]Pyrimidines Derivatives as Anti-Inflammatory Agents. Mol. Divers. 2022, 26, 27–38. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Wei, T.Z.Z.; Guo, H.W.F.; Piao, C.Z.H. Synthesis and Biological Evaluation of Ursolic Acid Derivatives Containing an Aminoguanidine Moiety. Med. Chem. Res. 2019, 28, 959–973. [Google Scholar] [CrossRef]
- Qi, Z.; Xie, P.; Wang, Z.; Zhou, H.; Tao, R.; Popov, S.A.; Yang, G.; Shults, E.E.; Wang, C. Synthesis of Novel Ursolic Acid-Gallate Hybrids via 1,2,3-Triazole Linkage and Its Anti-Oxidant and Anti-Inflammatory Activity Study. Arab. J. Chem. 2024, 17, 105762. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Hameedi, S.; Mousa, A. Design, Synthesis and Biological Evaluation of Novel Benzodioxole Derivatives as COX Inhibitors and Cytotoxic Agents. BMC Chem. 2020, 14, 54. [Google Scholar] [CrossRef]
- Xie, Y.D.; Xu, Y.H.; Liu, J.P.; Wang, B.; Shi, Y.H.; Wang, W.; Wang, X.P.; Sun, M.; Xu, X.Y.; Bian, X.L. 1,3-Benzodioxole-Based Fibrate Derivatives as Potential Hypolipidemic and Hepatoprotective Agents. Bioorg. Med. Chem. Lett. 2021, 43, 127898. [Google Scholar] [CrossRef]
- Li, C.; Zhang, T.; Zhang, Q.; Liu, X.; Zou, J.; Bai, X. Screening of Ursolic Acid Analogs with HIF-1α and COX-2-Inhibiting Effects. Chem. Nat. Compd. 2022, 58, 882–887. [Google Scholar] [CrossRef]
- Zhang, R.X.; Li, Y.; Tian, D.D.; Liu, Y.; Nian, W.; Zou, X.; Chen, Q.Z.; Zhou, L.Y.; Deng, Z.L.; He, B.C. Ursolic Acid Inhibits Proliferation and Induces Apoptosis by Inactivating Wnt/β-Catenin Signaling in Human Osteosarcoma Cells. Int. J. Oncol. 2016, 49, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Dahmani, F.Z.; Zhang, J.; Xiong, H.; Wu, Y.; Yin, L.; Zhou, J.; Yao, J. 2 Anti-angiogenic activity and antitumor efficacy of amphiphilic twin drug from ursolic acid and low molecular weight heparin. Nanotechnology 2017, 28, 075102. [Google Scholar] [CrossRef]
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. Smart pH-Responsive Nano-Carrier as a Drug Delivery System for the Targeted Delivery of Ursolic Acid: Suppresses Cancer Growth and Metastasis by Modulating P53/MMP-9/PTEN/CD44 Mediated Multiple Signaling Pathways. Nanoscale 2017, 9, 9428–9439. [Google Scholar] [CrossRef]
- Gupta, A.P. Anticancer Curcumin: Natural Analogues and Structure-Activity Relationship, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54. [Google Scholar]
- Prasad, S.; Yadav, V.R.; Sung, B.; Reuter, S.; Kannappan, R.; Deorukhkar, A.; Diagaradjane, P.; Wei, C.; Baladandayuthapani, V.; Krishnan, S.; et al. Ursolic Acid Inhibits Growth and Metastasis of Human Colorectal Cancer in an Orthotopic Nude Mouse Model by Targeting Multiple Cell Signaling Pathways: Chemosensitization with Capecitabine. Clin. Cancer Res. 2012, 18, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Nie, M.; Tian, Y.; Chen, X.; Chen, C.; Chen, H.; Liu, R. Ursolic Acid Derivative FZU-03,010 Inhibits STAT3 and Induces Cell Cycle Arrest and Apoptosis in Renal and Breast Cancer Cells. Acta Biochim. Biophys. Sin. 2017, 49, 367–373. [Google Scholar] [CrossRef]
- Wang, W.J.; Sui, H.; Qi, C.; Li, Q.; Zhang, J.; Wu, S.F.; Mei, M.Z.; Lu, Y.Y.; Wan, Y.T.; Chang, H.; et al. Ursolic Acid Inhibits Proliferation and Reverses Drug Resistance of Ovarian Cancer Stem Cells by Downregulating ABCG2 through Suppressing the Expression of Hypoxia-Inducible Factor-1α In Vitro. Oncol. Rep. 2016, 36, 428–440. [Google Scholar] [CrossRef]
- Zong, L.; Cheng, G.; Liu, S.; Pi, Z.; Liu, Z.; Song, F. Reversal of Multidrug Resistance in Breast Cancer Cells by a Combination of Ursolic Acid with Doxorubicin. J. Pharm. Biomed. Anal. 2019, 165, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Ubosi, N.I.; Obeagu, G.U.; Egba, S.I.; Bluth, M.H. Understanding Apoptosis in Sickle Cell Anemia Patients: Mechanisms and Implications. Medicine 2024, 103, E36898. [Google Scholar] [CrossRef]
- Moghadam, A.R.; da Silva Rosa, S.C.; Samiei, E.; Alizadeh, J.; Field, J.; Kawalec, P.; Thliveris, J.; Akbari, M.; Ghavami, S.; Gordon, J.W. Autophagy Modulates Temozolomide-Induced Cell Death in Alveolar Rhabdomyosarcoma Cells. Cell Death Discov. 2018, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, J.; Zeki, A.A.; Mirzaei, N.; Tewary, S.; Rezaei Moghadam, A.; Glogowska, A.; Nagakannan, P.; Eftekharpour, E.; Wiechec, E.; Gordon, J.W.; et al. Mevalonate Cascade Inhibition by Simvastatin Induces the Intrinsic Apoptosis Pathway via Depletion of Isoprenoids in Tumor Cells. Nat. Publ. Gr. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Chuang, W.; Lin, P.; Lin, H.; Chen, Y. The Apoptotic Effect of Ursolic Acid on SK-Hep-1 Cells is Regulated by the PI3K/Akt, p38, and JNK MAPK Signaling Pathways. Molecules 2016, 21, 460. [Google Scholar] [CrossRef]
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic Acid: A Systematic Review of its Pharmacology, Toxicity and Rethink on its Pharmacokinetics Based on PK-PD Model. Fitoterapia 2020, 147, 104735. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Tan, Z.J.; Hu, Y.P.; Shu, Y.J.; Bao, R.F.; Jiang, L.; Wu, X.S.; Li, M.L.; Ding, Q.; Wang, X.A.; et al. Ursolic Acid Induces Cell Cycle Arrest and Apoptosis of Gallbladder Carcinoma Cells. Cancer Cell Int. 2014, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Geng, L.; Yang, F.; Dong, X.; He, D.; Zhang, Y. Ursolic Acid Derivative Induces Apoptosis in Glioma Cells through Down-Regulation of cAMP. Eur. J. Med. Chem. 2019, 176, 61–67. [Google Scholar] [CrossRef]
- Mendes, V.I.S.; Bartholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A.R. Synthesis and Cytotoxic Activity of Novel A-Ring Cleaved Ursolic Acid Derivatives in Human Non-Small Cell Lung Cancer Cells. Eur. J. Med. Chem. 2016, 123, 317–331. [Google Scholar] [CrossRef]
- Nedopekina, D.A. Mitochondria-Targeted Betulinic and Ursolic Acid Derivatives: Synthesis and Anticancer Activity. MedChemComm 2017, 8, 1934–1945. [Google Scholar] [CrossRef]
- Liu, M.; Yang, S.; Hu, L.J.D.; Xue, W.; Eb, A.O. Synthesis and Evaluation as Potential Antitumor Agents of Novel Ursolic Acid Derivatives. Med. Chem. Res. 2016, 25, 2267–2279. [Google Scholar] [CrossRef]
- Gou, W.; Luo, N.; Wei, H.; Wu, H.; Yu, X.; Duan, Y.; Bi, C.; Ning, H.; Hou, W.; Li, Y. Ursolic Acid Derivative UA232 Evokes Apoptosis of Lung Cancer Cells Induced by Endoplasmic Reticulum Stress. Pharm. Biol. 2020, 58, 707–715. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, C.; Yu, T.; Li, W.; Li, Q. Synthesis and Antitumor Activity Evaluation of Ursolic Acid Derivatives. J. Asian Nat. Prod. Res. 2020, 22, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Chen, H.; Li, D.D.; Li, A.L.; Wang, W.Y.; Gu, W. Design, Synthesis, and Anticancer Evaluation of Novel Quinoline Derivatives of Ursolic Acid with Hydrazide, Oxadiazole, and Thiadiazole Moieties as Potent MEK Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 955–972. [Google Scholar] [CrossRef]
- Gu, W.; Jin, X.Y.; Li, D.D.; Wang, S.F.; Tao, X.B.; Chen, H. Design, Synthesis and In Vitro Anticancer Activity of Novel Quinoline and Oxadiazole Derivatives of Ursolic Acid. Bioorg. Med. Chem. Lett. 2017, 27, 4128–4132. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Zhang, L.F.; Liu, D.Y.; Liu, L.W.; Zhang, Y.; Zhao, M.J. Synthesis and Antitumor Activity Evaluation of Novel Ursolic Acid Derivatives. J. Asian Nat. Prod. Res. 2016, 18, 280–288. [Google Scholar] [CrossRef]
- Csuk, R.; Deigner, H.P. The Potential of Click Reactions for the Synthesis of Bioactive Triterpenes. Bioorg. Med. Chem. Lett. 2019, 29, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Frolova, T.S.; Shestopalov, M.A.; Wang, C.; Qi, Z.; Shults, E.E.; Turks, M. Synthesis and Cytotoxicity of Hybrids of 1,3,4- or 1,2,5-Oxadiazoles Tethered from Ursane and Lupane Core with 1,2,3-Triazole. Steroids 2020, 162, 108698. [Google Scholar] [CrossRef]
- Zhang, T.; He, B.; Yuan, H.; Feng, G.; Chen, F.; Wu, A.; Zhang, L.; Lin, H.; Zhuo, Z.; Wang, T. Synthesis and Antitumor Evaluation in Vitro of NO-Donating Ursolic Acid-Benzylidene Derivatives. Chem. Biodivers. 2019, 16, e1900111. [Google Scholar] [CrossRef]
- Wang, W.; Lei, L.; Liu, Z.; Wang, H.; Meng, Q. Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents. Molecules 2019, 24, 877. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.; Khalitova, R.; Nedopekina, D.; Dzhemileva, L.; Yunusbaeva, M.; Odinokov, V.; D’yakonov, V.; Dzhemilev, U. Synthesis and Evaluation of Anticancer Activities of Novel C-28 Guanidine-Functionalized Triterpene Acid Derivatives. Molecules 2018, 23, 3000. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Nie, M.; Wang, W.; Liu, Z.; Chen, C.; Chen, H.; Liu, R.; Baloch, Z.; Ma, K. A novel synthetic ursolic acid derivative inhibits growth and induces apoptosis in breast cancer cell lines. Oncol. Lett. 2018, 15, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the clinical pipeline of treatments for drug-resistant bacterial infections: Despite progress, more action is needed. Antimicrob. Agents Chemother. 2022, 66, e01991-21. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Rafailidis, P.I.; Konstantelias, A.A.; Falagas, M.E. Predictors of mortality in patients with infections due to multi-drug resistant Gram-negative bacteria: The study, the patient, the bug or the drug? J. Infect. 2013, 66, 401–414. [Google Scholar] [CrossRef]
- Chung, P.Y.; Navaratnam, P.; Chung, L.Y. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Máñez, S. New pharmacological opportunities for betulinic acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef]
- Chung, P.Y.; Chung, L.Y.; Navaratnam, P. Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus. Fitoterapia 2014, 94, 48–54. [Google Scholar] [CrossRef]
- e Silva, M.D.L.; David, J.P.; Silva, L.C.; Santos, R.A.; David, J.M.; Lima, L.S.; Reis, P.S.; Fontana, R. Bioactive oleanane, lupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef]
- Siewert, B.; Pianowski, E.; Csuk, R. Esters and amides of maslinic acid trigger apoptosis in human tumor cells and alter their mode of action with respect to the substitution pattern at C-28. Eur. J. Med. Chem. 2013, 70, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Chouaïb, K.; Hichri, F.; Nguir, A.; Daami-Remadi, M.; Elie, N.; Touboul, D.; Jannet, H.B. Semi-synthesis of new antimicrobial esters from the natural oleanolic and maslinic acids. Food Chem. 2015, 183, 8–17. [Google Scholar] [CrossRef]
- Swidorski, J.J.; Liu, Z.; Sit, S.Y.; Chen, J.; Chen, Y.; Sin, N.; Venables, B.L.; Parker, D.D.; Nowicka-Sans, B.; Terry, B.J.; et al. Inhibitors of HIV-1 maturation: Development of structure-activity relationship for C-28 amides based on C-3 benzoic acid-modified triterpenoids. Bioorg. Med. Chem. Lett. 2016, 26, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, R.-H.; Wang, M.; Xu, G.-B.; Liao, S.-G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017, 131, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, D.; Tichaczek-Goska, D.; Kicia, M. Pentacyclic triterpenes combined with ciprofloxacin help to eradicate the biofilm formed in vitro by Escherichia coli. Indian J. Med. Res. Suppl. 2015, 141, 343–353. [Google Scholar] [CrossRef]
- Liu, G.; Qin, P.; Cheng, X.; Wu, L.; Zhao, W.; Gao, W. Evaluation of the mechanistic basis for the antibacterial activity of ursolic acid against Staphylococcus aureus. Front. Microbiol. 2024, 15, 1389242. [Google Scholar] [CrossRef] [PubMed]
- Cunha, W.R.; de Matos, G.X.; Souza, M.G.M.; Tozatti, M.G.; Andrade e Silva, M.L.; Martins, C.H.; Silva, R.D.; Da Silva Filho, A.A. Evaluation of the antibacterial activity of the methylene chloride extract of Miconia ligustroides, isolated triterpene acids, and ursolic acid derivatives. Pharm. Biol. 2010, 48, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.M.; Arriaga, Â.M.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zan, K.; Wu, J.Y.; Gao, W.; Yang, J.; Ba, Y.Y.; Wu, X.; Chen, X.Q. Antibacterial triterpenoids from the leaves of Ilex hainanensis Merr. Nat. Prod. Res. 2019, 33, 2435–2439. [Google Scholar] [CrossRef]
- Wang, C.M.; Jhan, Y.L.; Tsai, S.J.; Chou, C.H. The pleiotropic antibacterial mechanisms of ursolic acid against methicillin-resistant Staphylococcus aureus (MRSA). Molecules 2016, 21, 884. [Google Scholar] [CrossRef]
- Pandey, P.; Garg, A.; Singh, V.; Shukla, A. Evaluation of anthelmintic and antimicrobial activity of ursolic acid obtained from Tulsi (Ocimum sanctum). Asian J. Pharm. Pharmacol. 2016, 2, 67–71. [Google Scholar]
- Park, S.N.; Lim, Y.K.; Choi, M.H.; Cho, E.; Bang, I.S.; Kim, J.M.; Ahn, S.J.; Kook, J.K. Antimicrobial mechanism of oleanolic and ursolic acids on Streptococcus mutans UA159. Curr. Microbiol. 2018, 75, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wang, W.; Zhang, J.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and antibiofilm activities of ursolic acid against carbapenem-resistant Klebsiella pneumoniae. J. Antibiot. 2020, 73, 382–391. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.N.S.; Primon-Barros, M.; Macedo, A.J.; Gnoatto, S.C.B. Triterpene Derivatives as Relevant Scaffold for New Antibiofilm Drugs. Biomolecules 2019, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.A.; Wang, C.; Qi, Z.; Shul’ts, E.E.; Turks, M. Synthesis and Antioxidant Activity of New N-Containing Hybrid Derivatives of Gallic and Ursolic Acids. Chem. Nat. Compd. 2021, 57, 1042–1046. [Google Scholar] [CrossRef]
- Chang, C.D.; Lin, P.Y.; Hsu, J.L.; Shih, W.L. Ursolic Acid Suppresses Hepatitis B Virus X Protein-Mediated Autophagy and Chemotherapeutic Drug Resistance. Anticancer Res. 2016, 36, 5097–5107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Du, X.N.; Li, Y.G.; Zhang, G.N.; Wang, J.X.; Wang, Y.C. Design, Synthesis and Biological Evaluation of Novel HIV-1 Protease Inhibitors with Pentacyclic Triterpenoids as P2-Ligands. Bioorg. Med. Chem. Lett. 2019, 29, 357–361. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Anti-HIV Activity Has Been Identified for a Variety of Pentacyclic Triterpenoids, as Well as Their Saponin or Modifying Agents. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent Progress in the Antiviral Activity and Mechanism Study of Pentacyclic Triterpenoids and Their Derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Yoo, S.J.; Kwon, T.; Lyoo, Y.S. Challenges of Influenza A Viruses in Humans and Animals and Current Animal Vaccines as an Effective Control Measure. Clin. Exp. Vaccine Res. 2018, 7, 1–15. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Wang, H.; Luo, Z.; Zhang, L.; Zhou, D. Incorporation of Privileged Structures into 3-O-β-Chacotriosyl Ursolic Acid Can Enhance Inhibiting the Entry of the H5N1 Virus. Bioorg. Med. Chem. Lett. 2019, 29, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Dai, Z.; Xu, Y.; Teng, Y.; Wu, B. Structure-Aided Optimization of 3-O-β-Chacotriosyl Ursolic Acid as Novel H5N1 Entry Inhibitors with High Selective Index. Bioorg. Med. Chem. 2019, 27, 4048–4058. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Dai, Z.; Xu, Y.; Teng, Y.; Wu, B. Synthesis, Stability and Pharmacological Evaluation of a Novel Codrug Consisting of Lamivudine and Ursolic Acid. Eur. J. Pharm. Sci. 2012, 45, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Kalinowska, M.; Gryko, K. Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials 2021, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Busuioc, A.C.; Costea, G.V.; Botezatu, A.V.D.; Furdui, B.; Dinica, R.M. Cucumis metuliferus L. Fruits Extract with Antioxidant, Anti-Inflammatory, and Antidiabetic Properties as Source of Ursolic Acid. Separations 2023, 10, 274. [Google Scholar] [CrossRef]
- Wu, P.; He, P.; Zhao, S.; Huang, T.; Lu, Y.; Zhang, K. Synthesis and Evaluation of Novel Triterpene Analogues of Ursolic Acid as Potential Antidiabetic Agent. PLoS ONE 2015, 10, e0138767. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; He, P.; Zhao, S.; Huang, T.; Lu, Y.; Zhang, K. Effects of Ursolic Acid Derivatives on Caco-2 Cells and Their Alleviating Role in Streptozocin-Induced Type 2 Diabetic Rats. Molecules 2014, 19, 12559–12576. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wu, P.; Cheng, A.; Qin, J.; Zhang, K.; Zhao, S. A Hydrophilic Conjugate Approach Toward the Design and Synthesis of Ursolic Acid Derivatives as Potential Antidiabetic Agent. RSC Adv. 2015, 5, 44234–44246. [Google Scholar] [CrossRef]











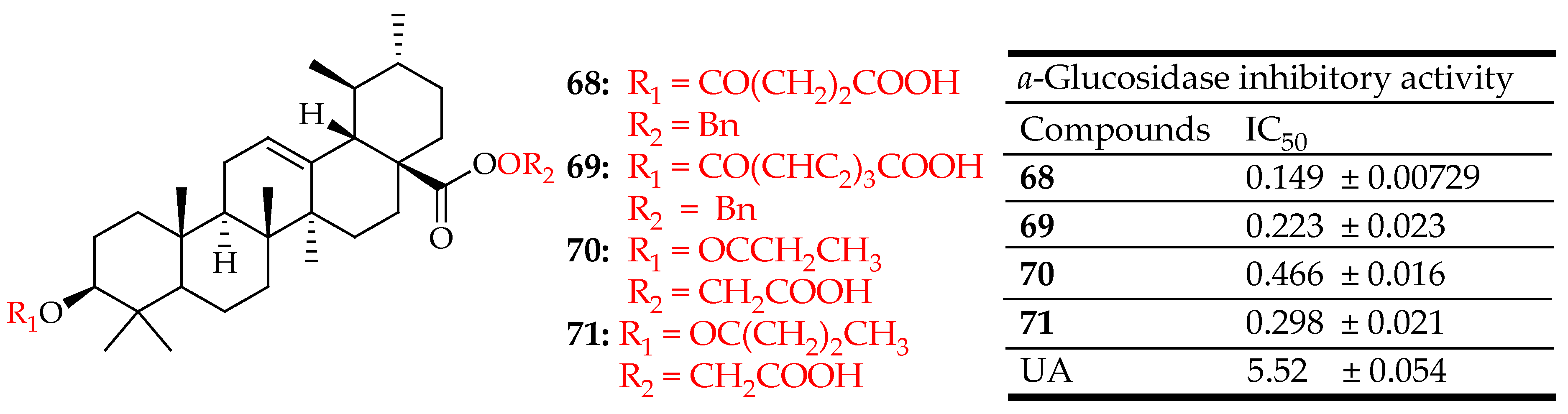
| Compounds | Modification Method | Tested Models/Assays | Observed Effects | Ref. |
|---|---|---|---|---|
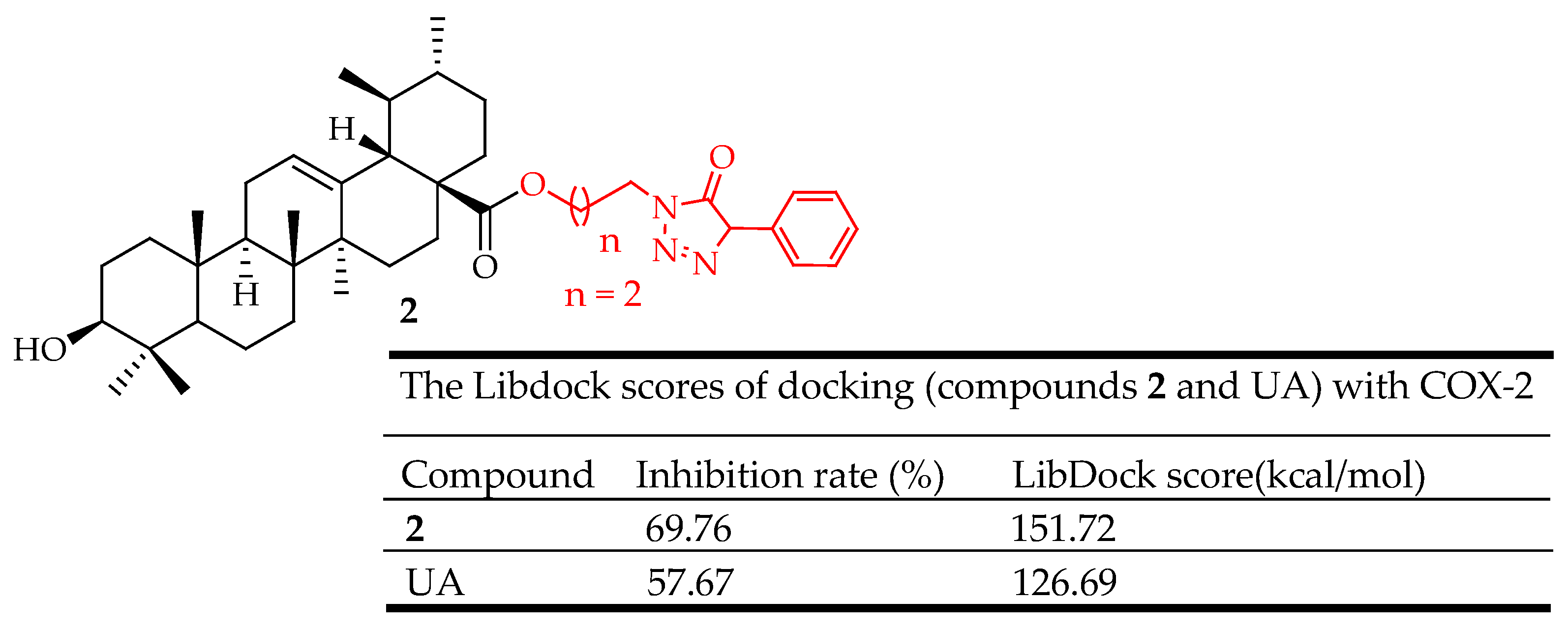
| 2 | Incorporated piperazine, triazolone, and oxadiazole groups at the C-3 position | Ear edema model | Decreased ear swelling, reduced COX-2 expression | [43] |
| 3 | attached 1,2,3-triazole groups at the C-28 position | Para-xylene-induced mice ear-swelling | Reduced inflammation, reduced COX-2 expression | [61] |
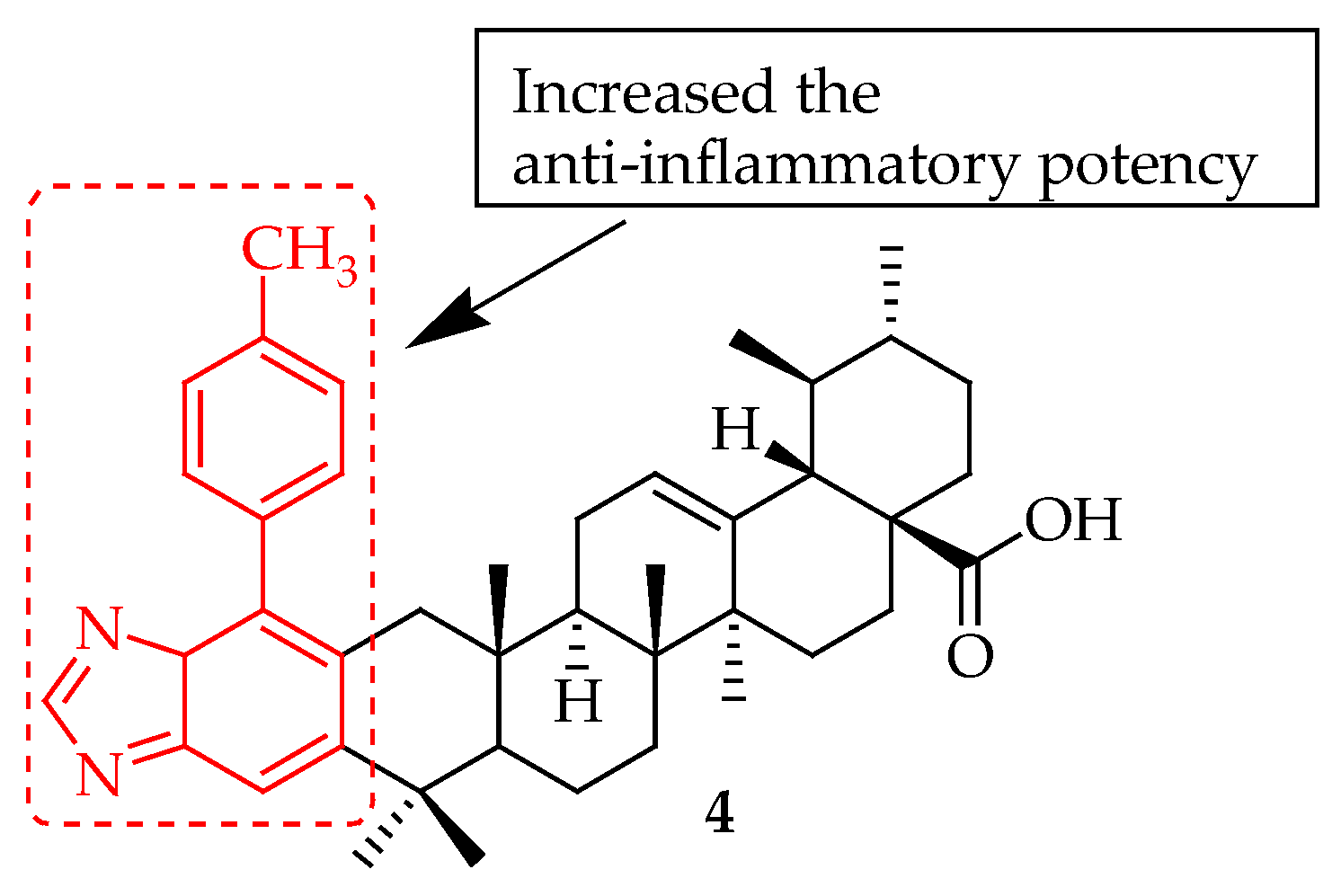
| 4 | Incorporated a 1,2,4-triazolo[1,5-a]pyrimidine group at C-28 position. | xylene-induced ear edema model | Decreased the production of the inflammatory factors, inhibited COX-2 | [61] |
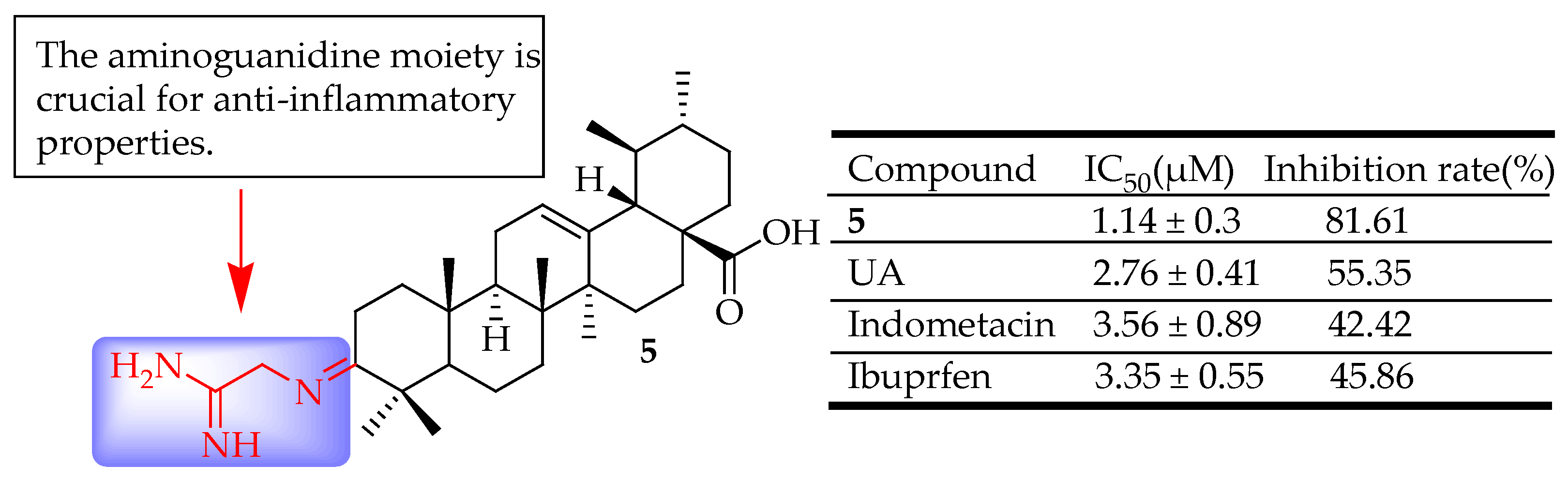
| 5 | Incorporated an aminoguanidine moiety | xylene-induced ear edema | Reduced inflammation | [62] |
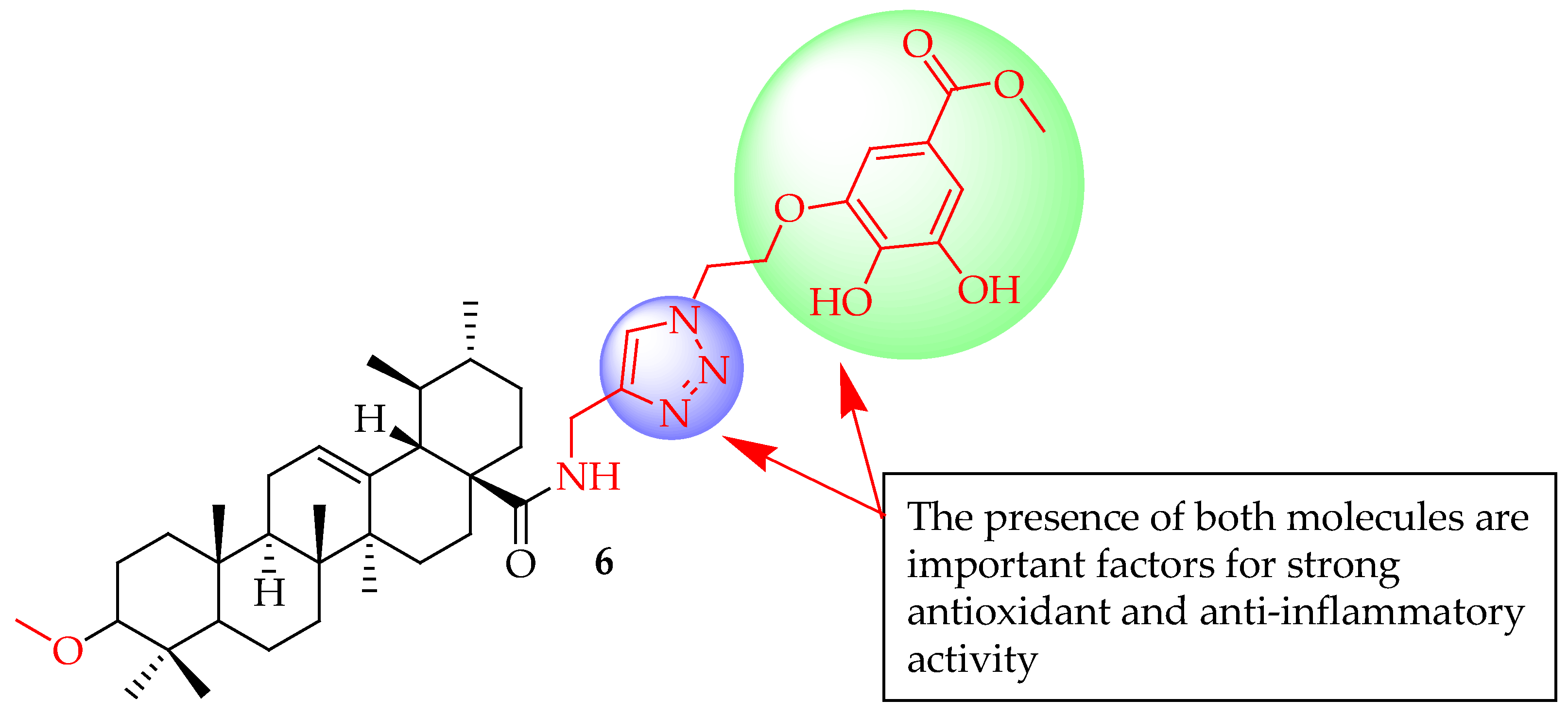
| 6 | Linked UA with modified gallate moieties through 1,2,3-triazole employing CuAAC 1,3-cycloaddition reactions | RAW 264.7 macrophages | Inhibited pro-inflammatory cytokines by suppressing the LPS-induced PI3K/Akt signalling pathway, suppressed mRNA levels of iNOS (p < 0.05) and COX-2) (p < 0.01). | [63] |
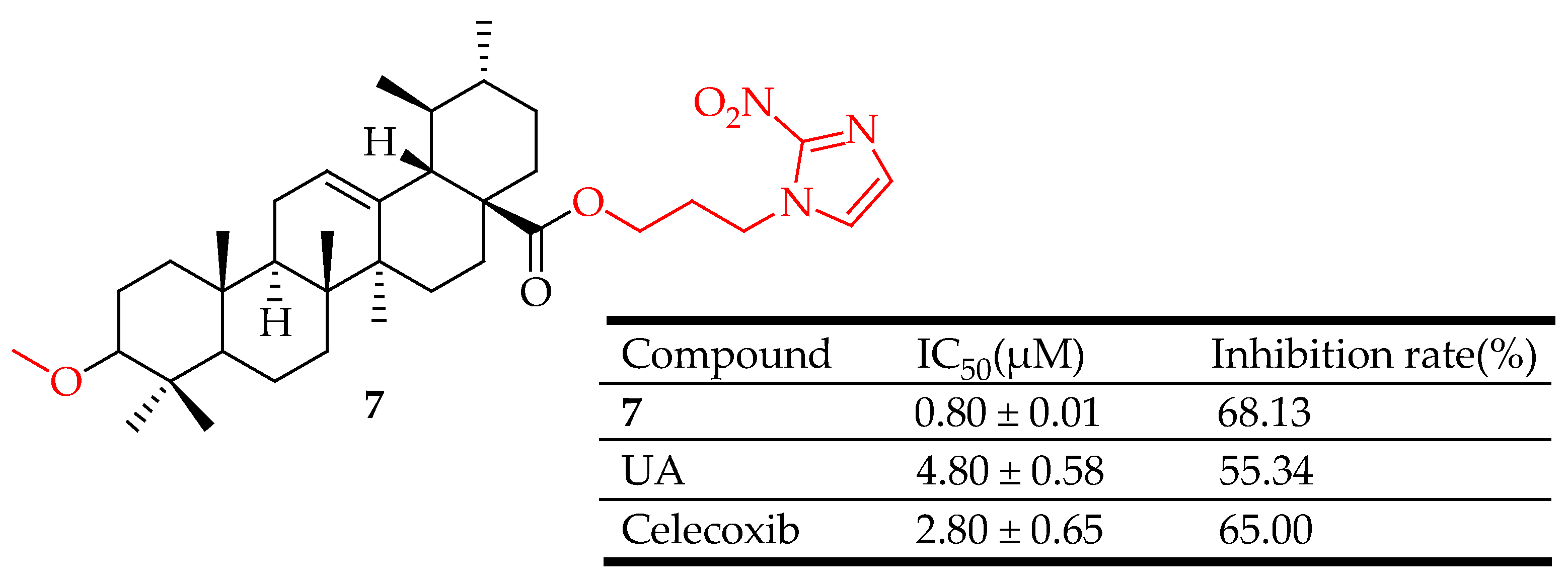
| 7 | introduced a 1,2,3-triazoles moiety, 1,2,4-triazoles moiety or a nitroimidazoles ring to the C-28 of UA nucleus | xylene-induced ear edema | Inhibited HIF-1α, and COX-2. | [66] |
| Compounds | Modification Method | Tested Cancer Cell Lines | Observed Effects | Ref. |
|---|---|---|---|---|
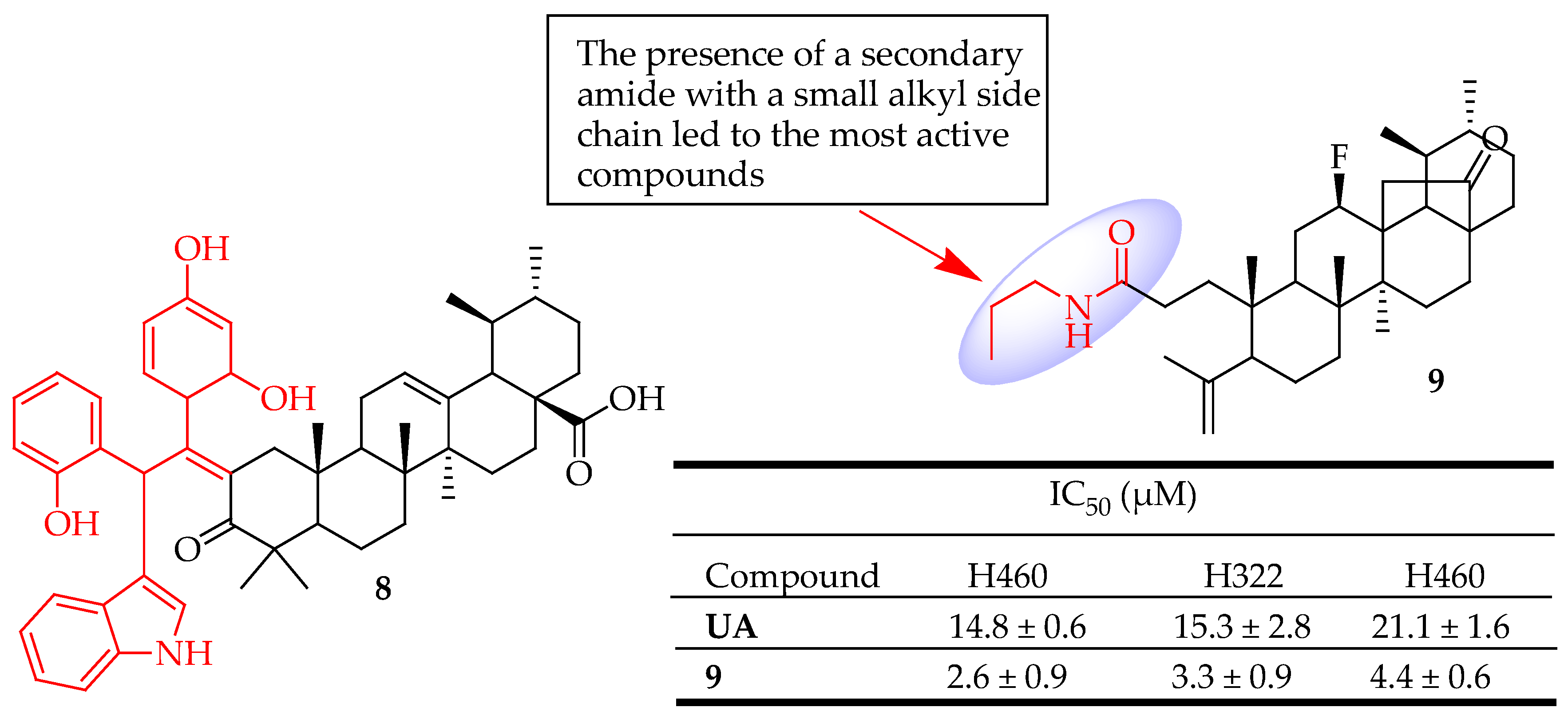
| 8 | Used Jones reagent to deliver the C-3 oxidized UA derivative then incorporated benzaldehyde and indole. | U251 (Glioblastoma) | Suppressed the growth of glioma cells, triggered apoptosis, and halted cell cycle progression by down-regulating metabolic pathways | [81] |
| 9 | Introduced a secondary amine at position C-3 of a cleaved ring-A | NSCLC (Lung cancer) | Induced apoptosis and autophagy | [82] |
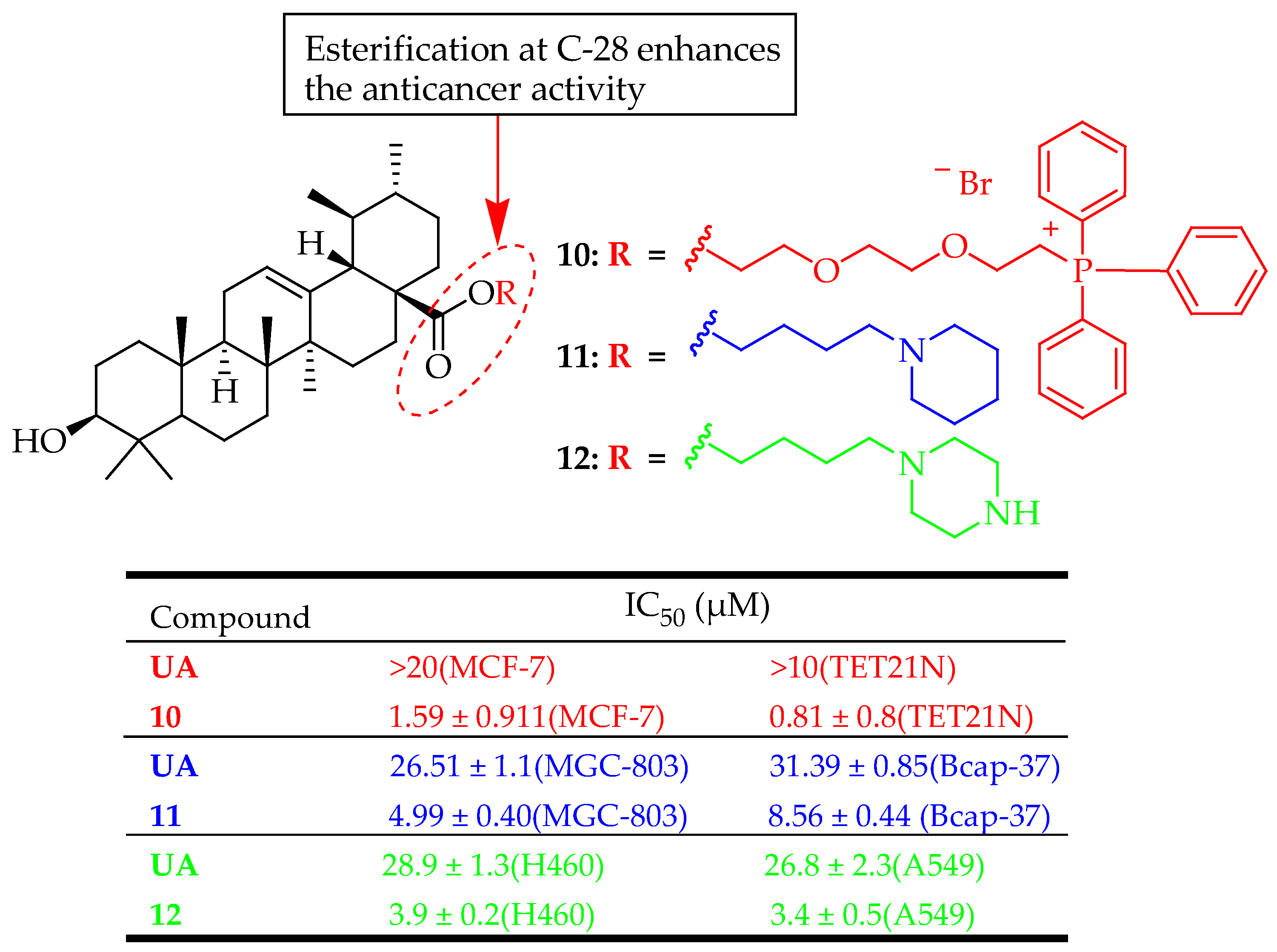
| 10 | Linked the triphenylphosphonium group to a UA at the C-28 position through the hydrophobic n-butyl or hydrophilic triethylene glycol spacer | MCF-7 (Breast adenocarcinoma) and TET21N (Neuroblastoma) | Induced mitochondria-dependent apoptosis | [83] |
| 11 | Reacted UA with 1,2-dibro-moethane, 1,3-dibromopropane, 1,4-dibromobutane or butyl bromide in DMF in the presence of K2CO3, and then reacted with corresponding amines to yield the targeted compounds. | Bcap-37 (Breast cancer) and MGC-803 (Gastric cancer) | Induced apoptosis on MGC-803 cells, | [84] |
| 12 | UA was coupled with 1,4-dibromo-butane in the presence of K2CO3 and KI in DMF. The resulting intermediate was subsequently reacted with piperazine. | A549 and H460 (Lung cancer) | Inhibited cell proliferation, induced apoptosis, Increased cell cycle arrest in the G0/G1 phase | [85] |
| 13–16 | They oxidized the UA via John reagent (PCC), introduced various bromo-alkanes at the C-28 position, and then added 1-(4-nitrophenyl)hydrazine at the C-3 position. | BEL7402 (liver cancer) and SGC7901 (Gastric cancer) | Reduced tumor growth, enhanced cytotoxicity, and inhibited the NF-kB pathway of tumor cells. | [86] |
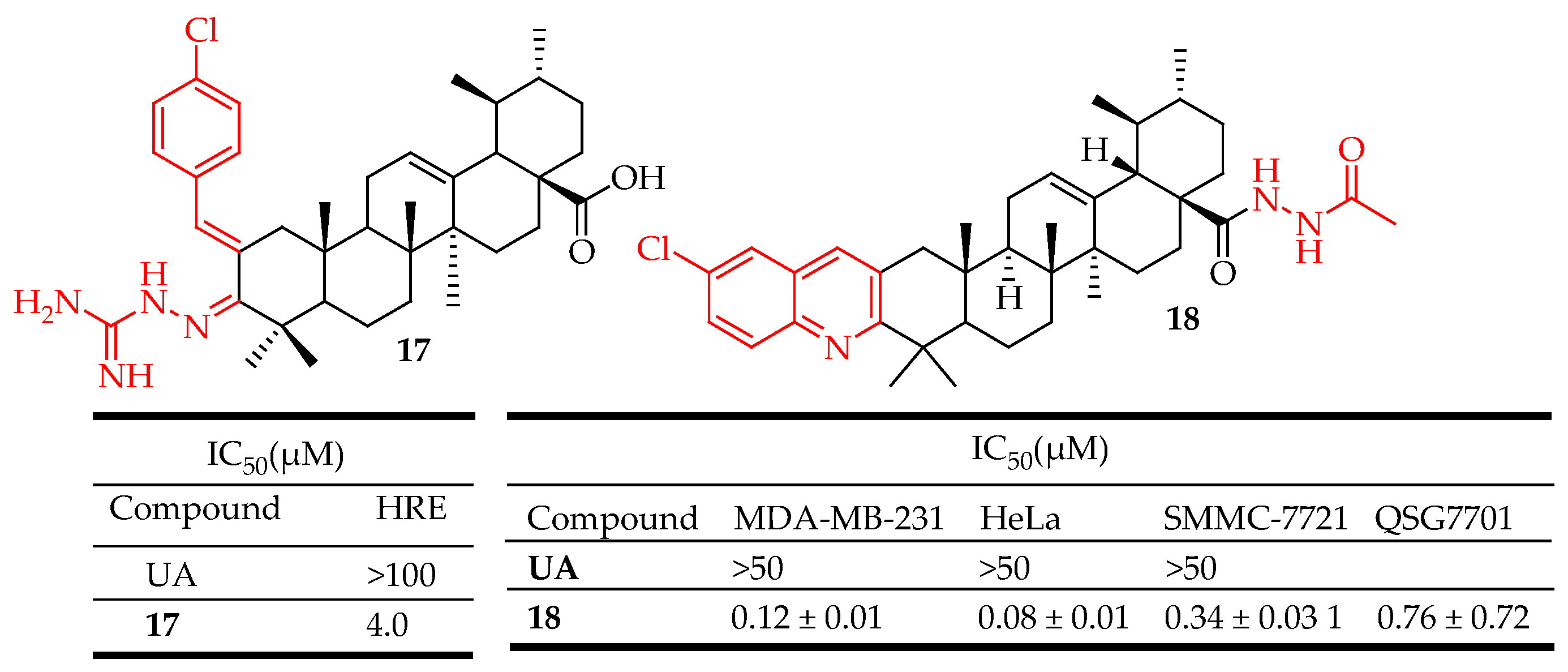
| 17 | Fused aminoguanidine moiety at the UA skeleton. | HCT116 (Colon cancer), A549: (Lung cancer), Hep3B (Liver cancer), HeLa (Cervical cancer) | reduced HIF-1α protein levels inhibited hypoxia-induced expression of VEGF at both the mRNA and protein levels and inhibited the proliferation of cancer cells in vitro. | [38] |
| 18 | Incorporated hydrazide, and oxadiazole moieties into UA structure. | SMMC-7721 (Liver cancer), HeLa (Cervical cancer), MDA-MB-231 (Breast cancer) | Enhanced cytotoxicity, Induced apoptosis of HeLa cells, arrested cell cycle at the G0/G1 phase, elevated intracellular reactive oxygen species level, decreased mitochondrial membrane potential, inhibited MEK1 kinase activity, and impeded Ras/Raf/ MEK/ERK transduction pathway | [87] |
| 21 | Incorporated hydrazide derivatives into UA structure. | SMMC-7721 (Liver cancer), HeLa (Cervical cancer), MDA-MB-231 (Breast cancer) | Induced apoptosis in MDA-MB-231 cell lines in a dose-dependent manner. Additionally, promoted G0/G1 phase arrest in MDA-MB-231 cell lines. | [88] |
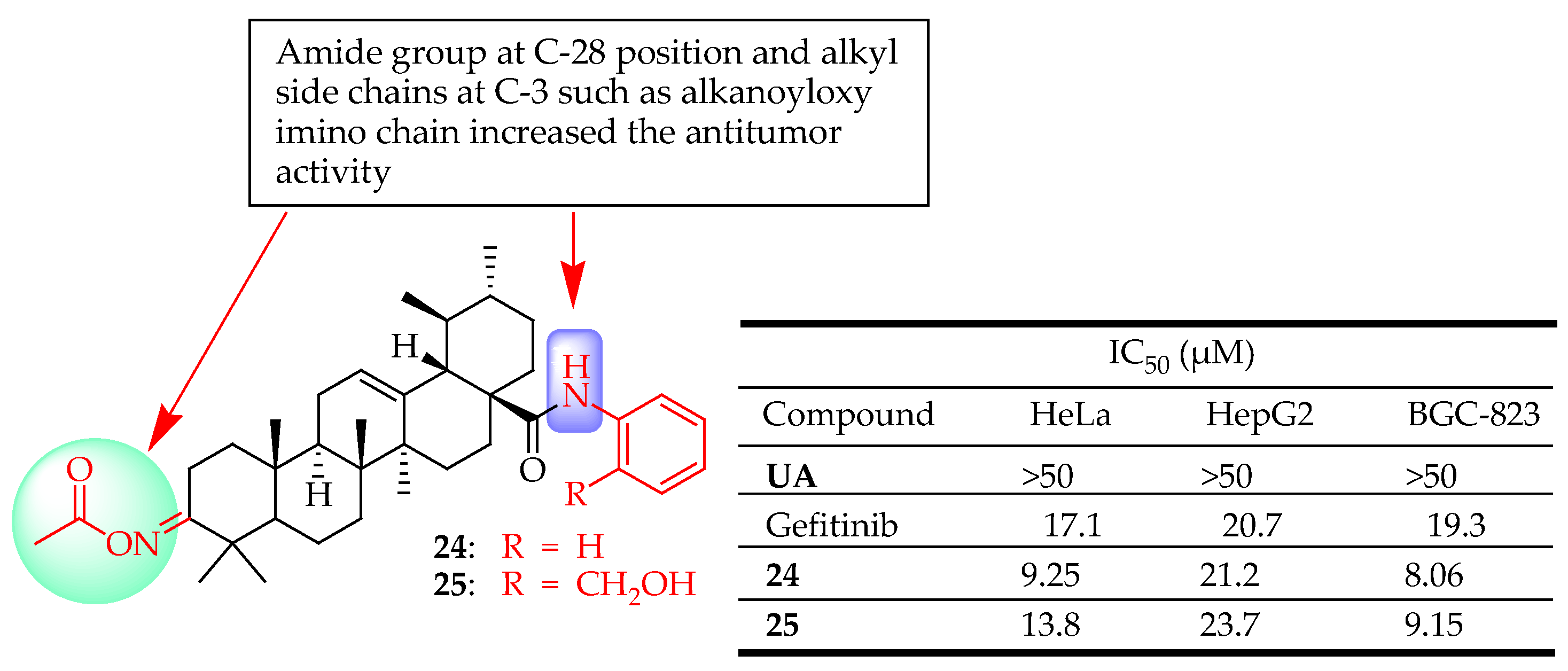
| 24, 25 | They oxidized UA using Jone’s reagent, followed by treatment with NH2-OH·HCl. The resulting intermediate was then reacted with Ac2O. This intermediate was subsequently condensed with suitable amino and phenol compounds in the presence of triethylamine. | HeLa (Cervical cancer), HepG2 (Liver cancer), BGC-823(Gastric cancer) | Inhibited cell proliferation, Enhanced cytotoxicity | [89] |
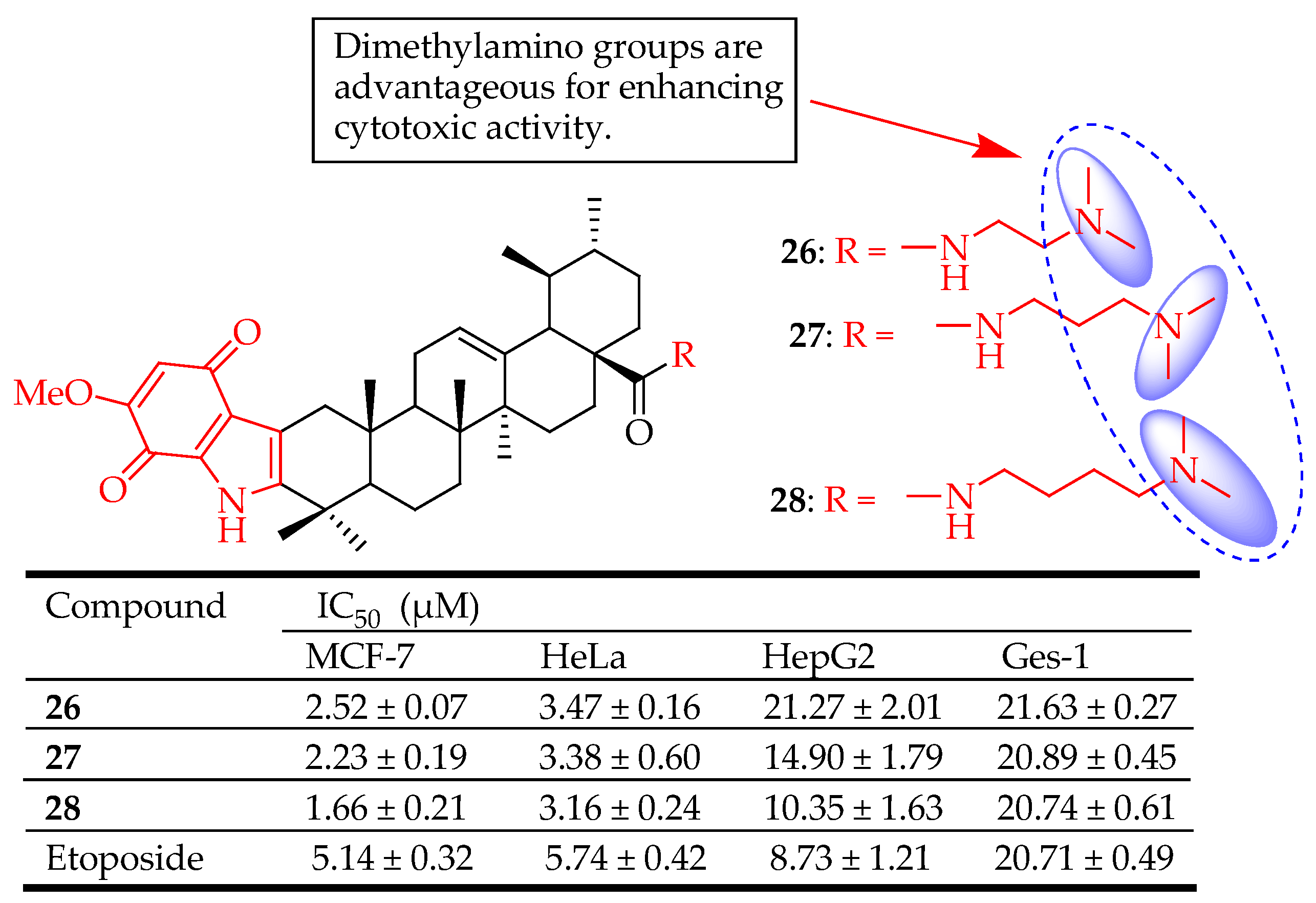
| 26–28 | Designed novel indolequinone derivatives of UA-bearing ester, hydrazide, or amide moieties | MCF-7 (Breast cancer), HeLa (Cervical cancer), HepG2 (Liver cancer) | Enhanced cytotoxicity, suppresses the migration of MCF-7 cells, elevates intracellular reactive oxygen species (ROS) levels, and decreases mitochondrial membrane potential. upregulated Bax, cleaved caspase-3/9, cleaved PARP levels and downregulated Bcl-2 level of MCF-7 cells, inhibited cell proliferation | [40] |
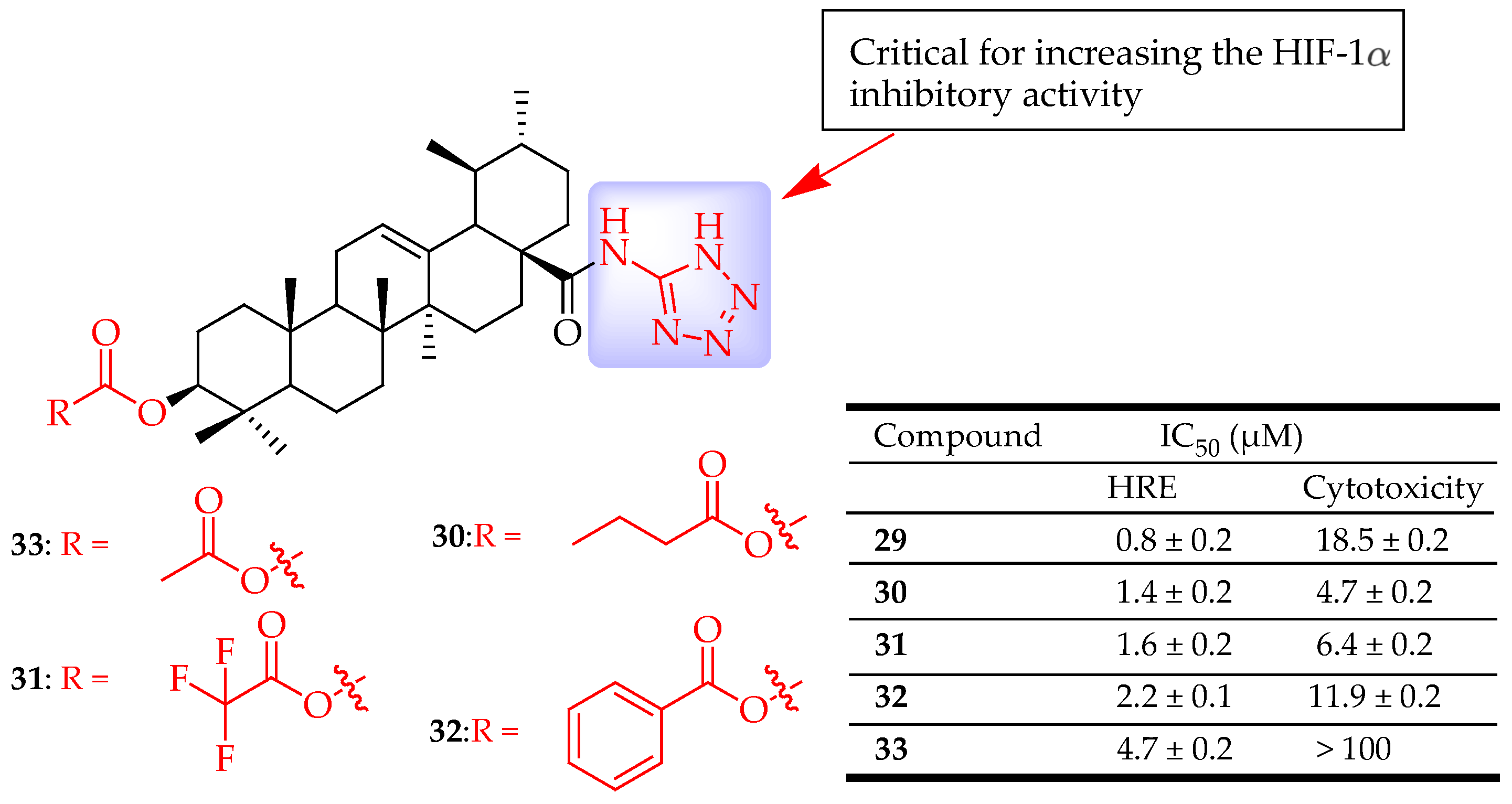
| 29–33 | UA was modified by introducing a tetrazole moiety, with the tetrazole group directly attached to the nitrogen atom of the amide group at the C-28 position. The C-3 hydroxy group was either left unmodified, oxidized, esterified, or converted to hydrazine | Hep3B cells (Liver cancer) | Inhibited the HIF-1α | [39] |
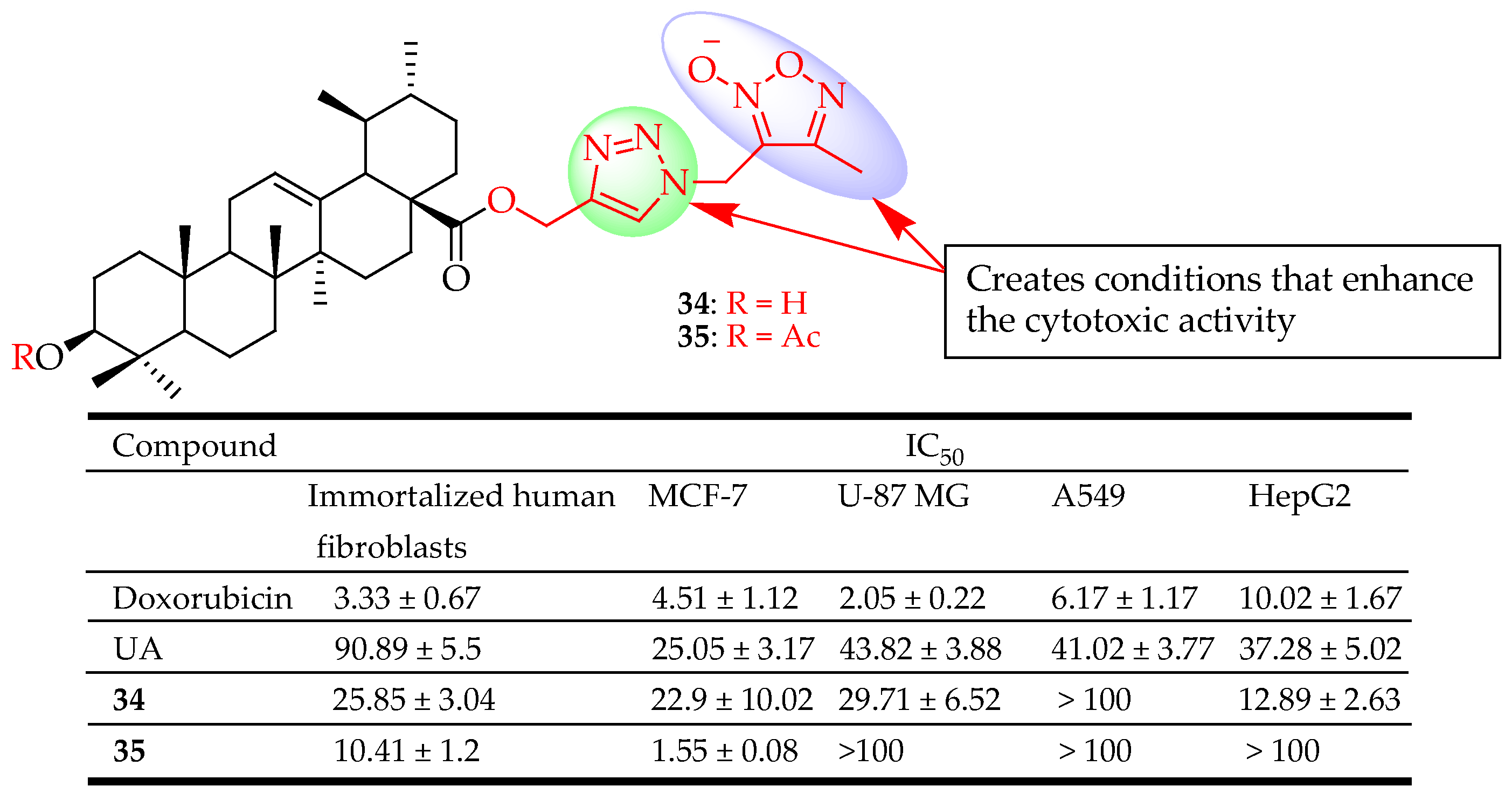
| 34, 35 | Combined UA with two different azole types (1,3,4- oxadiazole and 1,2,3- triazole or 1,2,5- oxadiazole and 1,2,3- triazole) at different positions of UA. | MCF-7 (Breast cancer), HepG2 (Liver cancer), A549 (Lung cancer), U-87MG (Glioblastoma) | Enhanced cytotoxicity | [90] |
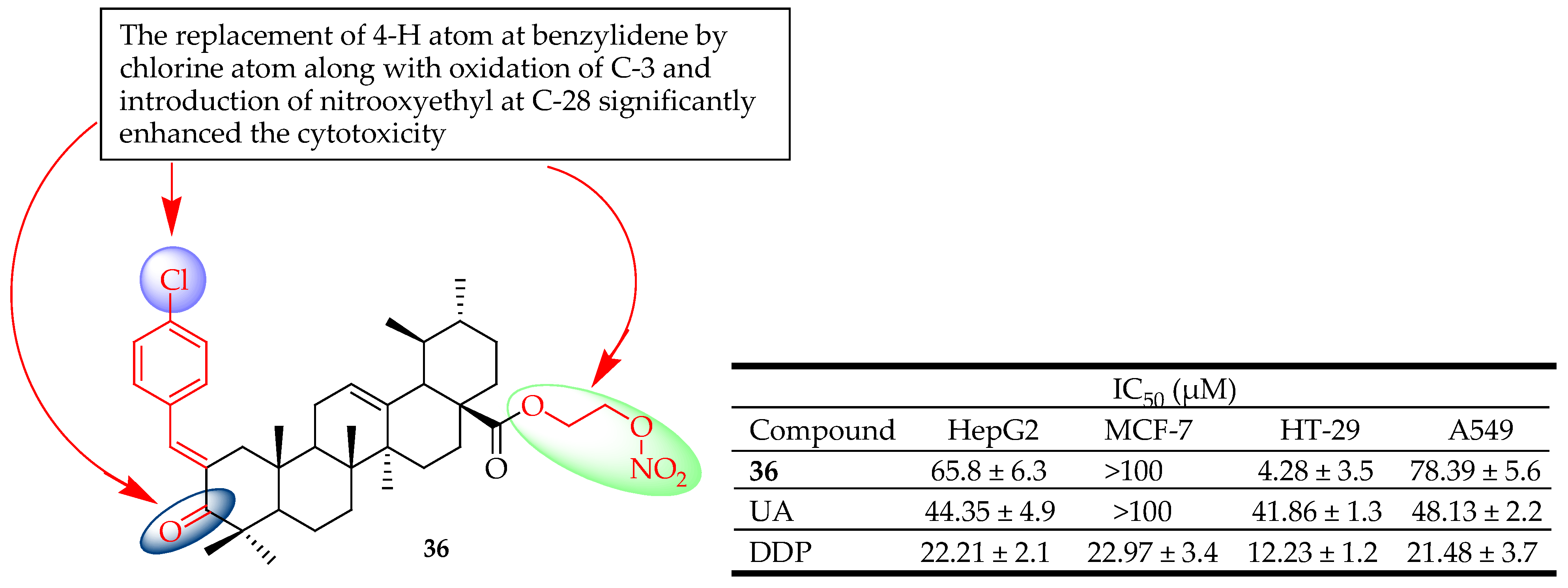
| 36 | Incorporated different constituents of the benzylidene at C-2 | HepG2 (Liver cancer), HT-29 (Colon cancer), A549 (Lung cancer) | Induced apoptosis via arrest of the cycle at the G1 phase and mitochondria-mediated pathway. Enhanced cytotoxicity | [92] |
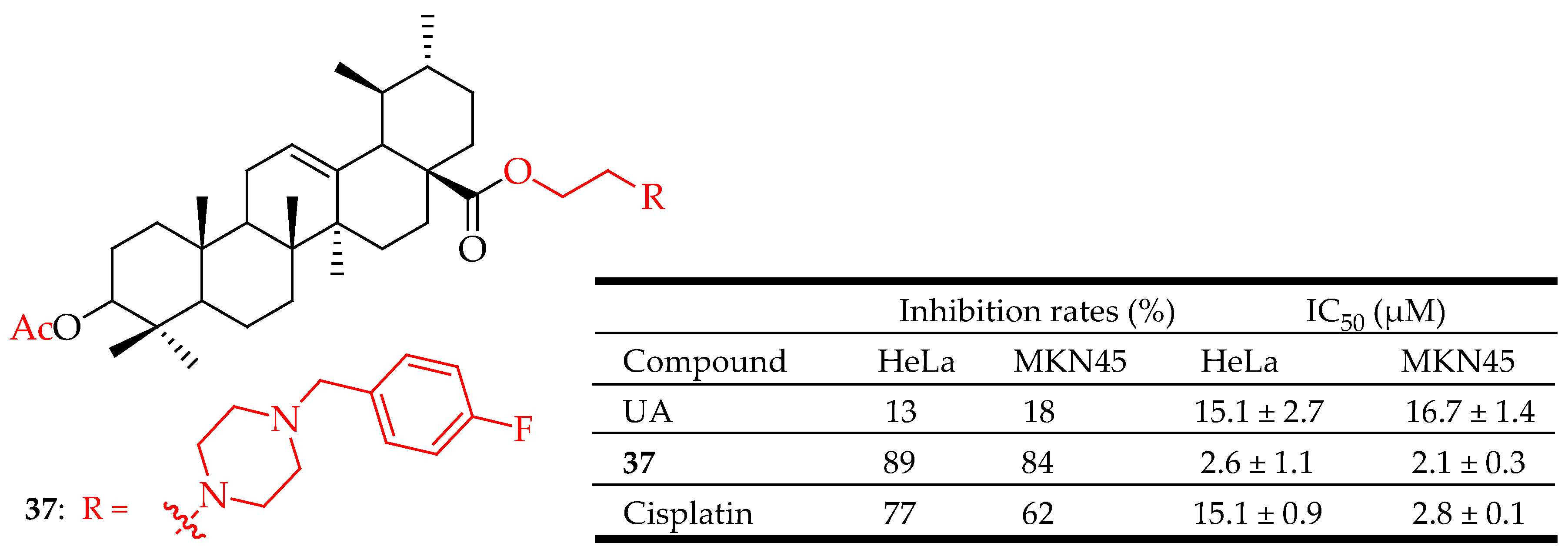
| 37 | Acetylation of the hydroxyl group at the C-3 position. Introduction of 2-chloroethanol at the C-28 position. Addition of methanesulfonyl chloride (MsCl) in pyridine. Reaction with piperazine. Oxidation with PCC. Introduction of 4-fluorobenzyl bromide at the piperazine moiety | MKN45 (Gastric cancer) | Decreased the apoptosis regulator (BCL2/BAX) ratio, disrupted mitochondrial potential, induced apoptosis, and suppressed the growth of Hela xenografts in nude mice. | [93] |
| 38, 39 | Converted the UA into C-28-amino-functionalized derivatives | HeLa, Jurkat, Hek293, K562, and U937 | Inducted the cell cycle arrest at the S-phase and apoptosis. | [94] |
| 40 | UA was treated with acetic anhydride in dry pyridine under the 4-dimethylamino pyridine. The 3-acetyl UA was treated with oxalyl chloride to produce an intermediary 28-acyl chloride. This compound was then mixed with piperazine to produce the targeted compound. | SUM149PT (Breast cancer), HCC1937(Breast cancer), | Suppressed cell proliferation and triggered apoptosis in both cell lines | [95] |
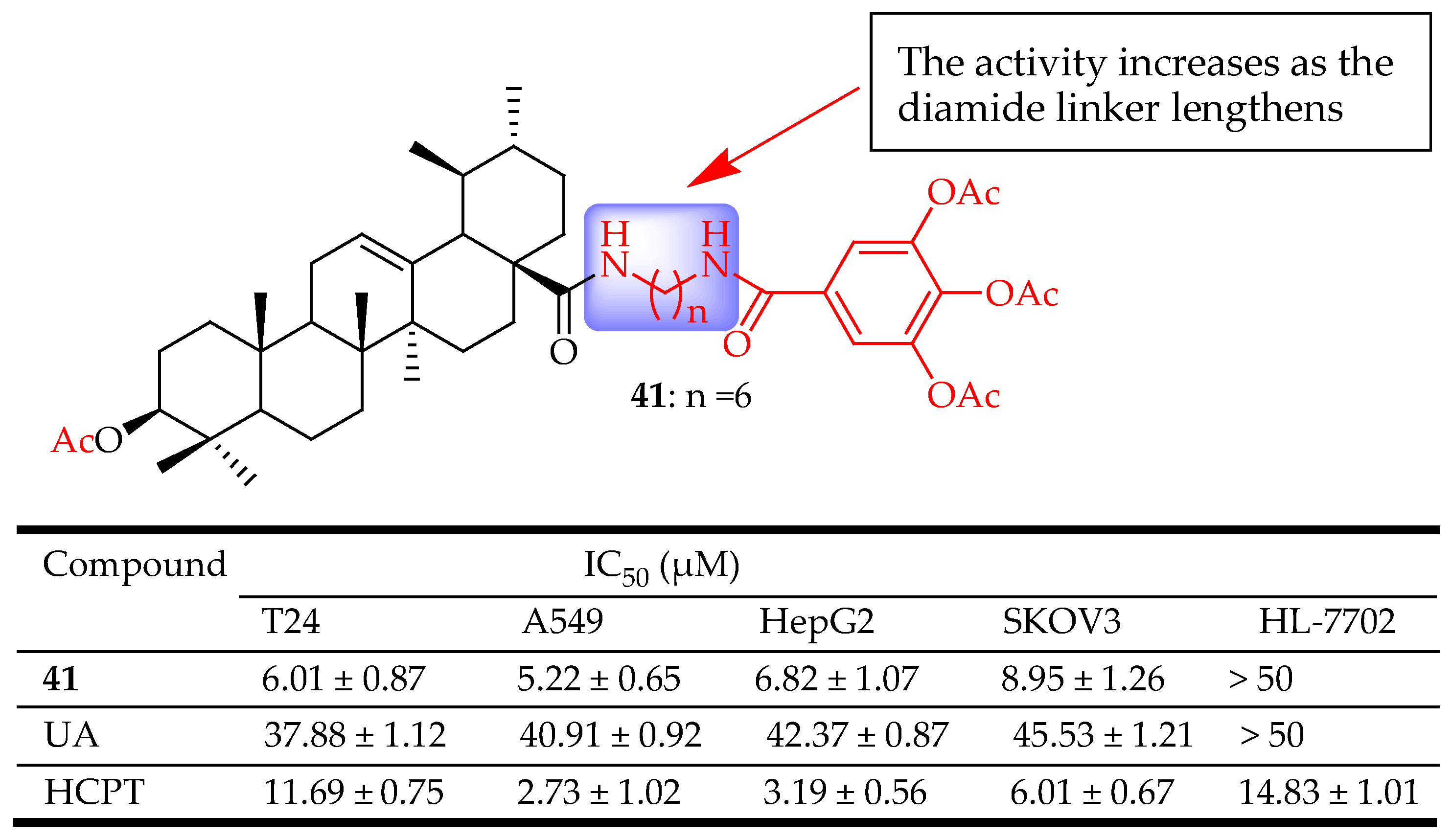
| 41 | Acylated the C-3(OH) position. Converted the carboxylic group at the C-8 position oxalyl chloride ((CO)2Cl2). The intermediated was reacted with hexamethylenediamine (H2N(CH2)6NH2). Then reacted with 3,4,5-triacetoxybenzoic acid to form the amide bond. | A549 (Lung cancer), HepG2 (Liver cancer) KOV3 (Ovarian cance) T24 (Bladder cancer) | Inhibited the binding of NF-κB to DNA, suppressed NF-κB activation, inhibited A549 cell migration in vitro, and arrested A549 cell line at the G1 phase. | [36] |
| 42 | Methylated the C-28 carboxylic group of UA using diazomethane to produce the methyl ester | HepG2, Hep3B and HA22T/VGH (Liver cancer) | Inhibited cell growth and induced an inhibition of NF-κB activation in hepatocellular carcinoma cell lines | [33] |
| Compounds | Modification Method | Bacterial Strain | Effects | Ref. |
|---|---|---|---|---|
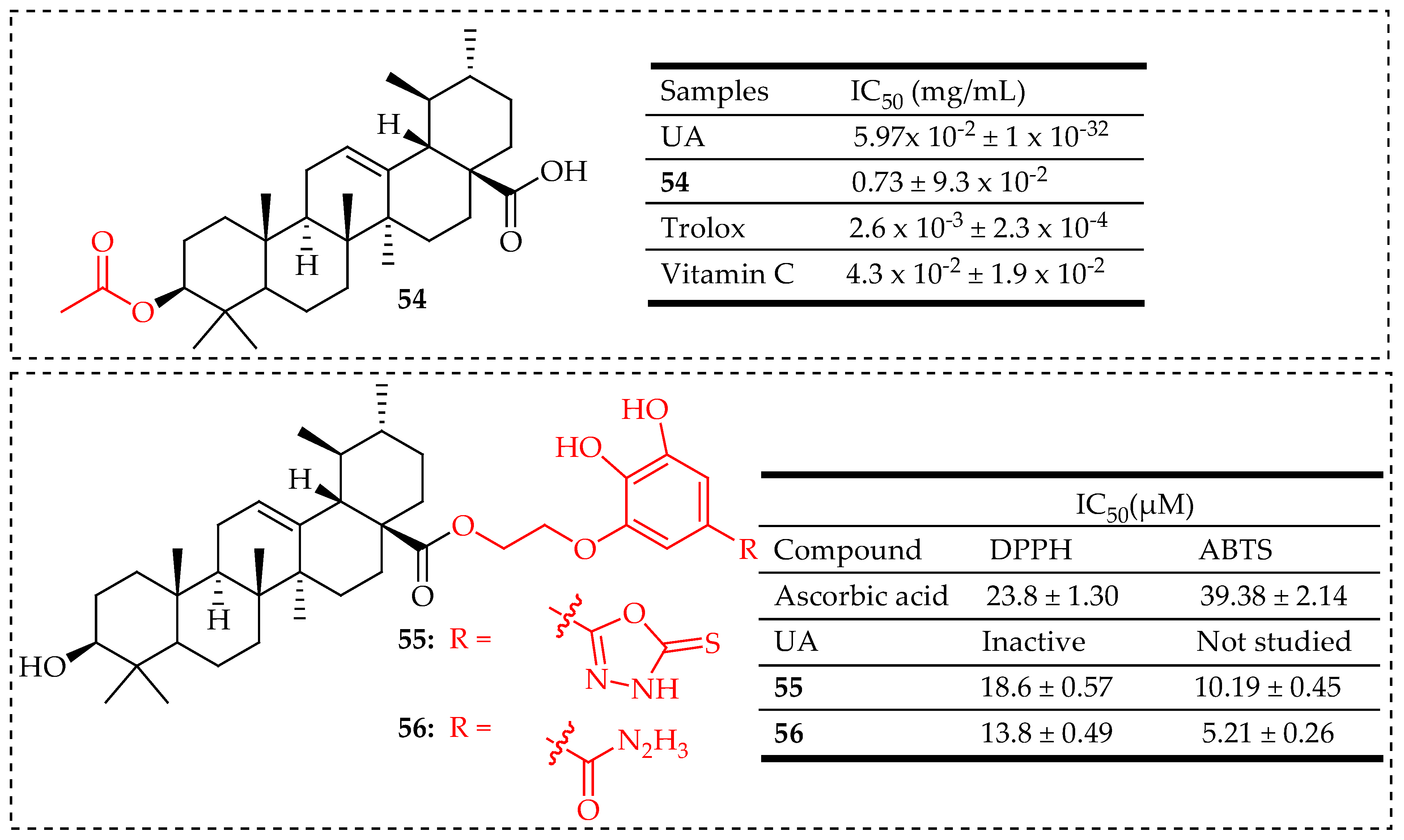
| 43 | UA was reacted with acetic anhydride (Ac2O) in pyridine at room temperature for 24 h to yield compound 54 | K. pneumoniae (ATCC 10031) Shigella flexneri (ATCC 12022) E. coli (ATCC 25922) | Enhanced antibacterial activity against Shigella flexneri and E. coli, a multidrug-resistant clinical isolate from sputum | [109] |
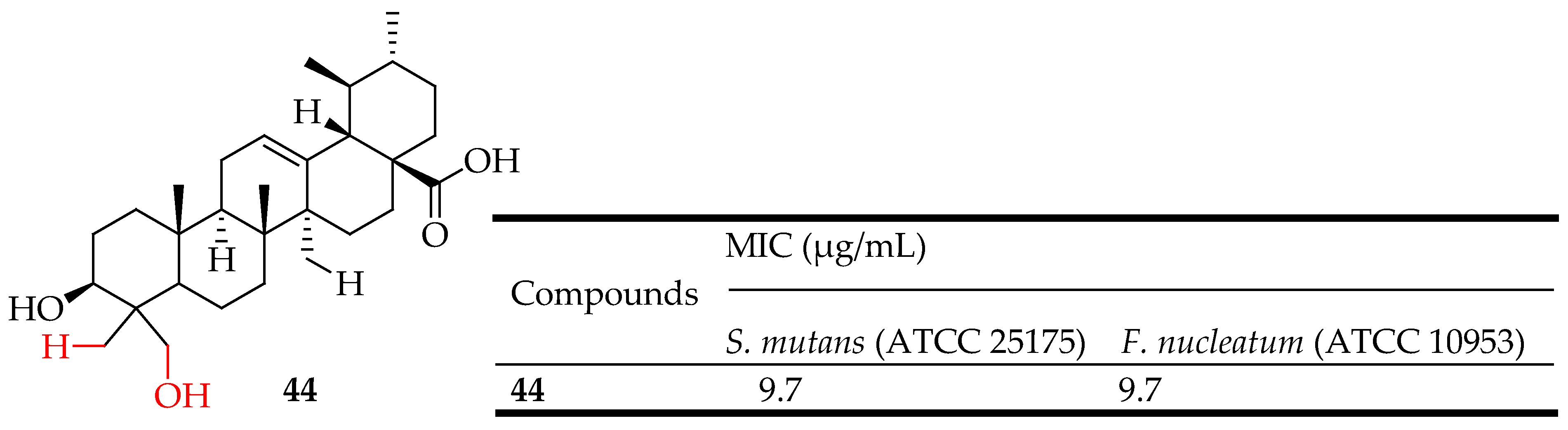
| 44 | Hybridization of UA with hydrazide and 1,3,4-oxadiazole groups | S. mutans ATCC 25175, Fusobacterium nucleatum ATCC 10953 | Showed significant antibacterial activity against S. mutans | [116] |
| 45 | The commercial anhydride was added to UA in pyridine (CH2Cl2, 2 mL) to form an ester derivative | E. faecalis, S. epidermidis and S. aureus | An antibiofilm activity against S. aureus without any effect on mammalian cells. | [115] |
| Compounds | Modification Method | Target Virus | Notes | Ref. |
|---|---|---|---|---|
| 48 | Modified UA as P2 ligands and phenylsulfonamide as P2′ ligands | HIV-1 | Demonstrated HIV-1 protease inhibition, exhibiting 67 times greater inhibitory activity compared to its precursor, UA | [118] |
| 49, 50 | Attached the privileged fragment 2-(piperidin-1-yl)ethan- 1-amine or its bioisosteric surrogate 2-(1,3- oxazinan-3-yl)ethan-1- amine into UA by a crucial amide linker | H5N1, PR/8 (H1N1), JX/312 (H3N2) | 50 Inhibited infection of H1-, H3-, and H5-typed influenza A viruses by interfering with the viral hemagglutinin | [122] |
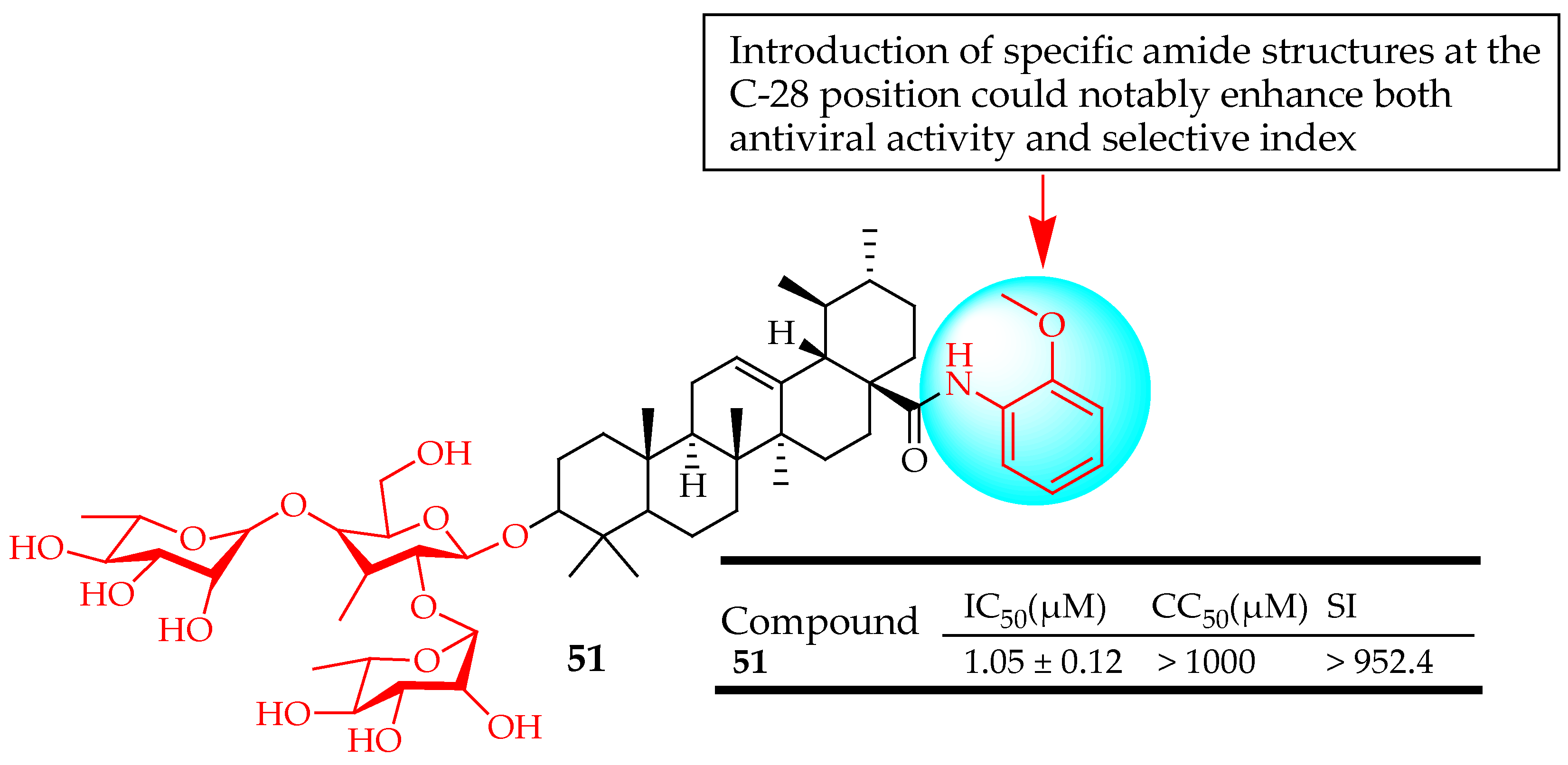
| 51 | Modified the C-28 position of UA saponins via conjugation with a series of amide derivatives | H5N1 | Inhibited influenza A virus replication | [123] |
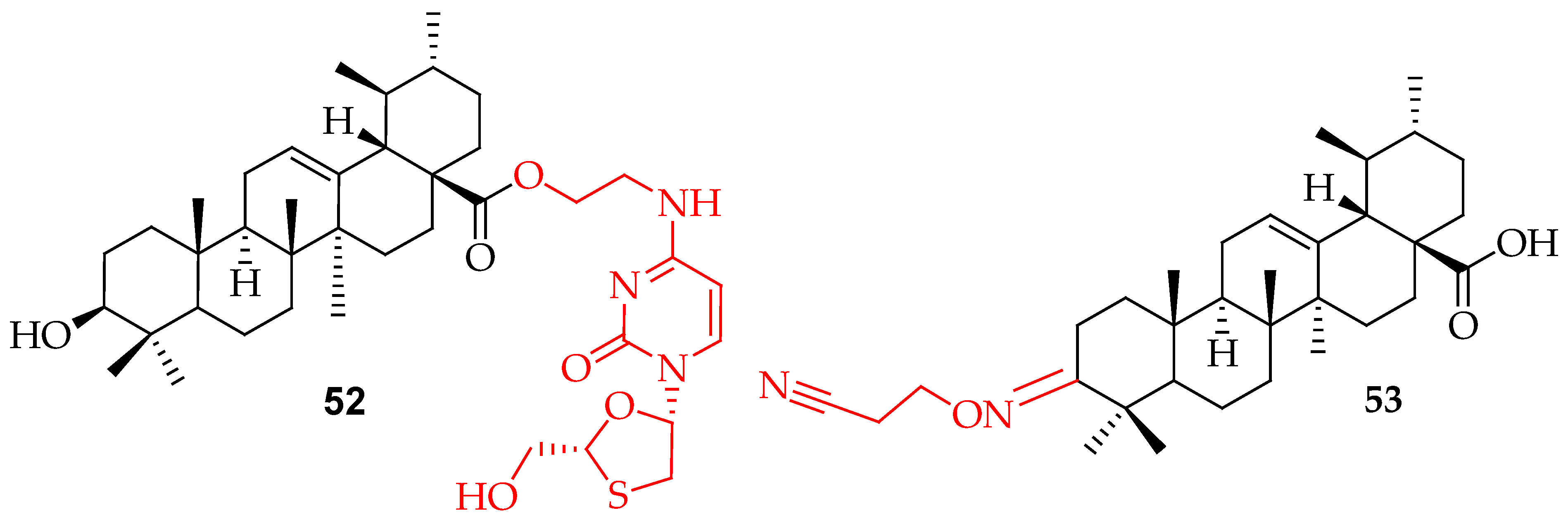
| 52 | Coupled lamivudine and UA with ethyl chloroacetate through an amide and ester linkage | Had the dual action of anti-hepatitis B virus activity and hepatoprotective effects against acute liver injury | [124] | |
| 53 | UA was oxidized using Jones’ reagent. The resulting compound was then reacted with hydroxylamine hydrochloride (NH2OH·HCl). The intermediate was further reacted with acrylonitrile (CH2CHCN). | Human papillomavirus type 11 | Inhibited human papillomavirus type 11 | [14] |
| Compounds | Modification Method | Antioxidant Assay | Notes | Ref. |
|---|---|---|---|---|
| 54 | UA was reacted with acetic anhydride (Ac2O) in pyridine at room temperature for 24 h to yield compound 54 | DPPH Radical Scavenging Assay | Strong antioxidant activity | [109] |
| 55, 56 | Hybridization of UA with hydrazide and 1,3,4-oxadiazole groups | DPPH Radical and ABTS Radical Scavenging Assay | High antioxidant activity compared to ascorbic acid | [116] |
| Compounds | Modification Method | Assay/Model Used | Notes | Ref. |
|---|---|---|---|---|
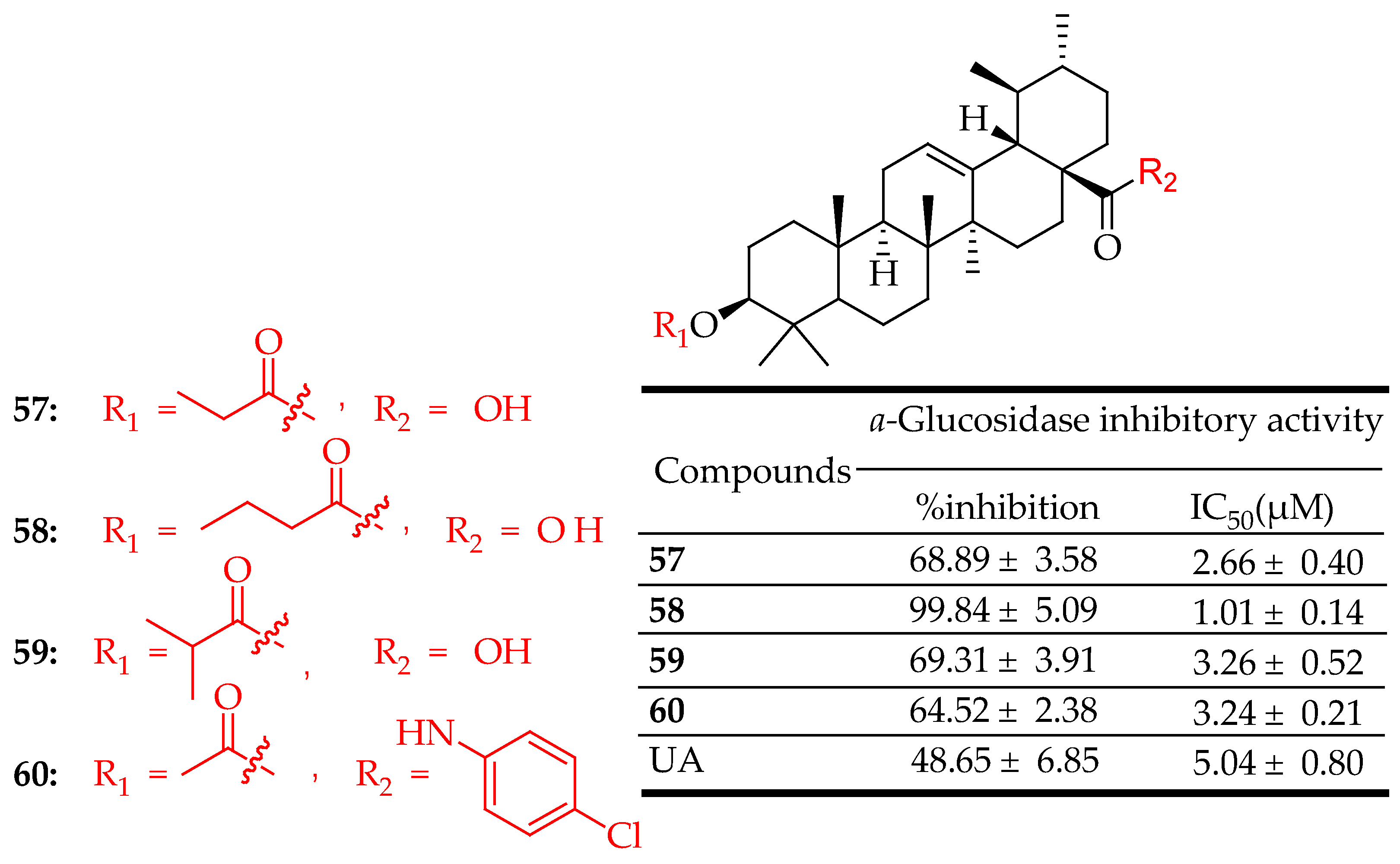
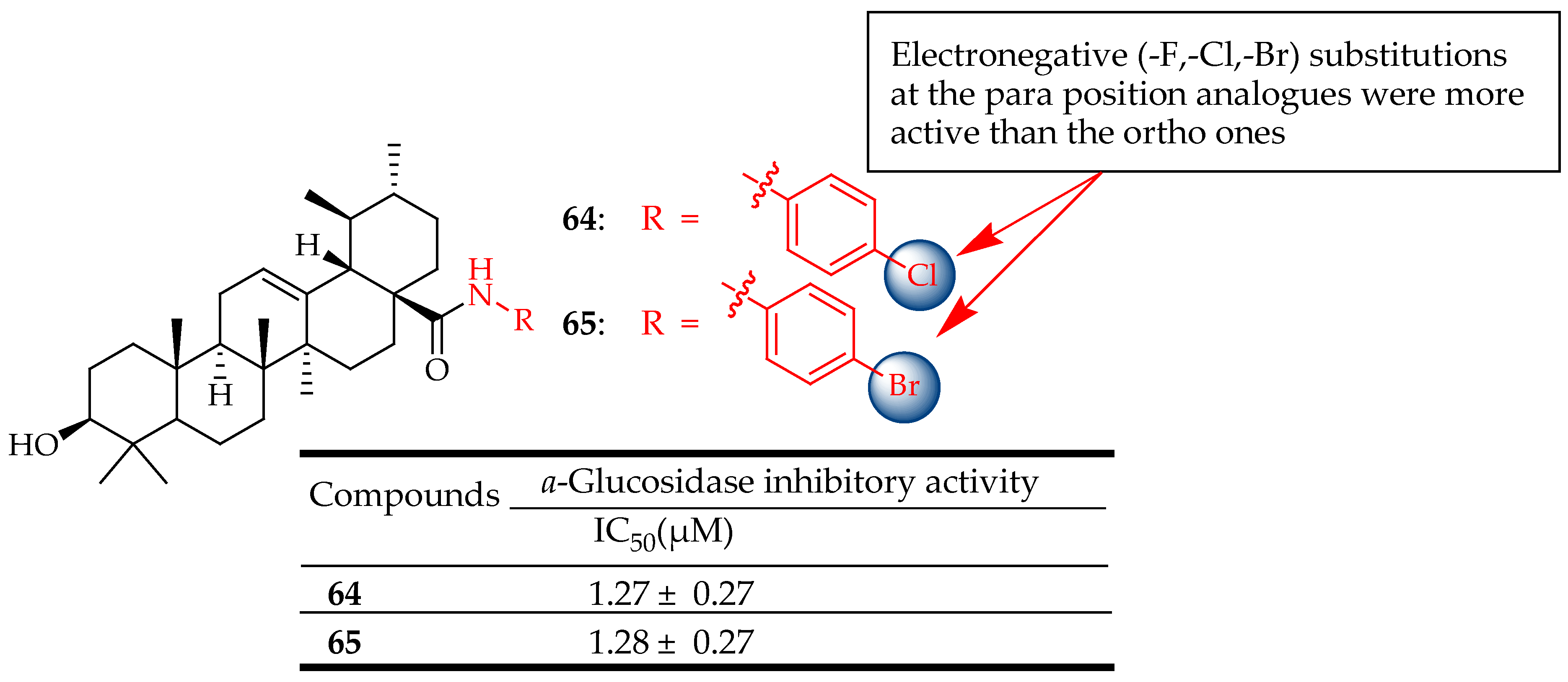
| 57–60 | UA was esterified in anhydrous pyridine with different anhydrides | α-Glucosidase Inhibition Assay | Strong inhibition of α-glucosidase compared to acarbose, the positive control | [28] |
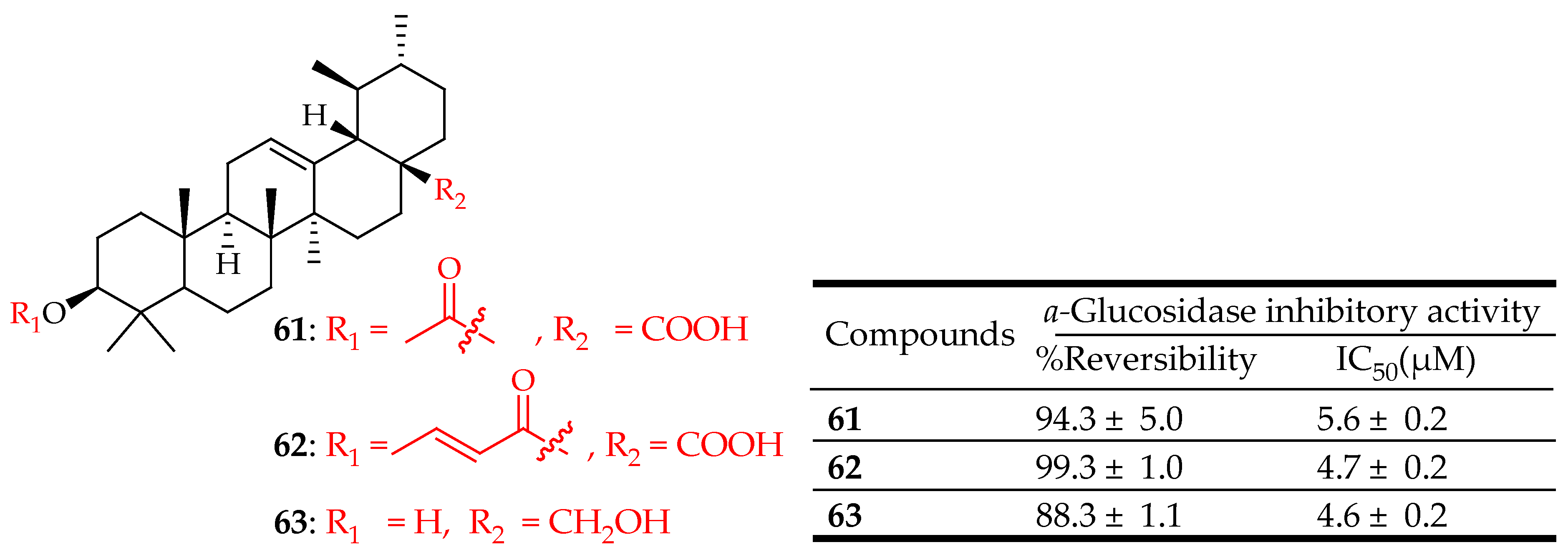
| 61–63 | For compounds 61 and 62 the reaction was initiated by adding a base to the UA at 0 °C in (CH2Cl2) or (THF). Then, an acyl or alkyl halide was added at C-3, and the mixture was subjected to microwave irradiation and refluxed.For compound 63, UA was reacted with (LiAlH4) in tetrahydrofuran (THF) for 8 h | PTP-1B inhibition assay | Significant inhibitory activity on PTP-1B enzyme in a reversible manner | [29] |
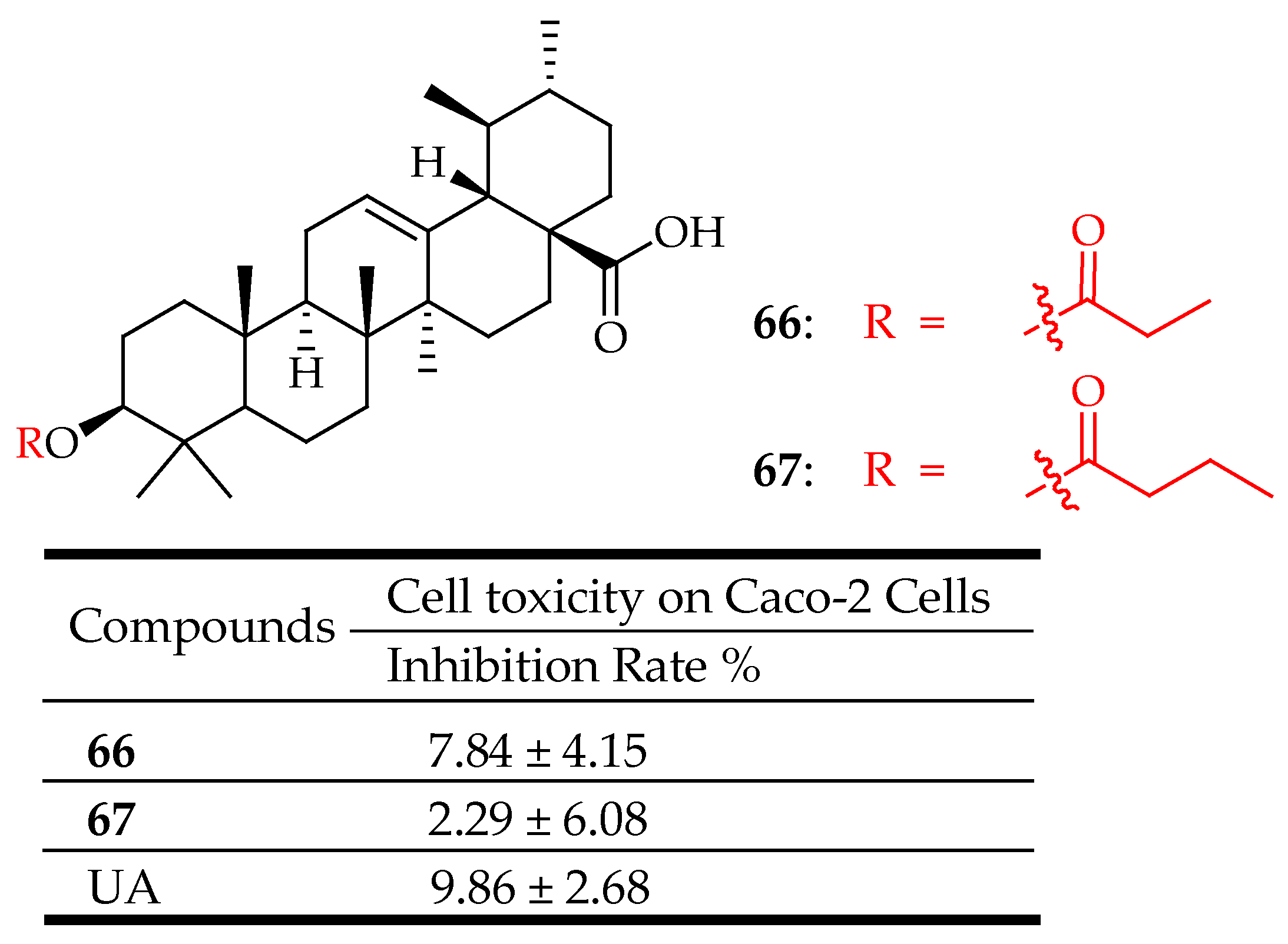
| 66, 67 | UA was esterified in anhydrous pyridine with different anhydrides | Glucose Uptake in L6 Myotubes | Displays an inhibitory effect on 2-NBDG uptake through inhibiting SGLT-1 and GLUT-2 transporter protein expression in Caco-2 cells | [128] |
| 68–71 | Conjugation of hydrophilic and polar groups at C-3 and/or C-28 position | α-Glucosidase Inhibition Assay | Inhibited α-glucosidase through a mixed-type inhibition, while compounds 70 and 71 exhibited a non-competitive inhibition mechanism | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khwaza, V.; Aderibigbe, B.A. Potential Pharmacological Properties of Triterpene Derivatives of Ursolic Acid. Molecules 2024, 29, 3884. https://doi.org/10.3390/molecules29163884
Khwaza V, Aderibigbe BA. Potential Pharmacological Properties of Triterpene Derivatives of Ursolic Acid. Molecules. 2024; 29(16):3884. https://doi.org/10.3390/molecules29163884
Chicago/Turabian StyleKhwaza, Vuyolwethu, and Blessing A. Aderibigbe. 2024. "Potential Pharmacological Properties of Triterpene Derivatives of Ursolic Acid" Molecules 29, no. 16: 3884. https://doi.org/10.3390/molecules29163884






