Fabrication Methods of Continuous Pure Metal–Organic Framework Membranes and Films: A Review
Abstract
1. Introduction
2. Fabrication Method of Pure MOF Membranes
2.1. In Situ Solvothermal Growth
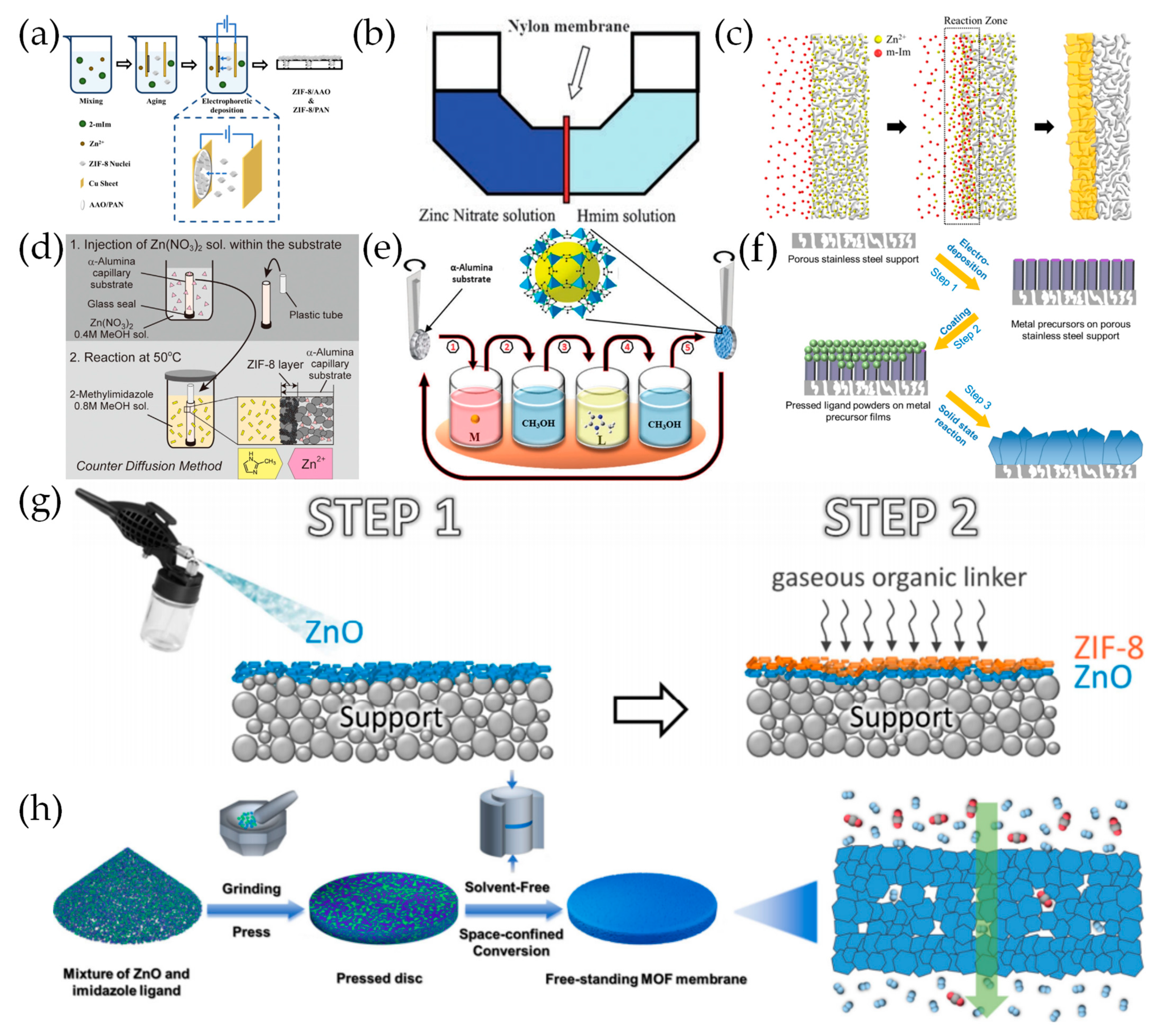
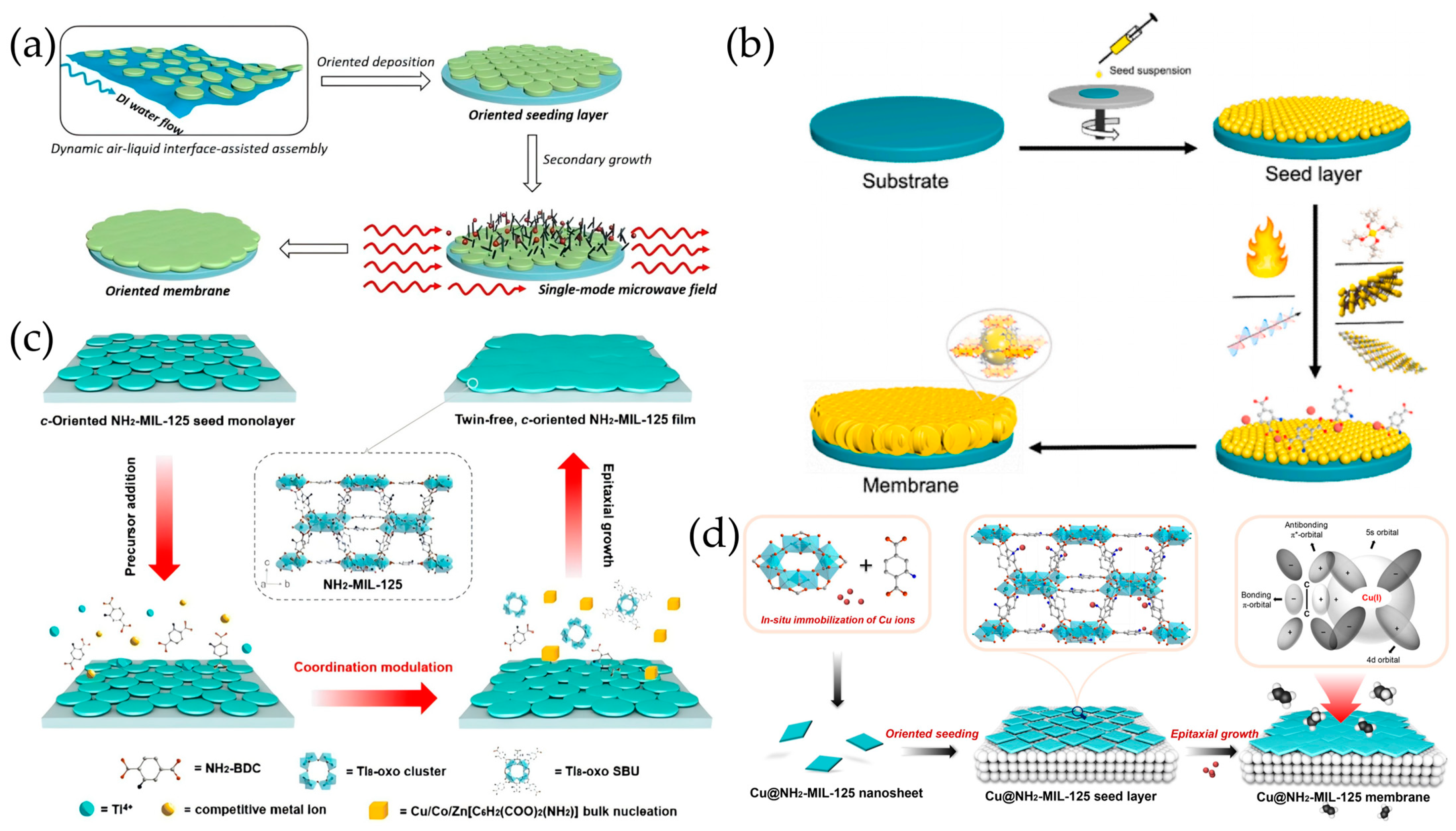
2.2. Secondary Growth of MOF Crystal Seeds
2.3. Electrochemical Deposition
2.4. Counter Diffusion Growth
2.5. Liquid Phase Epitaxy
2.6. Solvent-Free Synthesis
3. Fabrication of Zeolitic Imidazolate Framework Membranes
3.1. In Situ Solvothermal Synthesis
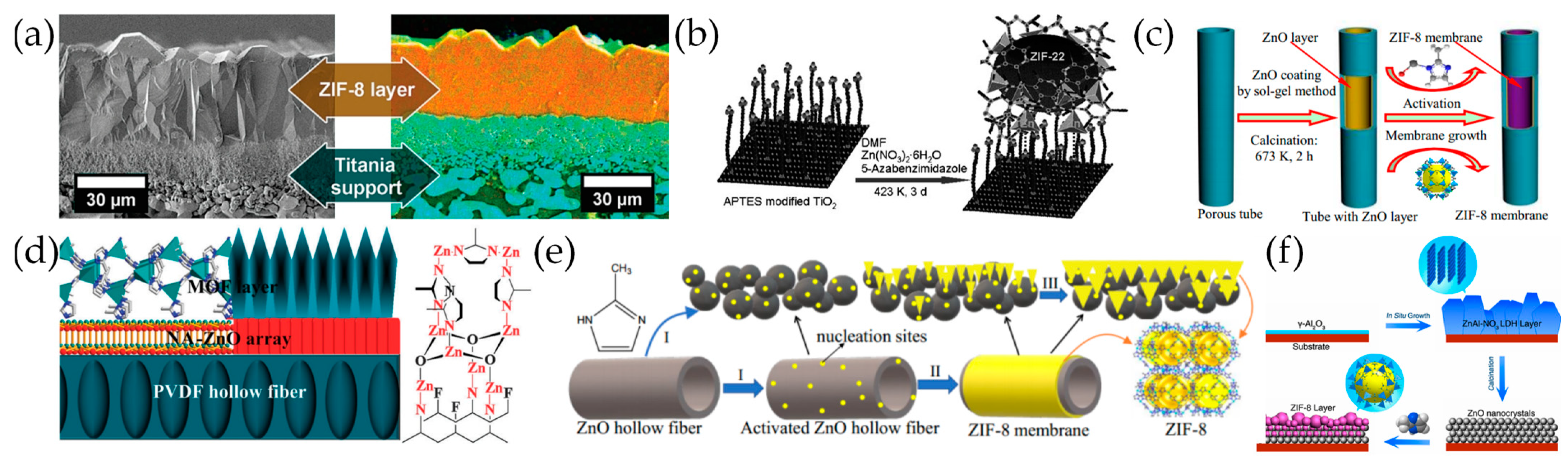
3.2. Secondary Growth

3.3. Electrochemical Deposition
3.4. Counter Diffusion Growth
3.5. Liquid Phase Epitaxy
3.6. Solvent-Free Synthesis
4. Fabrication of Cu-Based MOF Membranes
4.1. In Situ Solvothermal Synthesis
4.2. Secondary Growth
4.3. Electrochemical Deposition
4.4. Liquid Phase Epitaxy

4.5. Solvent-Free Synthesis
5. Fabrication of Zr-Based MOF Membranes
5.1. In Situ Solvothermal Synthesis
5.2. Secondary Growth
5.3. Electrochemical Deposition
5.4. Counter Diffusion Growth
5.5. Liquid Phase Epitaxy

5.6. Solvent-Free Synthesis
6. Fabrication of Al-Based MOF Membranes
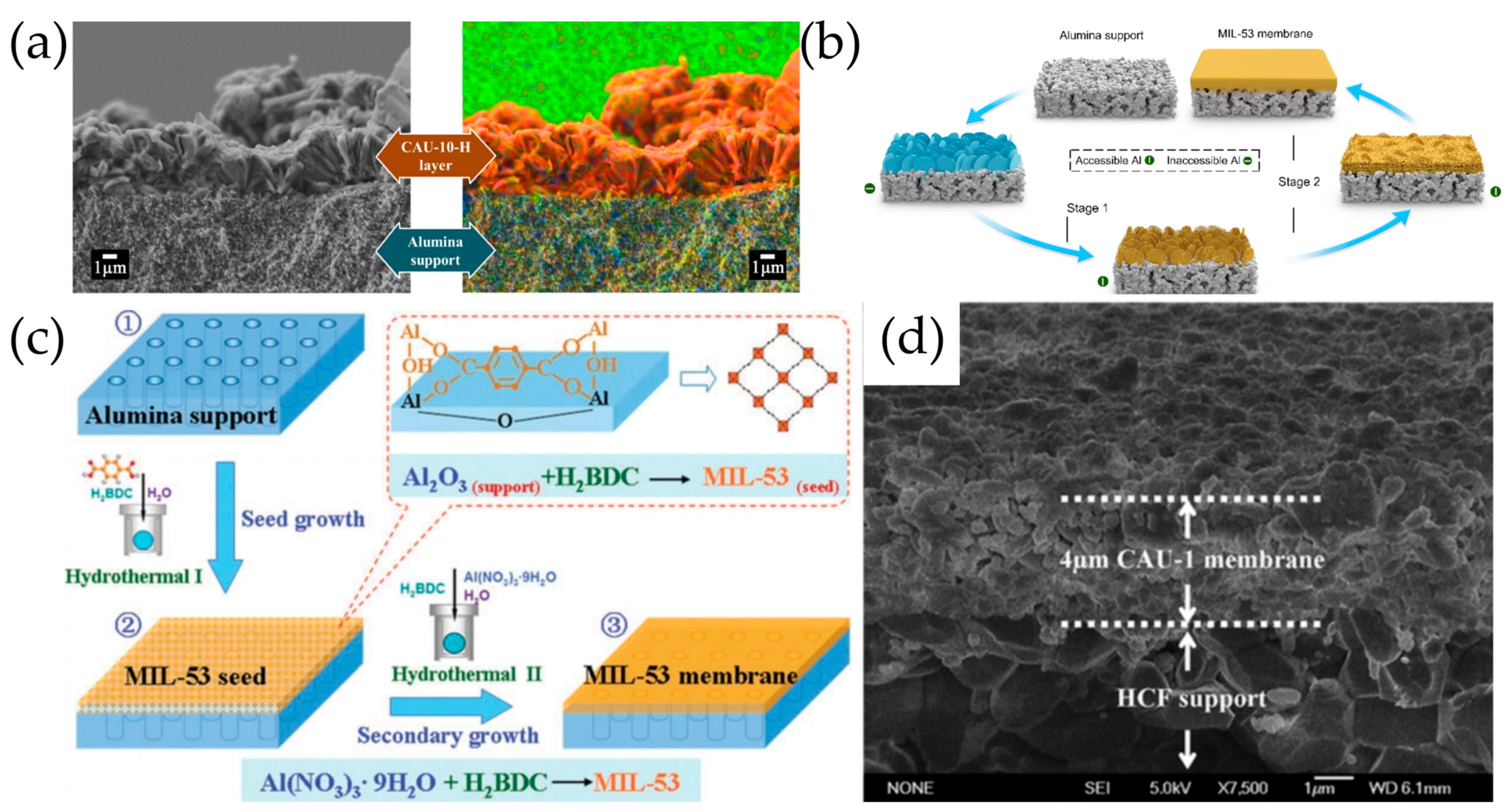
6.1. In Situ Solvothermal Synthesis
6.2. Secondary Growth
7. Fabrication of Ni-Based MOF Membranes
7.1. In Situ Solvothermal Synthesis
7.2. Secondary Growth
7.3. Counter Diffusion Growth

8. Fabrication of Ti-Based MOF Membranes
9. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A historical overview of the activation and porosity of metal-organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.; Besikiotis, V.; Blom, R. Application of metal–organic frameworks with coordinatively unsaturated metal sites in storage and separation of methane and carbon dioxide. J. Mater. Chem. 2009, 19, 7362–7370. [Google Scholar] [CrossRef]
- Carboni, M.; Abney, C.W.; Liu, S.; Lin, W. Highly porous and stable metal–organic frameworks for uranium extraction. Chem. Sci. 2013, 4, 2396–2402. [Google Scholar] [CrossRef]
- Demir, H.; Aksu, G.O.; Gulbalkan, H.C.; Keskin, S. MOF Membranes for CO2 Capture: Past, Present and Future. Carbon Capture Sci. Technol. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Shekhah, O.; Belmabkhout, Y.; Chen, Z.; Guillerm, V.; Cairns, A.; Adil, K.; Eddaoudi, M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 2014, 5, 4228. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A homochiral metal-organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Zhu, B.; Zou, R.; Xu, Q. Metal–Organic Framework Based Catalysts for Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801193. [Google Scholar] [CrossRef]
- Sun, F.; Li, Q.; Xue, H.; Pang, H. Pristine Transition-Metal-Based Metal-Organic Frameworks for Electrocatalysis. ChemElectroChem 2019, 6, 1273–1299. [Google Scholar] [CrossRef]
- Xiang, R.; Zhou, C.; Liu, Y.; Qin, T.; Li, D.; Dong, X.; Muddassir, M.; Zhong, A. A new type Co(II)-based photocatalyst for the nitrofurantoin antibiotic degradation. J. Mol. Struct. 2024, 1312, 138501. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, X.-Y.; Cheng, P.; Liao, D.-Z.; Yan, S.-P.; Jiang, Z.-H. Coordination Polymers Containing 1D Channels as Selective Luminescent Probes. J. Am. Chem. Soc. 2004, 126, 15394–15395. [Google Scholar] [CrossRef] [PubMed]
- Achmann, S.; Hagen, G.; Kita, J.; Malkowsky, I.M.; Kiener, C.; Moos, R. Metal-organic frameworks for sensing applications in the gas phase. Sensors 2009, 9, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, T.; Gao, Y.; Cai, Y.; Liu, J.; Ramachandraiah, K.; Mao, J.; Ke, F. Functional modification engineering of metal–organic frameworks for the contaminants detection in food. Coord. Chem. Rev. 2024, 516, 215990. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef]
- Huang, S.; Lu, H.; Chen, J.; Jiang, C.; Jiang, G.; Maduraiveeran, G.; Pan, Y.; Liu, J.; Deng, L.E. Advances in drug delivery-based therapeutic strategies for renal fibrosis treatment. J. Mater. Chem. B 2024, 12, 6532–6549. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, L.; Hu, W.; Luo, J.; Nezamzadeh-Ejhieh, A.; Ouyang, J.; Liu, X.; Pan, Y.; Cui, S.; Liu, J. Recent advances in NO-triggered gas therapy by metal-organic frameworks. Mater. Today Chem. 2024, 36, 101964. [Google Scholar] [CrossRef]
- Tu, K.; Ding, Y.; Keplinger, T. Review on design strategies and applications of metal-organic framework-cellulose composites. Carbohydr. Polym. 2022, 291, 119539. [Google Scholar] [CrossRef]
- Cheng, Y.; Datta, S.J.; Zhou, S.; Jia, J.; Shekhah, O.; Eddaoudi, M. Advances in metal–organic framework-based membranes. Chem. Soc. Rev. 2022, 51, 8300–8350. [Google Scholar] [CrossRef]
- Husna, A.; Hossain, I.; Jeong, I.; Kim, T.H. Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers 2022, 14, 655. [Google Scholar] [CrossRef]
- Shu, L.; Peng, Y.; Yao, R.; Song, H.; Zhu, C.; Yang, W. Flexible Soft-Solid Metal-Organic Framework Composite Membranes for H2/CO2 Separation. Angew. Chem. Int. Ed. 2022, 61, e202117577. [Google Scholar] [CrossRef]
- Arnold, M.; Kortunov, P.; Jones, D.J.; Nedellec, Y.; Kärger, J.; Caro, J. Oriented Crystallisation on Supports and Anisotropic Mass Transport of the Metal-Organic Framework Manganese Formate. Eur. J. Inorg. Chem. 2006, 2007, 60–64. [Google Scholar] [CrossRef]
- Zhou, S.; Shekhah, O.; Ramírez, A.; Lyu, P.; Abou-Hamad, E.; Jia, J.; Li, J.; Bhatt, P.M.; Huang, Z.; Jiang, H.; et al. Asymmetric pore windows in MOF membranes for natural gas valorization. Nature 2022, 606, 706–712. [Google Scholar] [CrossRef]
- Ma, C.; Liu, H.; Qiu, J.; Zhang, X. Bimetallic Zn/Co-ZIF tubular membrane for highly efficient pervaporation separation of Methanol/MTBE mixture. J. Membr. Sci. 2021, 638, 119676. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Wang, T.-Y.; Zou, C.; Chen, J.-J.; Lin, L.-C.; Kang, D.-Y. Highly-selective MOF-303 membrane for alcohol dehydration. J. Membr. Sci. 2022, 661, 120879. [Google Scholar] [CrossRef]
- Zhu, Y.; Gupta, K.M.; Liu, Q.; Jiang, J.; Caro, J.; Huang, A. Synthesis and seawater desalination of molecular sieving zeolitic imidazolate framework membranes. Desalination 2016, 385, 75–82. [Google Scholar] [CrossRef]
- Liu, Y.; Ng, Z.; Khan, E.A.; Jeong, H.-K.; Ching, C.-B.; Lai, Z. Synthesis of continuous MOF-5 membranes on porous α-alumina substrates. Microporous Mesoporous Mater. 2009, 118, 296–301. [Google Scholar] [CrossRef]
- Jia, S.; Ji, D.; Wang, L.; Qin, X.; Ramakrishna, S. Metal–Organic Framework Membranes: Advances, Fabrication, and Applications. Small Struct. 2022, 3, 2100222. [Google Scholar] [CrossRef]
- Shi, X.; Shan, Y.; Du, M.; Pang, H. Synthesis and application of metal-organic framework films. Coord. Chem. Rev. 2021, 444, 214060. [Google Scholar] [CrossRef]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly Water-Stable Zirconium Metal-Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef]
- Ranjan, R.; Tsapatsis, M. Microporous Metal Organic Framework Membrane on Porous Support Using the Seeded Growth Method. Chem. Mater. 2009, 21, 4920–4924. [Google Scholar] [CrossRef]
- Guo, H.; Liu, J.; Li, Y.; Caro, J.; Huang, A. Post-synthetic modification of highly stable UiO-66-NH2 membranes on porous ceramic tubes with enhanced H2 separation. Microporous Mesoporous Mater. 2021, 313, 110823. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Liu, H.; Qiu, J. Synthesis of a highly stable ZIF-8 membrane on a macroporous ceramic tube by manual-rubbing ZnO deposition as a multifunctional layer. J. Membr. Sci. 2015, 490, 354–363. [Google Scholar] [CrossRef]
- Wu, F.; Lin, L.; Liu, H.; Wang, H.; Qiu, J.; Zhang, X. Synthesis of stable UiO-66 membranes for pervaporation separation of methanol/methyl tert-butyl ether mixtures by secondary growth. J. Membr. Sci. 2017, 544, 342–350. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, X.; Nan, J.; Jin, W.; Ren, X.; Xu, N.; Lee, Y.M. Metal–organic framework membranes fabricated via reactive seeding. Chem. Commun. 2011, 47, 737–739. [Google Scholar] [CrossRef]
- Guerrero, V.V.; Yoo, Y.; McCarthy, M.C.; Jeong, H.-K. HKUST-1 membranes on porous supports using secondary growth. J. Mater. Chem. 2010, 20, 3938–3943. [Google Scholar] [CrossRef]
- Shi, E.; Zou, X.; Liu, J.; Lin, H.; Zhang, F.; Shi, S.; Liu, F.; Zhu, G.; Qu, F. Electrochemical fabrication of copper-containing metal-organic framework films as amperometric detectors for bromate determination. Dalton Trans. 2016, 45, 7728–7736. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, X.; Luo, X.; Hu, X.; Ji, L. Cathodic electrodeposited Cu-BTC MOFs assembled from Cu(II) and trimesic acid for electrochemical determination of bisphenol A. Mikrochim. Acta 2020, 187, 145. [Google Scholar] [CrossRef]
- Hod, I.; Bury, W.; Karlin, D.M.; Deria, P.; Kung, C.W.; Katz, M.J.; So, M.; Klahr, B.; Jin, D.; Chung, Y.W.; et al. Directed growth of electroactive metal-organic framework thin films using electrophoretic deposition. Adv. Mater. 2014, 26, 6295–6300. [Google Scholar] [CrossRef]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned Growth of Metal-Organic Framework Coatings by Electrochemical Synthesis. Chem. Mater. 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Li, M.; Dinca, M. Reductive electrosynthesis of crystalline metal-organic frameworks. J. Am. Chem. Soc. 2011, 133, 12926–12929. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, K.; Subramanian, P.; Xu, M.; Luo, J.; Fransaer, J. Electrochemical deposition of metal–organic framework films and their applications. J. Mater. Chem. A 2020, 8, 7569–7587. [Google Scholar] [CrossRef]
- Yao, J.; Dong, D.; Li, D.; He, L.; Xu, G.; Wang, H. Contra-diffusion synthesis of ZIF-8 films on a polymer substrate. Chem. Commun. 2011, 47, 2559–2561. [Google Scholar] [CrossRef]
- Karimi, A.; Vatanpour, V.; Khataee, A.; Safarpour, M. Contra-diffusion synthesis of ZIF-8 layer on polyvinylidene fluoride ultrafiltration membranes for improved water purification. J. Ind. Eng. Chem. 2019, 73, 95–105. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, X.; Zu, Y.; Sun, F.; Zhu, G. Interfacial growth of 2D MOF membranes via contra-diffusion for CO2 separation. Mater. Chem. Front. 2021, 5, 5150–5157. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.-F.; Yao, J.; Wang, H. Contra-diffusion synthesis of metal-organic framework separation membranes: A review. Sep. Purif. Technol. 2022, 300, 121837. [Google Scholar] [CrossRef]
- Wannapaiboon, S.; Sumida, K.; Dilchert, K.; Tu, M.; Kitagawa, S.; Furukawa, S.; Fischer, R.A. Enhanced properties of metal–organic framework thin films fabricated via a coordination modulation-controlled layer-by-layer process. J. Mater. Chem. A 2017, 5, 13665–13673. [Google Scholar] [CrossRef]
- Chernikova, V.; Shekhah, O.; Spanopoulos, I.; Trikalitis, P.N.; Eddaoudi, M. Liquid phase epitaxial growth of heterostructured hierarchical MOF thin films. Chem. Commun. 2017, 53, 6191–6194. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Liu, X.; Liu, Q.; Hao, M.; Wang, S.; Chen, Z.; Yang, H.; Wang, X. Design and application of metal–organic framework membranes for gas and liquid separations. Sep. Purif. Technol. 2024, 329, 125178. [Google Scholar] [CrossRef]
- Reif, B.; Somboonvong, J.; Fabisch, F.; Kaspereit, M.; Hartmann, M.; Schwieger, W. Solvent-free transformation of spray coated ZnO layers to ZIF-8 membranes. Microporous Mesoporous Mater. 2019, 276, 29–40. [Google Scholar] [CrossRef]
- Hou, J.; Hong, X.; Zhou, S.; Wei, Y.; Wang, H. Solvent-free route for metal–organic framework membranes growth aiming for efficient gas separation. AIChE J. 2018, 65, 712–722. [Google Scholar] [CrossRef]
- Stassen, I.; Campagnol, N.; Fransaer, J.; Vereecken, P.; De Vos, D.; Ameloot, R. Solvent-free synthesis of supported ZIF-8 films and patterns through transformation of deposited zinc oxide precursors. CrystEngComm 2013, 15, 9308–9311. [Google Scholar] [CrossRef]
- Ren, J.; Jen, T.-C. Atomic layer deposition (ALD) assisting the visibility of metal-organic frameworks (MOFs) technologies. Coord. Chem. Rev. 2021, 430, 213734. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K. Highly propylene-selective supported zeolite-imidazolate framework (ZIF-8) membranes synthesized by rapid microwave-assisted seeding and secondary growth. Chem. Commun. 2013, 49, 3854–3856. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Pan, Y.; Lai, Z. Synthesis of highly c-oriented ZIF-69 membranes by secondary growth and their gas permeation properties. J. Membr. Sci. 2011, 379, 46–51. [Google Scholar] [CrossRef]
- Huang, K.; Li, Q.; Liu, G.; Shen, J.; Guan, K.; Jin, W. A ZIF-71 Hollow Fiber Membrane Fabricated by Contra-Diffusion. ACS Appl. Mater. Interfaces 2015, 7, 16157–16160. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic Imidazolate Framework Membrane with Molecular Sieving Properties by Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Bux, H.; Steinbach, F.; Caro, J. Molecular-sieve membrane with hydrogen permselectivity: ZIF-22 in LTA topology prepared with 3-aminopropyltriethoxysilane as covalent linker. Angew. Chem. Int. Ed. Engl. 2010, 49, 4958–4961. [Google Scholar] [CrossRef]
- Huang, A.; Chen, Y.; Wang, N.; Hu, Z.; Jiang, J.; Caro, J. A highly permeable and selective zeolitic imidazolate framework ZIF-95 membrane for H2/CO2 separation. Chem. Commun. 2012, 48, 10981–10983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Q.; Caro, J.; Huang, A. Highly hydrogen-permselective zeolitic imidazolate framework ZIF-8 membranes prepared on coarse and macroporous tubes through repeated synthesis. Sep. Purif. Technol. 2015, 146, 68–74. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, D.; Liu, Z.; Zhong, C. Synthesis of Zeolitic Imidazolate Framework Membrane Using Temperature-Switching Synthesis Strategy for Gas Separation. Ind. Eng. Chem. Res. 2016, 55, 7164–7170. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, N.; Caro, J.; Huang, A. Bio-inspired polydopamine: A versatile and powerful platform for covalent synthesis of molecular sieve membranes. J. Am. Chem. Soc. 2013, 135, 17679–17682. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yue, T.; Qi, K.; Xia, B.Y.; Chen, Z.; Qiu, Y.; Guo, X. Probe into metal-organic framework membranes fabricated via versatile polydopamine-assisted approach onto metal surfaces as anticorrosion coatings. Corros. Sci. 2020, 177, 108949. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Qiao, Z.; Diestel, L.; Zhou, J.; Huang, A.; Caro, J. Polydopamine-based synthesis of a zeolite imidazolate framework ZIF-100 membrane with high H2/CO2 selectivity. J. Mater. Chem. A 2015, 3, 4722–4728. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Kong, L.; Liu, H.; Qiu, J.; Han, W.; Weng, L.-T.; Yeung, K.L.; Zhu, W. A simple and scalable method for preparing low-defect ZIF-8 tubular membranes. J. Mater. Chem. A 2013, 1, 10635–10638. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, S.; Kong, L.; Liu, H.; Li, Y.; Han, W.; Yeung, K.L.; Zhu, W.; Yang, W.; et al. New Membrane Architecture with High Performance: ZIF-8 Membrane Supported on Vertically Aligned ZnO Nanorods for Gas Permeation and Separation. Chem. Mater. 2014, 26, 1975–1981. [Google Scholar] [CrossRef]
- Li, W.; Meng, Q.; Li, X.; Zhang, C.; Fan, Z.; Zhang, G. Non-activation ZnO array as a buffering layer to fabricate strongly adhesive metal-organic framework/PVDF hollow fiber membranes. Chem. Commun. 2014, 50, 9711–9713. [Google Scholar] [CrossRef]
- Hara, N.; Hasegawa, Y.; Tanaka, H.; Yoshimune, M.; Yamaki, T.; Negishi, H. Development of ZIF-8 Membranes for Propylene/Propane Separation by Direct Growth on a ZnO-Modified Support without Activation. J. Chem. Eng. Jpn. 2020, 53, 616–625. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, X.; Meng, B.; Yang, N.; Tan, X.; Liu, S. Preparation of ZIF-8 Membranes on Porous ZnO Hollow Fibers by a Facile ZnO-Induced Method. Ind. Eng. Chem. Res. 2020, 59, 15576–15585. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Y.; Wang, N.; Li, Y.; Pan, J.H.; Yang, W.; Caro, J. Significantly Enhanced Separation using ZIF-8 Membranes by Partial Conversion of Calcined Layered Double Hydroxide Precursors. ChemSusChem 2015, 8, 3582–3586. [Google Scholar] [CrossRef]
- Ji, T.; Liu, L.; Wu, M.; Yu, K.; He, X.; Liu, Y. Subfreezing conversion of ALD-derived ZnO layer to ultra-thin ZIF-8 membrane for high-flux C3H6 production. Chem. Eng. Sci. 2023, 282, 119293. [Google Scholar] [CrossRef]
- Li, J.; Cao, W.; Mao, Y.; Ying, Y.; Sun, L.; Peng, X. Zinc hydroxide nanostrands: Unique precursors for synthesis of ZIF-8 thin membranes exhibiting high size-sieving ability for gas separation. CrystEngComm 2014, 16, 9788–9791. [Google Scholar] [CrossRef]
- Ji, T.; Yan, J.; He, Y.; Hu, W.; Peng, Y.; Yang, W.; Liu, Y. Towards ZIF-8 membranes with superb C3H6/C3H8 separation performances under varying conditions: Necessity of precise temperature control. J. Membr. Sci. 2023, 683, 121776. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Ma, Q.; Mo, K.; Mao, H.; Feldhoff, A.; Cao, X.; Li, Y.; Pan, F.; Jiang, Z. A MOF Glass Membrane for Gas Separation. Angew. Chem. Int. Ed. Engl. 2020, 59, 4365–4369. [Google Scholar] [CrossRef]
- Yang, Z.; Belmabkhout, Y.; McHugh, L.N.; Ao, D.; Sun, Y.; Li, S.; Qiao, Z.; Bennett, T.D.; Guiver, M.D.; Zhong, C. ZIF-62 glass foam self-supported membranes to address CH4/N2 separations. Nat. Mater. 2023, 22, 888–894. [Google Scholar] [CrossRef]
- Li, Y.S.; Liang, F.Y.; Bux, H.; Feldhoff, A.; Yang, W.S.; Caro, J. Molecular sieve membrane: Supported metal-organic framework with high hydrogen selectivity. Angew. Chem. Int. Ed. Engl. 2010, 49, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Venna, S.R.; Carreon, M.A. Highly permeable zeolite imidazolate framework-8 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2010, 132, 76–78. [Google Scholar] [CrossRef]
- Dong, X.; Huang, K.; Liu, S.; Ren, R.; Jin, W.; Lin, Y.S. Synthesis of zeolitic imidazolate framework-78 molecular-sieve membrane: Defect formation and elimination. J. Mater. Chem. 2012, 22, 19222. [Google Scholar] [CrossRef]
- Kasik, A.; Dong, X.; Lin, Y.S. Synthesis and stability of zeolitic imidazolate framework-68 membranes. Microporous Mesoporous Mater. 2015, 204, 99–105. [Google Scholar] [CrossRef]
- Li, L.; Yao, J.; Chen, R.; He, L.; Wang, K.; Wang, H. Infiltration of precursors into a porous alumina support for ZIF-8 membrane synthesis. Microporous Mesoporous Mater. 2013, 168, 15–18. [Google Scholar] [CrossRef]
- Fan, L.; Xue, M.; Kang, Z.; Wei, G.; Huang, L.; Shang, J.; Zhang, D.; Qiu, S. ZIF-78 membrane derived from amorphous precursors with permselectivity for cyclohexanone/cyclohexanol mixture. Microporous Mesoporous Mater. 2014, 192, 29–34. [Google Scholar] [CrossRef]
- Huang, K.; Dong, Z.; Li, Q.; Jin, W. Growth of a ZIF-8 membrane on the inner-surface of a ceramic hollow fiber via cycling precursors. Chem. Commun. 2013, 49, 10326–10328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, N.; Diestel, L.; Steinbach, F.; Caro, J. MOF membrane synthesis in the confined space of a vertically aligned LDH network. Chem. Commun. 2014, 50, 4225–4227. [Google Scholar] [CrossRef] [PubMed]
- Venna, S.R.; Zhu, M.; Li, S.; Carreon, M.A. Knudsen diffusion through ZIF-8 membranes synthesized by secondary seeded growth. J. Porous Mater. 2013, 21, 235–240. [Google Scholar] [CrossRef]
- Ma, Q.; Li, G.; Liu, X.; Wang, Z.; Song, Z.; Wang, H. Zeolitic imidazolate framework-8 film coated stainless steel meshes for highly efficient oil/water separation. Chem. Commun. 2018, 54, 5530–5533. [Google Scholar] [CrossRef]
- Song, M.; Zhao, Y.; Mu, S.; Jiang, C.; Li, Z.; Yang, P.; Fang, Q.; Xue, M.; Qiu, S. A stable ZIF-8-coated mesh membrane with micro-/nano architectures produced by a facile fabrication method for high-efficiency oil-water separation. Sci. China Mater. 2018, 62, 536–544. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, M.; Liang, X.; Wang, S.; Wang, H.; Zhu, Z.; Ren, Y.; Zhang, Z.; He, G.; Zhao, D.; et al. Growing ZIF-8 Seeds on Charged COF Substrates toward Efficient Propylene-Propane Separation Membranes. Angew. Chem. Int. Ed. Engl. 2023, 62, e202302355. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, J.; Ji, T.; Du, D.; Sun, Y.; Liu, L.; Zhang, X.; Liu, Y. Fabrication of highly (110)-Oriented ZIF-8 membrane at low temperature using nanosheet seed layer. J. Membr. Sci. 2022, 641, 119915. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, H.; Zhitomirsky, I.; Zhu, S. Preparation of metal–organic framework films by electrophoretic deposition method. Mater. Lett. 2015, 142, 19–22. [Google Scholar] [CrossRef]
- Ji, Y.; Song, Y.; Huang, Y.; Zhu, H.; Yue, C.; Liu, F.; Zhao, J. One-Step Synthesis of Ultrathin Zeolitic Imidazole Framework-8 (ZIF-8) Membrane on Unmodified Porous Support via Electrophoretic Deposition. Membranes 2022, 12, 1062. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K. In situ synthesis of thin zeolitic-imidazolate framework ZIF-8 membranes exhibiting exceptionally high propylene/propane separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef]
- Jang, E.; Kim, E.; Kim, H.; Lee, T.; Yeom, H.-J.; Kim, Y.-W.; Choi, J. Formation of ZIF-8 membranes inside porous supports for improving both their H2/CO2 separation performance and thermal/mechanical stability. J. Membr. Sci. 2017, 540, 430–439. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Diffusive separation of propylene/propane with ZIF-8 membranes. J. Membr. Sci. 2014, 450, 215–223. [Google Scholar] [CrossRef]
- Shekhah, O.; Eddaoudi, M. The liquid phase epitaxy method for the construction of oriented ZIF-8 thin films with controlled growth on functionalized surfaces. Chem. Commun. 2013, 49, 10079–10081. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Swaidan, R.; Belmabkhout, Y.; du Plessis, M.; Jacobs, T.; Barbour, L.J.; Pinnau, I.; Eddaoudi, M. The liquid phase epitaxy approach for the successful construction of ultra-thin and defect-free ZIF-8 membranes: Pure and mixed gas transport study. Chem. Commun. 2014, 50, 2089–2092. [Google Scholar] [CrossRef]
- Zvyagina, A.I.; Shiryaev, A.A.; Baranchikov, A.E.; Chernyshev, V.V.; Enakieva, Y.Y.; Raitman, O.A.; Ezhov, A.A.; Meshkov, I.N.; Grishanov, D.A.; Ivanova, O.S.; et al. Layer-by-layer assembly of porphyrin-based metal–organic frameworks on solids decorated with graphene oxide. New J. Chem. 2017, 41, 948–957. [Google Scholar] [CrossRef]
- Li, W.; Su, P.; Li, Z.; Xu, Z.; Wang, F.; Ou, H.; Zhang, J.; Zhang, G.; Zeng, E. Ultrathin metal-organic framework membrane production by gel-vapour deposition. Nat. Commun. 2017, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kumar, P.; Mittal, N.; Khlyustova, A.; Daoutidis, P.; Mkhoyan, K.A.; Tsapatsis, M. Zeolitic imidazolate framework membranes made by ligand-induced permselectivation. Science 2018, 361, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Bo, R.; Taheri, M.; Liu, B.; Ricco, R.; Chen, H.; Amenitsch, H.; Fusco, Z.; Tsuzuki, T.; Yu, G.; Ameloot, R.; et al. Hierarchical Metal-Organic Framework Films with Controllable Meso/Macroporosity. Adv. Sci. 2020, 7, 2002368. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, B.; Li, Z.; Yu, T.; Wang, S.; Fang, Q.; Qiu, S.; Xue, M. Free-Standing Metal-Organic Framework Membranes Made by Solvent-Free Space-Confined Conversion for Efficient H2/CO2 Separation. AACS Appl. Mater. Interfaces 2023, 15, 19241–19249. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Shi, Y.; Xu, Y.; Cao, J.; Meng, Y.; Chen, H. Metal-organic framework membranes with varying metal ions for enhanced water and wastewater treatment: A critical review. J. Environ. Chem. Eng. 2023, 11, 11468. [Google Scholar] [CrossRef]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, G.; Hewitt, I.J.; Qiu, S. “Twin copper source” growth of metal-organic framework membrane: Cu3(BTC)2 with high permeability and selectivity for recycling H2. J. Am. Chem. Soc. 2009, 131, 1646–1647. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.; Du, D.; Zhang, M.; Liu, Y.; Liu, L.; Ji, T.; He, G.; Liu, Y. Fabrication of c-oriented ultrathin TCPP-derived 2D MOF membrane for precise molecular sieving. J. Membr. Sci. 2021, 634, 119393. [Google Scholar] [CrossRef]
- Gascon, J.; Aguado, S.; Kapteijn, F. Manufacture of dense coatings of Cu3(BTC)2 (HKUST-1) on α-alumina. Microporous Mesoporous Mater. 2008, 113, 132–138. [Google Scholar] [CrossRef]
- Noh, S.-J.; Kwon, H.T.; Kim, J. Synthesis and Characterization of Cu3(BTC)2 Membranes by Thermal Spray Seeding and Secondary Growth. J. Nanosci. Nanotechnol. 2013, 13, 5671–5674. [Google Scholar] [CrossRef]
- Nan, J.; Dong, X.; Wang, W.; Jin, W.; Xu, N. Step-by-Step Seeding Procedure for Preparing HKUST-1 Membrane on Porous α-Alumina Support. Langmuir 2011, 27, 4309–4312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kusaka, S.; Hori, A.; Matsuda, R. Fabrication of a Kagomé-type MOF Membrane by Seeded Growth on Amino-functionalized Porous Al2O3 Substrate. Chemistry 2021, 16, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Cao, W.; Li, J.; Liu, Y.; Ying, Y.; Sun, L.; Peng, X. Enhanced gas separation through well-intergrown MOF membranes: Seed morphology and crystal growth effects. J. Mater. Chem. A 2013, 1, 11711–11716. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Chen, C.; Chen, Y.; Li, W.; Wang, J. Secondary-assembled defect-free MOF membrane via triple-needle electrostatic atomization for highly stable and selective organics permeation. J. Membr. Sci. 2022, 648, 120382. [Google Scholar] [CrossRef]
- Van de Voorde, B.; Ameloot, R.; Stassen, I.; Everaert, M.; De Vos, D.; Tan, J.-C. Mechanical properties of electrochemically synthesised metal–organic framework thin films. J. Mater. Chem. C 2013, 1, 7716–7724. [Google Scholar] [CrossRef]
- Guo, W.; Monnens, W.; Zhang, W.; Xie, S.; Han, N.; Zhou, Z.; Chanut, N.; Vanstreels, K.; Ameloot, R.; Zhang, X.; et al. Anodic electrodeposition of continuous metal-organic framework films with robust adhesion by pre-anchored strategy. Microporous Mesoporous Mater. 2023, 350, 112443. [Google Scholar] [CrossRef]
- Xie, S.; Monnens, W.; Wan, K.; Zhang, W.; Guo, W.; Xu, M.; Vankelecom, I.F.J.; Zhang, X.; Fransaer, J. Cathodic Electrodeposition of MOF Films Using Hydrogen Peroxide. Angew. Chem. Int. Ed. 2021, 60, 24950–24957. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Kowarik, S.; Schreiber, F.; Paulus, M.; Tolan, M.; Sternemann, C.; Evers, F.; Zacher, D.; Fischer, R.A.; et al. Step-by-Step Route for the Synthesis of Metal−Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 15118–15119. [Google Scholar] [CrossRef]
- Bétard, A.; Bux, H.; Henke, S.; Zacher, D.; Caro, J.; Fischer, R.A. Fabrication of a CO2-selective membrane by stepwise liquid-phase deposition of an alkylether functionalized pillared-layered metal-organic framework [Cu2L2P]n on a macroporous support. Microporous Mesoporous Mater. 2012, 150, 76–82. [Google Scholar] [CrossRef]
- Chang, L.-M.; An, Y.-Y.; Li, Q.-H.; Gu, Z.-G.; Han, Y.-F.; Zhang, J. N-Heterocyclic Carbene as a Surface Platform for Assembly of Homochiral Metal–Organic Framework Thin Films in Chiral Sensing. ACS Appl. Mater. Interfaces 2020, 12, 38357–38364. [Google Scholar] [CrossRef]
- Stavila, V.; Schneider, C.; Mowry, C.; Zeitler, T.R.; Greathouse, J.A.; Robinson, A.L.; Denning, J.M.; Volponi, J.; Leong, K.; Quan, W.; et al. Thin Film Growth of nbo MOFs and their Integration with Electroacoustic Devices. Adv. Funct. Mater. 2016, 26, 1699–1707. [Google Scholar] [CrossRef]
- Ikigaki, K.; Okada, K.; Tokudome, Y.; Toyao, T.; Falcaro, P.; Doonan, C.J.; Takahashi, M. MOF-on-MOF: Oriented Growth of Multiple Layered Thin Films of Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 6886–6890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rodewald, K.; Medishetty, R.; Rieger, B.; Fischer, R.A. Control of Water Content for Enhancing the Quality of Copper Paddle-Wheel-Based Metal–Organic Framework Thin Films Grown by Layer-by-Layer Liquid-Phase Epitaxy. Cryst. Growth Des. 2018, 18, 7451–7459. [Google Scholar] [CrossRef]
- Stassin, T.; Rodríguez-Hermida, S.; Schrode, B.; Cruz, A.J.; Carraro, F.; Kravchenko, D.; Creemers, V.; Stassen, I.; Hauffman, T.; De Vos, D.; et al. Vapour-phase deposition of oriented copper dicarboxylate metal–organic framework thin films. Chem. Commun. 2019, 55, 10056–10059. [Google Scholar] [CrossRef] [PubMed]
- Ahvenniemi, E.; Karppinen, M. Atomic/molecular layer deposition: A direct gas-phase route to crystalline metal–organic framework thin films. Chem. Commun. 2016, 52, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Gikonyo, B.; Liu, F.; De, S.; Journet, C.; Marichy, C.; Fateeva, A. Investigating the vapour phase synthesis of copper terephthalate metal organic framework thin films by atomic/molecular layer deposition. Dalton Trans. 2023, 52, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, C.; Wang, B.; Li, K. Novel Organic-Dehydration Membranes Prepared from Zirconium Metal-Organic Frameworks. Adv. Funct. Mater. 2017, 27, 1604311. [Google Scholar] [CrossRef]
- Wan, L.; Zhou, C.; Xu, K.; Feng, B.; Huang, A. Synthesis of highly stable UiO-66-NH2 membranes with high ions rejection for seawater desalination. Microporous Mesoporous Mater. 2017, 252, 207–213. [Google Scholar] [CrossRef]
- Wu, F.; Cao, Y.; Liu, H.; Zhang, X. High-performance UiO-66-NH2 tubular membranes by zirconia-induced synthesis for desulfurization of model gasoline via pervaporation. J. Membr. Sci. 2018, 556, 54–65. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Yang, Y.; Yang, T.; Li, C.; Kawi, S.; Wang, X. Salicylaldehyde-assisted ZrO2-induced conversion approach to prepare high performance hollow fiber-supported UiO-66-NH2 membrane for hydrogen separation. J. Membr. Sci. 2023, 684, 121851. [Google Scholar] [CrossRef]
- Wu, M.; Sun, Y.; Ji, T.; Yu, K.; Liu, L.; He, Y.; Yan, J.; Meng, S.; Hu, W.; Fan, X.; et al. Fabrication of water-stable MOF-808 membrane for efficient salt/dye separation. J. Membr. Sci. 2023, 686, 122023. [Google Scholar] [CrossRef]
- Liu, J.; Canfield, N.; Liu, W. Preparation and Characterization of a Hydrophobic Metal–Organic Framework Membrane Supported on a Thin Porous Metal Sheet. Ind. Eng. Chem. Res. 2016, 55, 3823–3832. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kohmura, S.; Iwatsuka, H.; Oumi, Y.; Uemiya, S. In situ solvothermal growth of highly oriented Zr-based metal organic framework UiO-66 film with monocrystalline layer. CrystEngComm 2015, 17, 3422–3425. [Google Scholar] [CrossRef]
- Friebe, S.; Geppert, B.; Steinbach, F.; Caro, J. Metal–Organic Framework UiO-66 Layer: A Highly Oriented Membrane with Good Selectivity and Hydrogen Permeance. ACS Appl. Mater. Interfaces 2017, 9, 12878–12885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Mu, S.; Jiang, C.; Song, M.; Fang, Q.; Xue, M.; Qiu, S.; Chen, B. UiO-66-Coated Mesh Membrane with Underwater Superoleophobicity for High-Efficiency Oil–Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 17301–17308. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Van Assche, T.; Campagnol, N.; Fransaer, J.; Denayer, J.; Tan, J.-C.; Falcaro, P.; De Vos, D.; Ameloot, R. Electrochemical Film Deposition of the Zirconium Metal–Organic Framework UiO-66 and Application in a Miniaturized Sorbent Trap. Chem. Mater. 2015, 27, 1801–1807. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Zhu, G.-L.; Mo, R.-J.; Wu, M.-Y.; Ding, X.-L.; Huang, L.-Q.; Wu, Z.-Q.; Xia, X.-H. Janus Metal–Organic Framework Membranes Boosting the Osmotic Energy Harvesting. ACS Appl. Mater. Interfaces 2023, 15, 23922–23930. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Monnens, W.; Zhang, W.; Guo, W.; Han, N.; Zhou, Z.; Xue, Z.; Vankelecom, I.F.J.; Zhang, X.; Fransaer, J. Control over cathodic deposition of continuous UiO-66 films for ion-selective transport. Cell Rep. Phys. Sci. 2023, 4, 101412. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Hang, Y.; Liu, G.; Mo, B.; Li, J.; Ji, W.; Liu, G.; Jin, W. Crystal seeds induced interfacial growth of zirconium metal–organic framework membranes towards efficient hydrogen purification. J. Membr. Sci. 2024, 689, 122171. [Google Scholar] [CrossRef]
- Semrau, A.L.; Wannapaiboon, S.; Pujari, S.P.; Vervoorts, P.; Albada, B.; Zuilhof, H.; Fischer, R.A. Highly Porous Nanocrystalline UiO-66 Thin Films via Coordination Modulation Controlled Step-by-Step Liquid-Phase Growth. Cryst. Growth Des. 2018, 19, 1738–1747. [Google Scholar] [CrossRef]
- Semrau, A.L.; Fischer, R.A. High-Quality Thin Films of UiO-66-NH2 by Coordination Modulated Layer-by-Layer Liquid Phase Epitaxy. Chemistry 2021, 27, 8509–8516. [Google Scholar] [CrossRef]
- Hashem, T.; Valadez Sánchez, E.P.; Weidler, P.G.; Gliemann, H.; Alkordi, M.H.; Wöll, C. Liquid-Phase Quasi-Epitaxial Growth of Highly Stable, Monolithic UiO-66-NH2 MOF thin Films on Solid Substrates. ChemistryOpen 2020, 9, 524–527. [Google Scholar] [CrossRef]
- Virmani, E.; Rotter, J.M.; Mähringer, A.; von Zons, T.; Godt, A.; Bein, T.; Wuttke, S.; Medina, D.D. On-Surface Synthesis of Highly Oriented Thin Metal–Organic Framework Films through Vapor-Assisted Conversion. J. Am. Chem. Soc. 2018, 140, 4812–4819. [Google Scholar] [CrossRef]
- Lausund, K.B.; Nilsen, O. All-gas-phase synthesis of UiO-66 through modulated atomic layer deposition. Nat. Commun. 2016, 7, 13578. [Google Scholar] [CrossRef]
- Luo, R.; Fu, H.; Li, Y.; Xing, Q.; Liang, G.; Bai, P.; Guo, X.; Lyu, J.; Tsapatsis, M. In Situ Fabrication of Metal–Organic Framework Thin Films with Enhanced Pervaporation Performance. Adv. Funct. Mater. 2023, 33, 2213221. [Google Scholar] [CrossRef]
- Jin, H.; Wollbrink, A.; Yao, R.; Li, Y.; Caro, J.; Yang, W. A novel CAU-10-H MOF membrane for hydrogen separation under hydrothermal conditions. J. Membr. Sci. 2016, 513, 40–46. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Guo, H.; Fan, S. A novel heterogeneous MOF membrane MIL-121/118 with selectivity towards hydrogen. Inorg. Chem. Commun. 2020, 111, 107637. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Wang, J.; Wang, Z.; Kapteijn, F.; Liu, X. Highly Water-Permeable Metal-Organic Framework MOF-303 Membranes for Desalination. J. Am. Chem. Soc. 2021, 143, 20055–20058. [Google Scholar] [CrossRef]
- Wang, Y.; Ban, Y.; Hu, Z.; Yang, W. A LDH Template Triggers the Formation of a Highly Compact MIL-53 Metal-Organic Framework Membrane for Acid Upgrading. Angew. Chem. Int. Ed. 2023, 62, e202302181. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, X.; Gao, X.; Fan, S.; Sun, F.; Ren, H.; Zhu, G. Hydrogen Selective NH2-MIL-53(Al) MOF Membranes with High Permeability. Adv. Funct. Mater. 2012, 22, 3583–3590. [Google Scholar] [CrossRef]
- Zhou, S.; Zou, X.; Sun, F.; Ren, H.; Liu, J.; Zhang, F.; Zhao, N.; Zhu, G. Development of hydrogen-selective CAU-1 MOF membranes for hydrogen purification by ‘dual-metal-source’ approach. Int. J. Hydrogen Energy 2013, 38, 5338–5347. [Google Scholar] [CrossRef]
- Fan, S.; Wu, S.; Liu, J.; Liu, D. Fabrication of MIL-120 membranes supported by α-Al2O3 hollow ceramic fibers for H2 separation. RSC Adv. 2015, 5, 54757–54761. [Google Scholar] [CrossRef]
- Jin, H.; Mo, K.; Wen, F.; Li, Y. Preparation and pervaporation performance of CAU-10-H MOF membranes. J. Membr. Sci. 2019, 577, 129–136. [Google Scholar] [CrossRef]
- Lv, J.; Zhou, X.; Yang, J.; Wang, L.; Lu, J.; He, G.; Dong, Y. In-situ synthesis of KAUST-7 membranes from fluorinated molecular building block for H2/CO2 separation. J. Membr. Sci. 2022, 658, 120585. [Google Scholar] [CrossRef]
- Lee, D.-J.; Li, Q.; Kim, H.; Lee, K. Preparation of Ni-MOF-74 membrane for CO2 separation by layer-by-layer seeding technique. Microporous Mesoporous Mater. 2012, 163, 169–177. [Google Scholar] [CrossRef]
- Kang, Z.; Xue, M.; Fan, L.; Huang, L.; Guo, L.; Wei, G.; Chen, B.; Qiu, S. Highly selective sieving of small gas molecules by using an ultra-microporous metal–organic framework membrane. Energy Environ. Sci. 2014, 7, 4053–4060. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Tan, W.; Sun, Y.; Wang, H.; Ma, J.; Zhao, X. Exceptional high selectivity of hydrogen/methane separation on a phosphonate-based MOF membrane with exclusion of methane molecules. Chem. Commun. 2017, 53, 9797–9800. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Caro, J.; Guo, X.; Song, C.; Liu, Y. In-Plane Epitaxial Growth of Highly c-Oriented NH2-MIL-125(Ti) Membranes with Superior H2/CO2 Selectivity. Angew. Chem. Int. Ed. Engl. 2018, 57, 16088–16093. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Guo, X.; Hong, S.; Choi, J.; Liu, Y. Microstructural optimization of NH2-MIL-125 membranes with superior H2/CO2 separation performance by innovating metal sources and heating modes. J. Membr. Sci. 2020, 616, 118615. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Ji, T.; Yan, J.; Liu, Y. Complete twin suppression in oriented NH2-MIL-125 film via facile coordination modulation. Chem. Commun. 2022, 58, 8822–8825. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, S.; Yan, J.; Ji, T.; Liu, L.; Wu, M.; Guo, X.; Liu, Y. Oriented Ultrathin pi-complexation MOF Membrane for Ethylene/Ethane and Flue Gas Separations. Angew. Chem. Int. Ed. Engl. 2023, 62, e202311336. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Li, L.; Krishna, R.; Ji, T.; Chen, S.; Yan, J.; Liu, Y. Titanium-Oxo Cluster Assisted Fabrication of a Defect-Rich Ti-MOF Membrane Showing Versatile Gas-Separation Performance. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203663. [Google Scholar] [CrossRef]
- Wang, C.; Ji, T.; Wu, Y.; He, G.; Liu, Y. Fabrication of MIL-125 Membrane with Unprecedented CO2/N2 Selectivity Using Ti6-oxo Cluster Source. ACS Mater. Lett. 2023, 5, 1311–1316. [Google Scholar] [CrossRef]
- Han, S.; Mullins, C.B. Current Progress and Future Directions in Gas-Phase Metal-Organic Framework Thin-Film Growth. ChemSusChem 2020, 13, 5433–5442. [Google Scholar] [CrossRef] [PubMed]
- Cacho-Bailo, F.; Caro, G.; Etxeberria-Benavides, M.; Karvan, O.; Tellez, C.; Coronas, J. High selectivity ZIF-93 hollow fiber membranes for gas separation. Chem. Commun. 2015, 51, 11283–11285. [Google Scholar] [CrossRef]
- Demir, H.; Keskin, S. A New Era of Modeling MOF-Based Membranes: Cooperation of Theory and Data Science. Macromol. Mater. Eng. 2023, 309, 2300225. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Q.; Xu, X.; Li, H.; Cui, Z.; Chu, B.; Xie, N.; Wang, Z.; Bai, P.; Guo, X.; Lyu, J. Fabrication Methods of Continuous Pure Metal–Organic Framework Membranes and Films: A Review. Molecules 2024, 29, 3885. https://doi.org/10.3390/molecules29163885
Xing Q, Xu X, Li H, Cui Z, Chu B, Xie N, Wang Z, Bai P, Guo X, Lyu J. Fabrication Methods of Continuous Pure Metal–Organic Framework Membranes and Films: A Review. Molecules. 2024; 29(16):3885. https://doi.org/10.3390/molecules29163885
Chicago/Turabian StyleXing, Qinglei, Xiangyou Xu, Haoqian Li, Zheng Cui, Binrui Chu, Nihao Xie, Ziying Wang, Peng Bai, Xianghai Guo, and Jiafei Lyu. 2024. "Fabrication Methods of Continuous Pure Metal–Organic Framework Membranes and Films: A Review" Molecules 29, no. 16: 3885. https://doi.org/10.3390/molecules29163885
APA StyleXing, Q., Xu, X., Li, H., Cui, Z., Chu, B., Xie, N., Wang, Z., Bai, P., Guo, X., & Lyu, J. (2024). Fabrication Methods of Continuous Pure Metal–Organic Framework Membranes and Films: A Review. Molecules, 29(16), 3885. https://doi.org/10.3390/molecules29163885





