Penicillides from Penicillium and Talaromyces: Chemical Structures, Occurrence and Bioactivities
Abstract
:1. Introduction
2. Chemical Structures
3. Occurrence
4. Biological Properties
5. Biosynthesis of Penicillide and Purpactin A
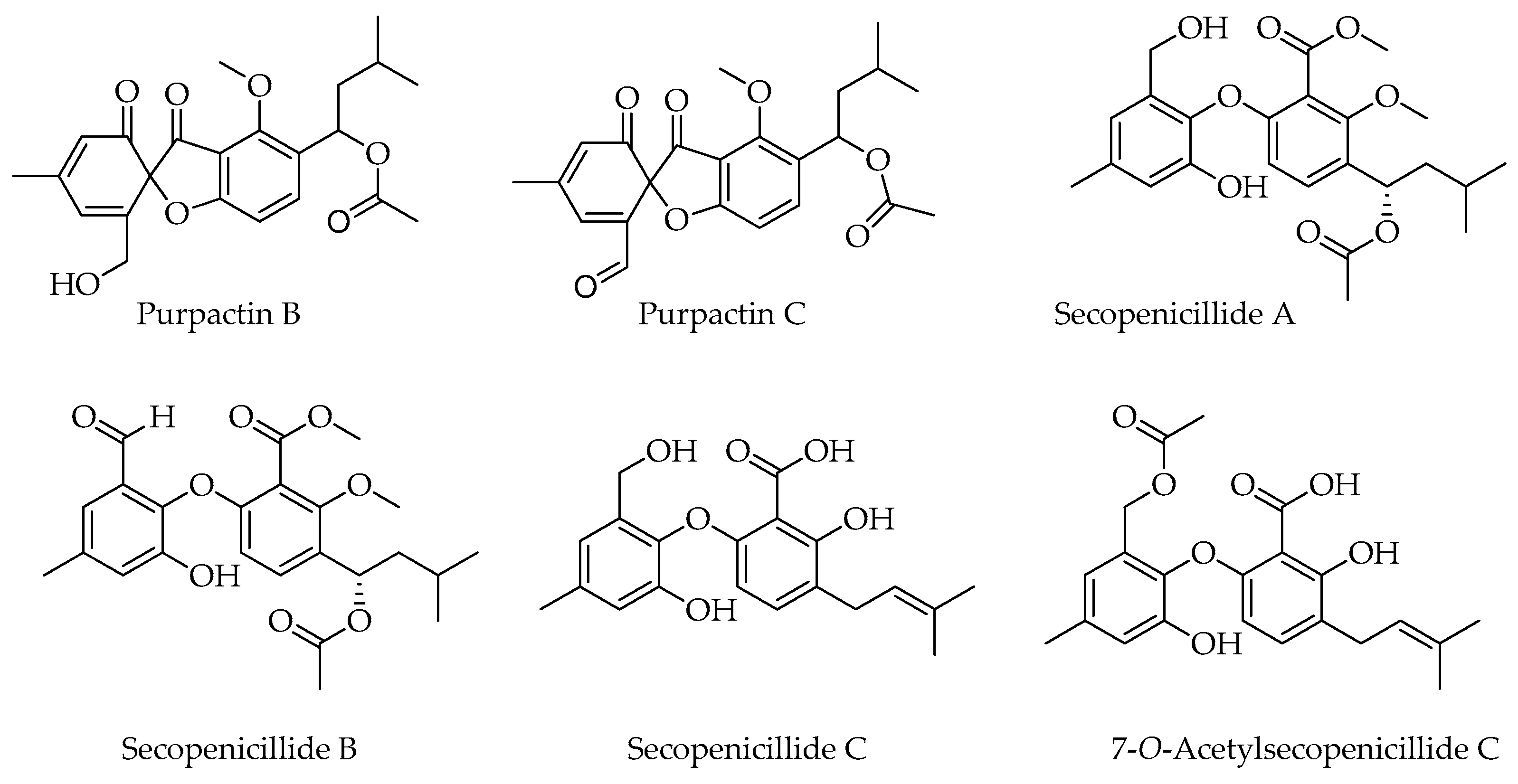
6. Related Products
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gosio, B. Richerche batteriologiche e chemiche sulle alterazoni del mais. Riv. Igiene Sanità Pub. 1896, 7, 825–849. [Google Scholar]
- Frisvad, J.C. Taxonomy, chemodiversity and chemoconsistency of Aspergillus, Penicillium and Talaromyces species. Front. Microbiol. 2015, 5, 773. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Crous, P.W.; Redhead, S.A.; Reynolds, D.R.; Samson, R.A.; Seifert, K.A.; Taylor, J.W.; Wingfield, M.J.; Abaci, Ö.; Aime, C.; et al. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2011, 2, 105–111. [Google Scholar] [CrossRef]
- Samson, R.A.; Yilmaz, N.; Houbraken, J.; Spierenburg, H.; Seifert, K.A.; Peterson, S.W.; Varga, J.; Frisvad, J.C. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2011, 70, 159–183. [Google Scholar] [CrossRef]
- Sassa, T. Structure of penicillide, a new metabolite produced by a Penicillium species. Tetrahedron Lett. 1974, 15, 3941–3942. [Google Scholar] [CrossRef]
- Tomoda, H.; Nishida, H.; Masuma, R.; Cao, J.; Okuda, S.; Omura, S. Purpactins, new inhibitors of acyl-CoA: Cholesterol acyltransferase produced by Penicillium purpurogenum I. Production, isolation and physico-chemical and biological properties. J. Antibiot. 1991, 44, 136–143. [Google Scholar] [CrossRef]
- Proksa, B.; Uhrínová, S.; Adamcová, J.; Fuska, J. Hydrogenation of vermistatin. Monatshefte Chem. Chem. Mon. 1992, 123, 251–256. [Google Scholar] [CrossRef]
- Lan, D.; Wu, B. Chemistry and bioactivities of secondary metabolites from the genus Talaromyces. Chem. Biodivers. 2020, 17, e2000229. [Google Scholar] [CrossRef]
- Lei, L.R.; Gong, L.Q.; Jin, M.Y.; Wang, R.; Liu, R.; Gao, J.; Liu, M.D.; Huang, L.; Wang, G.Z.; Wang, D.; et al. Research advances in the structures and biological activities of secondary metabolites from Talaromyces. Front. Microbiol. 2022, 13, 984801. [Google Scholar] [CrossRef]
- Nicoletti, R.; Salvatore, M.M.; Andolfi, A. Secondary metabolites of mangrove-associated strains of Talaromyces. Mar. Drugs 2018, 16, 12. [Google Scholar] [CrossRef]
- Nicoletti, R.; Andolfi, A.; Salvatore, M.M. Endophytic fungi of the genus Talaromyces and plant health. In Microbial Endophytes and Plant Growth; Accademic Press: London, UK, 2023; pp. 183–213. [Google Scholar]
- Nicoletti, R.; Manzo, E.; Ciavatta, M.L. Occurence and bioactivities of funicone-related compounds. Int. J. Mol. Sci. 2009, 10, 1430–1444. [Google Scholar] [CrossRef]
- Salvatore, M.M.; DellaGreca, M.; Andolfi, A. New insights into chemical and biological properties of funicone-like compounds. Toxins 2022, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Kaneko, T.; Koshino, H.; Esumi, Y.; Uzawa, J.; Sugawara, F. Penicillides from Penicillium sp. isolated from Taxus cuspidata. Nat. Prod. Lett. 2000, 14, 477–484. [Google Scholar] [CrossRef]
- Chung, M.C.; Lee, H.J.; Chun, H.K.; Kho, Y.H. Penicillide, a nonpeptide calpain inhibitor, produced by Penicillium sp. F60760. J. Microbiol. Biotechnol. 1998, 8, 188–190. [Google Scholar]
- Wang, C.; Gao, Y.K.; Lei, F.H.; Tan, X.C.; Shen, L.Q.; Gao, C.H.; Yi, X.X.; Li, X.Y. A new glycosyl ester isolated from marine-derived Penicillium sp. Chin. Tradit. Herb. Drugs 2019, 50, 2518–2523. [Google Scholar]
- Zhang, Y.; Li, X.M.; Shang, Z.; Li, C.S.; Ji, N.Y.; Wang, B.G. Meroterpenoid and diphenyl ether derivatives from Penicillium sp. MA-37, a fungus isolated from marine mangrove rhizospheric soil. J. Nat. Prod. 2012, 75, 1888–1895. [Google Scholar] [CrossRef]
- Jeon, H.; Shim, S.H. Chemical constituents of the endophyte Penicillium sp. isolated from Artemisia princeps. Chem. Nat. Compd. 2020, 56, 122–124. [Google Scholar] [CrossRef]
- Kong, F.D. Secondary metabolites from marine fungus Penicillium sp. SCS-KFD16. Chin. Tradit. Herb. Drugs 2018, 24, 5029–5033. [Google Scholar]
- Gao, H.; Zhou, L.; Li, D.; Gu, Q.; Zhu, T.J. New cytotoxic metabolites from the marine-derived fungus Penicillium sp. ZLN29. Helv. Chim. Acta 2013, 96, 514–519. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, B.W.; Yang, H.Y.; Jiang, F.F.; Ding, N.; Wu, Y.; Liu, X.; Tu, P.F.; Shi, S.P. Two new diphenyl ether derivatives from Penicillium chrysogenum MT-12, an endophytic fungus isolated from Huperzia serrata. Chin. Tradit. Herb. Drugs 2018, 49, 2496–2501. [Google Scholar]
- Kuroda, K.; Morishita, Y.; Saito, Y.; Ikuina, Y.; Ando, K.; Kawamoto, I.; Matsuda, Y. As-186 Compounds, new inhibitors of Acyl-Coa: Cholesterol acyltransferase from Penicillium asperosporum KYI635. J. Antibiot. 1994, 47, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Komai, S.I.; Hosoe, T.; Itabashi, T.; Nozawa, K.; Yaguchi, T.; Fukushima, K.; Kawai, K.I. New penicillide derivatives isolated from Penicillium simplicissimum. J. Nat. Med. 2006, 60, 185–190. [Google Scholar] [CrossRef]
- Hwang, H.; Kwon, C.; Kwon, J. Chemical constituents isolated from the Moss-derived fungus Talaromyces sp. J. Korean Magn. Reson. Soc. 2020, 24, 123–128. [Google Scholar]
- Song, F.; Dong, Y.; Wei, S.; Zhang, X.; Zhang, K.; Xu, X. New antibacterial secondary metabolites from a marine-derived Talaromyces sp. strain BTBU20213036. Antibiotics 2022, 11, 222. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.-M.; Yang, S.-Q.; Wang, B.-G.; Li, X. Antibacterial polyketides from the deep-sea cold-seep-derived fungus Talaromyces sp. CS-258. Mar. Drugs 2024, 22, 204. [Google Scholar] [CrossRef]
- Hui, Z.; Baocong, H.; Yaoyao, Z.; Jinqiu, M.; Xiahao, Z.; Chengyun, W. Secondary metabolites and antibacterial activity of the marine-derived fungus, Talaromyces sp. HK1-18. Chin. Mar. Med. 2022, 42, 67–72. [Google Scholar]
- Carrillo-Jaimes, K.; Fajardo-Hernández, C.A.; Hernández-Sedano, F.; Cano-Sánchez, P.; Morales-Jiménez, J.; Quiroz-García, B.; Rivera-Chávez, J. Antibacterial activity and AbFtsZ binding properties of fungal metabolites isolated from mexican mangroves. Rev. Bras. Farmacogn. 2024, 34, 564–576. [Google Scholar] [CrossRef]
- Wu, B.; Ohlendorf, B.; Oesker, V.; Wiese, J.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Acetylcholinesterase inhibitors from a marine fungus Talaromyces sp. strain LF458. Mar. Biotechnol. 2015, 17, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xia, J.; Chen, Z.; Lin, F.; Shao, Z.; Wang, W.; Hong, X. Cytotoxic and antibacterial meroterpenoids isolated from the marine-derived fungus Talaromyces sp. M27416. Mar. Drugs 2024, 22, 186. [Google Scholar] [CrossRef]
- Chen, M.; Han, L.; Shao, C.L.; She, Z.G.; Wang, C.Y. Bioactive diphenyl ether derivatives from a gorgonian-derived fungus Talaromyces sp. Chem. Biodivers. 2015, 12, 443–450. [Google Scholar] [CrossRef]
- Lv, H.; Su, H.; Xue, Y.; Jia, J.; Bi, H.; Wang, S.; Zhang, J.; Zhu, M.; Emam, M.; Wang, H.; et al. Polyketides with potential bioactivities from the mangrove-derived fungus Talaromyces sp. WHUF0362. Mar. Life Sci. Technol. 2023, 5, 232–241. [Google Scholar] [CrossRef]
- Yilmaz, N.; López-Quintero, C.A.; Vasco-Palacios, A.M.; Frisvad, J.C.; Theelen, B.; Boekhout, T.; Samson, R.A.; Houbraken, J. Four novel Talaromyces species isolated from leaf litter from Colombian Amazon rain forests. Mycol. Progr. 2016, 15, 1041–1056. [Google Scholar] [CrossRef]
- Daengrot, C.; Rukachaisirikul, V.; Tadpetch, K.; Phongpaichit, S.; Bowornwiriyapan, K.; Sakayaroj, J.; Shen, X. Penicillanthone and penicillidic acids A-C from the soil-derived fungus Penicillium aculeatum PSU-RSPG105. RSC Adv. 2016, 6, 39700–39709. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef]
- Yilmaz, N.; Houbraken, J.; Hoekstra, E.S.; Frisvad, J.C.; Visagie, C.M.; Samson, R.A. Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia 2012, 29, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Salim, R.G.; Fadel, M.; Youssef, Y.A.; Taie, H.A.A.; Abosereh, N.A.; El-Sayed, G.M.; Marzouk, M. A local Talaromyces atroroseus TRP-NRC isolate: Isolation, genetic improvement, and biotechnological approach combined with LC/HRESI-MS characterization, skin safety, and wool fabric dyeing ability of the produced red pigment mixture. J. Genet. Eng. Biotechnol. 2022, 20, 62. [Google Scholar] [CrossRef]
- Suzuki, K.; Nozawa, K.; Udagawa, S.; Nakajima, S.; Kawai, K. Penicillide and dehydroisopenicillide from Talaromyces derxii. Phytochemistry 1991, 30, 2096–2098. [Google Scholar] [CrossRef]
- Proksa, B.; Uhrin, D.; Adamcova, J.; Fuska, J. Vermixocins A and B, two novel metabolites from Penicillium vermiculatum. J. Antibiot. 1992, 45, 1268–1272. [Google Scholar] [CrossRef]
- Salituro, G.M.; Pettibone, D.J.; Clineschmidt, B.V.; Williamson, J.M.; Zink, D.L. Potent, non-peptidic oxytocin receptor antagonists from a natural source. Bioorgan. Med. Chem. Lett. 1993, 3, 337–340. [Google Scholar] [CrossRef]
- Cai, J.; Wang, L.; Zhang, Z. Study on the secondary metabolites and bioactivities of a medicinal mangrove endophytic fungus Talaromyces flavus TGGP34. Chin. J. Mar. Drugs 2021, 40, 37–43. [Google Scholar]
- Visagie, C.M.; Yilmaz, N.; Frisvad, J.C.; Houbraken, J.; Seifert, K.A.; Samson, R.A.; Jacobs, K. Five new Talaromyces species with ampulliform-like phialides and globose rough walled conidia resembling T. verruculosus. Mycoscience 2015, 56, 486–502. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Frisvad, J.C.; Kirk, P.M.; Lim, H.J.; Lee, H.B. Discovery and extrolite production of three new species of Talaromyces belonging to sections helici and purpurei from freshwater in Korea. J. Fungi 2021, 7, 722. [Google Scholar] [CrossRef]
- Zhai, M.M.; Niu, H.T.; Li, J.; Xiao, H.; Shi, Y.P.; Di, D.L.; Crews, P.; Wu, Q.X. Talaromycolides A-C, novel phenyl-substituted phthalides isolated from the green chinese onion-derived fungus Talaromyces pinophilus AF-02. J. Agric. Food Chem. 2015, 63, 9558–9564. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Abe, T.; Iwatsuki, M.; Mori, M.; Yamamoto, T.; Shiomi, K.; Ômura, S.; Masuma, R. Enhancement of metabolites productivity of Penicillium pinophilum FKI-5653, by co-culture with Trichoderma harzianum FKI-5655. J. Antibiot. 2011, 64, 769–774. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, Y.; Chen, R.; Liu, D.; Liu, M.; Proksch, P.; Guo, P.; Lin, W. Phenolic metabolites from mangrove-associated Penicillium pinophilum fungus with lipid-lowering effects. RSC Adv. 2016, 6, 21969–21978. [Google Scholar] [CrossRef]
- Salvatore, M.M.; DellaGreca, M.; Nicoletti, R.; Salvatore, F.; Vinale, F.; Naviglio, D.; Andolfi, A. Talarodiolide, a new 12-membered macrodiolide, and GC/MS investigation of culture filtrate and mycelial extracts of Talaromyces pinophilus. Molecules 2018, 23, 950. [Google Scholar] [CrossRef]
- He, F.; Li, X.; Yu, J.H.; Zhang, X.; Nong, X.; Chen, G.; Zhu, K.; Wang, Y.Y.; Bao, J.; Zhang, H. Secondary metabolites from the mangrove sediment-derived fungus Penicillium pinophilum SCAU037. Fitoterapia 2019, 136, 104177. [Google Scholar] [CrossRef]
- Zhao, D.L.; Shao, C.L.; Zhang, Q.; Wang, K.L.; Guan, F.F.; Shi, T.; Wang, C.Y. Azaphilone and diphenyl ether derivatives from a Gorgonian-derived strain of the fungus Penicillium pinophilum. J. Nat. Prod. 2015, 78, 2310–2314. [Google Scholar] [CrossRef]
- Pandit, S.G.; Puttananjaiah, M.H.; Serva Peddha, M.; Dhale, M.A. Safety efficacy and chemical profiling of water-soluble Talaromyces purpureogenus CFRM02 pigment. Food Chem. 2020, 310, 125869. [Google Scholar] [CrossRef]
- Shaaban, M.; El-Metwally, M.M.; Laatsch, H. New bioactive metabolites from Penicillium purpurogenum MM. Zeit. Naturforsch.—Sect. B J. Chem. Sci. 2016, 71, 287–295. [Google Scholar] [CrossRef]
- Cai, R.; Chen, S.; Long, Y.; Li, C.; Huang, X.; She, Z. Depsidones from Talaromyces stipitatus SK-4, an endophytic fungus of the mangrove plant Acanthus ilicifolius. Phytochem. Lett. 2017, 20, 196–199. [Google Scholar] [CrossRef]
- Ningsih, B.N.S.; Rukachaisirikul, V.; Phongpaichit, S.; Muanprasat, C.; Preedanon, S.; Sakayaroj, J.; Intayot, R.; Jungsuttiwong, S. Talarostatin, a vermistatin derivative from the soil-derived fungus Talaromyces thailandensis PSU-SPSF059. Nat. Prod. Res. 2024, 38, 2535–2542. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Q.H.; Jiang, T.; Cai, Y.W.; Huang, G.L.; Sun, X.P.; Zheng, C.J. Secondary metabolites from the mangrove-derived fungus Penicillium verruculosum and their bioactivities. Chem. Nat. Compd. 2021, 57, 588–591. [Google Scholar] [CrossRef]
- Yu, M.; Chen, X.; Jiang, M.; Li, X. Two marine natural products, penicillide and verrucarin J, are identified from a chemical genetic screen for neutral lipid accumulation effectors in Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2020, 104, 2731–2743. [Google Scholar] [CrossRef] [PubMed]
- Chaiyosang, B.; Kanokmedhakul, K.; Yodsing, N.; Boonlue, S.; Yang, J.X.; Wang, Y.A.; Andersen, R.J.; Yahuafai, J.; Kanokmedhakul, S. Three new indole diterpenoids from Aspergillus aculeatus KKU-CT2. Nat. Prod. Res. 2022, 36, 4973–4981. [Google Scholar] [CrossRef]
- de Sá, J.D.; Pereira, J.A.; Dethoup, T.; Cidade, H.; Sousa, M.E.; Rodrigues, I.C.; Costa, P.M.; Mistry, S.; Silva, A.M.; Kijjoa, A. Anthraquinones, diphenyl ethers, and their derivatives from the culture of the marine sponge-associated fungus Neosartorya spinosa KUFA 1047. Mar. Drugs 2021, 19, 457. [Google Scholar] [CrossRef]
- Song, F.; Lin, R.; Yang, N.; Jia, J.; Wei, S.; Han, J.; Li, J.; Bi, H.; Xu, X. Antibacterial secondary metabolites from marine-derived fungus Aspergillus sp. IMCASMF180035. Antibiotics 2021, 10, 377. [Google Scholar] [CrossRef]
- Tapfuma, K.I.; Uche-Okereafor, N.; Sebola, T.E.; Hussan, R.; Mekuto, L.; Makatini, M.M.; Green, E.; Mavumengwana, V. Cytotoxic activity of crude extracts from Datura stramonium’s fungal endophytes against A549 lung carcinoma and UMG87 glioblastoma cell lines and LC-QTOF-MS/MS based metabolite profiling. BMC Complement. Altern. Med. 2019, 19, 330. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.K.; Liu, F.; She, Z.G.; Yang, L.G.; Li, M.F.; Vrijmoed, L.L.P.; Lin, Y.C. 1H and 13C NMR assignments for 6-demethylvermistatin and two penicillide derivatives from the mangrove fungus Guignardia sp. (No. 4382) from the South China Sea. Magn. Reson. Chem. 2008, 46, 693–696. [Google Scholar] [CrossRef]
- Sy-Cordero, A.A.; Figueroa, M.; Raja, H.A.; Meza Aviña, M.E.; Croatt, M.P.; Adcock, A.F.; Kroll, D.J.; Wani, M.C.; Pearce, C.J.; Oberlies, N.H. Spiroscytalin, a new tetramic acid and other metabolites of mixed biogenesis from Scytalidium cuboideum. Tetrahedron 2015, 71, 8899–8904. [Google Scholar] [CrossRef]
- Primahana, G.; Narmani, A.; Surup, F.; Teponno, R.B.; Arzanlou, M.; Stadler, M. Five tetramic acid derivatives isolated from the iranian fungus Colpoma quercinum CCTU A372. Biomolecules 2021, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Arunpanichlert, J.; Rukachaisirikul, V.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. Meroterpenoid, isocoumarin, and phenol derivatives from the seagrass-derived fungus Pestalotiopsis sp. PSU-ES194. Tetrahedron 2015, 71, 882–888. [Google Scholar] [CrossRef]
- Xia, X.; Kim, S.; Liu, C.; Shim, S.H. Secondary metabolites produced by an endophytic fungus Pestalotiopsis sydowiana and their 20S proteasome inhibitory activities. Molecules 2016, 21, 944. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hu, J.Y.; Sun, H.D.; Zhao, X.; Zhong, W.T.; Duan, D.Z.; Wang, L.; Wang, X.L. Sinopestalotiollides A–D, cytotoxic diphenyl ether derivatives from plant endophytic fungus Pestalotiopsis palmarum. Bioorgan. Med. Chem. Lett. 2018, 28, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Guo, X.; Li, C.; Li, H.; Lou, H.; Ren, D. Chemical constituents from Phyllanthus emblica and the cytoprotective effects on H2O2-induced PC12 cell injuries. Arch. Pharm. Res. 2016, 39, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Sharma, P.; Singh, K.; Rana, D.; Kumar, V. Phyllanthus emblica seed extract as corrosion inhibitor for stainless steel used in petroleum industry (SS-410) in acidic medium. Chem. Phys. Impact 2021, 3, 100038. [Google Scholar] [CrossRef]
- Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues, and challenges. Microorganisms 2021, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Bae, H. Horizontal gene transfer and endophytes: An implication for the acquisition of novel traits. Plants 2020, 9, 305. [Google Scholar] [CrossRef]
- Bielecka, M.; Pencakowski, B.; Nicoletti, R. Using next-generation sequencing technology to explore genetic pathways in endophytic fungi in the syntheses of plant bioactive metabolites. Agriculture 2022, 12, 187. [Google Scholar] [CrossRef]
- Caradus, J.R.; Johnson, L.J. Epichloë fungal endophytes—From a biological curiosity in wild grasses to an essential component of resilient high performing ryegrass and fescue pastures. J. Fungi 2020, 6, 322. [Google Scholar] [CrossRef]
- Mamangkey, J.; Mendes, L.W.; Mustopa, A.Z.; Hartanto, A. Endophytic Aspergillii and Penicillii from medicinal plants: A focus on antimicrobial and multidrug resistant pathogens inhibitory activity. Biotechnologia 2024, 105, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Hazra, A.; Bhattacharya, E.; Bose, R.; Mandal Biswas, S. Characterization and metabolomic profiling of two pigment producing fungi from infected fruits of Indian gooseberry. Arch. Microbiol. 2023, 205, 141. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, P.; Wu, H.; Liu, M.; Liu, H.; Zeng, Q.; Wu, D.; Wang, X. Aqueous extract of Sargentodoxa cuneata alleviates ulcerative colitis and its associated liver injuries in mice through the modulation of intestinal flora and related metabolites. Front. Microbiol. 2024, 15, 1295822. [Google Scholar] [CrossRef]
- Nishida, H.; Tomoda, H.; Cao, J.; Araki, S.; Okuda, S.; Omura, S. Purpactins, new inhibitors of Acyl-Coa: Cholesterol acyltransferase produced by Penicillium purpurogenum III. Chemical modification of purpactin A. J. Antibiot. 1991, 44, 152–159. [Google Scholar] [CrossRef]
- Sturdikova, M.; Proksa, B.; Fuska, J.; Stancikova, M. Vermilutin, an elastase inhibitor produced by Penicillium vermiculatum. Biologia 1995, 50, 233–236. [Google Scholar]
- Yimnual, C.; Satitsri, S.; Ningsih, B.N.S.; Rukachaisirikul, V.; Muanprasat, C. A fungus-derived purpactin A as an inhibitor of TMEM16A chloride channels and mucin secretion in airway epithelial cells. Biomed. Pharmacother. 2021, 139, 111583. [Google Scholar] [CrossRef]
- Nishida, H.; Tomoda, H.; Okuda, S.; Omura, S. Biosynthesis of purpactin A. J. Org. Chem. 1992, 57, 1271–1274. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosyntheic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhang, Q.; Deng, C.; Fang, L.; Xu, W.; Zhao, Q.; Zhang, J.; Wang, Y.; Lei, X. Synthesis and evaluation of the analogues of penicillide against cholesterol ester transfer protein. Chin. J. Chem. 2013, 31, 355–370. [Google Scholar] [CrossRef]
- Tomoda, H.; Omura, S. Potential therapeutics for obesity and atherosclerosis: Inhibitors of neutral lipid metabolism from microorganisms. Pharmacol. Ther. 2007, 115, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Zeukang, R.D.; Siwe-Noundou, X.; Fotsing, M.T.; Kuiate, T.T.; Mbafor, J.T.; Krause, R.W.M.; Choudhary, M.I.; de Théodore Atchadé, A. Cordidepsine is a potential new anti-HIV depsidone from Cordia millenii, Baker. Molecules 2019, 24, 3202. [Google Scholar] [CrossRef]
- Khayat, M.T.; Ghazawi, K.F.; Samman, W.A.; Alhaddad, A.A.; Mohamed, G.A.; Ibrahim, S.R.M. Recent advances on natural depsidones: Sources, biosynthesis, structure-activity relationship, and bioactivities. PeerJ 2023, 11, e15394. [Google Scholar] [CrossRef] [PubMed]
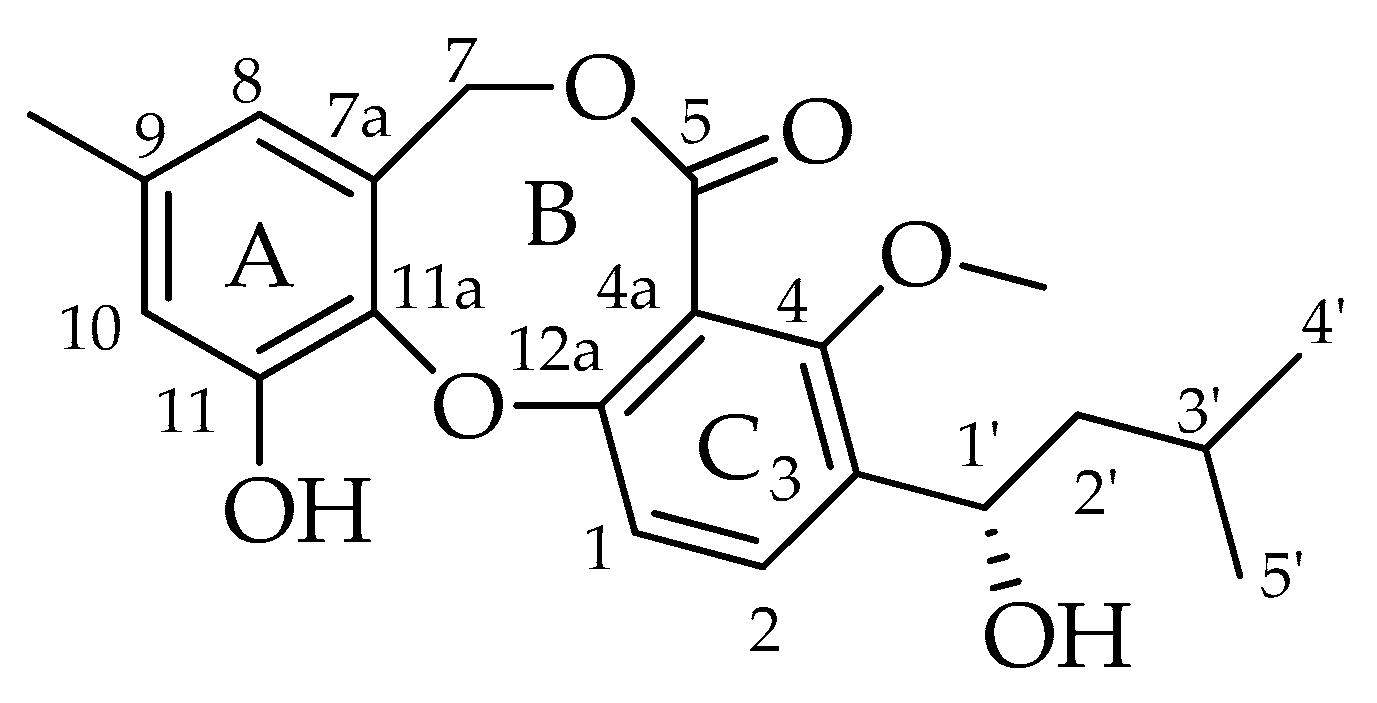

| Code | Compounds | Chemical Structure | Nominal Mass | Formula |
|---|---|---|---|---|
| 1 | Penicillide (= vermixocin A) |  | 372 | C21H24O6 |
| 2 | Purpactin A (= vermixocin B) |  | 414 | C23H26O7 |
| 3 | Δ1′,3′-1-Dehydroxypenicillide | 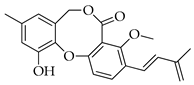 | 352 | C21H20O5 |
| 4 | 1′2′-Epoxy-3′,4′-didehydropenicillide |  | 368 | C21H20O6 |
| 5 | Dehydroisopenicillide (= MC-141, 1,2-dehydropenicillide) | 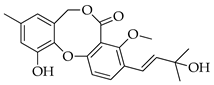 | 370 | C21H22O6 |
| 6 | 2′-Hydroxy-3′,4′-didehydropenicillide |  | 386 | C21H22O7 |
| 7 | 5′-Hydroxypenicillide |  | 388 | C21H24O7 |
| 8 | Hydroxypenicillide | 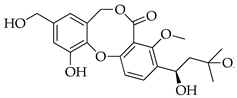 | 404 | C21H24O8 |
| 9 | Isopenicillide |  | 388 | C21H24O7 |
| 10 | 3′-O-Methyldehydroisopenicillide (= 3-methoxy-1′2-dehydropenicillide) | 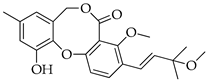 | 384 | C22H24O6 |
| 11 | Prenpenicillide | 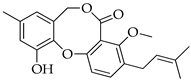 | 354 | C21H22O5 |
| Species | Substrate | Location | Compounds | Ref. |
|---|---|---|---|---|
| Penicillium sp. | - | Japan | 1 | [5] |
| Penicillium sp. | endophytic in Taxus cuspidata | Gunma (Japan) | 4, 6, 5, 10 | [14] |
| Penicillium sp. F60760 | soil | South Korea | 1 | [15] |
| Penicillium sp. H1 | marine | China | 1 | [16] |
| Penicillium sp. MA-37 1 | rhizosphere of Bruguiera gymnorrhiza | Hainan (China) | 1, 3, 5, 10 | [17] |
| Penicillium sp. PF9 | endophytic in Artemisia princeps | South Korea | 1, 10 | [18] |
| Penicillium sp. SCS-KFD16 | marine | China | 1 | [19] |
| Penicillium sp. ZLN29 | marine sediment | Jiazhou Bay (China) | 1, 11 | [20] |
| P. chrysogenum MT-12 | endophytic in Huperzia serrata | China | 1, 2, 8, 9 | [21] |
| P. montanense KYI635 2 | soil | Kanagawa (Japan) | 1, 2 | [22] |
| P. simplicissimum IFM53375 | unspecified | Japan | 1, 2 | [23] |
| Talaromyces sp. | moss (Climacium dendroides) | Gangneung (South Korea) | 1, 2 | [24] |
| Talaromyces sp. BTBU20213036 | mud from intertidal zone | Qingdao (China) | 1 | [25] |
| Talaromyces sp. CS-258 | mussel from cold seep | South China Sea | 1, 2, 5 | [26] |
| Talaromyces sp. HK1-18 | mangrove soil | Hainan (China) | 1 | [27] |
| Talaromyces sp. IQ-567 | endophytic in Rhizophora mangle | Tecomate Lagoon (Mexico) | 1 | [28] |
| Talaromyces sp. LF458 | sponge (Axinella verrucosa) | Elba island (Italy) | 3, 10 | [29] |
| Talaromyces sp. M27416 | sea water | Dongshan island (China) | 1 | [30] |
| Talaromyces sp. Wangcy005 | gorgonian (Subergorgia suberosa) | Weizhou island (China) | 1, 2, 3 | [31] |
| Talaromyces sp. WHUF0362 | mangrove soil | Hainan (China) | 2 | [32] |
| T. aculeatus IBT23209 | soil | Araracuara (Colombia) | 1, 2 | [33] |
| T. aculeatus IBT23210 | 2 | |||
| T. aculeatus IBT23211 | 2 | |||
| T. aculeatus IBT23212 | 2 | |||
| T. aculeatus PSU-RSPG105 | soil | Rajjaprabha Dam (Thailand) | 1, 2 | [34] |
| T. albobiverticillius IBT4466 | imported pomegranate | Denmark | 2 | [35] |
| T. albobiverticillius CBS113167 | air in cake factory | - | 2 | |
| T. amestolkiae CBS433.62 | ground domestic waste | Verona (Italy) | 2 | [36] |
| T. amestolkiae CBS436.62 | alum solution | - | 2 | |
| T. atroroseus IBT3933 | - | - | 2 | [35] |
| T. atroroseus TRP-NRC | agricultural waste | Egypt | 2 | [37] |
| T. derxii NHL2981 | soil | Kurashiki (Japan) | 1, 5 | [38] |
| T. flavus CCMF-276 | soil | Jàchymov (Czechia) | 1, 2 | [39] |
| T. flavus ATCC74110 | - | - | 1 | [40] |
| T. flavus TGGP34 | endophytic in Acanthus ilicifolius | China | 1, 2, 5 | [41] |
| T. fuscoviridis CBS193.69 | soil | the Netherlands | 2 | [42] |
| T. gwangjuensis CNUFC-WT19-1 | freshwater | Yeosu (South Korea) | 2 | [43] |
| T. koreana CNUFC-YJW2-13 | freshwater | Yeosu (South Korea) | 2 | |
| T. pinophilus AF-02 | endophytic in Allium fistulosum | Lanzhou (China) | 1 | [44] |
| T. pinophilus FKI-5653 | soil | Hachijo island (Japan) | 1, 5 | [45] |
| T. pinophilus H608 | mangrove sediment | Xiamen (China) | 1, 2, 3, 5, 7, 9 | [46] |
| T. pinophilus LT6 | tobacco rhizosphere | Lecce area (Italy) | 1 | [47] |
| T. pinophilus SCAU037 | rhizosphere of Rhizophora stylosa | Techeng isle (China) | 1, 9, 10 | [48] |
| T. pinophilus XS-20090E18 | unidentified gorgonian | Xisha islands (China) | 1, 2, 8, 9 | [49] |
| T. purgamentorum CBS113145 | forest leaf litter | Peña Roja (Colombia) | 1, 2 | [33] |
| T. purpureogenus CFRM02 | unspecified | Karnataka (India) | 1 | [50] |
| T. purpureogenus FO-608 | soil | Japan | 2 | [6] |
| T. purpureogenus ATCC44445 | corn kernel | Georgia (USA) | 2 | [36] |
| T. purpureogenus ATCC20204 | - | Japan | 2 | |
| T. purpureogenus CBS286.36 | - | Japan | 2 | |
| T. purpureogenus IBT17540 | barley | Winnipeg (Canada) | 2 | |
| T. purpureogenus IBT11632 | imported marjoram | Denmark | 2 | |
| T. purpureogenus IBT12779 | marine | France | 2 | |
| T. purpureogenus IMI112715 | rhizosphere of Trifolium alexandrinum | Egypt | 2 | |
| T. purpureogenus IMI136126 | molded corn | Wisconsin (USA) | 2 | |
| T. purpureogenus IMI136127 | molded corn | Wisconsin (USA) | 2 | |
| T. purpureogenus NRRL3290 | - | Georgia (USA) | 2 | |
| T. purpureogenus MM | cotton textile | Egypt | 1, 10 | [51] |
| T. ruber CBS237.93 | unknown | Unknown | 2 | [36] |
| T. ruber CBS113160 | - | - | 2 | |
| T. ruber CBS132699 | sandy soil | Sousse (Tunisia) | 2 | |
| T. ruber FRR1503 | preserved wood | Australia | 2 | |
| T. ruber IBT31167 | - | - | 2 | |
| T. stellenboschiensis CBS135665 | soil | Stellenbosch (South Africa) | 2 | [42] |
| T. stipitatus SK-4 | leaf of Acanthus ilicifolius | Guangxi (China) | 1, 2 | [52] |
| T. thailandensis PSU-SPSF059 | soil | Thailand | 2 | [53] |
| T. veerkampii CBS500.78 | soil | Meta (Colombia) | 1, 2 | [42] |
| T. veerkampii CBS136668 | soybean seed | Matou (Taiwan) | 1, 2 | [42] |
| T. verruculosus TGM14 | mangrove (Xylocarpus granatum) | Hainan (China) | 1 | [54] |
| Biological Activity | Concentration | Results and Further Details | Ref. |
|---|---|---|---|
| Penicillide (1) | |||
| Antibacterial | 100 µg mL−1 | Acinetobacter baumannii (40% inhibition) | [28] |
| 50 µg mL−1 | Clostridium perfringens (MIC) | [44] | |
| 64 µg mL−1 | Escherichia coli (MIC) | [26] | |
| 64 µg mL−1 | Klebsiella pneumoniae (MIC) | [26] | |
| 50 µg mL−1 | Micrococcus tetragenus (MIC) | [44] | |
| 32 µg mL−1 | Pseudomonas aeruginosa (MIC) | [26] | |
| 100 µg mL−1 | Staphylococcus aureus (MIC) | [25] | |
| 0.78 µg mL−1 | MRSA S. aureus (MIC) | [27] | |
| 64 µg mL−1 | Vibrio alginolyticus (MIC) | [26] | |
| 32 µg mL−1 | Vibrio parahaemolyticus (MIC) | [26] | |
| Antifouling | 2.6 µg mL−1 | Balanus amphitrite (EC50) | [49] |
| Antifungal | 128 µg mL−1 | Cryptococcus neoformans (MIC) | [34] |
| Anti-inflammatory | 11.5 µM | RAW264.7 (IC50) | [18] |
| Antimalarial | 16.41 µM | Plasmodium falciparum (IC50) | [34] |
| Brine shrimp lethal | 158.5 µM | LD50 | [17] |
| Cholesterol acyltransferase inhibition | 22.9 µM | rabbit liver microsomes (IC50) | [22] |
| Cytotoxic | 50 µg mL−1 | P388 | [39] |
| 9.7 µM | Hep G2 (IC50) | [20] | |
| 6.7 µM | HEp-2 (IC50) | [49] | |
| 43.77 µM | KB (IC50) | [34] | |
| 7.8 µM | RD (IC50) | [49] | |
| 53.73 µM | Vero (IC50) | [34] | |
| 50 µg mL−1 | incorporation of uridine, thymidine and valine in P388 | [39] | |
| m-Calpain inhibition | 7.1 µM | SLLVY-AMC (IC50) | [15] |
| Oxytocin binding inhibition | 67 µM | IC50 | [40] |
| α-Glucosidase inhibition | 78.4 µM | IC50 | [48] |
| Purpactin A (2) | |||
| Antibacterial | 8 µg mL−1 | Aeromonas hydrophila (MIC) | [26] |
| 4 µg mL−1 | E. coli (MIC) | ||
| 2.42 µmol L−1 | Helicobacter pylori 129 (MIC) | [32] | |
| 4.83 µmol L−1 | H. pylori G27 (MIC) | ||
| 64 µg mL−1 | K. pneumoniae (MIC) | [26] | |
| 64 µg mL−1 | MRSA S. aureus (MIC) | ||
| 8 µg mL−1 | Micrococcus luteus (MIC) | ||
| 32 µg mL−1 | P. aeruginosa (MIC) | ||
| 16 µg mL−1 | Vibrio anguillarum (MIC) | ||
| 8 µg mL−1 | Vibrio harveyi (MIC) | ||
| 32 µg mL−1 | V. parahaemolyticus (MIC) | ||
| Antifouling | 4.8 µg mL−1 | B. amphitrite (IC50) | [31] |
| 10 µg mL−1 | B. amphitrite (EC50) | [49] | |
| Antimalarial | 5.69 µM | P. falciparum (IC50) | [34] |
| Cholesterol acyltransferase inhibition | 120 µM | rat microsomes (IC50) | [6,75] |
| 1.2 µM | J774 (IC50) | ||
| 8.2 µM | rabbit liver microsomes (IC50) | [22] | |
| Cytotoxic | 15.1 µM | HCT-116 (IC50) | [31] |
| 38.95 µM | Hep G2 (IC50) | [29] | |
| 50 µg mL−1 | incorporation of uridine, thymidine and valine in P388 | [39] | |
| 9.7 µM | J774 (IC50) | [6,75] | |
| 52.5 µM | KB (IC50) | [34] | |
| 16.4 µM | MCF-7 (IC50) | [31] | |
| 75.28 µM | MCF-7 (IC50) | [34] | |
| 41.21 µM | NIH 3 T3 (IC50) | [29] | |
| 32.57 µM | Vero (IC50) | [34] | |
| Elastase inhibition | 37.2 µg mL−1 | IC50 | [76] |
| α-Glucosidase inhibition | 80.9 µM | IC50 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, M.M.; Nicoletti, R.; Fiorito, F.; Andolfi, A. Penicillides from Penicillium and Talaromyces: Chemical Structures, Occurrence and Bioactivities. Molecules 2024, 29, 3888. https://doi.org/10.3390/molecules29163888
Salvatore MM, Nicoletti R, Fiorito F, Andolfi A. Penicillides from Penicillium and Talaromyces: Chemical Structures, Occurrence and Bioactivities. Molecules. 2024; 29(16):3888. https://doi.org/10.3390/molecules29163888
Chicago/Turabian StyleSalvatore, Maria Michela, Rosario Nicoletti, Filomena Fiorito, and Anna Andolfi. 2024. "Penicillides from Penicillium and Talaromyces: Chemical Structures, Occurrence and Bioactivities" Molecules 29, no. 16: 3888. https://doi.org/10.3390/molecules29163888
APA StyleSalvatore, M. M., Nicoletti, R., Fiorito, F., & Andolfi, A. (2024). Penicillides from Penicillium and Talaromyces: Chemical Structures, Occurrence and Bioactivities. Molecules, 29(16), 3888. https://doi.org/10.3390/molecules29163888










